Abstract
Functional magnetic resonance imaging (fMRI) technology has not been used to investigate the frontal lobe function of subcortical ischemic vascular cognitive impairment (SIVCI). In this study 11 healthy elderly controls, 12 patients with subcortical ischemic vascular cognitive impairment no dementia (SIVCIND), and 12 patients with subcortical ischemic vascular dementia (SIVD) underwent fMRI examination on a SIEMENS Trio 3.0 Tesla scanner during Stroop task performance. Compared to the controls, participants with SIVCIND showed markedly increased activation in prefrontal cortex. By contrast, participants with SIVD exhibited decreased fMRI responses in the regions described above. A close correlation was found between the cognitive score in Montreal Cognitive Assessment test and the activation area of frontal and parietal lobule of patients with SIVD. Our results suggest that the alterations of cortical activation in SIVCI were bidirectional. There is a prefrontal dysfunction in SIVD and a compensation in SIVCIND. These findings might help guide a clinical differentiation among normal controls, SIVCIND, and SIVD.
Keywords: SIVCIND, SIVD, stroop task, fMRI
Introduction
Dementia is a syndrome with a number of symptoms, including loss of memory, judgment, reasoning, and changes in mood, behaviors, and communication abilities. 1 Vascular dementia is the second most common type of dementia after Alzheimer’s disease (AD), accounting for up to one-third of all dementias. It is thought to be due to impaired blood supply to the brain, and it is divided into different subtypes depending on the nature of the vascular diseases. 2 Subcortical Ischemic vascular dementia (SIVD) is one of the subtypes and affects the inner parts of the brain consisting of the basal ganglia, thalamus, and white matter, which is made up of nerve cell fibers. Subcortical ischemic vascular cognitive impairment no dementia (SIVCIND) is an early stage of SIVD. 3
The recent development of functional magnetic resonance imaging (fMRI) allows researchers to detect neural activity in real time and provides valuable insight into the causes of dementia. 4 Previously, severe cortex function lesions were found in the hippocampal, parahippocampal regions, and medial temporal lobe when patients with AD perform working memory and visual spatial tasks. 5 -7 Attention and executive deficits are prominent in the patients with SIVD. The main clinical manifestation of SIVD is a “dysexecutive syndrome” resulting from the interruption of prefrontal–subcortical loops. Patients with SIVD have worse cognitive impairment in executive and attention functions than those with other causes of dementia. 8 -10 Nevertheless, to date, no fMRI studies have been performed to investigate the prefrontal cortical functions in patients with SIVCIND and SIVD. We supposed that patients with SIVCIND and SIVD have special and different blood–oxygen-level dependence (BOLD) response in the brain regions responsible for attention and executive function.
Stroop task is a typical paradigm in cognitive neuroscience to detect attention and executive processes. 11 -15 Successful performance requires concentration to process relevant tasks and inhibition of irrelevant tasks. This task has been used as a classic measure of frontal lobe function. In this study, we observed and analyzed the activation of the cortex evoked by the Stroop task in patients with SIVCIND and SIVD using BOLD fMRI technology to help the clinical differentiation among normal controls, patient with SIVCIND, and SIVD.
