Abstract
trans-2′-Carboxybenzalpyruvate hydratase-aldolase was purified from a phenanthrene-degrading bacterium, Nocardioides sp. strain KP7, and characterized. The purified enzyme was found to have molecular masses of 38 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and 113 kDa by gel filtration chromatography. Thus, the homotrimer of the 38-kDa subunit constituted an active enzyme. The Km and kcat values of this enzyme for trans-2′-carboxybenzalpyruvate were 50 μM and 13 s−1, respectively. trans-2′-Carboxybenzalpyruvate was transformed to 2-carboxybenzaldehyde and pyruvate by the action of this enzyme. The structural gene for this enzyme was cloned and sequenced; the length of this gene was 996 bp. The deduced amino acid sequence of this enzyme exhibited homology to those of trans-2′-hydroxybenzalpyruvate hydratase-aldolases from Pseudomonas putida PpG7 and Pseudomonas sp. strain C18.
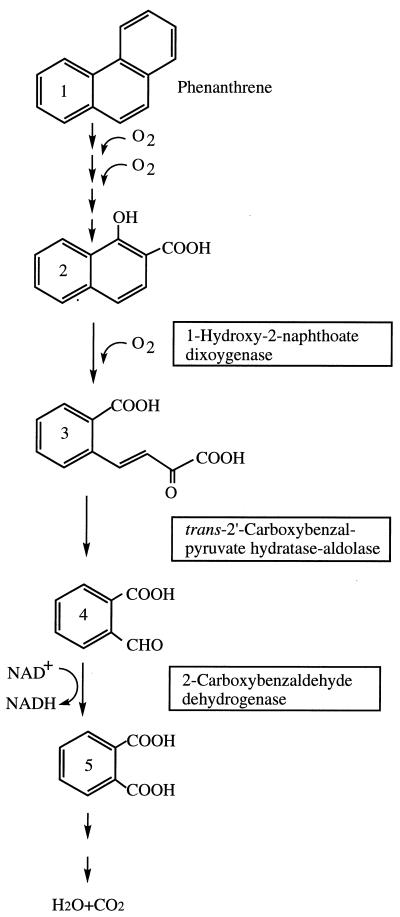
Phenanthrene is a polyaromatic hydrocarbon and has been used as a model for the biodegradation of polyaromatic hydrocarbons (4, 10, 11). In bacteria, phenanthrene is degraded via 1-hydroxy-2-naphthoate (2, 8, 18–20) (Fig. 1). The enzymatic steps involved in the transformation of phenanthrene to 1-hydroxy-2-naphthoate have been characterized (21, 29, 30). They are very similar to those involved in the transformation of naphthalene to salicylate (5–7, 11, 12, 23, 25, 31). In many bacteria, one ring of 1-hydroxy-2-naphthoate is cleaved by 1-hydroxy-2-naphthoate dioxygenase (2, 16, 18, 20, 24) (Fig. 1), yielding trans-2′-carboxybenzalpyruvate (1). Although the formation of 2-carboxybenzaldehyde and pyruvate during the degradation of phenanthrene has been demonstrated in an Aeromonas strain and gram-negative bacterial strain B156 (2, 18), enzymes involved in the transformation of trans-2′-carboxybenzalpyruvate to 2-carboxybenzaldehyde have not yet been characterized. In the 2-naphthoate catabolic pathway in Burkholderia strain JT 1500, the initial substrate was shown to be transformed to 2-carboxybenzaldehyde via 1-carboxy-2-naphthoate, and two enzymatic steps were proposed for the conversion of the ring cleavage product of 1-hydroxy-2-naphthoate to 2-carboxybenzaldehyde (24). In the present study, we purified and characterized the enzyme involved in the transformation of trans-2′-carboxybenzalpyruvate from a phenanthrene-degrading organism, Nocardioides sp. strain KP7 (17). Furthermore, the structural gene for this enzyme was cloned and sequenced.
FIG. 1.
