Abstract
Purpose:
To compare self-reported quality-of-life (QoL) outcomes of patients diagnosed as normal, glaucoma suspect, and glaucoma based on an objective reference standard for glaucomatous optic neuropathy (GON).
Design:
Cross-sectional study.
Participants:
1884 eyes of 1019 patients were included in the study.
Methods:
The data was sourced from the Duke Glaucoma Registry. Eyes were classified according to the presence and topographic correspondence of functional and structural damage, as assessed by parameters from standard automated perimetry (SAP) and spectral-domain OCT (SD-OCT). The objective diagnosis of the worse eye was used to define patient-level diagnosis. To assess QoL in the diagnostic groups, 14 unidimensional vision-related items of the National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) were used to assess QoL in the diagnostic groups. Association between NEI VFQ-25 Rasch-calibrated scores and diagnostic groups was assessed through multivariable regression that controlled for confounding demographic and socioeconomic variables such as age, sex, race, income, marriage status, insurance status, and highest education level.
Main Outcome Measures:
NEI VFQ-25 Rasch scores compared with objective criteria diagnosis based on SAP mean deviation (MD) and SD-OCT retinal nerve fiber layer (RNFL) thickness.
Results:
Overall, eyes classified as normal, glaucoma suspect, and glaucoma had decreasing mean scores in SAP MD (0.2 ± 1.0 dB, −0.9 ± 2.4 dB, −6.2 ± 7.0 dB, respectively; P < 0.001) and SD-OCT RNFL thickness (97.8 ± 9.5 μm, 89.0 ± 13.1 μm, 64.5 ± 12.8 μm, respectively; P < 0.001). The mean Rasch-calibrated NEI VFQ-25 score was significantly different among normal, suspect, and glaucoma groups (82.9 ± 13.0, 78.2 ± 14.8, and 72.6 ± 16.2, respectively; P < 0.001). When adjusted for confounding socioeconomic variables, glaucoma patients had significantly worse QoL than those classified as normal (β = −6.8 Rasch score units; P < 0.001).
Conclusion:
A glaucoma diagnosis, based on an objective reference standard for GON, was significantly associated with worse Rasch-adjusted scores of QoL. Utilization of such objective criteria may provide clinically relevant metrics with potential to improve comparability of research findings and validation of newly proposed diagnostic tools.
Keywords: Quality of life, Primary open angle glaucoma, Objective criteria, Glaucomatous optic neuropathy, Standard automated perimetry, Spectral-domain OCT
Glaucoma is the leading cause of irreversible blindness in the world. Although blindness occurs late in the course of the disease, gradual changes in visual function may decrease vision-related quality of life (QoL), leading to important social and economic losses.1,2 With over 111.8 million people projected to live with glaucoma by 2040, there is a pressing need for better standardized diagnostic tools that can be applied and replicated in different clinical settings and in research.
At present, the reliance on subjective human assessment for the diagnosis of glaucoma is laborious and expensive, leading to a large number of individuals with the disease remaining undiagnosed, particularly in medically under-served areas of the world, whereas many healthy subjects are misdiagnosed and receive unnecessary treatment.3,4 In a previous publication, we proposed a reference standard for glaucomatous optic neuropathy (GON) that incorporates recent advances in both structural and functional assessment of glaucoma.5 The criteria account for the presence and correspondence of retinal nerve fiber layer (RNFL) thinning and visual field loss, defined by parameters from spectral-domain OCT (SD-OCT) and standard automated perimetry (SAP), respectively. The proposed criteria may have multiple benefits over traditional subjective methods of diagnosing glaucoma in the research setting by improving diagnosis accuracy and reproducibility.
It is important to ensure that any proposed method to diagnose or classify glaucomatous damage shows clinical relevance from the patient standpoint, such as by bearing a significant relationship with the metrics of QoL. Quality of life in glaucoma has been largely investigated through the use of patient-reported outcomes, such as the 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25). Several previous studies have shown that patients with glaucoma, defined in traditional ways involving subjective clinical assessment of visual fields and optic nerve damage, have lower QoL scores in the NEI VFQ-25 than healthy subjects.6–10 In the Los Angeles Latino Eye Study, glaucoma diagnosis was based on clinical examination of optic disc photos and visual field tests, and NEI VFQ-25 composite scores were found to be poorer for glaucoma patients with visual field loss based on the better eye.10 In the Early Manifest Glaucoma Trial, a similar trend of lower NEI VFQ-25 scores for glaucoma patients was reported.11 However, these studies have used different definitions of glaucomatous damage, which make it difficult for comparison of results or any attempt to meta-analyze their data. At present, no such assessment of the relationship between QoL and glaucoma has been performed using proposed objective definitions of GON.
In the present study, we investigated the relationship between an objective reference standard to classify GON and patient-reported outcomes, as measured by the NEI VFQ-25. We hypothesized that patients diagnosed with GON based on the proposed definition would exhibit lower QoL scores compared with suspects and healthy subjects, providing further validation of the clinical utility of such a diagnostic tool.
Methods
This was a cross-sectional analysis of data from subjects enrolled in a prospective study designed to evaluate functional impairment in glaucoma and a database of electronic research records developed by the Vision, Imaging, and Performance Laboratory at Duke University. The Duke Health Institutional Review Board approved the study, and written informed consent was obtained from all participants. The methodology followed the tenets of the Declaration of Helsinki and of the Health Insurance Portability and Accountability Act.