Materials and Methods
Participants
Totally, 12 patients with SIVD and 12 with SIVCIND were recruited from the Department of Neurology in Xinqiao Hospital and Southwest Hospital of Third Military Medical University (TMMU) from December 2007 to December 2010 (Table 1). National Institute of Neurological and Communicative Disorders and Stroke Data Bank criteria were used by clinicians to identify ischemic stroke of candidates for this study. Clinical diagnosis of SIVD was made by a senior neurologist according to the criteria made by Erikinjuntti et al 16 and by Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition [DSM-IV]) at the time of recruitment. All patients with SIVD completed a formal neuropsychological assessment administered by a trained neuropsychological technician under the supervision of a neuropsychologist. Examination included the following tests: Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR), Neuropsychiatric Inventory, Montreal Cognitive Assessment (MoCA), Activities of Daily Living Scale, Hachinski and Hamilton test, Wechsler Memory Scale, Verbal and categorical fluency, Figural Recognition Test, and the Informant Questionnaire on Cognitive Decline in the Elderly. The MoCA was a rapid screening instrument and were used to assess different cognitive domains including attention and concentration, executive functions, memory, language, visual–constructional skills, conceptual thinking, calculations, and orientation. Participants were excluded from the study if their MMSE score was less than 12, their CDR score was greater than 2.0, or if they had a history of brain injury or alcoholism. Additionally, all participants with SIVD were required to complete an unstructured neurologic interview to determine a history of onset, symptoms, and recovery from stroke and a history of memory loss and other symptoms of dementia. The clinical diagnosis of SIVCIND was made based on the criteria set by Rockwood at the time of recruitment. 17 Disease severity was assessed using the MoCA score, which were from 20 to 25. Exclusion criteria of SIVD and SIVCIND included patients with aphasia or the patients who otherwise could not complete psychological tests because of language disorder, diagnosis with a chronic or degenerative diseases, active substance abuse disorders, intracranial hemorrhage, difficulty in controlling epilepsy that could present with cognitive impairment, or temporal lobe epilepsy. However, past or present medication for the treatment of cognitive impairment and infarction was not an exclusion criterion.
Table 1.
Characteristics of Study Participantsa
| Groups | SIVD | SIVCIND | Controls | χ 2 /F | P Value |
|---|---|---|---|---|---|
| N | 12 | 12 | 11 | ||
| Age | 65.5 (5.7) | 66.2 (4.9) | 64.9 (4.2) | 1.56 | >.05 |
| Female/male | 6/6 | 6/6 | 6/5 | 1.72 | >.05 |
| Education, years | 7.6 (3.2) | 6.9 (3.6) | 6.8 (4.2) | 2.82 | >.05 |
| MMSE | 16.5 (4.7) | 26.6 (1.7) | 28.0 (2.2) | 44.36 | <.01 |
| NPI | 26.54 (11.9) | 6.5 (3.3) | |||
| ADL | 39.5 (8.4) | 24.5 (5.2) | |||
| Hachinski | 12.3 (4.0) | 8.8 (1.3) | |||
| Hamilton | 14.2 (5.6) | 9.6 (2.5) | |||
| MoCA | 14.6 (3.8) | 22.3 (1.5) |
Abbreviations: MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; ADL, Activities of Daily Living scale; MoCA, Montreal Cognitive Assessment; SIVCIND, subcortical ischemic vascular cognitive impairment no dementia; SIVD, subcortical ischemic vascular dementia.
a Data were expressed as mean ± SD.
Eleven healthy, normally aged volunteers were recruited as controls. They had no cognitive complaints and did not have neurological or psychiatric disorders. At the time of inclusion, all normal participants were free of medication that could noticeably affect brain function, and none of them had received an acetylcholinesterase inhibitor. All the participants with SIVD, SIVCIND, and control groups were right-handed. Each participant gave written informed consent to participate in the study. The study was performed under the guidance and approval of the institutional review board at Southwest Hospital in China.
Functional MRI Studies
The MR imaging for all participants was performed on a 3.0-T whole body system (Magnetom Trio, Siemens Healthcare, Erlangen, Germany). Images were obtained with a T2-sensitive gradient-recalled, single-shot, echo-planar pulse sequence in Southwest Hospital, TMMU. The head coil was fitted with a bite bar to minimize motion during the sessions. Twenty T1-weighted axial slices (echo time [ET] TE = 2.78 milliseconds, TR = 250 milliseconds, field of view = 230 × 230 mm, and 291 × 448 data matrix) were obtained parallel to the anterior commissure and commissural posterior line, which was identified with the aid of a sagittal localizer anatomical image. 18 Functional images were obtained by a single-shot, echo-planar gradient-echo sequence (64 × 64 matrix, 192 × 192 mm field of view, TE 30 milliseconds, volume repetition time (TR) 3000 milliseconds, and flip angle 90°]. The voxel size was 3 × 3 × 5 mm. In addition, high-resolution structural imaging of the whole brain was performed with a T1-weighted sequence. The parameters were as follows 256 × 256 matrix, TE 2.52 milliseconds, TR 1900 milliseconds, and flip angle 9° were acquired for each participant for the purpose of landmark selection and 3-dimensional (3D) reconstruction.
