Abstract
Novel interventions for sickle cell disease (SCD) bring hope to patients, yet concern about thfe associated economic costs exist. Cost-effectiveness analysis (CEA) uses standardized methods, with robust underpinnings in health economics, to estimate the value of these interventions compared to usual care. However, due to the complexity and lifetime trajectory of SCD, CEAs are challenging to conduct. The objectives of this rapid review are to summarize the main characteristics, components and results of published CEAs of existing interventions for SCD, identify research gaps, and provide directions for future analyses. We identified records through searches of bibliographic databases, from reference lists of relevant review articles, and through consultation with experts. Thirteen CEAs met our inclusion criteria and were qualitatively synthesized. These evaluated blood transfusions (n=2), hematopoietic stem cell transplantation (n=1), pharmaceuticals (n=2), hypothetical cell or genetic therapy (n=1), screening programs (n=4), and interventions for SCD treatment complications (n=3). A limited number of potential SCD complications and treatment complications were evaluated. No study adopted a societal perspective in the base case, six studies examined lifetime cost-effectiveness, seven studies employed a Markov or discrete event simulation model, and eight studies used an outcome metric that captures both quality and length of life. To better compare the value of emerging and current therapies, future CEAs should adopt a societal perspective incorporating both medical and non-medical costs, comprehensively model SCD complexity using robust health economic simulation models over patient’s entire lifespan, and capture the intervention’s effect on both survival and quality of life.
Keywords: cost-effectiveness analysis, sickle cell disease, critical review
1. INTRODUCTION
Sickle cell disease (SCD) refers to a group of inherited red blood cell disorders, affecting over 20 million people throughout the world. In the United States (U.S.), the prevalence of SCD is approximately 100,000 and the majority of those affected are Black or African-American [1]. SCD can lead to a series of acute and chronic complications, such as acute pain episodes, chronic pain, stroke, acute chest syndrome, symptoms of anemia, and an increased risk of infections and organ damage [1]. These complications significantly impact patients’ life expectancy and quality of life [2–4]. Moreover, the economic costs due to SCD are considerable, with annual average healthcare costs ranging from $15,000 to $30,000 [5–7], placing a large burden on individuals, their caregivers, and on the healthcare system.
Several treatments are currently available for treating SCD. Hydroxyurea, an anti-metabolite, and for certain indications, transfusion, are accepted as standard of care [8,9]. Allogeneic hematopoietic stem cell transplantation (AlloHCT) is the only accepted treatment with curative intent [10]. However, these treatments can cause a wide range of complications. For example, blood transfusions may cause iron overload, alloimmunization and infections [9], and transplantation may cause graft versus host disease, graft failure, and transplantation-related organ toxicities and mortality [10]. Additional healthcare resources are sometimes needed to treat these complications.
Aside from the conventional treatments, several new therapies including crizanlizumab (a monoclonal antibody), voxelotor (a small molecule) and L-glutamine (a naturally occurring amino acid) have recently been approved by the U.S. Food and Drug Administration (FDA) [11]. Moreover, initial results of clinical trials of genetic therapy for SCD have been promising [12]. Indeed, the Cure Sickle Cell Initiative funded by the National Heart Lung and Blood Institute (NHLBI; https://www.nhlbi.nih.gov/science/cure-sickle-cell-initiative) is a large collaborative research effort intended to accelerate the development of genetic therapies to cure SCD.While the development of novel pharmacologic and stem cell therapies are providing hope to many SCD patients, the accompanying high costs warrant attention [12]. For instance, the average costs of crizanlizumab and voxelotor range from approximately US$80,000 to US$110,000, and US$100,000 to US$250,000 every year respectively [13,14]. The cost of one current genetic therapy for beta-thalassemia, another hemoglobin disorder, is approximately US$1.8 million per treatment [15].
The emergence of expensive SCD therapies makes the application of cost-effectiveness analysis (CEA) in this field timely. However, accurately capturing all the significant costs and outcomes in a CEA for SCD can be challenging. Doing so requires creation of a detailed model that simulates patients’ experience over their lifetime and reflects the complex natural history of the disease. It also requires data to inform model inputs for a disease that is relatively rare. The data may include not only the medical costs and health outcomes associated with SCD, its complications, and treatments, but also non-medical burden such as the impacts on patients’ education attainment and work productivity, and their caregiver’s burden. Despite these limitations, lessons can be learned from published CEAs in SCD.
We conducted this rapid review of published CEAs in SCD as one of a series of landscape analyses we performed as investigators within the Cure Sickle Cell Initiative.The aim of this review is to qualitatively synthesize and evaluate the main characteristics, components and results of published CEAs of interventions for SCD. We identify current research gaps and provide directions for valuing emerging gene therapies for SCD.
2. METHODS
2.1. Search Methods and Sources
We conducted a rapid literature review following methods of the Agency for Healthcare Research and Quality guidance for rapid reviews, and adopted the population, intervention, comparator, outcomes, timing and setting/study design (PICOTS) framework to establish eligibility criteria (Appendix 2) [16]. The adopted PICOTS framework reflects deliberations and decisions made over a three month period in late 2019 by an expert panel that included a molecular biologist, clinicians who care for patients with SCD, health economists, evidence synthesis scientists, and librarians. These stakeholders represent academia, clinical practice, and the federal government. The framework was executed as a search strategy in PubMed, EMBASE, the National Health System Economic Evaluation Database, the Tufts University CEA Registry, and EconLit by two experienced health sciences librarians and one health economist with expertise in evidence synthesis (search terms can be found in Appendix 3). Additional articles were identified from the reference lists of relevant review articles and through consultations with experts. The content of this report aligns with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement for reporting of systematic reviews [17].
2.2. Eligibility Criteria
We included English language articles published in peer-reviewed journals from January 2008 to June 2021 and white papers published from January 2018 to June 2021. Eligible articles included CEAs of SCD interventions with costs, effectiveness and incremental cost-effectiveness ratios (ICERs) as the outcomes. All types of interventions for SCD patients, treatment complications, and screening programs targeting newborns or pregnant women were included, while treatments targeting patients with sickle cell trait were excluded (Appendix 2).
2.3. Study Selection
Records identified through the databases, found from reference lists of relevant review articles, and from consultations with experts were merged. After duplicate records were removed, the primary reviewer (B.J.) independently screened the titles and abstracts of all references, and assessed the full text of all remaining articles for eligibility. The second reviewer (D.Q.) reviewed 10% of randomly selected references. Discrepancies between reviewers’ judgements were discussed and resolved through consensus.
2.4. Data Extraction
The primary reviewer (B.J.) extracted the main characteristics of included studies (intervention type, study design, geographic region and perspective), main components of each study (time horizon, model type, cost type, effectiveness measure, source of health utilities, discounting), disease characteristics (SCD complications and treatment complications) and study results (costs, effectiveness and ICER). To covert countries’ currencies to $US, we applied the average annual exchange rates for the fiscal years [18–20]. The second reviewer (D.Q.) verified the extracted data.
2.5. Critical Appraisal
We evaluated the adherence of the cost-effectiveness studies to the health economic evaluation reporting guidelines – The Consolidated Health Economic Evaluation Reporting Standards (CHEERS), which was developed by a task force supported by the International Society for Pharmacoeconomics and Outcomes Research [21]. Eighteen items from the CHEERS Statement were used to assess the proper description of methods and the complete presentation of results (Appendix 1). Each item was judged using ‘yes’, ‘no’, ‘partially or implied’, or ‘not applicable” options.
