Abstract
Infectious diseases are a leading contributor to death in the United States, and racial differences in clinical outcomes have been increasingly reported. Clostridioides difficile infection (CDI) is a growing public health concern, as it causes nearly half a million infections per year and considerable excess hospital costs. Concurrent with other infectious diseases, recent literature denotes racial disparities in CDI incidence rates, mortality, and associated morbidity. Of note, investigations into CDI and causative factors suggest that inequities in health-related social needs and other social determinants of health (SDoH) may cause disruption to the gut microbiome, thereby contributing to the observed deleterious outcomes in racially and ethnically minoritized individuals. Despite these discoveries, there is limited literature that provides context for the recognized racial disparities in CDI, particularly the influence of structural and systemic barriers. Here, we synthesize the available literature describing racial inequities in CDI outcomes and discuss the interrelationship of SDoH on microbiome dysregulation. Finally, we provide actionable considerations for infectious diseases professionals to aid in narrowing CDI equity gaps.
Keywords: Clostridioides difficile, microbiome, health disparities, social determinants of health, infectious diseases
Despite worldwide advancements in biomedical technology, treatments, and public health interventions, inequitable distribution of disease and socioeconomic health outcome disparities continue [1]. As defined by the Office of Disease Prevention and Health Promotion, health disparities are adverse health differences experienced by socially disadvantaged groups, whereas health equity focuses on how individuals from diverse social backgrounds, including those from historically marginalized communities (race/ethnicity, gender, sexual orientation, able status), can achieve their optimal health without encountering barriers [2]. Consequently, inequities in social determinants of health (SDoH), or health-related social needs, in addition to structural racism, can further exacerbate deleterious outcomes across marginalized groups. Social determinants that impact health include economic stability, education access and quality, healthcare access and quality, neighborhood and built environment, and social and community context [3]. Achievement of the Healthy People 2030 goal of eliminating health disparities requires a deep understanding of SDoH (eg, structural racism, systemic bias) and identifying novel biological pathways (eg, microbiome) that contribute to inequities [2, 3].
Health inequities have led to observed disparities in disease incidence, prevalence, mortality, and financial burden, particularly across persons from racially and ethnically minoritized (REM) groups [3]. Of note, infection is the second leading contributor (21.1%) to racial disparities in all-cause mortality when compared with other major categories of disease (eg, cardiovascular disease, trauma, cancer) [4]. More prevalently, disparities among REM groups have been observed in human immunodeficiency virus, viral hepatitis, sexually transmitted infections, tuberculosis, and coronavirus disease 2019 [5].
Recent studies have also highlighted disparities in Clostridioides difficile infection (CDI) risk and health outcomes among REM groups (Table 1). These studies have documented higher risk of incident CDI among White patients compared with REM individuals, even after stratifying by likelihood of antibiotic exposure [6, 7]. However, REM groups appear to be disproportionately affected by poor CDI health outcomes. Multiple studies have found that patients who are Black are more likely to experience recurrent CDI [8, 9]. Patients who are Black or Hispanic may also more commonly experience severe CDI and incur higher treatment-related costs [6, 9], which may be partially mediated by underlying chronic conditions [10]. In parallel with these findings, Argamany et al [6] found that patients with CDI who are Black had higher mortality and experienced longer hospital stays compared with patients who are White. Importantly, several studies included certain SDoH factors. Mao et al [7] found that CDI rates increase with higher income levels and were higher for hospitalization paid by Medicaid or classified as self-pay or free care. In contrast, Hudspeth et al [11] reported that census track–level socioeconomic measures, such as a high percentage of individuals without health insurance, were predictive of community-associated CDI (CA-CDI). Similarly, Skrobarcek et al [12] found that low socioeconomic status (SES), as defined by “poverty” and “foreign-born” factors, was positively associated with CA-CDI incidence, whereas “high income” was negatively associated with CA-CDI incidence.
Table 1.
