Abstract
INTRODUCTION
Bone-targeted therapies (BTTs) are integral to the management of bone metastases in metastatic castration-resistant prostate cancer (mCRPC). BTTs vary considerably in referral and drug access pathways and optimal BTT use requires multispecialty consultation and supervision. Health quality improvement (HQI) has become the predominant framework to improve patient care in multidisciplinary settings.
METHODS
HQI initiatives on use of BTT in mCRPC were developed and evaluated in five centers of a provincial cancer center network using plan-do-study-act (PDSA) methodology. Multidisciplinary teams (MDTs) completed a common quality assessment form and an HQI template and then implemented an HQI initiative. Feedback and findings were shared and discussed at regional events. It was subsequently determined whether to adopt, adapt, or abandon initiatives.
RESULTS
Patterns of unmet needs varied across types of BTT. Gaps in use of radium-223 were mostly referral and education issues that could be directly addressed at the local level by participating clinician teams. Conversely, most supportive BTT gaps were related to coverage and resourcing support. HQI initiatives selected by each site consisted of implementation or expansion of local MDT meetings, referral documents, databases, and improvement charters. The main HQI initiative was completed in four sites and was adapted or adopted in three. Improvements in BTT use were observed in two of three centers with data on HQI process measures.
CONCLUSIONS
Despite the overall heterogenous structure of the groups and metrics used, this study demonstrated that the PDSA framework provides the needed structure for improvements in BTT use in mCRPC across multiple sites.
INTRODUCTION
Health quality improvement (HQI) aims to improve the value, safety, standardization, and quality of care for patients in both acute and chronic disease settings.1–5 HQI initiatives use the continued collection and analyses of prespecified data to enhance patient care and disease management by eliminating errors and improving practice standards, as well as communication among caregivers. 4 HQI has, therefore, become the predominant framework used to improve patient outcomes and system performance in a multidisciplinary healthcare setting.6,7
Prostate cancer (PCa) is the second most common malignancy diagnosed in men and was the fifth leading cause of cancer-related mortality among men in 2020.8 Although nearly all metastatic patients respond to initial androgen deprivation therapy (ADT ), castration-resistant PCa (CRPC) invariably develops, leading to disease progression. Bone is a common site of progression in metastatic CRPC (mCRPC), occurring in approximately one-third of patients within two years of castration resistance and in over 90% of patients over the course of disease.9 This is a substantial cause of PCa-related morbidity and reduced quality of life, resulting in pain, fractures, spinal cord compression, and reduced mobility.10,11
Treatment of bone metastases from PCa can involve the use of palliative radiation therapy, antineoplastic, and bone-protective agents, with the goals of extending survival, alleviating pain, and reducing risk of skeletal-related events.12,13 Approved anti-neoplastic therapies for treatment of mCRPC, irrespective of metastatic site, include ADT, chemotherapy, androgen receptor pathway inhibitors (ARPI), poly (ADP-ribose) polymerase inhibitors, and prostate-specific membrane antigen ligand-targeted radiopharmaceuticals.14 Bone-targeted therapies (BTTs) include the α-emitting radionucleotide, radium-223, palliative external beam radiotherapy, and bone-protective agents, such as denosumab and zoledronic acid. Despite widespread guideline recommendations for BTT use in patients with bone metastases,15–19 issues related to BTT underutilization have been reported in multiple jurisdictions.20–24
Management of PCa is a dynamic, multidisciplinary process involving a number of specialties, including nursing, pathology, urology, radiation oncology, and medical oncology. Multidisciplinary collaboration is integral to optimally managing bone health. While bone-protective agents can generally be prescribed by urologists and oncologists, referrals to specialist centers for bone health assessment and management are recommended. 25 In our jurisdiction, radium-223 can only be prescribed by radiation oncologists and is administered in conjunction with nuclear medicine (NM ) experts.26–28 As cancer care at oncology centers can easily become siloed, the multidisciplinary collaboration needed to optimally manage bone metastases can be challenging. Common issues include a lack of coordination and communication among healthcare specialists, oncologist workload, lack of established referral pathways, and a lack of access to treatments.
BC Cancer is one of the largest networks of cancer centers in Canada and manages approximately 3600 new cases of PCa per year.29,30 The BeTTer Outcomes Workgroup (BOW) HQI project was initiated as a means of improving the care of mCRPC patients with bone metastases across five major BC cancer centers. The primary goals of the project were to facilitate discussions on use of BTTs and the development of a bone care improvement initiative in each site that, if successful, could be shared at the provincial level and adopted by other centers.
