Abstract
Background
Lipids are essential components of the structure and for the function of brain cells. The intricate balance of lipids, including phospholipids, glycolipids, cholesterol, cholesterol ester, and triglycerides, is crucial for maintaining normal brain function. The roles of lipids and lipid droplets and their relevance to neurodegenerative and neuropsychiatric disorders (NPDs) remain largely unknown.
Summary
Here, we reviewed the basic role of lipid components as well as a specific lipid organelle, lipid droplets, in brain function, highlighting the potential impact of altered lipid metabolism in the pathogenesis of Alzheimer’s disease (AD) and NDPs.
Key Messages
Brain lipid dysregulation plays a pivotal role in the pathogenesis and progression of neurodegenerative and NPDs including AD and schizophrenia. Understanding the cell type-specific mechanisms of lipid dysregulation in these diseases is crucial for identifying better diagnostic biomarkers and for developing therapeutic strategies aiming at restoring lipid homeostasis.
Keywords: Lipids, Cholesterol, Lipid droplets, Dysfunction, Alzheimer’s disease, Schizophrenia, Neuropsychiatric disorders
Introduction
Neuropsychiatric disorders (NPDs), including schizophrenia (SZ), bipolar disorder (BD), autism spectrum disorder (ASD), major depressive disorder (MDD), and attention deficit hyperactivity disorder (ADHD), are mental illnesses that impact brain function, emotion, and mood. These illnesses are prevalent (∼0.64% SZ, 2.8% BD, 4.4% ADHD, and 8.4% MDD in adults, and 2.8% ASD in children) and highly debilitating (www.nimh.nih.gov/health/statistics), which led to an approximately USD 5 trillion economic burden in 2019 [1], and is projected to rise to USD 6 trillion in 2030 [2]. Another major NPD is Alzheimer’s disease (though in this review, we usually refer to it separately as AD), a devastating neurodegenerative disorder that also elicits some neuropsychiatric symptoms. AD is the most common cause of dementia and afflicts more than 6.5 million Americans with a projected growth to 13.8 million by 2060 [3], and there are an estimated 40 million people worldwide living with dementia [4]. Despite decades of research, mostly on β-amyloid (Aβ) and tau lesions, effective treatment for AD is still lacking. The lack of major breakthroughs in understanding the pathogenic mechanisms and subsequent development of effective treatments of these NPDs, including AD, supports a need for paradigmatic change in understanding the possible disease pathogenesis. These NPDs all have a complex etiology, each involving polygenic and environmental risk factors, some possibly interacting. Recent genome-wide association studies (GWAS) and exome sequencing studies have identified hundreds of reproducible risk loci for NPD [5–17] and AD [18–23], providing unprecedented opportunities to better understand the disease biology of NPD and AD. However, most biological follow-up of these genetic findings in the field are aspects that have been explored for decades, i.e., focusing on neurodevelopmental deficits or synaptic dysfunction in neurons for NPD and on classical neuropathic Aβ and tau lesions for AD. On the other hand, available effective new treatments for these disorders remain scarce, and the therapeutic armamentarium largely relies on old drugs. For instance, most antipsychotic drugs for treating positive symptoms of SZ still target dopamine receptor D2, an approach taken due to a discovery made almost half a century ago [24]. Thus, a paradigm change in understanding the novel biology of NPD and AD is highly needed, which may help develop more accurate biomarkers for clinical diagnosis and identify more effective treatment through identifying druggable targets.
Dysregulation of lipid synthesis and metabolism in the brain may represent one such novel biological mechanism for influencing disease progression in NPD and AD. Brain is a lipid-rich organ, and 50% of brain dry weight is constituted by lipids [25]. The brain lipids consist of approximately 50% phospholipids, 40% glycolipids, 10% cholesterol and cholesterol ester, and a small amount of triglycerides [26]. Accumulating evidence, both genetic and biological, suggests that lipid systems play critical roles in NPD such as SZ and AD [27, 28]. In fact, Alois Alzheimer originally described “lipoid granules” in the AD brain, suggesting that irregular lipid metabolism may be a driving factor [29]. In this review, we will first discuss the supportive genetic evidence for linking lipids to NPD and AD and then address the cell type-specific biosynthesis, metabolism, and function of the main lipid components, including the lipid organelle named lipid droplets (LDs). Each section is followed by a discussion of the pathophysiological role of different types of lipids in developing AD and NPD. We also critically reviewed the possible link between sex-specific lipids and sex differences of these disorders and outlined potential disease treatment approaches targeting lipids.
Genetic Evidence That Supports the Link of Lipids with AD and NPD
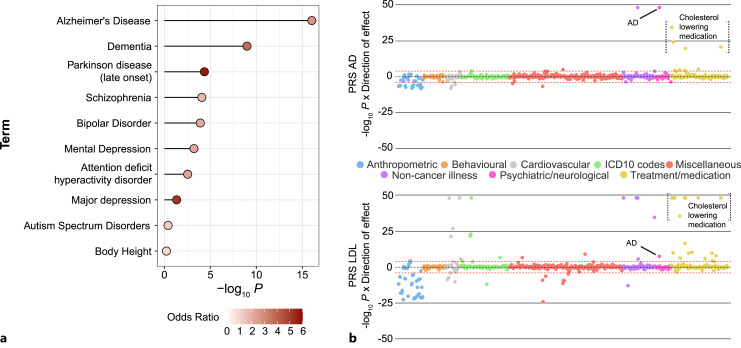
In recent years, lipid dysmetabolism has drawn much attention for contributing to the development of AD [27]. Besides strong supporting evidence from the “lipoid granules” originally found in the AD brain by Alois Alzheimer [29], perhaps the clearest evidence is that many lipid-related genes have been identified as some of the strongest risk factors for AD by GWAS studies, such as ApoE, ApoJ (clusterin), and ABCA7 [23, 30, 31]. Elevated plasma levels of low-density lipoprotein-cholesterol (LDL-C) and ApoB are significantly associated with increased risk of early-onset AD, and this effect is only partially mediated by the ApoE E4 genotype [32]. It was also found that plasma lipids can differentiate AD patients from healthy controls with cholesterol esters, phosphatidylethanolamines (PEs), and triacylglycerols showing differential associations with separate AD genes [33]. Collectively, with all the 568 genes curated in Reactome with function related to metabolism of lipids and lipid proteins (reactome.org/PathwayBrowser/#/R-HSA-556833), our disease risk gene enrichment analysis using DisGeNET [34] identified the most significant enrichment for AD and dementia among all major neurodegenerative and NPDs or traits (Fig. 1a).