Proposed routes for the degradation of phenanthrene in Nocardioides sp. strain KP7. Compound names: 1, phenanthrene; 2, 1-hydroxy-2-naphthoate; 3, trans-2′-carboxybenzalpyruvate; 4, 2-carboxybenzaldehyde; 5, o-phthalate. 1-Hydroxy-2-naphthoate dioxygenase and its structural gene were characterized previously (16). The ring cleavage product of 1-hydroxy-2-naphthoate was determined to be trans-2′-carboxybenzalpyruvate (1). 2-Carboxybenzaldehyde dehydrogenase and its structural gene were characterized previously (15). In this study, trans-2′-carboxybenzalpyruvate hydratase-aldolase and its structural gene were characterized.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
The phenanthrene-degrading bacterium, Nocardioides sp. strain KP7, used in this study was previously described (17). This strain was cultivated in marine broth (Difco Laboratories, Detroit, Mich.) in the presence or absence of 0.1% (wt/vol) phenanthrene at 30°C.
Preparation of trans-2′-carboxybenzalpyruvate.
trans-2′-Carboxybenzalpyruvate was prepared enzymatically in 50 mM potassium phosphate (KP) buffer (pH 7.5) containing 45 μM 1-hydroxy-2-naphthoate and 5 μg of 1-hydroxy-2-naphthoate dioxygenase ml−1; the latter was purified from Escherichia coli containing recombinant plasmid pMKT290 by a previously described method (15).
Enzyme assay.
As described in the Results section, trans-2′-carboxybenzalpyruvate was converted to 2-carboxybenzaldehyde and pyruvate by the enzyme purified in this study. We call this enzyme trans-2′-carboxybenzalpyruvate hydratase-aldolase. Under standard conditions, the activity of trans-2′-carboxybenzalpyruvate hydratase-aldolase was spectrophotometrically measured at 300 nm in 50 mM KP buffer (pH 7.5) containing 45 μM trans-2′-carboxybenzalpyruvate. The difference between the extinction coefficient of trans-2′-carboxybenzalpyruvate and that of the reaction products (an equimolar mixture of 2-carboxybenzaldehyde and pyruvate) at 300 nm was determined to be 17.3 mM−1 cm−1. The Michaelis-Menten kinetic parameters of trans-2′-carboxybenzalpyruvate hydratase-aldolase for trans-2′-carboxybenzalpyruvate were determined by measuring the initial velocity of transformation under standard conditions, except that various concentrations of the substrate were used. The reverse reaction was spectrophotometrically measured in 50 mM KP buffer (pH 7.5) containing 100 μM 2-carboxybenzaldehyde and 100 μM pyruvate at 25°C.
Analysis of the products formed from trans-2′-carboxybenzalpyruvate.
Five micrograms of purified trans-2′-carboxybenzalpyruvate hydratase-aldolase was added to 1 ml of KP buffer (pH 7.5) containing 52.1 nmol of trans-2′-carboxybenzalpyruvate, and the mixture was incubated at 25°C for 5 min. Aliquots (400 μl each) of this reaction mixture were used to determine the amounts of 2-carboxybenzaldehyde and pyruvate produced by applying 2-carboxybenzaldehyde dehydrogenase purified from Nocardioides sp. strain KP7 (15) and l-lactate dehydrogenase (Boehringer GmbH, Mannheim, Germany) (26), respectively. Both enzymatic reactions were carried out at 25°C, and the formation of NADH from NAD+ was spectrophotometrically measured.
2-Carboxybenzaldehyde produced from trans-2′-carboxybenzalpyruvate by the action of purified trans-2′-carboxybenzalpyruvate hydratase-aldolase was also analyzed with an octyldecyl silane column (type SG120 CapcellPak-C18; 4.6 by 250 mm; Shiseido, Tokyo, Japan) fitted to a high-performance liquid chromatography (HPLC) system (Tosoh, Tokyo, Japan). The product was analyzed at 25°C with a linear gradient of 50 to 90% (vol/vol) methanol in 30 ml of ultrapure water containing 0.1% (wt/vol) H3PO4 at a flow rate of 1 ml min−1. In this experiment, 2-carboxybenzaldehyde (Tokyo Chemical, Tokyo, Japan) was used as a standard.
