Abstract
Functional traits are phenotypic characteristics that contribute to fitness of individuals in dynamic and changing environments. In mammals, both categorical and continuous (e.g., quantitative) functional traits have been extensively utilized as proxies for diet, locomotion, and other aspects of species ecology, but there has been less focus on form and function of soft tissues. This is particularly true for the digestive system, which varies in size and complexity across Class Mammalia and plays a major role in the energetics of species. To guide more effective utilization of gastrointestinal (GI) morphology as a functional proxy in small mammal ecology, we examined how GI tracts (lengths and masses of four GI sections) varied within a population of deer mice (Peromyscus maniculatus) in the Southern Appalachian Mountains of North Carolina, United States. We collected samples of adult P. maniculatus monthly for 1 year and measured GI tracts to quantify variation with respect to seasonality and trophic level, providing insight into plasticity in this soft tissue trait over time. We found that season had a significant effect on the total length and wet mass of the GI tract, with January mice having the longest GI tracts and lengths being shortest in the summer. The relative shortening of the GI tract in summer corresponded with a partial trophic increase detected by stable isotope signatures. GI length and wet mass also were affected by reproduction, but males and females responded in sex-specific ways to demands of reproduction, with reproductively active males having shorter and lighter GI tracts than nonreproductively active males. Our study provides proof-of-concept for understanding population-level plasticity in a rarely collected soft tissue trait, which may also be complementary to standard craniodental measurements as a functional dietary proxy to understand mammalian ecology and community assembly.
Keywords: functional traits, gastrointestinal tract, morphology, North American deer mouse, phenology, stable isotope
In this study, we examine seasonal and sex-specific changes in the gastrointestinal tracts of a common North American small mammal. We show that the gastrointestinal tracts of winter mice are up to 35% longer and 18% heavier than those of summer mice. This was directly coincident with decreased dietary quality in the winter months.
Abstract
Los rasgos funcionales son características fenotípicas que contribuyen a la aptitud de los individuos en entornos dinámicos y cambiantes. En los mamíferos, los rasgos funcionales categóricos y continuos (por ejemplo, cuantitativos) se han utilizado ampliamente como indicadores de la dieta, la locomoción y otros aspectos de la ecología de las especies, a la vez que se ha prestado menos atención a la forma y función de los tejidos blandos. Este es particularmente el caso del sistema digestivo, que varía en tamaño y complejidad a través de la Clase Mammalia, jugando un papel importante en la energética de las especies. Para propiciar una utilización más efectiva de la morfología gastrointestinal (GI) como un rasgo funcional en la ecología de los pequeños mamíferos, examinamos cómo los tractos GIs (longitudes y masas de cuatro secciones GI) variaban dentro de una población de ratones ciervos (Peromyscus maniculatus) de las Montañas Apalaches del sur de Carolina del Norte, Estados Unidos. Mensualmente, durante un año, recolectamos especímenes adultos de P. maniculatus y medimos sus tractos gastrointestinales para cuantificar la variación con respecto a la estacionalidad y el nivel trófico, brindando información sobre la plasticidad en estos rasgos de tejido blando a lo largo del tiempo. Descubrimos que la estacionalidad tiene un efecto significativo en la longitud total y la masa húmeda del tracto gastrointestinal, ya que los ratones de enero tienen los tractos gastrointestinales más largos y las longitudes son más cortas durante el verano. El acortamiento relativo del tracto GI en verano se correspondió con un aumento trófico parcial detectado por las cuantificación de isótopos estables. La longitud GI y la masa húmeda también se vieron afectadas por la reproducción; pero los machos y las hembras respondieron de manera diferente a las demandas de la reproducción. Los machos reproductivamente activos tienen tractos gastrointestinales más cortos y ligeros que los machos no activos reproductivamente. Nuestro estudio constituye un avance para comprender la plasticidad a nivel de población en un rasgo de tejido blando raramente recolectado, que también puede ser complementario a las mediciones craneodentales estándar como un proxy dietético funcional para entender la ecología de los mamíferos y el ensamblaje de la comunidad.
Functional traits are defined as the physical or behavioral attributes that influence the ability of a species to persist in specific community and ecosystem contexts (McGill et al. 2006; Kohli and Rowe 2019; Gallagher et al. 2020). Some functional traits used by researchers in mammalian ecological and evolutionary research are continuous metrics, such as body mass and body length, but many are categorical, such as the diet guild or habitat guild of a species (Kohli and Rowe 2019; Verde Arregoitia and D’Elía 2021). However, the latter categorizations are coarse and overlook the exceptional levels of intraspecific variation that exist in ecomorphological, behavioral, and life history properties (Parins-Fukuchi 2018; Kohli and Rowe 2019) that mediate individual fitness in dynamic and changing environments. One reason for this shortcoming is that assembling continuous trait databases at the individual or population levels requires intensive field sampling or measurement of existing material (e.g., museum specimens; Kohli and Rowe 2019). Refining exact functional roles of traits is also important for their use in ecological studies, but this requires evaluating trait variation not only within taxa, but across meaningful (e.g., spatial or temporal) environmental gradients. Nevertheless, developing more detailed, continuous metrics as functional traits is critical to facilitate precise functional characterizations and improve ability to monitor and detect global change response (Laureto et al. 2015; Kissling et al. 2018; Parins-Fukuchi 2018; Kohli and Rowe 2019).
Changing climate and land use contexts can impact the quantity and quality of food available to vertebrates in different regions and times of the year (Ayres 1993; Schradin and Pillay 2006; Mayor et al. 2017; Soares et al. 2019). For example, life history events such as reproduction are often timed to coincide with resource pulses, so changing timing of peak resource availability may cause temporal mismatches with population-level consequences such as increased infant mortality (Post and Forchhammer 2008) or altered litter size and recruitment (Kerby and Post 2013). In these contexts, metrics of gastrointestinal (hereafter, GI) tract morphological and physiological function thus may be informative regarding the ability of a species to cope with changing environments (Naya et al. 2007, 2008a). It is well known that GI morphology varies significantly with diet across mammals (Chivers and Hladick 1980; Schieck and Millar 1985; Chivers 2003; Naya 2008; Duque-Correa 2021). Generally speaking, herbivores tend to have relatively larger and more complex digestive systems with larger numbers of compartments (Dehority 2002), including a larger cecum and a longer and heavier large intestine, which extend transit time of foodstuffs and are useful for processing poor-quality plant material. Conversely, omnivores and granivores tend to have smaller ceca and a lighter large intestine (Schieck and Millar 1985). Additional morphological adaptations to high-fiber diets exist that allow species to reprocess digesta and maintain sufficient microbial diversity in the gut, prior to defecation (Björnhag 1994). Unfortunately, detailed GI measurements at the intraspecific level have been reported for only a small proportion of mammal species, limiting ability to link GI form to individual diet.
One means of better assessing the functional signal in GI measurements is by temporal sampling, for example, across annual cycles of resource availability and energy demand. For example, GI morphology is known to vary seasonally with food availability and breeding activity in some temperate species (Derting and Austin 1998; Hammond et al. 1999; Derting and Hornung 2003; Naya et al. 2007, 2008b; Naya 2008). Limited access to high-quality diets during the winter, coupled with the increased energy demand of thermoregulation at this time (Hammond and Wunder 1995; Korslund and Steen 2006), appear to favor specific GI morphological changes that maximize food retention times and nutrient absorption (Hammond and Wunder 1995; Koteja 1996). Gut mass increased during winter in populations of both Peromyscus leucopus and Microtus pennsylvanicus (Derting and Noakes 1995). Another study of M. pennsylvanicus demonstrated increases in small intestine and cecum mass (wet and dry) with increased energetic demands (Derting and Bogue 1993). Laboratory populations of M. ochrogaster kept at cold temperatures also showed an increase in cecum length and mass, along with an increase in the total GI tract length (Gross et al. 1985). Hammond and Wunder (1995) demonstrated GI length increases in M. ochrogaster as well as Dicrostonyx groenlandicus acclimated to lower temperatures in the lab, which matched an increase in the amount of digesta present in the GI tract (Hammond and Wunder 1995). In P. maniculatus populations kept in seminatural conditions, the dry mass of the small intestine was heavier in cold-acclimated mice than in warm-acclimated mice (Hammond et al. 2001).