Fig. 1.
Genetic evidence that supports the link of lipids to AD and NPDs. a Disease gene set enrichment analysis by using DisGeNET in Enrichr (maayanlab.cloud/Enrichr/). Lipid- and lipid protein-related genes are from Reactome (reactome.org/PathwayBrowser/#/R-HSA-556833). Shown are the adjusted enrichment p values (in –log10 scale). b PheWAS-polygenic burden associations with human phenome in the UK Biobank (mrcieu.mrsoftware.org/PRS_atlas/). PRS of AD is calculated using a GWAS dataset with 74,046 individuals [21], and PRS of LDL is calculated using a GWAS dataset with 173,082 individuals [35]. PRS, polygenic risk score.
Furthermore, an unbiased phenome-wide association analysis between individual polygenic burden (i.e., polygenic risk score; PRS) and a variety of disease phenotypes or traits in the UK Biobank found that PRS of AD is strongly associated with high plasma cholesterol levels (Fig. 1b) [36]. Conversely, PRS of LDL levels is also significantly associated with AD [36], suggesting a potentially shared genetic risk liability (i.e., pleiotropy) between AD and lipid disorders. Such genetic pleiotropy is further supported by a recent coding-wide association study (a GWAS for protein-coding SNPs only) that showed strong correlation of the risk effect sizes of AD and lipid disorders [37]. Interestingly, a rare missense SNP, rs150484293 (minor allele frequency, MAF = 0.3%) in CLPTM1, a gene at the same ApoE risk locus that has a known role in GABA type A receptor forward trafficking [38], showed independent association to both AD and lipid disorders with effect sizes similar to those of ApoE [37, 39].
Compared to the strong genetic evidence for the role of lipids in AD or dementia, the genetic evidence, especially directly from GWAS, for linking lipids to NPD is lacking. However, our disease risk gene enrichment analysis using DisGeNET [34] for all 568 genes curated in Reactome with function related to metabolism of lipids and lipid proteins (reactome.org/PathwayBrowser/#/R-HSA-556833) identified much less robust (compared to AD), but still significant, enrichment for most NPDs (SZ, BD, ADHD, and MDD) (Fig. 1a). Among those enriched NPDs, SZ showed the strongest enrichment. Genetic study of ASD also suggests potential involvement of lipid metabolism in disease pathogenesis. ASD children and their close relatives are more likely to carry genetic mutations within genes known to regulate lipid metabolism [40]. Nonetheless, the lack of strong risk genes directly involved in lipid pathways for NPD does not exclude the importance of lipids in the pathogenesis of NPD; for instance, a mouse model of the SZ-associated CNV 3q29 deletion (40-fold increase of SZ risk) showed strong global alteration of genes related to fatty acid synthesis and other lipid metabolisms, suggesting a mechanistic link between abnormal lipid levels and NPD.
Role of Glycerophospholipids and Sphingolipids in AD and NPD
Glycerophospholipids (GPLs) and sphingolipids are the two types of phospholipids that are essential components of the cellular membrane. Phosphatidylcholine (PC), PE, and phosphatidylserine are the three most common GPL species. Sphingolipids, together with cholesterol, form lipid rafts [41] that are also considered key membrane components. Sphingolipids encompass a broad spectrum of lipids derived from their precursor ceramide, and the main bioactive ones include sphingomyelin, sphingosine, sphingosine-1-phosphate (S1P), ceramide-1-phosphate (C1P), dihydroceramide, and glycosphingolipids [42]. Phospholipids utilize the long chain (>20 carbons) polyunsaturated fatty acids to constitute hydrophobic membrane bilayers. As for their biosynthesis and metabolism, phosphatidic acid (PA) and diacylglycerol are two common precursors for GPLs with the final products being PC, PE, PS, and phosphatidylinositol (PI). The biosynthesis of sphingolipids starts from sphingosine, which is converted to dihydroceramide by ceramide synthase enzymes and further to ceramide by ceramidase [43]. Phospholipids are degraded in the lysosome, where GPLs are catabolized by phospholipases into second messengers such as DAG, inositol 1,4,5-trisphosphate, lysoglycerophospholipid, platelet-activating factor, and long-chain polyunsaturated fatty acids [44, 45], while sphingolipids are hydrolyzed to ceramide then to sphingosine [43]. Despite their fundamental role as building blocks of cell membranes and their conserved cellular mechanism of biosynthesis and metabolism, phospholipids show brain cell type-specific functions.
Function of Phospholipids in Neurons
Phospholipids play an important role in regulating dendrite branch formation, neurite outgrowth, and synaptic vesicle shuttling in neurons. Phosphoinositides (PPIs), the phosphorylated derivatives of PI, are the docking sites of actin cytoskeleton regulators (Wiskott-Aldrich syndrome proteins) [46]. PPIs can also bind with lipid-binding proteins (e.g., Bin-amphiphysin-Rvs), which further interact with actin regulators and contribute to neurite branching [47]. PA supports neurite elongation by stimulating vesicle fusion for plasma membrane expansion, although it only represents a small fraction of the membrane [48]. As major types of glycosphingolipids mainly present in neurons [49] and highly enriched at synapses [50], gangliosides and other sphingolipids are essential for driving neural stem cell (NSC) proliferation and differentiation and neuronal synaptogenesis, myelination, and axonal arborization during CNS development [43, 51]. For instance, depletion of GD3, a minor ganglioside that is converted from ganglioside GM3 by the enzyme α-2,8-sialyltransferase (GD3 synthase), impairs neurogenesis and reduces dendritic complexity and spine density [52]. In addition, GM1 interacts with neurotrophic factors and their receptors, further promoting neuron growth and survival [53, 54].