The stimuli of the Stroop task was designed and administered on a computer with the software E-Prime (Psychology Software Tools, Inc, 2002, Pittsburgh, PA). It was presented on a projector screen, which was mounted onto the patient’s bed in the MR scanner. An angled mirror, positioned above the participants’ eyes, provided a full view of the screen. 19,20 Participants were presented with a list of words “red,” “green,” and “blue,” however, the ink colors of the words were discordant with the colors of the presented words (eg, the word “red” printed in blue ink). Participants pressed 1 of the 3 buttons to indicate whether the ink color of the word was blue, yellow, or green regardless of the meaning of the word. Each stimulus was presented for 1250 milliseconds, followed by an interstimulus interval lasting 250 milliseconds. Generally, 2 to 3 runs of 60 stimuli were presented. The color names or presented colors were repeated randomly. Reaction time was recorded from stimulus onset. Trials in which the participants did not respond within 1250 milliseconds were counted as leaking errors. The control task consisted of a solid white crosscentered on a black background, during which the participants keep static in the magnet. The time of the resting and stimulation state presentations within each block was 30 seconds, which included 10 TR. Prior to the scanning, all participants were fitted with MRI-compatible corrective lenses to correct for refractive errors. All participants were trained to practice the task for 1 or 2 runs on the same device as in the magnet. 21
Image Processing and Analysis
Image processing and analyses were performed with Analysis of Functional Neuroimages software. 22 All data were motion corrected for 3 translational directions and 3 possible rotations. 23 Runs with head motion in excess of 1.5 mm displacement and 1° rotation were rejected. The first 6 volumes of each run were discarded to allow the MR signal to reach steady state. Echo planar images were coregistered to the image that minimized image translation and rotation relative to all other images and were intensity normalized, convolved with a 3D Gaussian kernel (FWHM = 8 mm, kernel width = 5).
The block design time series was convolved to represent the target hemodynamic response function. For each voxel, a correlation coefficient was calculated to indicate the strength of correlation between the participants BOLD signal and target reference function. The correlation coefficient for each participant was then compared with a value of 0 at each pixel using an unpaired t statistic. These t maps were analyzed using a threshold with a P value <.01 and a cluster filter of 9 adjacent pixels. The functional and anatomical images for each participant were transformed into the Talairach and Tournoux coordinate system, 24 and group composite t maps for fMRI signal change associated with the Stroop task were obtained. The activation of the whole brain of 3 groups were analyzed and compared. The activation area of all regions and MoCA score of patients with SIVD were correlated using SPSS statistical software (version 13.0, SPSS Inc, Chicago, Illinois). P values of .05 were considered statistically significant.
Results
When performing the Stroop color–word task, the patients with SIVD had a higher leaking response percentage (7.33 ± 3.45%) and longer reaction time (714.63 ± 145.61 milliseconds) than those with SIVCIND (5.41 ± 1.26%, 627.18 ± 139.55 milliseconds, P < .05), which were also higher and longer than that of the control groups (3.68 ± 1.51%, 531.46 ± 98.76 milliseconds, P < .05). The patients with SIVD had a higher error response percentage (5.24± 2.17%) than those with SIVCIND (3.37± 1.35%, P < .05), which was not different from normal controls (3.47 ±1.93%, P > .05; Table 2).
Table 2.