Purification of trans-2′-carboxybenzalpyruvate hydratase-aldolase.
trans-2′-Carboxybenzalpyruvate hydratase-aldolase was purified from cells of strain KP7 that had been grown for 48 h at 30°C in 20 liters of marine broth (Difco) containing 0.1% (wt/vol) phenanthrene. The cells were pelleted by centrifugation at 10,000 × g for 20 min at 4°C, resuspended in 200 ml of 10 mM Tris-H2SO4 buffer (pH 7.5), and disrupted by two passages through a precooled French pressure cell (Ohtake Works, Tokyo, Japan) at a pressure of 76 MPa. The resulting cell extract was centrifuged at 27,700 × g for 30 min at 4°C, and the supernatant fluid was recentrifuged at 250,000 × g for 60 min at 4°C. The resulting supernatant was passed through a Sterivex-HV filter (0.45-μm pore size; Millipore, Bedford, Mass.) and loaded into an anion-exchange column (TSKgel DEAE-5PW; 21.5 by 150 mm; Tosoh) fitted to an HPLC system (Tosoh). The protein was eluted with a linear gradient of 0 to 0.5 M Na2SO4 in 150 ml of 10 mM Tris-H2SO4 buffer (pH 7.5) at a flow rate of 5 ml min−1. The eluate was collected in 5-ml fractions on ice. trans-2′-Carboxybenzalpyruvate hydratase-aldolase was eluted at a salt concentration of 0.26 M. Pooled fractions containing the trans-2′-carboxybenzalpyruvate hydratase-aldolase activity were dialyzed against 20 mM KP buffer (pH 7.5). The dialyzed fractions were adjusted to a 0.6 M saturation of ammonium sulfate at 4°C, and the precipitated proteins were removed by centrifugation at 27,700 × g for 30 min at 4°C. The trans-2′-carboxybenzalpyruvate hydratase-aldolase activity was recovered in the supernatant fluid. This supernatant was passed through a Millex-GV filter (0.45-μm pore size; Millipore) and loaded into a hydrophobic interaction column (TSKgel Phenyl-5PW; 7.5 by 75 mm; Tosoh) that had been preequilibrated with 20 mM KP buffer (pH 7.5) containing 0.6 M ammonium sulfate. Proteins were eluted from the column with a linear gradient of 0.6 to 0 M ammonium sulfate in 60 ml of 20 mM KP buffer (pH 7.5) at a flow rate of 1 ml min−1. Pooled fractions containing the trans-2′-carboxybenzalpyruvate hydratase-aldolase activity were dialyzed against 10 mM Tris-H2SO4 buffer (pH 8.0). The dialyzed sample was passed through a Millex-GV filter (0.45-μm pore size; Millipore) and loaded into an anion-exchange column (MonoQ HR5/5; 5 by 50 mm; Pharmacia, Uppsala, Sweden) fitted to an HPLC system (Tosoh). The protein was eluted with a linear gradient of 0 to 0.25 M Na2SO4 in 60 ml of 10 mM Tris-H2SO4 buffer (pH 8.0) at a flow rate of 1 ml min−1. trans-2′-Carboxybenzalpyruvate hydratase-aldolase was eluted at a salt concentration of 0.13 M.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with a preformed polyacrylamide slab gel (Multigel Gel 4/20; Daiichi Pure Chemicals, Tokyo, Japan) as described previously (14). Gel filtration chromatography (TSKgel G3000SWXL; 7.8 by 300 mm; Tosoh) was carried out with a mobile phase of 20 mM Tris-H2SO4 buffer (pH 6.8) containing 100 mM Na2SO4 at a flow rate of 0.5 ml min−1. The molecular mass of the enzyme was determined from the mobility relative to that of protein molecular mass standards (β-galactosidase, 465 kDa; immunoglobulin G, 150 kDa; immunoglobulin G Fab fragment, 50 kDa; and myoglobin, 17 kDa) (Boehringer). The protein concentration was determined by the method of Bradford (3) by use of an assay kit (Bio-Rad Laboratories, Richmond, Calif.) with bovine serum albumin as a standard.
Amino acid sequencing.
The amino-terminal sequence of the purified enzyme was determined by Edman degradation with an automated protein sequencer (model 477; Perkin Elmer Applied Biosystems, Branchburg, N.J.).
Gene cloning, sequencing, and expression in E. coli.
In a previous study, a 6.2-kb BamHI DNA fragment carrying the structural gene for 1-hydroxy-2-naphthoate dioxygenase (phdI) was cloned into pACYC184 to construct pMKT280 (15, 16). The downstream region of phdI was sequenced by use of a Taq DyeDeoxy terminator cycle sequencing kit and a model 373A DNA sequencer (Perkin Elmer Applied Biosystems).