3. RESULTS
3.1. Study Selection
The PRISMA flow diagram outlines study selection and reasons for exclusion (Figure 1). Our search identified 166 references. One additional study was identified through consultation with experts. No additional studies that were not already contained in our search were identified from the reference lists of relevant literature review articles. After removing the duplicate articles, we screened the titles and abstracts of the remaining 128 articles and included 44 articles for full-text assessment. Thirteen articles met our final inclusion criteria and data from these were extracted.
Figure 1.
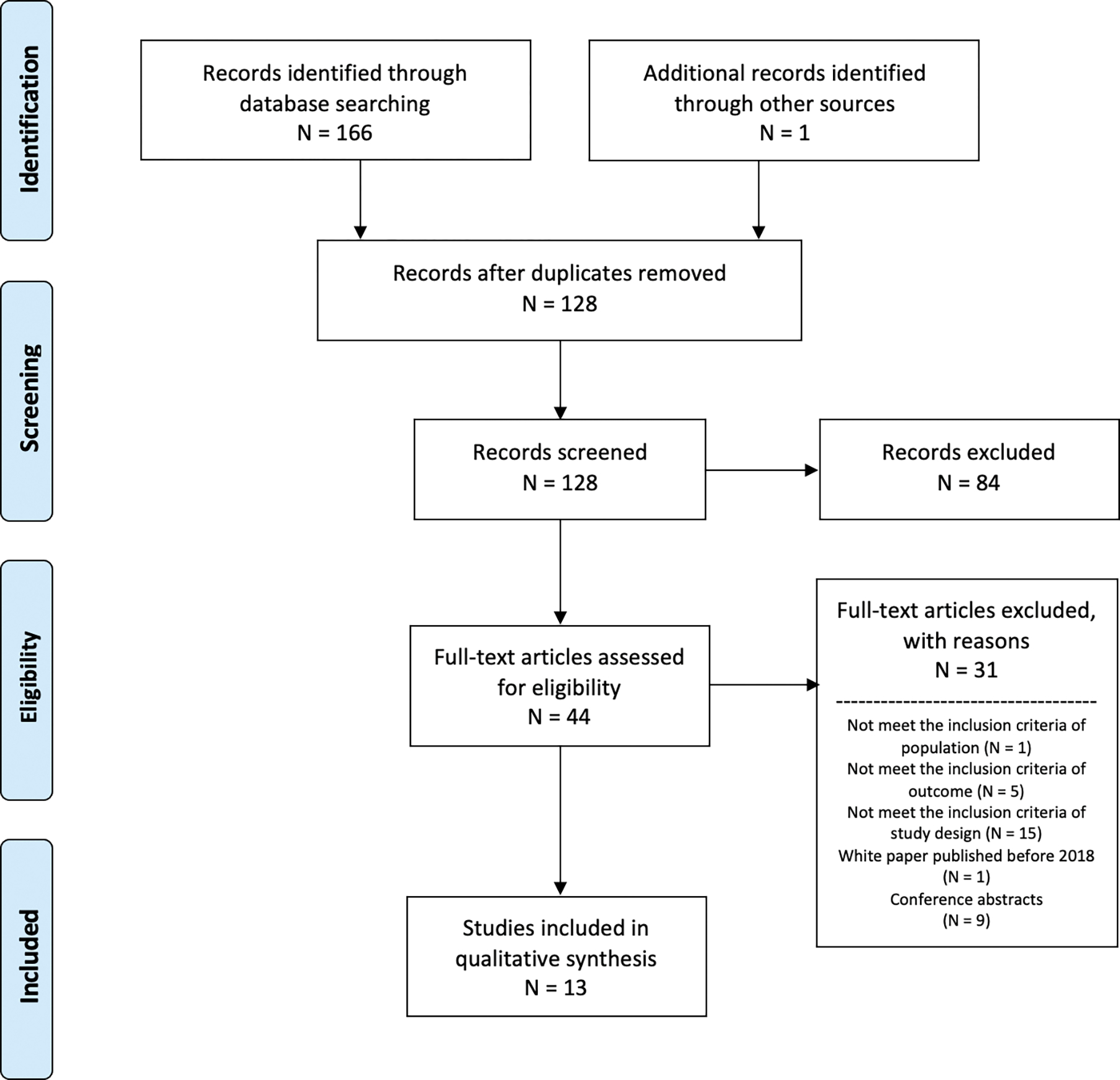
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow diagram of studies included in this systematic review and reasons for exclusion
3.2. Overview of Included Studies
The main characteristics of the included studies are presented in Table 1. Two studies estimated the value of the intervention of blood transfusion [22,23], one study evaluated transplantation [24], two studies evaluated pharmaceuticals for SCD (hydroxyurea, crizanlizumab, voxelotor and L-glutamine) [25,26], one study evaluated a hypothetical cell or genetic therapy [27], four studies examined screening programs [28–31], and the remaining three studies evaluated interventions for SCD treatment complications [32–34].
Table 1.
Main characteristics of included cost-effectiveness analyses
| Author, Year | Study Design | Region | Perspective | Intervention Type |
|---|---|---|---|---|
| Cherry et al., 2012[22] | • Model-based study | • U.K. | • Healthcare system | • Blood transfusion |
| Spackman et al., 2014[23] | • Model-based study | • U.K. | • Healthcare system | • Blood transfusion |
| Arnold et al., 2015[24] | • Observational study | • U.S. | • Healthcare institution or hospital | • Transplantation |
| Cunningham-Myrie et al., 2015[25] | • Observational study | • Jamaica | • Healthcare system | • Pharmaceuticals |
| Bradt et al., 2020[26] | • Model-based study | • U.S. | • Healthcare system • Modified societal |
• Pharmaceuticals |
| Salcedo et al., 2021[27] | • Model-based study | • U.S. | • Healthcare system | • Hypothetical cell or genetic therapy |
| Castilla-Rodríguez et al., 2016[28] | • Model-based study | • Spain | • Healthcare system | • Screening |
| Kuznik et al., 2016[29] | • Model-based study | • Sub-Saharan Africa | • Healthcare system | • Screening |
| McGann et al. 2015[30] | • Model-based study | • Angola | • N/A | • Screening |
| Bryan et al., 2011[31] | • Model-based study | • U.K. | • Healthcare system | • Screening |
| Kacker et al., 2014 (a)[32] | • Model-based study | • U.S. | • Hospital | • Intervention for treatment complications |
| Kacker et al., 2014 (b)[33] | • Model-based study | • U.S. | • Hospital | • Intervention for treatment complications |
| McLeod et al., 2009[34] | • Model-based study | • U.K. | • Healthcare system | • Intervention for treatment complications |
N/A = not available; U.K. = United Kingdoms; U.S. = United States
Five studies were set in the context of the U.S. [24,26,27,32,33], four in the United Kingdom (U.K.) [22,23,31,34], one in Jamaica [25], one in Spain [28], one in Angola [30], and one in Sub-Saharan Africa [29]. In the base case scenario, nine studies adopted the health care system perspective [22,23,25–29,31,34], three studies adopted the healthcare institution or hospital perspective [24,32,33], and one study did not mention the perspective [30]. One study also adopted a modified societal perspective as a scenario analysis [26].
