Abstract
Dopamine is a key neurotransmitter in the signaling cascade controlling ocular refractive development, but the exact role and site of action of dopamine D1 receptors (D1Rs) involved in myopia remains unclear. Here, we determine whether retinal D1Rs exclusively mediate the effects of endogenous dopamine and systemically delivered D1R agonist or antagonist in the mouse form deprivation myopia (FDM) model. Male C57BL/6 mice subjected to unilateral FDM or unobstructed vision were divided into the following four groups: one noninjected and three groups that received intraperitoneal injections of a vehicle, D1R agonist SKF38393 (18 and 59 nmol/g), or D1R antagonist SCH39166 (0.1 and 1 nmol/g). The effects of these drugs on FDM were further assessed in Drd1-knock-out (Drd1-KO), retina-specific conditional Drd1-KO (Drd1-CKO) mice, and corresponding wild-type littermates. In the visually unobstructed group, neither SKF38393 nor SCH39166 affected normal refractive development, whereas myopia development was attenuated by SKF38393 and enhanced by SCH39166 injections. In Drd1-KO or Drd1-CKO mice, however, these drugs had no effect on FDM development, suggesting that activation of retinal D1Rs is pertinent to myopia suppression by the D1R agonist. Interestingly, the development of myopia was unchanged by either Drd1-KO or Drd1-CKO, and neither SKF38393 nor SCH39166 injections, nor Drd1-KO, affected the retinal or vitreal dopamine and the dopamine metabolite DOPAC levels. Effects on axial length were less marked than effects on refraction. Therefore, activation of D1Rs, specifically retinal D1Rs, inhibits myopia development in mice. These results also suggest that multiple dopamine D1R mechanisms play roles in emmetropization and myopia development.
SIGNIFICANCE STATEMENT While dopamine is recognized as a “stop” signal that inhibits myopia development (myopization), the location of the dopamine D1 receptors (D1Rs) that mediate this action remains to be addressed. Answers to this key question are critical for understanding how dopaminergic systems regulate ocular growth and refraction. We report here the results of our study showing that D1Rs are essential for controlling ocular growth and myopia development in mice, and for identifying the retina as the site of action for dopaminergic control via D1Rs. These findings highlight the importance of intrinsic retinal dopaminergic mechanisms for the regulation of ocular growth and suggest specific avenues for exploring the retinal mechanisms involved in the dopaminergic control of emmetropization and myopization.
Keywords: dopamine D1 receptor, gene knock-out mice, myopia, retina
Introduction
Myopia (near-sightedness) is the most common refractive error worldwide. The prevalence of myopia has steadily increased in recent years, particularly in East and Southeast Asia (Dolgin, 2015; Holden et al., 2016). Ocular pathologies associated with high myopia (refraction more negative than −6D) can lead to severe complications, including retinal detachment, glaucoma, and even blindness (Ohno-Matsui et al., 2016). Better understanding of the mechanisms underlying this condition is needed, to make possible better methods for controlling its progression.
A wide range of myopia research has identified dopamine (DA) in the retina as a key neurotransmitter involved in the signaling cascade that regulates refractive development and ocular growth. Retinal levels of DA are reduced during form deprivation myopia (FDM), and administration of DA, or the nonselective DA agonist apomorphine, attenuates FDM development in various animal models (Iuvone et al., 1989, 1991; Dong et al., 2011; Yan et al., 2015; Lan et al., 2016). These findings suggest that the development of FDM is mediated by insufficient release of DA in the retina, as the release of DA or the addition of DA agonist prevents myopia progression.
DA exerts its effects through two groups of DA receptors: D1-like [D1 receptor (D1R) and D5R] and D2-like (D2R, D3R, and D4R). The role of D1Rs in FDM development is currently controversial, since the results of several early studies in chickens suggested that only D2Rs take part in the regulation of ocular growth (Rohrer et al., 1993; McCarthy et al., 2007; Nickla and Totonelly, 2011). However, the importance of D1Rs in regulating myopia development was suggested by the findings that D1Rs play a predominant role in modulating visual acuity and refractive development in mammals, combined with a recent genome-wide association study identifying Drd1 as one of the genes associated with refractive error in humans (Hysi et al., 2020).
Studies conducted in chicks revealed that daily intravitreal injection of D1R antagonist SCH23390 inhibited the ameliorating effects of periods of unobstructed vision on lens-induced myopia (Nickla et al., 2010) and blocked the development of FDM (Schaeffel et al., 1995). By contrast, we found that inhibition of FDM in mice by the nonselective DA agonist apomorphine was blocked by knockout of the Drd1 gene (Drd1-KO) but not of the Drd2 gene (Drd2-KO; Huang et al., 2018), suggesting that activation of D1Rs specifically inhibited myopia in mice. In line with this, activation of D1Rs by SKF38393 inhibited spontaneous or experimentally induced myopia in guinea pigs (Jiang et al., 2014; Zhang et al., 2018). These discrepancies reflect the complexity of D1R signaling in myopia development, which tends to vary with differences in the pharmacokinetics and specificity of D1R drugs in different animal species. Many variables influence the pharmacokinetics of drug delivery, and little is known about the intraocular distribution of locally or systemically administered drugs. As the D1Rs are widely expressed in a variety of ocular tissues including retina, iris, ciliary body, uveoscleral tissue, trabecular meshwork, and cornea (Bucolo et al., 2019), all of these structures are potential sites of action of D1R-targeted pharmacological intervention in ocular growth and myopia. Furthermore, the D1R drugs also appear to interact with other neurotransmitter receptors at multiple sites (Seeman and Van Tol, 1993), and these also are plausible candidates for myopia control. Therefore, to explore the D1R-mediated mechanisms involved in myopia, the exact role and site of action of D1Rs in control of visual growth and myopia development need to be evaluated using a targeted approach.
As an extension of our previous study on the site of action of D2Rs in myopia development (Huang et al., 2022), this study further tests the hypothesis that the effects of endogenous dopamine and systemically administered D1R agonist or antagonist on FDM in mice are mediated exclusively by D1Rs in the retina using transgenic mouse models combined with pharmacological intervention.
Materials and Methods
Experimental design
Animals.
Animals were reared and treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and all procedures were approved by the Animal Care and Use Committees of Wenzhou Medical University. Mice were maintained under a 12 h light/dark cycle at an ambient illumination of ∼300 lux provided by fluorescent light (36 W; TL-D LIFEMAX, PHILIPS) during the daytime with abundant food and water. Male C57BL/6 mice were purchased from GemPharmatech.
Drd1-KO mice.
The Drd1-KO mice (Drd1tm1a(KOMP)Wtsi; catalog #4451251, MGI; The Knockout Mouse Project, Mouse Biology Program, University of California, Davis; Skarnes et al., 2011; Assali et al., 2021) were backcrossed for up to 10 generations with C57BL/6J to maintain a C57BL/6J genetic background. Briefly, an IRES:lacZ trapping cassette and a floxed promoter-driven neocassette were inserted upstream of the critical exon 2 to generate a germline KO allele, resulting in constitutive disruption of the translational frame of the gene and premature termination at the neo-poly A site (http://www.informatics.jax.org/allele/MGI:4451251). Heterozygous mice were bred to generate homozygous Drd1-KO and wild-type (Drd1-WT) littermates.
Retina-specific conditional Drd1-KO mice.
Drd1fl/fl mice were generated by removal of the gene-trap cassette from the above Drd1-KO mice by Flp recombinase, leaving loxP sites on either side of the critical exon 2. Further information on targeting strategies used for this allele can be found at http://www.informatics.jax.org/mgihome/nomen/IKMC_schematics.shtml. Conditional Drd1-KO (Drd1-CKO) mice (Six3creDrd1fl/fl) were then generated by crossing the Drd1fl/fl mice with Six3-Cre (a retinal cre, C57BL/6J * DBA/2 background; stock #019755, The Jackson Laboratory), resulting in deletion of the critical exon 2 to induce a frameshift mutation and trigger nonsense-mediated decay of the mutant transcript (Furuta et al., 2000). The Cre-negative (Drd1fl/fl) littermate mice served as controls.
Gene expression analyses
The Drd1 and Drd2 mRNA expression levels in the cornea, lens, neural retina, and sclera of Drd1-CKO and their Drd1fl/fl littermates were determined by quantitative real-time PCR (qRT-PCR) analysis, as previously described (Huang et al., 2022). The primer sequences were (1) for Drd1 (targeting the transcripts that were deleted in Drd1-CKO mice): 5′-CACGGCATCCATCCTTAACCT-3′ (forward) and 5′-TGCCTTCGGAGTCATCTTCCT-3′ (reverse); (2) for Drd2: 5′-CCCTGGGTCGTCTATCTGGAG-3′ (forward) and 5′-GCGTGTGTTATACAACATAGGCA-3′ (reverse); and (3) for 18s rRNA: 5′-CGGACACGGACAGGATTGAC-3′ (forward) and 5′-GTTCAAGCTGCCCGTCTCCTCATC-3′ (reverse).
RNAscope in situ hybridization
In situ hybridization (ISH) was performed on frozen sections of retinas fixed for 24 h with 4% paraformaldehyde in 0.1 m PBS at 4°C and embedded in optimal cutting temperature (OCT) compound (Sakura). Sections were labeled using an RNAscope Kit [RNAscope Multiplex Fluorescent Reagent Kit v2; catalog #323100, Advanced Cell Diagnostics (ACD)], as directed by the manufacturer. After hybridization and amplification, the samples were counterstained with RNAscope Probe-Mm-Drd1 (catalog #461901, ACD), which was designed to detect the deleted target region (521–1524 bp) in both Drd1-KO and Drd1-CKO mice. Positive and negative controls were conducted simultaneously in each experiment to verify the reliability of the RNAscope analysis (positive control probe Mm-Polr2a; catalog #320881, ACD; and negative control probe DapB; catalog #320871, ACD). The images were obtained with a microscope (model LSM 880, Zeiss).