METHODS
Study design and setting
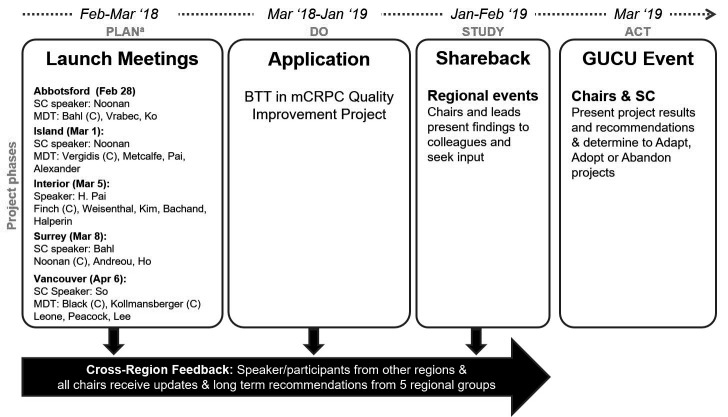
This multicenter study assessed the impact of HQI initiatives directed toward use of BTT in mCRPC in a large, provincial cancer center network — BC Cancer. The study was conducted in all centers considered to have sufficient capacity to undertake a multidisciplinary HQI initiative (Surrey, Abbotsford, Kelowna, Vancouver, and Victoria). The initiative used plan-do-study-act (PDSA) methodology and workflow (Figure 1), as outlined by the Institute for Healthcare Improvement.31 The project aimed to optimize BTT prescription and referral patterns in patients with mCRPC in British Columbia. Process measures included pharmacy records of drug administration and physician recommendations, as well as number of bone health-related specialist consultations. Balancing measures included increased workload on physicians, as well as side effects from BTT, such as osteonecrosis of the jaw.
Figure 1.
Visual diagram of the BeTTer Outcomes Workgroup plan-do-study-act (PDSA) cycle. aQuality improvement principles and steps. BTT: bone-targeted therapy; C: chair; GUCU event: provincial genitourinary tumor group meeting; MDT: multidisciplinary team; mCRPC: metastatic castration-resistant prostate cancer; SC: steering committee.
PDSA process
The plan step involved the completion of health quality assessment and PDSA flowsheet forms (Supplementary Figure 1; available at cuaj.ca) by multidisciplinary teams (MDTs) at all five participating sites. This was followed by a retrospective chart review in select sites to confirm initial health quality assessments. The do step consisted of implementation of the selected HQI project by each group. In the study step, feedback and findings arising from initiatives and investigations conducted at each site were shared and discussed at regional events. In the final act step, chairs and steering committee members determined whether to adopt, adapt, or abandon initiatives, along with a concurrent expansion to other genitourinary (GU) disease settings, such as malignancies of the kidney, bladder, and testes, after review of project results. Additional details of each PDSA step, including assessments performed and tools developed are available in the Appendix (at cuaj.ca).
Analysis
In the analysis of needs assessment feedback patterns, met needs were categorized as either access and awareness, outcome measures, triage, diagnosis and followup process, confidence in data, MDT communication, or other. Unmet needs were categorized as patient barriers, resourcing, access/coverage, MDT communication, education, application of standards of care, or referral issues. Survey and retrospective chart audit data were summarized using descriptive statistics and are reported in the form of tables and plots (pie charts) and in text. Assessment of HQIs was also performed descriptively, without pre-established statistical thresholds to define minimally significant improvement.
Ethics approval
Ethics board approval for the HQI initiative was not procured, as no patient identifiable information was used for the purpose of the outcome evaluation. Retrospective chart audits conducted at each site were approved by the respective ethics boards.
RESULTS
Overall
The BOW was composed of 41 participants from different specialties (urology, medical oncology, radiation oncology, urologic oncology, internal medicine, endocrinology, and nursing) and distributed across multiple locations in BC (Figure 2, Supplementary Table 1; available at cuaj.ca). Participants were organized in teams, one for each BC Cancer center (n=5) and included team members, guests, and community partners. The PDSA process was conducted across all sites over a period of 14 months — from February 2018 to March 2019 (Figure 1).
Figure 2.
Composition of the BeTTer Outcomes Workgroup participants by specialty (top) and location (bottom).
Table 1.