3.3. Study Design
The key components of the study design can be found in Table 2. Eleven studies were model-based [22,23,26–34], six used a Markov model [22,26,27,29,32,33], one used a discrete event simulation model [28], one used a life table model [30], and three did not explicitly mention the model type [23,31,34]. The lifetime horizon was used in five of the model-based studies [22,26,27,29,30]. The remaining two studies summarized the costs and effectiveness outcome directly, based on longitudinal data without building a decision model [24,25]. One study followed the patients over one year and also predicted the lifetime cost-effectiveness [24]. The mean follow-up time of the other study was approximately four years [25].
Table 2.
Summary of components of included cost-effectiveness analyses
| Author, Year | Study population | Mean Age (years) | Intervention | Time Horizon | Model Type | Cost Type | Effectiveness Measure | Source of Health Utility | Discounting |
|---|---|---|---|---|---|---|---|---|---|
| Cherry et al., 2012[22] | • Children with SCD • Had no prior history of stroke |
• 2 | • TCD scans followed by blood transfusion where the scan revealed a blood velocity of > 200 cm/second | • Lifetime | • Markov model | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• QALY | • Empirically elicited for non-SCD specific population • Assumed by the authors without referencing empirical study. |
• 3.5% |
| Spackman et al., 2014[23] | • SCD patients • Undergoing low- or medium-risk surgery |
• 17.3 | • Preoperative transfusion | • 1 year | • N/A | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• QALY | • Empirically elicited from SCD population • Assumed by the authors without referencing empirical study. |
• Not discounted |
| Arnold et al., 2015[24] | • Children with SCD | • Intervention: 9.10 (SD: 6.25) • Comparator: 4.23 (SD: 3.74) |
• alloHCT | • 1 year • Lifetime |
• Not a modeling study | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• QALY | • Empirically elicited for SCD population • Empirically elicited for non-SCD specific population |
• Not discounted |
| Cunningham-Myrie et al., 2015[25] | • Children with SCD • Had a first clinical stroke |
• N/A | • Hydroxyurea | Mean study duration • 3.82 years for intervention group • 4.47 years for control group |
• Not a modeling study | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• Stroke • Death |
• Utilities not used | • Not discounted |
| Bradt et al., 2020[26] | • SCD patients • Had a baseline rate of 3 acute pain crises per year |
• 24 | • Voxelotor • Crizanlizumab • L-glutamine |
Lifetime | • Markov model | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention Costs within the informal health care sector • Caregiver-time costs Costs within the non-health care sector • Productivity • Education |
• LY • QALY • evLYG |
• Empirically elicited for SCD population • Empirically elicited for non-SCD specific population • Assumed by the authors without referencing empirical study. |
• 3% |
| Salcedo et al., 2021[27] | • Newborns with SCD | • At birth | • Hypothetical cell or genetic therapy | • Lifetime | • Markov model | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• LY • QALY |
• Empirically elicited for SCD specific population • Empirically elicited for non-SCD specific population |
• 3% |
| Castilla-Rodríguez et al., 2016[28] | • Newborns | • At birth | • Newborn screening program | • 10 years | • Discrete event simulation model | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• LY | • Utilities not used | • 3% |
| Kuznik et al., 2016[29] | • Newborns | • At birth | • Newborn screening program | • Lifetime | • Markov model | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• DALY | • Utilities not used | • 3% |
| McGann et al. 2015[30] | • Newborns | • At birth | • Newborn screening program | • Lifetime | • Life table | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• HLY | • Utilities not used | • 3% |
| Bryan et al., 2011[31] | • Pregnant women (biological mothers) • Their partners (biological fathers) |
• N/A | • Primary care parallel • Primary care sequential |
• Pregnancy to conclusion | • N/A | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• Woman screened | • Utilities not used | • Not discounted |
| Kacker et al., 2014 (a)[32] | • SCD patients • Undergoing chronic blood transfusion |
• Equivalent to mean age of the U.S. population | • Prospective antigen-matching • Perfectly informed antigen-matching • Imperfectly informed antigen-matching |
• 10 years • 20 years |
• Markov model | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• Alloimmunization event | • Utilities not used | • 3% |
| Kacker et al., 2014 (b)[33] | • SCD patients • Undergoing chronic blood transfusion |
• N/A | • Prospective limited matching • Prospective extensive matching |
• 10 years • 20 years |
• Markov model | Costs within the formal healthcare sector • Costs of the intervention • Costs of consequences due to the intervention |
• Alloimmunization event | • Utilities not used | • 3% |
| McLeod et al., 2009[34] | • Patients with beta-thalassemia major or SCD | • Stratified by age ranging from 2 to 18+ | • Deferasirox | • 1 year | • N/A | Costs within the formal healthcare sector • Cost of interventions |
• QALY | • Empirically elicited for non-SCD specific population • Assumed by the authors without referencing empirical study |
• Not discounted |
alloHCT = Allogeneic hematopoietic cell transplantation; evLYG = equal value of life year gain; DALY = disability-adjusted life year; HLY = health life year; ICER = incremental cost-effectiveness ratio; LY = life year; N/A = not available; QALY = quality-adjusted life year; SCD = sickle cell disease; SD = standard deviation; TCD = transcranial doppler ultrasound
3.4. Cost Inputs
Table 2 displays the types of cost inputs and effectiveness measures found in each study. All 13 studies included costs of the healthcare intervention. Twelve studies also included costs of the healthcare consequences due to the intervention (i.e. downstream healthcare resource use) [22–33]. One studies considered costs outside the formal healthcare sector in their scenario analysis, such as the effect of SCD on caregiver burden, education, and productivity [26].
3.5. Utility Inputs
Six studies employed the quality-adjusted life year (QALY) as the effectiveness measure [22–24,26,27,34]. Whilst four of the studies used health utility values that were empirically elicited from an SCD population [23,24,26,27], all the six also relied on assumptions by the authors without referencing an empirical study or values empirically elicited for non-SCD specific populations [22–24,26,27,34].
3.6. Clinical Inputs – Disease and Treatment Complications
Seven studies considered SCD complications [22,24–28,30]. The most common complications in those studies were stroke (six studies) [22,24–26,28,30], vaso-occlusive crisis or pain crisis (five studies) [22,24,26,27,30], and acute chest syndrome (four studies) [22,24,26,30] (Appendix 4). Seven studies considered treatment complications [22–24,26,32–34]. Treatment complications from blood transfusions were most common, such as iron overload (three studies) [22,26,34], and alloimmunization (three studies) [22,32,33]. One study considered complications of allogeneic hematopoietic cell transplantation (alloHCT), such as acute and chronic graft-versus-host disease, cytomegalovirus reactivation and primary graft failure [24] (Appendix 4).
3.7. Effectiveness Measures
Apart from QALY as the effectiveness measure in six studies [22–24,26,27,34], other similar measures capturing both quality and length of life included: disability-adjusted life year (DALYs; one study) [29], healthy life years (HLY; one study) [30], and equal value of life years gained (evLYG; one study) [26]. DALYs combine years of life lost due to early death and years lost due to disability. HLYs measure disability-free life expectancy. The metric of evLYG combines quality and length of life, as do QALYs, during the baseline survival period, adding gains in length of life (not considering quality of life during added life years). Three studies used life years gained (LYG) as the measure of effectiveness [26–28]. Three studies measured health events, such as stroke, death and alloimmunization [25,32,33]. One antenatal screening study measured number of women screened [31].
