Abstract
A common neuroscience application of Pavlovian fear conditioning is to manipulate neuron-type activity, pair a cue with foot shock, then measure cue-elicited freezing in a novel context. If the manipulation reduces freezing, the neuron type is implicated in Pavlovian fear conditioning. This application reduces Pavlovian fear conditioning to a single concept. In this Viewpoint, I describe experiments supporting the view that Pavlovian fear conditioning refers to three distinct concepts: procedure, process, and behavior. An experimenter controls procedure, observes behavior, but infers process. Distinguishing these concepts is essential because: (1) a shock-paired cue can engage numerous processes and behaviors; (2) experimenter decisions about procedure influence the processes engaged and behaviors elicited; and (3) many processes are latent, imbuing the cue with properties that only manifest outside of the original conditioning setting. This means we could understand the complete neural basis of freezing, yet know little about the neural basis of fear. Neuroscientists can choose to use a variety of procedures to study a diversity of processes and behaviors. Manipulating neuron-type activity in multiple procedures can reveal specific, general, or complex neuron-type contributions to cue-elicited processes and behaviors. The results will be a broader and more detailed neural basis of fear with greater relevance to the spectrum of symptoms defining anxiety and stressor-related disorders.
Introduction
When a neuroscientist thinks about Pavlovian fear conditioning, the following scenario comes to mind. A mouse is placed in a chamber with a grid floor underfoot. An auditory cue can be played from an overhead speaker. Foot shock can be delivered through the floor. Playing the cue initially produces little change in overt behavior. The experimenter pairs the two events by playing the cue, then delivering a strong foot shock. After this pairing, the cue will elicit overt behavior, such as freezing (Blanchard and Blanchard, 1969; Bolles and Collier, 1976). Observing cue-elicited behavior serves as evidence a cue–shock association was formed.
A common neuroscience application of Pavlovian fear conditioning is to manipulate the activity of a neuron type, pair a cue with shock, then measure cue-elicited freezing. If the manipulation reduces freezing, the neuron type is ascribed a role in Pavlovian fear conditioning. A prominent example is that manipulating the activity of BLA neurons reduces cue-elicited freezing (LeDoux et al., 1988; Helmstetter, 1992; Maren, 1999; Goosens and Maren, 2001; H. J. Lee et al., 2001; Nader et al., 2001; Choi and Brown, 2003; Gale et al., 2004; Koo et al., 2004; Anglada-Figueroa and Quirk, 2005; J. L. Lee et al., 2005; Petrovich et al., 2009; McDannald, 2010; Liu et al., 2022; Williams-Spooner et al., 2022). Observing reduced cue-elicited freezing is central to the historical claim that the amygdala is the site of plasticity for Pavlovian fear conditioning (Fanselow and LeDoux, 1999), as well as to modern claims that the amygdala is a hub for fear learning and expression (Ressler et al., 2022).
I find common neuroscience thinking about Pavlovian fear conditioning to be fuzzy and oversimplistic. Think again about the scenario above. What does Pavlovian fear conditioning refer to? Is it the experimenter-arranged pairing of cue and shock? Is it the display of cue-elicited behavior? Or is it a process engaged within the mouse? From an associative learning theory perspective, the answer can be all three. Pavlovian fear conditioning can be conceptualized as a procedure, a process, or a behavioral outcome (Rescorla, 1980; Dickinson, 1981). This means that Pavlovian fear conditioning (procedure) can engage Pavlovian fear conditioning (process) to produce Pavlovian fear conditioning (behavioral outcome) (paraphrased from Peter C. Holland). Fuzzy thinking blurs these three concepts, while oversimplistic thinking reduces Pavlovian fear conditioning to a single concept.
If Pavlovian fear conditioning consisted of a single procedure that engaged a single process to produce a single behavioral outcome (Fig. 1A), then distinctions between these three concepts would be meaningless. Here I discuss experimental findings showing that these distinctions are not only meaningful but are essential to uncovering a complete brain basis for fear. The important points are: (1) a shock-paired cue can engage dissociable behaviors and processes; (2) experimenter decisions about procedure influence the behaviors and processes engaged; and (3) many processes are latent, unnecessary for overt behavior but imbuing a cue with properties that only manifest outside of the original conditioning setting (Fig. 1B). Fear is a collection of processes and behaviors. This means that neuroscientists could describe the complete brain basis for a single behavior, such as freezing, but still know little about the brain basis of fear. We will only reveal a complete brain basis for fear by combining increasingly sophisticated neuroscience tools (Jung et al., 2023; Massi et al., 2023) with sophisticated thinking about procedure, process, and behavioral outcome in Pavlovian conditioning (Rescorla, 1988; Holland, 1990).
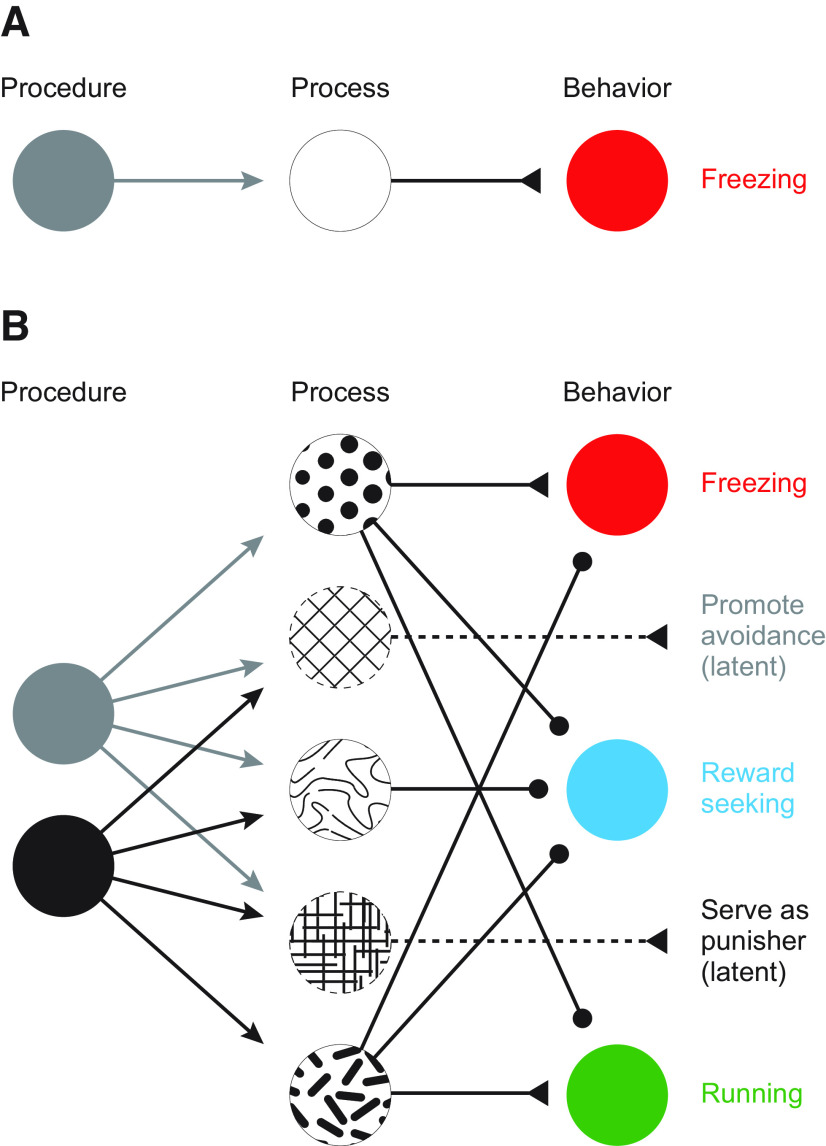
Figure 1.
