Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment for patients with acute leukemia. Despite this, studies have shown that only a minority of patients ultimately proceed to allo-HCT. The primary objective of this prospective, observational study was to identify the rate of allo-HCT in patients for whom it was recommended, and reasons why patients deemed appropriate and eligible for HCT did not subsequently undergo transplant. Between 04/2016 – 04/2021, adult patients with newly diagnosed or relapsed/refractory acute leukemia were enrolled at the time of induction/reinduction therapy. Initial transplantation workup and allo-HCT recommendations were made during the early phase of induction/reinduction. Of the 307 enrolled patients, allo-HCT was recommended to 85% (n=259), of whom 66% (n=170) underwent transplant. Donor sources comprised 54% human-leukocyte-antigen (HLA)-matched unrelated donors, 20% HLA-matched sibling donors and HLA-mismatched graft sources with 15% umbilical cord blood units, 8% HLA-mismatched unrelated donors and 4% HLA-haploidentical donors. The most common reason for transplant disqualification in the 89 patients in whom it was initially recommended was persistent/relapsed disease (70%), followed by early patient death (10%). In this prospective study, we report a high allo-HCT rate, which may be due to early transplant referral and workup. The main allo-HCT barrier was disease control, followed by early patient death. With the increasing availability of HLA-mismatched graft sources, lack of donor availability was not a transplant barrier. Further development of novel transplant strategies for patients not achieving remission, and improvements in induction regimens could result in increased allo-HCT utilization. ClinicalTrials.gov: NCT02677064.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative therapy for acute myelogenous leukemia (AML) and acute lymphoblastic leukemia (ALL), with AML being the most common allo-HCT indication for adult recipients in the United States1. Despite allo-HCT providing the best chance of long-term disease-free survival in acute leukemia, prior studies have suggested that allo-HCT is underutilized with only a minority of patients who may benefit from transplant ultimately proceeding to allo-HCT2. The last prospective study evaluating allo-HCT rates is now over 15-years ago3. In this study, Estey et al. reported that only a quarter of patients with AML and high-risk myelodysplastic syndrome (MDS) who achieved a first complete remission (CR) and were referred for transplant ultimately proceeded to allo-HCT3.
Historically, a key barrier to transplantation had been lack of a suitable stem cell donor, particularly in patients with a non-European ancestry4. However, this should no longer be a major limitation given the increasing availability of human leukocyte antigen (HLA) mismatched graft sources, which include umbilical cord blood units, HLA-haploidentical and HLA-mismatched unrelated donors1,5–7. Furthermore, with an expanded donor pool, advances in transplantation-specific conditioning regimens, and novel peri-transplant strategies, allo-HCT is now being offered to a wider group of patients including those of an advanced age, and in some instances, also considered in those not achieving CR1,8–10.
Given the potentially curative nature of allo-HCT for acute leukemia, it is important to determine the proportion of patients with newly diagnosed or relapsed disease proceeding to transplant, and to ascertain barriers to transplantation in the current era. In this prospective observational study, all newly diagnosed or relapsed adult patients with acute leukemia were enrolled at the time of induction or reinduction therapy and followed throughout their treatment journey to determine the rate of allo-HCT in patients for whom transplant was initially recommended. We also identified the reasons why patients initially deemed appropriate and eligible for allo-HCT did not subsequently proceed to transplant.
METHODS
Study Design and Patients
In this single-center, prospective observational study we enrolled adult patients aged ≥ 18 years with newly diagnosed or relapsed/refractory acute leukemia and admitted as an inpatient to the Memorial Sloan Kettering Cancer Center (MSK) for induction/reinduction therapy. This analysis reports on patients enrolled between April 2016 and April 2021 at MSK. Patients with relapsed acute leukemia post-allo-HCT, and those who received induction/re-induction therapy at an outside center or in the outpatient setting were excluded. The primary objective of the study was to prospectively determine the rate of allo-HCT in patients with newly diagnosed or relapsed acute leukemia who were initially recommended for transplant, and to identify factors that prevented patients from proceeding to allo-HCT. The secondary objective was to describe the stem cell donor sources and the concordance between the initially recommended and subsequently utilized stem cell source. Additional details of the study design and eligibility requirements are available through the ClinicalTrials.gov Identifier: NCT02677064.
All patients consented to the study and were enrolled at the time of commencement of induction/reinduction therapy during their inpatient stay at MSK. Each patient was assessed on three separate visits (Supplemental Figure 1). Visit number one occurred between the date of diagnosis and day 14 of induction/reinduction therapy. During visit number one, HLA-typing of patients and any available siblings was performed, as well as a preliminary unrelated donor search with the intention of identifying all potential donor sources early in the disease course. Information on patient ancestry as previously defined (European or non-European) was also collected11. However, no decision was made regarding whether the patient should proceed to allo-HCT given that it was too early in the disease course.