Function of Phospholipids in Glial Cells
For astrocytes and microglia, brain cell types that may be more relevant to neurodegenerative disorders, lipids are also instrumental for normal cellular function. Inflammatory response is one of key capacities of astrocytes and microglia, which need inflammatory cell signaling to induce cytokine secretion. An important inflammatory cell signaling molecule is PPI 3-kinase (PI3K) that can phosphorylate the 3-position hydroxyl group of the inositol ring of PI. Lipid secondary signaling molecules including PPIs and lactosylceramide are either directly or indirectly involved in the PI3K signaling process. Specifically, PIP2 is the substrate of PI3K that activates the downstream AKT pathway [55], while lactosylceramide interacts with inflammatory pathways and induces gliosis, pro-inflammatory cytokines, and inducible nitric oxide synthase secretion [56]. PPIs also regulate microglial phagocytosis [57]. In addition, sphingomyelin, galactosylceramide, and its sulfatide are highly enriched in oligodendrocytes, where the myelin sheath is used to wrap the axon and accelerate neurotransmission and support neuronal function [58]. Sphingomyelin also interacts with cholesterol to regulate ion channel function [58].
GPLs and Sphingolipids in AD
Consistent with the strong genetic evidence that supports the involvement of abnormal lipids in AD pathogenesis (Fig. 1), membrane lipid composition and integrity along with neuroinflammation and oxidative stress have been suggested to play an important role in AD pathogenesis [59–61]. Decreased phospholipids (PC, PE, and PI) have been reported in AD cohorts (reviewed in [62]). The phospholipid composition determines the biolayer’s phase, fluidity, charge, and thickness, which directly affects Aβ binding and permeation and further alters Aβ aggregation [63, 64]. For instance, specifically disturbing PE (with an intrinsic negative curvature of the membrane) disrupts Aβ membrane binding and blocks its toxicity [65]. In addition, phospholipase A2 (PLA2), which hydrolyzes fatty acid from membrane phospholipids, and its derivative product, arachidonic acid (AA), have been reported to mediate the Aβ-induced excitotoxicity in AD and contribute to the observed learning and memory deficits in mouse models of AD [66].
As precursors of sphingolipids, ceramide, C1P, and S1P are reported as signaling molecules involving stress response, cell proliferation, and differentiation [67, 68]. In an early stage of AD, ceramide, which assists pro-apoptotic signaling, is elevated; conversely, the antiapoptotic S1P level is decreased [69, 70]. Based on these observations, the C1P/SIP pro-survival signaling pathway has been proposed as a therapeutic target for AD [71]. In addition, ceramide facilitates Aβ production through stabilizing β-secretase, which produces Aβ by proteolytic cleavage [72]. Lipid raft regions that are rich in cholesterol and sphingolipid could serve as an anchor for β-secretase and γ-secretase and are directly associated with Aβ production [73]. Besides the effects of lipids on the classical Aβ lesion in AD, the glial function of lipid secondary signaling in glial cell activation and oxidative stress is strongly congruent with the well-established neuroinflammatory pathogenic theory in AD [74].
GPLs and Sphingolipids in NPD
Given that and synaptic dysfunction are the major postulated pathophysiological mechanisms of NPD, it is conceivable that dysregulation of phospholipids in neurons plays an important role in developing NPD. Despite the much weaker genetic evidence for the role of lipids in NPD than in AD (Fig. 1), abnormal lipid levels in the brain have been implicated in multiple NPDs. In postmortem brain, lower levels of lipids including free fatty acids (FFAs), PC, and ceramides, and decreased expression levels of lipid metabolism-related genes are detected in SZ cohorts in different brain regions [75–77]. Along with decreased phospholipids, PLA2 overactivation and reduced AA levels have been found in SZ [78]. Increased PLA2 activity is also found in MDD, BD, and substance use [79]. Such changes of PLA2 and AA are found to be associated with monoaminergic neurotransmission dysregulation [78]. It is noteworthy that the reduced AA level in SZ is expected to result in neural hypofunction, which is opposite to the AA-mediated Aβ-induced neural hyperexcitability in AD [66].
Consistently, a recent study of lipidome composition of prefrontal cortex gray matter in 396 cognitively healthy individuals and 67 adult individuals diagnosed with ASD, SZ, and Down syndrome found that 95% of the 5,024 detected lipids showed significant age-dependent concentration differences, and a concentration decrease of those in the category of GP metabolism was found for all three disorders [80]. Interestingly, the lipid concentration decrease in SZ can be further linked to genetic risk variants in genes involved in PI signaling [80], indicating the relevance of the brain lipidomic decrease for SZ. The PI3K-AKT pathway, which uses PI as a substrate, has also been postulated to be involved in the pathophysiological development of MDD and suicide, with significantly reduced activity of PI3K and AKT in the patients’ brains [81, 82].
The observed alteration of PI-related function in NPD also seems to be supported by animal studies of stress models that mimic the depressive state of MDD, which consistently showed reduced PI in the prefrontal cortex and some other brain regions [83]. In addition to PI, other lipids like PC and PE were also reduced, while LPC, LPE, ceramide, DAG, and TAG levels were elevated in induced depressive states [83]. In addition, the elevated activity of acid sphingomyelinase and the increase of its product ceramide were also found in BD and MDD patients’ brain or peripheral samples [76, 84]. Such studies suggest a plausible pathway that may link chronic stress, lipidomic dysregulation, and the development of MDD and other NPDs [83]. However, the emerging pattern of the reduced PC, PE, and PI and the increased LPC, LPE, ceramide, TAG, and DAG levels in response to depressed states in NPD needs to be replicated in more independent studies.
Role of Cholesterol in AD and NPD
Cholesterol accounts for about 20% of the entire body weight of human and around 25% of the body’s cholesterol is found in the brain [85]. In the brain, there are two major locations of cholesterol: myelin sheath and cell membranes. About 70% of cholesterol is made from de novo synthesis in local organs themselves rather than being directly transported from the liver, and almost all brain cholesterol is synthesized locally due to the separation of brain from peripheral sources of cholesterol by the blood-brain barrier (BBB) [86]. Brain cholesterol mostly exists in an unesterified form, with only about 1% esterified cholesterol stored in LD (see below) [42]. Brain cholesterol biosynthesis, metabolism, and transport are highly cell-type-specific, contributing to NPD and AD in a cell-type-specific manner.