Procedure, process, and behavior in Pavlovian fear conditioning. A, Schematic of Pavlovian fear conditioning in which a single procedure (gray) engages a single internal process (white), to elicit a single behavioral outcome (freezing, red). B, Schematic of Pavlovian fear conditioning in which different procedures (black vs gray), engage unique and overlapping processes (abstract patterns), to elicit (triangles) or inhibit (circles) a suite of behaviors (red represents freezing; cyan represents reward seeking; green represents running).
Pavlovian fear conditioning as procedure
Pavlovian fear conditioning as procedure refers to the experimenter-arranged relationship between events. A common arrangement consists of an auditory cue preceding foot shock. A procedure is Pavlovian, as opposed to instrumental, when the subject's behavior has no impact on the event–event relationship. The cue will be played, and the shock will be delivered no matter what the subject does. A longstanding goal of learning theorists is to describe event–event relationships that support conditioning (Rescorla and Wagner, 1972; Pearce and Hall, 1980; Esber and Haselgrove, 2011). Although the goal is to identify principles that apply to many behavioral settings, foundational experiments have used foot shock as one of the events (Estes and Skinner, 1941). The results of these experiments make clear that conditioning does not occur anytime a cue is followed by foot shock. Pavlovian fear conditioning as procedure is about contingent, event–event relationships (Rescorla, 1968; Kamin, 1969), and is constrained by behavioral relevance (Garcia and Koelling, 1966), belongingness, and relative validity (Colwill et al., 2022).
Demonstrating the importance of contiguity, Mowrer and Aiken (1954) arranged for different groups of rats to receive different temporal relationships between a flashing light and foot shock: Group I, light preceded shock (forward pairing); Group II, light was concurrent with shock onset; Group III, light was concurrent with shock termination; and Group IV, light followed shock termination (backward pairing). When tested, light responding systematically differed between groups (I > II > III > IV). The forward-paired Group I showed the greatest evidence of a light-shock association, while the backward-paired Group IV showed little evidence (Mowrer and Aiken, 1954). The authors concluded that Pavlovian fear conditioning, as procedure, occurs when a cue precedes and is contiguous with foot shock.
Rescorla (1968) hypothesized that conditioning depends not just on the number of times a cue is paired with shock (contiguity), but also on the likelihood of shock delivery outside of cue presentation (contingency). Conditioning should occur when shock delivery is more likely during a cue's presence than during a cue's absence. To demonstrate the necessity of contingency, Rescorla (1968) systematically manipulated the probability of foot shock in the presence and absence of a tone. Many groups of rats were tested, but the key finding can be summarized by comparing two groups. Groups I and II were given identical, contiguous tone–shock relationships: the probability that shock would follow tone was 0.4. Groups I and II were given different contingent relationships. The probability of receiving foot shock in the absence of tone was 0.0 for Group I, but 0.4 for Group II. If conditioning was based on contiguity, both groups should form a tone–shock association. If conditioning was based on contingency, only Group I should form a tone–shock association. Group I, but not Group II, showed robust evidence of a tone–shock association. Pavlovian fear conditioning, as procedure, occurs when shock delivery is contingent on cue presentation.
Demonstrating a requirement of behavioral relevance, Garcia and Koelling (1966) paired tasty water (flavored) or bright/noisy water (delivery coincided with light illumination and clicker presentation) with foot shock. Despite pairing, rats receiving tasty water showed no evidence of a taste–shock association. By contrast, rats receiving bright/noisy water showed strong evidence of a light/clicker–shock association (Garcia and Koelling, 1966). The same results were obtained in experiments that minimized instrumental confounds associated with voluntary drinking (Domjan and Wilson, 1972). Sights and sounds are more relevant to external threats than are tastes. Therefore, conditioning with shock outcome is more readily obtained with procedures using external cues. Similarly, belongingness (Hamm et al., 1989) and relatively validity (Le Pelley et al., 2014) effects are observed in people. Not all stimuli are equally effective in supporting cue–shock associations.
Pavlovian fear conditioning as behavioral outcome
Pavlovian fear conditioning as behavioral outcome refers to a subject's measurable response to a shock-paired cue. From the experimenter's viewpoint, the difference between procedure and behavioral outcome could not be starker. An experimenter is controlling the event–event relationships with procedure but is observing the subject's response to those events with behavioral outcome.
It was recognized early in the neuroscience of fear that a shock-paired cue elicits a collection of overt behaviors and autonomic responses in rats (Davis, 1992). This includes the ability to elicit or alter: freezing (Blanchard and Blanchard, 1969; Bolles and Collier, 1976), suppression of reward seeking (Estes and Skinner, 1941), heart rate (Kapp et al., 1979), body temperature (Godsil et al., 2000), blood pressure (LeDoux et al., 1988), defecation (Seligman et al., 1971; Mikulka et al., 1972), micturition (Antoniadis and McDonald, 1999), piloerection (Moore, 1956), and hyperventilation (Kappauf and Schlosberg, 1937). A shock-paired cue can also elicit jumping (Chu et al., 2022), running/locomotion/darting (Bolles and Collier, 1976; Gruene et al., 2015; Totty et al., 2021; Chu et al., 2022; Mitchell et al., 2022), and rearing (Holland, 1979; Chu et al., 2022). Most of these observations predate the neuroscientific study of Pavlovian fear conditioning.
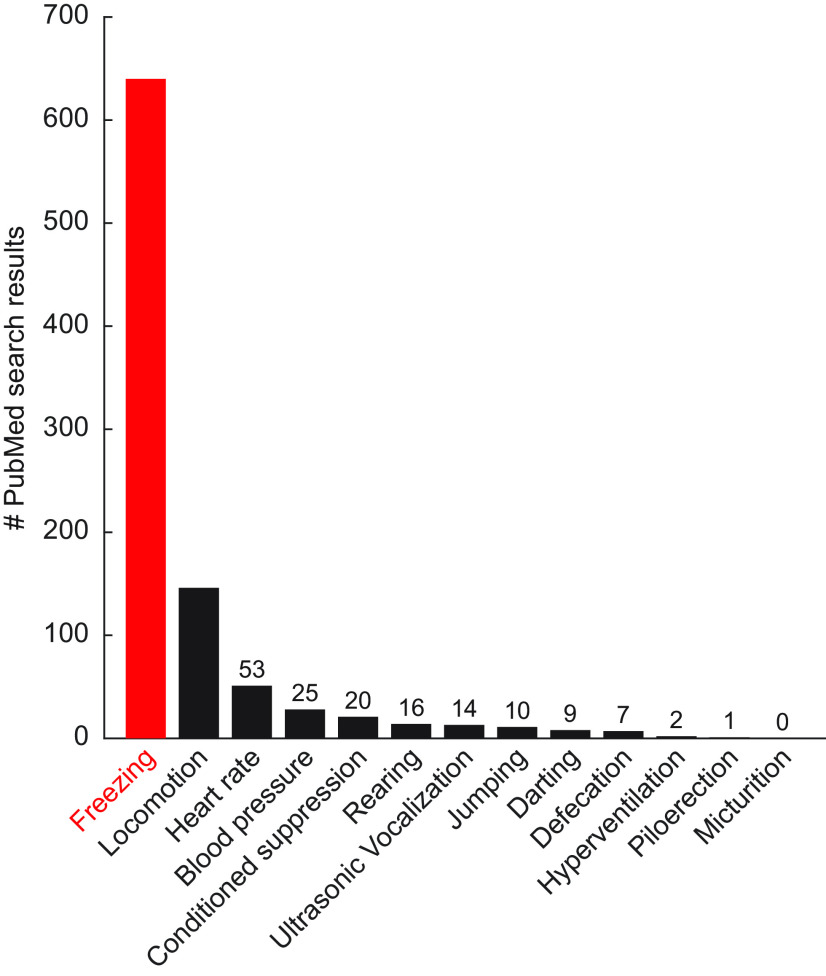
The diversity of behavioral outcomes observed to a shock-paired cue may not be obvious to students new to the neuroscience of fear because freezing dominates. A PubMed literature search for fear and each of the 13 behavioral outcomes listed above, spanning the last 10 years, returned more results for freezing (630) than for the other 12 behaviors combined (298; Fig. 2). Freezing as a behavioral outcome of Pavlovian fear conditioning is so ubiquitous, it is easy to think fear and “freezing” are interchangeable terms.