Other studies have directed manipulated diet quality, mimicking changes that may be experienced byindividuals in the wild. In a laboratory study of P. maniculatus, diet quality was manipulated and the length and mass of the small intestine and the masses of the large intestine and cecum increased with increasing amounts of fiber (Green and Millar 1987). Another study found that when populations of M. ochrogaster and M. pennsylvanicus switched from a low to high-fiber diet, the GI tract became longer and heavier (Young Owl and Batzli 1998). However, any temporal analysis that spans climate or dietary differences must also account for reproduction and different levels of energetic investment in reproduction between the sexes, as this can have an exceptionally large effect on GI morphology (Millar et al. 1990; Derting and Noakes 1995; Derting and Hornung 2003; Naya et al. 2007), especially in gestating and lactating females (Hammond 1997; Naya 2008; Naya et al. 2008b; Speakman 2008; Canul-Medina and Fernandez-Mejia 2019). Previous work on P. leucopus and M. pennsylvanicus has shown that increased energy investments in reproduction, particularly lactation in females, result in a larger GI tract due to the higher energetic demand during this period (Millar et al. 1990).
While these and other studies confirm that GI tract form is strongly related to function and that it can be plastic within individuals and populations, rapid postmortem degradation of these tissues means that measurements must be obtained from fluid-preserved museum specimens or through intensive new field sampling. Unfortunately, the vast majority of small mammal specimens in natural history collections are preserved as skins and skeletons, with soft internal tissues not preserved (Quay 1974; Greiman et al. 2018). Of a total of 2.3 million small mammal specimens stored in the 10 largest mammal archives in North America, only around 500,000 are fluid-preserved with GI tracts or organs (Greiman et al. 2018). Moreover, fluid preservation causes shrinkage that changes the morphology and leads to inaccurate measurements (Kingston 2018). This occurs regardless of the solution used to preserve tissues, although different solutions and concentrations may result in different degrees of shrinkage (Kingston 2018).
The goal of this study was to use new field sampling to expand knowledge of intraspecific GI morphology to additional wild mammal populations and better understand how this organ system is shaped by extrinsic (food quality) and intrinsic (age, reproduction) factors. To do this, we monitored a population of the Eastern Deer mouse (P. maniculatus) for 1 year in the Southern Appalachian Mountains and performed detailed measurements of 15 GI traits (lengths and masses). Concomitantly, we performed diet analysis using a coarse metric of dietary quality (trophic position, via stable isotope analysis), providing insight into the magnitude of diet changes across this temporal gradient. Finally, we analyzed GI data with respect to metrics of age, body size, and reproductive activity to more conclusively link potential GI morphological changes to these potential underlying drivers.
Materials and Methods
Field sampling.
We sampled P. maniculatus at five plots in the Nantahala and Pisgah National Forests in western North Carolina, United States (Table 1) monthly during 2021. The plots were characterized by similar mature, mixed forest habitats and did not differ in any meaningful way. For each of the monthly trapping events, at least five adult P. maniculatus were collected using Sherman live traps evenly distributed throughout the plot (with the exception of only two adult individuals collected in July and December). Trapping effort rotated among different combinations of plots each month in order to mitigate potential demographic effects that could bias our sampling, specifically, by changing the age ratio of populations. Plots were generally not sampled for more than three consecutive months each. Traps were baited with dilute vanilla extract, set at dusk, and checked at dawn the following morning. The nonedible bait ensured that any stomach contents or fecal pellets collected were a true representation of natural diet. All animals were euthanized in the field following approved institutional protocols (UNCG IACUC #20-008) and recommendations of the American Society of Mammalogists Animal Care and Use Committee (Sikes et al. 2016). Immediately after euthanization, each GI tract was removed and frozen at −20°C for a maximum of 2 weeks until processing. Individuals were assigned to age-class based on pelage (color and presence of molt lines) and standard external measurements. Pregnancy or lactation (for females) and maintenance of scrotal testes (for males) were considered evidence of reproductive activity. All individuals were preserved as voucher specimens in the UNC Greensboro Mammal Collection, with associated data, multiple tissue samples, and details of age, sex, and reproductive condition (McLean 2023).
Table 1.
Characteristics of the five plots sampled in Western North Carolina, United States, including the county name, U.S. National Forest name, latitude, longitude, and elevation (m). The maximum linear distance between any two plots was 5.97 km.
| Plot | County | National Forest | Latitude, longitude | Elevation (m) |
|---|---|---|---|---|
| 1 | Transylvania | Pisgah | 35.25335, −82.91935 | 1,122 |
| 2 | Transylvania | Pisgah | 35.27768, −82.91628 | 1,398 |
| 3 | Jackson | Nantahala | 35.26771, −82.98082 | 1,116 |
| 4 | Jackson | Nantahala | 35.27771, −82.97295 | 1,114 |
| 5 | Jackson | Nantahala | 35.25959, −82.95141 | 1,234 |
Species identifications.
In our study region, P. maniculatus and P. leucopus occur sympatrically and, often, syntopically. To ensure only P. maniculatus was represented in our data set, we performed DNA barcoding of specimens at the mitochondrial DNA cytochrome b gene (1,140 bp) for all individuals with a tail length of less than 75 mm, a threshold above which individuals in North Carolina can be confidently assigned to P. maniculatus. We extracted DNA from freshly frozen liver tissue using Purelink Genomic DNA kits (ThermoFisher Scientific, High Point, NC) following manufacturer’s protocols. Standard polymerase chain reaction (PCR) was performed in 25 µl reactions using a combination of the primers MSB05 and MSB14 (Hope et al. 2014) and annealing temperatures of 51°C on a Veriti PCR thermocycler (Applied Biosystems, Waltham, MA). Products were cleaned using EXOSAP-IT and sequenced on an ABI 3730 sequencer at the NC State University Genomic Sciences Laboratory (Raleigh, North Carolina) using Big Dye Terminator 3.1 technology. Sequences were manually edited in Geneious v2022.1.1 and identifications were confirmed using the BLAST tool in Geneious.
GI tract measurements.
Each GI tract was processed by thawing and separating into four constituent sections; stomach, cecum, small intestine, and large intestine. Each section was gently cleared of fat and connective tissue and placed into a petri dish with a small amount of physiological saline (to prevent desiccation), and three measurements were taken: length, wet mass, and dry mass. Lengths were obtained to the nearest millimeter using a flexible measuring tape by gently extending each section. For curved sections (i.e., stomach and cecum), the length measurement was taken along the outer curve. To obtain wet masses, we emptied each section by cutting the organ open lengthwise, rinsing with physiological saline, gently scraping out any remaining contents, blotting to remove excess fluid, and weighing to the nearest milligram using a Torbal AGZN120 Analytical Balance. Any stomach contents, cecal contents, and/or fecal pellets were collected and frozen. To obtain dry masses, each section was dried at 57°C to a constant mass (approximately 24 h for all sections) and weighed. We calculated mean length, wet mass, and dry mass for each GI section across all individuals in the data set, as well as for individuals by month. We binned individuals temporally by either month or season for some analyses. Seasons (Winter, Spring, Summer, and Fall) were delineated according to the winter and summer solstices, and the spring and fall equinoxes. Each of our analyses described below was performed on raw and scaled measurements. To scale GI length measurements, raw values were divided by the head-body length of each individual (i.e., total length minus tail length). To scale mass measurements, the cubic root of raw values was divided by the head-body length of an individual, to prevent scaling a linear measure by a volumetric measure. Finally, we explored ratios of large intestine:small intestine (hereafter, LI:SI) length and mass as proxies of adaptation to poor-quality (high-fiber) diet (Snipes 1994).