Cholesterol in Neurons
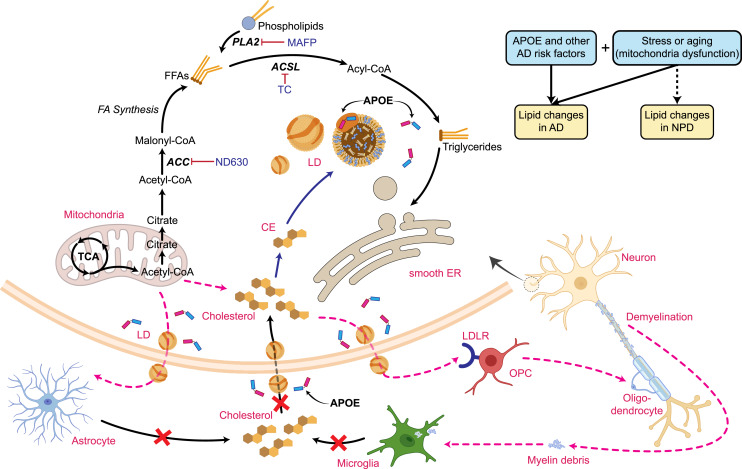
Mature neurons gradually abandon cholesterol synthesis after the embryogenesis stage and rely on it being supplied from astrocytes. However, upon cellular stress, neurons can regain the ability of cholesterol synthesis to a limited extent [87] (Fig. 2). Cholesterol is synthesized in the subcellular organelle, endoplasmic reticulum (ER), involving more than 15 enzymes in about 30 reactions [88]. This process can be further divided into two main steps: (1) from acetyl-CoA to lanosterol, which involves two rate-limiting enzymes, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) and squalene monooxygenase (SM) and (2) through two cell type-dependent pathways to complete final cholesterol synthesis, the K-R (neurons) and Bloch (astrocytes) pathways [88–90]. In human-induced pluripotent stem cell (hiPSC)-derived neurons, suppression of cholesterol levels by HMGCR inhibition from statins can impair synaptic function in a way that is similar to the effect of γ-secretase suppression, which increases synapse numbers but decreases synaptic transmission by lowering neuronal cholesterol levels [91]. Neuronal cholesterol can also mediate the neural-glial interaction; neuronal de novo cholesterol synthesis was recently suggested to be essential for remyelination of damaged neurons in aging and AD, likely due to cholesterol-mediated oligodendrocyte progenitor cell proliferation [92–94] (Fig. 2).
Fig. 2.
Schematic diagram of neuronal LD formation and cholesterol synthesis and lipids transport between brain cell types. Also shown are genetic risk factors and other risk factors (stress or aging) that may affect neural lipid synthesis, metabolism, and transport in developing AD or NPDs. OPC, oligodendrocyte precursor cells; CE, cholesterol ester; FFA, free fatty acids; LDs, lipid droplets; TC, triacsin C; AD, Alzheimer’s disease; NPDs, non-AD neuropsychiatric disorders. The figure was partially created with www.biorender.com.
Generally, cholesterol cannot be degraded in mammals and is usually excreted or locally recycled. One exception is that a small amount of cholesterol can be hydroxylated to 24-hydroxycholesterol by cholesterol 24-hydroxylase in some neurons such as pyramidal neurons in the hippocampus and cortex or Purkinje cells in the cerebellum. The addition of a hydroxyl group at the 24-position makes it more lipophilic with easier penetration of the BBB [95], thereby promoting a rapid diffusion to systematic circulation and cerebrospinal fluid (CSF). Despite some inconsistent findings, several studies suggest 24-hydroxycholesterol as a biomarker of the dysregulated cholesterol in AD and other neurodegenerative disorders [96, 97].
In the periphery, cholesterol transport is carried out mainly through the formation of chylomicrons, very low-density lipoprotein, intermediate-density lipoprotein, LDL, and high-density lipoprotein (HDL) with assistance of apolipoproteins. However, only a few LDL and HDL can cross the BBB through LDL receptor or scavenger receptor class B type I in normal conditions, and thus are usually considered negligible sources of cholesterol [98]. As a separate pool, the brain has different lipoproteins for cholesterol trafficking, named “HDL-like” particles with a wider range of sizes (10–22 nm) and similar density. Although the main apolipoproteins such as ApoE, ApoA-I, and ApoJ (clusterin, also known as CLU) are all highly expressed in astrocytes and presumably function in astrocytes, ApoJ also shows robust expression in neurons, and neural ApoE also seems to play an important role in lipid transfer from neurons to astrocytes under stress [99]. As one of the main lipoprotein endocytic receptors responsible for lipoprotein particle metabolism in the CNS, LRP1 is mainly expressed in neurons and metabolizes the ApoE-containing lipoproteins [100]. Moreover, among the ATP-binding cassette (ABC) transporters that are also involved in cholesterol transport in the brain [101], ABCA1 is the most studied and predominately expressed in neurons to remove excess cholesterol [100]. The ABCA1 expression level is tightly controlled by liver X receptors, a sterol-sensitive transcription factor from a nuclear receptor family [88]. ABCA1 expression can also be similarly regulated by another steroid hormone nuclear receptor, retinoid X receptor [102]. It would be interesting to test whether the steroid-related cholesterol transport regulation through ABCA1 in neurons may mediate the sex-specific role of lipids in AD and NPD.
Cholesterol in Glial Cells
Astrocytes are the main source of cholesterol, which can be further transported by lipoproteins to support neurite maintenance and synapse connectivity [103]. Although the cholesterol synthesis process in astrocytes shares some steps with neurons such as HMGCR-mediated conversion of acetyl-CoA to lanosterol, the final cholesterol synthesis follows an astrocyte-specific Bloch pathway [88–90]. For cholesterol trafficking, the “HDL-like” particles are mainly generated from astrocytes and cycled to the CSF [104]. The most abundant apolipoproteins found in the CSF are ApoE and ApoA-I, both of which can constitute lipoprotein particles independently from each other [100, 105]. ApoJ is also found in the CSF but is specifically enriched there in smaller sized (10–12 nm) lipoprotein particles that have limited lipid content without ApoE or ApoA-I [105]. Lipoprotein particle metabolism in astrocytes is mediated by LDLR and heparan sulfate proteoglycan, which mainly metabolize ApoE-containing lipoproteins [100]. Among ABC transporters that are also involved in cholesterol transport in the brain [101], unlike the major role of ABCA1 in neurons, ABCA7, ABCA8, ABCG1, and ABCG4 tend to be more involved in astrocytes [101].