Figure 2.
PubMed search for behavioral outcomes in neuroscience-publishing journals in the last decade. A PubMed search was performed on July 14, 2023 for 13 behavioral outcomes (listed on x axis). The search terms were the behavioral outcome (any field) and fear (title/abstract). The search was restricted to the last 10 years and to 27 behavioral-neuroscience publishing journals: Behavioral Neuroscience, Behavioural Brain Research, Biological Psychiatry, Cell, Cell Reports, Communications Biology, Current Biology, eLife, eNeuro, European Journal of Neuroscience, Frontiers in Behavioral Neuroscience, Journal of Neuroscience, Learning & Memory, Molecular Psychiatry, Nature, Nature Communications, Nature Neuroscience, Neurobiology of Learning and Memory, Neuron, Neuropharmacology, Neuropsychopharmacology, Neuroscience, Physiology & Behavior, Psychopharmacology, Science, Science Advances, and Translational Psychiatry. The number of search results is reported for each behavioral outcome (y axis). The specific number of results is indicated for behavioral outcomes returning <100.
Experimenter decisions about procedure influence behavioral outcome
Freezing is not the inevitable behavioral outcome of a Pavlovian fear conditioning procedure. Decisions made by experimenters influence the behaviors demonstrated to a shock-paired cue. Freezing is ubiquitous because neuroscientists make procedural decisions that bias behavior toward freezing. Two of the earliest experiments to quantify behavior during a Pavlovian fear conditioning procedure illustrate this point. Both studies varied experimental factors and measured freezing as well as an opposing behavior: activity (Bolles and Collier, 1976) or rearing (Holland, 1979).
Bolles and Collier (1976) performed one of the first experiments on the ability of shock-predictive cues to direct behavior. A long box and a short box were used as “cues.” During conditioning, half of the rats received foot shock in the long box, and half in the short box. Half of the rats in each group were tested in the long box, and half in the short box. The most-cited finding is that rats trained and tested in the short box freeze, accounting for ∼64% of observed behavior. Less cited is that rats trained and tested in the long box freeze (∼37% of behavior) and increase activity (∼40% of behavior). Even more, rats trained in the short box and tested in the long box exclusively increase activity (∼71% of behavior). Bolles and Collier (1976) concluded that the geometry of the long box seemed to “invite” running, outcompeting freezing.
Contemporary to Bolles and Collier (1976), Holland (1979) was demonstrating that appetitive Pavlovian cue-elicited behaviors can be CS-driven or US-driven. For example, a visual cue paired with food will elicit rearing (standing up on hind legs, CS-driven) and will also elicit food cup behavior (going to the site of food delivery, US-driven) (Holland, 1977). Holland (1979) asked whether shock intensity can bias the competition between CS-driven and US-driven behaviors. For the most pertinent finding, four separate groups of rats had a visual cue paired with unique foot shock intensities, ranging from low to high (in mA): 0.1, 0.25, 0.35, or 0.5. Rearing (CS-driven behavior) and freezing (US-driven behavior) to the shock-paired, visual cue were measured over six sessions. The lowest foot shock intensity supported neither light-elicited rearing nor freezing. The highest foot shock intensity supported only freezing (∼83% of cue-elicited behavior). An intermediate foot shock intensity supported freezing (∼21% of behavior) but a greater amount of rearing (∼29%). Rearing to a shock-paired visual cue is masked by freezing at high shock intensities. As for why rearing is observed, there are several possibilities. Rearing may reflect vigilance (Dielenberg et al., 2001), a component of an escape response (Cain and LeDoux, 2007), attention for purposes of learning, or attention for purposes of acting (Gallagher and Holland, 1994).
Flash forward to the present. Neuroscientists commonly use a short (cubical) box, auditory cue, and intense foot shock. These procedural choices bias cue-elicited behavior toward freezing. One can imagine an alternate universe in which common procedural choices include a long box, visual cue, and intermediate foot shock intensity. These procedural choices would bias behavior toward running and rearing. In this alternate universe, neuroscientists may think of fear and running as interchangeable terms. Neither the common choices made in our universe nor the alternate universe are wrong. Instead, common procedural choices invite narrow thinking that equates fear to a small subset of behaviors elicited by a shock-paired cue.
Pavlovian fear conditioning as process
Pavlovian fear conditioning as process refers to a change within the subject as the result of cue–shock pairings. A process can also be thought of as an acquired cue property. Of the three concepts, process is the most difficult to study because it cannot be controlled by the experimenter, nor can it be directly observed. The existence of a process must be inferred. If a neutral cue cannot elicit freezing, then after shock pairings will elicit robust freezing, I infer something has changed within the subject. This internal change can persist for a long time. Rats given cue–shock pairings, then tested over a year later, show marked cue-elicited freezing (Gale et al., 2004). If processes only served to elicit behavior, then distinguishing process from behavioral outcome might not be meaningful. Pavlovian fear conditioning as process is meaningful because a shock-paired cue can engage latent processes. Latent processes are not obvious during cue-elicited responding but are revealed by testing the cue in a new setting or by postconditioning manipulations. Further, a shock-paired cue can engage a variety of processes, imbuing the cue with a variety of properties.
Second-order conditioning can reveal the ability of a shock-paired cue to support new learning. In this procedure, subjects are first given pairings of light and foot shock (light → shock, called first-order conditioning). When responding to the light is tested, subjects will suppress reward seeking (McDannald, 2010) and freeze (Holland, 1979). Neither suppression of reward seeking nor freezing requires the shock-paired light to engage a process to support new learning (similar to foot shock in first-order conditioning). To test for the existence of such a process, a second-order relationship is arranged in which a novel tone is paired with light (tone → light). Critically, no foot shock is presented during second-order pairings. This means that any responding to the tone must be because of some association between that stimulus and either a representation of its past associate (i.e., the shock) and/or the light-elicited responses (e.g., freezing, running, or other). A final test reveals the tone has acquired the ability to suppress reward seeking (Davenport, 1966; Kamil, 1968; Rizley and Rescorla, 1972) and elicit freezing (Nader and LeDoux, 1999; McDannald and Galarce, 2011), behaviors similar to those elicited by the first-order light. Testing the light in a new setting revealed its ability to support learning, similar in some ways to the learning supported by foot shock.
Conditioned punishment reveals the ability of a shock-paired cue to serve as an instrumental punisher. In a standard punishment procedure, two actions are available. Both actions lead to reward, but one action also leads to foot shock. Subjects reduce responding to the action leading to foot shock, biasing responding to the action leading to only reward. In conditioned punishment, subjects first receive tone–shock pairings. Next, subjects are placed in a new setting in which two actions are available. Both actions lead to reward, but one action also leads to the tone. No foot shocks are presented during these sessions. Demonstrating conditioned punishment, subjects will reduce responding to the action leading to the shock-paired tone (Hake and Azrin, 1965). Testing the tone in a new, operant setting revealed its ability to support punishment.
A shock-paired cue can contain information about intensity (Rescorla, 1974) and number (Dickinson et al., 1976). This information can only be revealed through postconditioning manipulations. Revealing intensity information, Rescorla (1974) gave rats pairing of a tone followed by an intermediate foot shock intensity. Next, half of the rats received an inflated foot shock (longer and more intense). Control rats received the same foot shock as during conditioning. During a test session in which the tone was presented alone, rats receiving the inflated shock increased tone responding relative to controls (Rescorla, 1974). Thus, during initial tone–shock pairings, rats had formed an intensity prediction. When intensity was subsequently increased, rats were able to integrate new intensity information with the original prediction to increase tone responding.