Subjects in the study underwent a comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP), gonioscopy, dilated ophthalmoscopic examination, stereoscopic optic disc photography, SAP with Humphrey Field Analyzer (Carl Zeiss Meditech, Inc), and SD-OCT testing with the Spectralis SD-OCT (Heidelberg Engineering, GmbH). To apply our definition for glaucoma, subjects with concurrent conditions, such as nonglaucomatous optic neuropathies, retinal detachment, retinal or malignant choroidal tumors, nonglaucomatous disorders of the optic nerve and visual pathways, uveitis, and venous or arterial retinal occlusion according to International Classification of Diseases codes, were excluded. Additionally, subjects with spherical refraction within ±5.0 diopters and cylinder correction less than 3.0 diopters were included. International Classification of Diseases and Current Procedural Terminology codes used for inclusion and exclusion in the study have been described in detail previously.12
The Spectralis SD-OCT (software version 5.8) was used to measure RNFL thickness in the present study. The device has been described previously.13 The SD-OCT RNFL thickness parameters used were the global circumpapillary RNFL thickness (i.e., corresponding to the average of all 768 points distributed equidistantly around the optic nerve head) and the sectorial RNFL thicknesses (i.e., averages of the points in each sector) as provided by the built-in software. Tests with a quality score lower than 15 were excluded, following the manufacturer recommendations. Monocular visual fields were performed using the 24–2 Swedish Interactive Thresholding Algorithm standard strategy with the Humphrey Field Analyzer. Standard automated perimetry tests were excluded if they had more than 33% fixation losses or more than 15% false-positive errors. False-negative errors were not used for exclusion because they are expected in later disease and have been recently demonstrated to be more related to variability in diseased locations and have less of an impact in visual field reliability than false-positive errors.14,15 Visual fields were also excluded in the presence of eyelid or rim artifacts or fatigue effects. For each eye, SD-OCT peripapillary RNFL scans and SAP were paired, with tests acquired within 6 months of each other.
Objective Definition of GON
All patients were categorized as either normal, glaucoma suspect, or glaucoma based on an objective definition of GON described by Mariottoni et al.5 This objective reference standard utilizes the presence and correspondence of structural and functional defects defined by parameters from SD-OCT and SAP.
For a diagnosis of glaucoma, subjects needed to meet the criteria for global or localized loss in at least one eye. An eye was considered to have GON if any of the following was present:
Global RNFL thickness outside normal limits and abnormal SAP as defined by Glaucoma Hemifield Test outside normal limits or Pattern Standard Deviation with P < 5%,
At least one sector in the superior RNFL thickness (temporal-superior or nasal-superior) outside normal limits with a corresponding abnormality on SAP inferior hemifield, defined as inferior hemifield mean deviation (MD) with P < 5%,
At least one sector in the inferior RNFL thickness (temporal-inferior or nasal-inferior) outside normal limits with a corresponding abnormality on SAP superior hemifield, defined as superior hemifield MD with P < 5%. The superior and inferior SAP hemifield MD were calculated as the average of the total deviation values in each hemifield.
To be considered normal, both SD-OCT and SAP results were required to be normal in both eyes. This meant that the SD-OCT–SAP pairs met all of the following criteria:
Global RNFL thickness within normal limits,
RNFL thickness within normal limits for all sectors,
SAP Glaucoma Hemifield Test within normal limits,
SAP Pattern Standard Deviation probability “not significant.”
Finally, subjects that did not meet the criteria for normal or glaucoma were classified as glaucoma suspects. These included eyes with either only SD-OCT or with SAP abnormality, or with abnormalities with no structuralefunctional correspondence. The eye with the worse objective diagnosis was used to define patient-level diagnosis of GON. Because our study was concerned with the diagnosis of GON, using the worse eye for diagnosis prevents misclassifying a subject as “normal” in cases where a subject had a “normal” better eye and a “glaucomatous” worse eye, for example.
25-Item National Eye Institute Visual Function Questionnaire and Rasch Analysis
Vision-related QoL was evaluated using the NEI VFQ-25.16 The NEI VFQ-25 is a set of 25 questions that represent 11 subscales with an additional single-item general health rating question.13 The subscales include general vision, near and distance vision activities, ocular pain, vision-related mental health, vision-related dependency, driving difficulties, color vision, and peripheral vision.13 For our study, we excluded items related to dependency, mental health, and role limitations, which have been previously shown to belong to a separate socioemotional dimension not directly related to visual functioning.17 In total, 14 question items were used to assess QoL for each patient. Previous publications have utilized a similar approach when investigating the relationship between SAP and vision-related QoL measured using the NEI VFQ-25.18–20
Rasch analysis was conducted to obtain final estimates of person measures, summarizing the NEI VFQ-25 responses. Rasch modeling procedures have been previously published in detail.21–23 In brief, Rasch analysis locates where each respondent falls on a linear scale representing the degree of impairment in QoL as measured by the NEI VFQ-25. These scores were then rescaled to range from 0 to 100 to be used for subsequent parametric statistical analyses, with the higher scores reflecting better QoL.24
Demographics, Clinical, and Socioeconomic Variables
Socioeconomic questionnaires were administered to patients containing survey questions about demographics, history of ocular and medical conditions, marital status, health insurance coverage, education level, and income. Patients were categorized based on their responses to the survey questions. Data for all races were collected. Insurance status was sorted into those that had insurance, those that did not have insurance, and those that did not report. Marriage was categorized as single, married, divorced/separated, widowed, and not reported. Education was sorted as did not complete high school, completed high school, some college, college graduate, postgraduate or professional degree, and not reported. Income was categorized as follows: <$24 999, $25 000 to $49 999, $50 000 to $74 999, $75 000 to $124 999, > $125 000, and those that declined from reporting. For the purpose of univariable and multivariable regression analysis, participants were recoded into binary categories to adjust for the impact of socioeconomic status in the QoL outcomes: race was redefined to reference Black or African American vs other racial groups, marital status as married vs other status, education as at least a high school education or not, and annual per capita income below or over $25 000.
Data Analysis
Statistically significant differences between diagnostic groups for demographics and clinical data, as determined by the objective criteria, were analyzed using 2 different statistical methods. For categorical variables, chi-square analysis was conducted; these included race, sex, insurance status, marriage status, education level, and income level. The KruskaleWallis test was used for continuous variables encompassing age, SAP MD, SD-OCT global RNFL thickness, and Rasch-corrected NEI VFQ-25 scores.
Multivariable ordinary least square regression models were used to assess the relationship between NEI VFQ-25 Rasch scores and diagnostic groups, while adjusting for potentially confounding variables. Covariates included in multivariable analyses were age, sex, race, marital status, income, and insurance status. To address missing socioeconomic values for the regression analysis, we utilized multiple imputation with joint random logistic categorical imputation for the reorganized socioeconomic variables. This method has been shown to produce valid statistical inferences that address uncertainty in the data because of the presence of missing values.25
Statistical analyses were performed using Stata version 17 (StataCorp LLC). The a level (type I error) was set at 0.05.