Visit number two occurred between days 15 to 21 of induction/reinduction therapy. A decision regarding the timing of allo-HCT was made at this visit through a MSK Leukemia and adult bone marrow transplant (BMT) consensus conference, which usually coincided with the weekly inpatient leukemia or BMT case conference meetings, attended by a large number of medical staff. A minimum of two faculty members from both the MSK leukemia and adult BMT service were required for the consensus decision. The allo-HCT recommendation took into consideration patient and disease characteristics, the 2015 National Comprehensive Cancer Network (NCCN) and 2017 European Leukemia Net (ELN) guidelines, and patient response and initial tolerance to the induction/reinduction therapy12–14. The final recommendation from the consensus conference at visit number two allowed for three options: (1) do not proceed with allo-HCT at this time (2) proceed with consolidative allo-HCT within the next few weeks (3) defer decision until next assessment at visit three. During visit number two, a description of the suitable stem cell donor source and procurement process was also discussed based on results from the preliminary unrelated donor search, HLA-typing of the patient and any available sibling(s), and umbilical cord blood unit search.
Visit number three occurred between days 28 to 42 of induction/reinduction therapy. At this time, the recommendation from the consensus conference was communicated to the patient, and if a decision was deferred during visit number two, it was rediscussed, and a recommendation was made. If a patient recommended for allo-HCT agreed to the proposed plan, an official BMT consultation to organize and plan the transplant was made. Regardless of the decision to proceed with allo-HCT, the research team developed a potential donor priority score for each patient at visit three to provide a recommendation on an optimal stem cell donor. The optimal donor choice was based on the availability of HLA-matched related or unrelated donors, umbilical cord blood units and other HLA-mismatched donors, the MSK donor selection algorithm (Supplemental Figure 2) and whether there was a need to urgently proceed to allo-HCT.
Follow-up assessments were completed by the research team at 6- and 12-months following induction/reinduction therapy to determine whether the patient proceeded to allo-HCT, and whether the preferred stem cell donor source was used. Amongst patients who did not follow with the recommendations, the reasons for not proceeding or using an alternative donor were obtained. The reasons for not proceeding to allo-HCT included persistent/relapsed disease, patient preference, physician preference, new comorbidities, early death, and the lack of a suitable stem cell donor.
Study Oversight
The clinical trial (NCT02677064) was approved by the MSK Institutional Review Board and conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization guidelines for Good Clinical Practice. Written informed consent was obtained from patients prior to enrollment. All authors had access to the data and were involved in the analysis of results and vouch for the data and adherence to the protocol.
Study Endpoints
The primary endpoint of the study was the proportion of patients who proceeded to allo-HCT within those who were initially recommended for transplant. Key secondary endpoints included identifying factors that prevented patients recommended for allo-HCT from proceeding with transplant, a description of the utilized stem cell donor sources within patients who proceeded to allo-HCT, and the overall survival of patients with newly diagnosed acute leukemia recommended for transplant.
Statistical Analysis
Patient baseline characteristics were descriptively summarized with median (interquartile range, IQR) for continuous measures, and frequency (percentage) for categorical variables. The primary and secondary endpoints were descriptively summarized with frequency (percentage). Median (95% confidence interval, CI) follow-up was estimated from the initial diagnosis using the reverse Kaplan-Meier method. For the subset of newly diagnosed patients who were recommended transplant, overall survival was estimated and compared between the patients who had proceeded to allo-HCT per the recommendation versus those who did not proceed to allo-HCT using a log-rank test. A landmark timepoint of 6-months after the initial diagnosis was used, as this is the time by which patients would be expected to have received the recommended transplant. All analyses were done in R v4.1.3 and statistical significance defined at two-sided P < 0.05.
RESULTS
Patients
Of the 444 patients that were pre-screened, 307 patients met eligibility criteria and consented to the study. The median follow-up for the cohort was 4.0 years (95% CI, 3.7 – 4.4 years). Baseline characteristics of the patients are summarized in Table 1. The median age was 58 years (IQR, 46 – 66 years). Twenty-three percent of the study population had a non-European ancestry.
Table 1.
Baseline characteristics of study participants
| Characteristic | Overall, N = 3071 |
AML, N = 2241 |
B-cell ALL, N = 491 |
MPAL, N = 151 |
T-cell ALL, N = 181 |
Ambiguous lineage, N = 11 |
|---|---|---|---|---|---|---|
| Age | 58 (46, 66) | 60 (51, 67) | 55 (40, 63) | 55 (42, 63) | 38 (30, 54) | 27 (27, 27) |
| Age ≥ 65 | 88 (29%) | 77 (34%) | 8 (16%) | 2 (13%) | 1 (5.6%) | 0 (0%) |
| Age ≥ 70 | 46 (15%) | 44 (20%) | 2 (4.1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Sex | ||||||
| Female | 127 (41%) | 102 (46%) | 16 (33%) | 5 (33%) | 4 (22%) | 0 (0%) |
| Male | 180 (59%) | 122 (54%) | 33 (67%) | 10 (67%) | 14 (78%) | 1 (100%) |
| Broad Ancestry | ||||||
| European | 184 (60%) | 133 (59%) | 32 (65%) | 9 (60%) | 9 (50%) | 1 (100%) |
| Non-European | 72 (23%) | 52 (23%) | 11 (22%) | 4 (27%) | 5 (28%) | 0 (0%) |
| Missing | 51 (17%) | 39 (17%) | 6 (12%) | 2 (13%) | 4 (22%) | 0 (0%) |
| Diagnosis type | ||||||
| Newly diagnosed | 251 (82%) | 185 (83%) | 36 (73%) | 13 (87%) | 16 (89%) | 1 (100%) |
| Refractory | 19 (6.2%) | 16 (7.1%) | 3 (6.1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Relapsed | 37 (12%) | 23 (10%) | 10 (20%) | 2 (13%) | 2 (11%) | 0 (0%) |
data summarized as median (IQR) and n (%); AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MPAL, mixed phenotypic acute leukemia; IQR, interquartile range.