Diet analysis.
For a subset of P. maniculatus analyzed (n = 53 total, average of 4.4 per month), a subsample of liver was subjected to stable isotope analysis to assess relative trophic position. We did this because trophic shifts (e.g., consuming more arthropods in summer), which have been recorded in our general study region (Linzey and Linzey 1973), also represent diet quality shifts with bearing on individual energetics. Liver tissue has a relatively rapid turnover time, and should therefore be a good indicator of intra-seasonal diet (Post et al. 2007). One study quantified the half-life of carbon in liver tissue as 6.4 days, meaning complete turnover would occur in less than 2 weeks (Tieszen et al. 1983). Each liver subsample was lyophilized for approximately 24 h to remove excess water, and 0.4–0.6 mg of each was ground and packaged into tin capsules. Samples were analyzed for organic C and N signatures via mass spectroscopy at the University of New Mexico Center for Stable Isotopes analysis (http://csi.unm.edu). Measurements were made using a Costech ECS 4010 Elemental Analyzer coupled to a ThermoFisher Scientific Delta V Advantage mass spectrometer via a CONFLO IV interface. Isotope ratios are reported using the standard delta (δ) notation relative to V-AIR and to Vienna Pee Dee Belemnite, respectively. Three internal laboratory standards were run at the beginning, at intervals between samples, and at the end of analytical sessions. Analytical precision was calculated as ±0.1‰ (1σ standard deviation) for both δ15N and δ13C.
Fecal pellets collected from individuals (whenever present) were also preprocessed in order to perform fiber content analysis, which would also help to identify diet quality shifts, both among and within trophic levels (e.g., consuming different plant parts). Fecal pellets were dried for approximately 48 h at 57°C, ground to a powder, and weighed. However, total masses were insufficient for fiber analyses (>2 g required) even when pooling all individuals into two coarse time bins (Summer and Winter); therefore, these analyses were not performed and are not discussed further.
Statistical analysis.
To more confidently link GI plasticity to dietary change, we first tested whether trophic position (as reflected by δ15N and δ13C content) varied by season. Because lipid content can bias carbon isotope signatures, prior to analysis we assessed potential for lipid bias by regressing δ13C on C:N ratios (Post et al. 2007). We found evidence for lipid bias in δ13C, so we corrected for this using equation 6 from Post et al. (2007). We then performed separate one-way analysis of variances (ANOVAs) on δ15N and δ13C, with season as treatment.
We next tested the effects of season, sex, and breeding condition on GI traits using a three-way ANOVA. We incorporated a sex × breeding interaction in the tests to account for potential sex-specific differences in GI response to energetic demands of breeding. The ANOVA was performed separately for each trait (length, wet mass, and dry mass) for each GI section, as well as for the total length, wet mass, and dry mass. Seasons were delineated as previously stated. The distributions of the scaled total lengths and wet masses were each right-skewed, so we log10-transformed these specific metrics prior to analysis. However, qualitatively identical results were obtained from ANOVAs performed on untransformed data.
Analyses of total GI length and mass provide a holistic view of GI size but could obscure potential trade-offs occurring among individual GI sections. To explore this possibility, we calculated the LI:SI ratios for all individuals to track how these metrics changed with varying food availability and quality. The LI:SI length ratio has been used previously to classify species to dietary guild (Snipes 1994) because the large intestine (including cecal compartments) in small mammals is intimately involved in processing poor-quality foods; a higher LI:SI ratio thus indicates greater relative functional investment in large intestine size. The large intestine in our calculated ratio included the cecum. We calculated the ratio for all three traits (length, wet mass, and dry mass) and ran an ANOVA on each of these metrics to test the effects of season (we did not include sex and reproduction as predictors for analyses of the ratio).
Finally, to visualize annual cycles in GI proportions, we conducted polynomial linear regressions that predicted GI traits as a function of Julian day, sex, breeding condition, and the sex x breeding interaction, as above. Regressions were only performed for GI traits with significant seasonal changes recovered in the three-way ANOVA. We evaluated models that used a second-, third-, and fourth-order polynomial on time (Julian day) and compared these models section-wise using Akaike information criterion scores. All analyses were conducted in R v4.0.2 (R Development Core Team. 2012).
Results
Field sampling results.
A total of 3,913 trap-nights resulted in 122 P. maniculatus captures (3.12% trap success). Of those, 87 were collected as vouchers and the GI tracts measured. A total of 79 individual adult P. maniculatus were included in the analyses after excluding juveniles and subadults, as well as individuals identified as P. leucopus using DNA barcoding. We chose to omit juveniles and subadults in this study because there is evidence for ontogenetic variation in the GI tract, and certain organs may grow disproportionally relative to the rest of the body (O’Connor 1966; Munn et al. 2021). There was a mean of 6.58 mice per month included in subsequent analyses, with a range from 2 (July, December) to 11 (January).
Dietary analysis results.
The δ15N values of liver tissue ranged from 0.62 to 5.7 with a mean of 2.39. The δ13C values ranged from −26.84 to −22.74 with a mean of −24.22. Seasonal averages for δ15N signatures were highest in the summer and lowest in the winter (2.93 and 1.46, respectively; Fig. 1). The ANOVA that tested the effect of season on δ15N was highly significant (P < 0.001, F = 7.573), supporting partial, upward trophic shifts in summer. Conversely, season averages for δ13C were highest in the winter and lowest in the summer (−23.97 and −24.44, respectively; Fig. 1), although season had no significant effect on δ13C based on ANOVA.
Fig. 1.
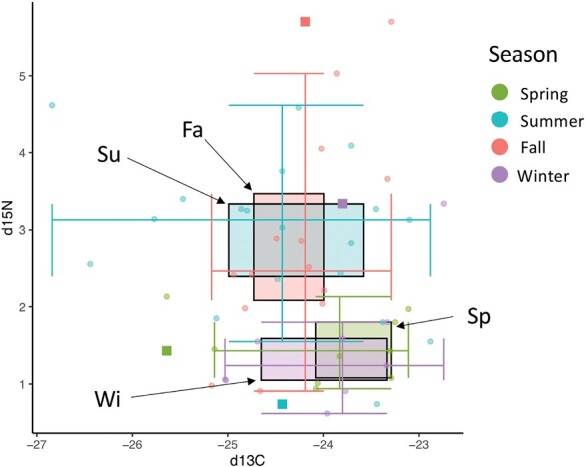
Stable isotope signatures (δ15N and δ13C) of Peromyscus maniculatus liver tissue, binned by season.
Effects of sex, reproduction, and season.
Summary statistics for all GI measurements taken in this study are reported in Table 2. Season had a significant effect on total GI length (P < 0.001, F = 14.529) and wet mass (P < 0.001, F = 6.786), but not dry mass (Table 3), with GI tracts being relatively longest and heaviest in winter (Figs. 2 and 3). For the two former traits, season had the single greatest effect size of any predictor. Sex did not have an effect on any trait, but reproduction had a significant effect on GI length (P < 0.001, F = 13.255) and wet mass (P = 0.039, F = 4.853; Table 3). Notably, the sex × reproduction interaction was significant for all three traits (P < 0.05 for all; Table 3), indicating there are sex-specific responses in GI tracts with breeding in P. maniculatus. Post hoc analyses of the data indicated that reproductively active males had shorter and lighter GI tracts than nonreproductive males, while there was no difference between breeding and nonbreeding females (Fig. 2).
Table 2.