Unmet needs, quality assessment investigations, HQI initiatives, measures, and outcomes across participating BC cancer center MDTs
| BC cancer center | Most frequent types of unmet needs | Additional quality assessment investigationsa | Main HQI initiative(s) | HQI MDT meetings (n) | HQI measure and predicted outcome | Measured outcome | Adopt, adapt, or abandon HQI initiative |
|---|---|---|---|---|---|---|---|
| Surrey | NM BTTs – Referral (n=3, 60%) and education (n=1, 20%) issues Supportive BTTs – Poor coverage (n=3, 43%) and resourcing support (n=2, 29%) |
None | MDT mCRPC patient review meetings | 20 | BTT recommendation patterns Increase |
Additional BTT recommendations (n=48) in 29 patients, including mCRPC patients (n=20) | Adapt by merging with Abbottsford team |
| Abbotsford | NM BTTs – Referral (n=5, 50%) and MDT communication (n=2, 20%) issues Supportive BTTs – poor coverage (n=5, 63%) and education (n=25%) issues |
Retrospective audit of all patients treated with radium-223 in 2017 | MDT mCRPC case review meetings and instituted regular GU MDT meetings in the area | 15 | Use of BTTs Increase (≥20%) |
NA | Adapt by merging with Surrey team |
| Kelowna | NM BTTs – Referral issues (n=3, 43%) and poor access (n=2, 29%) Supportive BTTs – Resourcing support (n=3, 38%) and standards of care not followed (n=3, 38%) |
Prospective survey of medical oncologists on use of BTTs for mCRPC | GP mCRPC bone health letter | 6 | Patients on ADT starting BTT treatment Increase |
NA | Abandon – project canceled due to lack |
| Vancouver | NM BTTs – Referral (n=6, 50%) and education (n=6, 50%) issues Supportive BTTs – Poor coverage (n=7, 64%) and education (n=2, 18%) issues |
None | Radium-223 community referral form and checklist | 5 | Radium-223 referral rates Increase |
New patients referred for radium-223 treatment (n=7)b | Abandonc |
| Victoria | NM BTTs – Referral (n=2, 33%) and access (n=2, 33%) issues Supportive BTTs – Standards of care not followed (n=4, 50%) and poor coverage (n=3, 38%) |
Retrospective audit on BTT use in radium-223 patients | Bone health referral and materials (referral guidelines and forms; bone health order and assessment forms) | 6 | Percentage of patients supported with bone protective agents Increase |
55 patients (60.4%) prescribed with bone health agents | Adoptd |
In addition to the common PDSA needs assessment form completed by participating MDTs from all sites.
This number was not considered sufficient for formal analysis and conclusion by the local MDT.
Continued MDT collaboration beyond the PDSA study period did, however, result in development of a new initiative – the Vancouver inter-professional clinic for advanced prostate cancer (VICAP).
With introduction of process refinements.
BC: British Columbia; BTT: bone-targeted therapies; GP: general practitioner; GU: genitourinary; HQI: health quality improvement; mCRPC: metastatic castration-resistant prostate cancer; MDT: multidisciplinary team; NA: not available or applicable; NM BTT: nuclear medicine bone-targeted therapy (i.e., radium-223).
A total of 33 BOW members from working groups in Surrey, Abbotsford, Kelowna, Vancouver, and Victoria participated in a survey on patterns of BTT use as part of the initial quality assessment. A total of 190 needs assessment comments were generated from structured discussions, 42 of which were submitted as out-of-scope. Of those in-scope, the comments describing unmet needs (n=82) outweighed those describing needs that were considered to be met (n=66). Met needs were most often categorized as access and awareness to treatment (34.2% and 32.1%), MDT communication (23.7% and 35.7%), and triage process (21.1% and 17.9%) for both types of BTTs (NM and general/supportive, respectively). Referral (47.5%) and education (22.5%) issues were the most common unmet needs identified for NM BTTs, while coverage (47.6%) and standards of care not followed (19.0%) were the most common for general/supportive BTTs (Figure 3).
Figure 3.
Feedback patterns from BeTTer Outcomes Workgroup participants on met (top panels) and unmet (bottom panels) needs regarding delivery of nuclear medicine (i.e., radium-223; left panels) and general/supportive (right panels) BTTs. BC: British Columbia; BTT: bone-targeted therapy; MDT: multidisciplinary team; NM: nuclear medicine; SOC: standard of care.
Surrey
Unmet needs identified by Surrey participants that were actionable by the site’s BOW team were related to referrals and physician education (n=6). As such, the group selected improving BTT workflow and collaboration between BC Cancer and the community via MDT meetings to review mCRPC patients as their main HQI project (Table 1). The group predicted that after 4–6 months of meetings there would be improvements in at least one workflow and/or clinical parameter and, if successful, this would be apparent in a narrow patient population (mCRPC with bone disease) after a short test period. Bimonthly mCRPC MDT tumor board meetings (n=20) involving team members and community partners were initiated to improve collaboration and assess proper initiation of BTTs. Patients referred for BTT by any physician in the area were eligible for discussion. An online tool was created to track outcomes from the MDT meetings (Supplementary Figure 2A; available at cuaj.ca).
Impact of the HQI initiative in Surrey was evaluated by changes in BTT recommendation patterns from MDT case reviews after the start of the project. Results of the analysis (as of June 14, 2018; after 15 meetings) showed that of the 29 patients discussed, 20 (69.0%) were mCRPC and recommendations for radium-223, denosumab, zoledronic acid, radiotherapy, and calcium/vitamin D were issued in 17 (58.6%), 14 (48.3%), one (3.4%), seven (24.1%), and nine (31.0%) of the cases, respectively. In addition, bone clinic referrals increased by 24.1% (n=7) relative to baseline. Since previous recommendations for BTT were not changed, these represented net increases in BTT recommendation rates relative to start of the HQI initiative; therefore, the conditions for a successful HQI were met. The Surrey team subsequently merged with the Abbotsford team to conduct ongoing multisite collaborative MDTs with an expanded GU focus.