Two-hundred and fifty-one patients (82%) had newly diagnosed acute leukemia, 37 (12%) had relapsed, and 19 (6%) had refractory disease. The most common histologic subtype of acute leukemia was AML (n = 224 patients; 73%), followed by B-cell ALL (n = 49; 16%), T-cell ALL (n = 18; 5.9%), mixed phenotypic acute leukemia (n = 15; 4.9%) and acute leukemia of ambiguous lineage (n = 1; 0.3%). Within patients with AML, 51% had adverse risk disease, 27% intermediate risk and 22% favorable risk disease as per the ELN 2017 risk stratification14. The breakdown of ELN 2017 risk stratification by AML diagnosis type (newly diagnosed vs. relapsed vs. refractory) is provided in Supplemental Table 1. Of the patients with B-cell ALL, 18% were Philadelphia chromosome positive (Ph+). Further details on the clinical subtypes of the acute leukemias and available cytogenetic and molecular profiles are provided in Supplemental Table 2.
Transplant Recommendations
Within the entire study cohort, allo-HCT was recommended to 259 patients (85%). No transplant recommendation was made in two patients who died early prior to visit number two. The median age of patients recommended for allo-HCT was significantly higher compared to those who were not recommended for allo-HCT (59 vs. 52 years, respectively; P = 0.007) – Supplemental Figure 3. There was no significant difference in the ancestries of patients recommended for allo-HCT compared to those who were not recommended for allo-HCT (non-European ancestry; 24% vs. 20%, respectively P = 0.2). For patients with AML, the distribution of ELN risk was significantly different between those who were recommended allo-HCT vs. not (favorable: 14 vs. 76%, intermediate: 30 vs. 6.9%, adverse: 56 vs. 17%, P < 0.001) – Supplemental Figure 4. Further details on the characteristics of the patients recommended for versus not recommended for allo-HCT are provided in Supplemental Table 3.