Comparison of Reaction Time, Leaking, and Error Response Percentage of SIVD, SIVCIND, and Normal Control Participants
| Groups | Reaction Time, ms | Leaking Response, % | Error Response, % |
|---|---|---|---|
| Normal controls | 531.46 ± 98.76 | 3.68 ± 1.51 | 3.37 ± 1.35 |
| SIVCIND group | 627.18 ± 139.55a | 5.41 ± 1.26a | 3.437 ± 1.93 |
| t = 5.882 | t = 10.02 | t = 0.975 | |
| SIVD group | 714.63 ± 145.61a | 7.33 ± 3.45a | 5.24 ± 2.17a |
| t = 6.012 | t = 18.43 | t = 11.036 |
Abbreviations: SIVCID, subcortical ischemic vascular cognitive impairment no dementia; SIVD, subcortical ischemic vascular dementia.
a P < .05 as compared with the normal control participants.
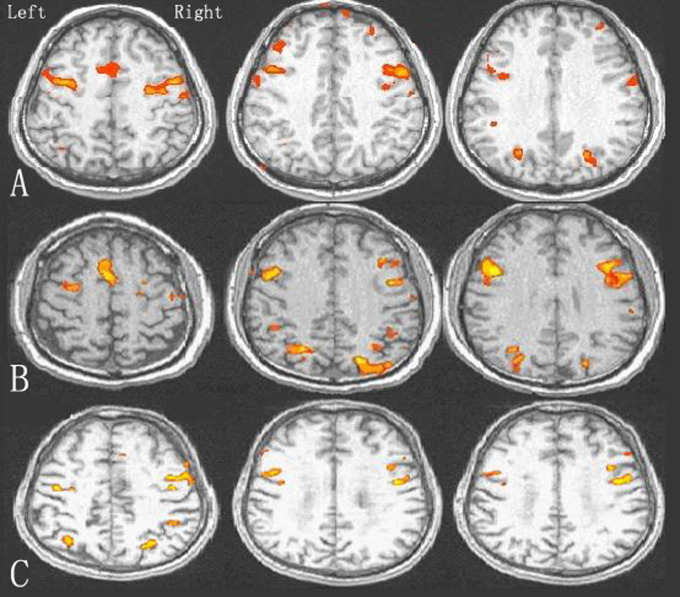
Following the Stroop color–word stimulation, the participants in the 3 groups produced different prefrontal activations. Activated brain regions in the control and participants with SIVCIND included the dorsal anterior cingulate, bilateral middle and inferior frontal gyri, inferior parietal lobule, insular and basal ganglia, which indicated a brain network of attention and execution. By contrast, most of the activation regions in patients with SIVD consisted of the bilateral middle, inferior frontal gyri, insular and inferior parietal lobule. Compared to controls, increased cortical activation was seen bilaterally in all the brain regions activated except the basal ganglia in patients with SIVCIND (t = 12.25−33.24, P < .05). Decreased cortical activation was seen in all the brain regions in patients with SIVD, and the reduction reached statistical significance (t = 6.35−35.65, P < .05; Figure 1, Table 3). Thus, patients with SIVD, SIVCIND, and the controls were distinguished by differences in brain activation.
Figure 1.
Differences in prefrontal cortical activation of SIVCIND, SIVD, and controls during performance of Stroop color–word task. A, Control participants B, Patients with SIVCIND. C, Patients with SIVD. SIVCIND indicates subcortical ischemic vascular cognitive impairment no dementia; SIVD, subcortical ischemic vascular dementia.
Table 3.