Revealing number information, Dickinson et al. (1976) had control rats undergo a blocking procedure in which a tone was first paired with foot shock (tone → shock). Next, a tone/light compound was paired with the same foot shock (tone/light → shock), a procedure that normally “blocks” conditioning to the added light (Kamin, 1969). An experimental group also received initial tone–shock pairings, but during the compound phase received additional foot shocks (tone/light → shock → shock). Increasing the number of shocks resulted in “unblocking”: learning to the added light (Dickinson et al., 1976). Although theoretical accounts vary (Pearce and Hall, 1980), one explanation is that a prediction of foot shock number was formed during initial tone–shock pairings. Violating this number prediction during the blocking phase permitted new learning to the added light (Rescorla and Wagner, 1972).
A shock-paired cue will enhance avoidance. Avoidance is an instrumental procedure in which a foot shock will be delivered unless the subject performs a response to prevent it. Avoidance is unsignaled when there is no explicit cue instructing when the response must be performed. Rescorla and LoLordo (1965) first trained subjects to jump over a barrier to prevent foot shock delivery. After avoidance responding was established, subjects separately received pairings of tone and foot shock. The critical test came when subjects were returned to the avoidance setting and the tone presented. Tone presentation increased barrier jumping behavior, enhancing avoidance (Rescorla and LoLordo, 1965). A shock-paired cue can also act as a surrogate for a signal instructing avoidance (Overmier and Leaf, 1965). The ability of a shock-paired cue to enhance avoidance is even more striking considering that presenting the same cue in a neutral setting can elicit freezing (McCue et al., 2014).
A shock-paired cue will potentiate startle (Brown et al., 1951; Davis et al., 1993). In a basic demonstration, a light is paired with foot shock. Rather than testing overt behavioral responding to the light, the critical test comes when the light is precisely illuminated before a startle stimulus. Startle is enhanced by the light, compared with presentations of the startle stimulus alone and numerous control conditions (Davis et al., 1989). Pairing with shock imbued the light with a new property, the ability to enhance startle.
A shock-paired cue can engage dissociable behaviors and processes
In theory, a shock-paired cue could engage a single process that is responsible for the total properties acquired, and the complete spectrum of behaviors elicited. If this were the case, then brain manipulations that disrupt one process/behavioral outcome should disrupt all processes and behavioral outcomes. In support of a single process, LeDoux et al. (1990) gave rats sham or neurotoxic lesions of the lateral amygdala, gave tone–shock pairings, and measured multiple behavioral outcomes. During test, sham rats froze, increased arterial blood pressure, and suppressed drinking during the shock-paired tone. Neurotoxic lesions of the lateral amygdala diminished all three behavioral outcomes (LeDoux et al., 1990). Similarly, H. J. Lee et al. (2001) found that pairing a tone with shock, then antagonizing NMDA receptors during an extinction test, reduced cue-elicited freezing, ultra-sonic vocalizations, and defecation. Both results support the interpretation that a single, BLA-dependent process is responsible for a suite of cue-elicited behaviors.
Deviating from procedures commonly used by neuroscientists can reveal dissociable behaviors and processes. Petrovich et al. (2009) gave rats sham or neurotoxic lesions of the BLA. Following recovery, rats were food-restricted, then given alternating sessions in appetitive and aversive contexts. In the appetitive context, rats freely ate food. In the aversive context, half of the rats in each surgical group had tone paired with shock. The remaining half of each group had tone and shock unpaired. Testing took place in the appetitive context where food was freely available, and tones were intermittently played. Time spent freezing and grams of food eaten were measured. Sham paired rats froze and suppressed eating during tone presentation, while Sham unpaired rats did not freeze and readily ate. Basolateral paired rats failed to freeze during tone presentation but nevertheless suppressed eating (Petrovich et al., 2009).
There are several possible interpretations for these results. Fear could be a single process engaged at different levels, with high fear eliciting freezing and suppressing eating, but low fear only suppressing eating. I favor the interpretation that the shock-paired tone engaged two processes: a basolateral-dependent process for freezing and a basolateral-independent process for suppression of eating. Indeed, high fear could be the result of engaging several processes at once, but low fear the result of engaging a single process.
McDannald and Galarce (2011) gave rats sham or neurotoxic lesions of the BLA. A visual CS+ was paired with foot shock, while a visual CS– was unpaired. Pairings took place over a baseline of rewarded lever pressing. The experimental design permitted conditioned freezing and conditioned suppression (reductions in rewarded lever pressing during cue presentation) to be concurrently measured. Sham rats acquired CS+ freezing and suppression, and showed discriminative CS+/CS– freezing and suppression. BLA lesions impaired CS+ freezing and abolished discriminative CS+/CS– freezing. However, these same lesioned rats acquired CS+ suppression, and demonstrated discriminative CS+/CS– suppression (McDannald and Galarce, 2011). The results could reflect different levels of fear differentially affecting freezing and suppression of reward seeking (as for Petrovich et al., 2009). However, the results are consistent with the interpretation that the shock-paired visual cue engaged two processes. A basolateral-dependent process engaged freezing, and a more basolateral-independent process suppressed reward seeking. The results of McDannald and Galarce (2011) are consistent with prior work showing a dissociable contribution of the BLA to conditioned freezing and conditioned suppression with extended training (J. L. Lee et al., 2005), and intact conditioned suppression in rats with BLA lesions undergoing a dual conditioned suppression/conditioned punishment procedure (Killcross et al., 1997).
Studies that measure multiple behavior outcomes, or assess an additional acquired cue property, often find dissociable contributions of a brain region. Lateral hypothalamus lesions that diminish a conditioned arterial pressure response leave conditioned freezing intact (Iwata et al., 1986; LeDoux et al., 1988). Periaqueductal gray lesions that abolish conditioned freezing leave a conditioned arterial pressure response intact (LeDoux et al., 1988). Ventrolateral periaqueductal gray lesions (Amorapanth et al., 1999; McDannald, 2010) or central amygdala lesions (McDannald, 2010) that abolish conditioned freezing leave conditioned suppression intact. BLA lesions that disrupt the acquisition of freezing leave US-inflation intact (Rabinak and Maren, 2008; Rabinak et al., 2009). Medial amygdala lesions that abolish cue-potentiated avoidance leave cued freezing intact (McCue et al., 2014).
Using the Killcross et al. (1997) procedure to assess conditioned punishment and conditioned suppression in large cohorts of rats (Jean-Richard-dit-Bressel et al., 2019) and healthy people (Jean-Richard-dit-Bressel et al., 2021) reveals differing sensitivity distributions. Rats and people show unimodal distribution of conditioned suppression sensitivity: all subjects are sensitive. These same subjects show a bimodal distribution of conditioned punishment sensitivity: a subset of subjects are insensitive to punishment. Further distinguishing the two processes, medial orbitofrontal cortex lesions that impair conditioned punishment leave conditioned suppression intact (Ma et al., 2020).
Although a minority of studies are designed to reveal dissociative contributions of a brain region to cue-elicited behaviors and processes, dissociations abound. As a field, we are very far from a complete picture of the independent and overlapping neural mechanisms underlying the total processes and behaviors elicited by a shock-paired cue. Nevertheless, these studies work toward that complete picture, and demonstrate that progress will only be made by assessing neuron-type contributions to a variety of processes and behaviors.