Mean values and standard deviations for six measurements taken on adult Peromyscus maniculatus gastrointestinal tracts. The sample size was 79, and any deviations from this are noted in parentheses next to measurements. Scaled measurements represent the raw measurement divided by head-body length (lengths) or cube root of head-body length (masses).
| Gastrointestinal section | Length (mm) | Scaled length | Wet mass (g) | Scaled wet mass | Dry mass (g) | Scaled dry mass |
|---|---|---|---|---|---|---|
| Stomach | 32.304 ± 3.657 | 0.395 ± 0.053 | 0.259 ± 0.063 | 0.008 ± 0.001 | 0.055 ± 0.013 | 0.005 ± 0.0004 |
| Cecum | 38.97 ± 6.123 (78) | 0.478 ± 0.084 (78) | 0.118 ± 0.033 | 0.006 ± 0.001 | 0.017 ± 0.006 | 0.003 ± 0.0004 |
| Small intestine | 302.064 ± 42.162 (78) | 3.696 ± 0.640 (78) | 0.327 ± 0.034 (78) | 0.006 ± 0.001 (78) | 0.058 ± 0.021 (78) | 0.005 ± 0.0005 (78) |
| Large intestine | 86.544 ± 15.749 | 1.056 ± 0.193 | 0.014 ± 0.101 | 0.008 ± 0.001 | 0.018 ± 0.006 | 0.003 ± 0.0004 |
| Total | 459.636 ± 55.600 | 5.626 ± 0.852 | 0.809 ± 0.178 | 0.011 ± 0.001 | 0.148 ± 0.001 | 0.006 ± 0.0005 |
Table 3.
Results of analysis of variance tests examining effects of season and individual-level variables on scaled gastrointestinal (GI) measurements. df = degrees of freedom. Significance codes denotes 0: ***; 0.001: **; 0.01: *.
| df | Sum sq | Mean sq | F-value | Pr(>F) | |
|---|---|---|---|---|---|
| Scaled total GI length | |||||
| Sex | 2 | 0.00799 | 0.00399 | 1.661 | 0.197535 |
| Reproductively active | 1 | 0.03004 | 0.03004 | 12.493 | 0.000734*** |
| Season | 3 | 0.10364 | 0.03455 | 14.367 | 2.27e-07*** |
| Sex:Reproductively active | 1 | 0.01866 | 0.01866 | 7.759 | 0.006896** |
| Residuals | 69 | 0.16591 | 0.00240 | ||
| Scaled total GI wet mass | |||||
| Sex | 2 | 0.00089 | 0.000446 | 0.325 | 0.723755 |
| Reproductively active | 1 | 0.00609 | 0.006092 | 4.441 | 0.038674* |
| Season | 3 | 0.02757 | 0.009190 | 6.698 | 0.000486*** |
| Sex:Reproductively active | 1 | 0.00832 | 0.008324 | 6.067 | 0.016232* |
| Residuals | 70 | 0.09603 | 0.001372 | ||
| Scaled total GI dry mass | |||||
| Sex | 2 | 4.440e-07 | 2.219e-07 | 0.870 | 0.4234 |
| Reproductively active | 1 | 2.730e-07 | 2.729e-07 | 1.070 | 0.3044 |
| Season | 3 | 6.010e-07 | 2.004e-07 | 0.786 | 0.5058 |
| Sex:Reproductively active | 1 | 1.481e-06 | 1.481e-06 | 5.809 | 0.0186* |
| Residuals | 70 | 1.785e-05 | 2.550e-07 | ||
Fig. 2.
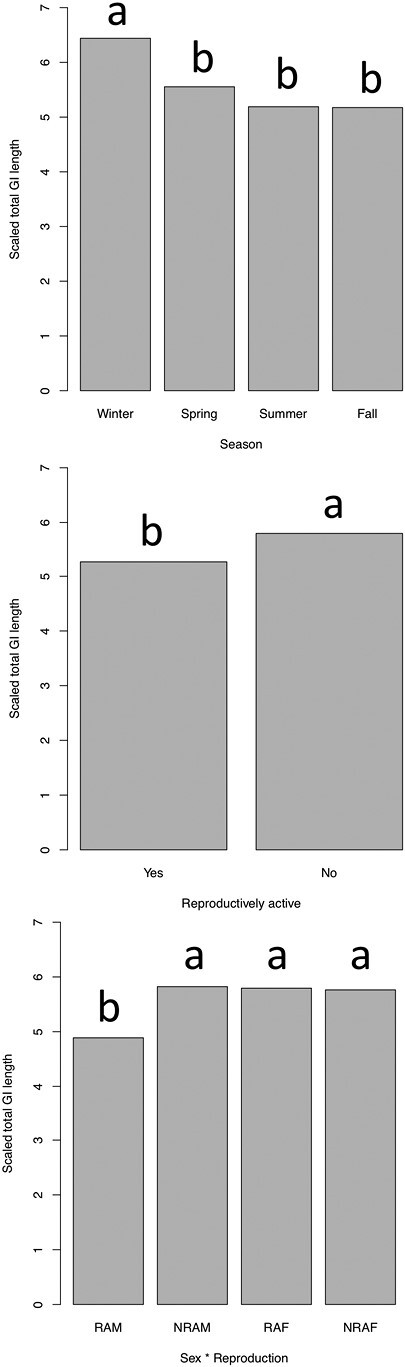
Effects of season, reproductive activity, and the interaction between sex and reproductive activity on scaled total gastrointestinal length in Peromyscus maniculatus. The fixed term for sex was not significant and is not included individually here. Letters within terms represent statistically significant groups. “RAM,” reproductively active males; “NRAM,” nonreproductively active males; “RAF,” reproductively active females; “NRAF,” nonreproductively active females.
Fig. 3.
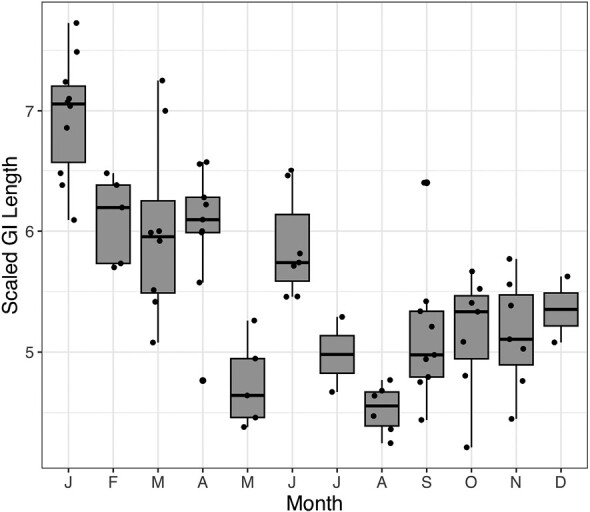
Boxplots of Peromyscus maniculatus scaled gastrointestinal tract length by month. Each boxplot reflects the median (center line), first and third quartile (box edges), and ±1.58 time the interquartile range (whiskers).
Among the individual GI sections, effects of season and life history traits were variable. For stomach, season had a significant effect on the length, wet mass, and dry mass (Supplementary Data SD1). For cecum, season had a significant effect on the length and wet mass (Supplementary Data SD1). The results of the ANOVA for the small intestine showed that season had a significant effect on the length (Supplementary Data SD1). Finally, the ANOVA for the large intestine showed that season had a significant effect on the wet mass and dry mass (Supplementary Data SD1).
We found that LI:SI ratios also changed seasonally, as expected given the observed shifting quality of diet consumed in different seasons. However, length and mass ratios changed in opposite directions (Fig. 4). The LI:SI length ratio shifted among seasons (P = 0.01, F = 4.096), with winter having the lowest mean value (Fig. 4). The LI:SI mass ratios also changed seasonally (P < 0.001, F = 13.16 and 12.5 for wet and dry mass), with winter having the highest mean values (Fig. 4).
Fig. 4.