Electroretinographic recording
Electroretinograms (ERGs) were measured with a RETI-port ERG system (Ganzfeld Q450 SC, Roland Consult) as previously reported (Huang et al., 2018). Initially, white LED stimuli of five flashing light stimuli of relatively low intensities (−3.699, −2.201, −0.699, 0.301, and 0.799 log cd · s/m2) were used to induce scotopic responses. Subsequently, stronger illumination (1.398 log cd/m2) was applied for 10 min for light adaptation, and then five stimuli of higher intensity (−0.699, −0.201, 0.301, 0.799, and 1.301 log cd · s/m2) were used to induce photopic responses.
Form deprivation myopia and drug administration
To induce FDM, a handmade translucent occluder was attached to the fur around the left eye, using glue, at postnatal day 28 (Huang et al., 2018, 2022); the contralateral fellow eye was untreated. A plastic collar was fitted around the neck to prevent the mouse from removing the occluder. D1R agonist SKF38393 (18 and 59 nmol/g body weight) and D1R antagonist SCH39166 (0.1 and 1 nmol/g body weight; both from Tocris Bioscience) were dissolved in distilled H2O containing 6 μmol/ml ascorbic acid (ICN Biomed) and 1 μmol/ml dimethylsulfoxide (DMSO; Sigma-Aldrich), before daily intraperitoneal injections. The pharmacological doses were chosen to achieve effective drug concentrations in the CNS, on the basis of our study (Chen et al., 2017) and other studies (Bratcher et al., 2005; Servonnet et al., 2021). All injections (10 µl/g body weight of the animal) were administered at 9:00 A.M. Noninjected control group and groups injected with vehicle alone (6 μmol/ml ascorbic acid or 1 μmol/ml DMSO) are abbreviated as “Con” and “Veh,” respectively. The two different doses of D1R agonist and D1R antagonist are abbreviated as “18SKF” or “59SKF,” and “0.1SCH” or “1SCH,” respectively.
Measurement of refractive error and ocular biometry
Refractive error in both eyes of each animal was measured in a darkened room, as previously described (Zhou et al., 2008), with an eccentric infrared photorefractor designed by Schaeffel et al. (2004) that is customized for the mouse eye and was calibrated regularly. Briefly, each unanesthetized mouse was placed on a small, elevated platform and gently restrained by holding its tail, and its position was adjusted until the first Purkinje image was clearly shown in the center of the pupil, indicating an on-axis measurement. The reported refractive error of each eye is the average of values from at least three measurements. Interocular differences in every form-deprived (FD) animals but only absolute values of the right eyes in the visually unobstructed animals were used for statistical analyses.
A custom-made, ultra-long depth, spectral-domain optical coherence tomography instrument was used to measure anterior chamber depth, lens thickness, vitreous chamber depth (VCD), axial length (AL; from front of the cornea to front of the retina), and anterior corneal radius of curvature (Huang et al., 2018, 2022). After being anesthetized with a mixture of 0.6% pentobarbital and 0.2% xylazine (10 µl/g body weight) delivered intraperitoneally, the mouse was placed on a stage in front of a modified slit lamp and scanned along the optical axis of the eye with an X-Y cross-scanning system. The raw data were exported and analyzed using custom-designed software to obtain the axial components and the anterior corneal radius of curvature. The value of each parameter was taken as the mean of values in three recorded OCT images.
High-performance liquid chromatography
Levels of retinal and vitreal DA and its major metabolite, dihydrophenylacetic acid (DOPAC), were quantified using high-performance liquid chromatography (HPLC) with electrochemical detection (Thermo Fisher Scientific). All samples for HPLC analysis were collected under strictly controlled illumination levels of 300 lux provided by LED lights (15 W; TL-D LIFEMAX, PHILIPS) at zeitgeber 1 (ZT1) to ZT2 (daytime, 1–2 h into the light period, when retinal DA levels reach the daily peak; Doyle et al., 2002). Retinas and vitreous bodies (collected using an Eppendorf pipette with a 10 μl pipette tip) were harvested quickly under a dissecting microscope and immediately frozen in liquid nitrogen. Each frozen sample was transferred into 100 μl (for the retina) or 15 μl (for the vitreous body) of ice-cold 0.1 m perchloric acid, containing 10 μm ascorbic acid, 0.1 mm EDTA disodium salt, and 0.02 μm 3,4-dihydroxybenzylamine. DA and DOPAC levels were assessed with an neurotransmitter analyzer (1200 series, Agilent Technologies) as previously reported (Zhang et al., 2018). The data were collected and analyzed by ChemStation (Agilent Technologies). In addition, the DOPAC/DA ratio was calculated as a measure of DA turnover.
Statistics
The Shapiro–Wilk normality test was used to analyze the distribution of all datasets. For comparisons of two groups, independent datasets were compared by unpaired two-tailed t tests or nonparametric Mann–Whitney U tests, according to the results of normality testing. For multiple comparisons with repeated measures, two-way or three-way repeated-measures ANOVA was performed with or without Geisser–Greenhouse correction, depending on conditions for sphericity statistics, and Bonferroni corrections were applied in post hoc analyses. p < 0.05 was considered significant (GraphPad Prism 8.0). Data are presented as the mean ± SEM.
Results
D1R agonist SKF38393 prevents FDM but has no effect on normal refractive development
Effects of SKF38393 in C57BL/6 mice under unobstructed vision and unilateral FD
Intraperitoneal injections of SKF38393 (18SKF or 59SKF), administered daily for 4 weeks (Fig. 1A,B), did not significantly affect body weight, refraction, or other ocular biometric parameters in the visually unobstructed group (p > 0.05, two-way repeated-measures ANOVA; Fig. 1C–G). Similarly nonsignificant were the baseline values of these parameters across all FD groups (all p values > 0.05; Fig. 1H–K). After 4 weeks of treatment, the FD-induced myopic shift was similar in the FD-Con and FD-Veh groups (p > 0.05), indicating that vehicle injections had no effects on myopia development. In contrast, the myopic shift was significantly less in the FD-18SKF and FD-59SKF groups than in the FD-Veh group (p < 0.001). In parallel, VCD (p = 0.035 and p = 0.004) and AL (p = 0.010 and p = 0.001) elongations were also less in the FD-18SKF and FD-59SKF groups, respectively, than in the FD-Veh group. No significant interocular differences in corneal radius of curvature, anterior chamber depth, or lens thickness were observed among different FD groups after treatment with SKF38393 for 4 weeks (p > 0.05; Fig. 1K, data for anterior chamber depth or lens thickness not shown). Thus, treatment with this D1R agonist reliably and partially attenuated the FD-induced changes in refraction, VCD and AL.
Figure 1.
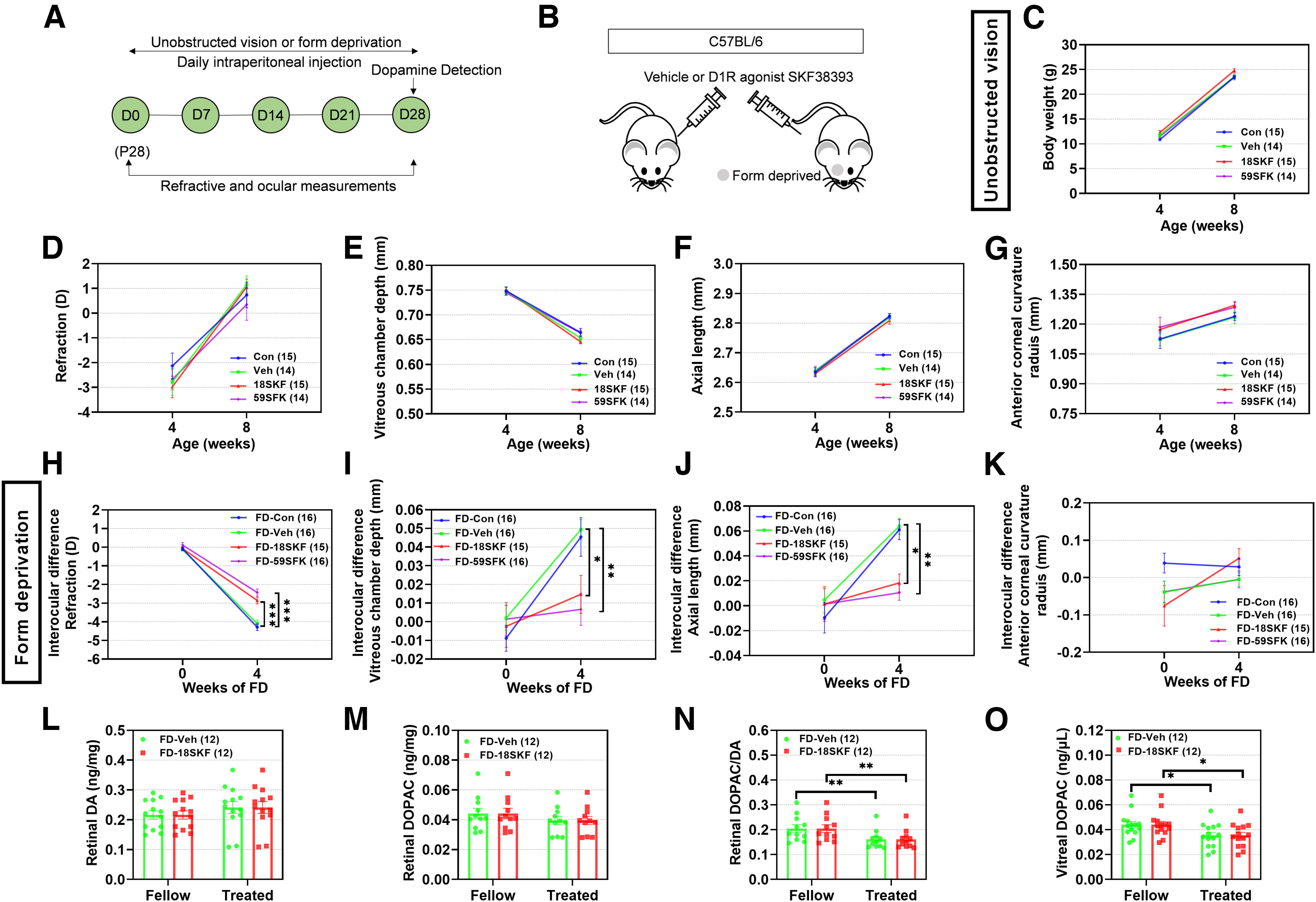
D1R agonist SKF38393 prevents FDM but has no effect on normal refractive development in C57BL/6 mice with unobstructed vision. A, Timeline of the experimental procedure and data collection. D, Day of experimental treatment (e.g., D0). B, Sketch illustrating the experimental design for testing the effect of SKF38393 (18 and 59 nmol/g) on C57BL/6 mouse refractive development under unobstructed vision and FDM. C–G, Under unobstructed vision, absolute values for body weight (C), refraction (D), vitreous chamber depth (E), axial length (F), and corneal radius of curvature (G) were not affected by SKF38393. H–K, Under FDM, interocular differences in eyes treated with FD-SKF38393, compared with FD-Veh and FD-Con eyes, revealed significantly lower myopia (H), with smaller vitreous chamber depth (I), shorter axial length (J), and no difference in corneal radius of curvature (K). L–O, Assayed amounts of retinal and vitreal DA and DOPAC: retinal DA (L), DOPAC (M), DOPAC/DA ratio (N), and vitreal DOPAC (O) were unaffected by treatment with FD-SKF38393. Numbers of animals (pairs of eyes) are shown in parentheses for each treatment condition. *p < 0.05, **p < 0.01, and ***p < 0.001, two-way repeated-measures ANOVA.