Abbotsford
Most of the unmet needs in NM BTT identified by the Abbotsford team were issues related to referrals and MDT communication (n=7, 70%) that could be potentially addressed with interventions at the local level (Table 1). In contrast, supportive BTT gaps were predominantly related to coverage/access (n=5, 63%), issues that often require changes at provincial and national levels. The Abbotsford group opted to conduct further quality assessment using an informal, retrospective chart review of all patients treated with radium-223 in the previous year (2017; n=24) and predicted that this would find a pattern of late referrals. The analysis showed that a median of three prior treatments (range 1–7) were undertaken prior to use of radium-223, with a median time from mCRPC to radium-223 initiation of two years. Notably, radium-223 was never used as initial therapy for mCRPC in this small patient cohort and the vast majority of patients (n=20) required palliative radiotherapy at some point but only three during or after radium-223 treatment. The results indicated that patients had more prior lines of therapy at radium-223 initiation relative to the average in the BC Cancer area from September 2013 to February 2016 (median of two, range 0–5)32 and in the pivotal ALSYMPCA trial.33 The group presented their audit data and sequencing insight to the Abbotsford GU oncology team to seek advice on moving forward with HQI projects.
In order to address referral and MDT communication issues, the Abbotsford group took a leading role in mCRPC case reviews in MDT meetings (n=15, at times in collaboration with the Surrey team) and instituted periodic GU meetings in the area. The team also created a triage referral document for treating physicians to address workflow issues and an improvement charter to track quality improvement. A 20% increase in use of both NM and supportive BTTs by the end of 2019 was predicted and set as goal for the initiative. As of July 2020, the Abbotsford team merged their MDT meetings with the Surrey site to form a multisite collaborative MDT with an expanded GU focus.
Kelowna
Issues related to referrals, MDT communication, and compliance with standards of care (n=7, 47%) were common BTT gaps identified by the Kelowna team. Other unmet needs that were identified were related to access and coverage, as well as resourcing support (n=8, 53%). In particular, the group identified a need for a regional GU multidisciplinary tumor board where urologists, medical oncologists, and radiation oncologists could meet to discuss GU oncology cases, including patients with mCRPC.
Additional quality assessment was performed by surveying medical oncologists treating mCRPC patients (n=11) to assess use of concomitant bone health agents alongside radium-223, and to understand the knowledge level of bone health support in patients receiving radium-223 (Table 1). Results showed that six patients were supplementing with vitamin D, while no patients were receiving calcium, denosumab, or a bisphosphonate (Supplementary Table 2A; available at cuaj.ca). Only one patient confirmed that they had undergone bone mineral density (BMD ) screening in the last three years, and the majority (n=8) could not recall. The team identified the need for a systemic way to recommend supplementation with vitamin D and calcium, as well as an increase in early BMD screening.
General practitioners (GPs) were identified as care providers that may be able to best address the need, however, prior data demonstrated that they had been less involved. After holding six meetings, the Kelowna team created an educational letter to GPs outlining information on bone health in order to address workflow and treatment recommendation issues and to increase rates of vitamin D and calcium supplementation and early BMD screening. The letter included specific recommendations for supporting bone health, as well as fields for patient-specific information (Supplementary Figure 2B; available at cuaj.ca). However, the project was subsequently cancelled due to lack of resources.
Vancouver
The initial needs assessment performed by the Vancouver project group was challenging due to a small number of BTT referrals. Gaps in use of NM BTT identified by the group were workflow issues either related to physician education (n=6) or referrals (n=6) (Table 1). Conversely, most unmet needs in use of supportive BTTs were due to coverage or resourcing support (n=8, 73%) that required changes in infrastructure. The group selected as the primary project goal to prepare a radium-223 referral form/checklist to optimize the referral process and to ensure the patients being referred met eligibility criteria. An increase in radium-223 referral rate relative to the start of the project was predicted. The checklist was finalized and implemented in July 2018 (Supplementary Figure 2C; available at cuaj.ca) and seven new patients were referred for treatment; however, this number was not considered sufficient to perform formal analysis and draw conclusions. After five team meetings, the project was deferred to 2020, with the goal of achieving a total of 10 referrals. Although the tool was never officially adopted, the core MDT group continued to meet to discuss a spinoff multidisciplinary clinic for PCa patients called the Vancouver Inter-Professional Clinic for Advanced Prostate Cancer (VICAP), which was launched in late 2020.