Cholesterol biosynthesis in microglia is usually deemed an emergent response to abnormal conditions, i.e., during myelin damage (Fig. 2). In this case, microglia engulf and digest myelin debris and myelin associated cholesterol, and simultaneously synthesize desmosterol, an immediate precursor of cholesterol from the Bloch pathway. Microglia then excrete cholesterol, activate liver X receptor-signaling, and facilitate the cholesterol recycling process to assist remyelination [106]. As mentioned above, one of main uses of cholesterol is constituted myelin (∼80%). Oligodendrocytes are the main cell type that synthesizes cholesterol for myelination by itself, only with some complements from astrocytes during the developmental stages [85, 107, 108]. Once myelination is completed, oligodendrocytes only keep low rates of cholesterol synthesis for myelin maintenance, while astrocytes continue to synthesize and supply cholesterol to support all neighboring cells [107, 108]. Lipoprotein particle metabolism in microglia is dominated by the lipoprotein endocytic receptor, triggering receptor expressed on myeloid cells 2, which mainly mediates myelin phagocytosis [100].
Cholesterol in AD
Cholesterol plays a central role in AD by affecting the two main pathogenic hallmarks of AD: Aβ plaque and tau accumulation. As discussed above, AD risk genes ApoE, ApoJ, and ABCA7 are all involved in cholesterol trafficking. As the strongest AD risk variant, ApoE4 is associated with higher Aβ plaque burden and more severe tau pathology in the postmortem brain, whereas the opposite effect was observed for ApoE2, a protective isoform [109–111]. Some mechanistic studies show that ApoE4 directly dysregulates the cholesterol pathway in different cell types, which further impairs myelination of oligodendrocytes [112], induces malfunction of astrocytes and microglia [113–115], and promotes pathogenesis of AD [111, 116, 117]. ApoE4 also affects sphingolipid metabolism [118] and LD storage [115, 116, 119–122] to facilitate AD progression. ApoJ, besides its direct involvement in lipid transport and metabolism, can bind to Aβ oligomers and interfere with Aβ aggregation, which further induces neurotoxicity with excess amounts of Aβ [123]. ApoJ can also enhance tau aggregates, promoting tau pathology [124]. For ABCA7, different splicing isoforms and methylation levels of its CpG sites are all strongly associated with AD [125]. At a functional level, studies show that ABCA7 mediates phagocytosis and immune responses, which could contribute to AD development [126, 127].
Although it remains largely unclear how abnormal brain cholesterol leads to AD pathogenesis, recent studies seem to suggest the involvement of different brain cell types. Studying AD postmortem with different numbers of copies of the ApoE4 allele found that ApoE4 affected gene expression across all assayed brain cell types [112]. Specifically for cholesterol changes, the APOE4 allele was associated with increased expression of cholesterol-manufacturing genes and dysregulated cholesterol-transporting genes in oligodendrocytes, which may explain the observed accumulation of abnormal amounts of cholesterol in oligodendrocytes rather than using it to make healthy myelin sheaths around axons [112]. As the main source of cholesterol in the CNS, astrocytes also play an important role in mediating the effect of cholesterol on AD. For instance, compared to ApoE3 astrocytes, ApoE4 astrocytes express higher levels of genes involved in cholesterol biosynthesis and displayed cholesterol accumulation in lysosomes [113]. The role of neuronal cholesterol in AD is relatively understudied, but neuronal de novo cholesterol synthesis was recently suggested to be essential for remyelination of damaged neurons in aging and AD, likely due to cholesterol-mediated oligodendrocyte progenitor cell proliferation [92–94].
It is interesting to note that the peripheral cholesterol level is similarly associated with AD. High levels of cholesterol, particularly LDL-C, are a well-established risk factor for coronary artery disease and stroke, and numerous studies have implicated a high blood cholesterol level as the risk factor for AD [128]. The link between the elevated level of blood cholesterol and AD is further confirmed by a recent large study with 1.8 million UK adults aged over 40 who had a blood cholesterol measurement between 1992 and 2009 [129]. Both total cholesterol levels and LDL-C in midlife were found associated with an increased risk of developing dementia and AD over a decade later, although the association with total cholesterol level was much weaker than LDL-C [129]. Whether or how the elevated peripheral cholesterol level is correlated with the accumulation of abnormal amounts of cholesterol in oligodendrocytes or other brain cell types in AD [112] remains to be addressed.
Cholesterol in NPD
In the cell membrane, cholesterol is enriched in lipid rafts that serve as hubs to cluster proteins such as neurotransmitter receptors, ion channels, and synaptic proteins [130]. Cholesterol homeostasis is thus essential for maintaining neuronal activity. Furthermore, cholesterol constitutes the basic cellular components for synaptic outgrowth and myelin formation. In addition, cholesterol is the substrate of steroid hormones acting on the hypothalamic-pituitary-adrenal axis, and an aberrant hypothalamic-pituitary-adrenal axis has been suggested to contribute to the pathophysiology of NPD, especially for MDD and anxiety disorders [131]. It is thus conceivable that abnormal cholesterol in the brain plays an important role in developing NPD. However, reports on brain cholesterol and NPD have been scarce. The most relevant studies are on the role of apolipoproteins in NPD and have yielded inconsistent results. Increased ApoE expression has been reported in the brains of SZ patients [132, 133], supporting the association of ApoE allele with SZ [134], but this was not confirmed by others [135]. Similarly inconsistent, several studies reported the decline of APOA1 level in SZ [136, 137], while others supported its elevation in CNS [132].
Compared to the lack of studies on the role of brain cholesterol in NPD, there have been numerous studies of the link between blood cholesterol levels and multiple NPDs. Higher levels of serum lipids including cholesterol and TAGs in SZ cohorts have been found in multiple studies [138, 139]. However, for the first-episode psychosis, a recent meta-analysis found that while blood triglycerides were greater in patients, the mean total cholesterol and HDL-C were reduced in patients [140]. Consistently, lower total cholesterol and HDL-C were also found in a cohort of drug-naïve patients with BD [141]. These observations in drug-naïve patients with SZ and BD seem to be supported at a molecular level by a recent transcriptomic profiling of blood samples of SZ and BD, in which the expression of a disease-associated innate immunity signature gene was found to show a positive correlation with triglyceride and a negative correlation with HDL cholesterol [142]. These studies strongly suggest that lipid levels including cholesterol may be a credible blood biomarker for the disease progression of NPD.