Overview of Published Literature Identifying Clostridioides difficile Infection Health Disparities
| Study | Population | Outcome | Key Findings | P Value |
|---|---|---|---|---|
| Freedberg et al 2013 | Single-center hospital (n = 894 CDI patients) |
CDI recurrence | Black versus White race Hazard ratio, 1.66; 95% confidence interval, 1.05–2.63 |
|
| Mao et al 2015 | National sample of US hospital discharges that included antibiotic exposure (n = 178 000 CDI discharges) | CDI incidence | Black race: 0.75% Asian race: 0.25% Hispanic ethnicity: 0.50% White race: 1.0%; |
.001 .003 .059 .001 |
| CDI complications | Black race: 7.8% Asian race: 9.0% Hispanic ethnicity: 7.8% White race: 8.25% |
1.000 .824 .824 1.000 |
||
| Inpatient mortality | Black race: 1.0% Asian race: 0.20% Hispanic ethnicity: 1.5% White race: 1.2% |
1.000 | ||
| Argamany et al 2016 | National sample of US hospital discharges (n = 1.7 million CDI discharges) | CDI incidence | Black race: 4.9 per 1000 White race: 7.7 per 1000 |
<.001 |
| Severe CDI | Black race: 24% White race: 19% |
<.001 | ||
| Inpatient mortality | Black race: 7.4% White race: 7.2% |
<.001 | ||
| LOS >7 days | Black race: 57% White race: 52% |
<.001 | ||
| Young et al 2022 | National sample of US healthcare system (n = 45 331 CDI encounters) | Severe CDI | Black race: aOR 1.85 (1.71–2.00) Hispanic ethnicity: aOR 1.22 (1.08–1.38) |
|
| Recurrent CDI | Black race: aOR 1.17 (1.03–1.34) Hispanic ethnicity: aOR 0.94 (0.76–1.17) |
|||
| Inpatient mortality | Black race: aOR 0.96 (0.82–1.13) Hispanic ethnicity: aOR 0.93 (0.72–1.21) |
|||
| LOS >7 days | Black race: aOR 1.29 (1.19–1.41) Hispanic ethnicity: aOR 0.89 (0.78–1.02) |
|||
| Treatment costs (recurrent CDI) | Black race: $12 352, Hispanic ethnicity: $11 019 White race: $8502 |
<.001 < .001 | ||
| Lee et al 2023 | Single hospital system (n = 219 CDI patients) | Severe CDI | REM: 40.0% Non-REM: 38.1% |
.779 |
| Intensive care unit admission | REM: 42.2% Non-REM: 26.2%, |
.016 | ||
| Inpatient mortality | REM: 10.4% Non-REM: 13.1%, |
.537 | ||
| LOS >10 days | REM: 74.0% Non-REM: 32.0% |
.016 | ||
| Skrobarcek et al 2021 | CDI surveillance in 10 US states (n = 9413 CA-CDI cases) | “Poverty” factor | RR: 1.19 (1.15–1.22) | .0001 |
| “Foreign-born” factor | RR: 1.05 (1.02–1.08) | <.001 | ||
| “High-income” factor | RR: 0.95 (0.92–0.97) | <.001 | ||
| Hudspeth et al 2019 | CDI surveillance in Bernalillo County, New Mexico (n = 1672 CA-CDI cases) | >20% no health insurance | Incidence rate ratio: 1.718 (1.4–2.108) | <.001 |
| CDI incidence | White women: Reference Black women: 5.98 (3.379–10.567) Asian race: 9.353 (5.444–16.067) White men: Reference Black men: 27.681 (12.931–59.256) American Indian/Alaskan Native men: 11.657 (5.781–23.507) |
<.001 <.001 <.001 <.001 |
Abbreviations: aOR, adjusted odds ratio; CA-CDI, community-associated Clostridioides difficile infection; CDI, Clostridioides difficile infection; LOS, hospital length of stay; REM, persons from racial or ethnic minority groups; RR, relative risk.
Many factors likely influence the observed differences among REM persons in CDI incidence and health outcomes including multimorbidity, health care exposure, access to care, quality of care, and socioeconomic factors. Additionally, CDI pathogenesis is tightly linked to disruption of the healthy gut microbiome, and emerging evidence links microbiome changes with individual (eg, health and lifestyle) and population-level determinants of health. None of the currently published literature on CDI disparities has explicitly evaluated the impact of the gut microbiome or the gut microbiome–SDoH interaction. In this review, we use CDI as a framework to describe the complex relationship between the gut microbiome and the many factors that may contribute to health disparities among persons of REM groups in infectious diseases.