Activation Area of Different Brain Regions in the Patients With SIVD, SIVCIND, and the Control Participants During Performance of Incongruent Stroop Color-Word Task (mm3)
| Brain Region | Control | SIVCIND | SIVD | |||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| Inferior parietal lobule | 1027 ± 155 | 916 ± 171 | 3211 ± 458a | 2399 ± 398a | 868 ± 151a | 690 ± 124a |
| t = 30.68 | t = 27.65 | t = 6.35 | t = 8.17 | |||
| Anterior cingulate | 2398 ± 87 | 3454 ± 326a | - | |||
| t = 33.24 | ||||||
| Middle frontal gyri | 3854 ± 511 | 3542 ± 472 | 4868 ± 536a | 4375 ± 388a | 1366 ± 183a | 1011 ± 109a |
| t = 16.35 | t = 18.21 | t = 28.69 | t = 35.65 | |||
| Inferior frontal gyri | 1216 ± 197 | 1186 ± 202 | 1533 ± 231a | 1365 ± 148a | 667 ± 83a | 732 ± 75a |
| t = 13.32 | t = 12.25 | t = 20.31 | t = 22.64 | |||
| Insula | 563 ± 86 | 311 ± 64 | 722 ± 91a | 432 ± 74a | 272 ± 47a | 174 ± 32a |
| t = 16.32 | t = 14.55 | t = 19.63 | t = 21.17 | |||
Abbreviations: SIVCID, subcortical ischemic vascular cognitive impairment no dementia; SIVD, subcortical ischemic vascular dementia.
a P < .05 as compared with the control participants.
A close correlation was found between cognitive score in MoCA test and activation area in the bilateral middle, inferior frontal gyri, insular and inferior parietal lobules in patients with SIVD (Table 4; R = 0.366−0.565, P < .05).
Table 4.
Correlation Between Cortical Activation and Cognitive Score (MoCA) in Patients With SIVD
| Brain Activation Versus Cognitive Score | Left Hemisphere R/P Valuea | Right Hemisphere R/P Valuea |
|---|---|---|
| Inferior parietal lobule | 0.492/.001 | 0.433/.003 |
| Middle frontal gyri | 0.483/.001 | 0.565/0 |
| Inferior frontal gyri | 0.375/.011 | 0.366/.013 |
| Insula | 0.503/.0005 | 0.452/.012 |
Abbreviation: SIVD, subcortical ischemic vascular dementia.
a P < .05.
Discussion
In the present study, the functions of the prefrontal cortex associated with attention and execution were compared among the patients with SIVCIND, SIVD, and normal controls. The brain activities in the prefrontal cortex were largely decreased in patients with SIVD compared with those of the controls in the dorsal anterior cingulate, bilateral middle and inferior frontal gyri, inferior parietal lobules, and insular. 19,20 A close correlation was found between cortical activation of areas above and cognitive score in MoCA. These findings suggest dysfunction in these brain regions, and the cortical dysfunction is the cause or outcome of cognitive impairments. The SIVCIND is an early stage of SIVD. 25 In this study, compared with the patients with SIVD and controls, patients with SIVCIND showed distinctly increased activation in the prefrontal cortex. Considering that the cortical activation of these areas was significantly reduced in SIVD, the increased cortical activation in SIVCIND may be a compensation. These findings suggest that brain regions as described above may be the most affected areas in SIVCI, and the bidirectional alterations are a possible characteristic of them. The measurement of cortical activation in these regions might have great potential value in the diagnosis and differential diagnosis of these impairments.