Results
Study Subject Characteristics
The study included 1884 eyes of 1019 patients. Based on the objective diagnostic criteria, 169 patients were classified as normal, 481 patients as glaucoma suspects, and 369 patients were considered glaucomatous. Table 1 reports demographic and clinical characteristics of eyes included in the study. Normal patients were on average younger (54 ± 15 years) compared with the other diagnostic groups and glaucoma subjects were the oldest (69 ± 11 years; P < 0.001). Diagnostic groups had statistically significant differences with respect to socioeconomic variables including insurance status (P < 0.001), sex (P = 0.005), marital status (P = 0.007), and education level (P = 0.005); the majority of subjects in all diagnostic groups were married, had insurance, and were at least college graduates.
Table 1.
Demographics and Clinical Characteristics of Patients Included in the Study
| Diagnosis at Baseline |
|||||
|---|---|---|---|---|---|
| Characteristics | Overall | Normal | Suspect | Glaucoma | P |
| Patients, n (%) | 1019 (100.0) | 169 (16.6) | 481 (47.2) | 369 (36.2) | |
| Age (y), mean ± SD | 63.4 ± 14.3 | 53.9 ± 14.5 | 62.5 ± 14.6 | 69.0 ± 11.0 | <0.001 a |
| Race/ethnicity | 0.016 b | ||||
| Black or African American, n (%) | 365 (35.8) | 49 (29.0) | 193 (40.1) | 123 (33.3) | |
| Non–Black or African American, n (%) | 654 (64.2) | 120 (71.0) | 288 (59.9) | 246 (66.7) | |
| Sex | 0.005 b | ||||
| Female, n (%) | 596 (58.5) | 110 (65.1) | 294 (61.1) | 192 (52.0) | |
| Male, n (%) | 423 (41.5) | 59 (34.9) | 187 (38.9) | 177 (48.0) | |
| Insurance status | < 0.001 b | ||||
| Yes, n (%) | 903 (90.9) | 141 (84.4) | 421 (90.9) | 341 (93.7) | |
| No, n (%) | 69 (6.9) | 24 (14.4) | 35 (7.6) | 10 (2.8) | |
| Not reported, % | 22 (2.2) | 2 (1.2) | 7 (1.5) | 13 (3.6) | |
| Marriage status | 0.007 b | ||||
| Single, n (%) | 163 (16.4) | 41 (24.6) | 78 (16.9) | 44 (12.1) | |
| Married, n (%) | 533 (53.6) | 83 (49.7) | 250 (54.0) | 200 (55.0) | |
| Divorced/separated, n (%) | 179 (18.0) | 33 (19.8) | 81 (17.5) | 65 (17.9) | |
| Widowed, n (%) | 99 (10.0) | 8 (4.8) | 47 (10.2) | 44 (12.1) | |
| Not reported, n (%) | 20 (2.0) | 2 (1.2) | 7 (1.5) | 11 (3.0) | |
| Education level | 0.005 b | ||||
| Did not complete high school, n (%) | 35 (3.5) | 4 (2.4) | 13 (2.8) | 18 (5.0) | |
| Completed high school, n (%) | 147 (14.8) | 19 (11.4) | 74 (16.0) | 54 (14.8) | |
| Some college, n (%) | 23 (2.3) | 1 (0.6) | 5 (1.1) | 17 (4.7) | |
| College graduate, n (%) | 468 (47.1) | 82 (49.1) | 232 (50.1) | 154 (42.3) | |
| Postgraduate or professional degree, n (%) | 312 (31.4) | 59 (35.3) | 137 (29.6) | 116 (31.9) | |
| Not reported, n (%) | 9 (0.9) | 2 (1.2) | 2 (0.4) | 5 (1.4) | |
| Income | 0.244b | ||||
| <$24,999, n (%) | 150 (15.1) | 22 (13.2) | 83 (17.9) | 45 (12.4) | |
| $25,000–$49,999, n (%) | 178 (17.9) | 35 (21.0) | 88 (19.0) | 55 (15.1) | |
| $50,000–$74,999, n (%) | 154 (15.5) | 29 (17.4) | 66 (14.3) | 59 (16.2) | |
| $75,000–$124,999, n (%) | 158 (15.9) | 24 (14.4) | 70 (15.1) | 64 (17.6) | |
| > $125,000, n (%) | 169 (17.0) | 24 (14.4) | 80 (17.3) | 65 (17.9) | |
| Declined, n (%) | 185 (18.6) | 33 (19.8) | 76 (16.4) | 76 (20.9) | |
| Baseline NEI Rasch VFQ-25, mean ± SD | 76.9 ± 15.5 | 82.9 ± 13.0 | 78.2 ± 14.8 | 72.6 ± 16.2 | <0.001 a |
| Eye, n (%) | 1884 (100.0) | 531 (28.2) | 838 (44.5) | 515 (27.3) | |
| MD (dB), mean SD | −2.6 ± 5.3 | 0.2 ± 1.0 | −0.9 ± 2.4 | −6.2 ± 7.0 | <0.001 a |
| RNFL (μm), mean SD | 84.8 ± 17.7 | 97.8 ± 9.5 | 89.0 ± 13.1 | 64.5 ± 12.8 | <0.001 a |
dB = decibels; MD = mean deviation; NEI VFQ-25 items score = National Eye Institute Visual Function Questionnaire 25; RNFL = retinal nerve fiber layer; SD = standard deviation.
Boldface indicates P-value of significance < 0.05.
KruskaleWallis.
Chi-square.
As expected, mean SAP MD and global RNFL thickness values were significantly different among groups (P < 0.001), because they were used in the proposed classification (Table 1). On average, eyes classified as normal, glaucoma suspect, and glaucoma had decreasing mean values in SAP MD (0.2 ± 1.0 dB, −0.9 ± 2.4 dB, and −6.2 ± 7.0 dB, respectively) and SD-OCT mean RNFL thickness (97.8 ± 9.5 μm, 89.0 ± 13.1 μm, and 64.5 ± 12.8 μm, respectively). A wide range of disease severity was included in the study, as observed in Figure 1.