THE ROLE OF THE MICROBIOME IN INFECTIOUS DISEASES AND CDI PATHOGENESIS
The human microbiome refers to the collection of microbial genomes located in or on the body. Microorganisms are found in nearly every anatomical location, though the gastrointestinal tract (gut microbiome) harbors the highest microbial concentration. The gut microbiome’s functions and relationship with human health and disease have been extensively reviewed previously [13, 14]. In infectious diseases, the microbiome protects against pathogens through production of antimicrobial compounds, competition for resources, and modulation of host–microbe–immune interactions [15]. Dysbiosis, broadly defined as a change in commensal microbiota community structure compared with healthy individuals [16], has been associated with enteric infections and antimicrobial resistance gene colonization (gut), bacterial vaginosis and sexually transmitted infections (vaginal), and skin and soft tissue infections (skin and nasal) [17]. One of the most well-established relationships between the microbiome and disease is with CDI. Dysbiosis, primarily related to antimicrobial exposure, results in significant reduction in gut microbial diversity and a loss of core microbiota from the Firmicutes and Bacteroides phyla. Ultimately, these structural changes create an environment conducive to C. difficile colonization and germination to the toxin-producing form, whereby there is a shift in abundance of anti-germinant secondary bile acids to pro-germinant primary bile acids [18].
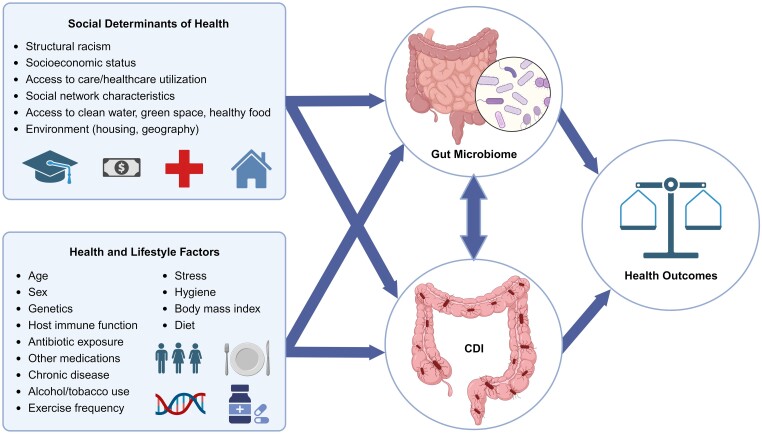
The primary, direct contributors to dysbiosis and CDI pathogenesis, such as antimicrobial use, healthcare exposures, older age, and underlying disease, are well established; however, there are relatively fewer investigations of the relationships between race/ethnicity, SDoH, and upstream contributions to gut microbiome dysbiosis. Numerous studies have documented microbiome structure differences between REM groups compared with nonminoritized groups [19–21]. In a large cross-sectional study of Americans, Brooks et al [21] documented 12 microbial genera and families that reproducibly vary by self-declared ethnicity. While race and ethnicity are considered individual-level factors, they are not biological constructs but rather social constructs that may serve as a proxy for socioeconomic variation. The disparity in microbiome composition and CDI-related outcomes is likely related to systematic exposure to poorer SDoH. The reasons for these systematic differences are complex; however, it is critical to emphasize that the observed differences are likely the result of systems of oppression (eg, racism, sexism, ableism). With this caveat, the following sections describe the CDI–gut microbiome–SDoH triad using direct evidence and theoretical frameworks (Figure 1).
Figure 1.
Complex relationships between the microbiome, social determinants of health, and health inequities in CDI. This figure demonstrates how social and structural determinants of health, as well as health and lifestyle factors, shape the gut microbiome and its influence on CDI. These relationships demonstrate that different patient populations may experience health disparities, providing valuable insights for addressing CDI outcomes and promoting health equity. Abbreviation: CDI, Clostridioides difficile infection. (Created with BioRender.com)
RACIAL/ETHNIC DISPARITIES RELATED TO THE MICROBIOME AND INDIVIDUAL-LEVEL FACTORS
Comorbidities
REM individuals are disproportionately affected by chronic and immunocompromising conditions, which may place them at higher risk for CDI and poor health outcomes. For example, certain comorbidities have been identified to increase CDI risk, notably cancer, diabetes mellitus, heart failure, and chronic kidney disease [22]. Many of these factors also have a demonstrated relationship with the microbiome either directly or indirectly related to medication use and healthcare exposures. Cancer is one of the strongest comorbidity risk factors for CDI that also has notable racial/ethnic disparities and robust evidence for microbiome associations. Other chronic comorbidities may play a role as well. Obesity, for example, is more prevalent among persons of racial and ethnic minorities [23]. Obesity has been associated with notable changes in the microbiome [24] and CDI risk independent of antimicrobial or healthcare exposures [25].