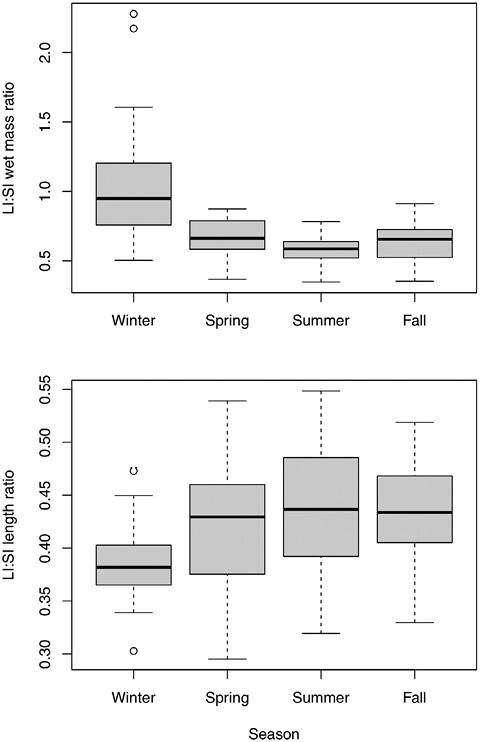
Boxplots of the ratio of large intestine: small intestine length (top) and wet mass (bottom), binned by season.
When visualizing the annual trend in GI proportions, the best-fitting polynomial regression across all traits (including total length and mass) was a second-order polynomial. The population-level trend is one in which winter mice have the longest GI tracts, lengths decrease into the summer, and then rise again in the fall and early winter. The highest seasonal mean for scaled GI tract length occurred in winter (6.44), while the lowest seasonal mean for scaled GI tract length occurred in fall (5.17), a decrease of approximately 20%. Accordingly, the extremes of monthly mean for scaled GI length occurred in January (6.95) and August (4.53), a decrease of approximately 35% (Fig. 5). As with length, the greatest seasonal mean scaled wet mass occurred in winter, while the lowest seasonal mean scaled wet mass occurred in fall, with a decrease of approximately 11%. The greatest monthly mean scaled wet mass occurred in January and the lowest occurred in August, decreasing by approximately 18% (Supplementary Data SD1). Conversely, scaled dry masses were relatively constant throughout the year, changing by a maximum of just 4.5% among seasons (the greatest and least mean values occurred in winter and summer) and 8% among months (the greatest and least mean values occurred in June and September; Supplementary Data SD1).
Fig. 5.
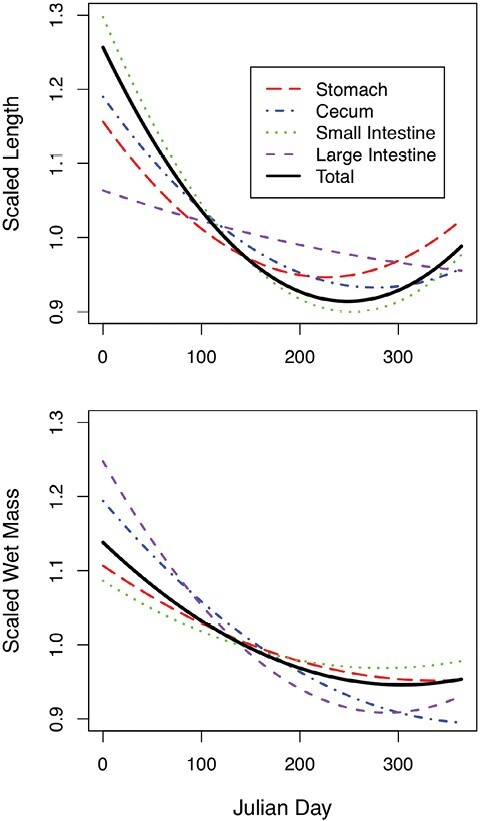
Top-ranking polynomial regressions of scaled length (top) and wet mass (bottom) of four gastrointestinal tract sections. Regressions were evaluated for each section (stomach, small and large intestines, and cecum) and the total scaled gastrointestinal length. Each regression was scaled by the mean value of each section.
Finally, there was a clear annual pattern in body size (head-body lengths and masses) of the 79 adult mice used in this study. Head-body lengths were lowest in the winter and increased into the summer and fall, before decreasing again in the beginning of winter (Supplementary Data SD2). The body masses displayed a similar pattern, with the lowest monthly average body masses occurring in December and January (12.13 and 14.18 g, respectively) and the largest monthly average body mass occurring in September (20.5 g, Supplementary Data SD2). While body mass alone may be partly confounded by reproductive activity, especially pregnancy, a similar annual trend in head-body lengths suggests that demographic effects are present in our data and that use of scaled traits is essential in any research that utilizes GI metrics.
Discussion
Refining the functional roles of soft tissue traits is crucial for their expanded use in small mammal ecology. Craniodental traits are often used as predictors of dietary guild in mammals, but these are unlikely to exhibit adaptive plasticity in the face of changing individual diets. Our study shows that GI morphology, an underutilized soft tissue proxy, can be a more effective functional trait for this purpose given its intimate linkages to energy supply (e.g., diet quality) and demand (e.g., maintenance of homeothermy, reproductive activity). Specifically, we show that GI length and mass change significantly across seasons, with mice caught in the winter having the longest and heaviest GI tracts. These changes occur simultaneously with a shift to lower dietary quality (based on δ15N) in the winter months, suggesting that partial trophic changes are a driver of GI tract variation. In addition, we found evidence that reproduction affects GI length and mass, although these changes are sex-specific. In general, these findings are consistent with the hypothesis that GI phenotypic plasticity helps to mitigate increased energetic demand and poorer quality of diets during the winter period.
The P. maniculatus species group has one of the largest geographic ranges of any North American mammal (Kurta 1995) and is common throughout much of the continent, being abundant in many different types of habitats (Greenbaum et al. 2019). Our plots were located in a seasonal, high-elevation part of North Carolina where temperatures regularly dropped below freezing during the winter sampling sessions, with occasional snowpack. It is important to note that our results may not be representative of plasticity in all P. maniculatus lineages, particularly those that occur in more aseasonal areas, which are exposed to different energetic extremes and display differing breeding phenologies and life history parameters (Millar 1989; McLean et al. 2019; McLean and Guralnick 2021; Wilsterman and Cunningham 2022). However, most P. maniculatus lineages do inhabit regions of high seasonality and thus our results, combined with those of previous studies, suggest that this species is characterized by substantial potential flexibility in GI morphology, with seasonal dietary changes being one main driver.
The climatic variability hypothesis (CVH) states that, due to an increase in climatic fluctuation at higher latitudes, individuals living in such areas should exhibit increased phenotypic flexibility (Naya 2008; Naya et al. 2008a; Molina-Montenegro and Naya 2012). This increased phenotypic flexibility should in turn allow phenotypically flexible populations to thrive and become more widely distributed throughout those habitats (Naya et al. 2008a). Naya et al. (2008a) took a meta-analytic approach to evaluating the CVH as manifested in small intestine length in rodents, and found that plasticity in small intestine length was positively related to latitude and the number of different habitats occupied by those species (Naya et al. 2008a). A 2010 study of field mice in Chile (Abrothrix olivaceus) also showed that GI tract plasticity was greater in mice from higher latitudes (Bozinovic et al. 2010).