Our study is the first fMRI study of brain activation in the patients with SIVD and SIVCIND. In previous studies, fMRI has been used to observe functional lesions in the cortex of AD and patients with mild cognitive impairment (MCI). Patients with AD have decreased activation in the hippocampal and parahippocampal regions compared with the controls during episodic encoding tasks. 5 -7 Using a face–name associative paradigm, Petrella et al found no differences between MCI and controls in medial temporal lobe activation during encoding, however, they observed hypoactivation in the left hippocampus during retrieval conditions. 26 Additionally, Johnson et al found similar hypoactivation in the right hippocampal in patients with MCI when compared with the controls using an item-based old and new recognition retrieval paradigm. 27
Our findings demonstrated that performing Stroop task highly activated the prefrontal cortex including the anterior cingulate cortex (ACC) and the dorsolateral prefrontal cortex (DLPFC), which is consistent with a previous study showing that the prefrontal regions act in an attention and executive manner. 28 The ACC has been hypothesized to play an important role in cognitive control, detecting conflict, and engaging the DLPFC to resolve the conflict. 29 The levels of activation of ACC and DLPFC have also been found to reflect conflict and control, respectively. 30 Based on experimental evidence from monkeys, the ACC and DLPFC are important not only for memory buffering to permit “online” processing but also for prepotent response inhibition. 31,32 Therefore, lesions of the prefrontal cortex could result in impaired performance in Stroop tasks. For example, patients with schizophrenia have abnormalities in the ACC and DLPFC, presenting with impairments in attention conflict paradigms. 33,34 In our study, a number of parietal regions were activated by the Stroop tasks as well. These regions appeared to be related to word processing. For example, the left inferior parietal area that exhibited increased activation in this study has been reported to become activated when processing words, compared to letter strings. 35 The significantly greater activation of the lateral left inferior region is likely to reflect the translation of orthography to phonology, as this area has been reported to be more active when viewing or naming words than the pictures. 36
The present study is the first fMRI study of SIVCI. Different prefrontal cortex activation characteristics were observed among normal aging and different states of participants with SIVCI during Stroop task performance. There is a dysfunction in SIVD and an compensation in SIVCIND. These findings might help guide a clinical differentiation among normal controls, patients with SIVCIND, and SIVD. We are aware of several limitations in this study. First, all the patients with SIVCIND included in this study were typical with very near MoCA score, so we could not correlate it with the BOLD response. Second, in the training session prior to the scanning we did not let participants meet some performance standard to ensure that they understood the task. Future investigations should address the limitations of this present study by using larger, more diverse sample groups, and by correlating MoCA score with brain activity of all stages of patients with SIVCI.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: the National Natural Science Foundation of China (Grant No. 81000607).
References
- 1. Kawas CH, Corrada MM. Alzheimer’s and dementia in the oldest: a century of challenges. Curr Alzheimer Res. 2006;3(5):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Frontal lobe dementia and subcortical vascular dementia: a neuropsychological comparison. Psychol Rep. 2005;96(1):141–151. [DOI] [PubMed] [Google Scholar]
- 3. luzzi S, Sheu C-F, Zanetti O, Frisoni GB. Distinctive clinical features of mild cognitive impairment with subcortical cerebrovascular disease. Dement Geriatr Cogn Disord. 2005;19(4):196–203. [DOI] [PubMed] [Google Scholar]
- 4. Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. [DOI] [PubMed] [Google Scholar]
- 5. Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61(4):500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kato T, Knopman D, Liu H. Dissociation of regional activation in mild Alzheimer’s disease during visual encoding: a functional MRI study. Neurology. 2001;57(5):812–816. [DOI] [PubMed] [Google Scholar]
- 7. Prvulovic D, Hubl D, Sack AT, et al. Functional imaging of visuospatial processing in Alzheimer’s disease. Neuroimage. 2002;17(3):1403–1414. [DOI] [PubMed] [Google Scholar]