3.8. Cost-Effectiveness Results
The costs, effectiveness and ICER of each intervention versus its comparator can be found in Table 3. If the studies presented the total costs and effectiveness for the entire cohort, the costs and effectiveness were converted to per person values (calculated as total costs or effectiveness divided by cohort size). The original numbers from the references can be found in Appendix 5.
Table 3.
Results of the base case scenario in included economic evaluations of interventions for sickle cell disease
| Author, Year | Study Population | Time Horizon | Intervention vs. Comparator | Currency (fiscal year); Effectiveness Measure | Costs (per person) of Intervention vs. Comparator* | Effectiveness (per person) of Intervention vs. Comparator* | ICER as Reported by Authors of Original Publication |
|---|---|---|---|---|---|---|---|
| Blood Transfusion | |||||||
| Cherry et al., 2012[22] | • Children with SCD • Had no prior history of stroke |
• Lifetime | • Intervention: TCD scans followed by blood transfusion where the scan revealed a blood velocity of > 200 cm/second • Comparator: TCD scans only |
• £(2010); QALY | • 52,472 • 38,720 |
• 14.87 • 14.30 |
• £24,075 (US$37,316) per QALY gained |
| Spackman et al., 2014[23] | • SCD patients • Undergoing low- or medium-risk surgery |
• 1 year | • Intervention: Preoperative transfusion • Comparator: No transfusion |
• £(2011); QALY | • 1,706 • 2,442 |
• 0.71 • 0.70 |
• Less costly, more effective |
| Transplantation | |||||||
| Arnold et al., 2015[24] | • Children with SCD | • 1 year | • Intervention: alloHCT • Comparator: Referred but without alloHCT |
• US$(N/A); QALY | • 430,861 • 8,245 |
• 0.78 • 0.91 |
• More costly, less effective† |
| • Lifetime | • N/A • N/A |
• N/A • N/A |
• N/A† | ||||
| Pharmaceuticals | |||||||
| Cunningham-Myrie et al., 2015[25] |
• Children with SCD • Had a first clinical stroke |
Mean study duration • 3.82 years for intervention group • 4.47 years for control group |
• Intervention: Hydroxyurea • Comparator: No hydroxyurea |
• J$(2009); stroke | • 86,710 • 69,941 |
• 0.03 • 0.13 |
• J$169,238 (US$1,917) per Stroke averted |
| • J$(2009); death | • 0.00 • 0.00 |
• J$635,843 (US$7,203) per death averted | |||||
| Bradt et al., 2020 2020[26] |
• SCD patients • Had a baseline rate of 3 acute pain crises per year |
• Lifetime | • Intervention: L-glutamine • Comparator: Optimal usual care alone |
• US$(2019); LY | • 1,414,000 • 1,174,000 |
• 15.35 • 14.34 |
• US$238,000 per LY gained |
| • US$(2019); evLYG | • 8.96 • 8.07 |
• US$270,000 per evLYG gained | |||||
| • US$(2019); QALY | • 8.47 • 8.07 |
• US$604,000 per QALY gained | |||||
| • Intervention: Crizanlizumab • Comparator: Optimal usual care alone |
• US$(2019); LY | • 2,046,000 • 1,174,000 |
• 16.36 • 14.34 |
• US$432,000 per LY gained | |||
| • US$(2019); evLYG | • 9.78 • 8.07 |
• US$509,000 per evLYG gained | |||||
| • US$(2019); QALY | • 8.87 • 8.07 |
• US$1,086,000 per QALY gained | |||||
| • Intervention: Voxelotor • Comparator: Optimal usual care alone |
• US$(2019); LY | • 2,291,000 • 1,174,000 |
• 16.37 • 14.34 |
• US$550,000 per LY gained | |||
| • US$(2019); evLYG | • 9.96 • 8.07 |
• US$589,000 per evLYG gained | |||||
| • US$(2019); QALY | • 9.10 • 8.07 |
• US$1,082,000 per QALY gained | |||||
| Hypothetical cell or genetic therapy | |||||||
| Salcedo et al, 2021[27] | • Newborns with SCD | • Lifetime | • Intervention: Hypothetical cell or genetic therapy • Comparator: Standard of care |
• US$(2018); LY | • 2,372,482 • 1,175,566 |
• 29.9 • 26.2 |
• N/A‡ |
| • US$(2018); QALY | • 26.4 • 17.9 |
• US$140,877 per QALY gained | |||||
| Screening | |||||||
| Castilla-Rodríguez et al., 2016[28] | • Newborns | • 10 years | • Intervention: Newborn screening program • Comparator: No screening |
• €(2013); LY | • 5 • 6 |
• 8.66 • 8.66 |
• €34,169(US$45,445) per LY gained |
| Kuznik et al., 2016[29] | • Newborns | • Lifetime | • Intervention: Newborn screening and prophylactic intervention • Comparator: No screening |
• US$(2014); DALY | • 16 • 0 |
N/A§ N/A§ |
• US$213 per DALY averted |
| McGann et al., 2015[30] | • Newborns | • Lifetime | • Intervention: Newborn screening and treatment program for sickle cell anemia • Comparator: No screening |
• US$(N/A); HLY | • 44 • 0 |
N/A‖• N/A‖ | • US$2,214 per HLY gained – US$2,824 per HLY gained |
| Bryan et al., 2011[31] | • Pregnant women (biological mothers • Their partners (biological fathers) |
• Pregnancy to conclusion | • Intervention: Primary care sequential • Comparator: Midwife care |
• £(2010); woman screened | • 18 • 15 |
• 0.29 • 0.03 |
• £13(US$20) per woman screened |
| • Intervention: Primary care parallel • Comparator: Midwife care |
• 20 • 15 |
• 0.26 • 0.03 |
• £25(US$39) per woman screened | ||||
| • Intervention: Primary care parallel • Comparator: Primary care sequential |
• 20 • 20 |
• 0.26 • 0.29 |
• More costly, less effective | ||||
| Intervention for SCD complications | |||||||
| Kacker et al., 2014 (a)[32] | • SCD patients • Undergoing chronic blood transfusion |
• 10 years | • Intervention: Prospective antigen-matching • Comparator: History-based antigen-matching |
• US$(2012); alloimmunization event | • 247,201 • 162,623 |
• 0.25 • 0.46 |
• US$412,132 per alloimmunization event averted |
| • Intervention: Perfectly informed antigen-matching • Comparator: History-based antigen-matching |
• 164,866 • 162,623 |
• 0.25 • 0.46 |
• US$10,934 per alloimmunization event averted | ||||
| • Intervention: Imperfectly informed antigen-matching • Comparator: History-based antigen-matching |
• 164,556 – 185,700 • 162,623 |
• 0.25 – 0.31 • 0.46 |
• US$12,558 per alloimmunization event averted – US$147,915 per alloimmunization event averted | ||||
| • 20 years | • Intervention: Prospective antigen-matching • Comparator: History-based antigen-matching |
• 433,064 • 287,313 |
• 0.41 • 0.60 |
• US$759,799 per alloimmunization event averted | |||
| • Intervention: Perfectly informed antigen-matching • Comparator: History-based antigen-matching |
• 288,721 • 287,313 |
• 0.41 • 0.60 |
• US$7,344 per alloimmunization event averted | ||||
| • Intervention: Imperfectly informed antigen-matching • Comparator: History-based antigen-matching |
• 288,619 – 325,057 • 287,313 |
• 0.41 – 0.46 • 0.60 |
• US$9,082 per alloimmunization event averted – US$261,638 per alloimmunization event averted | ||||
| Kacker et al., 2014 (b)[33] | • SCD patients • Undergoing chronic blood transfusion • Had been transfusion naive initially |
• 10 years | • Intervention: Prospective limited matching • Comparator: History-based limited matching |
• US$(2012); alloimmunization event | • 456,376 • 236,978 |
• 0.08 • 0.36 |
• US$369,479 per alloimmunization event averted |
| • Intervention: Prospective extensive matching • Comparator: History-based extensive matching |