Figure 1.
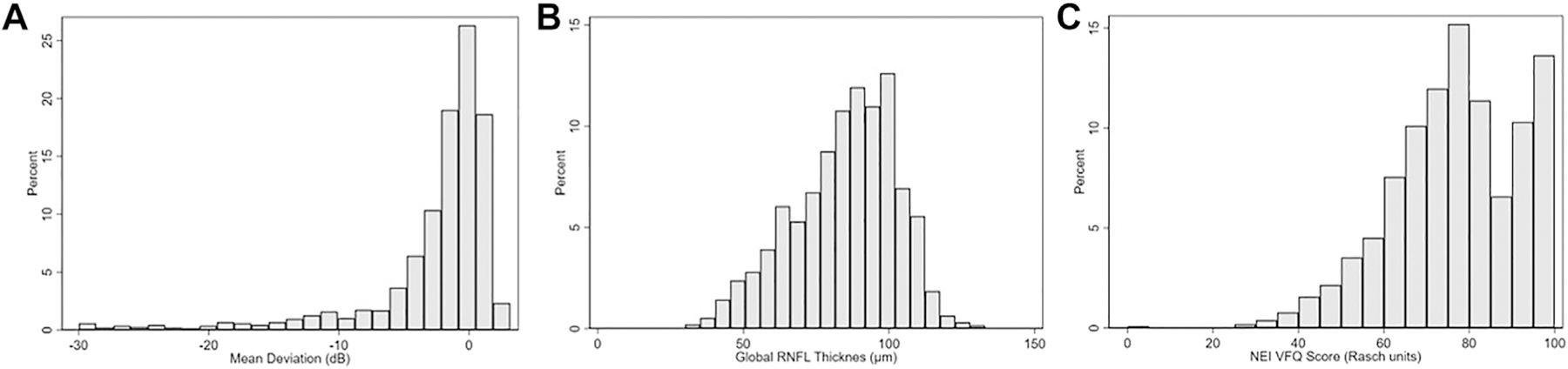
Distribution of (A) mean deviation (MD) (B) global retinal nerve fiber layer (RNFL) thickness, and (C) standardized Rasch scores for the National Eye Institute Visual Function Questionnaire (NEI VFQ-25) in the study sample.
Rasch Scores and Regression Models
Mean NEI VFQ-25 Rasch-adjusted scores were significantly different among diagnostic groups, with progressively lower mean scores for normal, suspect, and glaucoma patients (82.9, 78.2, and 72.6, respectively; P < 0.001). Pairwise comparisons using Wilcoxon rank-sum test for QoL scores between groups demonstrated a significant difference for glaucoma vs normal, glaucoma vs suspect, and normal vs suspect (P < 0.001 for all comparisons). Figure 2 illustrates the relationship between Rasch NEI VFQ-25 scores vs MD and global RNFL thickness, with eyes color-coded for the diagnosis at the patient level, as given by the objective criteria. In the figure, it can be observed that lower levels of Rasch-adjusted NEI VFQ-25 scores were associated with lower MD and thinner mean RNFL thickness, corresponding to worsening disease diagnosis. Figure 3 further shows violin plots with the distribution of MD, RNFL, and NEI VFQ-25 scores within each diagnostic group, demonstrating decreasing median values for all metrics going from normal to glaucoma.
Figure 2.
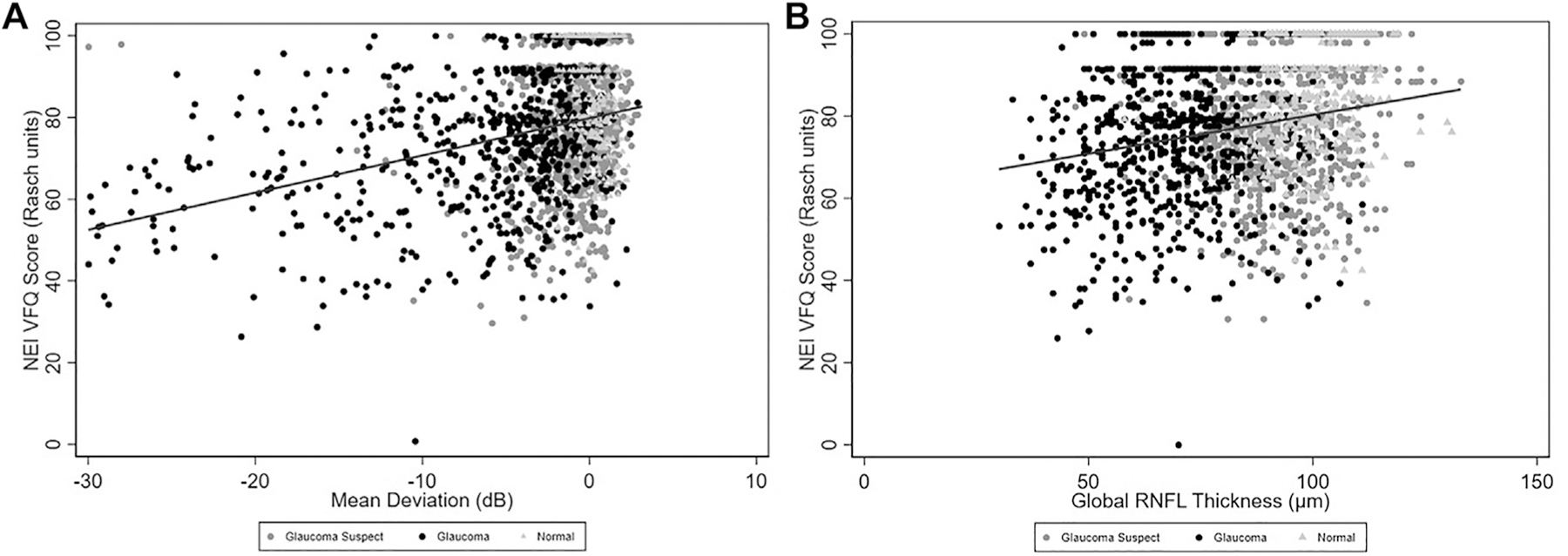
Scatterplots demonstrating the relationships between National Eye Institute Visual Function Questionnaire (NEI VFQ-25) Rasch scores and (A) mean deviation (MD) and (B) global retinal nerve fiber layer (RNFL) thickness. Eyes were color-coded for the diagnosis based on the objective criteria at the patient level.