Victoria
Gaps identified for optimal BTT treatment by the Victoria working group included both workflow challenges (n=8), such as issues related to referrals, MDT communication, physician education, and compliance with standards of care; and infrastructure issues (n=6), such as barriers to drug access and resourcing support (Table 1). A retrospective chart review was performed to assess the use of concomitant bone health agents in patients receiving radium-223. A total of 70 patients who had received treatment between January 2015 and December 2017 were reviewed (Supplementary Table 2B; available at cuaj.ca). Similar to the results of the chart review in Abbottsford, the degree of pretreatment was higher than the pivotal ALSYMPCA trial,33 with nearly all patients (n=69, 99%) receiving at least one line of systemic therapy prior to radium-223. Results also showed that only 10% of patients were treated with concurrent bone health agents (denosumab or zoledronate; compared to 41% bisphosphonate use in the pivotal ALSYMPCA trial33), all of which were started before commencing radium-223. In patients who were receiving a bone health agent, 57% experienced a symptomatic skeletal event, compared to 76% in patients who did not receive a bone health agent.
A bone health referral network was proposed to streamline access to bone health consultations. It was predicted that the initiative would increase the percentage of patients receiving bone-protective agents. The bone health referral network was initiated by identifying specialists from endocrinology and internal medicine with a special interest in bone health for cancer patients and establishing patterns of referral through dialogue and continuing medical education events. Referral documents were created, including referral guidelines, referral forms for bone health specialists, and pre-printed order and assessment forms for bone health management (Supplementary Figure 2D; available at cuaj.ca). A followup analysis performed between June 2018 and January 2019 showed that 91 cancer patients (prostate, n=56; breast, n=24; other, n=11) were referred, of whom 15 had bony metastasis. A total of 55 patients (60.4%) ultimately were prescribed bone health agents, including 29 with low-dose denosumab, 13 with high-dose denosumab, nine with zoledronic acid, and four with oral bisphosphonates, in addition to conservative measures. As improvements in the referral process and increase in volume of BTT prescriptions were quite apparent, the group continued to use the referral process after the PDSA study period, along with implementing process refinements.
DISCUSSION
Quality assessment is a key component of HQI projects34–37 and it was initially performed in this study using a common needs assessment template. Although clinicians felt their centers were doing well in addressing many BTT needs related to access and awareness, MDT communication, and patient triage, use of BTT in mCRPC presents multiple, concurrent issues. Patterns of unmet needs identified by project participants varied across types of BTT. Gaps in use of NM BTTs (i.e., radium-223) were mostly referral and education issues that could be directly addressed at the local level by participating clinician teams. As a result, many of the initiatives undertaken were focused on NM -delivered BTTs. Conversely, most gaps identified for supportive BTTs were related to coverage and resourcing support. The project framework allowed for communication of these issues at provincial level in the form of comments and reports to the BC GU tumor group and to the steering committee, which included provincial and Canadian Urological Association leads.
The main HQI initiative selected by each site was completed in four sites and was adapted or adopted in three. Among the three centers with available data on process measures, improvements in BTT use were observed in two and data was not considered sufficient for formal analysis in the third site. Key challenges to implementing HQI projects of multidisciplinary nature are clinician availability, adequate center capacity and resourcing, coordination of team efforts across multiple areas of expertise, and need for strong team leadership. 38–43 Limited resourcing and capacity resulted in premature cancellation of the project in one center (Kelowna) and failure to implement the main HQI initiative in another (Vancouver). The merge of MDTs from two centers (Surrey and Abbottsford) was an adaptation mechanism used that is associated with increased capacity and reductions in overall and individual participant burden.
This descriptive study also provides support for the use of group discussions as a tool to facilitate multidisciplinary collaboration. Multidisciplinary meetings provide an appropriate setting to discuss complex issues, such as those related to patient selection, treatment initiation and sequencing, optimization of lines of therapy, and management of multiple morbidities.44–46 This is especially important as management of advanced PCa patients becomes increasingly complex, with new genetic testing tools and more treatment options across multiple lines of treatment.47,48
Improvements in multidisciplinary interaction were apparent during the HQI project by the creation and expansion of MDTs and implementation of periodic meetings in some centers. MDT discussions often addressed topics related to recurrent feedback patterns of late or absent BTT referrals, such as when to stop ARPI treatment, patient triage and followup, referral pathways, and initiation of funding requests. Timely initiation of treatment is particularly important for BTTs in mCRPC with bone metastases, as both the oncologic condition and some of its treatments threaten bone health and BTT eligibility may be affected by disease progression (e.g., radium-223). For example, late referral to radium-223 was a frequently reported unmet need and both retrospective chart reviews found a relatively high number of previous lines of therapy at radium-223 initiation. Although the PDSA study did not allow to evaluate changes in timing of BTT initiation, HQI initiatives undertaken were often designed to improve MDT communication and resulted in apparent increases in BTT referral and prescription rates. Multidisciplinary collaboration continued beyond the PDSA study, as evident by adoption or adaptation of project initiatives (in Surrey/Abbottsford and Victoria) or development of new ones (VICAP in Vancouver).