Advanced Age
Older adults disproportionately develop CDI and experience higher rates of hospitalization and mortality [22]. The diversity and complexity of the microbiome develop over the human life span. The infant microbiome is shaped by a variety of environmental exposures, including delivery method, feeding practices (eg, breastfeeding), and antibiotic exposure. Disparities in these exposures may lead to microbiome dysbiosis and CDI risk. For example, non-Hispanic Black persons are 14.9% less likely to breastfeed infants compared with non-Hispanic White individuals [26], and breastfeeding is associated with lower rates of C. difficile colonization in infants [27]. In general, older adults tend to lose microbial diversity and abundance of core microbial taxa, similar to the trends seen among patients with CDI [28]. Importantly, older adults also can experience a low-grade systemic inflammation and decline in immune system function (ie, immunosenescence). The causes of these age-associated changes are a subject of continued investigation; however, emerging evidence suggests that this relationship may be partially mediated by changes in microbiome function [29]. Alternations in immune and inflammatory response may then predispose patients to develop clinical infection and more severe infection, though further research is needed to validate this theory. While the relative impact of the aging microbiome by race and ethnicity is largely unknown, these data and the disproportionate impact of individual and public health factors among minorities highlight the complex interplay between age and the development of aging-related health conditions over time that may disproportionately affect minoritized groups.
Diet and Lifestyle
While the relationship between diet and CDI has been largely unexplored, numerous studies have documented the impact of diet and lifestyle factors on the microbiome and how these factors vary for minoritized groups. Diet is considered one of the primary drivers of microbiome structure through regulation of microbial taxa diversity and abundance and their individual or community functions [30]. However, minoritized groups may have less access to foods that positively impact microbiome structure and function. For example, low-fat, high-fiber (LFHF) diets positively impact gut microbiome diversity and function [30]. However, LFHF diets tend to be less accessible to low socioeconomic and minoritized groups [31].
In addition to diet, other lifestyle factors may impact the gut microbiome. For example, higher levels of stress experienced by minoritized populations due to systemic bias and oppression [32] may alter microbiome composition [33]. Alcohol intake, tobacco use, exercise frequency and intensity, and personal hygiene all play a role in shaping microbiome composition [34]. It is important to emphasize that the diet and lifestyle differences seen between racial and ethnic groups are likely heavily influenced by multiple factors, especially access to healthy food, knowledge about healthy food, and other SDoH that are often outside of individuals’ control, as described below.
RACIAL/ETHNIC DISPARITIES RELATED TO THE MICROBIOME AND PUBLIC HEALTH LEVEL FACTORS
Healthcare Access and Quality
The clearest impact of SDoH on the microbiome and CDI is related to healthcare access and quality. Currently in the United States, the proportion of insured individuals is lower in REM groups compared with non-REM groups [35]. The insurance gap has several potential population-level consequences including worse management of chronic conditions (see above), higher use of emergency department services, and increased risk of hospitalization. The latter 2 items have been directly linked to increased risk of C. difficile acquisition [36]. When patients in minoritized communities become infected with CDI, they also may have less access to primary care physicians [37]. This may lead to delays in care that can cause disease progression prior to treatment. The cumulative effect of lower access appears to be an indirect but potentially significant upstream contributor to gut microbiome health. The direct impact of healthcare access on microbiome health still requires future research.
Healthcare quality may also vary by REM community membership. A specific example of these differences is the quality of antibiotic use. These differences are seen across treatment settings and age groups. For example, a nationwide cross-sectional study of more than 7.0 billion outpatient visits demonstrated that Black and Hispanic patients had the highest inappropriate antibiotic prescribing rates [38]. Since quality of antibiotic prescribing is a well-established risk factor for C. difficile, it is expected that this effect would translate to an increased risk of worse gut microbiome outcomes, including CDI.