• 286,025 • 195,959 |
• 1.06 • 1.30 |
• US$769,344 per alloimmunization event averted | ||||
| • 20 years | • Intervention: Prospective limited matching • Comparator: History-based limited matching |
• 502,938 • 344,544 |
• 1.86 • 2.10 |
• US$640,814 per alloimmunization event averted | |||
| • Intervention: Prospective extensive matching • Comparator: History-based extensive matching |
• 799,565 • 15,649 |
• 0.13 • 0.41 |
• US$1,364,247 per alloimmunization event averted | ||||
| • SCD patients • Undergoing chronic blood transfusion • Included patients with a prior history of transfusion and possible alloimmunization |
• 10 years | • Intervention: Prospective limited matching • Comparator: History-based limited matching |
• 169,720 • 127,562 |
• 0.64 • 0.81 |
• US$252,708 per alloimmunization event averted | ||
| • Intervention: Prospective extensive matching • Comparator: History-based extensive matching |
• 271,488 • 162,016 |
• 0.04 • 0.25 |
• US$541,939 per alloimmunization event averted | ||||
| • 20 years | • Intervention: Prospective limited matching • Comparator: History-based limited matching |
• 271,272 • 201,192 |
• 1.05 • 1.25 |
• US$355,630 per alloimmunization event averted | |||
| • Intervention: Prospective extensive matching • Comparator: History-based extensive matching |
• 433,806 • 254,993 |
• 0.07 • 0.30 |
• US$775,069 per alloimmunization event averted | ||||
| McLeod et al., 2009[34] | • Patients with beta-thalassemia major or SCD • Stratified by age ranging from 2 to 18+ years |
• 1 year | • Intervention: Deferasirox • Comparator: Deferoxamine/desferrioxamine |
• £(2007); QALY | • 4,386 – 18,594 • 2,733 – 7,219 |
• 0.84 • 0.66 |
• £9,232 (US$18,464) per QALY gained – • £63,195 (US$126,390) per QALY gained |
| • Intervention: Deferasirox • Comparator: Deferiprone |
• 4,386 – 18,594 • 2,194 – 5,565 |
• 0.84 • 0.66 |
• £12,224(US$24,448) per QALY – • £72,386(US$144,772) per QALY gained |
||||
alloHCT = allogeneic hematopoietic cell transplantation; evLYG = equal value of life year gain; DALY = disability-adjusted life year; HLY = health life year; ICER = incremental cost-effectiveness ratio; N/A = not available; SCD = sickle cell disease; TCD = transcranial doppler ultrasound
The costs and effectiveness were converted to per person value if not reported. The original number from the references can be found in Appendix 5.
The ICER provided by Arnold et al. was not calculated as incremental costs divided by incremental effectiveness. The original ICER can be found in Appendix 5.
Salcedo et al. provided the ICER measured as costs per QALY gained but did not provide the ICER measured as costs per LY gained.
Kuznik et al. provided the DALYs averted but did not provide the mean DALYs for intervention and for comparator.
McGann et al. provided the HLYs gained but did not provide the mean HLYs for intervention and for comparator.
Blood transfusion:
The ICER for blood transfusion for primary stroke prevention in one study was estimated to be £24,075 (US$37,316) per QALY gained versus no transfusion over a lifetime horizon (fiscal year 2010) [22]. The other study found that preoperative blood transfusion was less costly and more effective (dominant) than no transfusion over one year (fiscal year 2011) [23].
Transplantation:
The study assessing alloHCT versus no alloHCT presented an ‘ICER’ over one-year post transplantation and another over a lifetime horizon [24]. However, the ‘ICER’ presented was not calculated as incremental costs divided by incremental effectiveness, as is standard for CEAs. This issue was also addressed in a letter to the editor by Thielen et al [35]. The results of this study revealed that alloHCT was more costly than the comparator (median: US$430,816 vs. US$8,245) and produced fewer QALYs (mean: 0.78 vs. 0.91) over the post-alloHCT year (fiscal year not available). The lifetime cost and effectiveness values of the intervention and comparator were not available. The original ‘ICER’ can be found in Appendix 5.
Pharmaceuticals:
One study, conducted in Jamaica, estimated the ICER for hydroxyurea versus no hydroxyurea at J$169,238 (US$1,917) per stroke averted and J$635,843 (US$7,203) per death averted over an approximate four-year mean follow-up (fiscal year 2009) [25]. Separately, the lifetime ICER of newer drugs (crizanlizumab, voxelotor and L-glutamine) versus optimal usual care (e.g. hydroxyurea, blood transfusion) ranged from US$604,000 per QALY gained to US$1,086,000 per QALY gained in the U.S. (fiscal year 2019), under the assumption that the treatment effects of those new therpies do not wane over time [26].
Hypothetical cell or genetic therapy:
One U.S.-based study examined the cost-effectiveness of a hypothetical one-time administration cell or genetic therapy for newborns with SCD, relative to standard of care (including antibiotics, vaccinations, pain-relief medications, hydroxyurea, transfusions, and transplatation) [27]. In the base case, they assumed a lifetime durability of cure and a price of US$2,100,000 for the hypothetical therapy. The ICER was $140,877 per QALY gained (fiscal year 2018) under these assumptions.
Screening:
The effectiveness measure varies among the screening studies. The ICER for newborn screening versus no screening was €34,169 (US$45,445) per life year gained in Spain over ten years (fiscal year 2013) [28], US$213 per DALY averted in Sub-Saharan Africa (fiscal year 2014)[29] and US$2,214 per HLY gained to US$2,824 per HLY gained in Angola over a lifetime horizon (fiscal year not available) [30]. The primary care parallel strategy (testing mother and father at the same time in primary care) and primary care sequential strategy (testing mother in primary care and then subsequently testing the father if the mother is a carrier) led to an ICER of £25 (US$39) and £13 (US$20) per woman screened, respectively compared to a midwife care strategy (sequential testing at the first midwife consultation) over ten weeks in the U.K. (fiscal year 2010) [31].
Interventions for treatment complications:
The ICER for a prospective antigen-matching strategy versus history-based antigen-matching strategy to prevent alloimmunization following transfusion ranged from US$10,934 per alloimmunization event averted to US$769,344 per alloimmunization event averted over ten years, and from US$9,082 per alloimmunization event averted to US$1,364,247 per alloimmunization event averted over 20 years in the U.S. (fiscal year 2012) [32,33]. The ICER for deferasirox, the drug used to treat iron overload ranged from £9,232(US$18,464) per QALY gained to £63,195 (US$126,390) per QALY gained versus deferoxamine/desferrioxamine. When compared with deferiprone, the ICER for deferasirox ranged from £12,224 (US$24,448) per QALY gained to £72,386 (US$144,772) per QALY gained over one year in the U.K. (fiscal year 2007) [34].