A DNA fragment carrying the gene for trans-2′-carboxybenzalpyruvate hydratase-aldolase (phdJ) was amplified by use of Vent DNA polymerase (New England BioLabs, Beverly, Mass.) with a set of primers. One PCR primer, 5′-ACGATCAGGACGACATATGACGTCACC-3′, was designed to add an NdeI site in the −16 to +11 region of phdJ, while the other PCR primer, 5′-TGGCGGCAGGATCCTTCGATGATG-3′, was designed to add a BamHI site in the +1055 to +1078 region of phdJ. The amplified DNA fragment was digested with NdeI and BamHI and cloned into pET-22b(+) (27) to construct pMKT310. The DNA fragment cloned into pMKT310 was sequenced to confirm the absence of any mutation in the amplified fragment. E. coli BL21(DE3) containing pMKT310 was cultivated in 100 ml of L broth containing 50 μg of ampicillin ml−1 until the A660 reached 0.6. After the addition of 1 ml of 100 mM isopropyl-β-d-thiogalactopyranoside (IPTG), the cells were further cultivated for 2 h at 37°C. The resulting cells were pelleted by centrifugation at 10,000 × g for 20 min at 4°C, resuspended in 5 ml of 10 mM Tris-H2SO4 buffer (pH 7.5), and disrupted by two passages through a precooled French pressure cell (Ohtake Works) at a pressure of 76 MPa. The cell extract was centrifuged at 27,700 × g for 30 min at 4°C, and the resulting supernatant fluid was recentrifuged at 250,000 × g for 60 min at 4°C. The residual supernatant fluid was passed through a Sterivex-HV filter (0.45-μm pore size; Millipore). Measurement of the trans-2′-carboxybenzalpyruvate hydratase-aldolase activity in the cell extract was carried out as described earlier.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the DDBJ/GenBank/EMBL DNA databases as accession number D89988.
RESULTS AND DISCUSSION
Expression of trans-2′-carboxybenzalpyruvate hydratase-aldolase in strain KP7.
The expression of trans-2′-carboxybenzalpyruvate hydratase-aldolase was examined in strain KP7 grown in marine broth in the presence and absence of phenanthrene. The specific activities of trans-2′-carboxybenzalpyruvate hydratase-aldolase were 0.008 μmol min−1 mg of protein−1 in the extract of strain KP7 grown in the absence of phenanthrene and 0.08 μmol min−1 mg of protein−1 in the presence of phenanthrene. This result indicated that trans-2′-carboxybenzalpyruvate hydratase-aldolase of strain KP7 was induced by phenanthrene or its metabolite(s).
Purification and characterization of trans-2′-carboxybenzalpyruvate hydratase-aldolase.
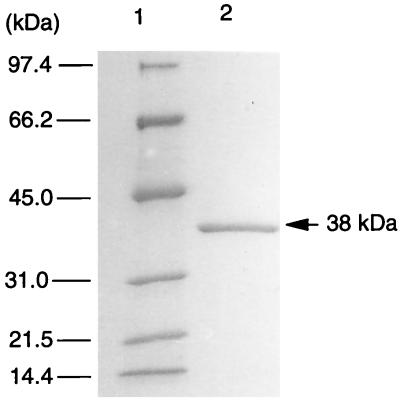
The purification procedure for trans-2′-carboxybenzalpyruvate hydratase-aldolase from strain KP7 grown on phenanthrene is summarized in Table 1. The enzyme activity was eluted from the first anion-exchange column at an Na2SO4 concentration of 0.26 M. In hydrophobic interaction chromatography, the enzyme activity was eluted at an ammonium sulfate concentration of 0.35 M. In the second anion-exchange column chromatography, the activity was eluted at an Na2SO4 concentration of 0.13 M. The purified sample after the second anion-exchange column chromatography gave a single protein band in SDS-PAGE. The molecular mass of trans-2′-carboxybenzalpyruvate hydratase-aldolase evaluated by gel filtration chromatography was 113 kDa (data not shown), and that determined by SDS-PAGE was 38 kDa (Fig. 2). Thus, the homotrimer of the 38-kDa subunit constituted an active enzyme.
TABLE 1.