Retinal dopamine levels and metabolism
Neither FD nor SKF38393 injections affected the DA and DOPAC levels in the retina (p > 0.05, two-way repeated-measures ANOVA; Fig. 1L,M). The calculated retinal DOPAC/DA ratio is a measure of DA turnover, which is a surrogate for DA release in the retina, while vitreal DOPAC content is a direct indicator of retinal DA release. There was a significant decrease in both the retinal DOPAC/DA ratio and vitreal DOPAC levels after 4 weeks of FD (main effects of FDM: for retinal DOPAC/DA: F(1,22) = 25.83, p < 0.001; for vitreal DOPAC, F(1,22) = 14.72, p < 0.001; Fig. 1N,O). However, there were no significant differences in these levels when the FDM mice were injected with SKF38393, compared with those in the vehicle group (p > 0.05). Overall, endogenous DA release and turnover were unchanged by SKF38393 injections.
D1R antagonist SCH39166 promotes FDM but has no effect on normal refractive development
Effects of SCH39166 in C57BL/6 mice under unobstructed vision and unilateral FD
Four weeks of treatment with D1R antagonist (SCH39166: 0.1SCH or 1SCH; Fig. 2A,B) did not affect ocular refraction or biometric parameters under unobstructed vision, in any groups (p > 0.05; Fig. 2C–G). Under FD, the myopic shifts in refraction in the FD-Veh and FD-Con groups were not significantly different (p > 0.05; Fig. 2H). However, the myopic shift was significantly greater in the FD-0.1SCH group than in the vehicle group (p = 0.001). The myopic shift was even higher in the FD-1SCH group than in the vehicle-injected group (p < 0.001), indicating that D1R antagonist treatments dose-dependently enhanced the myopic shift in refraction. However, the increases of interocular differences in VCD (Fig. 2I) or AL (Fig. 2J) in the FD-0.1SCH or FD-1SCH mice were greater but not significantly different from those in the FD-Veh mice (p > 0.05). There were also no significant interocular differences in other biometric parameters among the different FD groups (p > 0.05; Fig. 2K).
Figure 2.
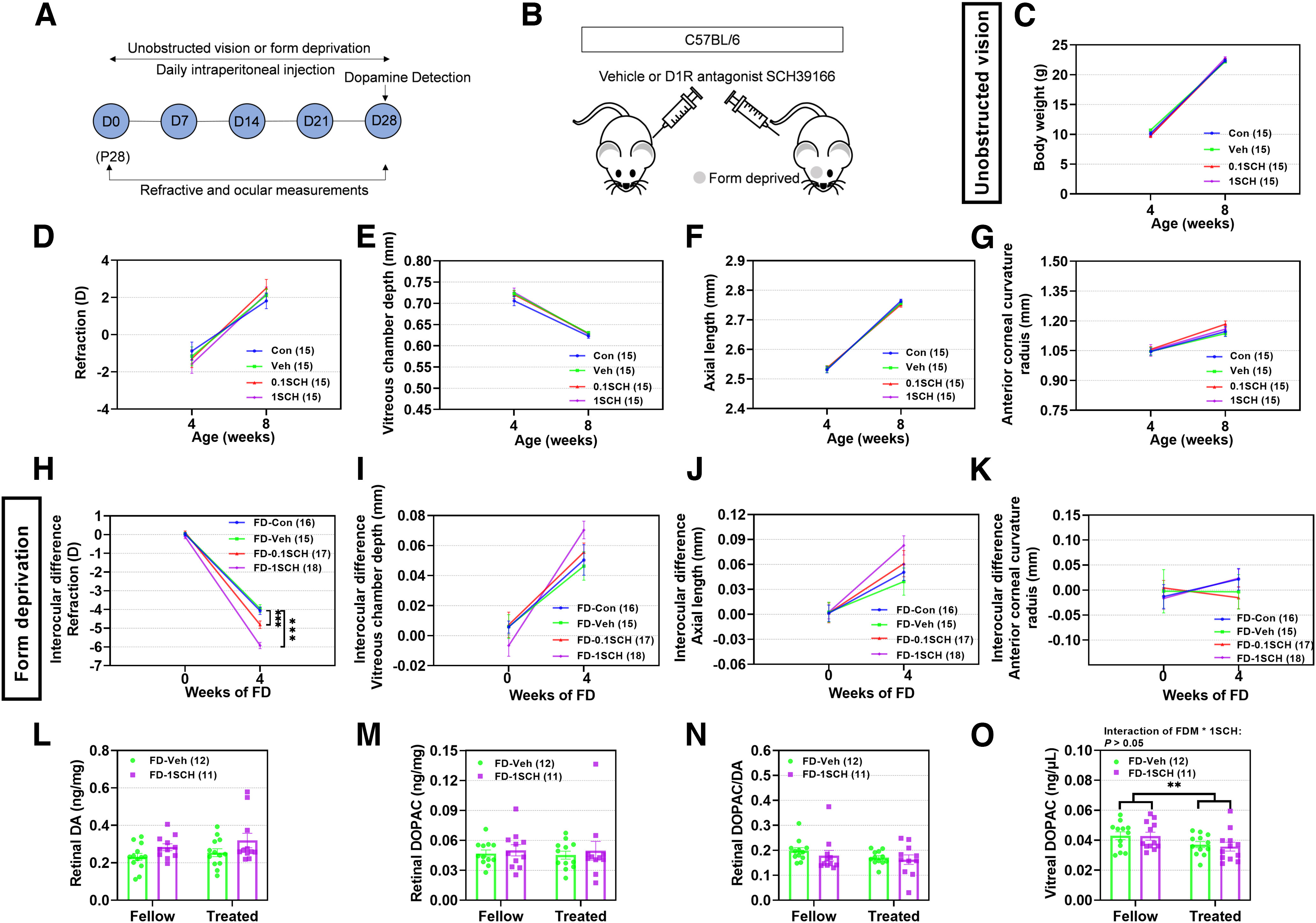
D1R antagonist SCH39166 promotes FDM but has no effect on normal refractive development, in C57BL/6 mice with unobstructed vision. A, Timeline of the experimental procedure and data collection. D, Day of experimental treatment (e.g., D0). B, Sketch illustrating the experimental design for testing the effect of SCH39166 (0.1 and 1 nmol/g) on C57BL/6 mouse refractive development under unobstructed vision and FDM. C–G, Under unobstructed vision, absolute values for body weight (C), refraction (D), vitreous chamber depth (E), axial length (F), and corneal radius of curvature (G) were not affected by SCH39166. H–K, Under FDM, interocular differences between eyes treated with FD-CH39166 and eyes treated with FD-Veh or FD-Con revealed significantly greater myopia (H), with trends toward increases in vitreous chamber depth (I) and axial length (J), and no difference in corneal radius of curvature (K). L–O, Assayed amounts of retinal and vitreal DA and DOPAC: retinal DA (L) and DOPAC (M) concentrations, DOPAC/DA ratio (N), and vitreal DOPAC concentration (O) were unaffected by treatment with FD-SCH39166. Numbers of animals (pairs of eyes) are shown in parentheses for each treatment condition. **p < 0.01, and ***p < 0.001, two-way repeated-measures ANOVA.
Retinal dopamine levels and metabolism
The DA and DOPAC levels and DOPAC/DA ratios in the retinas of form-deprived eyes were not different from those in the untreated fellow eyes, and they were unchanged by SCH39166 injections (p > 0.05; Fig. 2L–N). After 4 weeks of treatment, vitreal DOPAC levels in the FDM-treated eyes were significantly lower than those in the untreated fellow eyes (main effects of FDM: F(1,21) = 9.759, p = 0.005; Fig. 2O). However, there were no significant differences between these levels in SCH39166-injected and vehicle-injected animals (p > 0.05). Thus, SCH39166 injections, like those of SKF38393, had no significant effect on retinal DA release or turnover.
Suppression of FDM by SKF38393 is mediated by activation of D1Rs
The receptor specificities of these DA drugs on FDM remain unclear, as both SKF38393 and SCH39166 interact with other subtypes of D1-like receptor D5R or even other neurotransmitter receptors (Seeman and Van Tol, 1993; Jiang et al., 2014). Thus, we used a D1R subtype-specific knock-out mouse, Drd1-KO, and a lower dose of SKF38393 (18 nmol/g), which inhibited FDM just as well as the higher dose, to determine whether D1Rs alone mediated these effects of DA drugs on FDM.
First, to verify the efficiency of deletion in the Drd1-KO retina, we used Drd1 RNAscope chromogenic ISH on sections of mouse retinas. In C57BL/6 mice, Drd1 transcripts were detected in the inner retina—almost exclusively throughout all levels of the INL—but were absent from photoreceptor nuclei and cell bodies in the ONL (Fig. 3A), which is in agreement with previous findings (Popova, 2014; Farshi et al., 2016; Travis et al., 2018). It is noteworthy, however, that strong labeling was observed at the level of photoreceptor inner segments, just external to the ONL and outer limiting membrane for the positive control (Mm-Polr2a; data not shown). Importantly, no signal was detected when the same Drd1 probe was applied to the retinas of Drd1-KO mice, indicating that the target region is not expressed in the retinas of Drd1-KO mice.
