Abstract
Background:
Patients with cirrhosis and portal hypertension face a high risk of complications. Besides their anti-inflammatory and antifibrotic effects, statins may reduce portal pressure and thus the risk of complications and mortality. We aimed to investigate the effects of atorvastatin on hospital admissions, mortality, inflammation, and lipidomics in cirrhosis with portal hypertension.
Methods:
We performed a double-blinded, randomized, placebo-controlled clinical trial among patients with cirrhosis and portal hypertension. Atorvastatin (10–20 mg/d) was administered for 6 months. We measured splanchnic hemodynamics, analyzed inflammatory markers, and performed lipidomics at baseline and after 6 months.
Results:
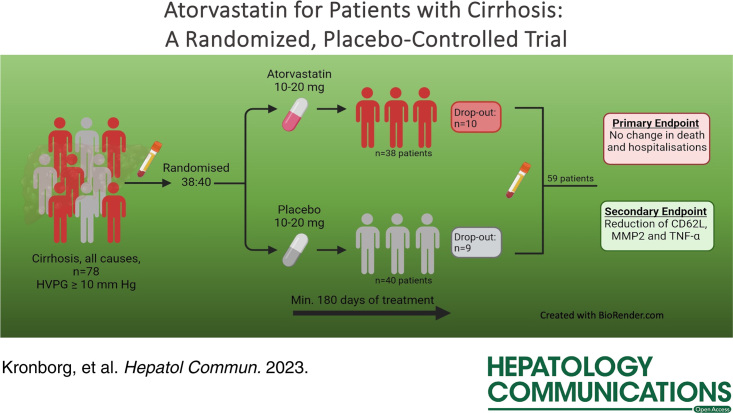
Seventy-eight patients were randomized, with 38 patients allocated to atorvastatin and 40 patients to placebo. Fifty-nine patients completed 6 months of intervention. Comparisons between changes in each group were calculated. Liver-related complications and mortality were similar between the groups. The HVPG and Model for End-stage Liver Disease score did not change between groups (p=0.95 and 0.87, respectively). Atorvastatin decreased 3 of 42 inflammatory markers, CD62-L-selectin, matrix metalloproteinases-2, and TNF-α (p-values: 0.005, 0.011, and 0.023, respectively), while lipidomics was not significantly changed.
Conclusions:
In patients with cirrhosis, atorvastatin was safe to use, but did not reduce mortality, the risk of liver-related complications, or the HVPG. Atorvastatin induced minor anti-inflammatory effects and minor effects on lipids during a 6-month treatment period.
INTRODUCTION
The incidence of cirrhosis is increasing worldwide, and its prevalence in Europe ranges from 447 to 1100 per 100,000.1 In the late stages of the disease, complications, such as ascites, esophageal variceal bleeding, HE, and infections, increase morbidity and mortality.2–4 In recent years, it has become apparent that, besides portal hypertension, also systemic inflammation plays a pivotal role in the development of these complications and predisposes patients to an increased risk of mortality.5–7 Systemic inflammation is involved in driving the disease course of acute decompensation and predisposes patients to acute-on-chronic liver failure (ACLF).6
Current management of cirrhosis focuses on the treatment of complications, but disease-modifying drugs are lacking.
Statins may be a potential drug candidate, and data from both experimental and human studies have shown lowering effects on portal pressure and reduced mortality due to pleiotropic effects.8,9 Statins decrease oxidative stress and inflammation in the vessel walls and improve endothelial dysfunction by increasing the availability of nitric oxide, possibly inhibiting the development of portal hypertension in cirrhosis.10–14 In experimental models, statins decrease intrahepatic vascular resistance through relaxation of HSC and might also inhibit fibrogenesis and hepatic inflammation.15–18 Prior clinical studies have assessed the effects of simvastatin on portal hypertension and the risk of variceal bleeding in selected patients and demonstrated a reduction of portal pressure, advantageous effects on endothelial cells, hepatic resistance and hepatosplanchnic output, and an antioxidative potential, as well as improvements in survival after variceal bleeding.19–23 Atorvastatin may inhibit and delay the development of cirrhosis and HCC and may have beneficial effects on portal hypertension.24,25 These findings need confirmation in randomized and controlled trials.
In this randomized, placebo-controlled trial in patients with cirrhosis and portal hypertension, we aimed to investigate the safety of atorvastatin and its effects on survival, hospital admissions, and biomarkers of systemic inflammation and lipidomics compared to placebo.
METHODS
The StatLiver trial was investigator-initiated and approved by the Scientific Ethics Committee of the Capital Region of Denmark (H-19030643) and the European Medicines Agency (EudraCT 2019-001806-40) and registered on clinicaltrials.gov (NCT04072601). All research was conducted in accordance with both the Declarations of Helsinki and Istanbul. This was a multicenter, randomized, double-blinded, placebo-controlled clinical trial. The Good Clinical Practice Unit, Copenhagen University, served as an external trial monitor, and all participants gave informed written consent. The trial protocol is available in the Supplemental Material, http://links.lww.com/HC9/A672, and is published.26
Inclusion and exclusion criteria
Inclusion criteria were patients aged 18–80 years with cirrhosis diagnosed by liver biopsy, ultrasound, elastography or CT scan of the liver, and clinical biochemistry compatible with cirrhosis; portal hypertension with a pressure gradient ≥10 mm Hg; ability to read and understand project information in Danish; and written, informed consent to participate.
Exclusion criteria were continuous treatment with statins in the preceding year; clinically verified infection in the preceding 4 weeks; pregnancy or lactation; HCC, HIV infection, and/or treatment with protease inhibitors; signs of hepatorenal syndrome in the preceding 14 days; a Model for End-stage Liver Disease (MELD) score above 23 or a Child-Pugh score above 13; HE grade 2 or above; or reasonable doubt of compliance with the trial protocol, as determined by the clinician responsible for standard care.
Study design
We performed 1:1 randomization as a block randomization by 6 (3:3) with a computer-generated sequence provided by the Hospital Pharmacy of the Capital Region of Denmark. A unique 4-digit number identified participants. Participants, personnel, and data assessors were blinded to the treatment.
Intervention and blinding
Study medication was packed and delivered according to the randomization list by the Hospital Pharmacy of the Capital Region of Denmark. One laboratory pharmacist, responsible for the packing and blinding of medication, was unblinded to the randomization code. This person was physically located at the Hospital Pharmacy of the Capital Region of Denmark and was otherwise not involved in trial-specific routines.
Patients in the intervention group received atorvastatin as 10 mg capsules daily. The placebo group received capsules similar in size, color, shape, and identical packaging. The dose was increased to 20 mg (2 capsules) after 4 weeks of treatment if the initial dose was tolerated. The treatment continued for at least 24 weeks (180 d) and a maximum of 18 months, in addition to standard treatment. The trial medication was handed out at the outpatient clinic, and surplus capsules were collected and counted at each visit. For reasons of safety, unblinding of a participant was permitted if they experienced severe toxicity in the case of a life-threatening serious adverse event. Only the principal investigator and the sponsor in unison were able to unblind the patient and data.
Laboratory analyses and comparisons between groups were performed without revealing the intervention allocation. Scientific evaluation and clinical translation of study results and data were performed after the unblinding of which group belonged to the active and the placebo arms.
Outcome assessment
The primary outcomes were (1) a composite of mortality with time to liver transplantation (LTX) within 1.5 y and (2) the number of hospitalizations. Secondary outcomes were safety and changes in standard biochemistry and portal hypertension. The ACLF score was calculated at every hospitalization.
Exploratory outcomes were effects on inflammation and lipidomics. Secondary outcomes of macrophage activity, HSC activity, and explorations of the proteome and microRNAs will be reported elsewhere.
Sample size
The sample size was estimated based on the median survival time in prior studies, an estimated increased survival in the statin group, and a follow-up time of 24 months.23,27 Given an HR of placebo participants relative to experimental participants of 0.52, a minimum of 70 patients in each group was needed. The expected dropout rate was 15%, and we planned to enroll 162 participants in total.
For the primary outcome, survival and LTX, we aimed to enroll a total of 162 participants.
We initiated the first part of the trial as a pilot study of the exploratory outcomes and aimed to include 24 participants in each treatment arm, registering survival, time to first hospitalization, number of hospitalizations, and LTX within 180 days.
Data and procedures
We registered demographic data, comorbidities, and standard biochemistry every 3 months. Transjugular liver vein catheterization with assessment of portal pressure and liver biopsy sampling was performed at baseline and after 6 months. A balloon catheter was inserted into the hepatic venous system by means of the right jugular vein, and the correct position was ensured by injection of contrast and transillumination agents, as described elsewhere.28,29
Peripheral blood sampling was performed for standard biochemistry and specialized analyses. Samples of whole blood and EDTA plasma were stored at −80 °C until analysis.
Analysis of inflammatory markers
Inflammatory markers were measured in EDTA plasma samples from peripheral and hepatic venous blood collected at baseline and after 6 months. Analytes were measured on customized Luminex Human Discovery X-plex immunoassays30 (Bio-Techne, Wiesbaden, Germany) using the Luminex 200 system (Bio-Techne, Wiesbaden, Germany) according to the manufacturer’s instructions. For a description of the sample analysis, see Supplemental Material, http://links.lww.com/HC9/A671.
Lipidomics analysis
Lipidomics were analyzed in plasma from a peripheral vein, as described elsewhere,31,32 and were modified to fit the specific sample set and instrumentation. Samples were analyzed randomly, without knowledge of disease or outcomes. Chromatographic separation was carried out with an Agilent 1290 Infinity II ultra-HPLC system (Agilent, Waldbronn, Germany). For details about the chromatographic separation of lipidomics and mass detection, see Supplemental Material, http://links.lww.com/HC9/A671.
Statistical analysis
Comparisons between groups were initially performed by blinded allocation, meaning analyses were performed as group A versus group B, without knowledge of which group received the active drug. Intention to treat analyses of differences between the atorvastatin and the placebo groups were assessed by unpaired t tests of mean difference (delta values of baseline minus 6 months) of both clinical and hemodynamic variables. For the exploratory outcomes, comparisons between baseline and six months were performed in the subgroup of participants who completed 6 months of follow-up as per protocol. Concentration values of inflammatory biomarkers below the lower limit of detection were stated as the lower limit of detection divided by 2. Concentration values above the upper limit were replaced by the upper limit. Data were evaluated for normal distribution assumptions.
Lipidomics data were log2-transformed before analysis. Changes in lipid levels in the placebo and atorvastatin groups were compared using t tests. The changes were calculated as baseline values minus 6-month values. p-values were adjusted using the Benjamini-Hochberg procedure to control the false discovery rate. Time to events end points were analyzed by plotting the survival functions by group. Participants without admission were followed until day 180. Log-rank tests were computed for comparisons of groups. For the clinical parameters, the analysis at month 6 was done by using measurements as observed. The last observation carried forward approach was applied in which data at month 3 were carried forward to month 6 if the measurement was missing at month 6. The results obtained with these 2 different approaches were similar, showing that the results from the “as observed analyses” were robust. Biomarker data were only measured at baseline and month 6, that is, a complete case analysis was performed.
A p-value of ≤0.05 was considered statistically significant. Adjusted p-values are provided in the Supplemental Material, http://links.lww.com/HC9/A671. All analyses were computed using R version 4.2.1.
RESULTS
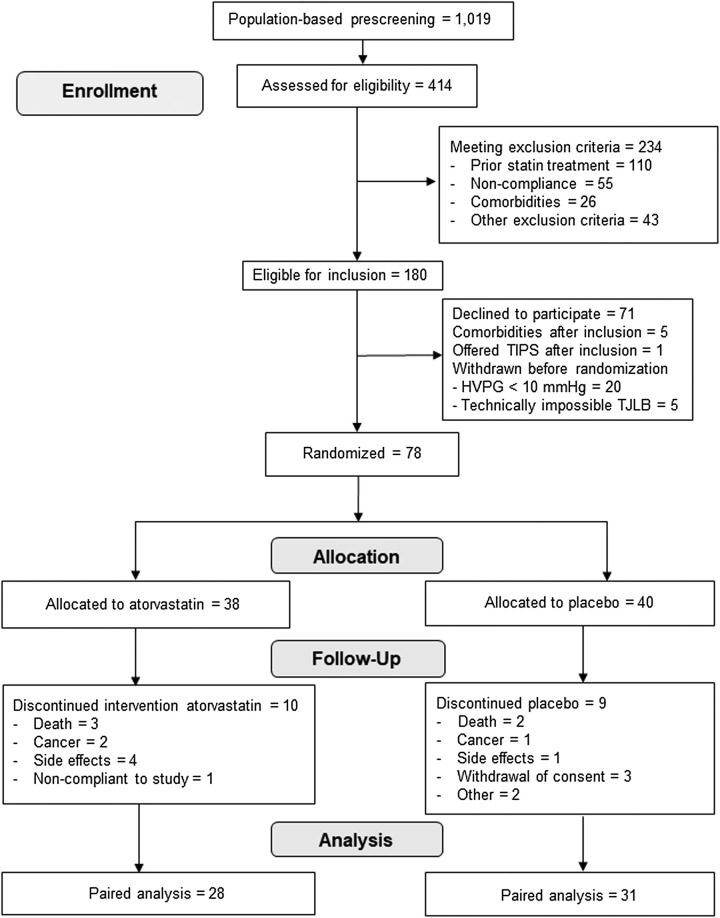
Between February 1, 2020, and December 22, 2021, a population of 1019 patients from the Gastro Unit, Medical Division, Copenhagen University Hospital, Hvidovre, Herlev University Hospital, and Aarhus University Hospital Denmark were prescreened for eligibility. Of these, 414 were eligible for inclusion and were screened further. Of these, 234 were excluded, the majority due to statin use (n=110) and a history of noncompliance with hospital attendance (n=55). Seventy-one patients declined to participate, and 31 patients were not suitable for randomization after inclusion; hence 78 participants were included and allocated to atorvastatin or placebo (Figure 1). Due to COVID-19 restrictions and recruitment difficulties at the study sites, it was decided to stop the study after recruiting 78 participants. At this point, 24 participants in each study arm had completed at least 180 days of treatment, and the first part of the study was completed with a median follow-up time of 362 days (range from 10 to 553 d). Of the 78 participants, 59 participants completed >180 days of the intervention as per protocol and were included in the exploratory analyses.
FIGURE 1.
Consort flowchart. Abbreviation: TJLB, transjugular liver biopsy.
The median age was 60.5 years (range 37–79 y), and 33 (42.3%) were female. The groups were comparable at baseline, besides a statistically significant difference in MELD score, bilirubin, and international normalized ratio (Table 1).
TABLE 1.
Baseline characteristics of atorvastatin and placebo groups
| Atorvastatin group | Placebo group | |
|---|---|---|
| N | 38 | 40 |
| Sex=male (%) | 21 (55.3) | 24 (60.0) |
| Age, years | 59.0 [46; 79] | 63.5 [37; 73] |
| HVPG, mm Hg | 16.3 [10; 23] | 15.0 [10; 25] |
| Mean arterial pressure, mm Hg | 104 [80; 119] | 94.5 [86; 110] |
| Systolic blood pressure, mm Hg | 124 [105; 198] | 122.5 [98; 163] |
| Diastolic blood pressure, mm Hg | 73 [53; 103] | 76 [52; 105] |
| Child-Pugh score | 7.0 [5; 11] | 7.0 [5; 12] |
| A | 12 | 18 |
| B | 21 | 20 |
| C | 5 | 2 |
| Ascites, number (%) | ||
| None | 14 (36.8) | 14 (35) |
| Mild | 21 (55.3) | 24 (60) |
| Moderate to severe | 3 (7.9) | 2 (5) |
| Alcohol | 30 (78.9) | 34 (85.0) |
| Fatty liver | 3 (7.9) | 1 (2.5) |
| HBV | 0 | 1 (2.5) |
| AIH | 0 | 1 (2.5) |
| PBC | 2 (5.3) | 1 (2.5) |
| Methotrexate-induced | 1 (2.6) | 1 (2.5) |
| Unknown | 4 (10.5) | 1 (2.5) |
| Primary sclerosing cholangitis | 1 (2.5) | 0 |
| MELD score | 12.5 [7; 21] | 10.0 [6; 21] |
| Hemoglobin, mmol/L | 7.9 [4.9; 10.4] | 7.6 [5; 10.4] |
| Platelets, 109/L | 111 [44; 314] | 148.5 [44; 394] |
| INR | 1.4 [1.0; 2.0] | 1.3 [0.8; 2.2] |
| Leukocytes, 109/L | 5.9 [2.7; 15.8] | 6.7 [3.0; 12.1] |
| C-reactive protein, mg/L | 8.0 [0.6; 46] | 4.7 [0.6; 44] |
| Albumin, g/L | 30 [21; 43] | 33 [15; 41] |
| Bilirubin, μmol/L | 29 [7; 125] | 17 [7; 89] |
| Creatinine, μmol/L | 60.5 [24; 113] | 65 [43; 211] |
| Sodium, mmol/L | 136 [126; 145] | 135 [127; 143] |
| Beta-blockers | 5 (13.2) | 13 (32.5) |
| Spironolactone | 20 (52.6) | 25 (62.5) |
| Furosemide | 17 (44.7) | 15 (37.5) |
| Furosemide+spironolactone | 13 (34.2) | 14 (35.0) |
Note: Results are reported as a median [range], unless otherwise stated.
Abbreviations: AIH, autoimmune hepatitis; INR, international normalized ratio; MELD, Model for End-stage Liver Disease; PBC, primary biliary cholangitis.
Eighteen of the patients received nonselective beta-blockers during the intervention (5 in the atorvastatin group, 13 in the placebo group), and 1 patient began treatment with nonselective beta-blockers during the intervention (atorvastatin group). Patients remained on stable doses throughout the study period. All participants who continued beyond 4 weeks had their dose increased from 10 to 20 mg of the trial medication. Adherence to medication was 98% (range 56%–100%), consistent with the trial protocol.
Primary outcomes
Mortality
Five participants died within the first 6 months of the trial, 3 in the atorvastatin group and 2 in the placebo group. The deaths were related to liver disease (n=2), occurred outside the hospital (n=2), or were related to alcohol abuse (n=1).
In the total study period, 8 patients died: 4 from the atorvastatin group and 4 from the placebo group. Three participants underwent LTX after 265, 290, and 336 days, respectively, and all were in the atorvastatin group. Hence, the composite end point for 180 days did not include any LTX. There was no significant difference between survival in the atorvastatin and the placebo groups (p=0.54).
Hospital admissions
Seven patients were admitted with an ACLF episode during the study period: 4 patients in the atorvastatin group and 3 patients in the placebo group.
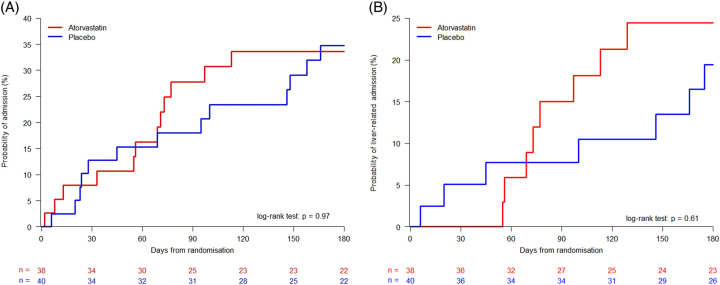
In total, 25 participants were admitted to the hospital within 180 days: 12 in the atorvastatin group and 13 in the placebo group. The median time to admission was 62.5 days in the atorvastatin group and 69 days in the placebo group (Figure 2A and Table 2). No statistical difference was found between the groups regarding total hospital admissions (p=0.97) or liver-related admissions (p=0.61; Figure 2B). Adjusting for the MELD score at baseline did not reveal statistically significant differences between the groups (p=0.85 for total admissions and p=0.78 for liver-related admissions).
FIGURE 2.
(A) Admissions within 180 days of inclusion in the atorvastatin and placebo groups, (B) Liver-related admissions. Comparison of groups by log-rank tests.
TABLE 2.
Admissions within 180 days of inclusion in the study
| Atorvastatin (33) | Placebo (34) | |
|---|---|---|
| No. admissions | 23 (among 12 patients) | 22 (among 13 patients) |
| No. liver-related admissions | 17 (among 8 patients) | 13 (among 7 patients) |
| Time to first admission, median of days [range] | 62.5 [2; 113] | 69.0 [6; 166] |
| Time to first liver-related admission, median of days [range] | 75 [55; 129] | 100 [6; 175] |
The admissions within 180 days were mainly due to complications of cirrhosis (66.7%), including ascites, HE, electrolyte disturbances, edema, icterus, infections, alcohol-associated hepatitis, bleeding esophageal varices, and hepatorenal syndrome (Supplemental Table S1, http://links.lww.com/HC9/A671). There was no significant difference between the number of admissions between the atorvastatin and the placebo groups (p=0.97).
Secondary outcomes
Safety
Plasma creatine kinase increased to a maximum value of 552 U/L in only 1 atorvastatin-treated participant, and the dose was subsequently reduced to 10 mg after 3 months. No participants developed toxic hepatitis due to atorvastatin, which would have triggered an immediate discontinuation of the intervention. One participant in the placebo group was withdrawn from the trial due to alcohol-associated hepatitis and concern of risk by continuing the trial, but creatine kinase was not measured. One participant in the atorvastatin group was admitted to the hospital due to suspected adverse effects of the study drug (muscle pain) and was withdrawn from the trial. Two participants in the placebo group wished to withdraw from the trial due to possible adverse effects related to the study drug; the symptoms were visual disturbance and abdominal pain. Three participants in the atorvastatin group wished to withdraw from the trial due to possible adverse effects, all reporting muscle pain and tiredness, and 1 reporting nausea. None of the adverse effects caused hospital admissions and were not suspected of representing known side effects caused by the study drug.
Adverse events
No participants experienced treatment-related serious adverse events, and no deaths were related to the study drug during the total study period. We registered 76 serious adverse events in 31 (39.7%) participants. In total, 136 adverse events occurred in 48 (61.5%) participants: 39 were mild, 21 were moderate, and 76 were severe. Eighteen participants experienced 38 serious adverse events in the placebo group, 32 of which were related to complications of cirrhosis. Thirteen participants experienced 38 serious adverse events in the atorvastatin group; 32 were related to cirrhosis (Supplemental Table S2, http://links.lww.com/HC9/A671). After 180 days, there was no difference found in the occurrence of adverse events in the groups (p=0.99; Supplemental Figures S1 and S2, http://links.lww.com/HC9/A671).
Changes in HVPG, clinical variables, and biochemistry
We found no significant differences between the atorvastatin and placebo groups in their mean changes (delta values) in clinical variables and HVPG from baseline to 6 months (Supplemental Table S3, http://links.lww.com/HC9/A671). The change in HVPG, MELD, and biochemical parameters of platelets, white blood cells, and CRP from baseline to 6 months of follow-up were similar in the 2 groups.
Exploratory outcomes
Inflammation
Fifty-nine participants completed 6 months of treatment as per protocol and were included in the exploratory analyses.
We explored 42 inflammatory markers (Supplemental Tables S4 and S5, http://links.lww.com/HC9/A671). The mean difference of 3 markers (delta values), CD62-L-selectin (CD62L), matrix metalloproteinase 2 (MMP-2), and TNF-α, was significantly different between the study groups in peripheral vein blood. CD62-L decreased by 250.3 ng/mL and increased by 266.8 ng/mL (p-value of 0.005) in the atorvastatin and placebo groups, respectively. MMP-2 decreased by 26.5 and increased by 48.2 ng/mL (p-value of 0.011) in the atorvastatin and placebo groups, respectively. TNF-α decreased by 0.7 and increased by 1.3 pg/mL (p-value of 0.023) in the atorvastatin and placebo groups, respectively. No significant mean differences between the groups from baseline to 6 months were found in liver vein blood.
Lipidomics
We compared the mean differences of level changes in 20 lipid classes (388 distinct lipids) in peripheral vein blood between the atorvastatin and the placebo groups after 6 months (Supplemental Table S6, http://links.lww.com/HC9/A671 and Supplemental Figure S3, http://links.lww.com/HC9/A671). Lipidomics showed 34 statistically significant differences between groups (p-values ranging from 0.002 to 0.045), with 31 decreases in the atorvastatin group and 3 decreases in the placebo group, where most lipids were triglycerides and hexosylceramides. Intragroup differences were more numerous, showing more decreases of statin-relevant lipids [triglycerides, free fatty acids, and cholesterol esters (CE)] in the atorvastatin group (Supplemental Figure S4, http://links.lww.com/HC9/A671).
When adjusting for multiple testing by Benjamini-Hochberg, no significant differences were found between groups.
Cholesterol was measured as 11 CEs, showing 1 significant decrease in the atorvastatin compared to the placebo group (CE 18:0, p=0.034).
DISCUSSION
In this randomized, placebo-controlled trial, we investigated the safety of atorvastatin and its clinical, hemodynamic, and explorative effects in patients with cirrhosis and portal hypertension. There were no differences between atorvastatin and placebo with respect to survival, hospital admissions, or changes in portal pressure after 6 months of treatment. Atorvastatin proved safe, with few adverse events and hospital admissions among groups. Atorvastatin reduced the concentrations of 3 of 42 inflammatory markers. Thirty-four lipids (mostly triglycerides and hexosylceramides) in peripheral vein blood also differed between the groups, although they were not further analyzed. The study suggested a lipid-lowering effect of atorvastatin and suggested an anti-inflammatory potential, in agreement with previous findings.15–18,35,36
Clinical end points
A meta-analysis of randomized controlled trials of simvastatin found lower mortality, concurring with a registry-based cohort study where simvastatin treatment lowered mortality by 8.0%–8.7% in patients with cirrhosis and Child-Pugh stages A and B.37,38 Earlier studies showed its positive effects on hemodynamic and clinical end points, such as HVPG, survival rate, and decompensation events in long-term follow-up.39 A recent study showed atorvastatin (20 mg/d) had a significant effect on HVPG when combined with propranolol, compared to propranolol alone, suggesting that combination therapies should be investigated further.24 In our study, participants receiving atorvastatin had higher baseline MELD scores, which could have influenced hospitalizations and mortality for patients randomized to atorvastatin. The lack of effect on survival and hospital admissions might, further, be explained by the limited number of study participants and the short follow-up period.20 We did not see an effect of atorvastatin or placebo on HVPG. Studies using statins explicitly to treat cirrhosis are rare, and evidence to support the use of statins to prevent portal hypertension and decompensation is not yet sufficiently established.23. Overall, we can recommend the use of statins in patients with cirrhosis with an approved indication for statins according to the Baveno VII guidelines.33
Adverse events
The number and severity of adverse events were similar between the study groups. Cirrhosis may change the pharmacokinetics of atorvastatin, and vigilant monitoring is recommended in patients with decompensation.40 Rhabdomyolysis has been reported as a side effect of simvastatin,34,40,41 especially in the dose of 40 mg as reported in the LiverHOPE study.34 The adverse effects of atorvastatin seen in patients with liver dysfunction are likely dose-dependent as pharmacokinetics of atorvastatin seem to increase in patients with Child-Pugh B cirrhosis compared to Child-Pugh A cirrhosis.40,41 However, we observed no liver or muscle toxicity, and if possible benefits of statins are further established, the low risk of hepatotoxicity can be overruled.42
Inflammatory signaling
CD62L, MMP-2, and TNF-α were reduced in the atorvastatin group compared to the placebo group. In previous studies, these markers were elevated in patients with cirrhosis.43–50 CD62L is a cell adhesion protein, MMP-2 is an enzyme involved in the degradation of extracellular matrix proteins, while TNF-α is a proinflammatory cytokine.43–49 TNF-α induces the expression of MMP-2, and MMPs increase the activity of TNF-α.46 MMP-2 seems elevated in cirrhosis (increasing in line with Child-Pugh class) and during HSC activation and after partial hepatectomy, while CD62L is increased in patients with acute decompensation and positively correlates with MELD scores.46,48–50 The knowledge of TNF-α is more established, which increases early in chronic liver diseases and correlates with the disease stage.51,52 TNF-α is also involved in the development and progression of cirrhosis, and it continues to be produced in established cirrhosis, and further increases in decompensation and ACLF.43–45 Only 3 of 42 markers showed significant differences among groups, which might be explained by too few participants, a missed optimal window for statins’ anti-inflammatory effects in the trajectory of cirrhosis or too short an intervention period.
Cytokines (being VCAM-1, VEGF-A, Fractalkine, MIP-1α, eotaxin, IP-10, RANTES, GM-CSF, IL-1β, IL-2, ICAM-1, and MCP-1) are formerly reported to be related to the severity and prognosis of cirrhosis, and have been used to differentiate patients with decompensation from patients with ACLF.53 Reducing the ongoing inflammation in cirrhosis might reduce the risk and number of ACLF episodes, as inflammation activity is a crucial predictor of ACLF and bleeding and determines the progression from acute decompensation to ACLF and death.54,55 A prior study found that statins reduced the frequency of developing ACLF and lowered mortality with a hazard reduction of 38%.56 However, the present study could not reproduce those results as the number of ACLF episodes was limited, and the retrospective design of the trial increases the risk of bias.
Lipidomics
Significant lipid changes were mainly observed in triglycerides and hexosylceramides. Triglycerides have formerly been described as decreased in cirrhosis.57 Atorvastatin has well-known lipid-lowering effects, including triglycerides, which might explain our results.58 Hexosylceramides have not been investigated thoroughly in cirrhosis but seem positively correlated with the degree of fibrosis in patients with HCV.59 Further explorations of the lipidome might reveal statins’ pleiotropic effects.
Summary
This is the first study to explore inflammation and lipidomics in a randomized trial with atorvastatin. We found a small tendency of reduced inflammatory activity and atorvastatin’s positive effects on lipidomics. The major strength of the present trial is its randomized, double-blinded design, performed in 3 dedicated liver centers in Denmark. The trial included both clinical and extensive exploratory measures. A limitation of the study is its sample size, which prevented optimal assessment of the true effects of atorvastatin, limited our calculations on primary end points, and hindered the adjustments for age and sex variables. In conclusion, atorvastatin had no effect on clinical end points after 6 months of treatment but proved to be very safe. Our exploratory results suggest that atorvastatin may have anti-inflammatory effects. To elucidate the effects of atorvastatin at all stages of cirrhosis, especially its earlier stages, including fibrosis and prefibrosis,60 further research on the pleiotropic effects of statins in clinical trials is needed, preferably in larger studies with longer follow-up.
Supplementary Material
AUTHOR CONTRIBUTIONS
Nina Kimer and Flemming Bendtsen: conceptualized and designed the study. Nina Kimer, Thit M. Kronborg, Rasmus H. Gantzel, Mette L. Andersen, Ane S. Teisner, Henning Grønbæk, Søren Møller, and Lise Hobolth: recruited participants and performed study investigations. Robert Schierwagen, Kajetan Trošt, and Thit M. Kronborg: curated the data, Robert Schierwagen: conducted the inflammation analysis, Kajetan Trošt: conducted the lipidomics analysis, and Qian Gao: conducted the formal analyses of lipidomics data. Nina Kimer, Flemming Bendtsen, Søren Møller, Thomas Moritz, Lise Hobolth, Jonel Trebicka, and Thit M. Kronborg: interpreted the results. All authors had access to all data and reviewed and approved the final manuscript.
FUNDING INFORMATION
The study received funding from the Hvidovre University Hospital Research Foundation (AHH-2019-Nina Kimer and AHH-2020- Thit M. Kronborg); from the Danish Regions Foundation for Medical Treatment (EMM-2018-01114); the Danish Medical Association Research Fund (2019-3780/1); and the Beckett Foundation (20-2-5630). Nina Kimer conducted the study as part of her employment as a postdoctoral fellow at the University of Copenhagen (Bridge.ku.dk), financed by the Novo Nordisk Foundation (NNF18SA0034956). Qian Gao, Kajetan Trošt, and Thomas Moritz were supported by the Novo Nordisk Foundation (grant no. NNF18CC0034900). Jonel Trebicka received funding from the Hessian Ministry of Higher Education, Research and the Arts (HMWK) for the ACLF-I cluster projects and by the German Research Council: SFB 1382 (ID 403224013, project A09), by the German Federal Ministry of Education and Research (BMBF) for the DEEP-HCC project, from the European Union’s Horizon 2020 research and innovation program for MICROB-PREDICT (project ID 825694), DECISION (project ID 847949), GALAXY (project ID 668031), LIVERHOPE (project ID 731875), and IHMCSA (project ID 964590) projects. The manuscript reflects only the authors’ views, and the funders are not responsible for any use that may be made of the information it contains.
ACKNOWLEDGMENTS
The authors thank the StatLiver Study Group of Malene Barfod O’Connell, Karen Lisa Hilsted, Christian Mortensen, Niels Kristian Aagaard, Lisbet Gerdes, Toto Nairn, Raissa Rodrigues, Marlen Klepper, and Søren Lophaven for support with conducting the study. In addition, the authors thank the nurses in the Gastro Unit, Medical Division, and the Department of Clinical Biochemistry at Hvidovre University Hospital, especially Annette Rank, for an indispensable collaboration. The authors thank the staff at the Department of Clinical Physiology for skillful assistance during liver vein catheterization and obtaining transjugular liver biopsies.
CONFLICTS OF INTEREST
Henning Grønbæk consults for AstraZeneca and Novo Nordisk. He received grants from AbbVie and Intercept. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: ACLF, acute-on-chronic liver failure; AIH, autoimmune hepatitis; CD62L, CD62-L-selectin; CE, cholesterol ester; ICAM-1, intercellular adhesion molecule 1; IP-10, Interferon inducible protein; LTX, liver transplantation; MCP-1, monocyte chemoattractant protein-1; M-CSF, macrophage colony-stimulating factor; MELD, Model for End-stage Liver Disease; MIP-1-, macrophage inflammatory; MMP, matrix metalloproteinases; RANTES, regulated on activation, normal T cell expressed and secreted; VCAM, vascular cell adhesion molecule.
Contributor Information
Thit M. Kronborg, Email: thit.mynster.kronborg@regionh.dk.
Robert Schierwagen, Email: robert.schierwagen@ukmuenster.de.
Kajetan Trošt, Email: kajetan.trost@sund.ku.dk.
Qian Gao, Email: qian.gao@sund.ku.dk.
Thomas Moritz, Email: thomas.moritz@sund.ku.dk.
Flemming Bendtsen, Email: flemming.bendtsen@regionh.dk.
Rasmus H. Gantzel, Email: ragant@rm.dk.
Mette L. Andersen, Email: mette.lehmann.andersen@regionh.dk.
Ane S. Teisner, Email: ane.soegaard.teisner@regionh.dk.
Henning Grønbæk, Email: henning.gronbaek@aarhus.rm.dk.
Lise Hobolth, Email: lise.hobolth.01@regionh.dk.
Søren Møller, Email: Soeren.Moeller@regionh.dk.
Jonel Trebicka, Email: Jonel.Trebicka@ukmuenster.de.
Nina Kimer, Email: nina.kimer@regionh.dk.
REFERENCES
- 1. Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: A population-based cohort study. Liver Int. 2012;32:79–84. [DOI] [PubMed] [Google Scholar]
- 3. Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: Observational study. BMJ. 2018;362:2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deleuran T, Vilstrup H, Jepsen P. Decreasing mortality among Danish alcoholic cirrhosis patients: A nationwide cohort study. Am J Gastroenterol. 2016;111:817–22. [DOI] [PubMed] [Google Scholar]
- 5. Costa D, Simbrunner B, Jachs M, Hartl L, Bauer D, Paternostro R, et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J Hepatol. 2021;74:819–28. [DOI] [PubMed] [Google Scholar]
- 6. Arroyo V, Angeli P, Moreau R, Jalan R, Clària J, Trebicka J, et al. The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74:670–85. [DOI] [PubMed] [Google Scholar]
- 7. Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097–108. [DOI] [PubMed] [Google Scholar]
- 8. Pose E, Trebicka J, Mookerjee RP, Angeli P, Ginès P. Statins: Old drugs as new therapy for liver diseases? J Hepatol. 2019;70:194–202. [DOI] [PubMed] [Google Scholar]
- 9. Bang UC, Benfield T, Bendtsen F. Reduced risk of decompensation and death associated with use of statins in patients with alcoholic cirrhosis. A nationwide case-cohort study. Aliment Pharmacol Ther. 2017;46:673–80. [DOI] [PubMed] [Google Scholar]
- 10. Kalinowski L, Dobrucki LW, Brovkovych V, Malinski T. Increased nitric oxide bioavailability in endothelial cells contributes to the pleiotropic effect of cerivastatin. Circulation. 2002;105:933–8. [DOI] [PubMed] [Google Scholar]
- 11. Lefer DJ. Statins as potent antiinflammatory drugs. Circulation. 2002;106:2041–2042. [DOI] [PubMed] [Google Scholar]
- 12. McGirt MJ, Lynch JR, Parra A, Sheng H, Pearlstein RD, Laskowitz DT, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–6. [DOI] [PubMed] [Google Scholar]
- 13. Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926–31. [DOI] [PubMed] [Google Scholar]
- 14. Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: Endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–51. [DOI] [PubMed] [Google Scholar]
- 15. Abraldes JG, Rodríguez-Vilarrupla A, Graupera M, Zafra C, García-Calderó H, García-Pagán JC, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46:1040–6. [DOI] [PubMed] [Google Scholar]
- 16. Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–53. [DOI] [PubMed] [Google Scholar]
- 17. Chong L-W, Hsu Y-C, Lee T-F, Lin Y, Chiu Y-T, Yang K-C, et al. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol. 2015;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tripathi DM, Vilaseca M, Lafoz E, Garcia-Calderó H, Viegas Haute G, Fernández-Iglesias A, et al. Simvastatin prevents progression of acute on chronic liver failure in rats with cirrhosis and portal hypertension. Gastroenterology. 2018;155:1564–77. [DOI] [PubMed] [Google Scholar]
- 19. Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernández M, García-Pagán JC, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–755. [DOI] [PubMed] [Google Scholar]
- 20. Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: A randomized controlled trial. Gastroenterology. 2009;136:1651–8. [DOI] [PubMed] [Google Scholar]
- 21. Cash WJ, O’Neill S, O’Donnell ME, Mccance DR, Young IS, Mceneny J, et al. Randomized controlled trial assessing the effect of simvastatin in primary biliary cirrhosis. Liver Int. 2013;33:1166–74. [DOI] [PubMed] [Google Scholar]
- 22. Pollo-Flores P, Soldan M, Santos UC, Kunz DG, Mattos DE, da Silva AC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: A randomized controlled trial. Dig Liver Dis. 2015;47:957–63. [DOI] [PubMed] [Google Scholar]
- 23. Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology. 2016;150:1160–70.e3. [DOI] [PubMed] [Google Scholar]
- 24. Bishnu S, Ahammed SKM, Sarkar A, Hembram J, Chatterjee S, Das K, et al. Effects of atorvastatin on portal hemodynamics and clinical outcomes in patients with cirrhosis with portal hypertension: A proof-of-concept study. Eur J Gastroenterol Hepatol. 2018;30:54–59. [DOI] [PubMed] [Google Scholar]
- 25. Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016;64:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimer N, Grønbæk H, Fred RG, Hansen T, Deshmukh AS, Mann M, et al. Atorvastatin for prevention of disease progression and hospitalisation in liver cirrhosis: Protocol for a randomised, double-blind, placebo-controlled trial. BMJ Open. 2020;10:e035284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: A Danish population-based cohort study. Hepatology. 2010;51:1675–82. [DOI] [PubMed] [Google Scholar]
- 28. Kristensen H, Kimer N, Møller S. Indications and methods for measuring portal hypertension in cirrhosis. Scand J Gastroenterol. 2022;57:1149–57. [DOI] [PubMed] [Google Scholar]
- 29. Ferral H, Fimmel CJ, Sonnenberg A, Alonzo MJ, Aquisto TM. Transjugular liver biopsy with hemodynamic evaluation: Correlation between hepatic venous pressure gradient and histologic diagnosis of cirrhosis. J Clin Imaging Sci. 2021;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schierwagen R, Alvarez-Silva C, Madsen MSA, Kolbe CC, Meyer C, Thomas D, et al. Circulating microbiome in blood of different circulatory compartments. Gut. 2019;68:578–80. [DOI] [PubMed] [Google Scholar]
- 31. Israelsen M, Kim M, Suvitaival T, Madsen BS, Hansen CD, Torp N, et al. Comprehensive lipidomics reveals phenotypic differences in hepatic lipid turnover in ALD and NAFLD during alcohol intoxication. JHEP Reports. 2021;3:100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hansen CS, Suvitaival T, Theilade S, Mattila I, Lajer M, Trošt K, et al. Cardiovascular autonomic neuropathy in type 1 diabetes is associated with disturbances in TCA, lipid, and glucose metabolism. Front Endocrinol (Lausanne). 2022;13:831793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Abraldes JG, et al. Baveno VII—renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:31–41. [DOI] [PubMed] [Google Scholar]