Strengths and limitations
One of the strengths of our study was its multicenter nature, allowing for assessment of HQI initiatives in five large and diverse settings, thereby reducing the impact of site-specific bias and increasing confidence in the generalizability of the results. Network-wide meetings promoted knowledge and experience sharing, which set the foundation for implementation and homogenization of bone health practices at the provincial level. Two individuals have undergone formal HQI training, which proved to be very beneficial to implementing this framework in the respective centers. Moreover, HQI and bone healthcare expertise are readily applicable to disease settings other than mCRPC, including earlier PCa stages and other GU cancers. The MDT board in Surrey expanded to include BTT discussions for other GU cancers and the MDT discussions expanded to include urologists, as well as radiation oncologists; many of the patients referred to bone health experts in Victoria were non-mCRPC.
A significant limitation of the study is the overall heterogenous structure of the groups and metrics used. For example, outcome measures varied substantially across sites and data on balancing measures was only available from one site. Data collection was challenging due to the lack of centralized databases for referrals and prescriptions. We also acknowledge that there was some divergence from the initial project goals and protocols and less rigorous documentation of initiatives than had initially been planned. Our study shortcomings represent a lesson for the necessity of adaptability and empowerment of individual sites when conducting high-quality qualitative evaluations in lower resourced or geographically disparate settings.
CONCLUSIONS
Through this project, we were able to build knowledge and capacity for HQI, offering a promising strategy to address growing BTT needs from a population perspective. Insights from this work indicate that HQI methods may be a powerful approach for development of tailored initiatives aimed at improving multidisciplinary care across cancer center networks.
Supplementary Information
ACKNOWLEDGEMENT
The authors would like to thank Dan Pelletier, Jennifer Lowther, Ramona Foster, and Michele Glen for their administrative support; Chris Bitcon for his research support; Deanna McLeod and Liam Sturgess from Kaleidoscope Strategic Inc. for their research and editorial support; and Bayer and Amgen for supporting this work.
Footnotes
Appendix available at cuaj.ca
This paper has been peer-reviewed.
COMPETING INTERESTS: Dr. Noonan has served in a consultancy or advisory role for AstraZeneca, Astellas, Bayer, BMS, Eisai, EMD Serono, Ipsen, Janssen, Merck, and Pfizer. Dr. Ko has served in a consultancy or advisory role for, Astellas, AstraZeneca, Bayer, Ipsen, Janssen, Merck, Pfizer, and Takeda; has received honoraria from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, and Pfizer, and received research funding from Bayer and Janssen. Dr. Black has served in a consultancy or advisory role for AbbVie, Astellas, AstraZeneca, Bayer, BMS, EMD Serono, Ferring, Janssen, MDxHealth, Merck, Minogue, Nonagen, Nanology, Pfizer, Protara, QED, Roche, Sanofi, Sesen, STIMIT, Therelase, UroGen, and Verity; a speaker for Bayer, BioSyent, Pfizer, Sanofi, and TerSera; has participated in clinical trials supported by Roche; and holds a patent from Veracyte. Dr. Peacock has served in a consultancy or advisory role and received honoraria from Bayer; and received an CIHR grant for research funding. Dr. Finch has served in a consultancy or advisory role and received honoraria from Astellas, Bayer, and Janssen. Dr. Kollmannsberger has served in a consultancy or advisory role and received honoraria from Astellas, BMS, Eisai, Ipsen, Janssen, Merck, Novartis, and Pfizer. Dr. Pai has received honoraria from Bayer; has received research funding from Janssen and Tolmar; and served on the speakers’ bureau for AbbVie. All other authors do not report any competing personal or financial interests related to this work.
FUNDING: Funding for this project was provided through an unrestricted educational grant from Bayer Canada. The sponsor provided administrative support and played an active role in data collection.