Economic Stability, Education Access, and Educational Quality
Economic stability, education access, and educational quality have also been linked to gut microbiome health. These terms are combined here using the term “socioeconomic status,” but the term should be used with caution as there is no standardized definition. Studies directly or indirectly link socioeconomic status to microbiome differences in relative abundances of microbial taxa, alpha-diversity, and other measures of dissimilarity [20]. Socioeconomic status also has been associated with C. difficile acquisition. For example, in an active surveillance study of 9682 community-acquired CDI cases in 2474 census tracts, “poverty” (combination of income, educational attainment, and other covariates as 1 factor) was associated with an increased risk ratio for CA-CDI (1.19; 96% confidence interval, 1.15–1.22) [12]. Although a clear relationship between socioeconomic status stability exists, it is important to note the interrelatedness between economic stability and other factors such as food security, housing, and insurance status.
Social and Community Context
There is indirect evidence for the influence of social and community context, including health literacy and social support, on CDI health outcomes. First, and closely related to the section above, educational access and quality directly impact health literacy, which can then affect healthcare use and quality of care. For example, lower health literacy has been previously linked with lower knowledge of antibiotics [39]. Lack of knowledge of antibiotics and their side effects can, in turn, lead to failure to recognize CDI when it occurs and delayed treatment, which can perpetuate risk for poor health outcomes. Thus, individuals of low socioeconomic status have been shown to have lower levels of health literacy, and due to systemic and structural barriers, individuals are more likely to have a low SES designation. The community context regarding peer support also warrants examination because of social norms found within REM communities. For instance, due to perceived lower health literacy, Hispanic individuals were shown to be more likely to believe that an antibiotic would help them recover more quickly from a viral infection and more likely to seek and acquire antibiotics not prescribed by a clinician [40]. Patient desire to receive an antibiotic has been demonstrated to increase the probability of antibiotic prescribing, even when inappropriate [41]. In sum, these data would suggest that specific REM communities are at increased risk for inappropriate antibiotic use and CDI due to social and community contexts. These indirect impacts are likely to contribute to gut microbiome and CDI outcomes; however, additional research is needed to understand the full effect of social and community context on gut microbiome outcomes.
Neighborhoods and Built Environments
There are several potential impacts of neighborhoods and built environments on the microbiome and CDI. Time spent indoors and access to green spaces are believed to be associated with gut microbiome diversity [42]. However, low SES- and REM-identifying persons may have less access to safe, outdoor green spaces compared with other groups [43]. Access to clean water can also contribute to poorer outcomes across REM groups [36]. This lesser access to positive living environments is attributed to historical systemic housing segregation and the concentration of REM individuals in heavily disparaged living areas [44]. Although the United States currently is on target for the Healthy People 2030 goal of “the proportion of people whose water supply meets Safe Drinking Water Act regulations,” REM communities may drink less tap water on average in part due to decreased trust of municipal drinking water [45]. Hydration, access to clean water, and trust of municipal drinking water are important social determinants since hydration is a cornerstone of therapy in the management of diarrhea.
Crowded living conditions also contribute to gut microbiome health and CDI risk. Also resultant of housing barriers, it is estimated that REM individuals are more likely to live in crowded conditions and to live with healthcare workers [46]. Living in crowded conditions is a well-established risk factor for acquisition of communicable diseases [47], including CDI [36].
FUTURE PUBLIC HEALTH POLICY AND RESEARCH NEEDS
In this review, we highlight the growing body of evidence that supports the links between the gut microbiome, individual and public health-related factors, and health inequities in infectious diseases. However, significant gaps in research and health policy exist that must be addressed to advance science and promote health equity.
Public Health Policy
Public health policy can be leveraged to diminish health inequities in infectious diseases. Much of this work begins by engaging stakeholders at the individual (eg, patients, providers, healthcare institutions) and population levels (eg, government, regulatory bodies, medical associations, funding agencies) to measure and address health inequities. A recent commentary by Ma et al [48] provides a conceptual model for measuring healthcare equity, including prevention and access, transitions, quality of care, post-discharge, and social and structural equity. Given that disparities in CDI incidence and outcomes are likely linked to these factors, we provide an example of a CDI healthcare equity model. First, patients should have equitable access to preventative and acute care. While healthcare exposures may predispose to C. difficile colonization, preventative care can aid in preventing or mitigating underlying health conditions that may place patients at higher risk for clinical infection. Next, patients with CDI often require care in different settings, which makes coordination among patients, caregivers, and providers essential to minimize negative health outcomes. Patients suspected of having CDI should have equitable and timely admission to the appropriate site of care (eg, primary, hospital admission, intensive care unit, long-term care). Quality of care can then be measured using infection-relevant health outcomes, such as clinical cure, hospital length of stay, mortality, readmissions, and recurrences. Next, post-discharge planning and execution should be equitable and should include access to prescribed treatments in the outpatient setting, counseling on proper home cleaning procedures, medications that may increase their risk for recurrence, and symptoms that may require them to contact their provider and schedule follow-up. Finally, social and structural equity can be measured at the community level using socioeconomic and environmental factors as described above.