It is unclear how to reconcile decreased brain lipids and elevated serum lipids with reduced cholesterol in SZ and some other NPDs. One possibility is that serum lipid levels may be associated with antipsychotic treatments [139]. In this regard, it is noteworthy that antipsychotic clozapine can strongly upregulate cholesterol biosynthesis genes including dihydropyrimidinase like 2 in iPSC-derived neurons [143]. It would be interesting to test for possible “lipid deficiencies” in neural models derived from SZ donors and see whether they can be restored or reversed by clozapine.
Lipid Droplets
LDs are a conserved organelle that stores intracellular neutral lipids including TAGs, cholesteryl esters, and a small amount of retinyl esters [144–146]. Unlike other organelles with similar sizes such as lysosomes and endosomes, there is a phospholipid monolayer embedded with LDs-associated proteins, which can regulate LDs function [144] (Fig. 2). As a lipid reservoir, LDs play a significant role in membrane formation and energy metabolism [147]. Phase separation of hydrophobic lipids by LDs also protects the cell from toxic fatty acid accumulations [148]. LDs are found in all types of brain cells, but mostly in glial cells [149, 150]. Aging, inflammation, and oxidative stress are believed to be the major factors that trigger LDs accumulation in brain cells [149]. However, whether LDs are beneficial or detrimental remains controversial; for instance, heavily LDs-accumulated astrocytes or microglia often become inflammatory or toxic to neurons [150], and neurotoxic reactive astrocytes can secrete FFA and other lipids that kill neurons and oligodendrocytes [151]. Addressing this question will benefit from a better understanding of the cell type-specific LDs biogenesis, turnover, and function in the brain.
Neuronal LDs
LDs are formed de novo from the ER and budded off from its leaflet. Generally, TAGs and cholesteryl esters are synthesized between ER leaflets by recruiting and converting fatty acids and cholesterol. The formation of LDs is further facilitated by Seipin, a conserved ER membrane protein, and other LDs biogenesis factors such as lipid binding protein perilipin-1 localized at the ER interface. Finally, LDs are expanded and maturated through LD-LD fusion or via increasing TAG content by transferring TAGs from the ER or directly synthesized on the LDs surface [144]. Catabolism of LDs has two main mechanisms: lipolysis and lipophagy. Lipolysis is induced by lipase, which binds to the surface of LDs and hydrolyzes the TAGs into FFA and glycerol [150, 152]. Lipophagy is the autophagy of LDs, which involves the lysosome pathway [150, 152]. An important component of lipophagy is microtubule-associated protein 1A/1B-light chain 3, that help engulf and fuse LDs to the lysosome, and then catabolized it by lysosomal acid hydrolase [150, 152].
Unlike glial cells, neurons usually do not store and utilize neutral lipid as an energy source due to the limited capacity for fatty acid catabolism; instead, they constantly convert TAGs to phospholipids to maintain neurite integrity [150, 153, 154]. Thus, LDs accumulation is rarely detected in neurons. Usually, FFAs are transferred from neurons to astrocytes and stored as LDs, and ApoE plays the pivotal role during the process [99, 154]. However, emerging evidence suggests that hyperactive neurons under stress, such as in aging or AD, can synthesize lipids that are sequestered as LDs (Fig. 2). When these LDs cannot be efficiently cleaned up by dysfunctional astrocytes, they may lead to neuronal damage [99, 150, 154–156] (Fig. 2). In other stress conditions, such as overloading with fatty acid or carrying toxic ApoE4 [99, 154, 157], neurons can also accumulate LDs (Fig. 2). Due to different circumstances of the in vivo system, cultured neurons are considered a stress model that often leads to LDs formation in their cell body [150]. One possible mechanism for neuronal LDs formation is that due to the relatively poor antioxidant defense system in neurons, the mitochondrial reactive oxygen species (ROS) generation stimulated by the FFA β‐oxidation becomes toxic to neurons [158]. However, it is still controversial whether it is ROS generation that induces LDs or vice versa. The functional implications of neuronal LDs in normal aging or under pathogenic conditions such as AD or other NPDs remain largely unknown.
Glial LDs
LDs in astrocytes could serve as an alternative energy source in case glucose energy becomes exhausted [159]. It is estimated that around 20% of brain energy production comes from FFA metabolism [160, 161]. Astrocytes utilize this function to support neuronal energy consumption, particularly in starving conditions. LDs are also found in ependymal cells, a special glial cell type that forms the epithelial lining of the ventricles to maintain CSF homeostasis. It is believed that CSF lipoprotein particle internalization by ependymal cells facilitates LDs formation [150]. In the nonpathological in vivo context, abundant LDs are indeed observed in ependymal cells [162–164], which is even more pronounced in the AD brain [165].
LDs accumulation in microglia is often correlated with its phagocytic function. Many lipid-rich contents, including dead cells, myelin debris, lipoprotein particles, and neuron-derived lipid particles, can be engulfed by microglia to form LDs [150]. Recently, a special microglia subpopulation with LDs has been identified in aged brain and named as LD-accumulating microglia [166]. LD-accumulating microglia have a unique transcriptomic signature that further contributes to age-related neuronal inflammation and neurodegeneration [166]. In oligodendrocytes, LDs accumulation usually occurs during the developmental stage with a high rate of myelination and demyelination, when a considerable amount of cholesterol is synthesized [150].
Given that the LDs levels often rise in glial cells under stress or inflammation conditions, e.g., neurodegenerative disease, hypoxia, metabolic stress, cancer, and BBB leakage [166–171] but stays low under normal physiological conditions [172], it is conceivable to speculate that the main role of LDs in glial cells is protective. Such protective effects of LDs may be reflected by buffering the release of lipid-rich content induced by abnormal or pathogenic conditions, and/or serving as an energy resource. On the other hand, LDs in brain cells may also impair their normal cellular functions, e.g., lipopolysaccharide-treated microglia result in fatty acid accumulation that further leads to more LDs, which makes microglia lose their phagocytic capacity [166, 173]. A reasonable hypothesis could be that homeostasis of LDs in glial cells, astrocytes or microglia, is important for maintaining normal glial cell and neuron function.