Figure 3.
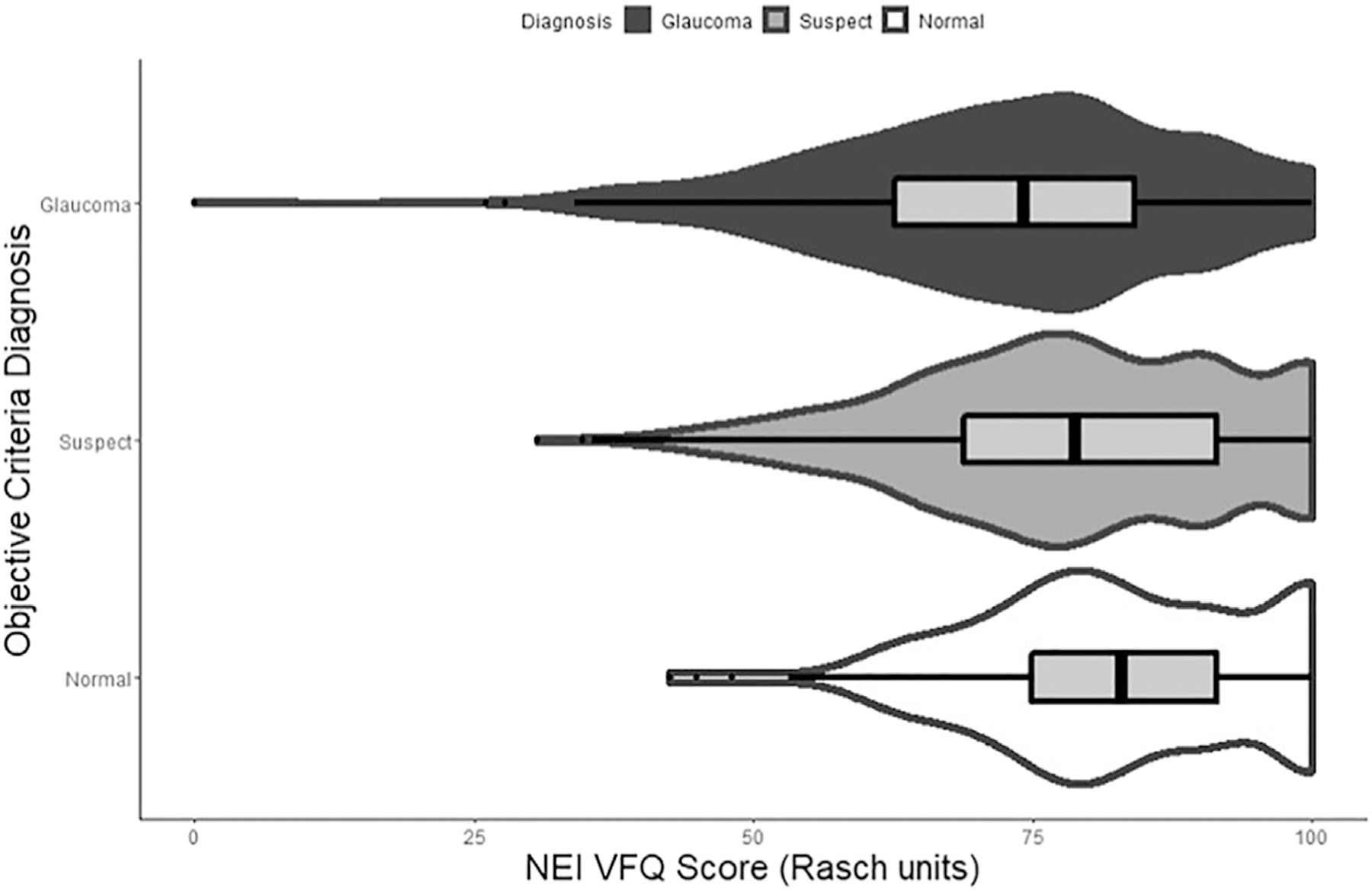
Violin plot demonstrating distribution of National Eye Institute Visual Function Questionnaire (NEI VFQ) Rasch scores for diagnostic groups based on the objective criteria. Eyes were color-coded for the diagnosis based on the objective criteria at the patient level.
Table 2 reports results of univariable and multivariable regression models explaining NEI VFQ-25 Rasch scores. Compared to normal subjects, glaucoma suspects had a lower Rasch score by 4.3 units (P < 0.001), whereas glaucoma subjects had a lower Rasch score by 10.1 units (P < 0.001). Age, sex, race, income, marital status, and insurance were also found to be significant predictors of vision-related QoL (all P < 0.05). In the multivariable analysis, subjects with glaucoma had a significant 6.8 Rasch units loss compared with normal subjects (P < 0.001), after adjustment for potentially confounding variables; but glaucoma suspects did not show a statistically significant difference compared with normal (P = 0.057; Table 2). When using the glaucoma diagnostic group as reference, glaucoma suspects had a significantly better score of 4.6 Rasch units (P < 0.001) than glaucoma subjects.
Table 2.