Consistent with the CVH and previous work, we found that scaled total GI lengths of P. maniculatus measured in this study varied by approximately 20% among seasons and 35% among months (model averages), while scaled small intestine lengths showed a maximum change of 23% among seasons and an identical maximum change of 35% among months. Although our plots were at an intermediate latitude, they are high elevation sites which results in increased levels of seasonality relative to adjacent areas. We also found that LI:SI ratios—a proxy for relative adaptation to low-quality food—changed seasonally, indicating that the relative roles of these two major GI organs vary with dietary shifts. We expected the length ratio to vary similarly as for total GI lengths (being greatest in winter), but found the opposite trend, while mass ratios were consistent with our expectations (being greatest in winter). We found that the small and large intestines themselves changed in the expected directions; however, the much longer length and higher absolute amount of change in the small intestine appear to result in a lower LI:SI ratio. It is possible that dietary shifts throughout the year included major changes in fiber content, which could lead to these relatively larger changes to the small intestine. Future studies should focus on similar quantification of levels of plasticity in GI tract morphology in wild populations located in less seasonal areas, in order to further test how the CVH applies when food quality is more stable throughout the year. Additional studies of variation in form (length, mass, or other proxy) among GI sections will also be critical to resolve actual functional changes in the gut.
Evaluating the proximate factor of diet is an important step in properly contextualizing GI flexibility; however, this can be difficult because different methods for quantifying diet contain different sources of bias. The pattern of increased summer δ15N indicates that this population of P. maniculatus incorporates more animal (likely adult or larval arthropods) or fungal matter in the summer than winter. A 1986 study on P. maniculatus in Montana showed that populations consumed the highest number of arthropods in the summer; however, they consumed the lowest number of arthropods in the spring (Sieg et al. 1986). Populations of P. maniculatus in Great Smoky Mountains National Park (North Carolina/Tennessee), which is reasonably close to our study site, may incorporate over three times more insect material in summer than winter (Linzey and Linzey 1973). Consistent with those studies, our data indicate a partial trophic increase in summer, with average δ15N signatures being 1.41‰ higher in summer than in winter. Our data are strongly suggestive of these lower-quality winter diets as a cause for GI tract lengthening, consistent with predictions from theory and empirical results (Young Owl and Batzli 1998), but additional, higher-resolution data on diet (e.g., micro histology or DNA metabarcoding) will be critical in future tests of GI tract remodeling in P. maniculatus and other small mammals.
Beyond diet, we found that reproduction mediated seasonal phenotypic plasticity of the GI tract. Reproductive activity had a significant effect on the length and wet mass of the GI tract, as well as lengths and masses of most constituent sections, while sex did not. There was also a strong interaction between the two terms, indicating that there are sex-specific responses of GI length to breeding activity. Post hoc tests indicate that reproductively active males had shorter and lighter GI tracts than nonreproductive males, but there was no difference in either trait for females. Since most reproductive males were collected in warmer months, the male effect could possibly just be a seasonal effect. However, results for females were discordant with our expectations for longer GI tracts, given that reproduction in females (especially lactation) carries relatively high energetic demand (Cripps and Williams 1975; Kenagy and Barnes 1988; Speakman 2008; Canul-Medina and Fernandez-Mejia 2019). This lack of change between reproductive and nonreproductive females is thus interesting and may reflect opposing effects of diet (e.g., favoring shorter GI tracts in summer) and breeding (e.g., requiring longer GI tracts in summer). Again, however, most reproductively active mice (80% for females and 85.72% for males) were captured in the spring and summer months, so it remains difficult to fully tease apart effects of seasonal diet and reproduction. Furthermore, only two females that classified as reproductively active were lactating (others were in various stages of pregnancy and/or with enlarged nipples), despite the fact that lactation represents the greatest energetic demand during female reproduction (Cripps and Williams 1975; Kenagy and Barnes 1988; Speakman 2008);this reflects a need for more balanced sampling design in future studies.
While the entire GI tract plays a unique role in food processing and energy acquisition, understanding which GI sections (and measurements) are maximally informative about capacity for dietary flexibility is essential. Total length of the GI tract was more variable than the total wet mass or dry mass in our study, indicating that the length trait may be more responsive to energetic demands than either of the mass traits. The wet mass showed a similar trend to length measurements, reaching a maximum in summer months, although the maximum percent change in the monthly averages was lower (18% for wet mass vs. 35% for length). The dry mass did not change significantly throughout the year, only showing a maximum change of 4.5% between seasons and 8% between months, suggesting that changes in mucosal thickness or cellular architecture are likely driving the wet mass changes. Conversely, Derting and Noakes (1995) showed that the wet mass of the stomach, cecum, and small intestine increased in the winter in both P. leucopus and M. pennsylvanicus and this increase in the small intestine was mainly attributed to an increase in the amount of mucosa, which is heavily involved in the absorption of nutrients (Jankowski et al. 1994; Doherty and Charman 2002). We recommend that future studies continue to focus on both lengths and masses of the GI trait, particularly because our results may not be representative of other populations and species.
As with single measurements, the LI:SI ratios also varied throughout the year. Ratios of both wet and dry masses were highest in the winter, indicating more of an investment into the large intestine during this time and likely higher capacity for processing low-quality diets. However, the LI:SI length ratio showed an opposite trend, being lowest in winter. This suggests that increases in large intestine size may not serve to increase retention times per se; instead, relative increases in the mass of the large intestine during periods of low food quality may increase absorptive capacity in the gut mucosa. We included the cecum in these ratios, and P. maniculatus have a relatively short and simple cecum, so it may be more effective to invest into increasing the mass of this particular section. It is also possible that this trend does not hold in all species; those with longer and more complex ceca may more easily benefit from increasing large intestine length as well as mass.
The clear trend in body size throughout the year at our plots suggests that any study of GI tract changes in wild mammals must utilize a relativized metric of GI morphology. Observed body size increases over the course of spring and summer could be due to the early-year (Spring) cohort reaching maturation, given that adult mice continue to grow throughout their life (Myers and Master 1983). The increase could additionally be driven by older, overwintered adults being in better condition in the growing season versus the winter. Conversely, a decrease in the average head-body length starting in October probably reflects weaning of the fall cohort and their recruitment into the population, although this cohort may experience slower maturation rates than spring cohorts. Since using a relativized metric of GI size is desirable, future studies might consider incorporating more precise indicators of age, such as dental features or telomere length. However, the body size changes we observed are not likely to be driving seasonal and sex-specific patterns. Intestines of lab mice grow disproportionally faster than the body, although this rate levels off as the mouse matures (O’Connor 1966). A similar effect was observed in western grey kangaroos (Munn et al. 2021). These ontogenetic trends would generate results opposite of that observed here; specifically, relatively longer GI tracts at times when new cohorts are entering the population (e.g., summer and fall), which is not what we observe here. Still, the arrival of new cohorts throughout the year may introduce statistical noise in the form of month-to-month trends, such as the significant jump in GI morphology from May to June (potentially reflecting recruitment of the Spring cohort). Considering individual age, population demography and ontogenetic trajectories of different soft tissues will be critical factors to consider when applying similar methods to other wild species.
Implications.
This study expands understanding of phenotypic plasticity in rodents by quantifying annual change in GI tract morphology of P. maniculatus inhabiting western North Carolina. Relative length and mass of the GI tract varied seasonally with shifting trophic level, with winter mice consuming poorer-quality diets and having significantly longer and heavier GI tracts than mice collected in the summer. GI morphology also varied with energetic demands of breeding, and we observed sex-specific effects of reproduction on GI tract lengths and wet masses. By sampling across a temporal gradient of dietary change, our results provide further evidence for the functional role of the GI tract and constituent sections in accommodating naturally occurring dietary variation, paving the way for more widespread use of GI proportions as continuous, soft tissue functional traits in ecological studies.
Supplementary Data
Supplementary data are available at Journal of Mammalogy online.
Supplementary Data SD1.—Boxplots of monthly scaled gastrointestinal tract wet masses (left) and dry masses (right) of adult Peromyscus maniculatus (n = 79) used in this study, binned by month.
Supplementary Data SD2.—Boxplots of monthly changes in head-body length (left) and body mass (right) of adult Peromyscus maniculatus (n = 79) used in this study.