Implications for the neurobiology of fear
Let's return to the Introduction scenario in which a mouse receives pairings of tone and strong shock. While receiving pairings, experimental mice have “Alpha” neuron types within a defined brain region chemogenetically inhibited (Armbruster et al., 2007). Control mice have Alpha neurons left intact. Presenting the tone in a novel context elicits freezing in control mice, but not in chemogenetically inhibited mice. The experimenters then demonstrate that chemogenetic inhibition does not disrupt the behavioral capacity to freeze. Historically, these results would lead to the conclusion that Alpha neurons are necessary for Pavlovian fear conditioning (Fig. 3A).
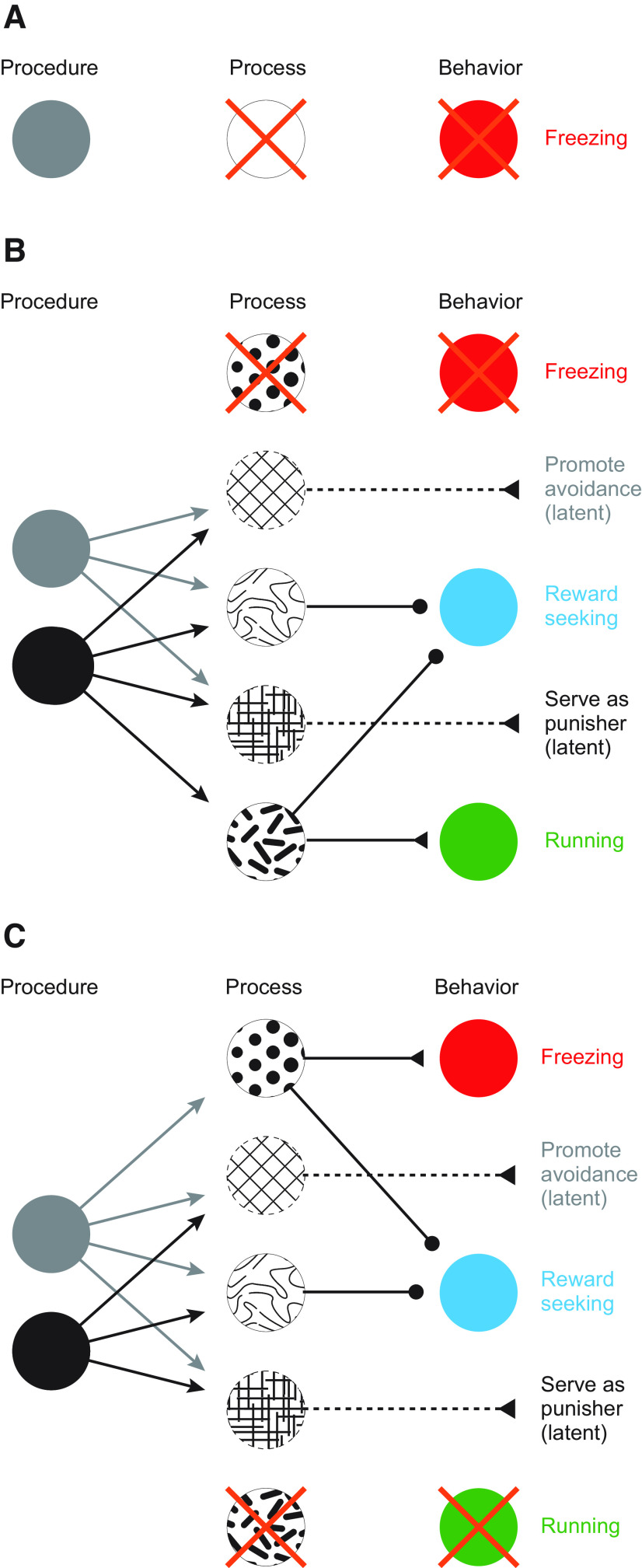
Figure 3.
Interpreting effects of neuron-type manipulation with procedure, process, and behavior. A, Equating procedure, process, and behavior can result in claims that Pavlovian fear conditioning failed to occur when a neuron-type manipulation abolishes a conditioned behavior (red represents freezing). B, Schematic of Pavlovian fear conditioning in which different procedures (black vs gray), engage unique and overlapping processes (abstract patterns), to elicit (triangles) or inhibit (circles) a suite of behaviors (red represents freezing; cyan represents reward seeking; green represents running) as well as latent processes manifesting outside of the original conditioning setting. A neuron-type manipulation that disrupts the process eliciting freezing may leave other processes and behavioral outcomes intact. C, Schematic same as in B. A neuron-type manipulation that disrupts the process eliciting running may leave other processes and behavioral outcomes intact.
Viewing Pavlovian fear conditioning through separate lenses of procedure, process, and behavioral outcome leads to a different conclusion. By abolishing cue-elicited freezing, but leaving intact the behavioral capacity to freeze, the results demonstrate that Alpha neurons are critical for a shock-paired cue to engage a process that elicits freezing. Absent additional observations or experiments, further conclusions cannot be made. Certainly, we cannot claim that Alpha neurons are necessary for Pavlovian fear conditioning. Why not? Disrupting Alpha neuron firing may leave intact cue-elicited processes that engage other behaviors, such as suppressing reward seeking or running. Further, latent processes engaged by the shock-paired cue may also be intact, such as the ability to promote avoidance or to serve as a conditioned punisher (Fig. 3B). A similar conclusion would be reached if chemogenetic inhibition disrupted cue-elicited running (but left intact the behavioral capacity to run). Mice that fail to run during a shock-paired cue may nevertheless freeze or suppress reward seeking, and latent processes may also be intact (Fig. 3C).
Now think about a scenario in which you pair a tone with strong shock (as above) while chemogenetically inhibiting “Beta” neuron types in a separate brain region. Presenting the tone in the novel context elicits freezing in control and chemogenetically inhibited mice, with no group differences observed. Surely, you can conclude that Beta neurons are not necessary for Pavlovian fear conditioning!? Again, I would argue no. The results demonstrate that Beta neurons are not necessary for a shock-paired cue to engage a process that elicits freezing. Despite this, Beta neurons may be necessary for processes that engage other cue-elicited behaviors or may be critical to engage latent processes observed outside of the original conditioning setting.
Progress toward revealing a complete and detailed neural basis for fear will come through linking neuron types, circuits, and networks to the spectrum of processes engaged by a shock-paired cue (Fig. 4). I make five recommendations for effective use of procedure, process, and behavior in Pavlovian fear conditioning.
Use procedures with appropriate control cues (Rescorla, 1967). The shock-paired cue must have a positive predictive relationship with foot shock, while a control cue should be physically similar but not have a predictive relationship. A truly random control may be possible in between-subjects behavioral designs. However, care should be taken to avoid early, incidental cue–shock pairings, which could result in a cue–shock association forming (Benedict and Ayres, 1972). Further, truly random controls with dense cue and shock presentations should be avoided (Kremer, 1971). When possible, use within-subjects designs that rule out nonassociative accounts of behavior change, such as sensitization or “psuedoconditioning” (Kamprath and Wotjak, 2004). Unpaired cues presented at times distant from foot shock can be used in both within- and between-subjects designs. Although this results in a negative predictive relationship, the burden of proof for claiming an inhibitory association is substantial (Rescorla, 1969).
Ensure behavioral relevance of the procedure. Use cues relevant to the outcome being predicted (Garcia and Koelling, 1966). Scrutinize findings in head-fixed preparations which may not reflect findings in freely moving preparations. For example, Cho et al. (2021) directly compared dorsal raphe dopamine neuron calcium transients during tone–shock pairings in a fixed versus freely moving behavioral setting. In both settings, dorsal raphe dopamine neurons show calcium transients to foot shock presentation. However, no calcium transients to the tone were observed in the head fixed setting but were readily observed in the freely moving setting. Had Cho et al. (2021) only imaged in the head fixed setting, they would have concluded no role for these neurons in processing a shock-paired cue.