Results of Univariable and Multivariable Regression Models Evaluating the Associations between Rasch-Adjusted Scores for the National Eye Institute Visual Function Questionnaire (NEI VFQ-25) and Diagnostic Classifications Based on the Proposed Objective Criteria for Glaucomatous Optic Neuropathy
| Characteristic | Univariable Model |
Multivariable Model |
||
|---|---|---|---|---|
| Coefficient (95% CI) | P | Coefficient (95% CI) | P | |
| 0. normal | 0 | Base | 0 | Base |
| 1. suspect | −4.3 (−6.0 to −2.6) | < 0.001 | −2.2 (−4.6 to 0.1) | 0.057 |
| 2. glaucoma | −10.1 (−12.0 to −8.3) | < 0.001 | −6.8 (−9.5 to −4.2) | < 0.001 |
| Age, per decade older | −3.0 (−3.6 to −2.4) | < 0.001 | −2.5 (−3.2 to −1.9) | < 0.001 |
| Sex, female | −3.5 (−5.4 to −1.5) | < 0.001 | −3.7 (−5.5 to −1.8) | < 0.001 |
| Race, African American | −3.4 (−5.4 to −1.4) | 0.001 | −3.2 (−5.2 to −1.2) | 0.002 |
| Marital status, married | 2.5 (0.5–4.4) | 0.014 | 1.5 (−0.6 to 3.5) | 0.156 |
| Insurance, yes | −3.8 (−7.2 to −0.4) | 0.030 | −0.9 (−4.6 to 2.7) | 0.619 |
| Education level, at least high school degree | 3.7 (−1.5 to 9.0) | 0.164 | ||
| Income, ≥ $25,000 | 3.0 (0.3 to 5.7) | 0.031 | 2.4 (−0.4 to 5.2) | 0.098 |
CI = confidence interval.
Boldface indicates significant (P < 0.05).
Discussion
In the present study, we demonstrated that a diagnostic group classification based on objective structural and functional metrics was significantly associated with metrics of QoL in glaucoma. Subjects with glaucoma, as defined by this objective reference standard, had significantly lower NEI VFQ-25 Rasch scores compared with normal and suspects, even after adjustment for confounding demographic and socioeconomic variables. These findings provide further validation of the clinical relevance of the proposed diagnostic tool by demonstrating its relationship with meaningful metrics measuring QoL.
The NEI VFQ-25 QoL questionnaire has been widely used in the literature to assess the impact of glaucoma on QoL.26–32 In our study, glaucoma subjects identified by the proposed objective reference standard had mean Rasch scores significantly lower than normal subjects (72.6 ± 16.2 vs 82.9 ± 13.0, respectively). In multivariable analysis adjusted for age, sex, race, and socioeconomic variables, this difference remained significant (β = −6.8; P < 0.001). This agrees with previous reports on the literature looking at QoL in patients diagnosed with glaucoma using subjective assessments.27,28,33,34
The validation of our proposed criteria by associating an objective classification of GON to measures of QoL further demonstrates the utility of the proposed set of objective metrics that assess for glaucomatous damage where currently no uniform and reliable standard exists. Glaucoma diagnosis is typically made based on subjective assessment of damage to the visual field and optic nerve, sometimes incorporating IOP. However, this approach can make it difficult to standardize and compare research findings. In our proposed criteria, we used objective measures of structure and function to classify such eyes but not IOP. Although elevated IOP is very often a factor driving a clinician’s judgment of higher probability of glaucoma, IOP alone is not part of the definition of glaucoma, but rather a risk factor for the disease.35,36 Using IOP cutoffs would lead to a number of patients with ocular hypertension being misdiagnosed as glaucoma and missing many low-tension glaucoma patients. Additionally, the subjective diagnostic evaluation of visual fields and optic disc for glaucoma relies on the identification of characteristic patterns of damage, which are essentially subjective. Such subjective evaluation may have limited accuracy, largely because of the wide variation in the appearance of optic discs and imprecise definitions of what “characteristic features of glaucoma” are.37–40 This lack of consensus prevents comparisons among research studies and may hamper the development of new diagnostic tools. For example, there has been great interest in the development of artificial intelligence algorithms that could improve recognition of glaucoma damage. However, training and validation of such algorithms require comparison to a reference standard. A definition of glaucomatous neuropathy that is objective and standardized, such as the one proposed here, could serve as a “ground truth” that can be applied to both train and validate artificial intelligence algorithms and facilitate comparison of research findings. A recent study by Mariottoni et al5 shows how the objective criteria for GON can be used to train and validate a deep learning model to detect glaucoma damage on fundus photographs. Of note, another study also proposed an objective criteria based on structural and functional testing to define GON by comparing to gradings of a large number of worldwide specialists.41 Although the authors did not evaluate the relationship with QoL metrics of their proposed definition, their findings also provide support for developing a standardized objective classification that can facilitate comparison of research findings in glaucoma.41
It is important not to overreach the bearing of such objective criteria, so as to prevent its application naïvely in practice. For example, patients with certain confounding diseases such as nonglaucomatous optic neuropathies or retinal diseases may show abnormalities on both OCT and SAP that would fit the criteria for glaucoma proposed by our objective criteria. Therefore, application of such criteria requires first exclusion of these potentially confounding abnormalities by clinical examination, which is usually a straightforward task.
Based on the objective criteria, eyes suspected for glaucoma were those with only SD-OCT or only SAP abnormality.5 The requirement for a topographic agreement in structure and function increases the specificity for detection of damage. This is particularly relevant in the context of using the proposed objective reference standard in the development or validation of proposed artificial intelligence screening tools, as described above. As glaucoma has a relatively low prevalence, screening approaches for the disease need to have very high specificity.42,43 The downside of such requirement, however, is that eyes with preperimetric glaucoma would potentially still be classified as “suspects.” However, focusing on this early stage of disease for screening is not only unnecessary, but also leads to problems related to the uncertainty in diagnosis. From a public health standpoint, an early diagnosis means diagnosing a patient at a stage earlier than the patient would have presented symptomatically.44,45 Given that symptomatic presentation of glaucoma generally occurs only at a late stage, almost any stage of glaucoma is early disease from the point of view of screening. Given the difficulties related to discriminating early glaucoma from normal variation, attempting to focus screening programs on detection of very early disease will likely lead to failure. Detection of well-established cases of glaucoma that would still be asymptomatic has a much better chance of leading to improved diagnostic accuracy and effectiveness.