3.9. Critical Appraisal
Appendix 1 presents the results of critical appraisal of each CEA. In general, most of the items in the reporting guideline were followed. Nonetheless, several studies did not explicitly present or correctly calculate the incremental costs and effectiveness [24,29,30], did not report the uncertainties [24,25], and did not report the heterogeneity of cost-effectiveness between subgroups with different characteristics [25,31].
4. DISCUSSION
Few CEAs of SCD treatments have been published to date, and existing studies have been limited in scope. There are some similarities across the 13 studies that met our inclusion criteria. For example, the studies adopted the perspective of the healthcare system and most included only costs within the formal healthcare sector. However, our results reveal that published CEAs in SCD are quite heterogeneous in terms of geographic setting, intervention type, SCD complications and treatment complications included, choice of model and time horizon, and effectiveness measures used. As a consequence, cost-effectiveness findings are inconsistent across studies.
Our rapid review found that most studies were limited to a very narrow subset of disease complications and treatments. Admittedly, modeling such a complex disease, with so many complications occurring over the lifetime horizon is challenging. Estimates of necessary model input parameters require data sources that include information about the trajectory of the disease burden, treatments, and treatment complications; these data sources are few in SCD. Nevertheless, models need to incorporate these elements, as many of the complications have significant implications for survival, quality of life, and economic costs. Unfortunately real-world datasets necessary to quantify SCD incidence, costs and outcomes are limited. There are no large comprehensive national registries and extraction from electronic medical records and claim data are fraught with complications, such as inaccurate or inconsistent coding, limited clinical information, and incomplete record of care received [36]. Nonethess, the estimates may be derived from the existing large cohort studies [37–39]. In addition to needing higher quality databases, eliciting input from stakeholders, especially patients on their perceptions about which of the complications are most troubling, is critical in guiding model development.
Over the past thirty years there have been ongoing efforts to bring increased rigor to, and standardize the methodological practices and improve the comparability and quality of CEA. Transparent and complete reporting of methods and findings remains critical to the CEAs in SCD, as we note that several included studies did not explicitly present or correctly calculate the incremental costs and effectiveness, and did not report the uncertainties or heterogeneity of the findings. The CHEERS statement can be a reliable tool to enhance the quality of reporting in future studies [21]. In 2016, the Second Panel on Cost-Effectiveness in Health and Medicine also provided guidance for future studies, which has been widely referenced since then [40]. The panel recommend that all studies report reference case analyses from a health care sector perspective and a societal perspective. The societal reference case analysis should consider all parties affected by the medical interventions and include all significant outcomes and costs (i.e. those in formal and informal health care sectors as well as non-health care sector). They also recommend that health effects should be measured in terms of QALY in the reference case analysis. Moreover, they recommend that the time horizon should be long enough to capture all relevant outcomes.
While the healthcare costs attributable to SCD are substantial, the non-healthcare costs are likely substantial as well, placing high burden on patients and families. Those costs can arise from, but are not be limited to the impact of SCD on work productivity, education, household activities, and caregiver’s time use [41–44]. Neglecting those costs may substantially underestimate the economic value of SCD interventions. Our review found that only one study adopted a modified societal perspective in a scenario analysis, incorporating a portion, but not all of the costs that may be incurred outside the health care system [26]. This study has shown that including such costs would substantially decrease the ICER estimates [26]. Consistent with the second panel’s recommendation, we recommend that future CEAs in SCD adopt a societal perspective, explicitly incorporating the costs outside the formal healthcare sectors.
Preventing SCD complications can not only lower the risk of death, but also promote improved quality of life [2–4]. Hence, we recommend that both of these effects should be captured in CEAs using outcome measures such as QALY. We found that only eight studies used QALY or similar measures. One possible explanation for this gap might be that health utility data in the SCD population are sparse. Most of the studies relied on utility values for non-SCD specific populations (to inform the utility decrement due to complications) or based on assumptions. Additionally, in the absence of QALY or DALY as outcome measures, it is difficult to draw a conclusion about whether the intervention is cost-effective, as most established willingness to pay thresholds are based on QALYs gained or DALYs averted, both metrics that can be compared across disease states [45].
Since SCD interventions are likely to have long-term health and economic impacts, using a lifetime horizon is recommended for future CEA in SCD. This is also in line with the second panel’s recommendation. Another important aspect of the natural history of SCD is that the rate and spectrum of complications varies throughout the patient’s lifespan [46]. Further, patients receive different types of medical care across the life stages (e.g. pediatric care versus adult care, primary care versus specialty care) [47]. To reflect the long-term and time-varying features of SCD, a simulation model (e.g. state-transition, microsimulation or discrete event model) with a lifetime horizon would be beneficial for valuing the emerging treatments.
Admittedly, the CEAs conducted to date in the context of SCD provide limited information. These analyses do not typically incorporate the value of a treatment in reducing inequity in resources and expenditures for patients with SCD [48]. Some argue that research funding and pharmaceutical investment for a rare disease like SCD are not commensurate with funding and investment for more prevalent diseases [49]. Inequity also reflects in the lack of access to necessary health care among underserved population, such as SCD patients. To incorporate the equity issue in economic evaluations, future studies will likely employ innovative, emerging methods, such as distributional CEA [50]. Additionally, it is clear that treatment approaches for SCD continue to evolve, and both newly approved therapeutics and those under investigation appear promising [51–53]. A single CEA has limited ability to reflect this dynamic treatment landscape. CEAs could be updated to reflect changes in treatment modalities.
We provide recommendations to increase rigor and comparability of future CEAs in SCD. Global heterogeneity, however, should be addressed. Our review identified the existing CEAs conducted globally; these countries have various SCD burden, financial resources, healthcare systems, as well as preferences for types of SCD interventions [54–56]. Thus, the model inputs should be customized to the local population using country specific information for input parameters.. Additionaly, our study reveals the issue of global inequity in terms of CEA researches. Although the prevalence and incidence of SCD are substantialy higher in Africa and Latin America than in North America and Europe [54], the former regions produced a much lower volume of CEA studies than the latter. The studies conducted in North America and Europe might shed light on the value of SCD interventions for the other regions, yet the relative ranking of cost-effectiveness of SCD interventions possibly differs across the regions. For example, screening program is likely to be more cost-effective in a region with higher incidence and prevalence of SCD. To better inform the healthcare resources allocation decisions in the countries with high SCD burden and poverty rates, it is imperative to provide them with more research resources.
Finally, we found that the ICER estimates were inconsistent across studies. This might be due not only to the properties of interventions assessed, but also the study designs (e.g. short-term versus long-term) and global heterogeneity. Of note, the study by Bradt and colleagues is part of the assessment of the Institute of Economic and Clinical Review in the U.S., revealing that the ICERs of those newly approved SCD therapies ranged from approximately $600,000 to $1million per QALY gained [26]. Although high, these estimates are lower than those for therapies for some other rare diseases. For instance, other assessments from the Institute for Clinical and Economic Review suggest that the ICERs for lanadelumab and the C1 esterase inhibitors for hereditary angioedema ranged from approximately $1million per QALY gained to $6 million per QALY gained [57], and ICERs for modulator treatments for cystic fibrosis were over $1million per QALY gained [58]. Caution is needed in drawing conclusions from these ‘high’ ICERs about whether the treatments are cost-effective, since there is a general recognition that a higher value-based price can be justified for rare diseases that have a catastrophic impact on health [59].