Figure 3.
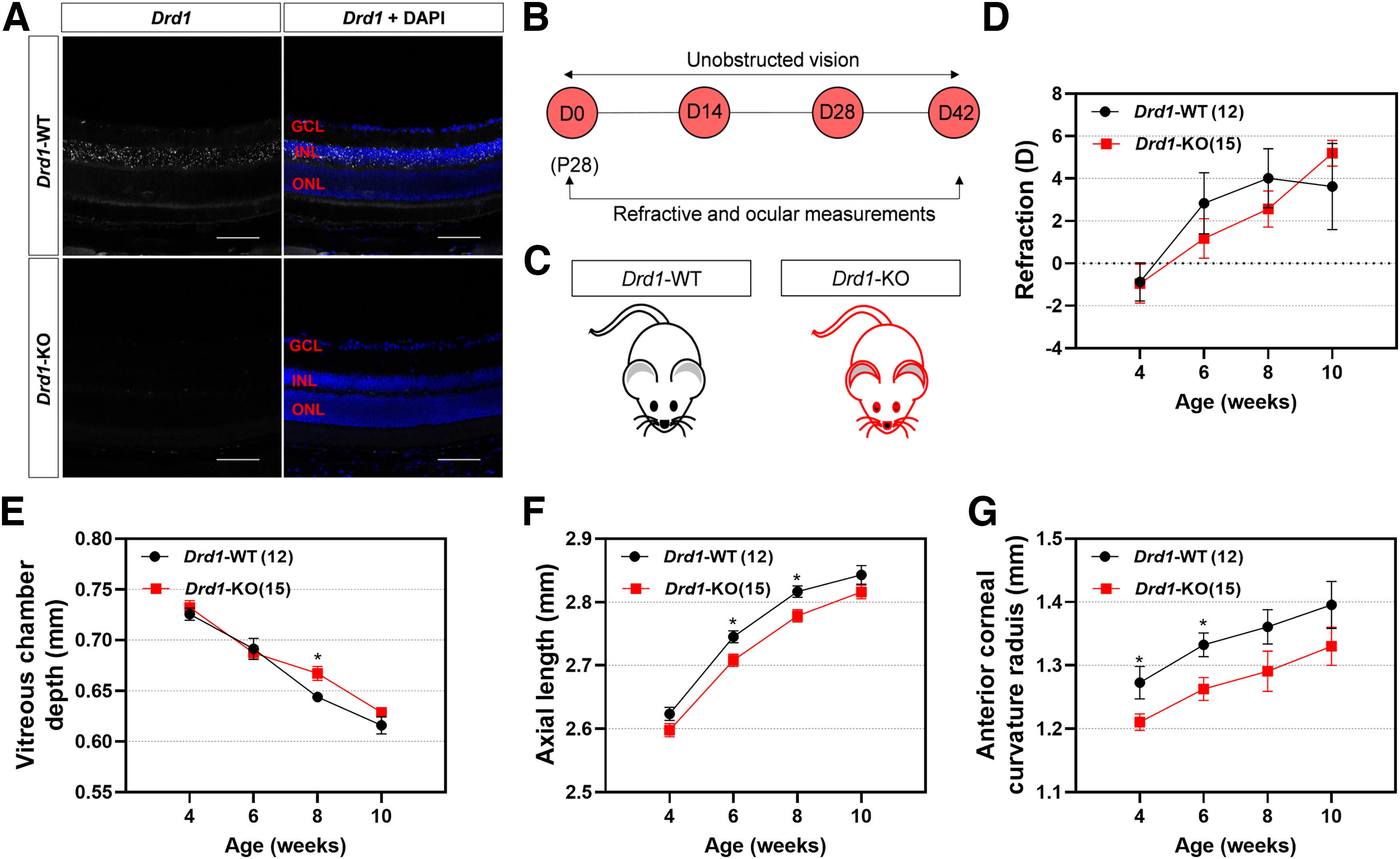
Effects of Drd1-KO on normal refractive development with unobstructed vision. A, Fluorescence in situ hybridization produced no detectable labeling of Drd1 mRNA in Drd1-KO retina. Scale bar, 100 µm. B, Timeline of the experimental procedure and data collection. C, Diagram of Drd1-KO and breed-matched Drd1-WT mice. D–G, Biometric measurements in Drd1-KO and Drd1-WT groups from 4 to 10 weeks old: refraction (D), vitreous chamber depth (E), axial length (F), and corneal radius of curvature (G). *p < 0.05, two-way repeated ANOVA.
Effects of Drd1-KO on normal refractive development
The refractive development was similar in Drd1-KO and Drd1-WT mice (p > 0.05; Fig. 3B–D). However, some ocular measurements also increased at slower rates in the Drd1-KO than in the Drd1-WT mice, as revealed by shorter AL and smaller corneal radius of curvature compared with Drd1-WT mice (Fig. 3E–G, details).
Effects of SKF38393 in Drd1-KO mice under unilateral FD
As previously reported (Huang et al., 2018), the body weights of Drd1-KO mice were less than those of Drd1-WT mice (p < 0.001; Fig. 4C). After 4 weeks of FD, the myopic shift in the Drd1-WT-18SKF group was significantly smaller than that in the Drd1-WT-Veh group (p < 0.001; Fig. 4D). Strikingly, there was no significant difference in refraction between the Drd1-KO-Veh and Drd1-KO-18SKF groups (p > 0.05). In agreement with the results of our previous study (Huang et al., 2018), the myopic shifts in the Drd1-WT-Veh and Drd1-KO-Veh groups were not significantly different (p > 0.05). The increase in AL in the Drd1-WT-18SKF group was smaller than that in the Drd1-WT-Veh group (p < 0.001; Fig. 4F), whereas the interocular differences in AL in the Drd1-KO-Veh and Drd1-KO-18SKF groups were not significantly different (p > 0.05). None of the treatments affected other biometric parameters (p > 0.05; Fig. 4G).
Figure 4.
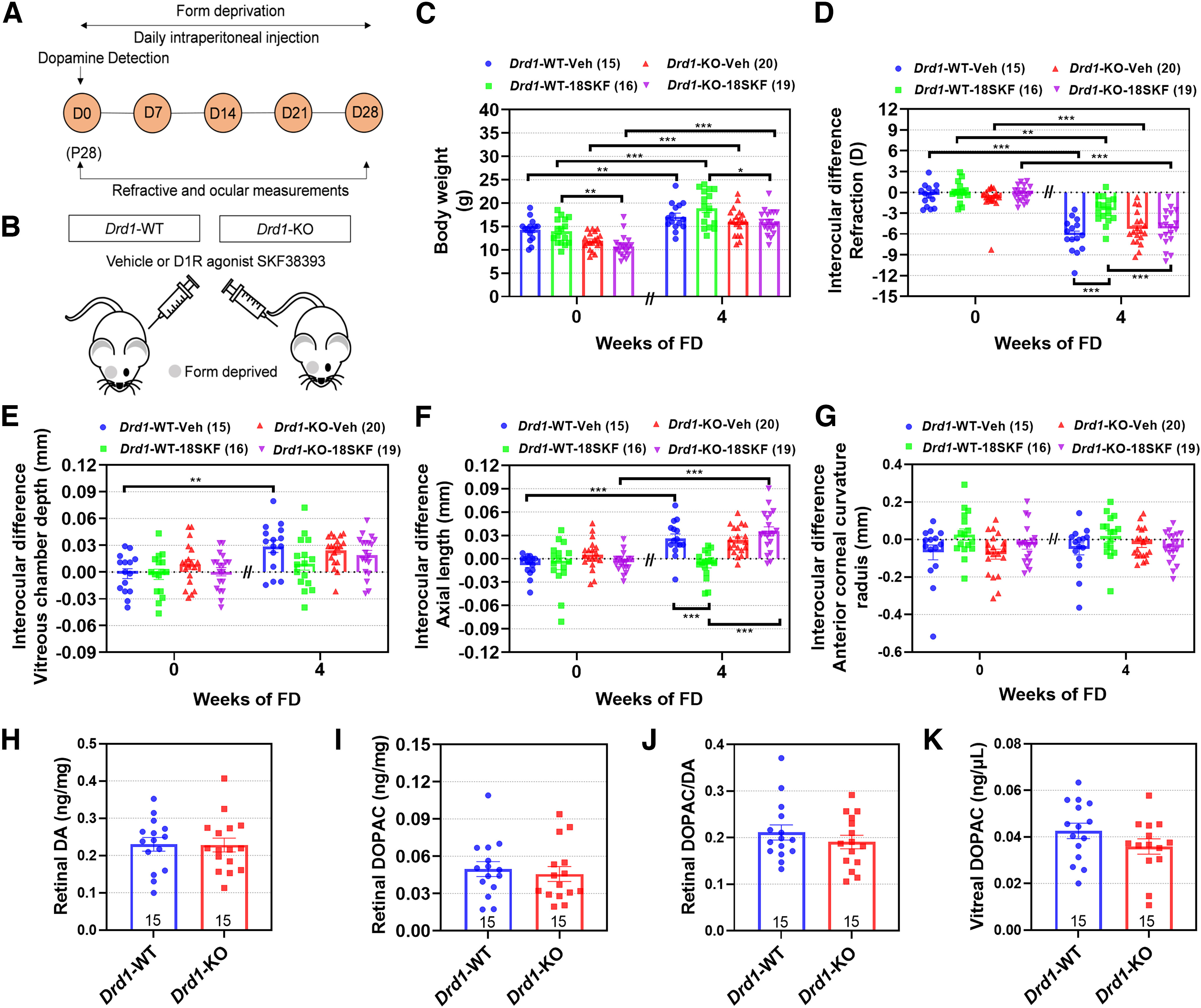
Activation of D1Rs by SKF38393 suppresses FDM development in Drd1-KO mice. A, Flow diagram of experimental procedure and data collection. D, Day of experimental treatment (e.g., D0). B, Schematic illustrating intraperitoneal injections of SKF38393 (18 nmol/g) in Drd1-KO mice with FDM. C–G, Biometric measurements in Drd1-WT-Veh, Drd1-WT-18SKF, Drd1-KO-Veh, and Drd1-KO-18SKF groups, before and after 4 weeks of each treatment (4 and 8 weeks old, respectively). C, Body weight. D–G, Interocular differences (FD – fellow eye) in the following: refraction (D), vitreous chamber depth (E), axial length (F), and anterior corneal radius of curvature (G). H–K, Quantification of retinal and vitreal DA and DOPAC: retinal DA (H) and DOPAC (I) concentrations, DOPAC/DA ratio (J), and vitreal DOPAC concentration (K) were all unaffected by Drd1-KO. Numbers of animals (pairs of eyes) are shown in parentheses (C–G) or at the bottom of the bar chart (H–K). *p < 0.05, **p < 0.01, and ***p < 0.001; three-way repeated ANOVA (C–G), Mann–Whitney U tests (I), or independent t tests (H, J, K) were applied as required.