Acknowledgments
We thank the many undergraduate and graduate students, especially Amanda Weller (UNCG Biology), who helped with both field and lab work. We also thank two anonymous reviewers; whose comments greatly improved the quality of this paper.
Contributor Information
Olivia S Chapman, Department of Biology, University of North Carolina at Greensboro, 325 McIver Street, Greensboro, North Carolina 27412, USA.
Bryan S McLean, Department of Biology, University of North Carolina at Greensboro, 325 McIver Street, Greensboro, North Carolina 27412, USA.
Conflict of Interest
The authors declare they have no conflict of interest.
Funding
This study was funded by the University of North Carolina at Greensboro Biology Department, including the 2021 W. John O’Brien Award for Field Research (to OSC) and faculty startup funds (to BSM). Dissemination was supported by the 2022 Annie M. Alexander Award (to OSC) awarded by the American Society of Mammalogists.
Author Contributions
OSC conceived the ideas and designed methodology, collected the data, analyzed the data, and led the writing of the manuscript. BSM conceived the ideas and designed methodology, analyzed the data, and led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Data Availability
Publicly available data sets utilized for this research include McLean (2023). University of North Carolina at Greensboro Mammal Collection (Arctos) Version 1.7 (GBIF occurrence data set https://doi.org/10.15468/nujf6d).
Literature Cited
- Ayres, M.P. 1993. Global change, plant defense, and herbivory. In: Kareiva P.M., Kingsolver J.G., and Huey R.B., editors. Biotic interactions and global change. Sinauer Associates, Sunderland, Massachusetts, USA; p. 75–94. [Google Scholar]
- Björnhag, G. 1994. Adaptations in the large intestine allowing small animals to eat fibrous foods. In: Chivers D.J. and Langer P., editors. The digestive system in mammals: food form and function. Cambridge University Press, Cambridge, United Kingdom; p. 287–310. [Google Scholar]
- Bozinovic F., Rojas J.M., Maldonado K., Sabat P., Naya D.E.. 2010. Between-population differences in digestive flexibility in the olivaceous field mouse. Zoology 113:373–377. [DOI] [PubMed] [Google Scholar]
- Canul-Medina G., Fernandez-Mejia C.. 2019. Morphological, hormonal, and molecular changes in different maternal tissues during lactation and post-lactation. The Journal of Physiological Sciences 69:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivers, D.J. 2003. The digestive system in mammals: food form and function. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Chivers D.J., Hladik C.M.. 1980. Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. Journal of Morphology 166:337–386. [DOI] [PubMed] [Google Scholar]
- Cripps A.W., Williams V.J.. 1975. The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. British Journal of Nutrition 33:17–32. [DOI] [PubMed] [Google Scholar]
- Dehority B.A. 2002. Gastrointestinal tracts of herbivores, particularly the ruminant: anatomy, physiology and microbial digestion of plants. Journal of Applied Animal Research 21:145–160. [Google Scholar]
- Derting T.L., Austin M.W.. 1998. Changes in gut capacity with lactation and cold exposure in a species with low rates of energy use, the Pine Vole (Microtus pinetorum). Physiological Zoology 71:611–623. [DOI] [PubMed] [Google Scholar]
- Derting T.L., Bogue B.A.. 1993. Responses of the gut to moderate energy demands in a small herbivore (Microtus pennsylvanicus). Journal of Mammalogy 74:59–68. [Google Scholar]
- Derting T.L., Hornung C.A.. 2003. Energy demand, diet quality, and central processing organs in wild white-footed mice (Peromyscus leucopus). Journal of Mammalogy 84:1381–1398. [Google Scholar]
- Derting T.L., Noakes E.B. III. 1995. Seasonal changes in gut capacity in the White-footed Mouse (Peromyscus leucopus) and Meadow Vole (Microtus pennsylvanicus). Canadian Journal of Zoology 73:243–252. [Google Scholar]
- Doherty M.M., Charman W.N.. 2002. The mucosa of the small intestine. Clinical Pharmacokinetics 41:235–253. [DOI] [PubMed] [Google Scholar]
- Duque-Correa M.J., Codron D., Meloro C., McGrosky A., Schiffmann C., Edwards M.S., Clauss M.. 2021. Mammalian intestinal allometry, phylogeny, trophic level and climate. Proceedings of the Royal Society of London, B: Biological Sciences 288:20202888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R.V., Falster D.S., Maitner B.S., Salguero-Gómez R., Vandvik V., Pearse W.D., Schneider F.D., Kattge J., Poelen J.H., Madin J.S., et al. 2020. Open science principles for accelerating trait-based science across the tree of life. Nature Ecology & Evolution 4:294–303. [DOI] [PubMed] [Google Scholar]
- Greenbaum I.F., Honeycutt R.L., Chirhart S.E.. 2019. Taxonomy and phylogenetics of the Peromyscus maniculatus species group. Special Publications, Museum of Texas Tech University 71:559–575. [Google Scholar]
- Green D.A., Millar J.S.. 1987. Changes in gut dimensions and capacity of Peromyscus maniculatus relative to diet quality and energy needs. Canadian Journal of Zoology 65:2159–2162. [Google Scholar]
- Greiman S.E., Cook J.A., Tkach V.V., Hoberg E.P., Menning D.M., Hope A.G., Sonsthagen S.A., Talbot S.L.. 2018. Museum metabarcoding: a novel method revealing gut helminth communities of small mammals across space and time. International Journal for Parasitology 48:1061–1070. [DOI] [PubMed] [Google Scholar]
- Gross J.E., Wang Z., Wunder B.A.. 1985. Effects of food quality and energy needs: changes in gut morphology and capacity of Microtus ochrogaster. Journal of Mammalogy 66:661–667. [Google Scholar]
- Hammond K.A. 1997. Adaptation of the maternal intestine during lactation. Journal of Mammary Gland Biology and Neoplasia 2:243–252. [DOI] [PubMed] [Google Scholar]
- Hammond K.A., Roth J., Janes D.N., Dohm M.R.. 1999. Morphological and physiological responses to altitude in deer mice Peromyscus maniculatus. Physiological and Biochemical Zoology 72:613–622. [DOI] [PubMed] [Google Scholar]
- Hammond K.A., Szewczak J., KrÓl E.. 2001. Altitude and temperature effects on phenotypic plasticity. The Journal of Experimental Biology 204:1991–2000. [DOI] [PubMed] [Google Scholar]
- Hammond K.A., Wunder B.A.. 1995. Effect of cold temperatures on the morphology of gastrointestinal tracts of two Microtine rodents. Journal of Mammalogy 76:232–239. [Google Scholar]
- Hope A.G., Panter N., Cook J.A., Talbot S.L., Nagorsen D.. 2014. Multilocus phylogeography and systematic revision of North American water shrews (genus: Sorex). Journal of Mammalogy 95:722–738. [Google Scholar]
- Jankowski J.A., Goodlad R.A., Wright N.A.. 1994. Maintenance of normal intestinal mucosa: function, structure, and adaptation. Gut 35:S1–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenagy G.J., Barnes B.M.. 1988. Seasonal reproductive patterns in four coexisting rodent species from the Cascade Mountains, Washington. Journal of Mammalogy 69:274–292. [Google Scholar]
- Kerby J., Post E.. 2013. Capital and income breeding traits differentiate tropic match-mismatch dynamics in large herbivores. Philosophical Transactions of the Royal Society of London, B: Biological Sciences 368:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston A.K. 2018. Longitudinal study of rat volar fat pad fixation and ethanol storage: implications for the use of fluid-preserved specimens in morphological studies. Journal of Anatomy 233:607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling W.D., Ahumada J.A., Bowser A., Fernandez M., Fernández N., García E.A., Guralnick R.P., Isaac N.J.B., Kelling S., Los W., et al. 2018. Building essential biodiversity variables (EBVs) of species distribution and abundance at a global scale. Biological Reviews 93:600–625. [DOI] [PubMed] [Google Scholar]