Considering the uncertainties in modeling the cost-effectiveness of SCD, making comparisons across existing studies can inform the accuracy and validity of results. Although limited in their scope, the estimates we summarized in this review may be a useful resource against which to compare the results of future CEAs. Specifically, if a future CEA compares a genetic therapy to a conventional treatment found in this review, the projected costs and effectiveness of the conventional treatment in that new study can be compared to the estimates for that same treatment summarized in this review. Of course, researchers must use caution when making these comparisons because, as seen here, studies vary widely in many ways.
Our review is subject to several limitations. First, only English articles were included in our review. Second, we included only peer-reviewed journal articles published since 2008 and white papers published since 2018. However, a previous study suggests that decision analytic studies were rarely published prior to 2009 [60]. Finally, a quantitative synthesis (meta-analysis) of the ICER estimates was not feasible due to the large degree of heterogeneity among the studies.
5. CONCLUSION
Our review provides direction for future research. Published CEAs of SCD are not comprehensive, yet may serve as a basis for comparisons with more robust CEAs conducted in the future. Specially, future studies should adopt a societal perspective, examine effects of interventions on both quality and length of life, and use an advanced simulation model design to capture a wide range of SCD complications and treatment complications over patients’ lifetime. These modeling strategies will be essential to accurately value emerging genetic therapies, as well as other novel agents under invetigation.
Supplementary Material
Key Points for Decision Makers.
This is the first literature review to identify published cost-effectiveness analyses (CEAs) of sickle cell disease (SCD) interventions. The indentified studies are heterogeneous in terms of geographic setting, intervention type, SCD complications and treatment complications evaluated, and choice of decision analytic model, time horizon, and outcome metrics. Consequently, the findings are inconsistent across the studies.
This review illuminates the gaps in the existing CEAs in SCD, such as a limited number of SCD complications and treatment complications are included, non-medical costs are not incorporated in the base case, a simulation model or a lifetime horizon is not frequently employed to reflect the complexity of disease natural history, and an outcome metric that captures both quality and length of life is not commonly used.
Future CEAs could incorporate the value of a SCD intervention in reducing inequity and reflect the fast evolving treatment landscape in SCD.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the following collaborators: N. DiFronzo (NHLBI); C. Henry, K. Johnson, D. Louden, A. Morgan, J. Rich (University of Wasington); W. Wright (Fred Hutchinson Cancer Research Center). The authors also appreciate the valuable insights and suggestions provided by the members of the Clinical and Economic Analysis Initiative Expert Panel.
Funding
This research was, in part, funded by the National Institutes of Health (NIH) Agreement OTA OT3HL152448, OT3HL151434. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH.
Footnotes
DECLARATIONS
Conflicts of interest/Competing interests
No contributing authors have a conflict of interest
Code availability
Not applicable
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
The authors give the consent for the manuscript to be published in PharmacoEconomics.
Availability of data and material
Not applicable
REFERENCES
- [1].National Heart, Lung, and Blood Institute. Sickle Cell Disease [Internet]. [cited 2020 Aug 2]. Available from: https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sickle-cell.
- [2].Dampier C, LeBeau P, Rhee S, et al. Health-related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. Am J Hematol. 2011;86:203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dampier C, Lieff S, LeBeau P, et al. Health-related quality of life in children with sickle cell disease: A report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatric Blood & Cancer. 2010;55:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lubeck D, Agodoa I, Bhakta N, et al. Estimated Life Expectancy and Income of Patients With Sickle Cell Disease Compared With Those Without Sickle Cell Disease. JAMA Netw Open. 2019;2:e1915374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shah N, Bhor M, Xie L, et al. Treatment patterns and economic burden of sickle-cell disease patients prescribed hydroxyurea: a retrospective claims-based study. Health Qual Life Outcomes. 2019;17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shah N, Bhor M, Xie L, et al. Medical Resource Use and Costs of Treating Sickle Cell-related Vaso-occlusive Crisis Episodes: A Retrospective Claims Study. J Health Econ Outcomes Res. 2020;7:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kauf TL, Coates TD, Huazhi L, et al. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84:323–327. [DOI] [PubMed] [Google Scholar]
- [8].McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert Opin Drug Saf. 2015;14:1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Howard J Sickle cell disease: when and how to transfuse. Hematology Am Soc Hematol Educ Program. 2016;2016:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shenoy S Hematopoietic stem-cell transplantation for sickle cell disease: current evidence and opinions. Ther Adv Hematol. 2013;4:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ballas SK. The Evolving Pharmacotherapeutic Landscape for the Treatment of Sickle Cell Disease. Mediterr J Hematol Infect Dis [Internet]. 2020. [cited 2020 Aug 2];12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6951351/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ikawa Y, Miccio A, Magrin E, et al. Gene therapy of hemoglobinopathies: progress and future challenges. Hum Mol Genet. 2019;28:R24–R30. [DOI] [PubMed] [Google Scholar]
- [13].Herity LB, Vaughan DM, Rodriguez LR, et al. Voxelotor: A Novel Treatment for Sickle Cell Disease. Ann Pharmacother. 2020;1060028020943059. [DOI] [PubMed] [Google Scholar]
- [14].Carvalho J Adakveo, 1st Sickle Cell Treatment for Pain Crises in Teens and Adults, Approved by FDA. Sickle Cell News; [Internet]. 2019. Nov 18 [cited 2020 Nov 7]; Available from: https://sicklecellanemianews.com/2019/11/18/fda-approves-adakveo-novartis-scd-pain-crises-treatment-adults-teens/. [Google Scholar]
- [15].Harrison C First gene therapy for β-thalassemia approved. Nature Biotechnology. 2019;37:1102–1103. [DOI] [PubMed] [Google Scholar]
- [16].Agency for Healthcare Research and Quality. Rapid Review Guidance Document [Internet]. 2019. Available from: https://libguides.library.kent.edu/ld.php?content_id=47021546. [Google Scholar]
- [17].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Macrotrends. Pound Dollar Exchange Rate (GBP USD) - Historical Chart [Internet]. [cited 2021 Jul 9]. Available from: https://www.macrotrends.net/2549/pound-dollar-exchange-rate-historical-chart.