Diversity in Clinical Trials and Observational Studies
REM participants have historically been underrepresented in clinical trials. Lack of representation could limit the generalizability of study findings to the general population but also limit access to potentially effective medical interventions and compound health disparities in these groups. Recently, US Food and Drug Administration–approved CDI therapies highlight this lack of diversity. The phase 3 clinical trials for both fidaxomicin and bezlotoxumab did not report participant race or ethnicity as part of their main study findings. More recently, 2 microbiome-targeted, live biotherapeutic products have been approved for prevention of recurrent CDI. The phase 3 trial for fecal microbiota, live-jslm (Rebyota) included approximately 5% Black and 2% Hispanic patients [49], and the phase 3 trial of fecal microbiota spores, live-brpk (Vowst) included 4% Black and 6% Hispanic patients [50]. These percentages are well below the population demographics of the United States: 12% Black and 19% Hispanic. Given the above-described differences in microbiome by SES, microbiome-targeted therapeutics may not be universally effective. Further studies using geographically or population-based precision microbiome therapeutics may be necessary to achieve optimal results.
Advances in Health Equity and Microbiome Research
Last, while there has been exponential growth in microbiome research in the past few decades, many studies have significant limitations, underrepresentation of minorities, and lack of measurements of social determinants of health or health inequities. In order to advance science in this field, future studies with adequate sample size should aim to integrate microbiome structure and function with health outcomes and SDoH. These studies could then control for confounding related to socioeconomic, environmental, psychosocial, and epigenetic factors that may be mediating the relationship between race/ethnicity and health outcomes. To do this, studies should include comprehensive data collection on patients, providers, healthcare facilities, and the community. Studies would also benefit from multidisciplinary teams, including basic, clinical, public/population health, social, and translational scientists, to optimize study design and execution and be inclusive of scientists from various backgrounds. Finally, most microbiome studies have focused on identifying associations between microbiome structure and disease, but fewer identify mechanistic/causal links. Advancements in whole-genome sequencing technology, multi-omics techniques, and epigenetics could play a critical role in linking functional changes in the gut ecosystem to disease and determinants of health.
CONCLUSIONS
Recent studies have highlighted health disparities in CDI incidence and health outcomes for racial and ethnic minorities. While there are numerous factors that contribute to health disparities in infectious diseases, a growing body of evidence indicates that the gut microbiome may play a role in responding to or perpetuating these disparities due to associations with underlying CDI risk factors and SDoH. This is particularly relevant in the CDI space, as CDI pathogenesis is tightly linked to ecological and functional changes in the gut microbiome, and more recent microbiome-targeted therapeutics aim to mitigate these effects. To achieve health equity in infectious diseases, advocacy that engages multiple levels of stakeholders to create public health policy is needed. Furthermore, future research should integrate social and structural determinants of health and advancing microbiome technologies.
Contributor Information
Kelly R Reveles, Pharmacotherapy Division, College of Pharmacy, University of Texas at Austin, Austin, Texas, USA; Pharmacotherapy Education & Research Center, University of Texas Health San Antonio, San Antonio, Texas, USA.
Kelsey A Strey, Pharmacotherapy Division, College of Pharmacy, University of Texas at Austin, Austin, Texas, USA; Pharmacotherapy Education & Research Center, University of Texas Health San Antonio, San Antonio, Texas, USA.
Jacinda C Abdul-Mutakabbir, Division of Clinical Pharmacy, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California–San Diego, La Jolla, California, USA; Division of the Black Diaspora and African American Studies, University of California–San Diego, La Jolla, California, USA.
V Mateo Mendoza, Pharmacotherapy Division, College of Pharmacy, University of Texas at Austin, Austin, Texas, USA; Pharmacotherapy Education & Research Center, University of Texas Health San Antonio, San Antonio, Texas, USA.
Joseph J Carreno, Department of Pharmacy Practice, Albany College of Pharmacy and Health Sciences, Albany, New York, USA.