Purification of trans-2′-carboxybenzalpyruvate hydratase-aldolase from Nocardioides sp. strain KP7
| Purification step | Volume (ml) | Protein (μg ml−1) | Total protein (μg) | Sp act (μmol min−1 mg of protein−1) | Total activity (μmol min−1) | Yield (%) |
|---|---|---|---|---|---|---|
| Cell extract | 110 | 2,450 | 270,000 | 0.08 | 21.6 | 100 |
| First anion exchange | 15 | 1,220 | 18,300 | 0.24 | 4.4 | 20 |
| Hydrophobic interaction | 4 | 136 | 544 | 4.8 | 2.6 | 12 |
| Second anion exchange | 2 | 76 | 152 | 11.5 | 1.7 | 8 |
FIG. 2.
SDS-PAGE of purified trans-2′-carboxybenzalpyruvate hydratase-aldolase (38 kDa). Lanes: 1, molecular mass markers; 2, pooled active fractions after the second anion-exchange column chromatography.
The spectrum of trans-2′-carboxybenzalpyruvate revealed a maximum absorbance at 300 nm, and the transformation of trans-2′-carboxybenzalpyruvate to the reaction products (2-carboxybenzaldehyde and pyruvate, as described below) by purified trans-2′-carboxybenzalpyruvate hydratase-aldolase resulted in the dissipation of the peak at 300 nm and the appearance of another peak at 252 nm, as has been described by Kiyohara and Nagao (18). The Km and kcat values of purified trans-2′-carboxybenzalpyruvate hydratase-aldolase for trans-2′-carboxybenzalpyruvate measured at 25°C were 50 μM and 13 s−1, respectively. Determination of the trans-2′-carboxybenzalpyruvate hydratase-aldolase activity in different buffers at different pH values indicated the optimum pH for this enzyme to be 7.5. The optimum temperature for this enzyme was 45°C.
The products formed from trans-2′-carboxybenzalpyruvate by the action of purified trans-2′-carboxybenzalpyruvate hydratase-aldolase were quantitatively determined with reactions catalyzed by 2-carboxybenzaldehyde dehydrogenase and l-lactate dehydrogenase. It was found that 19.1 nmol of 2-carboxybenzaldehyde and 21.3 nmol of pyruvate were formed from 20.8 nmol of trans-2′-carboxybenzalpyruvate in the reaction catalyzed by trans-2′-carboxybenzalpyruvate hydratase-aldolase. An HPLC analysis showed that one of the reaction products had the same retention time as 2-carboxybenzaldehyde (data not shown).
The reverse reaction of this enzyme was spectrophotometrically examined with 1 ml of 50 mM KP buffer (pH 7.5) containing 100 nmol of 2-carboxybenzaldehyde, 100 nmol of pyruvate, and 1.5 μg of the enzyme at 25°C. When the reaction had equilibrated, it was calculated from the change in the absorbance at 300 nm that 21 nmol of trans-2′-carboxybenzalpyruvate had been formed. Assuming 1:1 stoichiometry for 2-carboxybenzaldehyde and pyruvate consumption and the formation of trans-2′-carboxybenzalpyruvate, the equilibrium constant of the reaction was calculated to be [(100 − 21)(100 − 21)]/21 = 297. When this value was used to estimate the amounts of 2-carboxybenzaldehyde and pyruvate produced from 20.8 nmol of trans-2′-carboxybenzalpyruvate at equilibrium, the result was 19.5 nmol. This value was in agreement with those determined experimentally (19.1 and 21.3 nmol, respectively).
The activity of this enzyme for trans-2′-hydroxycinnamate, benzalpyruvate, and trans-2′-methoxybenzalpyruvate was spectrophotometrically examined at a substrate concentration of 50 μM in 50 mM KP buffer (pH 7.5) at 25°C. Under these conditions, this enzyme transformed none of these compounds.
Effects of metals, chelators, and borohydride.
Many bacterial aldolases require a divalent cation, such as Zn2+, for catalysis and are inhibited by EDTA (13, 28). Thus, the effects of metals and chelators on the activity of trans-2′-carboxybenzalpyruvate hydratase-aldolase were examined. EDTA at 10 mM, EGTA at 10 mM, Mn2+ at 1 mM, Mg2+ at 10 mM, and Ca2+ at 1 mM did not have any significant effect on the activity of trans-2′-carboxybenzalpyruvate hydratase-aldolase. Members of another type of aldolase, exemplified by the class I fructose-1,6-bisphosphate aldolase, form an intermediate with their substrate through a Schiff base, and borohydride irreversibly inhibits this type of aldolase by reducing the Schiff base (9). Preincubation of trans-2′-carboxybenzalpyruvate hydratase-aldolase in 50 mM KP buffer (pH 7.5) containing 20 mM sodium borohydride and 20 μM sodium pyruvate for 10 min at 25°C did not inhibit the activity of this enzyme. These observations indicated that the catalytic mechanism of this enzyme is different from those of metal-dependent and Schiff base-forming aldolases.