REFERENCES
- 1.Young C, Ignaszewski M, Wilson M. Roots of quality improvement in health care. BC Med J . 2017;59:517–22. [Google Scholar]
- 2.Anderson JC, Rungtusanatham M, Schroeder RG. A theory of quality management underlying the Deming management method. Acad Manage Rev . 1994;19:472–509. doi: 10.2307/258936. [DOI] [Google Scholar]
- 3.Walton M. The deming management method: The bestselling classic for quality management! Penguin. 1988 [Google Scholar]
- 4.Kheraj R, Tewani SK, Ketwaroo G, et al. Quality improvement in gastroenterology clinical practice. Clin Gastroenterol Hepatol . 2012;10:1305–14. doi: 10.1016/j.cgh.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reese D, Dolansky MA, Moore SM, et al. Quality improvement education innovation: Evaluation of Coursera MOOC ‘Take the Lead on Healthcare Quality Improvement’. J Res Nurs . 2021;26:62–78. doi: 10.1177/1744987120982644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batalden PB, Davidoff F. What is “quality improvement” and how can it transform healthcare? Qual Saf Health Care . 2007;16:2–3. doi: 10.1136/qshc.2006.022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backhouse A, Ogunlayi F. Quality improvement into practice. BMJ . 2020;368:m865. doi: 10.1136/bmj.m865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin . 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 9.Den RB, George D, Pieczonka C, et al. Ra-223 treatment for bone metastases in castrate-resistant prostate cancer: Practical management issues for patient selection. Am J Clin Oncol . 2019;42:399–406. doi: 10.1097/COC.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuzuki S, Park SH, Eber MR, et al. Skeletal complications in cancer patients with bone metastases. Int J Urol . 2016;23:825–32. doi: 10.1111/iju.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldessari C, Pipitone S, Molinaro E, et al. Bone metastases and health in prostate cancer: From pathophysiology to clinical implications. Cancers (Basel) . 2023;15:1518. doi: 10.3390/cancers15051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Oronzo S, Wood S, Brown JE. The use of bisphosphonates to treat skeletal complications in solid tumours. Bone . 2021;147:115907. doi: 10.1016/j.bone.2021.115907. [DOI] [PubMed] [Google Scholar]
- 13.Boopathi E, Birbe R, Shoyele SA, et al. Bone health management in the continuum of prostate cancer disease. Cancers (Basel) . 2022;14:4305. doi: 10.3390/cancers14174305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turco F, Gillessen S, Cathomas R, et al. Treatment landscape for patients with castration-resistant prostate cancer: Patient selection and unmet clinical needs. Res Rep Urol . 2022;14:339–50. doi: 10.2147/RRU.S360444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.So A, Chin J, Fleshner N, et al. Management of skeletal-related events in patients with advanced prostate cancer and bone metastases: Incorporating new agents into clinical practice. Can Urol Assoc J . 2012;6:465–70. doi: 10.5489/cuaj.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saad F, Aprikian A, Finelli A, et al. 2021 Canadian Urological Association (CUA)-Canadian Uro Oncology Group (CUOG) guideline: Management of castration-resistant prostate cancer (CRPC) Can Urol Assoc J . 2021;15:E81–e90. doi: 10.5489/cuaj.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman R, Hadji P, Body JJ, et al. Bone health in cancer: ESMO clinical practice guidelines. Ann Oncol . 2020;31:1650–63. doi: 10.1016/j.annonc.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Grávalos C, Rodríguez C, Sabino A, et al. SEOM clinical guideline for bone metastases from solid tumors. Clin Transl Oncol . 2016;18:1243–53. doi: 10.1007/s12094-016-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline PART II. J Urol . 2021;205:22–9. doi: 10.1097/JU.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 20.Freedland SJ, Richhariya A, Wang H, et al. Treatment patterns in patients with prostate cancer and bone metastasis among US community-based urology group practices. Urology . 2012;80:293–8. doi: 10.1016/j.urology.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Body JJ, von Moos R, Rider A, et al. A real-world study assessing the use of bone-targeted agents and their impact on bone metastases in patients with prostate cancer treated in clinical practice in Europe. J Bone Oncol . 2019;14:100212. doi: 10.1016/j.jbo.2018.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapierre M, Fraelic A. Utilization of radium-223 dichloride (Ra-223) in patients with metastatic castration-resistant prostate cancer in Canada: A real-world retrospective study. Value Health . 2016;19:A162. doi: 10.1016/j.jval.2016.03.1492. [DOI] [Google Scholar]
- 23.Poon D, Chan K, Chan T, et al. 219MO Real-world utilization pattern of bone-targeted agents for metastatic prostate cancer: Web-based questionnaire study by Hong Kong Society of Uro-Oncology (HKSUO) [abstract] Ann Oncol . 2020;31:S1326. doi: 10.1016/j.annonc.2020.10.439. [DOI] [Google Scholar]
- 24.Seesaghur A, Egger P, Warden J, et al. Assessment of bone-targeting agents use in patients with bone metastasis from breast, lung or prostate cancer using structured and unstructured electronic health records from a regional UK-based hospital. BMJ Open . 2023;13:e069214. doi: 10.1136/bmjopen-2022-069214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JE, Handforth C, Compston JE, et al. Guidance for the assessment and management of prostate cancer treatment-induced bone loss. A consensus position statement from an expert group. J Bone Oncol . 2020;25:100311. doi: 10.1016/j.jbo.2020.100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renzulli JF, 2nd, Collins J, Mega A. Radium-223 dichloride: Illustrating the benefits of a multidisciplinary approach for patients with metastatic castration-resistant prostate cancer. J Multidiscip Healthc . 2015;8:279–86. doi: 10.2147/JMDH.S81007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker C, Heidenreich A, Nilsson S, et al. Current approaches to incorporation of radium-223 in clinical practice. Prostate Cancer Prostatic Dis . 2018;21:37–47. doi: 10.1038/s41391-017-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng S, Arciero V, Goldberg H, et al. Population-based analysis of the use of radium-223 for bone-metastatic castration-resistant prostate cancer in Ontario, and of factors associated with treatment completion and outcome. Cancer Manag Res . 2019;11:9307–19. doi: 10.2147/CMAR.S213051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer BC. New prostate cancer treatment improves survival rate in men with advanced disease. 2021. [Accessed March 23, 2023]. [updated 2021 July 21]. Available at: http://www.bccancer.bc.ca/about/news-stories/stories/new-prostate-cancer-treatment-improves-survival-rate-in-men-with-advanced-disease.