Notes
Acknowledgments. The authors thank University of Texas at Austin College of Pharmacy student Hannah Angeles for reviewing the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Supplement sponsorship. This article appears as part of the supplement “The Microbiome and Human Health Perspective,” sponsored by Ferring Pharmaceuticals Inc., Seres Therapeutics Inc., and Nestlé Health Science.
References
- 1. Organization for Economic Co-operation and Development . Health at a glance, 2021. Available at: https://www.oecd.org/health/health-at-a-glance/. Accessed 19 September 2023.
- 2. Office of Disease Prevention and Health Promotion . Health equity and health disparities environmental scan. Available at: https://health.gov/sites/default/files/2022-04/HP2030-HealthEquityEnvironmentalScan.pdf. Accessed 1 June 2023.
- 3. Healthy People 2030 . Social determinants of health. Available at: https://health.gov/healthypeople/priority-areas/social-determinants-health. Accessed 1 June 2023.
- 4. Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med 2002; 347:1585–92. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . Health disparities in HIV, viral hepatitis, STDs, and TB. Available at: https://www.cdc.gov/nchhstp/healthdisparities/. Accessed 1 June 2023.
- 6. Argamany JR, Delgado A, Reveles KR. Clostridium difficile infection health disparities by race among hospitalized adults in the United States, 2001 to 2010. BMC Infect Dis 2016; 16:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao EJ, Kelly CR, Machan JT. Racial differences in Clostridium difficile infection rates are attributable to disparities in health care access. Antimicrob Agents Chemother 2015; 59:6283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freedberg DE, Salmasian H, Friedman C, Abrams JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection among inpatients. Am J Gastroenterol 2013; 108:1794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young EH, Strey KA, Lee GC, Carlson TJ, Koeller JM, Reveles KR. Clostridioides difficile infection treatment and outcome disparities in a national sample of United States hospitals. Antibiotics (Basel) 2022; 11:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JM, Zhou AY, Ortiz-Gratacos NM, Al Isso A, Tan KK, Abdul-Mutakabbir JC. Examining the impact of racial disparities on Clostridioides difficile infection outcomes at a southern California academic teaching hospital. Infect Control Hosp Epidemiol 2023:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hudspeth WB, Qeadan F, Phipps EC. Disparities in the incidence of community-acquired Clostridioides difficile infection: an area-based assessment of the role of social determinants in Bernalillo County, New Mexico. Am J Infect Control 2019; 47:773–9. [DOI] [PubMed] [Google Scholar]
- 12. Skrobarcek KA, Mu Y, Ahern J, et al. Association between socioeconomic status and incidence of community-associated Clostridioides difficile infection—United States, 2014-2015. Clin Infect Dis 2021; 73:722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021; 19:55–71. [DOI] [PubMed] [Google Scholar]
- 14. Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med 2018; 24:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ducarmon QR, Zwittink RD, Hornung BVH, van Schaik W, Young VB, Kuijper EJ. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol Mol Biol Rev 2019; 83:e00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 2014; 16:1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta A, Singh V, Mani I. Dysbiosis of human microbiome and infectious diseases. Prog Mol Biol Transl Sci 2022; 192:33–51. [DOI] [PubMed] [Google Scholar]
- 18. Antharam VC, Li EC, Ishmael A, et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol 2013; 51:2884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deschasaux M, Bouter KE, Prodan A, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med 2018; 24:1526–31. [DOI] [PubMed] [Google Scholar]
- 20. He Y, Wu W, Zheng HM, et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med 2018; 24:1532–5. [DOI] [PubMed] [Google Scholar]
- 21. Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol 2018; 16:e2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furuya-Kanamori L, Stone JC, Clark J, et al. Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2015; 36:132–41. [DOI] [PubMed] [Google Scholar]
- 23. Petersen R, Pan L, Blanck HM. Racial and ethnic disparities in adult obesity in the United States: CDC’s tracking to inform state and local action. Prev Chronic Dis 2019; 16:E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444:1027–31. [DOI] [PubMed] [Google Scholar]