- [19].Bank of Jamaica. Average Exchange Rates [Internet]. [cited 2021 Jul 9]. Available from: https://boj.org.jm/market/foreign-exchange/average-exchange-rates/. [Google Scholar]
- [20].Exchange Rates UK. Euro to US Dollar Spot Exchange Rates for 2013 [Internet]. [cited 2021 Jul 9]. Available from: https://www.exchangerates.org.uk/EUR-USD-spot-exchange-rates-history-2013.html. [Google Scholar]
- [21].Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. Value in Health. 2013;16:e1–e5. [DOI] [PubMed] [Google Scholar]
- [22].Cherry MG, Greenhalgh J, Osipenko L, et al. The clinical effectiveness and cost-effectiveness of primary stroke prevention in children with sickle cell disease: a systematic review and economic evaluation. Health Technol Assess. 2012;16:1–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Spackman E, Sculpher M, Howard J, et al. Cost-effectiveness analysis of preoperative transfusion in patients with sickle cell disease using evidence from the TAPS trial. Eur J Haematol. 2014;92:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arnold SD, Jin Z, Sands S, et al. Allogeneic Hematopoietic Cell Transplantation for Children with Sickle Cell Disease Is Beneficial and Cost-Effective: A Single-Center Analysis. Biol Blood Marrow Transplant. 2015;21:1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cunningham-Myrie C, Abdulkadri A, Waugh A, et al. Hydroxyurea use in prevention of stroke recurrence in children with sickle cell disease in a developing country: A cost effectiveness analysis. Pediatr Blood Cancer. 2015;62:1862–1864. [DOI] [PubMed] [Google Scholar]
- [26].Bradt P, Spackman E, Synnott P, et al. Crizanlizumab, Voxelotor, and L-Glutamine for Sickle Cell Disease: Effectiveness and Value. Institute for Clinical and Economic Review [Internet]. 2020. Available from: https://icer-review.org/material/sickle-cell-disease-draft-evidence-report/. [Google Scholar]
- [27].Salcedo J, Bulovic J, Young CM. Cost-effectiveness of a hypothetical cell or gene therapy cure for sickle cell disease. Sci Rep. 2021;11:10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Castilla-Rodríguez I, Cela E, Vallejo-Torres L, et al. Cost-effectiveness analysis of newborn screening for sickle-cell disease in Spain. Expert Opinion on Orphan Drugs. 2016;4:567–575. [Google Scholar]
- [29].Kuznik A, Habib AG, Munube D, et al. Newborn screening and prophylactic interventions for sickle cell disease in 47 countries in sub-Saharan Africa: a cost-effectiveness analysis. BMC Health Serv Res. 2016;16:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McGann PT, Grosse SD, Santos B, et al. A Cost-Effectiveness Analysis of a Pilot Neonatal Screening Program for Sickle Cell Anemia in the Republic of Angola. J Pediatr. 2015;167:1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bryan S, Dormandy E, Roberts T, et al. Screening for sickle cell and thalassaemia in primary care: a cost-effectiveness study. Br J Gen Pract. 2011;61:e620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kacker S, Ness PM, Savage WJ, et al. Economic evaluation of a hypothetical screening assay for alloimmunization risk among transfused patients with sickle cell disease. Transfusion. 2014;54:2034–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kacker S, Ness PM, Savage WJ, et al. Cost-effectiveness of prospective red blood cell antigen matching to prevent alloimmunization among sickle cell patients. Transfusion. 2014;54:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McLeod C, Fleeman N, Kirkham J, et al. Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation. Health Technol Assess. 2009;13:iii–iv, ix–xi, 1–121. [DOI] [PubMed] [Google Scholar]
- [35].Thielen FW, Blommestein HM, Uyl-de Groot CA. Using Appropriate Methods in Cost-Effectiveness Analyses: The Case of Allogeneic Hematopoietic Cell Transplantation in Sickle Cell Disease. Biol Blood Marrow Transplant. 22:2109–2110. [DOI] [PubMed] [Google Scholar]
- [36].Stein JD, Lum F, Lee PP, et al. Use of Health Care Claims Data to Study Patients with Ophthalmologic Conditions. Ophthalmology. 2014;121:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hankins JS, Estepp JH, Hodges JR, et al. Sickle Cell Clinical Research and Intervention Program (SCCRIP): A lifespan cohort study for sickle cell disease progression from the pediatric stage into adulthood. Pediatr Blood Cancer. 2018;65:e27228. [DOI] [PubMed] [Google Scholar]
- [38].Uyoga S, Macharia AW, Mochamah G, et al. The epidemiology of sickle cell disease in children recruited in infancy in Kilifi, Kenya: a prospective cohort study. Lancet Glob Health. 2019;7:e1458–e1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Serjeant GR, Chin N, Asnani MR, et al. Causes of death and early life determinants of survival in homozygous sickle cell disease: The Jamaican cohort study from birth. PLoS One. 2018;13:e0192710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Neumann PJ, Sanders GD, Russell LB, et al. Cost-effectiveness in health and medicine. Oxford University Press; 2016. [Google Scholar]
- [41].Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schatz J, Brown RT, Pascual JM, et al. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56:1109–1111. [DOI] [PubMed] [Google Scholar]
- [43].Jacob E, Miaskowski C, Savedra M, et al. Changes in sleep, food intake, and activity levels during acute painful episodes in children with sickle cell disease. J Pediatr Nurs. 2006;21:23–34. [DOI] [PubMed] [Google Scholar]
- [44].Moskowitz JT, Butensky E, Harmatz P, et al. Caregiving time in sickle cell disease: psychological effects in maternal caregivers. Pediatr Blood Cancer. 2007;48:64–71. [DOI] [PubMed] [Google Scholar]
- [45].Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action [Internet]. 2018. [cited 2020 Sep 24];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5930346/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Serjeant GR. The Natural History of Sickle Cell Disease. Cold Spring Harb Perspect Med [Internet]. 2013. [cited 2020 Mar 15];3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3784812/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Treadwell M, Telfair J, Gibson RW, et al. Transition from pediatric to adult care in sickle cell disease: Establishing evidence-based practice and directions for research. Am J Hematol. 2011;86:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lakdawalla DN, Doshi JA, Garrison LP, et al. Defining Elements of Value in Health Care—A Health Economics Approach: An ISPOR Special Task Force Report [3]. Value in Health. 2018;21:131–139. [DOI] [PubMed] [Google Scholar]
- [49].Lee L, Smith-Whitley K, Banks S, et al. Reducing Health Care Disparities in Sickle Cell Disease: A Review: Public Health Reports [Internet]. 2019. [cited 2020 Oct 12]; Available from: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Asaria M, Griffin S, Cookson R. Distributional Cost-Effectiveness Analysis. Med Decis Making. 2016;36:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Telen MJ. Beyond hydroxyurea: new and old drugs in the pipeline for sickle cell disease. Blood. 2016;127:810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Azar S, Wong TE. Sickle Cell Disease: A Brief Update. Med Clin North Am. 2017;101:375–393. [DOI] [PubMed] [Google Scholar]
- [53].Inusa BPD, Hsu LL, Kohli N, et al. Sickle Cell Disease-Genetics, Pathophysiology, Clinical Presentation and Treatment. Int J Neonatal Screen. 2019;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wastnedge E, Waters D, Patel S, et al. The global burden of sickle cell disease in children under five years of age: a systematic review and meta-analysis. J Glob Health [Internet]. [cited 2021 Mar 14];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6286674/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McGann PT, Hernandez AG, Ware RE. Sickle cell anemia in sub-Saharan Africa: advancing the clinical paradigm through partnerships and research. Blood. 2017;129:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Carey PJ. Addressing the global health burden of sickle cell disease. International Health. 2014;6:269–270. [DOI] [PubMed] [Google Scholar]
- [57].Institute for Clinical and Economic Review. Prophylaxis for Hereditary Angioedema with Lanadelumab and C1 Inhibitors: Effectiveness and Value [Internet]. 2018. Available from: https://icer-review.org/wp-content/uploads/2018/03/ICER_HAE_Final_Evidence_Report_111518-1.pdf.
- [58].Tice J, Kuntz K, Wherry K, et al. Modulator Treatments for Cystic Fibrosis: Effectiveness and Value [Internet]. 2020. Available from: https://icer-review.org/wp-content/uploads/2019/09/ICER_CF_Evidence_Report_042720.pdf. [Google Scholar]
- [59].Garrison LP, Jackson T, Paul D, et al. Value-Based Pricing for Emerging Gene Therapies: The Economic Case for a Higher Cost-Effectiveness Threshold. Journal of Managed Care & Specialty Pharmacy. 2019;25:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].O’Brien SH, Hankins JS. Decision analysis of treatment strategies in children with severe sickle cell disease. J Pediatr Hematol Oncol. 2009;31:873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable


