Abstract
Cervical cancer constitutes a significant health burden for women globally. While most patients with early-stage disease can be cured with radical surgery or chemoradiotherapy, patients with high-risk locally advanced disease or with recurrent/metastatic disease have a poor prognosis with standard treatments. Immunotherapies are a rational treatment for this HPV-driven cancer that commonly expresses programmed cell death ligand-1. Before 2021, pembrolizumab was the only United States Food and Drug Administration-approved immunotherapy in cervical cancer, specifically for the second-line recurrent or metastatic (r/m) setting. In late 2021, the antibody-drug conjugate tisotumab vedotin was approved for second-line r/m cervical cancer and pembrolizumab combined with chemotherapy ± bevacizumab was approved for first-line r/m disease based on results from KEYNOTE-826. Moreover, with at least 2 dozen additional immunotherapy clinical trials in the second-line and first-line r/m setting, as well as in locally advanced disease, the treatment landscape for cervical cancer may eventually encounter a potential paradigm shift. Pivotal trials of immunotherapies for cervical cancer that were recently approved or with the potential for regulatory consideration through 2024 are reviewed. As immunotherapy has the opportunity to establish new standards of care in the treatment of cervical cancers, new biomarkers to identify the ideal patient populations for these therapies may also become important. However, issues with access, affordability, and compliance in low- and middle-income countries are anticipated.
Keywords: Cervical cancer, Immunotherapy, Metastatic, Locally advanced
Introduction
Cervical cancer is preventable and treatable, yet remains a significant global health burden. In 2020, it was the fourth most diagnosed cancer and the fourth leading cause of cancer-related deaths in women [1]. The burden of disease is disproportionally felt by low- and middle-income countries (LMICs), with highest incidence and mortality rates occurring in Africa, Melanesia, South America, South East Asia, and South Central Asia (age-standardized rate per 100,000: incidence, 15.3–40.1; mortality, 7.8–28.6) [1]. By 2070, effective implementation of screening, HPV vaccination, and scale-up of treatment facilities are estimated to reduce the mortality rate by up to 92% in LMICs [2]. In most developed countries, screening and vaccination (note that Japan suspended recommendation of HPV vaccination in 2013 and reinstated it in 2022 [3]) have reduced the incidence of cervical cancer and patients are often treated at early stages with high survival rates. However, socioeconomic factors may contribute to disparity of cervical cancer incidence and mortality within developed nations.
Appropriate staging is important for diagnosis, with the International Federation of Gynecology and Obstetrics (FIGO) 2018 recommendations considered the standard internationally (Fig. 1) [4]. Early-stage disease comprises small tumors limited to the cervix or with minimal invasion beyond the uterus (stage IA-IB2, IIA1). Locally advanced cervical cancer (LACC; stage IB3, IIA2-IVA) includes larger tumors with invasion spanning to adjacent tissues and pelvic organs and any size tumor with lymph node involvement. Metastatic spread to distant organs is stage IVB. In many countries, cervical cancer is mainly diagnosed at stage I/II [5–9]; however, some countries (eg, LMICs) report the majority of diagnoses at stage III/IV [9–12]. Metastatic disease at initial diagnosis is rare (~2% of cases) [4]; it more commonly presents as recurrence in the pelvic or para-aortic regions after treatment for earlier-stage disease.
Fig. 1.

FIGO 2018 staging system for cervical cancer. *Early-stage cervical cancer includes stage IA1-IB2 and IIA1, and locally advanced disease includes stages IB3 and IIA2–IVA. Adapted from Table 1 in Bhatla et al, 2018 [4].
For patients with recurrent or metastatic cervical cancer (r/mCC) who cannot receive curative intent surgery or radiotherapy, platinum-based chemotherapy with bevacizumab, if not contraindicated, is standard [13,14]. This regimen provides a median overall survival (OS) of 14.3–18.3 months [15,16], and, until recently, there was little benefit with second-line systemic therapies.
Patients with LACC treated with curative intent have a better prognosis than those with r/mCC. Chemoradiotherapy (CRT) became the standard of care for LACC in 1999, providing significant improvement in disease-free survival (DFS) and OS over radiotherapy alone [17]; intracavitary brachytherapy following CRT further improved outcomes and is considered a critical component of CRT [4,18]. Despite definitive CRT, patients with LACC experience 5-year DFS and OS of 47–80% [19–21], with poorer survival for stage IIIB/IVA or with nodal involvement [21,22]. Recent attempts to enhance CRT have experienced setback; addition of adjuvant chemotherapy to CRT did not improve survival in the phase III OUTBACK trial [23].
Immunotherapy is a promising emerging option supported by multiple findings. First, nearly all cases of cervical cancer arise after persistent infection with a high-risk HPV subtype [24]. Integration of HPV E6 and E7 viral oncoproteins into the cellular genome allows for their unregulated expression, creating a conducive environment for further genetic mutations related to tumor cell survival and immune escape [25]. This immune system suppression is a key step in development of cervical cancer. Second, programmed cell death ligand-1 (PD-L1) is widely expressed in the tumor microenvironment [26]. Third, the presence or absence of tumor-infiltrating immune cells has prognostic significance in cervical cancer [27]. Finally, with respect to treatment for LACC, radiotherapy increases antigen generation and presentation, T-cell priming, dendritic activation, as well as levels of pro-inflammatory cytokines [28], and small clinical trials of radiotherapy-based treatment combined with immunotherapy show promise [29,30].
Immunotherapies that directly target cervical cancer and provide innate antitumor effects as well as those that function to reactivate the immune system against cervical tumors are under investigation. In June 2018, pembrolizumab was the first immunotherapy to receive US Food and Drug Administration (FDA) accelerated approval as second-line treatment for patients with PD-L1-positive persistent or r/mCC. In 2021, two additional FDA approvals were granted: tisotumab vedotin for second-line r/mCC and pembrolizumab combined with chemotherapy ± bevacizumab for first-line PD-L1-positive persistent or r/mCC. With at least 2 dozen additional immunotherapies in clinical trials (Table 1) [31–39], the treatment landscape for cervical cancer is on the verge of a paradigm shift. Multiple compounds are in various stages of development that examine the potential for improvement in efficacy and safety in different treatment lines and cervical cancer stages. With many of these in early stages, specific advantages and risks for these treatments remain to be seen. Here we review pivotal trials of immunotherapies that were recently approved or have the potential for regulatory consideration through 2024 (Fig. 2) [39,40] and discuss biomarkers and histologic type in relation to immunotherapy, as well as anticipated challenges.
Table 1.
Risks and benefits of selected therapies under investigation for treatment of locally advanced cervical cancer.
| Therapy | Class/Target Mechanism of action | Cervical Cancer Stage | Previous Therapies | Clinical Phase | Additional Insights |
|---|---|---|---|---|---|
| Balstimab | PD-1 | r/m SCC, AC, or ASC cervical cancer | Failed prior platinum-based chemotherapy | II | Targets PD-1/PD-L1 CTLA-4 axis Hypothyroidism, diarrhea, fatigue most common trAEs [35] |
| Cadonilimab | PD-1 and CTLA-4 | r/m SCC or ASC cervical cancer | Up to 2 prior systemic therapies | II | Targets PD-1/PD-L1 CTLA-4 axis Bispecific antibody may affect toxicity |
| Anemia, hypothyroidism, alanine aminotransferase increase most common trAEs [39] | |||||
| Camrelizumab | PD-1 | r/m SCC, AC, or ASC cervical cancer | Relapsed after platinum-based chemotherapy regimen | II | Targets PD-1/PD-L1 CTLA-4 axis |
| Cemiplimab | PD-1 | r/m or persistent SCC, AC, or ASC cervical cancer | Prior platinum-based regimen | III | Targets PD-1/PD-L1 CTLA-4 axis Fatigue, nausea, anemia, asthenia, and decreased appetite most common trAEs [37] |
| Gepatanolimab | PD-1 | r/m PD-L1-positive cervical cancer | Progressed or had recurrence after platinum-based chemotherapy | II | Targets PD-1/PD-L1 CTLA-4 axis |
| Nivolumab | PD-1 | Virus-associated cancers (including r/m SCC cervical cancer with HPV status positive or unknown) | Progressed after up to 2 prior systemic therapies | I/II | Targets PD-1/PD-L1 CTLA-4 axis |
| Pembrolizumab | PD-1 | r/m or persistent SCC, AC, or ASC cervical cancer | Untreated | III | Targets PD-1/PD-L1 CTLA-4 axis AEs included hypothyroidism and decreased white cell count in treatment arm [32] |
| High-risk SCC, AC, or ASC cervical cancer, FIGO 2014 stage IB2-IIB node positive or stage III-IVA any node status | Untreated | III | Common trAEs of concern were diarrhea, nausea, and vomiting [33] | ||
| Prolgolimab | PD-1 with FC silencing LALA mutation | r/m or persistent SCC cervical cancer | Untreated | III | Targets PD-1/PD-L1 CTLA-4 axis LALA mutation may reduce FcyRI, IIa, IIIa binding, and improve efficacy [38] |
| Sintilimab | PD-1 | r/m cervical cancer | Relapsed after platinum-based chemotherapy regimen | II | Targets PD-1/PD-L1 CTLA-4 axis |
| Zimberelimab | PD-1 | PD-L1-positive r/m cervical cancer | Progressed on ≥ 1 chemotherapy or are resistant to chemotherapy | II | Targets PD-1/PD-L1 CTLA-4 axis |
| Atezolizumab | PD-L1 | PD-L1-positive r/m or persistent SCC, AC, or ASC cervical cancer | Progressed after 1–2 prior systemic chemotherapy regimens | II | Targets PD-1/PD-L1 CTLA-4 axis |
| r/m or persistent SCC, AC, or ASC cervical cancer | Untreated | III | |||
| Durvalumab | PD-L1 | SCC, AC, or ASC cervical cancer, FIGO 2009 Stage IB2-IIB node positive or IIIA-IVA any node status | Untreated | III | Targets PD-1/PD-L1 CTLA-4 axis |
| IBI-310 | CTLA-4 | r/m cervical cancer | Relapsed after platinum-based chemotherapy regimen | II | Targets PD-1/PD-L1 CTLA-4 axis |
| Ipilimumab | CTLA-4 | Virus-associated cancers (including r/m SCC cervical cancer with HPV status positive or unknown | Progressed after up to 2 prior systemic therapies | I/II | Targets PD-1/PD-L1 CTLA-4 axis |
| Zafrelimab | CTLA-4 | r/m SCC, AC, or ASC cervical cancer | Failed a prior platinum-based chemotherapy regimen | II | Targets PD-1/PD-L1 CTLA-4 axis Hypothyroidism, diarrhea, fatigue most common trAEs [35] |
| Famitinib | VEGFR-2, PDGFR, c-kit, FGFR (small molecule rTKIrTKI) (list targets) [36] | r/m SCC, AC, or ASC cervical cancer | Relapsed after platinum-based chemotherapy regimen | II | Unique targets Multiple targets and lack of specificity may cause off-target or unanticipated effects |
| Lifileucel | Autologous tumor infiltrating lymphocytes | r/m, or persistent SCC, AC, or ASC cervical cancer | ≥1 prior systemic therapy | II | Unique mechanism of action Most common grade 3/4 AEs included anemia, thrombocytopenia, neutropenia, and febrile neutropenia [34] |
| Tiragolumab | TIGIT | PD-L1-positive r/m or persistent SCC, AC, or ASC cervical cancer | Progressed after 1–2 prior systemic chemotherapy regimens | II | Unique target |
| Tisotumab vetodin | Antibody drug conjugate targeting tissue factor, with tubulin inhibitor payload | r/m SCC, AC, or ASC cervical cancer | up to 2 prior systemic treatments, which included platinum-based chemotherapy +/− bevacizumab | II | Unique target and mechanism Most common trAEs include alopecia, epistaxis, nausea, conjunctivitis, fatigue, dry eye [31] |
| Z-100 | Immunomodulator (bacterial extract) | SCC cervical cancer, FIGO 2008 Stage IIIB | Untreated | III | Unique mechanism |
AC, adenocarcinoma; ASC, adenosquamous carcinoma; c-kit, stem cell factor receptor; CRT, chemoradiotherapy; FcγR, FC gamma receptor; FGFR, fibroblast growth factor receptor; FIGO, International Federation of Gynecology and Obstetrics; PDGFR, platelet-derived growth factor receptor; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; r/m, recurrent/metastatic; SCC, squamous cell carcinoma; rTKI, receptor tyrosine kinase inhibitor; TIGIT, T-cell immunoreceptor with immunoglobulin and ITIM domains; trAEs, treatment-related adverse events; VEGFR-2, vascular endothelial growth factor receptor 2.
Fig. 2.
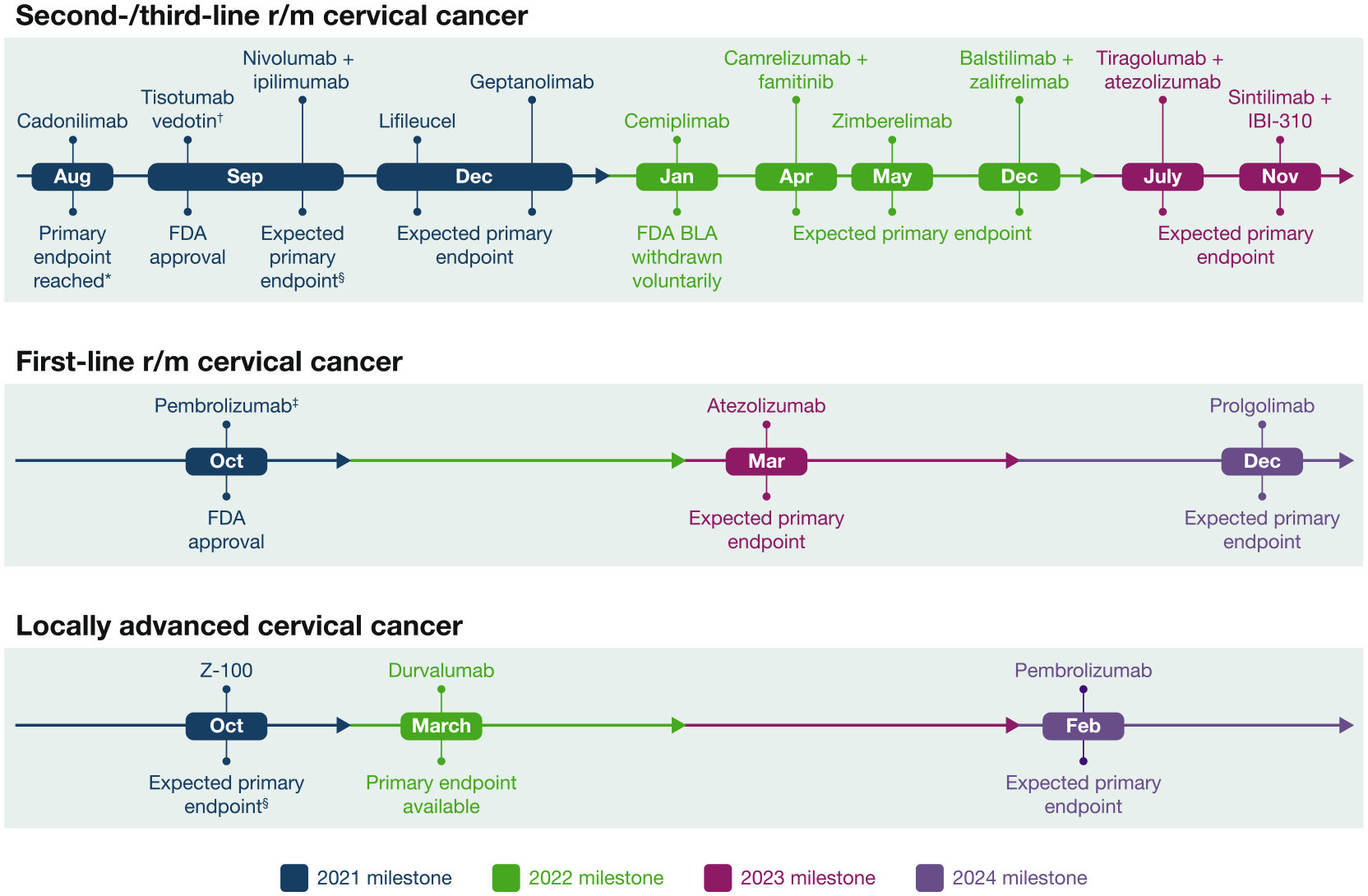
Health authority decisions and anticipated pivotal trial data for cervical cancer immunotherapies 2021–2024. *The cadonilimab primary endpoint data were released in an Akesobio investor presentation [40] and were presented at an academic congress [39]. †Tistumab vedotin monotherapy was given accelerated approval for treatment of adult patients with r/m cervical cancer that progressed on or after chemotherapy. §Final primary endpoint data have not been disclosed by the study sponsor nor presented at an academic congress or published in a peer-reviewed journal. ‡Pembrolizumab in combination with chemotherapy and with or without bevacizumab was approved for patients with untreated r/m or persistent cervical cancer that expresses PD-L1 (CPS ≥ 1%). BLA, biologic license application; CPS, combined positive score; FDA, United States Food and Drug Administration; PD-L1, programmed cell death ligand-1; r/m, recurrent/metastatic.
Key clinical trials of immunotherapy for cervical cancer
Second-line therapies for recurrent or metastatic cervical cancer
Numerous immunotherapies or immunotherapy combinations are under evaluation for second-line or greater treatment of r/mCC, with one antibody-drug conjugate FDA-approved in September 2021 (Table 2) [31,34,37,39,41–45].
Table 2.
Selected therapies under investigation for second-line or later treatment of recurrent/metastatic cervical cancer.
| Therapy | Mechanism of action | Cervical cancer-related health authority designations/decisions | Pivotal trial name/IDs | Study design | Patient population | Treatment arms | Primary endpoint(s) | Primary endpoint completion date | Available pivotal trial efficacy data |
|---|---|---|---|---|---|---|---|---|---|
| Tisotumab vedotin | Antibody drug conjugate targeting tissue factor, with tubulin inhibitor payload | FDA accelerated approval September 2021 [44] | innovaTV 204/GOG-3023/ENGOT-cx6 NCT03438396 |
Phase II Open-label Single Arm |
Adults with r/m SCC, AC, or ASC cervical cancer who had up to 2 prior systemic treatments, which included platinum-based chemotherapy +/− bevacizumab | Tisotumab vedotin monotherapy 2.0 mg/kg IV Q3W | ORR by IRC per RECIST v1.1 | June 2020 | Coleman, et al 2021:[31] (N = 101) Median 10.0 months F/U ORR, 24% DCR, 72% |
| Cemiplimab | Anti-PD-1 antibody | FDA BLA voluntarily withdrawn January 2022 [43] | EMPOWER-CERVICAL 1/GOG-3016/ENGOT-cs9 NCT03257267 |
Phase III Open-label Randomized 1:1 |
Adults with r/m or persistent SCC, AC, or ASC cervical cancer who had a prior platinum-based regimen | Experimental arm: Cemiplimab monotherapy 350 mg IV Q3W Comparator arm: Investigator’s choice chemotherapy (IC) |
OS in SCC population OS in overall population |
January 2021 | Tewari, et al 2022:[37] SCC population (N = 477) Cemiplimab arm OS, 11.1 months IC arm OS, 8.8 months HR 0.73; P = 0.006 Overall population (N = 608) Cemiplimab arm OS, 12.0 months IC arm OS, 8.5 months HR, 0.69; P < 0.001 |
| Cadonilimab (AK104) | Anti-PD-1/CTLA-4 bispecific antibody | FDA fast-track therapy 2020; China NMPA breakthrough therapy 2020; FDA orphan drug 2021 NDA submitted to China NMPA September 2021, with priority review |
AK104–201-AU NCT04380805 |
Phase II Open-label Single Arm |
Adults with r/m SCC, AC or ASC cervical cancer who had up to 2 prior systemic therapies | Cadonilimab monotherapy 6 mg/kg IV Q2W | ORR by IRC | August 2021 | SGO 2022[39] Overall population (N = 100) ORR, 33% CR, 12% PD-L1 + population (N = 64) ORR 44% |
| Nivolumab + ipilimumab | Anti-PD-1 antibody and an anti-CTLA-4 antibody | None | CheckMate358 NCT02488759 |
Phase I/II Open-label Multiple cohort Randomized |
Adults with virus-associated cancers (including r/m SCC cervical cancer with HPV status positive or unknown that progressed after up to 2 prior systemic therapies) [41] | Combo A: Nivolumab 3 mg/kg Q2W + ipilimumab 1 mg/kg Q6W Combo B: Nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3W for 4 doses, then nivolumab 240 mg Q2W |
ORR by investigator assessment | Expected September 2021 | ESMO 2019: [42] No PST Combo A (n = 19); ORR, 32% Combo B (n = 24); ORR, 46% With PST Combo A (n = 26); ORR, 23% Combo B (n = 22); ORR, 36% |
| Geptanolimab (GB226) | Anti-PD-1 antibody | None | Gxplore-008 NCT03808857 |
Phase II Open-label Single Arm |
Adults with r/m PD-L1-positive cervical cancer who progressed or had recurrence after platinum-based chemotherapy | Geptanolimab monotherapy 3 mg/kg infusion Q2W | ORR | Expected December 2021 | None |
| Lifileucel (LN-145) | Autologous tumor infiltrating lymphocytes | FDA orphan drug 2018; FDA breakthrough therapy 2019 | innovaTIL-04 NCT03108495 |
Phase II Open-label Multiple cohorts Nonrandomized |
Adults with r/m, or persistent SCC, AC, or ASC cervical cancer who had ≥ 1 prior systemic therapy Cohort 1: PD with 1–3 prior chemotherapies +/− bevacizumab Cohort 2: Same as Cohort 1, plus must have received prior ICI therapy Cohort 3 (US only): Only received prior CRT or surgery for locoregional disease; not treated with prior immunotherapy Cohort 4: Did not meet criteria for Cohorts 1 and 2 Cohort 5: Pts progressed on previous LN-145 treatment |
Adoptive cell transfer of autologous TILs Cohort 1, 2, 4: After non-myeloablative lymphodepletion (NMA), pts receive LN-145, then IL-2 Cohort 3: Pembro, then NMA, then pts receive LN-145 followed by IL-2; pembro – 3 wks after IL-2, for up to 24 months Cohort 5: pts receive a second treatment with LN-145 |
Cohort 1 and 2: ORR by IRC per RECIST v1.1 Cohort 3: Safety of pembro combo Cohort 4: Efficacy and safety Cohort 5: Efficacy and safety |
Expected December 2021 | ASCO 2019:* [34] (N = 27) Median F/U 7.4 months ORR, 44% DCR, 85% |
| Camrelizumab + famitinib | Anti-PD-1 antibody and a small molecule rTKI | China NMPA breakthrough therapy 2020 | SHR-1210-II-217 NCT04680988 |
Phase II Open-label Multiple arms Randomized |
Adults with r/m SCC, AC, or ASC cervical cancer who relapsed after platinum-based chemotherapy regimen | Arm 1: Camrelizumab IV Q3W + famitinib orally once daily Arm 2: Camrelizumab IV Q3W Arm 3 (active comparator): Investigator’s choice of: albumin-bound paclitaxel, pemetrexed, or gemcitabine |
PFS per RECIST v1.1 of Arm 1 vs Arm 2 OS of Arm 1 vs Arm 3 |
Expected April 2022 | None |
| Zimberelimab (GLS-010) | Anti-PD-1 antibody | China NMPA breakthrough therapy 2021 | YH-S001–05 NCT03972722 |
Phase II Open-label Single arm |
Adults with PD-L1-positive r/m cervical cancer who progressed on ≥ 1 chemotherapy or are resistant to chemotherapy | Zimberelimab monotherapy 240 mg Q2W | ORR by IRC per RECIST v1.1 | Expected May 2022 | IGCS 2020: [45] (N = 41) Median 5.2 months F/U ORR, 27% DCR, 54% |
| Balstilimab + zalifrelimab | Anti-PD-1 antibody and an anti-CTLA-4 antibody | FDA fast-track therapy 2020 | RaPiDS NCT03894215 |
Phase II Blinded Noncomparative Randomized 1:1 |
Adults with r/m SCC, AC, or ASC cervical cancer who failed a prior platinum-based chemotherapy regimen | Arm 1: Balstilimab 300 mg Q3W + placebo Arm 2: Balstilimab 300 mg Q3W + zalifrelimab 1 mg/kg Q6W |
ORR by IRC per RECIST v1.1 | Expected December 2022 | None |
| Tiragolumab + atezolizumab | Anti-TIGIT antibody and an anti-PD-L1 antibody | None | SKYSCRAPER- 04 NCT04300647 |
Phase II Open-label Parallel-cohort Randomized 3:1 |
Adults with PD-L1-positive r/m or persistent SCC, AC, or ASC cervical cancer who progressed after 1 −2 prior systemic chemotherapy regimens | Arm 1: Tiragolumab 600 mg IV Q3W + atezolizumab 1200 mg IV Q3W Arm 2: Atezolizumab monotherapy 1200 mg IV Q3W |
ORR by IRC | Expected July 2023 | None |
| Sintilimab + IBI-310 | Anti-PD-1 antibody and an anti-CTLA-4 antibody | None | CIBI310E201 NCT04590599 |
Phase II Double-blind Parallel-cohort Randomized |
Adults with r/m cervical cancer who relapsed after platinum-based chemotherapy regimen | Arm 1: Sintilimab 200 mg + placebo Arm 2: Sintilimab 200 mg + IBI-310 |
ORR by IRC per RECIST v1.1 | Expected November 2023 | None |
Cohorts were not specified in the ASCO poster or abstract; however, there were 4 patients who received prior anti-PD-1/PD-L1 therapy, suggesting a mix of cohorts is represented.
Trial design and anticipated primary endpoint completion dates were obtained from the respective clinicaltrials.gov webpages. Therapies are listed in order of primary endpoint completion date.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; ASCO, American Society of Clinical Oncology; BLA, biologic license application; CRT, chemoradiation; CR, complete response; DCR, disease control rate; ESMO, European Society for Medical Oncology; FDA, United States Food and Drug Administration; ICI, immune checkpoint inhibitor; IGCS, International Gynecologic Cancer Society; IRC, independent review committee; NMPA, China’s National Medical Products Administration; ORR, objective response rate; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; PST, prior systemic therapies; pts, patients; RECIST, Response Evaluation Criteria in Solid Tumors; rTKI, receptor tyrosine kinase inhibitor; SCC, squamous cell carcinoma; SGO, Society of Gynecologic Oncology; TIGIT, T-cell immunoreceptor with immunoglobulin and ITIM domains.
Cadonilimab
Cadonilimab (AK104) is a first-in-class anti-programmed cell death-1 (PD-1)/cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) bispecific monoclonal antibody being studied as monotherapy for patients with r/mCC after failed platinum-based chemotherapy. It received FDA fast-track and orphan drug designation as well as China’s National Medical Products Administration (NMPA) breakthrough therapy designation [46]. The pivotal trial is a phase II, single arm study of cadonilimab monotherapy in patients with previously treated r/mCC. Results showed 33.0% objective response rate (ORR) in 100 patients treated with cadonilimab (12.0% complete response) [39]. Patients considered PD-L1-positive (n = 64) had an ORR of 43.8%. Unpublished results from an August 2021 Akesobio investor meeting [40] were submitted in a new drug application to the China NMPA in September 2021 [47]. A second registrational trial in China (NCT04868708) is anticipated to complete its primary endpoint in April 2022 and a phase III trial in China of cadonilimab versus placebo combined with platinum-based chemotherapy ± bevacizumab for first-line persistent or r/mCC started in July 2021 (NCT04982237).
Cemiplimab
Cemiplimab is a high-affinity, hinge-stabilized, human anti-PD-1 IgG4 monoclonal antibody [48]. EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 is a phase III, open-label, randomized study of cemiplimab versus investigator’s choice chemotherapy in 608 patients with r/mCC that progressed after first-line platinum-based treatment. The trial stopped early due to the significant benefit of cemiplimab therapy. After discussions with the FDA regarding additional post-marketing studies, cemiplimab company sponsors voluntarily withdrew an FDA biologic license application in January 2022, though regulatory submissions outside of the US are being considered [43].
Median OS in the overall population (N = 304 for both arms) was 12.0 months with cemiplimab vs 8.5 months with chemotherapy (hazard ratio [HR], 0.69; P < 0.001) and ORR was 16.4% cemiplimab vs 6.3% chemotherapy [37]. Progression-free survival (PFS) in the overall and squamous cell carcinoma (SCC) populations also favored cemiplimab (2.8 vs 2.9 months for both populations; overall: HR, 0.75, P < 0.001; SCC: HR, 0.71, P < 0.001). Prespecified subgroup analysis favored cemiplimab over chemotherapy regardless of histology or Eastern Cooperative Oncology Group status, as well as for patients from Asia or regions other than North America, for those who had prior bevacizumab, or who received 1 prior line of therapy for r/mCC.
Treatment-related adverse events (AEs) occurred in 56.7% of patients receiving cemiplimab (n = 300) vs 81.4% receiving chemotherapy (n = 290), and no new toxicities were observed [37]. While the rate of treatment-related AEs of ≥ grade 3 was lower for cemiplimab (14.7% vs 40.3%), discontinuations due to any grade treatment-related AEs were more frequent (5.7% vs 3.4%). In both overall and SCC populations, treatment with cemiplimab resulted in statistically significant improvement in patient-reported global health status/quality of life and physical functioning scores versus chemotherapy, as well as clinically meaningful benefit in role functioning, pain, and appetite loss [37].
Geptanolimab
Geptanolimab (GB226) is an anti-PD-1 monoclonal antibody. The ongoing phase II pivotal trial, Gxplore-008, is evaluating the efficacy, safety, and immunogenicity of geptanolimab for second-line or later treatment of patients with PD-L1-positive r/mCC. Approximately 80 patients from China will be enrolled. The primary endpoint is ORR, which is estimated for completion in December 2021.
Zimberelimab
Zimberelimab (GLS-010) is a novel, fully-human anti-PD-1 monoclonal antibody being investigated as monotherapy for patients with PD-L1-positive (combined positive score [CPS] ≥ 1) r/mCC that failed ≥ 1 prior chemotherapy regimen. It received China’s NMPA breakthrough therapy designation in March 2021 [49]. The phase II registrational trial is enrolling approximately 89 patients, and primary endpoint (ORR) completion is expected in May 2022. In an interim analysis of 41 evaluable patients (April 2, 2020; median follow-up 5.2 months), investigator-assessed ORR was 27% and median DoR was not reached [45]. Treatment-related AEs ≥ grade 3 were experienced by 17 of 45 patients (38%), and 1 patient discontinued due to an AE [45].
Balstilimab + zalifrelimab
Balstilimab (bal) is a fully-human anti-PD-1 antibody. Zalifrelimab (zal) is a fully-human anti-CTLA-4 antibody. The bal + zal combination received FDA fast-track designation for r/mCC in March 2020. In final results from the phase II study, C-550–01, the ORR and median DoR were 25.6% and not reached for 125 patients who relapsed after one prior platinum-based treatment for r/mCC [35]. At a median follow up of 21.0 months, median PFS and OS were 2.7 and 12.8 months. Hypothyroidism (16.8%), diarrhea (14.2%), and fatigue (11.6%) were the most frequent treatment-related AEs, and 20% of patients experienced ≥ grade 3 treatment-related AEs. Toxicities were manageable as only 12.3% and 7.7% of patients had a treatment-related AE resulting in dose interruption or discontinuation.
RaPiDS is the ongoing pivotal, phase II randomized trial assessing the safety and efficacy of second-line bal monotherapy and bal + zal in patients with previously treated r/mCC. Approximately 200 patients from the United States, Mexico, Korea, Taiwan, and Thailand will be enrolled, and the trial is expected to complete in December 2022. The primary endpoint is ORR.
Nivolumab + ipilimumab
Combination therapy with anti-PD-1 antibody nivolumab, and anti-CTLA-4 antibody ipilimumab, is approved for treatment of several solid tumors [50]. In the Checkmate 358 study, nivolumab was tested alone and in combination with other anticancer agents for treatment of virus-associated tumors. Interim results were presented at ESMO 2019 for 2 regimens of nivolumab + ipilimumab (combo A: nivolumab 3 mg/kg Q2W + ipilimumab 1 mg/kg Q6W; combo B: nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3W (four doses), then nivolumab 240 mg Q2W) in 91 patients with HPV + or HPV status unknown r/mCC who were untreated or had failed up to two prior systemic therapies [42]. Patients who were not previously treated had numerically higher ORRs with both regimens (combo A and B untreated: 32% and 46%; previously treated: 23% and 36%). This trend was also evident with median PFS and OS (PFS combo A and B untreated: 13.8 and 8.5 months; previously treated: 3.6 and 5.8 months; OS untreated: not reached for both; previously treated: 10.3 and 25.4 months). The safety profile of both combinations was consistent with other indications. Primary endpoint completion was expected September 2021, but the results have not yet been published.
Sintilimab + IBI-310
Sintilimab is a fully-human anti-PD-1 IgG4 monoclonal antibody. IBI-310 is a biosimilar of ipilimumab. The ongoing phase II pivotal, randomized, double-blind trial is evaluating efficacy and safety of sintilimab + IBI-310 versus sintilimab + placebo in patients with r/mCC who failed prior platinum-based chemotherapy. Approximately 174 patients from China will be enrolled with completion expected in November 2023. The primary endpoint is ORR.
Camrelizumab + famitinib
Camrelizumab is a humanized anti-PD-1 IgG4 monoclonal antibody. Famitinib is a receptor tyrosine kinase inhibitor targeting c-Kit, vascular endothelial growth factor receptor-2 and -3, platelet-derived growth factor receptor, FMS-like tyrosine kinase-3 receptor, and Ret [51,52]. The combination received China’s NMPA breakthrough therapy designation [53]. The ongoing phase II, pivotal, randomized trial (SHR-1210-II-217) is evaluating the efficacy and safety of camrelizumab alone or in combination with famitinib versus chemotherapy in patients with r/mCC who failed prior platinum-based treatment. Approximately 250 patients from China will be enrolled. The dual primary endpoints are PFS for camrelizumab + famitinib versus camrelizumab alone and OS for camrelizumab + famitinib versus chemotherapy. Primary endpoint completion is estimated for April 2022.
Tiragolumab + atezolizumab
The inhibitory receptor, TIGIT (T-cell immunoreceptor with immunoglobulin and ITIM domains), is involved in dampening adaptive and innate immune responses to tumors and is being pursued as an immunotherapy target for multiple solid tumors [54]. Its expression has been observed in cervical cancer tumor infiltrating lymphocytes (TILs) [55]. In the ongoing phase II, international SKYSCRAPER-04 study, the safety and efficacy of the combination of tiragolumab (anti-TIGIT antibody), with atezolizumab (anti-PD-L1 antibody), or atezolizumab monotherapy is being evaluated in patients with PD-L1-positive r/mCC that progressed after ≥ 1 chemotherapy regimen. Enrollment completed in July 2021 with 172 patients. The primary endpoint of ORR is expected in July 2023.
Lifileucel
Lifileucel (LN-145) is an adoptive cell therapy, whereby TILs are harvested from the patient, expanded ex vivo, then infused after non-myeloablative lymphodepletion. Lifileucel received FDA breakthrough designation in 2019 [56] based on interim data from the ongoing phase II innovaTIL-04 (C-145–04) trial, which evaluated the efficacy and safety of lifileucel in 27 patients with persistent or r/mCC who had at least one prior line of chemotherapy [34,56]. At median follow-up of 7.4 months, ORR was 44.4%, disease control rate (DCR) was 85.2%, and the median DoR was not reached. Adverse events were consistent with the underlying advanced disease, lymphodepletion, and IL-2 regimens. The most common grade 3/4 AEs included anemia (55.6%), thrombocytopenia (44.4%), neutropenia (29.6%), and febrile neutropenia (29.6%). AE frequency was highest within ~ 12 days of receiving treatment, and was fairly infrequent afterward [34]. Primary endpoint completion is expected in December 2021.
Tisotumab vedotin
Tisotumab vedotin (TV) is a tissue factor-directed antibody-drug conjugate that releases the microtubule-disrupting agent monomethyl auristatin E (MMAE) after internalization into the target cell, which leads to cell cycle arrest and apoptotic cell death. MMAE may also leave the target cell and enter and kill neighboring tumor cells, known as bystander cytotoxicity [31]. In September 2021, TV was granted FDA accelerated approval to treat patients with r/mCC with disease progression on or after chemotherapy [44]. This is the first approved antibody-drug conjugate for cervical cancer. Approval was based on the pivotal phase II, single arm innovaTV 204/GOG-3023/ENGOT-cx6 trial [31]. After 10 months’ median follow-up of 101 women with previously treated r/mCC, the confirmed ORR was 24% and median duration of response (DoR) was 8.3 months. The most common treatment-related adverse events included alopecia, epistaxis, nausea, conjunctivitis, fatigue, and dry eye. Treatment-related serious and grade ≥ 3 AEs occurred for 13% and 28% of patients, respectively. A confirmatory phase III trial of TV versus chemotherapy for second- or third-line r/mCC was initiated in February 2021 (NCT04697628).
TV is also under investigation in combination with pembrolizumab for previously-treated r/mCC in a phase II study (innovaTV 205/ENGOT-cx8/GOG-3024) [57]. After median follow-up of 13 months, confirmed response rate was 38% and median DoR was 13.8 months. Forty-six percent of patients who received TV + pembrolizumab reported TV-related ≥ grade 3 AEs.
First-line therapies for recurrent and metastatic cervical cancer
Three immune checkpoint inhibitors (ICIs) are being tested in combination with chemotherapy regimens ± bevacizumab in phase III trials, with pembrolizumab approved in October 2021 for first-line treatment of PD-L1-positive disease (Table 3) [32,58].
Table 3.
Selected therapies under investigation for first-line treatment of recurrent/metastatic cervical cancer.
| Therapy | Mechanism of action | Cervical cancer-related health authority designations/decisions | Pivotal trial name/IDs | Study design | Patient population | Treatment arms | Primary endpoint(s) | Primary endpoint completion date | Available pivotal trial efficacy data |
|---|---|---|---|---|---|---|---|---|---|
| Pembrolizumab | Anti-PD-1 antibody | FDA approval October 2021: in combination with chemotherapy in patients with PD-L1 positive tumors (CPS ≥ 1) [58] | KEYNOTE-826 NCT03635567 |
Phase III, double-blind, randomized 1:1 | Adults with r/m or persistent SCC, AC, or ASC cervical cancer not previously treated; known PD-L1 status prior to randomization | Experimental arm: Pembrolizumab 200 mg IV + investigator choice of platinum-based chemotherapy regimen (IC) on day 1 per 21-day cycle Control arm: Placebo + IC on day 1 per 21-day cycle |
PFS by ICR per RECIST v1.1 OS |
November 2022 | Colombo et al. 2021 [32] ITT population (N = 617) Median PFS Pembro arm 10.4 months Placebo arm 8.2 months HR, 0.65; P < 0.001 24-month OS Pembro arm 50.4% Placebo arm 40.4% HR, 0.67; P < 0.001 PD-L1 CPS ≥ 1 (N = 548) Median PFS Pembro arm 10.4 months Placebo arm 8.2 months HR, 0.62; P < 0.001 24-month OS Pembro arm 53% Placebo arm 41.7% HR, 0.64; P < 0.001 |
| Atezolizumab | Anti-PD-L1 antibody | None | BEATcc/ENGOT-cx10 NCT03556839 |
Phase III, open-label, randomized 1:1 | Adults with r/m or persistent SCC, AC, or ASC cervical cancer not previously treated | Experimental arm: atezolizumab 1200 mg + chemotherapy (cisplatin 50 mg/m2 or carboplatin AUC 5 + paclitaxel 175 mg/m2) + bevacizumab 15 mg/kg IV on day 1 per 21-day cycle Control arm: chemotherapy + bevacizumab 15 mg/kg IV on day 1 per 21-day cycle |
OS | Expected March 2023 | None |
| Prolgolimab (BCD-100) | Anti-PD-1 antibody | None | FERMATA/ENGOT-cx13 NCT03912415 |
Phase III, double-blind randomized 1:1 | Adults with r/m or persistent SCC cervical cancer not previously treated; known PD-L1 status prior to randomization | Experimental arm: prolgolimab 3 mg/kg IV Q3W + chemotherapy (cisplatin or carboplatin + paclitaxel) +/− bevacizumab Control arm: placebo + chemotherapy +/− bevacizumab |
OS | Expected December 2024 | None |
Trial design and anticipated primary endpoint completion dates were obtained from the respective clinicaltrials.gov webpages. Therapies are listed in order of primary endpoint completion date.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; ICR, independent central review; IV, intravenous; OS, overall survival; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors; SCC, squamous cell carcinoma.
Pembrolizumab
Pembrolizumab is an anti-PD-1 antibody approved for treatment of many malignancies, including for PD-L1-positive (CPS ≥ 1) r/mCC that progressed on or after chemotherapy [59]. In October 2021, the FDA approved pembrolizumab combined with platinum-based chemotherapy ± bevacizumab as first-line treatment for patients with PD-L1-positive persistent or r/mCC (CPS ≥ 1) based on the first interim analysis of the phase III KEYNOTE-826 study [58].
Patients were randomized to receive pembrolizumab 200 mg (N = 308) or placebo (N = 309) every 3 weeks for up to 35 cycles + chemotherapy, with bevacizumab used at the investigator’s discretion [32]. Coprimary endpoints of PFS and OS were tested sequentially in the PD-L1 CPS ≥ 1 population, the intention-to-treat (ITT) population, and the PD-L1 CPS ≥ 10 population. PFS was significantly longer in the pembrolizumab versus placebo arms in patients with PD-L1 CPS ≥ 1 (median 10.4 vs 8.2 months; HR, 0.62; P < 0.001), the ITT population (10.4 vs 8.2 months, respectively; HR, 0.65; P < 0.001), and the PD-L1 CPS ≥ 10 group (10.4 vs 8.1 months; HR, 0.58; P < 0.001). Likewise, OS at 24 months was significantly longer in the pembrolizumab versus placebo groups among the three populations.
The safety profile in KEYNOTE-826 was consistent with known profiles of the individual therapies [32]. Discontinuations due to AEs were slightly higher with pembrolizumab (any agent: pembrolizumab 37.5% vs placebo 26.5%; all agents: 5.9% vs 4.9%). Adverse events that occurred in at least 10% of patients in the pembrolizumab arm included hypothyroidism (pembrolizumab 18.2% vs placebo 9.1%) and decreased white cell count (12.1% vs 7.1%). Overall, combination treatment did not intensify known toxicities associated with each agent. Patient-reported quality of life was improved or stable for more patients who received pembrolizumab versus placebo (78.3% vs 71.7%).
Prolgolimab
Prolgolimab (BCD-100) is an anti-PD-1 antibody with an Fc-silencing LALA mutation that is approved in Russia for the treatment of unresectable or metastatic melanoma [60]. The efficacy and safety of prolgolimab versus placebo in combination with platinum-based chemotherapy (cisplatin or carboplatin + paclitaxel) ± bevacizumab in patients with untreated r/mCC is being investigated in the ongoing phase III FERMATA trial. The trial is being conducted in China, Georgia, Russia, and Turkey, and aims to enroll approximately 316 patients with SCC histology. The primary endpoint is OS, which is estimated for completion in December 2024.
Atezolizumab
The ongoing, pivotal phase III BEATcc/ENGOT-Cx10/GEICO-68-C/JGOG-1084/GOG-3030 study is evaluating atezolizumab versus placebo in combination with a platinum-based chemotherapy regimen (cisplatin or carboplatin + paclitaxel + bevacizumab) for patients with untreated r/mCC [61]. Approximately 404 patients from Europe, Japan, and the United States were planned for enrollment; recruitment was completed in September 2021. The primary endpoint is OS, which is estimated for completion in March 2023.
Locally advanced cervical cancer
Three immunotherapies in combination with CRT or radiotherapy are in phase III studies with primary endpoint data expected to be published between 2022 and 2024 (Table 4) [62].
Table 4.
Selected therapies under investigation for treatment of locally advanced cervical cancer.
| Therapy | Mechanism of action | Cervical cancer-related health authority designations/decisions | Pivotal trial name/IDs | Study design | Patient population | Treatment arms | Primary endpoint(s) | Primary endpoint completion date | Available pivotal trial efficacy data |
|---|---|---|---|---|---|---|---|---|---|
| Z-100 | Immunomodulator (bacterial extract) | None | Z100–01 NCT02247232 |
Phase III, double-blind randomized | Adults with SCC cervical cancer, FIGO 2008 stage IIIB, not previously treated | Experimental arm:* Z-100 + radiotherapy Control arm: Placebo + radiotherapy |
OS | Expected October 2021 Enrollment completed July 2018 |
None |
| Durvalumab | Anti-PD-L1 antibody | None | CALLA NCT03830866 |
Phase III, double-blind, randomized 1:1 | Adults with SCC, AC, or ASC cervical cancer, FIGO 2009 Stage IB2-IIB node positive or IIIA-IVA any node status, not previously treated | Experimental arm: Durvalumab 1500 mg IV Q4W for 24 cycles + CRT (EBRT + cisplatin 40 mg/m2 IV or carboplatin AUC2 IV, followed by image-guided brachytherapy) once weekly for 5 weeks Control arm: Placebo Q4W for 24 cycles + CRT once weekly for 5 weeks |
PFS by investigator assessment per RECIST v1.1 | Expected October 2022 Enrollment completed January 2021 |
Primary endpoint not met [62] |
| Pembrolizumab | Anti-PD-1 antibody | None | KEYNOTE-A18/ENGOT-cx11/GOG-3047 NCT04221945 |
Phase III, double-blind, randomized 1:1 | Patients with high-risk SCC, AC, or ASC cervical cancer, FIGO 2014 stage IB2-IIB node positive or stage III-IVA any node status, not previously treated | Experimental arm: 5 cycles of pembro 200 mg Q3W + CRT (EBRT + cisplatin 40 mg/m2 IV QW, followed by brachytherapy), followed by 15 cycles of pembro 400 mg Q6W Control arm: Placebo + CRT, followed by placebo Q6W |
PFS by investigator assessment per RECIST v1.1 OS |
Expected February 2024 | None |
Dosing information was not available on the respective clinicaltrials.gov information webpage.
Trial design and anticipated primary endpoint completion dates were obtained from the respective clinicaltrials.gov webpages. Therapies are listed in order of primary endpoint completion date.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; AUC, area under the curve; CRT, chemoradiotherapy; EBRT, external beam radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; IV, intravenous; OS, overall survival; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors; SCC, squamous cell carcinoma.
Pembrolizumab
The phase III KEYNOTE-A18/ENGOT-Cx11 study is evaluating pembrolizumab versus placebo given during and after CRT for treatment-naïve LACC [63]. Patients must have stage IB2-IIB lymph node positive disease or stage III-IVA disease ± lymph node involvement per FIGO 2014 staging criteria. Approximately 980 patients from 30 countries across North America, South America, Europe, Asia, and Australia will be enrolled. The co-primary endpoints are PFS per investigator assessment and OS, with final data expected in February 2024.
Early safety data from a small phase II study of pembrolizumab given either during or after CRT for LACC support the safety and feasibility of this combination [33]. Of 52 evaluable patients, 88% experienced ≥ grade 2 treatment-related AEs; 11 patients had at least one grade 4 AE, 23 had at least one grade 3 AE, and no grade 5 AEs were reported. Median follow-up was 4.8 months. Common AEs of clinical concern considered related to pembrolizumab included diarrhea, nausea, and vomiting. All patients who received pembrolizumab during CRT completed cisplatin therapy.
Durvalumab
Durvalumab is a human anti-PD-L1 IgG1ĸ monoclonal antibody approved for use in NSCLC and extensive-stage small cell lung cancer [64]. In locally advanced NSCLC, durvalumab given as consolidation therapy after CRT improved outcomes compared with placebo (median PFS: 16.8 months vs 5.6 months; ongoing response for 72.8% vs 46.8% of patients at 18 months) [65]. In the phase III CALLA study, the efficacy and safety of durvalumab versus placebo given during and after CRT was investigated in immunotherapy-naïve patients who had no prior treatment for LACC [66]. Patients had high-risk LACC, defined as stages IB2-IIB disease with lymph node involvement or stages IIIA-IVA ± lymph node involvement according to FIGO 2009 staging criteria. Enrollment completed in January 2021 with 770 patients from 15 countries across North America, South America, Europe, Asia, and Africa. In March 2022, it was announced that the trial did not achieve statistical significance for the primary endpoint of PFS [62].
Z-100
Z-100 is an immunomodulatory extract from Mycobacterium tuberculosis strain Aoyama B used in Japan to treat leukopenia resulting from radiotherapy [67]. In a preclinical mouse model, Z-100 in combination with radiation augmented the antitumor effects of X-ray irradiation by inhibiting pulmonary metastasis and prolonging survival [67]. Z-100 also improved helper T-cell response from a type 2-dominant to a type 1-dominant state via upregulation of interferon-γ and IL-12, suggesting the potential to suppress tumor metastasis in preclinical studies [68,69].
In an earlier placebo-controlled, phase III trial of Z-100 in combination with radiotherapy or CRT (collectively referred to as RT) for patients with stage IIB-IVA cervical cancer, Z-100 did not provide a statistically significant improvement of 5-year OS compared with placebo (75.7% vs 65.8%; HR, 0.65, P = 0.07), and did not differ in recurrence-free survival [70]. However, there was an OS benefit for patients with stage III disease treated with Z-100 (HR, 0.51; P = 0.03). A phase III, placebo-controlled trial of Z-100 plus radiotherapy for patients with treatment-naïve stage IIIB (FIGO 2008) LACC was initiated in December 2014. The study is being conducted in seven countries within Asia. The trial has completed enrollment and the expected date for primary endpoint completion (OS) was October 2021. Published results are anticipated.
Are current immunotherapy biomarkers important for cervical cancer?
PD-L1 expression is commonly found in cervical cancer, although it may be influenced by histology and etiology of disease [26,48]. For example, PD-L1-high SCC was more likely to be HPV-18 than HPV-16 driven. PD-L1 expression may also be prognostic of survival: patients with SCC and diffuse PD-L1 expression or PD-L1-negative disease had worse DFS versus marginal PD-L1 expression (P = 0.022 and P = 0.029), and adenocarcinoma (AC) with versus without PD-L1-positive tumor-associated macrophages was associated with worse disease-specific survival (P = 0.014) [26].
PD-L1 expression appears to be a biomarker for response to some ICIs, though responses are seen in PD-L1-negative tumors. Approval of pembrolizumab + chemotherapy ± bevacizumab for first-line treatment of PD-L1-positive r/mCC reflects the survival benefits driven by this patient population in KEYNOTE-826 [32]. In contrast to the phase II KEYNOTE-158 trial of pembrolizumab monotherapy in the second-line or later setting where all responses were observed in patients with PD-L1-expressing tumors [71], some of the 69 patients with PD-L1-negative tumors in KEYNOTE-826 responded to pembrolizumab. However, there was no clear benefit in PFS (HR, 0.94) or OS (HR, 1.00). Similarly, bal + zal elicited a higher ORR in patients with PD-L1-positive second-line r/mCC versus the overall population (32.8% vs 25.6%), and 3 of 33 patients with PD-L1-negative tumors responded to treatment (9.1%) [35]. In the 254 patients from EMPOWER-Cervical 1 with valid PD-L1 samples (42% of randomized patients), survival benefits for cemiplimab versus chemotherapy were seen only in the PD-L1-positive group (OS HR, 0.7; PFS HR, 0.76) [37]. In the PD-L1-negative group, 5 of 44 patients responded to cemiplimab and 4 of 48 patients responded to chemotherapy.
Microsatellite instability (MSI)-high status and tumor mutational burden (TMB) are associated with response to immunotherapy in different cancer types; however, these biomarkers have not been extensively studied in cervical cancer. About 4–8% of patients with cervical cancer have MSI-high tumors [72,73], and the TMB rate is approximately 2.5–5.4 mutations/megabase [74,75]. In the KEYNOTE-158 TMB sub-analysis, 16 of 75 patients with advanced cervical cancer had high tissue TMB (≥10 mutations/megabase); 5 of the 16 patients responded to pembrolizumab monotherapy whereas 7 of the 59 non-TMB-high patients responded [76]. The population was small and requires validation in larger studies and with other ICIs. Further exploration of the known genomic features of cervical cancer, as reported in analysis on samples from The Cancer Genome Atlas [74], and their impact on outcomes from completed immunotherapy studies may also provide further clues to relevant predictive biomarkers.
How will histology affect immunotherapy treatment for cervical cancer?
Because HPV infection and malignant transformation of the cervix occur in the transformation zone, which is predominately composed of squamous epithelium, SCC histology comprises about 70% of cervical cancers. Adenocarcinoma, including adenosquamous (ASC) types, is the next most common histologic type, representing ~ 20% of cervical cancers. Nonsquamous tumors are more likely to be PD-L1 negative [26]. Some studies analyzing all stages of cervical cancer find AC histology is a statistically significant negative prognostic factor for survival compared with SCC [77,78]; however, studies of patients with LACC or r/mCC did not find a significant difference in survival between AC/ASC and SCC [79,80]. The HR for OS was 1.02 (P = 0.69) in a propensity-matched analysis of patients diagnosed with LACC from the SEER database 2010–2015 [79], and the HR for death was 1.13 (P = 0.23) in patients with r/mCC treated with chemotherapy doublets from pooled Gynecologic Oncology Group phase III studies [80].
Many of the immunotherapy trials discussed in this review allow for inclusion of patients with SCC, AC, and ASC histologic types. In EMPOWER-Cervical 1, median OS was 11.1 months with cemiplimab monotherapy versus 8.8 months with chemotherapy (HR, 0.73; P = 0.006) in patients with previously treated r/mSCC, and 13.3 versus 7.0 months in those with AC (HR, 0.56; P-value not given) [37]. Bal + zal elicited an ORR of 32.6% in patients with r/mSCC versus 8.8% in AC [35]. The ORR with TV was similar in patients with non-squamous (25%) and squamous (23%) r/mCC [31]. At present, the data suggest immunotherapies may provide benefit for SCC and AC/ASC histologic types in the r/mCC setting.
Neuroendocrine histology is an aggressive form of invasive cervical cancer occurring in approximately 1.4% of cases [81]. Little guidance on optimal therapy exists and patients commonly receive radiotherapy-based treatment or radical surgery combined with neoadjuvant/adjuvant chemotherapy, with recurrence averaging 16 months after treatment and with a mean OS duration of 40 months [81,82]. Small exploratory studies and case reports in patients with previously treated neuroendocrine tumors exhibit positive results with nivolumab combinations or monotherapy [83–85] and with adoptive immune cell therapy [86], but not with pembrolizumab monotherapy [87]. Further studies of immunotherapy in these rare tumors are needed.
Future directions
While the introduction of immunotherapies for cervical cancer may bring hope to many patients, the fact remains that those with greatest need may not have access. Nearly 84% of global cervical cancer cases and 88% of all deaths due to cervical cancer occur in countries with a low human development index [88]. Countries with high disease burden may lack sufficient access to basic treatments such as radiotherapy and/or chemotherapy. However, even countries with sufficient resources can have underserved populations with a higher incidence of cervical cancer and poorer outcomes. In addition to the introduction of immunotherapies, earlier diagnosis and treatment could help improve survival and cure rates. Concerns of affordability and compliance with immunotherapy, especially adoptive cell therapies, may impede immunotherapy use. Many patients may not be able to afford, be compliant with, or tolerate multiple years of maintenance therapy, which may reduce the effectiveness of these treatments. Furthermore, while some immunotherapies may be considered cost effective, governmental or insurance reimbursement for a high-cost treatment in a common disease may not be sustainable.
Understanding the toxicity of immunotherapy in a variety of ethnic groups and underserved populations will be important to help bring therapies to these regions. Newer trials are including patients from a variety of countries to address this [32,35,60,61,66], and stratification by region can aid in these analyses [66].
As the field evolves, clinical trials should also be inclusive of patients with HIV, who are at higher risk of HPV infection and are 4–5 times more likely to develop invasive cervical cancer [89]. Most immunotherapy trials across tumor types exclude patients living with HIV [90], though small retrospective and prospective studies show an overall acceptable safety profile with ICIs in people with HIV and cancer [91,92]. It remains to be seen if immunotherapy for cervical cancer is effective in this population.
Although not covered in this review, therapeutic HPV vaccines are also considered immunotherapies and great strides in clinical development have been achieved for treating cervical intraepithelial neoplasia and invasive cervical cancer [93]. One promising therapy, VGX-3100, is a DNA plasmid-based medicine which provided statistically significant regression of HPV-16/18-associated high-grade squamous intraepithelial lesions combined with virus clearance versus placebo; virus was undetected through the 52-week safety follow-up [94,95]. Additional phase III trials are ongoing [94].
Immunotherapy has the opportunity to establish new standard of cares in the treatment of cervical cancers. Biomarkers to assess response to immunotherapy will also likely be important to identify ideal candidates. However, issues with access, affordability, appropriate treatment delivery, and compliance are anticipated, especially in regions with a high burden of disease.
Role of the funding source
The authors had full control over the content and development of this article. Medical writing support, which was in accordance with Good Publication Practice (GPP3) guidelines, was provided by Nicole Seneca, PhD, of Parexel (Hackensack, NJ) and was funded by AstraZeneca.
Declaration of Competing Interest
BJM reports honoraria from Abbvie, Advaxis, Agenus, Amgen, AstraZeneca, Biodesix, Clovis, Conjupro, Genmab, Gradalis, Immunogen, Immunomedics, Incyte, Janssen/Johnson&Johnson, Mateon, Merck, Myriad, Perthera, Pfizer, Precision Oncology, Puma, Roche/Genentech, Samumed, Takeda, TESARO, Inc., and VBL. TE reports research grants and honoraria from Chugai; and honoraria from Takeda. WMK reports grants from Brooklyn ImmunoTherapeutics, AOV Therapeutics, Immunovaccine Inc., Nutcracker Biotechnologies, NIH R01 CA74397, and the Cancer Research Institute Clinic & Laboratory Integration Program; royalties/licenses from Nutcracker Biotechnologies; consulting fees from AOV Therapeutics, Nutcracker Biotechnologies, and Repertoire; and stock/stock options with Nutcracker Biotechnologies. MM reports non-financial support from Roche; and honoraria from AstraZeneca, Roche, Eisai, and GSK. DT reports grants and other from Singapore Ministry of Health’s National Medical Research Council [NMRC; NMRC Clinician Scientist Award (NMRC/CSA-INV/0016/2017) with grant and salary support]; grants from Singapore Ministry of Health’s National Medical Research Council (Centre Grant scheme to National University Cancer Institute, Singapore), Karyopharm Therapeutics; grants and personal fees from AstraZeneca and Bayer; personal fees and nonfinancial support from Eisai, MSD, and Roche; personal fees from Merck Serono; and charitable research funding from the Pangestu Family Foundation Gynaecological Cancer Research Fund. XW has nothing to disclose. AG-M reports research grants from GSK/Tesaro and Roche; and personal fees from AstraZeneca, Clovis, Eisai, Genmab, Pharmamar, Immunogen, MSD, Amgen, Oncoinvent, Merk/Pfizer, Pharmamar, Sotio, GSK/Tesaro, Siagen, and Roche.
Footnotes
CRediT authorship contribution statement
Bradley J. Monk: Conceptualization, Writing – original draft, Writing – review & editing. Takayuki Enomoto: Conceptualization, Writing – review & editing. W. Martin Kast: Conceptualization, Writing – review & editing. Mary McCormack: Conceptualization, Writing – review & editing. David S.P. Tan: Conceptualization, Writing – review & editing. Xiaohua Wu: Conceptualization, Writing – review & editing. Antonio González-Martín: Conceptualization, Writing – review & editing.
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- [2].Canfell K, Kim JJ, Brisson M, Keane A, Simms KT, Caruana M, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020;395(10224):591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Haruyama R, Obara H, Fujita N. Japan resumes active recommendations of HPV vaccine after 8.5 years of suspension. Lancet Oncol 2022;23:197–8. 10.1016/S1470-2045(22)00002-X. [DOI] [PubMed] [Google Scholar]
- [4].Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet 2018;143(Suppl 2):22–36. 10.1002/ijgo.12611. [DOI] [PubMed] [Google Scholar]
- [5].Henley SJ, King JB, German RR, Richardson LC, Plescia M, Centers for Disease C, et al. Surveillance of screening-detected cancers (colon and rectum, breast, and cervix) - United States, 2004–2006. MMWR Surveill Summ 2010;59:1–25. [PubMed] [Google Scholar]
- [6].Landy R, Pesola F, Castanon A, Sasieni P. Impact of cervical screening on cervical cancer mortality: estimation using stage-specific results from a nested case-control study. Br J Cancer 2016;115:1140–6. 10.1038/bjc.2016.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li S, Hu T, Lv W, Zhou H, Li X, Yang Ru, et al. Changes in prevalence and clinical characteristics of cervical cancer in the People’s Republic of China: a study of 10,012 cases from a nationwide working group. Oncologist 2013;18(10):1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lorin L, Bertaut A, Hudry D, Beltjens F, Roignot P, Bone-Lepinoy M-C, et al. About invasive cervical cancer: a French population based study between 1998 and 2010. Eur J Obstet Gynecol Reprod Biol 2015;191:1–6. [DOI] [PubMed] [Google Scholar]
- [9].Ryzhov A, Corbex M, Piñeros M, Barchuk A, Andreasyan D, Djanklich S, et al. Comparison of breast cancer and cervical cancer stage distributions in ten newly independent states of the former Soviet Union: a population-based study. Lancet Oncol 2021;22(3):361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dereje N, Gebremariam A, Addissie A, Worku A, Assefa M, Abraha A, et al. Factors associated with advanced stage at diagnosis of cervical cancer in Addis Ababa, Ethiopia: a population-based study. BMJ Open 2020;10(10). 10.1136/bmjopen-2020-040645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Renna NL Junior, Silva GAE. Tendencias temporais e fatores associados ao diagnostico em estagio avancado de cancer do colo uterino: analise dos dados dos registros hospitalares de cancer no Brasil, 2000–2012. [Temporal trend and associated factors to advanced stage at diagnosis of cervical cancer: analysis of data from hospital based cancer registries in Brazil, 2000–2012]. Epidemiol Serv Saude 2018;27:e2017285. 10.5123/s1679-49742018000200003. [DOI] [PubMed] [Google Scholar]
- [12].Sengayi-Muchengeti M, Joko-Fru WY, Miranda-Filho A, Egue M, Akele-Akpo MT, N’da G, et al. Cervical cancer survival in sub-Saharan Africa by age, stage at diagnosis and Human Development Index: a population-based registry study. Int J Cancer 2020;147:3037–48. 10.1002/ijc.33120. [DOI] [PubMed] [Google Scholar]
- [13].Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv72–83. [DOI] [PubMed] [Google Scholar]
- [14].National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer version 1.2022. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1426. accessed November 1, 2021.
- [15].Kitagawa R, Katsumata N, Shibata T, Kamura T, Kasamatsu T, Nakanishi T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol 2015;33(19):2129–35. [DOI] [PubMed] [Google Scholar]
- [16].Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014;370(8):734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008;26:5802–12. 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mayadev J, Klapheke A, Yashar C, Hsu I-C, Kamrava M, Mundt AJ, et al. Underutilization of brachytherapy and disparities in survival for patients with cervical cancer in California. Gynecol Oncol 2018;150(1):73–8. [DOI] [PubMed] [Google Scholar]
- [19].Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol 2004;22(5):872–80. [DOI] [PubMed] [Google Scholar]
- [20].Shrivastava S, Mahantshetty U, Engineer R, Chopra S, Hawaldar R, Hande V, et al. Cisplatin chemoradiotherapy vs radiotherapy in FIGO stage IIIB squamous cell carcinoma of the uterine cervix: a randomized clinical trial. JAMA Oncol 2018;4 (4):506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Potter R, Tanderup K, Schmid MP, Jurgenliemk-Schulz I, Haie-Meder C, Fokdal LU, et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol 2021;22: 538–47. 10.1016/S1470-2045(20)30753-1. [DOI] [PubMed] [Google Scholar]
- [22].Macdonald OK, Chen J, Dodson M, Lee CM, Gaffney DK. Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix. Am J Clin Oncol 2009;32:411–6. 10.1097/COC.0b013e31819142dc. [DOI] [PubMed] [Google Scholar]
- [23].Mileshkin LR, Moore KN, Barnes E, Gebski V, Narayan K, Bradshaw N, et al. Adjuvant chemotherapy following chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone: The randomized phase III OUTBACK Trial (ANZGOG 0902, RTOG 1174, NRG 0274). J Clin Oncol 2021;39(18_suppl). [Google Scholar]
- [24].Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189(1):12–9. [DOI] [PubMed] [Google Scholar]
- [25].Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomaviruses to escape the host immune response. Curr Cancer Drug Targets 2007;7:79–89. 10.2174/156800907780006869. [DOI] [PubMed] [Google Scholar]
- [26].Heeren AM, Punt S, Bleeker MCG, Gaarenstroom KN, van der Velden J, Kenter GG, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol 2016;29(7):753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang J, Li Z, Gao A, Wen Q, Sun Y. The prognostic landscape of tumor-infiltrating immune cells in cervical cancer. Biomed Pharmacother 2019;120:109444. 10.1016/j.biopha.2019.109444. [DOI] [PubMed] [Google Scholar]
- [28].Feng CH, Mell LK, Sharabi AB, McHale M, Mayadev JS. Immunotherapy with radiotherapy and chemoradiotherapy for cervical cancer. Semin Radiat Oncol 2020;30:273–80. 10.1016/j.semradonc.2020.05.003. [DOI] [PubMed] [Google Scholar]
- [29].Da Silva DM, Enserro DM, Mayadev JS, Skeate JG, Matsuo K, Pham HQ, et al. Immune activation in patients with locally advanced cervical cancer treated with ipilimumab following definitive chemoradiation (GOG-9929). Clin Cancer Res 2020;26(21):5621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mayadev JS, Enserro D, Lin YG, Da Silva DM, Lankes HA, Aghajanian C, et al. Sequential ipilimumab after chemoradiotherapy in curative-intent treatment of patients with node-positive cervical cancer. JAMA Oncol 2020;6(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Coleman RL, Lorusso D, Gennigens C, Gonzalez-Martin A, Randall L, Cibula D, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2021;22:609–19. 10.1016/S1470-2045(21)00056-5. [DOI] [PubMed] [Google Scholar]
- [32].Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med 2021;385(20):1856–67. [DOI] [PubMed] [Google Scholar]
- [33].Duska LR, Scalici JM, Temkin SM, Schwarz JK, Crane EK, Moxley KM, et al. Results of an early safety analysis of a study of the combination of pembrolizumab and pelvic chemoradiation in locally advanced cervical cancer. Cancer 2020;126(22): 4948–56. [DOI] [PubMed] [Google Scholar]
- [34].Jazaeri AA, Zsiros E, Amaria RN, Artz AS, Edwards RP, Wenham RM, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. JCO 2019;37(15_suppl). [Google Scholar]
- [35].O’Malley DM, Neffa M, Monk BJ, Melkadze T, Huang M, Kryzhanivska A, et al. Dual PD-1 and CTLA-4 checkpoint blockade using balstilimab and zalifrelimab combination as second-line treatment for advanced cervical cancer: an open-label phase II study. J Clin Oncol 2022;40(7):762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Qu Y-Y, Guo H, Luo H, Zou Q, Xing N, Sun Z, et al. Camrelizumab plus famitinib malate in patients with advanced renal cell cancer and unresectable urothelial carcinoma: A multicenter, open-label, single-arm, phase II trial. JCO 2020;38(15_suppl). [Google Scholar]
- [37].Tewari KS, Monk BJ, Vergote I, Miller A, de Melo AC, Kim H-S, et al. Survival with cemiplimab in recurrent cervical cancer. N Engl J Med 2022;386(6):544–55. [DOI] [PubMed] [Google Scholar]
- [38].Tjulandin S, Demidov L, Moiseyenko V, Protsenko S, Semiglazova T, Odintsova S, et al. Novel PD-1 inhibitor prolgolimab: expanding non-resectable/metastatic melanoma therapy choice. Eur J Cancer 2021;149:222–32. [DOI] [PubMed] [Google Scholar]
- [39].Wu X. Efficacy and safety of cadonilimab, an anti-PD-1/CTLA4 bi-specific antibody, in previously treated recurrent or metastatic (R/M) cervical cancer: a multicenter, open-label, single-arm, phase II trial. Presented at: Society of Gynecologic Oncology Annual Meeting on Womens’ Cancer; March 19, 2022; Phoenix, AZ. [Google Scholar]
- [40].Akesobio. 2021 Interim Results Presentation, August 2021. 2021. https://www.akesobio.com/media/1525/akeso-2021-ir-presentation-20210901_final_sj.pdf. accessed October 6, 2021.
- [41].Naumann RW, Hollebecque A, Meyer T, Devlin M-J, Oaknin A, Kerger J, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial. J Clin Oncol 2019;37(31):2825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Naumann RW, Oaknin A, Meyer T, Lopez-Picazo JM, Lao C, Bang Y-J, et al. Efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: Results from CheckMate 358. Ann Oncol 2019;30:v898–9. [Google Scholar]
- [43].Regeneron. Regeneron and Sanofi Provide Regulatory Update on Libtayo® (cemiplimab-rwlc) in Advanced Cervical Cancer. 2022. https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-regulatory-update-libtayor. accessed February 2, 2022.
- [44].US Food and Drug Administration. FDA grants accelerated approval to tisotumab vedotin-tftv for recurrent or metastatic cervical cancer. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tisotumab-vedotin-tftv-recurrent-or-metastatic-cervical-cancer accessed October 7, 2021.
- [45].Wu X, Xia L, Zhou Q, Zhu J, Wang K, Chen J, et al. 357 GLS-010 (zimberelimab), a novel fully human anti-PD-1 mAb in chinese patients with recurrent/metastatic cervical cancer: results from a multicenter, open-label, single-arm phase II trial. Int J Gynecol Cancer 2020;30:A147. 10.1136/ijgc-2020-IGCS.307. [DOI] [Google Scholar]
- [46].Akesobio. Cadonilimab (PD-1/CTLA-4 Bispecific Antibody) Obtained Orphan Drug Designation from FDA of the United States for Treating Cervical Cancer. 2021. https://www.akesobio.com/en/media/akeso-news/2021-3-3/. accessed July 6, 2021.
- [47].Akesobio. New drug application for cadonilimab (PD-1/CTLA-4 bi-specific antibody) for the treatment of relapsed or metastatic cervical cancer accepted by NMPA. 2021. https://portalvhds1fxb0jchzgjph.blob.core.windows.net/press-releases-attachments/1338370/HKEX-EPS_20210924_9946859_0.PDF. accessed October 6, 2021.
- [48].Rischin D, Gil-Martin M, González-Martin A, Braña I, Hou JY, Cho D, et al. PD-1 blockade in recurrent or metastatic cervical cancer: data from cemiplimab phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer. Gynecol Oncol 2020;159(2):322–8. [DOI] [PubMed] [Google Scholar]
- [49].Ligand’s partner Gloria Biosciences receives approval in China for zimberelimab for the treatment of recurrent or refractory classical Hodgkin’s lymphoma. 2021. Available at: https://www.businesswire.com/news/home/20210830005543/en/Ligand%E2%80%99s-Partner-Gloria-Biosciences-Receives-Approval-in-China-for-Zimberelimab-for-the-Treatment-of-Recurrent-or-Refractory-Classical-Hodgkin%E2%80%99s-Lymphoma. [accessed December 9, 2021].
- [50].Opdivo (nivolumab injection). US Prescribing information. Bristol Myers Squibb; September 2021. Accessed January 4, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f570b9c4-6846-4de2-abfa-4d0a4ae4e394. [Google Scholar]
- [51].Xie C, Zhou J, Guo Z, Diao X, Gao Z, Zhong D, et al. Metabolism and bioactivation of famitinib, a novel inhibitor of receptor tyrosine kinase, in cancer patients. Br J Pharmacol 2013;168(7):1687–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhou A, Zhang W, Chang C, Chen X, Zhong D, Qin Q, et al. Phase I study of the safety, pharmacokinetics and antitumor activity of famitinib. Cancer Chemother Pharmacol 2013;72(5):1043–53. [DOI] [PubMed] [Google Scholar]
- [53].Hengrui. Hengrui Medicine’s camrelizumab + famitinib malate combination therapy is included in the public announcement of the type of breakthrough treatment [Translated]. 2020. https://mp.weixin.qq.com/s/HTmMrJVkHaksBaW3Wk3BCw. accessed October 18, 2021.
- [54].Chauvin J-M, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer 2020;8(2):e000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li Xi, Wang R, Fan P, Yao X, Qin L, Peng Y, et al. A comprehensive analysis of key immune checkpoint receptors on tumor-infiltrating T cells from multiple types of cancer. Front Oncol 2019;9. 10.3389/fonc.2019.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Broderick JM. FDA Grants LN-145 Breakthrough Designation for Cervical Cancer [press release]. 2019. https://www.onclive.com/view/fda-grants-ln145-breakthrough-designation-for-cervical-cancer. accessed September 28, 2021.