Amino-terminal sequence of trans-2′-carboxybenzalpyruvate hydratase-aldolase.
The amino-terminal sequence of purified trans-2′-carboxybenzalpyruvate hydratase-aldolase was determined by automated Edman degradation to be Thr-Ser-Pro-Ala-Val-Thr-Ser-Ala-Asp-Ile-Thr-Gly-Leu-Val-Gly-Ile-Val-Pro-Thr-Pro-Ser-Lys-Pro-Gly.
Nucleotide sequence of the gene coding for trans-2′-carboxybenzalpyruvate hydratase-aldolase.
We had previously cloned and sequenced the gene coding for 1-hydroxy-2-naphthoate dioxygenase (phdI) from strain KP7 (16). An open reading frame had been found downstream of phdI. The amino-terminal sequence deduced from this open reading frame was in perfect agreement with the amino-terminal sequence determined for purified trans-2′-carboxybenzalpyruvate hydratase-aldolase. We then sequenced the open reading frame using primers designed from the determined sequence. The length of the trans-2′-carboxybenzalpyruvate hydratase-aldolase gene, called phdJ, was 996 bp, and the deduced amino acid sequence for the enzyme was 332 amino acids long. The molecular mass (35 kDa) of the enzyme calculated from the deduced amino acid sequence was in agreement with the size determined by SDS-PAGE (38 kDa).
The deduced amino acid sequence of this enzyme exhibited significant similarities (40% identity) to those of NahE and DoxI (5–7) (Fig. 3). NahE is encoded by the nahE gene on naphthalene catabolic plasmid NAH7 in Pseudomonas putida PpG7, while DoxI is encoded by the doxI gene in dibenzothiophene-degrading strain Pseudomonas sp. strain C18. NahE has been found to exhibit trans-2′-hydroxybenzalpyruvate hydratase-aldolase activity (6, 7). The amino-terminal sequence of trans-2′-hydroxybenzalpyruvate hydratase-aldolase from naphthalenesulfonate-degrading strain P. vesicularis BN6 (22) is also similar to that of trans-2′-carboxybenzalpyruvate hydratase-aldolase. trans-2′-Hydroxybenzalpyruvate hydratase-aldolase from strain BN6 has a molecular mass of 120 kDa and is composed of identical subunits of 38.5 kDa. Clearly, trans-2′-hydroxybenzalpyruvate hydratase-aldolases in these strains and trans-2′-carboxybenzalpyruvate hydratase-aldolase in strain KP7 are evolutionarily related. On the other hand, no significant sequence similarity was observed between trans-2′-carboxybenzalpyruvate hydratase-aldolase and other aldolases. Thus, trans-2′-carboxybenzalpyruvate hydratase-aldolase and trans-2′-hydroxybenzalpyruvate hydratase-aldolase constitute a novel enzyme family, and it may be of interest to clarify the reaction mechanism of these enzymes in future studies.
FIG. 3.
Alignment of the deduced amino acid sequences of trans-2′-carboxybenzalpyruvate hydratase-aldolase and trans-2′-hydroxybenzalpyruvate hydratase-aldolase. CBPA, trans-2′-carboxybenzalpyruvate aldolase from strain KP7; NahE, trans-2′-hydroxybenzalpyruvate hydratase-aldolase from naphthalene catabolic plasmid NAH7 in P. putida PpG7 (6); DoxI, trans-2′-hydroxybenzalpyruvate hydratase-aldolase from Pseudomonas sp. strain C18 (5). Conserved amino acids are shown by asterisks. Dashes indicate gaps.
Expression of the trans-2′-carboxybenzalpyruvate hydratase-aldolase gene in E. coli.