Measure multiple behavioral outcomes (Bolles and Collier, 1976; Holland, 1979). Even better, measure opposing behavioral outcomes (e.g., freezing vs running vs rearing). Multiple measures can be simultaneously analyzed with multivariate ANOVA. Individual measures can then be analyzed with univariate ANOVA, using a Bonferroni-corrected p value (Chu et al., 2022).
Choose procedures known to produce different behavioral outcomes (Bolles and Collier, 1976; Holland, 1979). If manipulating neuron Type A reduces freezing in a procedure biased toward freezing, determine whether manipulating neuron Type A reduces running in a procedure biased toward running. The results can allow the researcher to ascribe a more specific or general role for that neuron type in fear.
Assess latent processes engaged by a shock-paired cue. Do not assume the failure of a neuron-type manipulation to reduce freezing means the neuron type is unnecessary for Pavlovian fear conditioning (McCue et al., 2014).
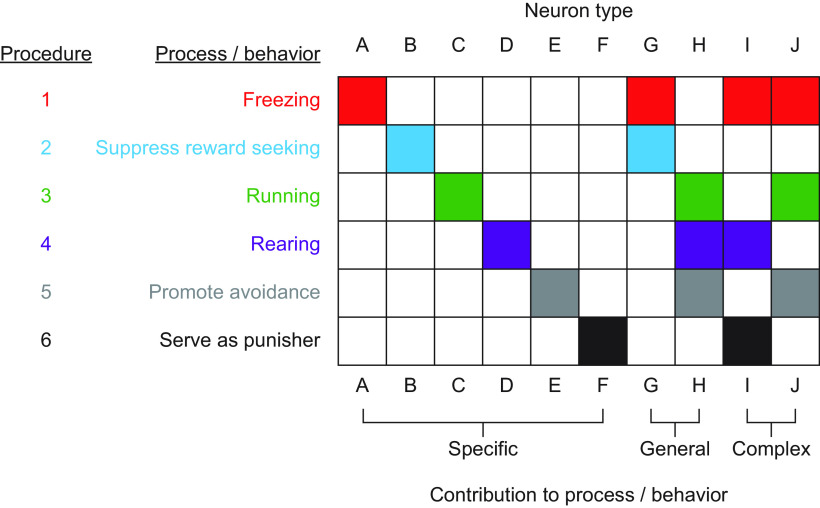
Figure 4.
Using procedure to link processes and behaviors to neuron types. Hypothetical results of studies using 6 different Pavlovian fear conditioning procedures (rows 1-6) that assess 4 distinct behaviors (red represents freezing; cyan represents reward seeking; green represents running; purple represents rearing) and 2 latent processes (gray represents promote avoidance; black represents serve as punisher). The 10 columns (A-J) represent 10 distinct neuron types nested in distinct brain regions. Each of the 60 cells (6 rows × 10 columns) represents a single experiment in which neuron-type activity is inhibited during a specific procedure (e.g., in cell F6, neuron Type F is inhibited during a conditioned punishment procedure). Filled cells represent that inhibiting neuron-type activity disrupts behavior or process (e.g., inhibiting activity of neuron Types A, G, I, and J disrupt cued freezing). Pattern across procedures can reveal specific (A–F), general (G and H), or complex (I and J) neuron-type contributions to processes and behaviors.
Applying these recommendations will lead to a broader and more detailed neural basis of fear. Take a scenario in which you use a single procedure that emphasizes freezing (Fig. 4, row 1 only) to examine roles for 10 neuron types (A-J). You find that inhibiting activity in four neuron types disrupts freezing (A, G, I, and J), concluding that these four neuron types alone are necessary for Pavlovian fear conditioning. Now you examine the same 10 neuron types using six procedural variations that emphasize four different behaviors (freezing, suppression of reward seeking, running, and rearing) and two latent processes (cue-potentiated avoidance and conditioned punishment). Your results reveal a broader neural basis for fear: inhibiting activity in each neuron type disrupts at least one behavior or process. Your results also reveal a more detailed basis for fear. You observe six neuron types contribute to a specific behavior/process (A-F), two neuron types correspond to general changes in activity (G, decreasing; and H, increasing), while two neuron types have complex contributions to behaviors and processes (I and J). Finally, you note that each behavior/process is characterized by a unique combination of neuron types.
Implications for the neurobiology of anxiety and stressor-related disorders
The popularity of Pavlovian fear conditioning is due in part to its many applications. Pavlovian fear conditioning is a good model to study learning and memory from the molecular to the cognitive level. Pavlovian fear conditioning continues to be an indispensable tool in the development of formal accounts of associative learning. One motivation to use Pavlovian fear conditioning in neuroscience is to help individuals with anxiety and stressor-related disorders. If disorders occur in part because of dysfunction in conserved neural mechanisms for fear, then understanding healthy neural function may allow us to ameliorate sources of neural dysfunction.
The Diagnostic and statistical manual of mental disorders recognizes 11 anxiety disorders and 5 trauma- and stressor-related disorders (American Psychiatric Association, 2013). The 16 disorders are similar in that they each cause clinical distress and impair normal functioning (e.g., school, work, social). The 16 disorders differ in the objects/settings that provoke them, associated cognitive ideation, and physical symptoms. Panic disorder is characterized by abrupt, short panic attacks that can include physical symptoms of shaking, pounding heart, heat sensations, and fear of “losing control.” Post-traumatic stress disorder is characterized by symptoms that can include intense physiological reactions to trauma-related cues, psychological distress at exposure to trauma-related cues, and avoiding external reminders of the traumatic event. Agoraphobia entails fear associated with being in public, open, or enclosed spaces that can be so overwhelming individuals may rarely leave home. These are a subset of symptoms from a subset of anxiety and stressor-related disorders. Even in this subset, it is clear that anxiety and stressor-related disorders span a spectrum of symptoms.
Pavlovian fear conditioning cannot directly model anxiety and stressor-related disorders. At best, Pavlovian fear conditioning allows neuroscientists to link basic processes and behaviors to neuron types, circuits, and networks. Viewing Pavlovian fear conditioning through the lens of procedure, process, and behavioral outcome maximizes its relevance to disorders. This view sees fear as a collection of diverse processes and behaviors. The full diversity of processes and behaviors engaged by a shock-paired cue better captures the heterogeneity of symptoms of anxiety and stressor-related disorders. Neuroscientists can make decisions about procedure and measurement to study a wider spectrum of behaviors and processes. We can uncover neural mechanisms for cue-control of heart rate, body temperature, running, jumping, and rearing, behaviors relevant to panic disorder. We can reveal neural mechanisms for latent cue processes, such as the ability to promote avoidance and act as an aversive event itself, processes relevant to post-traumatic stress disorder. Moreover, we can determine neural mechanisms by which a shock-paired cue inhibits necessary activities, such as eating and reward seeking, processes common to all anxiety and stressor-related disorders.
Studies simplifying Pavlovian fear conditioning to a single concept — or blurring the concepts of procedure, process, and behavior — have primarily uncovered neural mechanisms for cue-elicited freezing. Describing a complete neural basis of fear will mean describing neural mechanisms for the full diversity of processes and behaviors engaged by a shock-paired cue. This will require neuroscientists to use a wider range of procedures to assess the total diversity processes, and to quantify more types of behavior. A neural basis of fear that captures the total behaviors and processes engaged by shock-paired cue will necessarily have greater relevance to the symptom spectrum of anxiety and stressor-related disorders.
Footnotes
This work was supported by National Institutes of Health, National Institute of Mental Health Award MH117791. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. I thank Amanda Chu for discussions related to this Viewpoint.
The author declares no competing financial interests.