Retinal dopamine levels and metabolism
No significant differences were observed in retinal or vitreal DA or DOPAC levels, between Drd1-WT and Drd1-KO (p > 0.05, independent t tests for retinal DA and DOPAC/DA, and vitreal DOPAC; Mann–Whitney U tests for retinal DOPAC; Fig. 4H–K).
Promotion of FDM by SCH39166 is mediated by blockade of D1Rs
Since SCH39166 showed a dose dependency in inducing myopia, the higher dose (1 nmol/g) was injected in Drd1-KO mice to determine the receptor specificities of SCH39166 on FDM (Fig. 5A,B). As described above, body weights in the Drd1-KO mice were lower than those in Drd1-WT mice (p < 0.001; Fig. 5C), and the myopic shifts in Drd1-WT-Veh and Drd1-KO-Veh groups were not significantly different (p > 0.05). After 4 weeks of FD, the myopic shift was greater in Drd1-WT-1SCH than in Drd1-WT-Veh mice (p = 0.020; Fig. 5D). In contrast, there was no significant difference in refraction between the Drd1-KO-Veh and Drd1-KO-1SCH groups (p > 0.05). In parallel with the refractive changes, the interocular differences in VCD and AL tended to be greater in Drd1-WT-1SCH than in Drd1-WT-Veh mice, but these differences were not statistically significant (p > 0.05; Fig. 5E,F). After 4 weeks of either treatment, the interocular differences in other biometric parameters, between any two groups, were not significantly different (p values > 0.05; Fig. 5G). These results suggest that the augmentation of myopia by SCH39166 is mediated by antagonism of intrinsic DA action on D1Rs.
Figure 5.
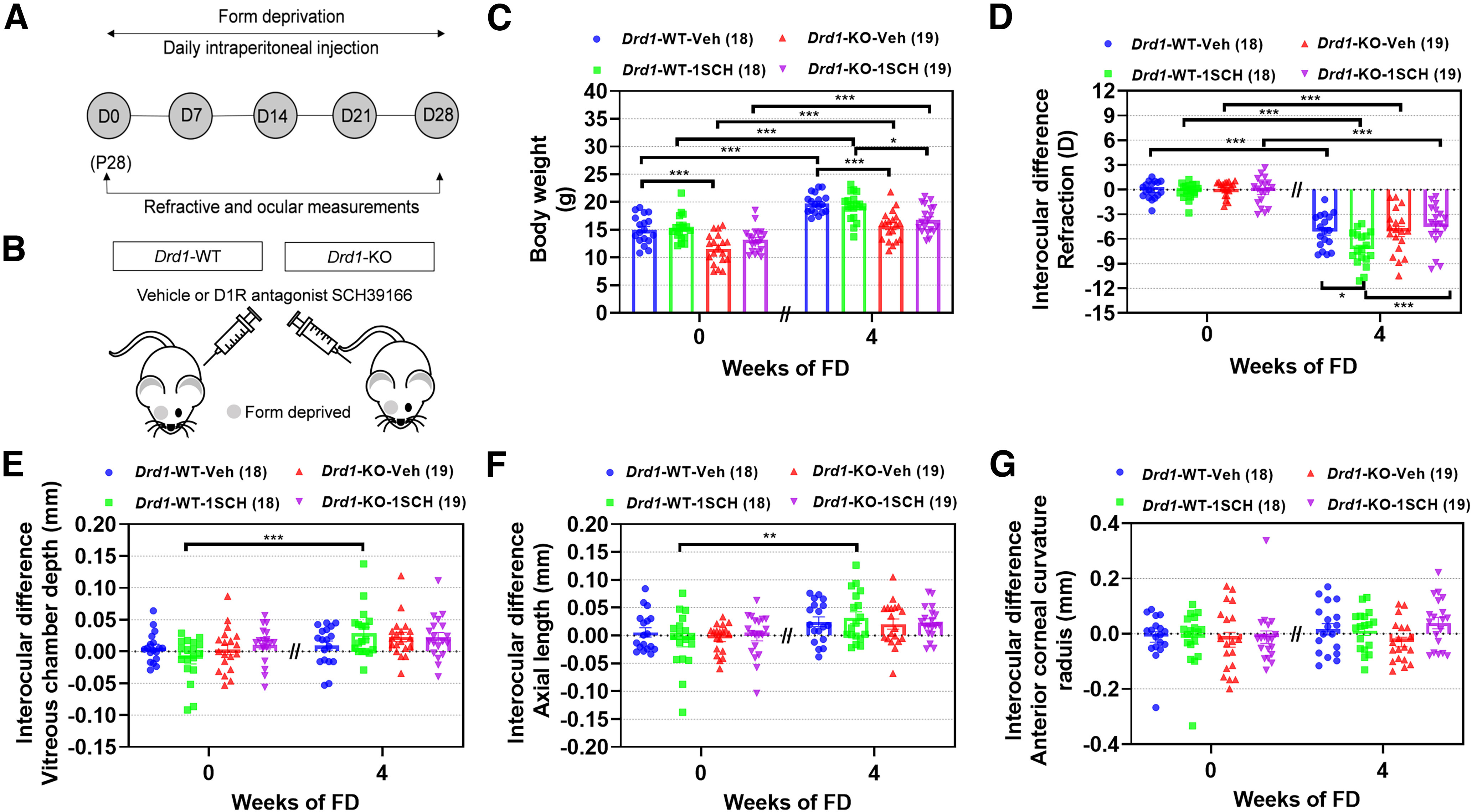
Blockade of D1R activation by SCH39166 promotes FDM development in Drd1-KO mice. A, Flow diagram of experimental procedure and data collection. D, Day of experimental treatment (e.g., D0). B, Schematic illustrating intraperitoneal injections of SCH39166 (1 nmol/g) in Drd1-KO mice with FDM. C–G, Biometric measurements in Drd1-WT-Veh, Drd1-WT-1SCH, Drd1-KO-Veh, and Drd1-KO-1SCH groups, before and after 4 weeks of each treatment (4 and 8 weeks old, respectively). C, Body weight. D–G, Interocular differences (FD – fellow eye) in the following: refraction (D), vitreous chamber depth (E), axial length (F), and anterior corneal radius of curvature (G). Numbers of animals (pairs of eyes) are shown in parentheses for each treatment condition. *p < 0.05, **p < 0.01, and ***p < 0.001, three-way repeated ANOVA.
D1Rs located in the retina mediate the prevention of FDM by SKF38393
The antimyopic effects of DA agonists could be mediated by the D1Rs in the retina or in other ocular tissues (e.g., choroid, sclera). Therefore, to further clarify whether the activation of specific retinal D1Rs inhibits myopia development, we tested the effects of SKF38393 on FDM in Drd1-CKO mice.
Analysis by qRT-PCR showed that retinal Drd1 gene expression was nearly abolished in Drd1-CKO mice and was significantly lower than that in the Drd1fl/fl littermates (p < 0.001; independent t tests for cornea, retina, and sclera; Mann–Whitney U tests for lens; Fig. 6A). In contrast, neither retinal Drd2 gene expression nor Drd1 expression level in the cornea, lens, or sclera was affected by Drd1-CKO (p > 0.05; Fig. 6A,B). Furthermore, no Drd1 transcripts were detected by Drd1 probe in the retinas of Drd1-CKO mice (Fig. 6C), whereas a prominent layer of Drd1 mRNA was detected in Drd1fl/fl retina, indicating that Drd1 silencing was specific to the retina in Drd1-CKO mice. Consistent with the findings in Drd1-KO or SCH39166-treated mice (Huang et al., 2018), ERG recordings showed that both scotopic and photopic responses were diminished in Drd1-CKO mice (p < 0.05, two-way repeated-measures ANOVA; Fig. 6D,E).
Figure 6.