Figure 1 summarizes the initial transplant recommendations for patients and their subsequent outcomes. Of the 259 patients recommended for allo-HCT, 170 (66%) underwent transplantation at MSK. The allo-HCT rate was similar between patients aged < 60 years (n = 134) versus ≥ 60 years (n = 125) at 66% and 65%, respectively. Of the 46 patients who were not recommended for allo-HCT, 10 (22%) patients subsequently proceeded to allo-HCT for either relapsed or persistent disease (including measurable residual disease positivity).
Figure 1.
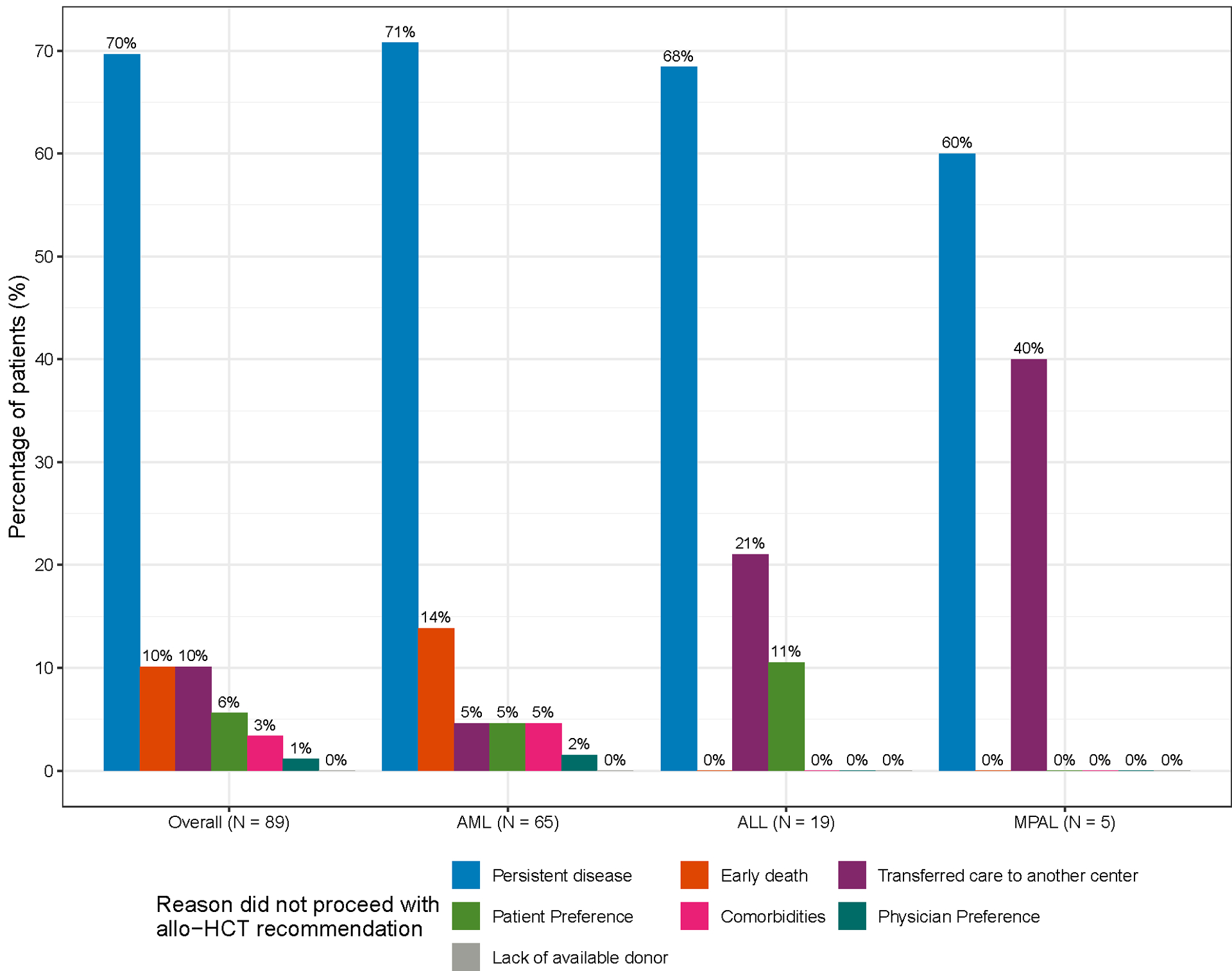
Allo-HCT recommendations and subsequent transplantation rates within the entire study population. * Two patients had early death before an allo-HCT recommendation could be made.
Transplant Barriers
The most common reason for failure to undergo transplantation in the 89 patients recommended for allo-HCT but who did not proceed was persistent/relapsed disease (70%). This was followed by early patient death (10%), patient preference (6%), new comorbidities (3%), and physician preference (1%) – Figure 2. Persistent/relapsed disease remained the most common reason for failure to proceed to allo-HCT in all acute leukemia subtypes. Nine patients (10%) were lost to follow-up due to transfer to another center and it is possible that some of these patients may have received an allo-HCT at an outside institution. No patient failed to proceed to allo-HCT due to the lack of an available donor as a suitable stem cell donor source was identified in all patients.
Figure 2.
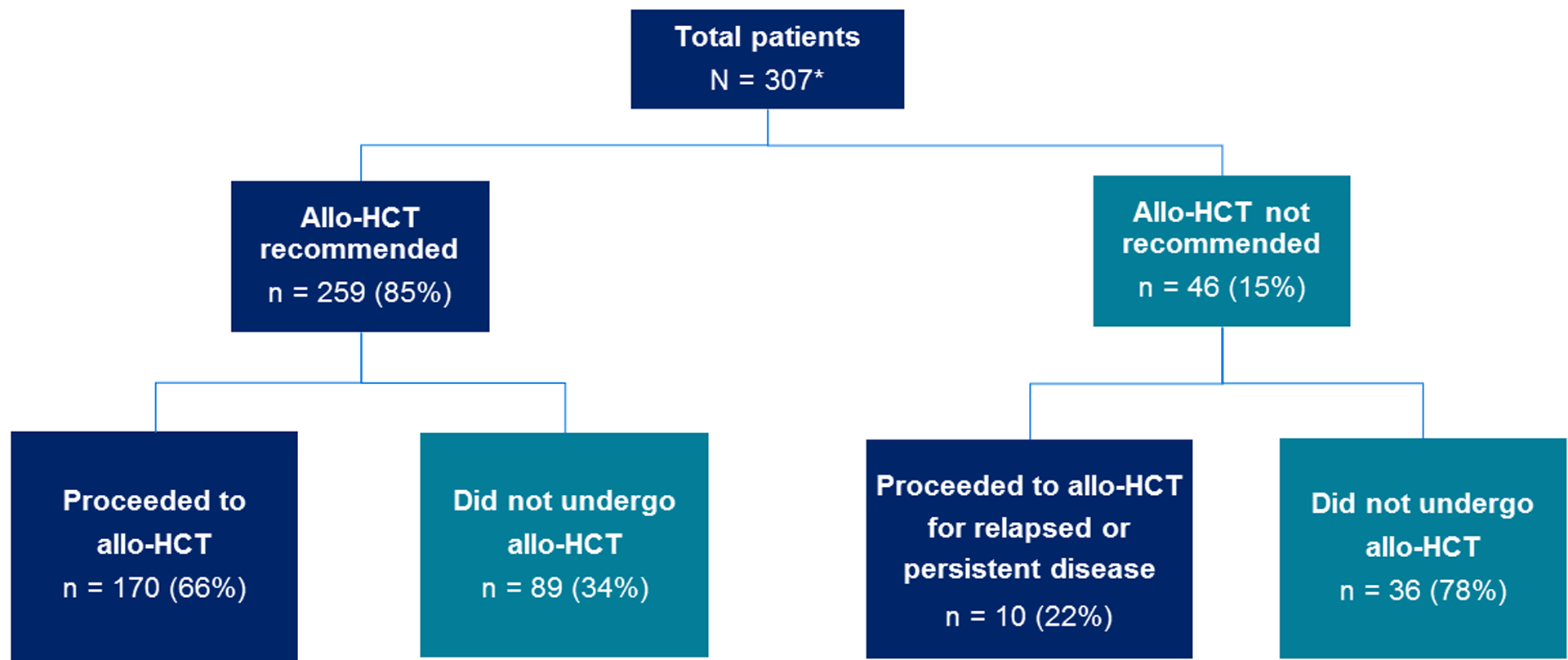
Barriers to allo-HCT in patients for whom it was recommended for but did not subsequently undergo transplant. AML; acute myeloid leukemia. ALL; acute lymphoblastic leukemia (both B-cell ALL and T-cell ALL); MPAL, mixed phenotypic acute leukemia. * It is possible that patients transferred to another center (n = 10 of overall population) may have subsequently received an allo-HCT at an outside institution.