A set of PCR primers was designed to amplify a DNA fragment containing phdJ, and a 1,094-bp DNA fragment was amplified by use of Vent DNA polymerase and these primers. The amplified fragment was cloned into pET-22b(+) to construct pMKT310. The nucleotide sequence of the cloned fragment was identical to that of the original phdJ sequence, except for the primer regions. E. coli BL21(DE3) containing pMKT310 was cultivated in the presence or absence of 0.1 mM IPTG in L broth at 37°C, and the trans-2′-carboxybenzalpyruvate hydratase-aldolase activity in the extracts of the cells thus cultivated was measured. The activity of trans-2′-carboxybenzalpyruvate hydratase-aldolase was not detected in the extract of noninduced L broth-grown cells (<0.001 μmol min−1 mg of protein−1), while it was expressed at 0.04 μmol min−1 mg of protein−1 in IPTG-induced cells. The results indicate that the 996-bp phdJ gene encodes trans-2′-carboxybenzalpyruvate hydratase-aldolase.
ACKNOWLEDGMENTS
We thank R. W. Eaton for generously providing benzalpyruvate and trans-2′-methoxybenzalpyruvate. We are also grateful for the support and valuable advice of Shigetoh Miyachi and Jun Inoue and for the technical assistance of Yukiko Itazawa and Yoshio Sasaki.
REFERENCES
- 1.Adachi, K., T. Iwabuchi, H. Sano, and S. Harayama. Submitted for publication.
- 2.Barnsley E A. Phthalate pathway of phenanthrene metabolism: formation of 2′-carboxybenzalpyruvate. J Bacteriol. 1983;154:113–117. doi: 10.1128/jb.154.1.113-117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Cerniglia C E, Heitkamp M A. Microbial degradation of polycyclic aromatic hydrocarbons (PAH) in the aquatic environment. In: Varanasi U, editor. Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 41–68. [Google Scholar]
- 5.Denome S A, Stanley D C, Olson E S, Young K D. Metabolism of dibenzothiophene and naphthalene in Pseudomonas strains: complete DNA sequence of an upper naphthalene catabolic pathway. J Bacteriol. 1993;175:6890–6901. doi: 10.1128/jb.175.21.6890-6901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton R W. Organization and evolution of naphthalene catabolic pathways: sequence of the DNA encoding 2-hydroxychromene-2-carboxylate isomerase and trans-o-hydroxybenzylidenepyruvate hydratase-aldolase from the NAH7 plasmid. J Bacteriol. 1994;176:7757–7762. doi: 10.1128/jb.176.24.7757-7762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton R W, Chapman P J. Bacterial metabolism of naphthalene: construction and use of recombinant bacteria to study ring cleavage of 1,2-dihydroxynaphthalene and subsequent reactions. J Bacteriol. 1992;174:7542–7554. doi: 10.1128/jb.174.23.7542-7554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans W C, Fernley H N, Griffiths E. Oxidative metabolism of phenanthrene and anthracene by soil pseudomonas. Biochem J. 1965;95:819–831. doi: 10.1042/bj0950819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fersht A. Enzyme structure and mechanism. W. H. New York, N.Y: Freeman & Co.; 1984. [Google Scholar]
- 10.Gibson D T, Subramanian V. Microbial degradation of aromatic hydrocarbons. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 181–252. [Google Scholar]
- 11.Harayama S. Polycyclic aromatic hydrocarbon bioremediation design. Curr Opin Biotechnol. 1997;8:268–273. doi: 10.1016/s0958-1669(97)80002-x. [DOI] [PubMed] [Google Scholar]
- 12.Harayama S, Rekik M. Bacterial aromatic ring-cleavage enzymes are classified into two different gene families. J Biol Chem. 1989;264:15328–15333. [PubMed] [Google Scholar]
- 13.Horecker B L, Tsolas O, Lai C Y. Aldolases. Enzymes. 1972;7:213–258. [Google Scholar]
- 14.Inoue J, Shaw J P, Rekik M, Harayama S. Overlapping substrate specificities of benzaldehyde dehydrogenase (the xylC gene product) and 2-hydroxymuconic semialdehyde dehydrogenase (the xylG gene product) encoded by TOL plasmid pWW0 of Pseudomonas putida. J Bacteriol. 1995;177:1196–1201. doi: 10.1128/jb.177.5.1196-1201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwabuchi T, Harayama S. Biochemical and genetic characterization of 2-carboxybenzaldehyde dehydrogenase, an enzyme involved in phenanthrene degradation by Nocardioides sp. strain KP7. J Bacteriol. 1997;179:6488–6494. doi: 10.1128/jb.179.20.6488-6494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwabuchi, T., and S. Harayama. Biochemical and molecular characterization of 1-hydroxy-2-naphthoate dioxygenase from Nocardioides sp. KP7. J. Biol. Chem., in press. [DOI] [PubMed]
- 17.Iwabuchi, T., Y. Yamauchi-Inomata, A. Katsuta, and S. Harayama. Isolation and characterization of marine Nocardioides capable of growing and degrading phenanthrene at 42°C. J. Mar. Biotechnol., in press.