References
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association. [Google Scholar]
- Amorapanth P, Nader K, LeDoux JE (1999) Lesions of periaqueductal gray dissociate-conditioned freezing from conditioned suppression behavior in rats. Learn Mem 6:491–499. 10.1101/lm.6.5.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ (2005) Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci 25:9680–9685. 10.1523/JNEUROSCI.2600-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, McDonald RJ (1999) Discriminative fear conditioning to context expressed by multiple measures of fear in the rat. Behav Brain Res 108:1–19. 10.1016/s0166-4328(99)00121-7 [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104:5163–5168. 10.1073/pnas.0700293104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict JO, Ayres JJ (1972) Factors affecting conditioning in the truly random control procedure in the rat. J Comp Physiol Psychol 78:323–330. 10.1037/h0032296 [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC (1969) Crouching as an index of fear. J Comp Physiol Psychol 67:370–375. 10.1037/h0026779 [DOI] [PubMed] [Google Scholar]
- Bolles RC, Collier AC (1976) The effect of predictive cues on freezing in rats. Anim Learn Behav 4:6–8. 10.3758/BF03211975 [DOI] [Google Scholar]
- Brown JS, Kalish HI, Farber IE (1951) Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. J Exp Psychol 41:317–328. 10.1037/h0060166 [DOI] [PubMed] [Google Scholar]
- Cain CK, LeDoux JE (2007) Escape from fear: a detailed behavioral analysis of two atypical responses reinforced by CS termination. J Exp Psychol Anim Behav Process 33:451–463. 10.1037/0097-7403.33.4.451 [DOI] [PubMed] [Google Scholar]
- Cho JR, Chen X, Kahan A, Robinson JE, Wagenaar DA, Gradinaru V (2021) Dorsal raphe dopamine neurons signal motivational salience dependent on internal state, expectation, and behavioral context. J Neurosci 41:2645–2655. 10.1523/JNEUROSCI.2690-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Brown TH (2003) Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. J Neurosci 23:8713–8721. 10.1523/JNEUROSCI.23-25-08713.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A, Michel CB, Gordon NT, Hanrahan KE, DuBois AM, Williams DC, McDannald MA (2022) A fear conditioned cue orchestrates a suite of behaviors. BioRxiv 502178. 10.1101/2022.08.05.502178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Delamater AR, Lattal KM (2022) Developments in associative theory: a tribute to the contributions of Robert A. Rescorla. J Exp Psychol Anim Learn Cogn 48:245–264. 10.1037/xan0000344 [DOI] [PubMed] [Google Scholar]
- Davenport JW (1966) Higher-order conditioning of fear (CER). Psychon Sci 4:27–28. 10.3758/BF03342157 [DOI] [Google Scholar]
- Davis M (1992) The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15:353–375. 10.1146/annurev.ne.15.030192.002033 [DOI] [PubMed] [Google Scholar]
- Davis M, Schlesinger LS, Sorenson CA (1989) Temporal specificity of fear conditioning: effects of different conditioned stimulus-unconditioned stimulus intervals on the fear-potentiated startle effect. J Exp Psychol Anim Behav Process 15:295–310. 10.1037/0097-7403.15.4.295 [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M (1993) Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res 58:175–198. 10.1016/0166-4328(93)90102-v [DOI] [PubMed] [Google Scholar]
- Dickinson A (1981) Contemporary animal learning theory. In: Problems in the behavioural sciences, Series 1. Cambridge: Cambridge UP. [Google Scholar]
- Dickinson A, Hall G, Mackintosh NJ (1976) Surprise and the attenuation of blocking. J Exp Psychol Anim Behav Process 2:313–322. 10.1037/0097-7403.2.4.313 [DOI] [Google Scholar]
- Dielenberg RA, Carrive P, McGregor IS (2001) The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Res 897:228–237. 10.1016/s0006-8993(01)02227-2 [DOI] [PubMed] [Google Scholar]
- Domjan M, Wilson NE (1972) Specificity of cue to consequence in aversion learning in the rat. Psychon Sci 26:143–145. 10.3758/BF03335461 [DOI] [Google Scholar]
- Esber GR, Haselgrove M (2011) Reconciling the influence of predictiveness and uncertainty on stimulus salience: a model of attention in associative learning. Proc Biol Sci 278:2553–2561. 10.1098/rspb.2011.0836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes KW, Skinner BF (1941) Some quantitative properties of anxiety. J Exp Psychol 29:390–400. 10.1037/h0062283 [DOI] [Google Scholar]
- Fanselow MS, LeDoux JE (1999) Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23:229–232. 10.1016/s0896-6273(00)80775-8 [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS (2004) Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci 24:3810–3815. 10.1523/JNEUROSCI.4100-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Holland PC (1994) The amygdala complex: multiple roles in associative learning and attention. Proc Natl Acad Sci USA 91:11771–11776. 10.1073/pnas.91.25.11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Koelling RA (1966) Relation of cue to consequence in avoidance learning. Psychon Sci 4:123–124. 10.3758/BF03342209 [DOI] [Google Scholar]
- Godsil BP, Quinn JJ, Fanselow MS (2000) Body temperature as a conditional response measure for Pavlovian fear conditioning. Learn Mem 7:353–356. 10.1101/lm.32800 [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S (2001) Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem 8:148–155. 10.1101/lm.37601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM (2015) Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4:e11352. 10.7554/eLife.11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake DF, Azrin NH (1965) Conditioned punishment. J Exp Anal Behav 8:279–293. 10.1901/jeab.1965.8-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Vaitl D, Lang PJ (1989) Fear conditioning, meaning, and belongingness: a selective association analysis. J Abnorm Psychol 98:395–406. 10.1037//0021-843x.98.4.395 [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ (1992) Contribution of the amygdala to learning and performance of conditional fear. Physiol Behav 51:1271–1276. 10.1016/0031-9384(92)90320-2 [DOI] [PubMed] [Google Scholar]
- Holland PC (1977) Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol Anim Behav Process 3:77–104. 10.1037//0097-7403.3.1.77 [DOI] [PubMed] [Google Scholar]
- Holland PC (1979) The effects of qualitative and quantitative variation in the US on individual components of Pavlovian appetitive conditioned behavior in rats. Anim Learn Behav 7:424–432. 10.3758/BF03209696 [DOI] [Google Scholar]
- Holland PC (1990) Event representation in Pavlovian conditioning: image and action. Cognition 37:105–131. 10.1016/0010-0277(90)90020-k [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Reis DJ (1986) Destruction of intrinsic neurons in the lateral hypothalamus disrupts the classical conditioning of autonomic but not behavioral emotional responses in the rat. Brain Res 368:161–166. 10.1016/0006-8993(86)91055-3 [DOI] [PubMed] [Google Scholar]
- Jean-Richard-dit-Bressel P, Lee JC, Liew SX, Weidemann G, Lovibond PF, McNally GP (2021) Punishment insensitivity in humans is due to failures in instrumental contingency learning. Elife 10:e69594. 10.7554/eLife.69594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Richard-dit-Bressel P, Ma C, Bradfield LA, Killcross S, McNally GP (2019) Punishment insensitivity emerges from impaired contingency detection, not aversion insensitivity or reward dominance. Elife 8:e52765. 10.7554/eLife.52765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Wang Y, Mocle AJ, Zhang T, Köhler S, Frankland PW, Josselyn SA (2023) Examining the engram encoding specificity hypothesis in mice. Neuron 111:1830–1845.e5. 10.1016/j.neuron.2023.03.007 [DOI] [PubMed] [Google Scholar]
- Kamil AC (1968) The second-order conditioning of fear in rats. Psychon Sci 10:99–100. 10.3758/BF03331426 [DOI] [Google Scholar]
- Kamin LJ (1969) Predictability, surprise, attention and conditioning. In: Punishment aversive behavior (Cambell BA, Church RM, eds), pp 279–296. New York: Appleton-Century-Crofts. [Google Scholar]
- Kamprath K, Wotjak CT (2004) Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem 11:770–786. 10.1101/lm.86104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR (1979) Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Behav 23:1109–1117. 10.1016/0031-9384(79)90304-4 [DOI] [PubMed] [Google Scholar]