LDs in AD
Despite Alois Alzheimer noticing LDs or “adipose saccules” in glial cells of the AD brain in 1907 [174], the importance of LDs in AD has been largely neglected until recently. LDs accumulation appears earlier than senile plaques and neurofibrillary tangles in an animal model of AD and was also found in AD postmortem brain [165], suggesting LDs may be a more upstream “causal” factor for AD pathogenesis. Transgenic mouse models of AD have yielded contradictory findings on the effect of LDs on NSC proliferation [165, 167]. While the relevance of NSC proliferation to AD remains to be established, these interesting findings on the cellular effects of LDs in AD mouse models highlight the potential significance of further exploring the role of LDs in AD pathogenesis.
The most direct supporting evidence for the link of LDs to AD comes from studying the effect of the strongest AD risk factor, ApoE4, on LD formation in glial cells and neurons (Fig. 2). In the absence of neurons, ApoE4 glial cells accumulate more LDs than ApoE3 or ApoE2 [99]. Similarly, ApoE4 microglia (hiPSC-derived) also accumulate more LDs than other APOE variants [116]. The observed upregulation of genes involved in lipid production such as acyl-CoA synthetase 1 in both iPSC-derived ApoE4 microglia and astrocytes [116, 119] suggests excess lipid production may also partially explain LDs accumulation in ApoE4 cells. Some other observations at the transcriptional level may also explain increased LDs in ApoE4 cells. For instance, ApoE4 microglia showed a dramatic decrease of expression of genes relating to mitochondrial oxidative phosphorylation [116], a process that may impair fatty acid oxidation and consequently lead to cellular lipid accumulation [155]. Moreover, ApoE4 microglia also showed significant downregulation of genes related to lipid catabolic processes [116], which would also contribute to the accumulation of lipids and LDs. Although the dysfunction of APOE, i.e., the impaired lipid transport capacity of the APOE4 allele, has been commonly used to explain lipid accumulation in glial cells, this would contradict the observations that knockdown of APOE in either the neurons or glia can reduce LDs formation [99, 154]. Therefore, mechanistically, it remains unclear how the APOE4 allele is linked to more LDs in glial cells or microglia.
The relevance of LDs to AD in neurons is less clear than in glial cells and microglia. Interestingly, when cultured in vitro in the absence of glial cells, mouse ApoE3 neurons exhibited stronger LDs accumulation than ApoE4 neurons; however, when cocultured with glial cells, mouse ApoE4 neurons retained more LDs than in ApoE3 neurons [99]. This observation seems to support the hypothesis that dysfunctional neuronal ApoE4 may impair neuronal cleanup of lipids by astrocytes. It would be interesting to see whether the neuronal ApoE4-induced LDs accumulation in mouse neurons can also be observed in human neurons and what would be the functional implications of neuronal LDs for AD pathogenesis.
From a functional perspective, cholesterol inclusion in LDs significantly increases p-tau levels, which can be reversed by inhibiting the cholesterol synthesis pathway [175]. Also, neuronal hyperactivity alone can sufficiently induce LDs accumulation in astrocytes and accelerate AD pathologies [175]. In microglia, LDs accumulation has been considered to represent a dysfunctional and pro-inflammatory state in aging brains [166]. Recently, ApoE4-induced LDs accumulation was found to render microglia weakly responsive to neuronal activity [116]. However, as addressed above, LDs accumulation may also be an adaptive process to neurotoxicity because blocking glial LDs formation worsens neurodegeneration [169]. Therefore, whether LDs are detrimental or beneficial in AD remains controversial.
LDs in NPD
The role of LDs in other (non-AD) NPDs has been understudied and is not well established. Despite the polygenic nature and complex etiology of NPDs, dysfunctional dopaminergic, glutamatergic, GABAergic, and/or serotoninergic neurotransmission have been implicated in these disorders [176]. Given the known role of sphingolipids and cholesterol in synaptogenesis and neurogenesis (see review [177]), it is conceivable that lipids and neuronal LDs may play a significant role in NPD pathogenesis. Furthermore, because inflammation and oxidative stress are among the major factors besides aging that trigger LD accumulation in brain cells [149] and given the well-established role of inflammation and oxidative stress in NPDs [178, 179], it is tempting to postulate that LDs in brain cells may contribute to the risk of developing NPDs.
Unlike neurodegenerative disorders such as AD, the neurodevelopmental feature of NPDs may point to different stages and brain cell types in which LDs may play a role in disease pathogenesis or act as a biomarker. For instance, for ASD that has the strongest neurodevelopmental aspect, abnormal LDs have been implicated in NSC or neural precursor cells (NPCs). It has been reported that NSC-autonomous insufficiencies in the activity of TMLHE, an ASD risk factor that supports long-chain fatty acid oxidation by catalyzing carnitine biosynthesis, may alter fatty acid mobilization from LDs, thereby reducing NSC pools in the mouse embryonic neocortex [180]. Similarly, NPCs in the subventricular zone and dentate gyrus niches of adult mouse brain express the LDs marker gene perilipin 2 (plin2) and, when cultured in vitro, they accumulate abundant Plin2+ LDs [181]. Interestingly, Plin2+ LDs content per NPC varies and correlates positively with oxygen consumption and cell proliferative ability [181]. In SZ, a proteomic screen of anterior cingulate cortex showed a 2.4-fold increase of the expression of Mover (also known as RP11-46F15.3 and TPRG1L) [182], a presynaptic protein that also serves as an LDs coat and induces accumulation of LDs in astrocytes [183].
As a neurodevelopmental cellular model, hiPSC-derived neuronal cells may help provide some novel insights on the role of LDs in NPD. The iPSC-derived neural cells from SZ patients often show alterations in oxidative phosphorylation and ROS levels (see review [184]), which may directly link to LDs formation. Furthermore, patient-specific iPSC models carrying a specific genetic risk factor of SZ, e.g., 22qdeletion, have suggested a link between SZ risk factors to dysfunctional mitochondria (see review [184]), which may induce LDs formation as a generalized response to stress or affect the mitochondria-LD bond [185, 186]. In this regard, it would be interesting to test whether such NPD genetic risk factors may affect LDs formation in iPSC-derived neurons and whether abnormal LDs may contribute to the altered neuronal phenotypic changes such as neuronal hyperexcitability [184, 187].