Given the exclusion of eyes with confounding pathologies that could affect the optic nerve or visual field, 154 subjects in our sample had only 1 eye included in the study. It is possible that QoL results in those subjects may have been affected by the statuses of the nonincluded eyes in ways unrelated to glaucoma damage, potentially confounding the results. Therefore, we conducted a subanalysis of the 1730 eyes of 865 subjects who had both eyes eligible for the study. Based on the objective diagnostic criteria, 144 patients were classified as normal, 319 patients had GON, and 402 patients were suspected for GON. Mean NEI VFQ-25 Rasch-adjusted scores for normal, suspects, and GON subjects were 83.3 ± 12.9, 79.5 ± 14.7, and 73.4 ± 15.2, respectively (P < 0.001). Similar to the main analyses, age, sex, race, and marital status were statistically significant socioeconomic and demographic predictors of vision-related QoL (all P < 0.05) in the univariable analyses (Table S3, available at www.ophthalmologyglaucoma.org). When adjusted for these significant confounding factors in the multivariable analysis, GON patients had a lower Rasch score of 6.6 units compared with normal subjects (P < 0.001). We also re-evaluated our original sample using the better eye to define patient-level diagnosis (Table S4, available at www.ophthalmologyglaucoma.org). When classified by the better eye status, both glaucoma and glaucoma suspect patients had significantly lower mean Rasch scores (9.2 and 2.9 units lower; P < 0.001 and P = 0.004, respectively) compared with normal subjects. Although using the better eye for diagnosis may cause misclassifications at the patient level (see Methods), this finding indicates some decrease in QoL even when the better eye is still not clearly glaucomatous, suggesting an impact of the worse eye in QoL outcomes.
The study has limitations. The glaucoma group was comprised of a significantly older population than the normal diagnostic group. Although we adjusted the analyses for age, such adjustment may not completely remove all the effects of the age difference between the 2 groups. However, the fact that the application of the objective criteria resulted in a glaucoma group of older age was to be expected in line with the well-known association between glaucoma and aging. Additionally, as discussed before, our objective criteria may not detect glaucoma in preperimetric or early glaucoma patients who may exhibit RNFL loss but not necessarily present any visual field defects by SAP testing. However, in such patients with preperimetric glaucoma, a significant reduction in QoL is not expected, as demonstrated previously.46 Finally, it is important to note that although patients in our study were not aware of their objective criteria diagnosis when they responded to the NEI VFQ-25 questionnaire, they were likely aware of their clinical diagnosis as determined by the attending ophthalmologist. Such a limitation is difficult to avoid unless questionnaires are given to newly diagnosed patients who are still completely uninformed of their disease.
In conclusion, our results indicate that the proposed objective criteria for the diagnosis of GON yields significant association with patient-reported QoL outcomes. Utilization of such criteria may provide clinically relevant metrics with potential to improve comparability of research findings and validation of newly proposed diagnostic tools.
Supplementary Material
Acknowledgments
Supported in part by National Institutes of Health/National Eye Institute grants EY029885 and EY031898 (F.A.M.). The funding organizations had no role in the design or conduct of this research.
Abbreviations and Acronyms:
- GON
glaucomatous optic neuropathy
- IOP
intraocular pressure
- MD
mean deviation
- NEI VFQ-25
National Eye Institute Visual Function Questionnaire 25
- QoL
quality of life
- RNFL
retinal nerve fiber layer
- SAP
standard automated perimetry
- SD-OCT
spectral-domain OCT
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): F.A.M.: Funding – National Eye Institute, Personal fees – Aerie Pharmaceuticals, Allergan, Annexon, Biogen, Carl Zeiss Meditec, Galimedix, Google Inc, Heidelberg Engineering, nGoggle Inc, Novartis, Stuart Therapeutics, Reichert.
HUMAN SUBJECTS: Human subjects were included in this study. The Duke Health Institutional Review Board approved the study and written informed consent was obtained from all participants. The methodology followed the tenets of the Declaration of Helsinki and of the Health Insurance Portability and Accountability Act.
No animal subjects were used in this study.
Supplemental material available at www.ophthalmologyglaucoma.org.
References
- 1.Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus 2020;12, e11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jammal AA, Ogata NG, Daga FB, et al. What is the amount of visual field loss associated with disability in glaucoma? Am J Ophthalmol 2019;197:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakata K, Sakata LM, Sakata VM, et al. Prevalence of glaucoma in a south Brazilian population: Projeto Glaucoma. Invest Ophthalmol Vis Sci 2007;48:4974–4979. [DOI] [PubMed] [Google Scholar]
- 4.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121:2081–2090. [DOI] [PubMed] [Google Scholar]
- 5.Mariottoni EB, Jammal AA, Berchuck SI, et al. An objective structural and functional reference standard in glaucoma. Sci Rep 2021;11:1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirneiss C, Kortüm K. Quality of life in patients with glaucoma [in German]. Klin Monbl Augenheilkd 2016;233:148–153. [DOI] [PubMed] [Google Scholar]
- 7.Haymes SA, Leblanc RP, Nicolela MT, et al. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci 2007;48:1149–1155. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EE, Muñoz B, West SK, et al. Glaucoma and quality of life: the Salisbury Eye Evaluation. Ophthalmology 2008;115:233–238. [DOI] [PubMed] [Google Scholar]
- 9.Hyman LG, Komaroff E, Heijl A, et al. Treatment and vision-related quality of life in the early manifest glaucoma trial. Ophthalmology 2005;112:1505–1513. [DOI] [PubMed] [Google Scholar]