- 18.Kiyohara H, Nagao K. Enzymatic conversion of 1-hydroxy-2-naphthoate in phenanthrene-grown Aeromonas sp. S45P1. Agric Biol Chem. 1977;41:705–707. [Google Scholar]
- 19.Kiyohara H, Nagao K. The catabolism of phenanthrene and naphthalene in bacteria. J Gen Microbiol. 1978;105:69–75. [Google Scholar]
- 20.Kiyohara H, Nagao K, Nomi R. Degradation of phenanthrene through o-phthalate by an Aeromonas sp. Agric Biol Chem. 1976;40:1075–1082. [Google Scholar]
- 21.Kiyohara H, Torigoe S, Kaida N, Asaki T, Iida T, Hayashi H, Takizawa N. Cloning and characterization of a chromosomal gene cluster, pah, that encodes the upper pathway for phenanthrene and naphthalene utilization by Pseudomonas putida OUS82. J Bacteriol. 1994;176:2439–2443. doi: 10.1128/jb.176.8.2439-2443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhm A E, Knackmuss H-J, Stolz A. Purification and properties of 2′-hydroxybenzalpyruvate aldolase from a bacterium that degrades naphthalenesulfonates. J Biol Chem. 1993;268:9484–9489. [PubMed] [Google Scholar]
- 23.Kurkela S, Lehvaslaiho H, Palva E T, Teeri T H. Cloning, nucleotide sequence and characterization of genes encoding naphthalene dioxygenase of Pseudomonas putida strain NCIB9816. Gene. 1988;73:355–362. doi: 10.1016/0378-1119(88)90500-8. [DOI] [PubMed] [Google Scholar]
- 24.Morawski B, Eaton R W, Rossiter J T, Guoping S, Griengl H, Ribbons D W. 2-Naphthoate catabolic pathway in Burkholderia strain JT 1500. J Bacteriol. 1997;179:115–121. doi: 10.1128/jb.179.1.115-121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon M J, Osslund T D, Saunders R, Ensley B D, Suggs S, Harcourt A, Suen W C, Cruden D L, Gibson D T, Zylstra G L. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816-4. Gene. 1993;127:31–37. doi: 10.1016/0378-1119(93)90613-8. [DOI] [PubMed] [Google Scholar]
- 26.Stolzenbach F. Lactic dehydrogenase (crystalline) Methods Enzymol. 1966;9:278–288. [Google Scholar]
- 27.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 28.Tack B F, Chapman P J, Dagley S. Purification and properties of 4-hydroxy-4-methyl-2-oxoglutarate aldolase. J Biol Chem. 1972;247:6444–6449. [PubMed] [Google Scholar]
- 29.Takizawa N, Kaida N, Torigoe S, Moritani T, Sawada T, Satoh S, Kiyohara H. Identification and characterization of genes encoding polycyclic aromatic hydrocarbon dioxygenase and polycyclic aromatic hydrocarbon dihydrodiol dehydrogenase in Pseudomonas putida OUS82. J Bacteriol. 1994;176:2444–2449. doi: 10.1128/jb.176.8.2444-2449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Chen R F, Shiaris M P. Metabolism of naphthalene, fluorene, and phenanthrene: preliminary characterization of a cloned gene cluster from Pseudomonas putida NCIB9816. J Bacteriol. 1994;176:2158–2164. doi: 10.1128/jb.176.8.2158-2164.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen K-M, Serdar C M. Genetics of naphthalene catabolism in pseudomonads. Crit Rev Microbiol. 1988;15:247–268. doi: 10.3109/10408418809104459. [DOI] [PubMed] [Google Scholar]