- 30.Cancer BC. Cancer statistics online dashboard - New cancer diagnoses, British Columbia, by cancer type, age at diagnosis and sex - genital organs, prostate, male, 2010–2018. 2022. [Accessed March 23, 2023]. [updated 2022 Oct 27]. Available at: https://bccandataanalytics.shinyapps.io/incidencecounts/
- 31.Institute for Healthcare Improvement. Quality improvement essentials toolkit. 2023. [Accessed June 21, 2023]. [updated 2023]. Available at: https://www.ihi.org/resources/Pages/Tools/Quality-Improvement-Essentials-Toolkit.aspx.
- 32.Parimi S, Tsang E, Alexander A, et al. A population-based study of the use of radium 223 in metastatic castration-resistant prostate cancer: Factors associated with treatment completion. Can Urol Assoc J . 2017;11:350–5. doi: 10.5489/cuaj.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 34.Bramesfeld A, Wensing M, Bartels P, et al. Mandatory national quality improvement systems using indicators: An initial assessment in Europe and Israel. Health Policy . 2016;120:1256–69. doi: 10.1016/j.healthpol.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Lohr KN, editor. Institute of Medicine Committee to Design a Strategy for Quality RAiM. Medicare: A Strategy for Quality Assurance. Vol. 1. Washington (DC): National Academies Press (US); 1990. Copyright © 1990 by the National Academy of Sciences. [Google Scholar]
- 36.World Health Organization. Performance Assessment Tool for Quality Improvement in Hospitals (PATH) 2007. [Accessed June 27, 2023]. Available at: https://apps.who.int/iris/handle/10665/107808. [DOI] [PubMed]
- 37.Veillard J, Champagne F, Klazinga N, et al. A performance assessment framework for hospitals: the WHO regional office for Europe PATH project. Int J Qual Health Care. 2005;17:487–96. doi: 10.1093/intqhc/mzi072. [DOI] [PubMed] [Google Scholar]
- 38.Brown GTF, Bekker HL, Young AL. Quality and efficacy of multidisciplinary team (MDT) quality assessment tools and discussion checklists: A systematic review. BMC Cancer . 2022;22:286. doi: 10.1186/s12885-022-09369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madge S, Khair K. Multidisciplinary teams in the United Kingdom: Problems and solutions. J Pediatr Nurs . 2000;15:131–4. doi: 10.1053/jn.2000.5516. [DOI] [PubMed] [Google Scholar]
- 40.Winters DA, Soukup T, Sevdalis N, et al. The cancer multidisciplinary team meeting: In need of change? History, challenges, and future perspectives. BJU Int . 2021;128:271–9. doi: 10.1111/bju.15495. [DOI] [PubMed] [Google Scholar]
- 41.Walraven JEW, Verhoeven RHA, Meulen RVD, et al. Facilitators and barriers to conducting an efficient, competent, and high-quality oncological multidisciplinary team meeting. BMJ Open Qual . 2023;12:e002130. doi: 10.1136/bmjoq-2022-002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madu A. Challenges in conducting quality improvement projects: reflections of a junior doctor. Future Healthc J . 2022;9:333–4. doi: 10.7861/fhj.2022-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arvidsson E, Dahlin S, Anell A. Conditions and barriers for quality improvement work: A qualitative study of how professionals and health centre managers experience audit and feedback practices in Swedish primary care. BMC Fam Pract . 2021;22:113. doi: 10.1186/s12875-021-01462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soukup T, Sevdalis N, Green JSA, et al. Quality improvement for cancer multidisciplinary teams: Lessons learned from the Anglian Germ Cell Cancer Collaborative Group. Br J Cancer . 2021;124:313–4. doi: 10.1038/s41416-020-01080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lammila-Escalera E, Greenfield G, Barber S, et al. A systematic review of interventions that use multidisciplinary team meetings to manage multimorbidity in primary care. Int J Integr Care . 2022;22:6. doi: 10.5334/ijic.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taberna M, Gil Moncayo F, Jané-Salas E, et al. The multidisciplinary team (MDT) approach and quality of care. Front Oncol . 2020;10:85. doi: 10.3389/fonc.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekhoacha M, Riet K, Motloung P, et al. Prostate cancer review: Genetics, diagnosis, treatment options, and alternative approaches. Molecules . 2022;27:5730. doi: 10.3390/molecules27175730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powers E, Karachaliou GS, Kao C, et al. Novel therapies are changing treatment paradigms in metastatic prostate cancer. J Hematol Oncol . 2020;13:144. doi: 10.1186/s13045-020-00978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.