- 10.McKean-Cowdin R, Wang Y, Wu J, et al. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology 2008;115: 941–948.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters D, Heijl A, Brenner L, Bengtsson B. Visual impairment and vision-related quality of life in the Early Manifest Glaucoma Trial after 20 years of follow-up. Acta Ophthalmol 2015;93:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jammal AA, Thompson AC, Mariottoni EB, et al. Impact of intraocular pressure control on rates of retinal nerve fiber layer loss in a large clinical population. Ophthalmology 2021;128:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gracitelli CP, Abe RY, Tatham AJ, et al. Association between progressive retinal nerve fiber layer loss and longitudinal change in quality of life in glaucoma. JAMA Ophthalmol 2015;133:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yohannan J, Wang J, Brown J, et al. Evidence-based criteria for assessment of visual field reliability. Ophthalmology 2017;124:1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardiner SK, Swanson WH, Goren D, et al. Assessment of the reliability of standard automated perimetry in regions of glaucomatous damage. Ophthalmology 2014;121:1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050–1058. [DOI] [PubMed] [Google Scholar]
- 17.Massof RW, Fletcher DC. Evaluation of the NEI visual functioning questionnaire as an interval measure of visual ability in low vision. Vision Res 2001;41:397–413. [DOI] [PubMed] [Google Scholar]
- 18.Alencar LM, Zangwill LM, Weinreb RN, et al. A comparison of rates of change in neuroretinal rim area and retinal nerve fiber layer thickness in progressive glaucoma. Invest Ophthalmol Vis Sci 2010;51:3531–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci 2011;52:5794–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medeiros FA, Zangwill LM, Girkin CA, et al. Combining structural and functional measurements to improve estimates of rates of glaucomatous progression. Am J Ophthalmol 2012;153:1197–1205.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medeiros FA, Gracitelli CPB, Boer ER, et al. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology 2015;122: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bond T, Fox C. Applying the Rasch Model: Fundamental Measurement in the Human Sciences 3rd edition. New York: Routledge; 2015. [Google Scholar]
- 23.Andrich D Rating scales and Rasch measurement. Expert Rev Pharmacoecon Outcomes Res 2011;11:571–585. [DOI] [PubMed] [Google Scholar]
- 24.Abe RY, Diniz-Filho A, Costa VP, et al. The impact of location of progressive visual field loss on longitudinal changes in quality of life of patients with glaucoma. Ophthalmology 2016;123:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddique J, Harel O, Crespi CM, Hedeker D. Binary variable multiple-model multiple imputation to address missing data mechanism uncertainty: application to a smoking cessation trial. Stat Med 2014;33:3013–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu M, Wang SY, Singh K, Lin SC. Association between visual field defects and quality of life in the United States. Ophthalmology 2014;121:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jampel HD, Schwartz A, Pollack I, et al. Glaucoma patients’ assessment of their visual function and quality of life. J Glaucoma 2002;11:154–163. [DOI] [PubMed] [Google Scholar]
- 28.Onakoya AO, Mbadugha CA, Aribaba OT, Ibidapo OO. Quality of life of primary open angle glaucoma patients in Lagos, Nigeria: clinical and sociodemographic correlates. J Glaucoma 2012;21:287–295. [DOI] [PubMed] [Google Scholar]
- 29.Knani L, Gatfaoui F, Mahjoub A, et al. Preliminary study of the quality of life of glaucoma patients in the district of Sousse (Tunisia): sociodemographic and clinical associations [in French]. J Fr Ophtalmol 2017;40:196–201. [DOI] [PubMed] [Google Scholar]
- 30.L Majerníková, Hudáková A, Obročníková A, et al. Quality of life of patients with glaucoma in Slovakia. Int J Environ Res Public Health 2021;18:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abe RY, Gracitelli CPB, Diniz-Filho A, et al. Frequency doubling technology perimetry and changes in quality of life of glaucoma patients: a longitudinal study. Am J Ophthalmol 2015;160:114–122.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labiris G, Katsanos A, Fanariotis M, et al. Psychometric properties of the Greek version of the NEI-VFQ 25. BMC Ophthalmol 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg I, Clement CI, Chiang TH, et al. Assessing quality of life in patients with glaucoma using the Glaucoma Quality of Life-15 (GQL-15) questionnaire. J Glaucoma 2009;18:6–12. [DOI] [PubMed] [Google Scholar]
- 34.van Gestel A, Webers CAB, Beckers HJM, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye (Lond) 2010;24:1759–1769. [DOI] [PubMed] [Google Scholar]
- 35.Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma Preferred Practice Pattern. Ophthalmology 2021;128: P71–P150. [DOI] [PubMed] [Google Scholar]
- 36.European Glaucoma Society Terminology and Guidelines for Glaucoma, Chapter 3: Treatment principles and options, Part 1: Treatment principles and options. 4th Edition. Supported by the EGS Foundation: Br J Ophthalmol 2017;101:130–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caprioli J, Coleman AL. Intraocular pressure fluctuation: a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008;115:1123–1129.e3. [DOI] [PubMed] [Google Scholar]
- 38.Musch DC, Gillespie BW, Niziol LM, et al. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 2011;118: 1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang X, Torres M, Varma R. Los Angeles Latino Eye Study Group. Variation in intraocular pressure and the risk of developing open-angle glaucoma: the Los Angeles Latino Eye Study. Am J Ophthalmol 2018;188:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harper R, Reeves B, Smith G. Observer variability in optic disc assessment: implications for glaucoma shared care. Ophthalmic Physiol Opt 2000;20:265–273. [PubMed] [Google Scholar]
- 41.Iyer JV, Boland MV, Jefferys J, Quigley H. Defining glaucomatous optic neuropathy using objective criteria from structural and functional testing. Br J Ophthalmol 2021;105: 789–793. [DOI] [PubMed] [Google Scholar]
- 42.Wormald RP, Rauf A. Glaucoma screening. J Med Screen 1995;2:109–114. [DOI] [PubMed] [Google Scholar]
- 43.Michelson G, Groh MJ. Screening models for glaucoma. Curr Opin Ophthalmol 2001;12:105–111. [DOI] [PubMed] [Google Scholar]
- 44.Stein JD, Khawaja AP, Weizer JS. Glaucoma in adults-screening, diagnosis, and management: a review. JAMA 2021;325:164–174. [DOI] [PubMed] [Google Scholar]
- 45.Quigley HA. Glaucoma. Lancet 2011;377:1367–1377. [DOI] [PubMed] [Google Scholar]
- 46.Daga FB, Gracitelli CPB, Diniz-Filho A, Medeiros FA. Is vision-related quality of life impaired in patients with pre-perimetric glaucoma? Br J Ophthalmol 2019;103:955–959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