- 8. Doddy RS, Massman PJ, Mawad M, Nance M. Cognitive consequences of subcortical magnetic resonance imaging changes in Alzheimer’s disease: comparison to small vessel ischemic vascular dementia. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11(4):191–199. [PubMed] [Google Scholar]
- 9. Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Clinical features of MRI-defined subcortical vascular disease. Alzheimer Dis Assoc Disord. 2003;17(4):236–242. [DOI] [PubMed] [Google Scholar]
- 10. Kramer JH, Reed BR, Mungas D, Weiner MW, Chui HC. Executive dysfunction in subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry. 2002;72(2):217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol-Bull. 1991;109(2):163–203. [DOI] [PubMed] [Google Scholar]
- 12. Scott AL, Kristy AN, Stephen MR. fMRI of healthy older adults during Stroop interference. Neuroimage. 2004;21(2):192–200. [DOI] [PubMed] [Google Scholar]
- 13. Dash J, Dash A. Cognitive developmental studies of the Stroop phenomena: cross-sectional and longitudinal data. Indian Psychol. 1982;1:24–33. [Google Scholar]
- 14. Diamond A, Taylor C. Development of an aspect of executive control: development of the abilities to remember what I said and to “do as I say, not as I do.” Dev Psychobiol. 1996;29(4):315–334. [DOI] [PubMed] [Google Scholar]
- 15. Gerstadt C, Hong YJ, Diamond A. The relationship between cognition and action: performance of children 2-7 years old on a Stroop-like day-night test. Cognition. 1994;53(2):129–153. [DOI] [PubMed] [Google Scholar]
- 16. Erikinjuntti T, Inzitari D, Pantoni L, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl. 2000;59:23–30. [DOI] [PubMed] [Google Scholar]
- 17. Rockwood K, Howard K, MacKnight C, Darvesh S. Spectrum of disease in vascular cognitive impairment. Neuroepidemiology. 1999;18(5):248–254. [DOI] [PubMed] [Google Scholar]
- 18. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156(12):1986–1988. [DOI] [PubMed] [Google Scholar]
- 19. Bondi MW, Serody AB, Chan AS, et al. Cognitive and neuropathologic correlates of Stroop Color-Word Test performance in Alzheimer’s disease. Neuropsychology. 2002;16(3):335–343. [DOI] [PubMed] [Google Scholar]
- 20. Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cereb Cortex. 2000;10(6):552–560. [DOI] [PubMed] [Google Scholar]
- 21. Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45(10):1237–1258. [DOI] [PubMed] [Google Scholar]
- 22. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. [DOI] [PubMed] [Google Scholar]
- 23. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 24. Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme; 1988. [Google Scholar]
- 25. Wentzel C, Rockwood K, MacKnight C, et al. Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology. 2001;57(4):714–716. [DOI] [PubMed] [Google Scholar]
- 26. Petrella JR, Krishnan S, Slavin MJ, Tran TT, Murty L, Doraiswamy PM. Mild cognitive impairment: evaluation with 4-T functional MR imaging. Radiology. 2006;240(1):177–186. [DOI] [PubMed] [Google Scholar]
- 27. Johnson SC, Schmitz TW, Moritz CH, et al. Activation of brain regions vulnerable to Alzheimer’s disease: the effect of mild cognitive impairment. Neurobiol Aging. 2006;27(11):1604–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yücel M, Pantelis C, Stuart GW, et al. Anterior cingulate activation during Stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am J Psychiatry. 2002;159(2):251–254. [DOI] [PubMed] [Google Scholar]
- 29. Carter CS, van-Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7(4):367–379. [DOI] [PubMed] [Google Scholar]
- 30. Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7(1):1–17. [DOI] [PubMed] [Google Scholar]
- 31. Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain Res Cogn Brain Res. 1996;5(1-2):175–181. [DOI] [PubMed] [Google Scholar]
- 32. Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Mountcastle VB, Plum F, eds. Handbook of Physiology: The Nervous System, Higher Functions of the Brain. Bethesda, MD: The American Physiological Society; 1987:373–417. [Google Scholar]
- 33. Nordahl TE, Carter CS, Salo RE, et al. Anterior cingulate metabolism correlates with stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology. 2001;25(1):139–148. [DOI] [PubMed] [Google Scholar]
- 34. Henik A, Salo R. Schizophrenia and the stroop effect. Behav Cogn Neurosci Rev. 2004;3(1):42–59. [DOI] [PubMed] [Google Scholar]
- 35. Jessen F, Erb M, Klose U, Lotze M, Grodd W, Heun R. Activation of human language processing brain regions after the presentation of random letter strings demonstrated within event-related functional magnetic resonance imaging. Neurosci Lett. 1999;270(1):13–16. [DOI] [PubMed] [Google Scholar]
- 36. Price CJ. The functional anatomy of word comprehension and production. Trends Cogn Sci. 1998;2(8):281–288. [DOI] [PubMed] [Google Scholar]