- Kohli B.A., Rowe R.J.. 2019. Beyond guilds: the promise of continuous traits for mammalian functional diversity. Journal of Mammalogy 100:285–298. [Google Scholar]
- Korslund L., Steen H.. 2006. Small rodent winter survival: snow conditions limit access to food resources. Journal of Animal Ecology 75:156–166. [DOI] [PubMed] [Google Scholar]
- Koteja P. 1996. Limits to the energy budget in a rodent, Peromyscus maniculatus: does gut capacity set the limit? Physiological Zoology 69:994–1020. [Google Scholar]
- Kurta, A. 1995. Mammals of the Great Lakes Region. The University of Michigan Press, Ann Arbor, Michigan, USA. [Google Scholar]
- Laureto L.M.O., Cianciaruso M.V., Samia D.S.M.. 2015. Functional diversity: an overview of its history and applicability. Natureza & Conservação 13:112–116. [Google Scholar]
- Linzey D.W., Linzey A.V.. 1973. Notes on food of small mammals from Great Smoky Mountains National Park. Journal of the Elisha Mitchell Scientific Society 89:6–14. [Google Scholar]
- Mayor S.J., Guralnick R.P., Tingley M.W., Otegui J., Withey J.C., Elmendorf S.C., Andrew M.E., Leyk S., Pearse I.S., Schneider D.C.. 2017. Increasing phenological asynchrony between spring green-up and arrival of migratory birds. Scientific Reports 7:1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill B., Enquist B., Weiher E., Westoby M.. 2006. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution 21:178–185. [DOI] [PubMed] [Google Scholar]
- McLean, B.S. 2023. University of North Carolina at Greensboro Mammal Collection (Arctos). Version 1.7. University of North Carolina at Greensboro. Occurrence dataset < 10.15468/nujf6d>. Accessed via GBIF.org on 16 June 2023. [DOI] [Google Scholar]
- McLean B.S., Barve N., Flenniken J., Guralnick R.P.. 2019. Evolution of litter size in North America’s most common small mammal: an informatics-based approach. Journal of Mammalogy 100:365–381. [Google Scholar]
- McLean B.S., Guralnick R.P.. 2021. Digital biodiversity data sets reveal breeding phenology and its drivers in a widespread North American mammal. Ecology 102:12. [DOI] [PubMed] [Google Scholar]
- Millar, J.S. 1989. Reproduction and development. In: Kirkland G. and Layne J., editors. Advances in the study of Peromyscus (Rodentia). Texas Tech University Press, Lubbock, Texas, USA; p.169–232. [Google Scholar]
- Millar J.S., Xia X., Norrie M.B.. 1990. Relationships among reproductive status, nutritional status, and food characteristics in a natural population of Peromyscus maniculatus. Canadian Journal of Zoology 69:555–559. [Google Scholar]
- Molina-Montenegro M.A., Naya D.E.. 2012. Latitudinal patterns in phenotypic plasticity and fitness-related traits: assessing the climatic variability hypothesis (CVH) with an invasive plant species. PLoS One 7:e47620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.J., Snelling E.P., Taggart D.A., Seymour R.S.. 2021. Ontogenetic scaling of the gastrointestinal tract of a marsupial foregut fermenter, the western grey kangaroo Macropus fuliginosus melanops. Journal of Comparative Physiology, B. Biochemical, Systematic, and Environmental Physiology 191:371–383. [DOI] [PubMed] [Google Scholar]
- Myers P., Master L.L.. 1983. Reproduction by Peromyscus maniculatus: size and compromise. Journal of Mammalogy 64:1–18. [Google Scholar]
- Naya D.E. 2008. Gut size flexibility in rodents: what we know, and don’t know, after a century of research. Revista Chilena de Historia Natural 81:599–612. [Google Scholar]
- Naya D.E., Bozinovic F., Karasov W.H.. 2008a. Latitudinal trends in digestive flexibility: testing the climatic variability hypothesis with data on the intestinal length of rodents. The American Naturalist 172:E122–E134. [DOI] [PubMed] [Google Scholar]
- Naya D.E., Ebensperger L.A., Sabat P., Bozinovic F.. 2008b. Digestive and metabolic flexibility allows female degus to cope with lactation costs. Physiological and Biochemical Zoology 81:186–194. [DOI] [PubMed] [Google Scholar]
- Naya D.E., Karasov W.H., Bozinovic F.. 2007. Phenotypic plasticity in laboratory mice and rats: a meta-analysis of current ideas on gut size flexibility. Evolutionary Ecology Research 9:1363–1374. [Google Scholar]
- O’Connor T.M. 1966. Cell dynamics in the intestine of the mouse from late fetal life to maturity. American Journal of Anatomy 118:525–536. [DOI] [PubMed] [Google Scholar]
- Parins-Fukuchi C. 2018. Use of continuous traits can improve morphological phylogenetics. Systematic Biology 67:328–339. [DOI] [PubMed] [Google Scholar]
- Post D.M., Layman C.A., Arrington D.A., Takimoto G., Quattrochi J., Montaña C.G.. 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. [DOI] [PubMed] [Google Scholar]
- Post E., Forchhammer M.C.. 2008. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philosophical Transactions of the Royal Society of London, B: Biological Sciences 363:2367–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay W.B. 1974. Bird and mammal specimens in fluid-objectives and methods. Curator: The Museum Journal 17:91–104. [Google Scholar]
- R Development Core Team. 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. <www.R-project.org/> [accessed 2020 Jun]. [Google Scholar]
- Schieck J.O., Millar J.S.. 1985. Alimentary tract measurements as indicators of diets of small mammals. Mammalia 49:93–104. [Google Scholar]
- Schradin C., Pillay N.. 2006. Female striped mice (Rhabdomys pumilio) change their home ranges in response to seasonal variation in food availability. Behavioral Ecology 17:452–458. [Google Scholar]
- Sieg C.H., Uresk D.W., Hansen R.M.. 1986. Seasonal diets of deer mice on bentonite mine spoils and sagebrush grasslands in southeastern Montana. Northwest Science 60:81–89. [Google Scholar]
- Sikes R.S., The Animal Care and Use Committee of the American Society of Mammalogists. 2016. 2016 guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97:663–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes, R.L. 1994. Morphometric methods for determining surface enlargement at the microscopic level in the large intestine and their application. In: Chivers D.J. and Langer P., editors. The digestive system in mammals: food form and function. Cambridge University Press, Cambridge, United Kingdom; p. 234–286. [Google Scholar]
- Soares J.C., Santos C.S., Carvalho S.M.P., Pintado M.M., Vasconcelos M.W.. 2019. Preserving the nutritional quality of crop plants under a changing climate: importance and strategies. Plant and Soil 443:1–26. [Google Scholar]
- Speakman J.R. 2008. The physiological costs of reproduction in small mammals. Philosophical Transactions of the Royal Society of London, B: Biological Sciences 363:375–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieszen L.L., Boutton T.W., Tesdahl K.G., Slade N.A.. 1983. Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57:32–37. [DOI] [PubMed] [Google Scholar]
- Verde Arregoitia L.D., D’Elía G.. 2021. Classifying rodent diets for comparative research. Mammal Review 51:51–65. [Google Scholar]
- Wilsterman K., Cunningham K.. 2022. Evolution in reproductive tempo and investment across the Peromyscus radiation. Journal of Experimental Zoology, Part A: Ecological and Integrative Physiology 339:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young Owl M., Batzli G.O.. 1998. The integrated processing response of voles to fibre content of natural diets: response of voles to fibre content of diet. Functional Ecology 12:4–13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available data sets utilized for this research include McLean (2023). University of North Carolina at Greensboro Mammal Collection (Arctos) Version 1.7 (GBIF occurrence data set https://doi.org/10.15468/nujf6d).