- Kappauf WE, Schlosberg H (1937) Conditioned responses in the white rat: III. Conditioning as a function of the length of the period of delay. Pedagogical Semin J Genet Psychol 50:27–45. 10.1080/08856559.1937.10534267 [DOI] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ (1997) Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature 388:377–380. 10.1038/41097 [DOI] [PubMed] [Google Scholar]
- Koo JW, Han JS, Kim JJ (2004) Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci 24:7654–7662. 10.1523/JNEUROSCI.1644-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer EF (1971) Truly random and traditional control procedures in CER conditioning in the rat. J Comp Physiol Psychol 76:441–448. 10.1037/h0031398 [DOI] [PubMed] [Google Scholar]
- Le Pelley ME, Beesley T, Griffiths O (2014) Relative salience versus relative validity: cue salience influences blocking in human associative learning. J Exp Psychol Anim Learn Cogn 40:116–132. 10.1037/xan0000006 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988) Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8:2517–2529. 10.1523/JNEUROSCI.08-07-02517.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM (1990) The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 10:1062–1069. 10.1523/JNEUROSCI.10-04-01062.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Choi JS, Brown TH, Kim JJ (2001) Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci 21:4116–4124. 10.1523/JNEUROSCI.21-11-04116.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Dickinson A, Everitt BJ (2005) Conditioned suppression and freezing as measures of aversive Pavlovian conditioning: effects of discrete amygdala lesions and overtraining. Behav Brain Res 159:221–233. 10.1016/j.bbr.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Liu J, Totty MS, Melissari L, Bayer H, Maren S (2022) Convergent coding of recent and remote fear memory in the basolateral amygdala. Biol Psychiatry 91:832–840. 10.1016/j.biopsych.2021.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Jean-Richard-dit-Bressel P, Roughley S, Vissel B, Balleine BW, Killcross S, Bradfield LA (2020) Medial orbitofrontal cortex regulates instrumental conditioned punishment, but not Pavlovian conditioned fear. Cereb Cortex Commun 1:tgaa039. 10.1093/texcom/tgaa039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (1999) Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci 19:8696–8703. 10.1523/JNEUROSCI.19-19-08696.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi L, Hagihara KM, Courtin J, Hinz J, Müller C, Fustiñana MS, Xu C, Karalis N, Lüthi A (2023) Disynaptic specificity of serial information flow for conditioned fear. Sci Adv 9:eabq1637. 10.1126/sciadv.abq1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue MG, LeDoux JE, Cain CK (2014) Medial amygdala lesions selectively block aversive Pavlovian–instrumental transfer in rats. Front Behav Neurosci 8:329. 10.3389/fnbeh.2014.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA (2010) Contributions of the amygdala central nucleus and ventrolateral periaqueductal grey to freezing and instrumental suppression in Pavlovian fear conditioning. Behav Brain Res 211:111–117. 10.1016/j.bbr.2010.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA, Galarce EM (2011) Measuring Pavlovian fear with conditioned freezing and conditioned suppression reveals different roles for the basolateral amygdala. Brain Res 1374:82–89. 10.1016/j.brainres.2010.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulka P, Kendall P, Constantine J, Porterfield L (1972) The effect of Pavlovian CS+ and CS– on exploratory behavior. Psychon Sci 27:308–310. 10.3758/BF03328973 [DOI] [Google Scholar]
- Mitchell JR, Trettel SG, Li AJ, Wasielewski S, Huckleberry KA, Fanikos M, Golden E, Laine MA, Shansky RM (2022) Darting across space and time: parametric modulators of sex-biased conditioned fear responses. Learn Mem 29:171–180. 10.1101/lm.053587.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY (1956) Some comments on the analysis of emotional behavior. Psychol Rec 6:51–56. 10.1007/BF03393276 [DOI] [Google Scholar]
- Mowrer OH, Aiken EG (1954) Contiguity vs drive-reduction in conditioned fear: temporal variations in conditioned and unconditioned stimulus. Am J Psychol 67:26–38. 10.2307/1418069 [DOI] [PubMed] [Google Scholar]
- Nader K, LeDoux J (1999) The dopaminergic modulation of fear: quinpirole impairs the recall of emotional memories in rats. Behav Neurosci 113:152–165. 10.1037//0735-7044.113.1.152 [DOI] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE (2001) Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem 8:156–163. 10.1101/lm.38101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmier JB, Leaf RC (1965) Effects of discriminative Pavlovian fear conditioning upon previously or subsequently acquired avoidance responding. J Comp Physiol Psychol 60:213–217. 10.1037/h0022340 [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G (1980) A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev 87:532–552. 10.1037/0033-295X.87.6.532 [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M (2009) Central, but not basolateral, amygdala is critical for control of feeding by aversive learned cues. J Neurosci 29:15205–15212. 10.1523/JNEUROSCI.3656-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Maren S (2008) Associative structure of fear memory after basolateral amygdala lesions in rats. Behav Neurosci 122:1284–1294. 10.1037/a0012903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Orsini CA, Zimmerman JM, Maren S (2009) The amygdala is not necessary for unconditioned stimulus inflation after Pavlovian fear conditioning in rats. Learn Mem 16:645–654. 10.1101/lm.1531309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA (1967) Pavlovian conditioning and its proper control procedures. Psychol Rev 74:71–80. 10.1037/h0024109 [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1968) Probability of shock in the presence and absence of CS in fear conditioning. J Comp Physiol Psychol 66:1–5. 10.1037/h0025984 [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1969) Pavlovian conditioned inhibition. Psychol Bull 72:77–94. 10.1037/h0027760 [DOI] [Google Scholar]
- Rescorla RA (1974) Effect of inflation of the unconditioned stimulus value following conditioning. J Comp Physiol Psychol 86:101–106. 10.1037/h0035964 [DOI] [Google Scholar]
- Rescorla RA (1980) Pavlovian second-order conditioning. London: Psychology. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1988) Pavlovian conditioning: it's not what you think it is. Am Psychol 43:151–160. 10.1037//0003-066x.43.3.151 [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Lolordo VM (1965) Inhibition of avoidance behavior. J Comp Physiol Psychol 59:406–412. 10.1037/h0022060 [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR (1972) A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Classical conditioning II: Current research and theory, pp 64–99. New York: Appleton-Century-Crofts. [Google Scholar]
- Ressler KJ, Berretta S, Bolshakov VY, Rosso IM, Meloni EG, Rauch SL, Carlezon WA (2022) Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol 18:273–288. 10.1038/s41582-022-00635-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizley RC, Rescorla RA (1972) Associations in second-order conditioning and sensory preconditioning. J Comp Physiol Psychol 81:1–11. 10.1037/h0033333 [DOI] [PubMed] [Google Scholar]
- Seligman ME, Maier SF, Solomon RL (1971) Unpredictable and uncontrollable aversive events. In: Aversive conditioning and learning (Brush FR, ed), pp 347–400. San Diego: Academic. [Google Scholar]
- Totty MS, Warren N, Huddleston I, Ramanathan KR, Ressler RL, Oleksiak CR, Maren S (2021) Behavioral and brain mechanisms mediating conditioned flight behavior in rats. Sci Rep 11:8215. 10.1038/s41598-021-87559-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Spooner MJ, Delaney AJ, Westbrook RF, Holmes NM (2022) Prediction error determines whether NMDA receptors in the basolateral amygdala complex are involved in Pavlovian fear conditioning. J Neurosci 42:4360–4379. 10.1523/JNEUROSCI.2156-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]