Patients with newly diagnosed acute leukemia recommended for allo-HCT
Of the 138 patients with newly diagnosed acute leukemia who were recommended for, and proceeded to allo-HCT, the median time from start of induction therapy to allo-HCT was 113 days (IQR 87 – 145). Within the subset of patients with newly diagnosed acute leukemia recommended for transplant (n = 205), a landmark analysis of overall survival was conducted, comparing patients who proceeded to transplant versus those who did not (n = 111 and 58, respectively). The landmark time was 6-months after initial diagnosis, resulting in exclusion of 36 patients either due to death (n = 33) or lost to follow-up (n = 3) within the 6-month interval. Given as persistent/relapsed disease was the most common reason for failure to proceed to allo-HCT, the estimated 3-year overall survival (OS) probability for patients who failed to proceed to allo-HCT was significantly lower at 27% (95% CI, 17 – 42%) compared to 69% (95% CI, 60 – 78%) for those who proceeded to allo-HCT (log-rank P < 0.001) – Supplemental Figure 5. Of the 58 patients in the landmark analysis that did not proceed to allo-HCT within the first 6-months, 22 (38%) did proceed to allo-HCT beyond the 6-months at a median time of 6.81 (IQR 6.33 – 7.52) months from initial diagnosis.
When comparing baseline characteristics of patients with newly diagnosed AML who were recommended for and proceeded to allo-HCT (n = 105) to those who were recommended for but did not to proceed to allo-HCT because of persistent disease (n = 34), patients who proceeded to allo-HCT were significantly younger (median age, 59 vs. 63 years, P = 0.016) compared to those who did not proceed to transplant because of persistent disease – Table 2. The AML types among the two groups were significantly different (p = 0.002) with lower proportion of patients who proceeded to allo-HCT having secondary AML (3 vs. 21%, respectively). The distribution of ELN 2017 risk was not statistically different between the two groups (favorable: 15 vs 12%, intermediate: 33 vs. 18%, adverse 71 vs. 51%, p = 0.13). The day-14 post-induction therapy bone marrow blast percentage (as measured morphologically and categorized as < 5% versus ≥ 5%) was not significantly different amongst the two groups (P > 0.9).
Table 2.
Comparison of patients with newly diagnosed AML who were either recommended for allo-HCT and proceeded to transplant, or recommended for allo-HCT but did not proceed due to persistent disease
| Characteristic | Overall, N = 1391 |
Proceeded to allo-HCT, N = 105 (76%)1 |
Persistent disease precluding allo-HCT N = 34 (24%)1 |
P value2 |
|---|---|---|---|---|
| Age | 61 (51, 67) | 59 (49, 65) | 63 (56, 71) | 0.016 |
| ELN risk stratification | 0.13 | |||
| Favorable | 20 (14%) | 16 (15%) | 4 (12%) | |
| Intermediate | 41 (29%) | 35 (33%) | 6 (18%) | |
| Adverse | 78 (56%) | 54 (51%) | 24 (71%) | |
| Day 14 bone marrow blast % | > 0.9 | |||
| < 5% | 38 (47%) | 32 (47%) | 6 (46%) | |
| ≥ 5% | 43 (53%) | 36 (53%) | 7 (54%) | |
| Not available | 58 | 37 | 21 | |
| AML subtype | 0.002 | |||
| Therapy-related | 19 (14%) | 14 (13%) | 5 (15%) | |
| AML – other3 | 54 (39%) | 47 (45%) | 7 (21%) | |
| Secondary AML | 10 (7.2%) | 3 (2.9%) | 7 (21%) | |
| AML with MDS related changes | 56 (40%) | 41 (39%) | 15 (44%) |
data summarized as median (IQR) and n (%);
Wilcoxon rank sum test; Fisher’s exact test; Pearson’s Chi-squared test;
AML – other includes AML with recurrent genetic abnormalities and AML, not otherwise specified. ELN, European Leukemia Net; allo-HCT, allogeneic hematopoietic cell transplantation; MDS, myelodysplasia
Stem Cell Donor Source
In the 170 patients who underwent allo-HCT, the most common stem cell donor source was HLA-matched unrelated donors in 54% followed by HLA-mismatched graft sources in 27% and HLA-matched sibling donors in 20%. HLA-mismatched graft sources comprised umbilical cord blood units in 15%, HLA 4–7/8 mismatched unrelated donors in 8% and haploidentical donors in 4%. Patients with a non-European ancestry had significantly higher utilization of HLA-mismatched graft sources than those with a European ancestry (50 vs. 16%, P < 0.001).
Eighty-five percent of patients had a consistent donor source between the initially recommended donor at visit number three, and the subsequently utilized donor. Reasons why patients received a different donor to their initial recommendation are summarized in Supplemental Table 4.
DISCUSSION
In this prospective analysis of 307 adult patients with newly diagnosed or relapsed/refractory acute leukemia, we demonstrate a high-rate of 66% of allo-HCT in patients who were initially recommended for transplantation (85% of the cohort). The transplant rate remained high in patients aged ≥ 60 years at 65%. Of the approximately one third of patients who did not proceed to transplant, persistent/relapsed disease remained the primary reason for transplant disqualification in 70%, and another 10% did not proceed due to early death. This was despite the study being carried out in a large comprehensive cancer center with access to novel therapies and clinical trials. Importantly, we demonstrate that with the increasing availability of HLA-mismatched donor sources, the lack of a suitable donor no longer represented a barrier to HCT. Based on these data, safely improving induction regimens, and developing novel allo-HCT strategies for patients with acute leukemia not achieving remission could result in increased HCT utilization and may ultimately improve long-term survival.
Prior studies have investigated rates of allo-HCT in patients with acute leukemia. In a prospective analysis from 2001 – 2003, Estey and colleagues reported that of the 38% of patients who achieved a CR to induction therapy, only 54% were referred for a transplant consultation, and of those, only 26% of patients proceeded to allo-HCT3. A retrospective analysis from the Fred Hutchinson Cancer Center of 116 newly diagnosed patients with high- or intermediate risk AML and in a first CR from 2013 demonstrated that 67% of eligible patients successfully proceeded to transplant15. However, a total of 182 patients had high- or intermediate risk AML, but 66 patients were excluded because of early death (n = 15), not achieving a CR (n = 44) and loss to follow-up (n = 7). As we had done in our study, if these patients were included in determining the allo-HCT rate (n = 182), the transplantation rate would have only been 48%15. The most common cause for patients failing to proceed to transplant in this study was disease relapse within six months15. There are several important differences between this study and ours. First, our study is a prospective analysis of a larger cohort which provided data on initial transplant recommendations and more reliably established subsequent transplantation rates in those recommended for allo-HCT. Second, our analysis of allo-HCT rates was not only limited to patients who achieved a CR after induction/reinduction therapy, and we did not exclude patients who had early death/loss to follow-up from our analysis. Third, we included patients diagnosed with all acute leukemia subtypes, as well as those presenting with relapsed/refractory disease. Fourth, in the Fred Hutchinson study, HLA typing was not performed on all patients and strong conclusions regarding donor availability could not be made, whereas we prioritized early HLA typing and performed our study in an era when HLA-mismatched graft sources are becoming increasingly available. In another retrospective single-center analysis, the allo-HCT rate observed in patients with acute leukemia and MDS was 57%, but reduced to 44% in those aged ≥ 60 years, and was lower than the allo-HCT rate of 65% in patients ≥ 60 years reported in our study16.
There are several reasons for the high transplant utilization seen in this study. The study design ensured a close collaboration between the leukemia and BMT services as well as early consensus review and early allo-HCT referral and transplant workup in those recommended for transplant. This supports a previous study that showed that beginning the transplant workup as early as possible improved the chances of consolidative allo-HCT (70 of 159, 44%) for patients aged ≤ 60 years in first CR for high-risk AML17. In this prior study, HLA-mismatched donor usage was 16%, but 18/37 patients in first CR did not receive allo-HCT due to either no donor identified (n = 1), other reasons (n = 9), or unknown reasons (n = 8). In our cohort, another reason for the increased transplant utilization is the increasing availability of HLA-mismatched donor sources. Over a quarter of transplanted patients had an HLA-mismatched graft source, and HLA-mismatched graft sources were more commonly used amongst patients with a non-European ancestry.
Our study does have some limitations. Given the prospective nature of the study, we only enrolled patients admitted as an inpatient to MSK for induction/reinduction therapy and not those who may have been initially treated for their leukemia at outside centers or treated with less intensive regimens in an outpatient setting. This is relevant given the favorable post-transplant outcomes that are seen in patients ≥ 60 years who receive lower-intensity induction therapies18. The study is currently undergoing modification to include patients treated at MSK in the outpatient setting. Nonetheless, based on these data, it will be important to develop strategies and partnerships with outside community centers to ensure patients with acute leukemia are referred early in their treatment journey to transplant centers for allo-HCT assessment.
In conclusion, in this prospective clinical trial we demonstrate that 66% of patients with acute leukemia recommended for allo-HCT ultimately proceeded to transplant. The main barrier to transplant was early disease progression followed by early patient death. With the availability of HLA-mismatched donor sources, the lack of a suitable stem cell donor was no longer a barrier to allo-HCT. Given as allo-HCT offers the best chance of cure for most patients with acute leukemia, these data suggests that future progress not only depends on improving transplant outcomes, but also on our ability to ensure early and efficient transplant workup, utilize HLA-mismatched donors, safely improve induction regimens, and optimize allo-HCT strategies for patients with acute leukemia that do not achieve remission.
Supplementary Material
ACKNOWLEDGEMENTS
All authors gratefully acknowledge the US National Cancer Institute Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center.
DISCLOSURES
Giralt: Research funding; Miltenyi Biotec, Takeda Pharmaceutical, Celgene, Amgen, Sanofi, Johnson & Johnson, Actinium Pharmaceuticals, and Omeros, and is a member on the advisory boards for Kite Pharma, Celgene, Sanofi, Novartis, Johnson & Johnson, Amgen, Takeda Pharmaceutical, Jazz Pharmaceuticals, Janssen, Actinium Pharmaceuticals, and Spectrum Pharma. Ponce: Seres Therapeutics: Research Funding; Incyte: Consultancy, Research Funding; Kadmon/Sanofi: Consultancy; Ceramidex: Consultancy; Evive: Consultancy; CareDx: Consultancy. Lin: Magenta Therapeutics: Consultancy; Kite, A Gilead Company: Consultancy. Shaffer: Miltenyi Biotec: Research Funding; Gamida Cell: Consultancy; Hansa Biopharma: Consultancy. Politikos: ExcelThera: Membership on an entity’s Board of Directors or advisory committees; PrecicionHeor: Honoraria; Merck: Research Funding. Park: Autolus Therapeutics: Consultancy; Artiva Biotherapeutics, Inc.: Membership on an entity’s Board of Directors or advisory committees; Amgen: Consultancy; Allogene Therapeutics: Membership on an entity’s Board of Directors or advisory committees; Affyimmune Therapeutics, Inc.: Consultancy; Juno: Research Funding; Genentech: Research Funding; AstraZeneca: Consultancy; Servier: Consultancy, Other: Provision of Services; Novartis: Consultancy; Kura Oncology: Consultancy; Kite, a Gilead Company: Consultancy; Intellia: Consultancy; Curocell Inc.: Consultancy; Innate Pharma: Consultancy; Bristol-Myers Squibb: Consultancy. Perales: Dr. Perales reports honoraria from Adicet, Allogene, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene, and Sellas Life Sciences, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune, Omeros and OrcaBio. He has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. Tallman: Abbvie: Research Funding; Orsenix: Research Funding; Biosight: Research Funding; Glycomimetics: Research Funding; Rafael Pharmaceuticals: Research Funding; Amgen: Research Funding; AbbVie: Membership on an entity’s Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity’s Board of Directors or advisory committees; Orsenix: Membership on an entity’s Board of Directors or advisory committees; KAHR-Adv Bd: Membership on an entity’s Board of Directors or advisory committees; Oncolyze: Membership on an entity’s Board of Directors or advisory committees; Jazz Pharma: Membership on an entity’s Board of Directors or advisory committees; Roche: Membership on an entity’s Board of Directors or advisory committees; Biosight: Membership on an entity’s Board of Directors or advisory committees; Novartis: Membership on an entity’s Board of Directors or advisory committees; Innate Pharmaceuticals: Membership on an entity’s Board of Directors or advisory committees; Kura: Membership on an entity’s Board of Directors or advisory committees; Syros Pharmaceuticals: Membership on an entity’s Board of Directors or advisory committees; Ipsen Biopharmaceuticals: Membership on an entity’s Board of Directors or advisory committees; UpToDate: Patents & Royalties: Royalties. Barker: Merck: Research Funding; New York Blood Center: Consultancy; Gamida Cell: Consultancy. Stein: Astellas Pharmaceutical, Agios Pharmaceuticals, and Genentech: Consultancy, Membership on an entity’s Board of Directors or advisory committees; PTC Therapeutics and Syros: Membership on an entity’s Board of Directors or advisory committees; Syndax: Consultancy, Research Funding; Amgen, AbbVie, Seattle Genetics, and Biotheryx: Consultancy; Daiichi-Sankyo, Celgene Pharmaceuticals, and Novartis: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Auron Therapeutics: Current equity holder in private company; PinotBio, Bristol Myers Squibb, Jazz Pharmaceuticals, Foghorn Therapeutics, Blueprint Medicines, Gilead Sciences, Janssen Pharmaceuticals: Consultancy; Bayer: Research Funding. Gyurkocza: Actinium Pharmaceuticals, Inc.: Research Funding.
Footnotes
Presented in part at the 64th ASH Annual Meeting and Exposition, New Orleans, LA 2022 (Oral Presentation)
DATA SHARING STATEMENT
Subject to patient privacy and confidentiality obligations, access to patient-level data and supporting clinical documents can be made available upon request and subject to review by the corresponding author. Such requests can be made to the corresponding author by email at gyurkocb@mskcc.org.
REFERENCES
- 1.Phelan R, Chen M, Bupp C, et al. Updated Trends in Hematopoietic Cell Transplantation in the United States with an Additional Focus on Adolescent and Young Adult Transplantation Activity and Outcomes. Transplantation and Cellular Therapy. 2022/07/01/ 2022;28(7):409.e1–409.e10. doi: 10.1016/j.jtct.2022.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao S, Hahn T, Zhang Y, et al. Unrelated donor allogeneic hematopoietic cell transplantation is underused as a curative therapy in eligible patients from the United States. Biol Blood Marrow Transplant. Oct 2013;19(10):1459–64. doi: 10.1016/j.bbmt.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 3.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood. Feb 15 2007;109(4):1395–400. doi: 10.1182/blood-2006-05-021907 [DOI] [PubMed] [Google Scholar]
- 4.Joshua TV, Rizzo JD, Zhang MJ, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. Jul 15 2010;116(14):3469–76. doi: 10.1002/cncr.25297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JN, Rocha V, Scaradavou A. Optimizing unrelated donor cord blood transplantation. Biol Blood Marrow Transplant. Jan 2009;15(1 Suppl):154–61. doi: 10.1016/j.bbmt.2008.10.020 [DOI] [PubMed] [Google Scholar]
- 6.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. Jun 2008;14(6):641–50. doi: 10.1016/j.bbmt.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw BE, Jimenez-Jimenez AM, Burns LJ, et al. National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol. Jun 20 2021;39(18):1971–1982. doi: 10.1200/jco.20.03502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stelljes M, Middeke JM, Bug G, et al. In Patients with Relapsed/Refractory AML Sequential Conditioning and Immediate Allogeneic Stem Cell Transplantation (allo-HCT) Results in Similar Overall and Leukemia-Free Survival Compared to Intensive Remission Induction Chemotherapy Followed By Allo-HCT: Results from the Randomized Phase III ASAP Trial. Blood. 2022;140(Supplement 1):9–11. doi: 10.1182/blood-2022-159962 [DOI] [Google Scholar]
- 9.Gyurkocza B, Nath R, Seropian SE, et al. 40 - High Rates of Transplantation in the Phase III Sierra Trial Utilizing Anti-CD45 (Iodine) 131I-Apamistamab (Iomab-B) Conditioning with Successful Engraftment and Tolerability in Relapsed Refractory (R/R) Acute Myeloid Leukemia (AML) Patients after Lack of Response to Conventional Care and Targeted Therapies. Transplantation and Cellular Therapy. 2022/03/01/ 2022;28(3, Supplement):S35–S36. doi: 10.1016/S2666-6367(22)00201-9 [DOI] [Google Scholar]
- 10.Schmid C, Labopin M, Schaap N, et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia – a matched pair analysis by the Acute Leukaemia Working Party of EBMT. British Journal of Haematology. 2019;184(5):782–787. doi: 10.1111/bjh.15691 [DOI] [PubMed] [Google Scholar]
- 11.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. Nov 2010;16(11):1541–8. doi: 10.1016/j.bbmt.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCCN. NCCN Clinical Practice Guidelines in Oncology - Acute Myeloid Leukemia. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf
- 13.NCCN. NCCN Clinical Practice Guidelines in Oncology - Acute Lymphoblastic Leukemia. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- 14.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. Jan 26 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mawad R, Gooley TA, Sandhu V, et al. Frequency of Allogeneic Hematopoietic Cell Transplantation Among Patients With High- or Intermediate-Risk Acute Myeloid Leukemia in First Complete Remission. Journal of Clinical Oncology. 2013/11/01 2013;31(31):3883–3888. doi: 10.1200/JCO.2013.50.2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bashey A, Zhang X, Morris LE, et al. Improved access to HCT with reduced racial disparities through integration with leukemia care and haploidentical donors. Blood Advances. 2023;7(15):3816–3823. doi: 10.1182/bloodadvances.2023009765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagel JM, Othus M, Garcia-Manero G, et al. Rapid Donor Identification Improves Survival in High-Risk First-Remission Patients With Acute Myeloid Leukemia. JCO Oncol Pract. Jun 2020;16(6):e464–e475. doi: 10.1200/jop.19.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Short NJ, Ong F, Ravandi F, et al. Impact of Type of Induction Therapy on Outcomes in Older Adults with AML after Allogeneic Stem Cell Transplantation. Blood Advances. 2023:bloodadvances.2022009632. doi: 10.1182/bloodadvances.2022009632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Subject to patient privacy and confidentiality obligations, access to patient-level data and supporting clinical documents can be made available upon request and subject to review by the corresponding author. Such requests can be made to the corresponding author by email at gyurkocb@mskcc.org.