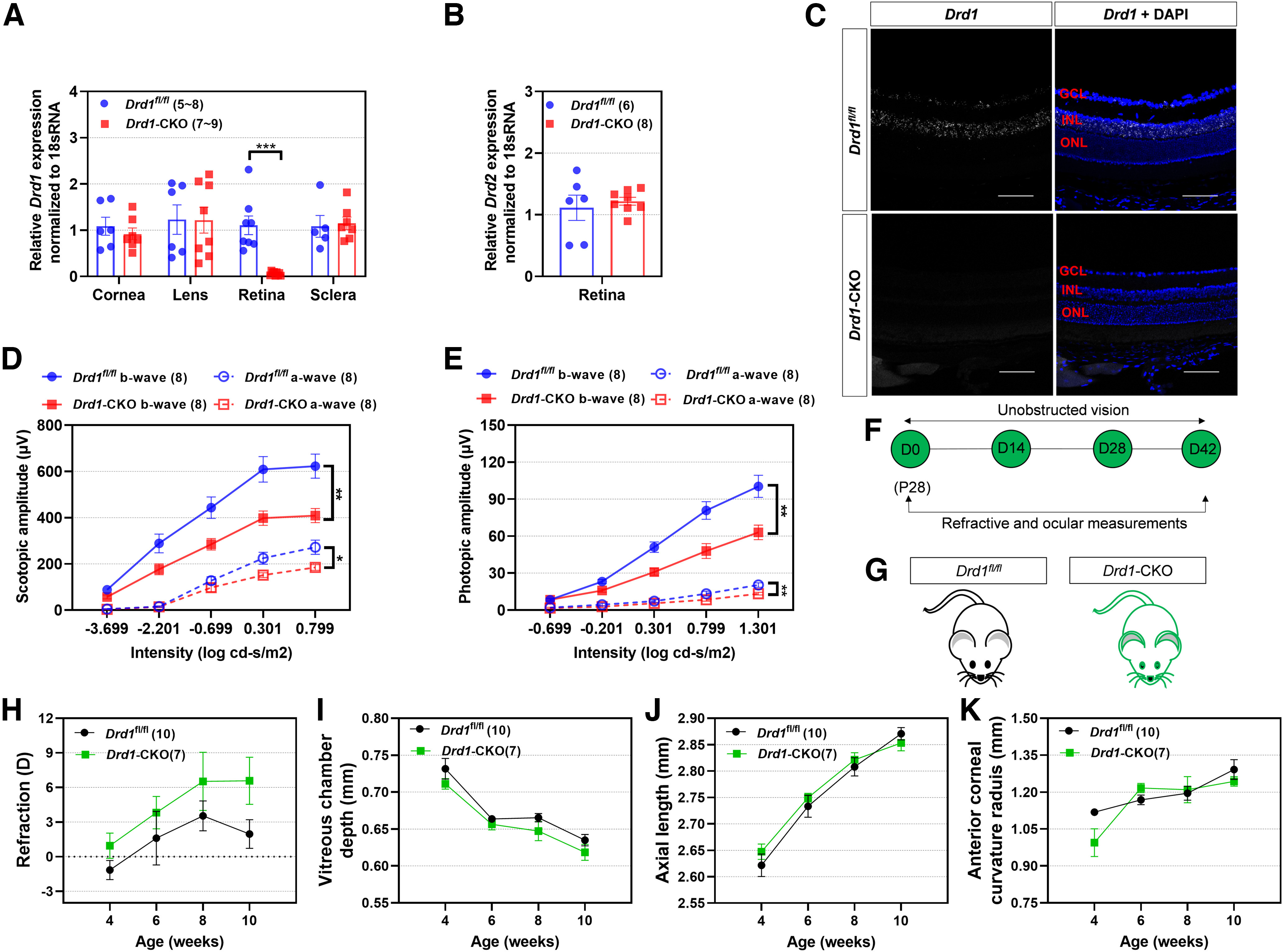
Effects of retina-specific Drd1-CKO on normal refractive development with unobstructed vision. A, qRT-PCR to assess relative expression levels of Drd1 mRNA in corneas, lenses, neural retinas, and sclera of Drd1-CKO and littermate control mice (Drd1fl/fl). B, Relative expression levels of Drd2 mRNA from retinas of Drd1-CKO and Drd1fl/fl mice. C, In situ hybridization produced no detectable labeling of Drd1 mRNA in Drd1-CKO retina, whereas a prominent layer of Drd1 mRNA was detected in Drd1fl/fl retina. Scale bar, 100 µm. D, E, Scotopic (D) and photopic (E) ERG a- and b-wave amplitudes in Drd1-CKO mice. F, Timeline of the experimental procedure and data collection. G, Diagram of Drd1-CKO and breed-matched Drd1fl/fl mice. H–K, Biometric measurements in Drd1-CKO and Drd1fl/fl groups from 4 to 10 weeks old, as follows: refraction (H), vitreous chamber depth (I), axial length (J), and corneal radius of curvature (K). *p < 0.05, **p < 0.01, and ***p < 0.001, Mann–Whitney U tests (lens in A) or independent t tests (cornea, retina, and sclera in A and B), two-way repeated-measures ANOVA (D, E, H–K).
Effects of Drd1-CKO on normal refractive development
The refractions showed small but statistically insignificant trends toward more hyperopia in retina-specific Drd1-CKO mice, with no significant differences in VCD, AL, or anterior corneal radius of curvature during development from 4 to 10 weeks of age postnatally (all p > 0.05; Fig. 6H–K). Thus, normal refractive development in mice was not affected by Drd1-CKO.
Effects of SKF38393 in Drd1-CKO mice under unilateral FD
In contrast with the Drd1-KO mice, which lacked D1Rs in all organs, tissues, and cell types, and had reduced body weights, the Drd1-CKO mice had body weights similar to those of control mice (p > 0.05; Fig. 7C). After 2 weeks of FD, the myopic shift in the Drd1fl/fl-18SKF mice was smaller than that recorded in the Drd1fl/fl-Veh mice (p < 0.001; Fig. 7D). In contrast, this inhibition of myopia by SKF38393 disappeared in Drd1-CKO mice, as no significant difference was observed between Drd1-CKO-Veh and Drd1-CKO-18SKF groups (p > 0.05); this suggests that the inhibitory effect of SKF38393 on FDM depends on the presence of functional D1Rs in the retina. In parallel, the mean interocular differences in both VCD and AL, in the Drd1fl/fl-18SKF mice, were slightly smaller but not significantly different from those recorded in the Drd1fl/fl-Veh mice (p > 0.05; Fig. 7E,F). In the Drd1-CKO mice, the interocular differences in VCD and AL were unaffected by SKF38393 injections (Drd1-CKO-Veh vs Drd1-CKO-18SKF, p > 0.05). The interocular differences in other biometric parameters among different FD groups, after 2 weeks of SKF38393 injections, were not significantly different (p > 0.05; Fig. 7G). These results further support the conclusion that D1Rs located in the retina are essential for dopaminergic regulation of myopia development in mice.
Figure 7.
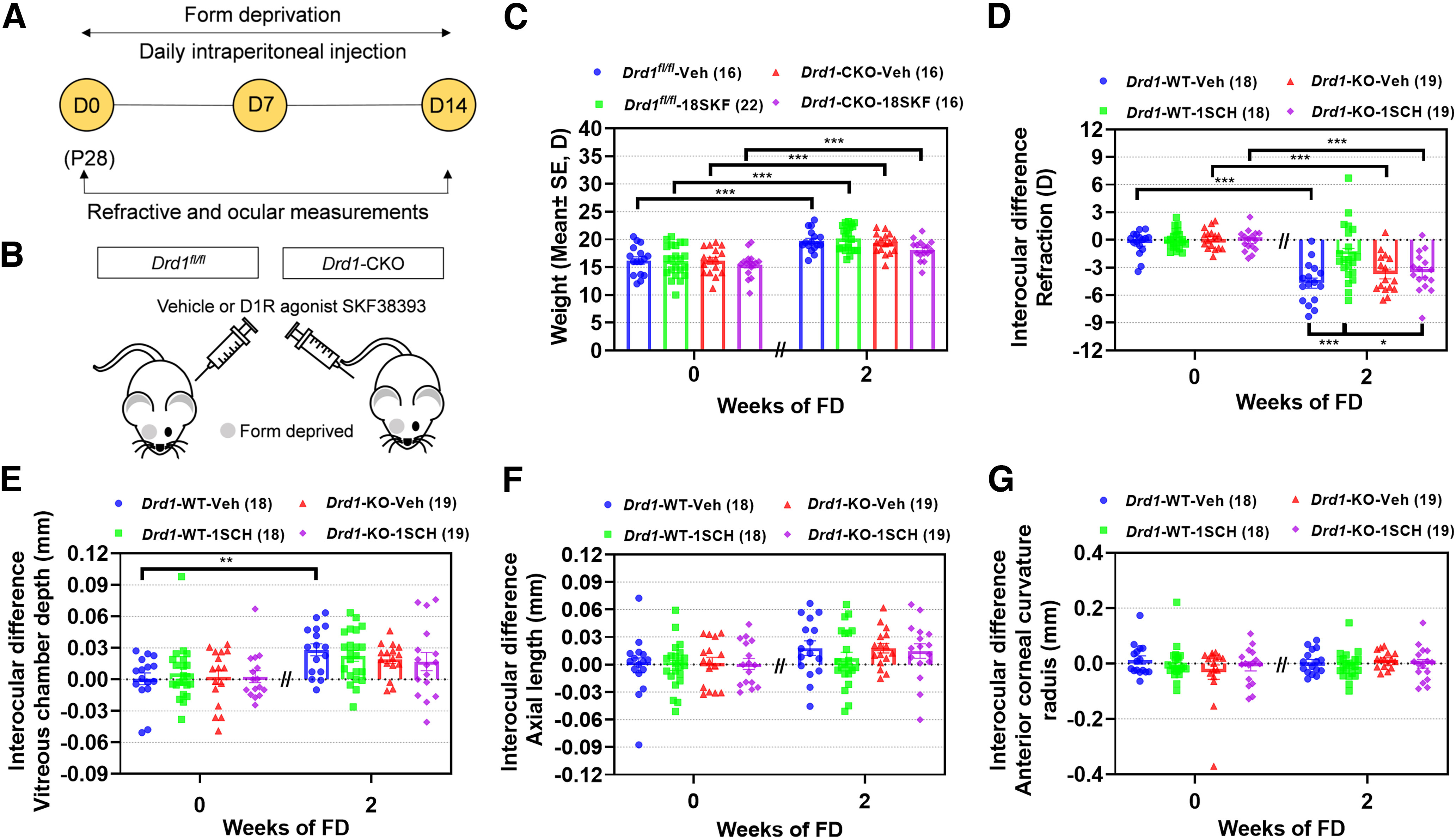
D1Rs located in the retina mediate the prevention of FDM by SKF38393 in Drd1-CKO mice. A, Experimental procedures and data collection flow diagram. D, Day of experimental treatment (e.g., D0). B, Scheme of Drd1-CKO mice with intraperitoneal injections of SKF38393 (18 nmol/g) and monocular form deprivation. C–G, Biometric measurements in Drd1fl/fl-Veh, Drd1fl/fl-18SKF, Drd1-CKO-Veh, and Drd1-CKO-18SKF groups before and after 2 weeks of each treatment (4 and 6 weeks old, respectively). C, Body weight. D–G, Interocular differences (FD – fellow eye) in the following: refraction (D), vitreous chamber depth (E), axial length (F), and anterior corneal radius of curvature (G). Numbers of animals (pairs of eyes) are shown in parentheses for each treatment condition. *p < 0.05, **p < 0.01, and ***p < 0.001, three-way repeated-measures ANOVA.