- 35. Chen F, Maridakis V, O’Neill EA, Hubbard BK, Strack A, Beals C, et al. The effects of simvastatin treatment on plasma lipid-related biomarkers in men with dyslipidaemia. Biomarkers. 2011;16:321–33. [DOI] [PubMed] [Google Scholar]
- 36. Bergheanu SC, Reijmers T, Zwinderman AH, Bobeldijk I, Ramaker R, Liem AH, et al. Lipidomic approach to evaluate rosuvastatin and atorvastatin at various dosages: Investigating differential effects among statins. Curr Med Res Opin. 2008;24:2477–87. [DOI] [PubMed] [Google Scholar]
- 37. Zhang H, Zhang Q, Li S, Xie B. Simvastatin is efficacious in treating cirrhosis: A meta-analysis. J Clin Gastroenterol. 2022;56:E303–12. [DOI] [PubMed] [Google Scholar]
- 38. Kaplan DE, Serper MA, Mehta R, Fox R, John B, Aytaman A, et al. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology. 2019;156:1693–706.e12. [DOI] [PubMed] [Google Scholar]
- 39. Gu Y, Yang X, Liang H, Li D. Comprehensive evaluation of effects and safety of statin on the progression of liver cirrhosis: A systematic review and meta-analysis. BMC Gastroenterol. 2019;19:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sung S, Al-Karaghouli M, Kalainy S, Cabrera Garcia L, Abraldes JG. A systematic review on pharmacokinetics, cardiovascular outcomes and safety profiles of statins in cirrhosis. BMC Gastroenterol. 2021;21:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cai T, Abel L, Langford O, Monaghan G, Aronson JK, Stevens RJ, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: Systematic review with pairwise, network, and dose-response meta-analyses. BMJ. 2021;374:n1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vargas JI, Arrese M, Shah VH, Arab JP. Use of statins in patients with chronic liver disease and cirrhosis: Current views and prospects. Curr Gastroenterol Rep. 2017;19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–76. [DOI] [PubMed] [Google Scholar]
- 44. Genesca J, Gonzalez A, Segura R, Catalan R, Marti R, Varela E, et al. Interleukin-6, nitric oxide, and the clinical and hemodynamic alterations of patients with liver cirrhosis. Am J Gastroenterol. 1999;94:169–77. [DOI] [PubMed] [Google Scholar]
- 45. Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249–64. [DOI] [PubMed] [Google Scholar]
- 46. Kurzepa J, Mdro A, Czechowska G, Kurzepa J, Celiński K, Kazmierak W, et al. Role of MMP-2 and MMP-9 and their natural inhibitors in liver fibrosis, chronic pancreatitis and non-specific inflammatory bowel diseases. Hepatobiliary Pancreat Dis Int. 2014;13:570–9. [DOI] [PubMed] [Google Scholar]
- 47. Calabro SR, Maczurek AE, Morgan AJ, Tu T, Wen VW, Yee C, et al. Hepatocyte produced matrix metalloproteinases are regulated by CD147 in liver fibrogenesis. PLoS One. 2014;9:e90571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gadd VL, Patel PJ, Jose S, Horsfall L, Powell EE, Irvine KM. Altered peripheral blood monocyte phenotype and function in chronic liver disease: Implications for hepatic recruitment and systemic inflammation. PLoS One. 2016;11:e0157771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cardoso CC, Matiollo C, Pereira CHJ, Fonseca JS, Alves HEL, da Silva OM, et al. Patterns of dendritic cell and monocyte subsets are associated with disease severity and mortality in liver cirrhosis patients. Sci Rep. 2021;11:5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang CY, Tseng KC, Lin MN, Tsai JP, Su CC. Plasma levels of matrix metalloproteinase-2 and -9 in male and female patients with cirrhosis of different aetiologies. J Clin Pathol. 2015;68:917–22. [DOI] [PubMed] [Google Scholar]
- 51. Tilg H. Cytokines and liver diseases. Can J Gastroenterol. 2001;15:661–8. [DOI] [PubMed] [Google Scholar]
- 52. Duan Y, Pan X, Luo J, Xiao X, Li J, Bestman PL, et al. Association of inflammatory cytokines with non-alcoholic fatty liver disease. Front Immunol. 2022;13:880298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Solé C, Solà E, Morales-Ruiz M, Fernàndez G, Huelin P, Graupera I, et al. Characterization of inflammatory response in acute-on-chronic liver failure and relationship with prognosis. Sci Rep. 2016;6:32341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zanetto A, Pelizzaro F, Campello E, Bulato C, Balcar L, Gu W, et al. Severity of systemic inflammation is the main predictor of ACLF and bleeding in individuals with acutely decompensated cirrhosis. J Hepatol. 2023;78:301–11. [DOI] [PubMed] [Google Scholar]
- 55. Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842–54. [DOI] [PubMed] [Google Scholar]
- 56. Mahmud N, Chapin S, Goldberg DS, Reddy KR, Taddei TH, Kaplan DE. Statin exposure is associated with reduced development of acute-on-chronic liver failure in a Veterans Affairs cohort. J Hepatol. 2022;76:1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Privitera G, Spadaro L, Marchisello S, Fede G, Purrello F. Abnormalities of lipoprotein levels in liver cirrhosis: Clinical relevance. Dig Dis Sci. 2018;63:16–26. [DOI] [PubMed] [Google Scholar]
- 58. Zhu YC, Jiang XZ, Bai QK, Deng SH, Zhang Y, Zhang ZP, et al. Evaluating the efficacy of atorvastatin on patients with carotid plaque by an innovative ultrasonography. J Stroke Cerebrovasc Dis. 2019;28:830–7. [DOI] [PubMed] [Google Scholar]
- 59. Li JF, Qu F, Zheng SJ, Ren F, Wu HL, Liu M, et al. Plasma sphingolipids: Potential biomarkers for severe hepatic fibrosis in chronic hepatitis C. Mol Med Rep. 2015;12:323–30. [DOI] [PubMed] [Google Scholar]
- 60. Sharma R, Simon TG, Hagström H, Lochhead P, Roelstraete B, Söderling J, et al. Statins are associated with a decreased risk of severe liver disease in individuals with non-cirrhotic chronic liver disease. Clin Gastroenterol Hepatol. 2023;S1542-3565:00317–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.