- 25. Eze P, Balsells E, Kyaw MH, Nair H. Risk factors for Clostridium difficile infections—an overview of the evidence base and challenges in data synthesis. J Glob Health 2017; 7:010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beauregard JL, Hamner HC, Chen J, Avila-Rodriguez W, Elam-Evans LD, Perrine CG. Racial disparities in breastfeeding initiation and duration among U.S. infants born in 2015. MMWR Morb Mortal Wkly Rep 2019; 68:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drall KM, Tun HM, Morales-Lizcano NP, et al. Clostridioides difficile colonization is differentially associated with gut microbiome profiles by infant feeding modality at 3-4 months of age. Front Immunol 2019; 10:2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Odamaki T, Kato K, Sugahara H, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 2016; 16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bosco N, Noti M. The aging gut microbiome and its impact on host immunity. Genes Immun 2021; 22(5-6):289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kolodziejczyk AA, Zheng D, Elinav E. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol 2019; 17:742–53. [DOI] [PubMed] [Google Scholar]
- 31. Zagorsky JL, Smith PK. The association between socioeconomic status and adult fast-food consumption in the U.S. Econ Hum Biol 2017; 27(Pt A):12–25. [DOI] [PubMed] [Google Scholar]
- 32. Harrell CJ, Burford TI, Cage BN, et al. Multiple pathways linking racism to health outcomes. Du Bois Rev 2011; 8:143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carson TL, Wang F, Cui X, et al. Associations between race, perceived psychological stress, and the gut microbiota in a sample of generally healthy Black and White women: a pilot study on the role of race and perceived psychological stress. Psychosom Med 2018; 80:640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Amato KR, Arrieta MC, Azad MB, et al. The human gut microbiome and health inequities. Proc Natl Acad Sci U S A 2021; 118:e2017947118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Savage RD, Fowler RA, Rishu AH, et al. The effect of inadequate initial empiric antimicrobial treatment on mortality in critically ill patients with bloodstream infections: a multi-centre retrospective cohort study. PLoS One 2016; 11:e0154944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Na’amnih W, Adler A, Miller-Roll T, Cohen D, Carmeli Y. Incidence and risk factors for community and hospital acquisition of Clostridium difficile infection in the Tel Aviv Sourasky Medical Center. Infect Control Hosp Epidemiol 2017; 38:912–20. [DOI] [PubMed] [Google Scholar]
- 37. Gaskin DJ, Dinwiddie GY, Chan KS, McCleary RR. Residential segregation and the availability of primary care physicians. Health Serv Res 2012; 47:2353–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Young EH, Strey KA, Lee GC, et al. National disparities in antibiotic prescribing by race, ethnicity, age group, and sex in United States ambulatory care visits, 2009 to 2016. Antibiotics (Basel) 2022; 12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Castro-Sanchez E, Chang PWS, Vila-Candel R, Escobedo AA, Holmes AH. Health literacy and infectious diseases: why does it matter? Int J Infect Dis 2016; 43:103–10. [DOI] [PubMed] [Google Scholar]
- 40. Francois Watkins LK, Sanchez GV, Albert AP, Roberts RM, Hicks LA. Knowledge and attitudes regarding antibiotic use among adult consumers, adult Hispanic consumers, and health care providers—United States, 2012–2013. MMWR Morb Mortal Wkly Rep 2015; 64:767–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sirota M, Round T, Samaranayaka S, Kostopoulou O. Expectations for antibiotics increase their prescribing: causal evidence about localized impact. Health Psychol 2017; 36:402–9. [DOI] [PubMed] [Google Scholar]
- 42. Roslund MI, Puhakka R, Gronroos M, et al. Biodiversity intervention enhances immune regulation and health-associated commensal microbiota among daycare children. Sci Adv 2020; 6:eaba2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roux AV D. Investigating neighborhood and area effects on health. Am J Public Health 2001; 91:1783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet 2017; 389:1453–63. [DOI] [PubMed] [Google Scholar]
- 45. Patel AI, Schmidt LA. Water access in the United States: health disparities abound and solutions are urgently needed. Am J Public Health 2017; 107:1354–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McCormack G, Avery C, Spitzer AK, Chandra A. Economic vulnerability of households with essential workers. JAMA 2020; 324:388–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lane AA, McGuire MK, McGuire MA, et al. Household composition and the infant fecal microbiome: the INSPIRE study. Am J Phys Anthropol 2019; 169:526–39. [DOI] [PubMed] [Google Scholar]
- 48. Ma SE, Agrawal S, Sahli R. Distinguishing health equity and health care equity: a framework measurement. NEJM Catalyst 2023; 4. [Google Scholar]
- 49. Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs 2022; 82:1527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med 2022; 386:220–9. [DOI] [PubMed] [Google Scholar]