Discussion
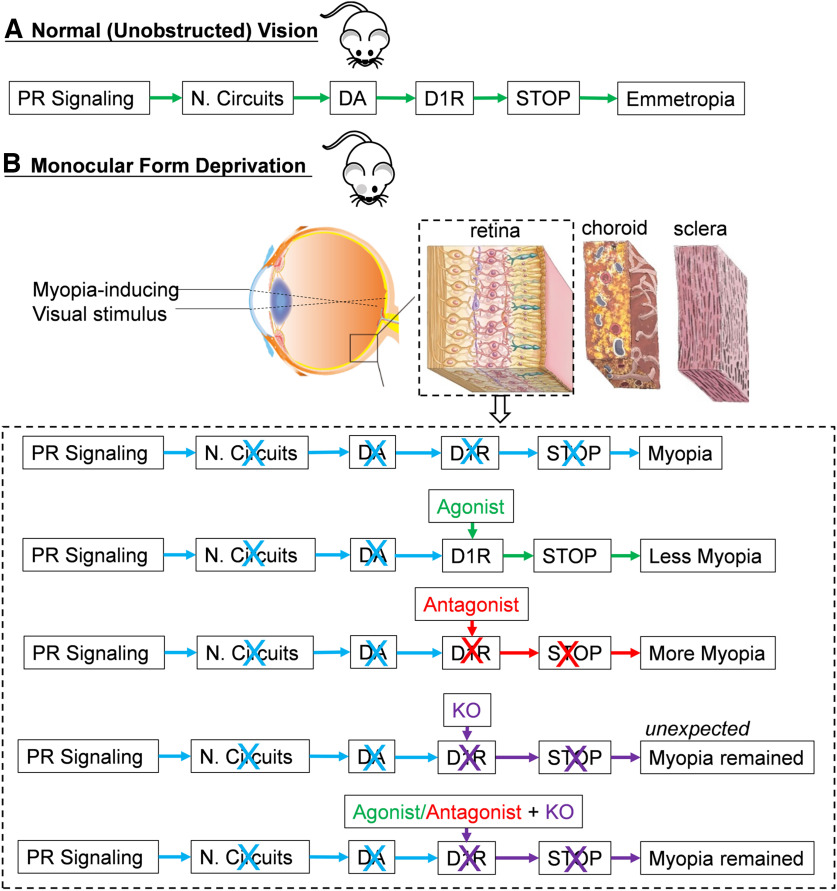
The role of D1Rs in the development of myopia is uncertain because the results of experiments using various animal species and procedures are not consistent with a unifying functional model. In the present study, we showed that while pharmacological activation of D1Rs attenuated the development of induced myopia, it had no effect on normal refractive development. We also showed that this myopia inhibition was eliminated in mice with Drd1-KO or Drd1-CKO, suggesting that activation of retinal D1Rs is obligatory for the suppression of myopia development by the D1R agonist. However, we found that neither normal refractive development nor FDM was significantly affected by genetic inactivation of Drd1 alone, in either Drd1-KO or Drd1-CKO mice, suggesting that the mere absence of D1Rs in the retina does not impede normal refractive development in a normal or form-deprived environment. Overall, these results implicate the involvement of more than one DA-D1R pathway or mechanism in ocular growth and myopia development (Fig. 8).
Figure 8.
Paradigm for intrinsic retinal dopaminergic mechanisms mediated by D1R, in the regulation of myopia. A, Under unobstructed vision, DA signaling, by activation of D1R, represents a “stop” signal that inhibits myopia development in mice. B, Under monocular form deprivation, these myopia-related visual signals decrease the endogenous DA release rate, thus leading to an insufficient activation of D1R-containing pathways in the retina and resulting in axial myopia. Working schematic for D1R agonist, antagonist, and KO (including Drd1-KO and Drd1-CKO) contributions on myopia development in the retina is shown in the dashed box. Words or arrows in green, blue, and red colors represent the signal transmission that related to emmetropia (or myopia inhibition), regular myopia, and myopia enhancement, respectively; those in purple represent the signal transmission that related to myopia remained by Drd1 KO. PR, Photoreceptors; N. Circuits, neural circuits.
Retinal dopamine levels and metabolism unaffected by D1R drugs or KO
The retinal and vitreal DA and/or DOPAC levels recorded in this study are similar to those reported previously in C57BL/6 mice (Wu et al., 2015; Liu et al., 2022). Experiments in different species have produced conflicting results; for example, retinal or vitreal DA and/or DOPAC levels were found to be reduced in FDM in chicks (Lan et al., 2016; Nickla et al., 2020) and primates (Iuvone et al., 1989), but were unaltered in FDM in C57BL/6 wild-type mice (Chakraborty et al., 2014; Wu et al., 2015; Chakraborty et al., 2019; Landis et al., 2021). We report here that the retinal DA and DOPAC levels were unchanged, but that the vitreal DOPAC and/or retinal DOPAC/DA levels were decreased, during the development of myopia in C57BL/6 mice; this indicates that retinal DA release and turnover are slightly reduced during FDM induction in mice. Furthermore, it has been shown that retinal DOPAC/DA ratios are more important than DA levels for determining susceptibility to myopia development in the mouse eye (Chakraborty et al., 2014, 2015; Chakraborty and Pardue, 2015). However, neither the D1R agonist or antagonist nor Drd1 KO further influenced retinal DA release or turnover; this suggests that the D1R drugs or KO induced these effects on FDM directly through the retinal D1R signaling pathway (i.e., downstream from the dopaminergic interneurons) rather than through changes in DA release or turnover at the level of those interneurons.
Modulation of D1R-targeted pharmacological agents on myopia development
We found that neither the D1R agonist nor antagonist was effective, except (1) when the endogenous DA release rate was low (i.e., under FD but not under unobstructed vision); and (2) when normal D1Rs were present in the retina (i.e., in WT but not in Drd1-KO or Drd1-CKO mice). First, the differences in ocular effects of DA drugs, under unobstructed vision and FDM, could be because of a higher affinity of the deprived eye for exogenous DA drugs, as the levels of retinal DA release and turnover are lower in form-deprived eyes than in those with unobstructed vision. Previous studies showed that as little as 2–3 h of FD interruption each day is adequate to restore normal retinal mechanisms and prevent myopia development in chicks (McCarthy et al., 2007; Nickla and Totonelly, 2011); in agreement with this, we found that daily D1R agonist or antagonist injections, whose effects are short lived, had no effect on normal refractive development in unobstructed vision. Second, myopia development was unaffected by either D1R agonist or antagonist when there were no functional D1Rs (Drd1-KO or Drd1-CKO) in the retina; this showed that D1Rs are essential for controlling ocular growth and preventing form deprivation myopia in mice, and for identifying the retina as the site of action for dopaminergic control via D1Rs.
These findings highlight the importance of D1Rs, in distinct retinal cell types and neural circuits, for the regulation of ocular growth in mouse models. Data from multiple studies using a variety of approaches including autoradiographic ligand binding, immunohistochemistry, and ISH, suggest that D1Rs are located mainly on inner retinal neurons—horizontal cells, cone (but not rod) bipolar cells, a few subtypes of amacrine cells, and ganglion cells (Popova, 2014; Farshi et al., 2016; Travis et al., 2018). The diverse localization of D1Rs within retinal neural networks suggests important modulatory roles for dopamine in the tuning of visual signaling pathways, probably mainly supporting and strengthening multiple cone-mediated motion and/or flicker-sensitive pathways. Evidence suggests that the activation of D1R-expressing cells contributes to the suppression of FDM development by photopic illumination in mice (Chen et al., 2017). However, as D1Rs are present on a wide variety of retinal neurons, it would be impossible at this time to say which cells expressing D1Rs in the mouse eye are involved in the control of emmetropization and myopia development.
Negative effects of mere genetic ablation of D1R on myopia development
Interestingly, in our study, the development of FDM in mice was not affected by mere genetic ablation of D1R in the retina, whereas it was attenuated by D1R-targeted pharmacological agonists. These results suggest that functional inactivation of existing D1Rs, but not their deletion, is sufficient and necessary for modulating the development of FDM. These differences between the pharmacological and genetic effects of D1R on ocular growth and FDM development could be because of differences in D1R kinetics (i.e., transient activation or inactivation in pharmacological treatments, versus sustained inactivation from conception onwards in genetic knockouts) and levels of D1R activation (i.e., upregulation/downregulation in pharmacological treatments vs complete absence in genetic deletions). One must be aware that the short-term effects of pharmacological agents are more similar to those of visual stimulation or physiological (e.g., circadian) variations in retinal DA metabolism, which change with time and visual conditions, than the long-term effects of gene silencing. Previous studies showed that differential D1R stimulation can initiate or modulate selectively different cellular signaling pathways (e.g., G-protein and arrestin pathways; Conroy et al., 2015; Kaya et al., 2020). Therefore, we suggest that the D1R agonist might act at D1Rs combined with other receptors or might act via separate cellular signaling pathways (i.e., biased signaling of D1Rs) to produce distinct effects, but only when administered and acting concurrently. The partial inhibition of FDM by a D1R agonist, as shown in the present study, also suggests either that the myopic shift does not entirely depend on the activity of retinal D1Rs or that other signaling pathways are also involved in the control of axial growth of the eye. These possibilities need to be clarified in future studies.
Limitations of the study
The main limitation of our study is that the Drd1-KO line was generated on a genetic background (C57BL/6J) very different from that of the Drd1-CKO mice (C57BL/6J * DBA/2). Consequently, not only the genetic deletion, but also the different genetic backgrounds in the two strains of mice, can be expected to influence the treatment outcomes. However, the strength of our experimental design is that our analysis compared the effects on experimentally treated versus untreated fellow eyes, in the same animal (interocular difference, FD – fellow eye), minimizing effects because of differences in genetic background or environmental exposure. Thus, the effects that might be ascribed to these differences in genetic background were minor, and this limitation does not diminish the strength of our conclusions.