- [57].Vergote IB, Monk BJ, O’Cearbhaill RE, Westermann AM, Banerjee S, Collins DC, et al. 723MO - Tisotumab vedotin (TV) + carboplatin (Carbo) in first-line (1L) or + pembrolizumab (Pembro) in previously treated (2L/3L) recurrent or metastatic cervical cancer (r/mCC): interim results of ENGOT-Cx8/GOG-3024/innovaTV 205 study. Ann Oncol 2021;32:S726–7. [Google Scholar]
- [58].Merck. FDA approves Merck’s KEYTRUDA® (pembrolizumab) plus chemotherapy, with or without bevacizumab, as treatment for patients with persistent, recurrent or metastatic cervical cancer whose tumors express PD-L1 (CPS ≥1). 2021. https://www.merck.com/news/fda-approves-mercks-keytruda-pembrolizumab-plus-chemotherapy-with-or-without-bevacizumab-as-treatment-for-patients-with-persistent-recurrent-or-metastatic-cervical-cancer-whose-tum/. accessed October 18, 2021.
- [59].Keytruda (pembrolizumab). US Prescribing information. Merck Sharp & Dohme Corp.; August 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9333c79b-d487-4538-a9f0-71b91a02b287. accessed September 29, 2021. [Google Scholar]
- [60].BIOCAD. Novel anti-PD-1 drug was approved for melanoma treatment. 2020. https://www.eurekalert.org/news-releases/904789. accessed August 13, 2021.
- [61].Grau JF, Farinas-Madrid L, Oaknin A. A randomized phase III trial of platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab versus platinum chemotherapy plus paclitaxel and bevacizumab in metastatic (stage IVB), persistent, or recurrent carcinoma of the cervix: the BEATcc study (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030). Int J Gynecol Cancer 2020;30:139–43. 10.1136/ijgc-2019-000880. [DOI] [PubMed] [Google Scholar]
- [62].AstraZeneca Press Release. Update on CALLA phase III trial of concurrent use of imfinzi and chemoradiotherapy in locally advanced cervical cancer, March 24, 2022. 2022. https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2022/update-on-calla-phase-iii-trial-for-imfinzi.html. accessed March 24, 2022.
- [63].Lorusso D, Xiang Y, Colombo N, Coleman R, Randall L, Duska L, et al. 254TiP - ENGOT-cx11/KEYNOTE-A18: a phase 3, randomized, double-blind study of pembrolizumab with chemoradiotherapy in patients with high-risk locally advanced cervical cancer. Ann Oncol 2020;31:S1341–2. [Google Scholar]
- [64].Imfinzi (durvalumab). US Prescribing information. AstraZeneca; July 2021. Accessed September 29, 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8baba4ea-2855-42fa-9bd9-5a7548d4cec3. [Google Scholar]
- [65].Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- [66].Mayadev J, Nunes AT, Li M, Marcovitz M, Lanasa MC, Monk BJ. CALLA: Efficacy and safety of concurrent and adjuvant durvalumab with chemoradiotherapy versus chemoradiotherapy alone in women with locally advanced cervical cancer: a phase III, randomized, double-blind, multicenter study. Int J Gynecol Cancer 2020;30: 1065–70. 10.1136/ijgc-2019-001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Oka H, Sasaki H, Shiraishi Y, Emori Y, Yoshinaga K, Takei M. Z-100, an immunomodulatory arabinomannan extracted from Mycobacterium tuberculosis strain Aoyama B, augments anti-tumor activities of X-ray irradiation against B16 melanoma in association with the improvement of type 1T cell responses. Biol Pharm Bull 2004;27:82–8. 10.1248/bpb.27.82. [DOI] [PubMed] [Google Scholar]
- [68].Oka H, Emori Y, Sasaki H, Shiraishi Y, Yoshinaga K, Kurimoto T. Anti-tumor mechanism of Z-100, an immunomodulatory arabinomannan extracted from Mycobacterium tuberculosis strain Aoyama B, on pulmonary metastases of B16F10 melanoma: restoration of helper T cell responses via suppression of glucocorticoid-genesis. Microbiol Immunol 2002;46:343–51. 10.1111/j.1348-0421.2002.tb02705.x. [DOI] [PubMed] [Google Scholar]
- [69].Oka H, Shiraishi Y, Sasaki H, Yoshinaga K, Emori Y, Takei M. Antimetastatic effect of an immunomodulatory arabinomannan extracted from Mycobacterium tuberculosis strain Aoyama B, Z-100, through the production of interleukin-12. Biol Pharm Bull 2003;26:1336–41. 10.1248/bpb.26.1336. [DOI] [PubMed] [Google Scholar]
- [70].Sugiyama T, Fujiwara K, Ohashi Y, Yokota H, Hatae M, Ohno T, et al. Phase III placebo-controlled double-blind randomized trial of radiotherapy for stage IIB-IVA cervical cancer with or without immunomodulator Z-100: a JGOG study. Ann Oncol 2014;25:1011–7. 10.1093/annonc/mdu057. [DOI] [PubMed] [Google Scholar]
- [71].Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2019;37: 1470–8. 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- [72].Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 2016;22:813–20. 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- [73].Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019;30:1232–43. 10.1093/annonc/mdz116. [DOI] [PubMed] [Google Scholar]
- [74].Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017;543:378–84. 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21: 1353–65. 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- [77].Lee JY, Kim YT, Kim S, Lee B, Lim MC, Kim JW, et al. Prognosis of cervical cancer in the era of concurrent chemoradiation from national database in Korea: a comparison between squamous cell carcinoma and adenocarcinoma. PLoS ONE 2015;10:e0144887. 10.1371/journal.pone.0144887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pan X, Yang W, Wen Z, Li F, Tong L, Tang W. Does adenocarcinoma have a worse prognosis than squamous cell carcinoma in patients with cervical cancer? A real-world study with a propensity score matching analysis. J Gynecol Oncol 2020;31: e80. 10.3802/jgo.2020.31.e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tian T, Gong X, Gao X, Li Y, Ju W, Ai Y. Comparison of survival outcomes of locally advanced cervical cancer by histopathological types in the surveillance, epidemiology, and end results (SEER) database: a propensity score matching study. Infect Agent Cancer 2020;15:33. 10.1186/s13027-020-00299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Seamon LG, Java JJ, Monk BJ, Penson RT, Brown J, Mannel RS, et al. Impact of tumour histology on survival in advanced cervical carcinoma: an NRG Oncology/Gynaecologic Oncology Group Study. Br J Cancer 2018;118:162–70. 10.1038/bjc.2017.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tempfer CB, Tischoff I, Dogan A, Hilal Z, Schultheis B, Kern P, et al. Neuroendocrine carcinoma of the cervix: a systematic review of the literature. BMC Cancer 2018;18:530. 10.1186/s12885-018-4447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Salvo G, Gonzalez Martin A, Gonzales NR, Frumovitz M. Updates and management algorithm for neuroendocrine tumors of the uterine cervix. Int J Gynecol Cancer 2019;29:986–95. 10.1136/ijgc-2019-000504. [DOI] [PubMed] [Google Scholar]
- [83].Sharabi A, Kim SS, Kato S, Sanders PD, Patel SP, Sanghvi P, et al. Exceptional response to nivolumab and stereotactic body radiation therapy (SBRT) in neuroendocrine cervical carcinoma with high tumor mutational burden: management considerations from the Center for Personalized Cancer Therapy at UC San Diego Moores Cancer Center. Oncologist 2017;22:631–7. 10.1634/theoncologist.2016-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Paterniti TA, Dorr K, Ullah A, White J, Williams H, Ghamande S. Complete response to combination nivolumab and ipilimumab in recurrent neuroendocrine carcinoma of the cervix. Obstet Gynecol 2021. 10.1097/AOG.0000000000004573. [DOI] [PubMed] [Google Scholar]
- [85].Paraghamian SE, Longoria TC, Eskander RN. Metastatic small cell neuroendocrine carcinoma of the cervix treated with the PD-1 inhibitor, nivolumab: a case report. Gynecol Oncol Res Pract 2017;4:3. 10.1186/s40661-017-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Goto S, Terao Y, Kamigaki T, Takimoto R, Naitoh K, Makita K, et al. Adoptive immune-cell therapy for the treatment of neuroendocrine carcinoma of the uterine cervix. Anticancer Res 2020;40(4741–8). [DOI] [PubMed] [Google Scholar]
- [87].Frumovitz M, Westin SN, Salvo G, Zarifa A, Xu M, Yap TA, et al. Phase II study of pembrolizumab efficacy and safety in women with recurrent small cell neuroendocrine carcinoma of the lower genital tract. Gynecol Oncol 2020;158: 570–5. 10.1016/j.ygyno.2020.05.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020;8:e191–203. 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].UN AIDS. Cervical cancer and HIV—two diseases, one response. 2018. Available at: https://www.unaids.org/en/resources/presscentre/featurestories/2018/october/cervical-cancer-and-hiv. [accessed October 19, 2021].
- [90].Vora KB, Awad MM. Exclusion rates of patients living with HIV from cancer immunotherapy clinical trials. J Clin Oncol 2020;38:e19035–e. 10.1200/JCO.2020.38.15_suppl.e19035. [DOI] [Google Scholar]
- [91].Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: a systematic review. JAMA Oncol 2019;5:1049–54. 10.1001/jamaoncol.2018.6737. [DOI] [PubMed] [Google Scholar]
- [92].Uldrick TS, Goncalves PH, Abdul-Hay M, Claeys AJ, Emu B, Ernstoff MS, et al. Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer-a phase 1 study. JAMA Oncol 2019;5:1332–9. 10.1001/jamaoncol.2019.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, et al. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett 2020;471:88–102. 10.1016/j.canlet.2019.11.039. [DOI] [PubMed] [Google Scholar]
- [94].Inovio. INOVIO highlights key updates on phase 3 program for VGX-3100, its DNA-based immunotherapy for the treatment of cervical HSIL caused by HPV-16 and/or HPV-18. 2021. https://ir.inovio.com/news-releases/news-releases-details/2021/INOVIO-Highlights-Key-Updates-on-Phase-3-Program-for-VGX-3100-its-DNA-based-Immunotherapy-for-the-Treatment-of-Cervical-HSIL-Caused-by-HPV-16-andor-HPV-18/default.aspx. accessed December 16, 2021.
- [95].Inovio. INOVIO announces positive results from REVEAL 1, a phase 3 pivotal trial evaluating VGX-3100, its DNA-based HPV immunotherapy for the treatment of high-grade precancerous cervical dysplasia caused by HPV-16 and/or HPV-18. 2021. https://ir.inovio.com/news-releases/news-releases-details/2021/INOVIO-Announces-Positive-Results-from-REVEAL-1-a-Phase-3-Pivotal-Trial-Evaluating-VGX-3100-its-DNA-based-HPV-Immunotherapy-for-the-Treatment-of-High-grade-Precancerous-Cervical-Dysplasia-Caused-by-HPV-16-andor-HPV-18/default.aspx. accessed December 16, 2021.