Conclusions
In conclusion, we have established conclusively that D1Rs are essential for controlling ocular growth and myopia development in mice, and we have identified the retina as the sufficient and necessary site of these dopaminergic actions. These findings further highlight the importance of intrinsic retinal dopaminergic mechanisms for the regulation of ocular growth. Although the exact mechanisms and sites of action of DA and related drugs in retinal pathways remain elusive, our results provide possible avenues for exploring the retinal mechanisms involved in the dopaminergic control of emmetropization and myopization.
Footnotes
This study was sponsored by Grants 81800860, 81830027, and 81970833 from the National Natural Science Foundation of China; and Grant 82025009 from the National Science Foundation for Distinguished Young Scholars of China. We thank Frank Schaeffel (Institute for Ophthalmic Research, Section of Neurobiology of the Eye, University of Tübingen, Tübingen, Germany) for providing support for our eccentric infrared photoretinoscope; and William K. Stell (Professor Emeritus, Department of Cell Biology and Anatomy, and Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada) for providing scientific advice and editorial support to improve the manuscript.
The authors declare no competing financial interests.
References
- Assali DR, Sidikpramana M, Villa AP, Falkenstein J, Steele AD (2021) Type 1 dopamine receptor (D1R)-independent circadian food anticipatory activity in mice. PLoS One 16:e0242897. 10.1371/journal.pone.0242897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratcher NA, Farmer-Dougan V, Dougan JD, Heidenreich BA, Garris PA (2005) The role of dopamine in reinforcement: changes in reinforcement sensitivity induced by D1-type, D2-type, and nonselective dopamine receptor agonists. J Exp Anal Behav 84:371–399. 10.1901/jeab.2005.82-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo C, Leggio GM, Drago F, Salomone S (2019) Dopamine outside the brain: the eye, cardiovascular system and endocrine pancreas. Pharmacol Ther 203:107392. 10.1016/j.pharmthera.2019.07.003 [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Pardue MT (2015) Molecular and biochemical aspects of the retina on refraction. Prog Mol Biol Transl Sci 134:249–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Park H, Aung MH, Tan CC, Sidhu CS, Iuvone PM, Pardue MT (2014) Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Mol Vis 20:1318–1327. [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Park HN, Hanif AM, Sidhu CS, Iuvone PM, Pardue MT (2015) ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res 137:79–83. 10.1016/j.exer.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Yang V, Park HN, Landis EG, Dhakal S, Motz CT, Bergen MA, Iuvone PM, Pardue MT (2019) Lack of cone mediated retinal function increases susceptibility to form-deprivation myopia in mice. Exp Eye Res 180:226–230. 10.1016/j.exer.2018.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhi Z, Ruan Q, Liu Q, Li F, Wan F, Reinach PS, Chen J, Qu J, Zhou X (2017) Bright light suppresses form-deprivation myopia development with activation of dopamine D1 receptor signaling in the ON pathway in retina. Invest Ophthalmol Vis Sci 58:2306–2316. 10.1167/iovs.16-20402 [DOI] [PubMed] [Google Scholar]
- Conroy JL, Free RB, Sibley DR (2015) Identification of G protein-biased agonists that fail to recruit β-arrestin or promote internalization of the D1 dopamine receptor. ACS Chem Neurosci 6:681–692. 10.1021/acschemneuro.5b00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E (2015) The myopia boom. Nature 519:276–278. 10.1038/519276a [DOI] [PubMed] [Google Scholar]
- Dong F, Zhi Z, Pan M, Xie R, Qin X, Lu R, Mao X, Chen JF, Willcox MD, Qu J, Zhou X (2011) Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis 17:2824–2834. [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M (2002) Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci 19:593–601. 10.1017/s0952523802195058 [DOI] [PubMed] [Google Scholar]
- Farshi P, Fyk-Kolodziej B, Krolewski DM, Walker PD, Ichinose T (2016) Dopamine D1 receptor expression is bipolar cell type-specific in the mouse retina. J Comp Neurol 524:2059–2079. 10.1002/cne.23932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Lagutin O, Hogan BL, Oliver GC (2000) Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis 26:130–132. [DOI] [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S (2016) Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123:1036–1042. 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Huang F, Zhang L, Wang Q, Yang Y, Li Q, Wu Y, Chen J, Qu J, Zhou X (2018) Dopamine D1 receptors contribute critically to the apomorphine-induced inhibition of form-deprivation myopia in mice. Invest Ophthalmol Vis Sci 59:2623–2634. 10.1167/iovs.17-22578 [DOI] [PubMed] [Google Scholar]
- Huang F, Shu Z, Huang Q, Chen K, Yan W, Wu W, Yang J, Wang Q, Wang F, Zhang C, Qu J, Zhou X (2022) Retinal dopamine D2 receptors participate in the development of myopia in mice. Invest Ophthalmol Vis Sci 63:24. 10.1167/iovs.63.1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysi PG, et al. (2020) Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat Genet 52:401–407. 10.1038/s41588-020-0599-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Fernandes A, Tigges J (1989) Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis Neurosci 2:465–471. 10.1017/s0952523800012360 [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM (1991) Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci 32:1674–1677. [PubMed] [Google Scholar]
- Jiang L, Long K, Schaeffel F, Zhou X, Zheng Y, Ying H, Lu F, Stell WK, Qu J (2014) Effects of dopaminergic agents on progression of naturally occurring myopia in albino guinea pigs (Cavia porcellus). Invest Ophthalmol Vis Sci 55:7508–7519. 10.1167/iovs.14-14294 [DOI] [PubMed] [Google Scholar]
- Kaya AI, Perry NA, Gurevich VV, Iverson TM (2020) Phosphorylation barcode-dependent signal bias of the dopamine D1 receptor. Proc Natl Acad Sci U S A 117:14139–14149. 10.1073/pnas.1918736117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Yang Z, Feldkaemper M, Schaeffel F (2016) Changes in dopamine and ZENK during suppression of myopia in chicks by intense illuminance. Exp Eye Res 145:118–124. 10.1016/j.exer.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Landis EG, Park HN, Chrenek M, He L, Sidhu C, Chakraborty R, Strickland R, Iuvone PM, Pardue MT (2021) Ambient light regulates retinal dopamine signaling and myopia susceptibility. Invest Ophthalmol Vis Sci 62:28. 10.1167/iovs.62.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AL, Liu YF, Wang G, Shao YQ, Yu CX, Yang Z, Zhou ZR, Han X, Gong X, Qian KW, Wang LQ, Ma YY, Zhong YM, Weng SJ, Yang XL (2022) The role of ipRGCs in ocular growth and myopia development. Sci Adv 8:eabm9027. 10.1126/sciadv.abm9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CS, Megaw P, Devadas M, Morgan IG (2007) Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res 84:100–107. 10.1016/j.exer.2006.09.018 [DOI] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K (2011) Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res 93:782–785. 10.1016/j.exer.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K, Dhillon B (2010) Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res 91:715–720. 10.1016/j.exer.2010.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Sarfare S, McGeehan B, Wei W, Elin-Calcador J, He L, Dhakal S, Dixon J, Maguire MG, Stone RA, Iuvone PM (2020) Visual conditions affecting eye growth alter diurnal levels of vitreous DOPAC. Exp Eye Res 200:108226. 10.1016/j.exer.2020.108226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Matsui K, Lai TY, Lai CC, Cheung CM (2016) Updates of pathologic myopia. Prog Retin Eye Res 52:156–187. 10.1016/j.preteyeres.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Popova E (2014) Role of dopamine in distal retina. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 200:333–358. 10.1007/s00359-014-0906-2 [DOI] [PubMed] [Google Scholar]
- Rohrer B, Spira AW, Stell WK (1993) Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci 10:447–453. 10.1017/s0952523800004673 [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Bartmann M, Hagel G, Zrenner E (1995) Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res 35:1247–1264. 10.1016/0042-6989(94)00221-7 [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Burkhardt E, Howland HC, Williams RW (2004) Measurement of refractive state and deprivation myopia in two strains of mice. Optom Vis Sci 81:99–110. 10.1097/00006324-200402000-00008 [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH (1993) Dopamine receptor pharmacology. Curr Opin Neurol Neurosurg 6:602–608. [PubMed] [Google Scholar]
- Servonnet A, Allain F, Gravel-Chouinard A, Hernandez G, Bourdeau Caporuscio C, Legrix M, Lévesque D, Rompré PP, Samaha AN (2021) Dopaminergic mechanisms underlying the expression of antipsychotic-induced dopamine supersensitivity in rats. Neuropharmacology 197:108747. 10.1016/j.neuropharm.2021.108747 [DOI] [PubMed] [Google Scholar]
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A (2011) A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474:337–342. 10.1038/nature10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis AM, Heflin SJ, Hirano AA, Brecha NC, Arshavsky VY (2018) Dopamine-dependent sensitization of rod bipolar cells by GABA Is conveyed through wide-field amacrine cells. J Neurosci 38:723–732. 10.1523/JNEUROSCI.1994-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XH, Li YY, Zhang PP, Qian KW, Ding JH, Hu G, Weng SJ, Yang XL, Zhong YM (2015) Unaltered retinal dopamine levels in a C57BL/6 mouse model of form-deprivation myopia. Invest Ophthalmol Vis Sci 56:967–977. 10.1167/iovs.13-13362 [DOI] [PubMed] [Google Scholar]
- Yan T, Xiong W, Huang F, Zheng F, Ying H, Chen JF, Qu J, Zhou X (2015) Daily injection but not continuous infusion of apomorphine inhibits form-deprivation myopia in mice. Invest Ophthalmol Vis Sci 56:2475–2485. 10.1167/iovs.13-12361 [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang J, Reinach PS, Wang F, Zhang L, Fan M, Ying H, Pan M, Qu J, Zhou X (2018) Dopamine receptor subtypes mediate opposing effects on form deprivation myopia in pigmented guinea pigs. Invest Ophthalmol Vis Sci 59:4441–4448. 10.1167/iovs.17-21574 [DOI] [PubMed] [Google Scholar]
- Zhou X, Shen M, Xie J, Wang J, Jiang L, Pan M, Qu J, Lu F (2008) The development of the refractive status and ocular growth in C57BL/6 mice. Invest Ophthalmol Vis Sci 49:5208–5214. 10.1167/iovs.07-1545 [DOI] [PubMed] [Google Scholar]