Sex Differences of Lipids in AD and NPDs
Lipid levels and lipid metabolism vary substantially between males and females and across the life span [188]. The sex differences of lipids have been commonly attributed to effects of sex hormones [188]; however, potential genetic contributions from sex chromosome differences between males and females are becoming more appreciated. For instance, a recent meta-analysis of GWAS on blood lipid levels with over 1.5 million participants identified over 2,000 lipid loci, including 21 novel lipid loci on the X chromosome [189]. Interestingly, the population effect sizes of those sex-specific lipid loci were often larger in females, i.e., “risk” variants often had stronger effects in females than in males [189]. It is thus likely that both sex hormones and genetic factors interactively contribute to the sex differences of lipids and, given the role of lipids in NPDs and AD, may partially explain the sex difference of these disorders or conditions.
For AD, it has been well established that females are more affected, with approximately two-thirds of the patients being female [190]. Although higher AD prevalence in females is partially explained by longer life expectancy of females, other sex-specific changes during aging and AD development such as lipid metabolism may also play an important role. The clearest evidence of sex-specific contributions has been built upon the interplay between sex hormone and the ApoE4 allele [191]. Specifically, estrogen elevates the expression of ApoE in the brain through estrogen receptor α activation [192, 193], providing a neuroprotective effect [194]. After menopause, the sudden drop of estrogen in women makes them more vulnerable to develop AD. This is in line with female ApoE4 carriers being more likely to develop LOAD and exhibit more severe Aβ, tau pathology, and worse cognitive decline [195–198]. In addition, ApoE4 directly impairs the beneficial effects of estrogen on anti-inflammatory effects of microglia and neurite growth [194, 199]. Moreover, ApoE4 regulates sex-specific transcriptional activity of genes involved in immune response, inflammation, oxidative stress, and aging, making females more vulnerable to develop AD [200]. In general, besides the interplay between sex hormones and ApoE risk loci, the drastic metabolic changes occurring during the critical period of perimenopause in women also increase oxidative stress in the brain [191], which may further lead to accumulation of LDs in specific brain cell types and contribute to sex-specific AD vulnerability. For example, female microglia show an increase of several long-chain species of TAGs with more LDs; such LD-laden microglia that are chronically exposed to Aβ exhibit a dysfunctional phagocytosis [201].
For NPDs, sex differences in prevalence and severity have been well documented. Those with neurodevelopmental aspects such as SZ and ASD affect more males and often with more severe symptoms than females, while some other NPDs such as MDD and anxiety are more prevalent in females (see review [202]). However, the link between sex differences of NPDs and lipids is not as well established as for AD. An earlier study with a small sample found increased blood cholesterol fractions of HDL and LDL during acute psychological stress, and interestingly, males had larger increases of LDL-C and blood pressure [203]. Another study of fatty acid levels in blood cells showed that SZ patients and men had higher levels of lipid peroxidation [204]. While it is not clear that these sex-specific blood lipids in SZ and under psychological stress conditions reflect a sex-specific response to stress in human brains, a recent cell type-specific single-cell transcriptomic profiling in mouse brains did find that oligodendrocytes in males showed a much stronger transcriptomic response than females under stress conditions [205]. Male mice also seem to more likely exhibit LPS-induced depressive behavior and an increase of oxidative stress in the hippocampus [206]. Given that oxidative stress is a major factor that may drive LD accumulation in brain cells [149], it would be interesting to test whether NPDs show sex-specific LD accumulation in human postmortem brains, iPSC-derived neurons, or mouse models. Independent from the sex-specific stress response, lower levels of docosahexaenoic acid (DHA) were found in blood cells of the first-episode male psychotic patients [207] and in the orbitofrontal cortex of male patients with SZ compared to females [208].
Treatment Strategies for AD and NPDs by Targeting Lipids
Because of the potential pathogenic role of lipids, including cholesterol and LDs, in developing AD and NPDs, it is tempting to explore the treatment of these disorders by modulating lipid levels. For AD that often exhibits elevated cholesterol levels (especially LDL-C) in the plasma and some brain cells, the use of statins, a class of drugs that are commonly used to lower blood cholesterol, seems to be a rational choice for reducing AD risk [209]. Some studies show that statins indeed can alleviate AD pathology, possibly through inhibition of cholesterol biosynthesis, thereby reducing Aβ production [210, 211]. However, some randomized trials find no beneficial effects of statins on AD symptoms, or even with a negative effect on cognition under high-dose statins [212, 213]. The negative impact of high-dose statins may be attributed to the excess reduction of cholesterol synthesis in some brain cell types where cholesterol is essential for neuronal function as discussed above. It is noteworthy that a recent study of the UK Biobank database finds potential beneficial effects of statins in ApoE4 carriers [214], suggesting the need for stratifying study participants by disease risk genotypes for evaluating the effects of cholesterol-lowering drugs for AD.
Compared to AD that seems to have consistent elevation of lipids in both serum and some brain cells, NPDs often show elevated serum lipids (TAGs) and cholesterol but reduced brain lipids (see above). Thus, cholesterol-lowering drugs like statins may not seem to be a rational choice for treating NPDs. Statins were found to be able to improve the depression score in MDD and alleviate the symptoms of SZ and BD in some studies [215–218], but not in others [219–221]. The beneficial effects of statins observed in some studies for NPDs may be due to its ability to reducing the serum cholesterol level, which subsequently positively influences brain function; alternatively, statins may confer neuroprotective effects by inhibiting neuroinflammatory and oxidative stress pathways that are involved in NPDs [130]. Besides targeting cholesterol, the possible deficit of DHA in the first-episode male psychotic patients or SZ [207, 208] also makes supplementing dietary marine omega-3 fatty acids (eicosapentaenoic acid [EPA] and DHA) a promising approach to reduce depression risk and promote favorable mood [222, 223].
Conclusion and Perspective
Lipids constitute the cell membrane, transduce cell signaling, and store energy. Lipids also regulate neuronal growth and synaptic plasticity, mediate cell toxicity and apoptosis, and assist in coping with stress, inflammation, and aging process. All these functions make lipids a prominent target for understanding the etiology of NPD and AD. However, the roles of cell type-specific lipids and LDs, and their relevance to neurodegenerative and NPDs remain largely unknown. Lipids can directly or indirectly affect chromatin accessibility and subsequently gene expression; for instance, a transcription factor, brain acid soluble protein 1, can recruit cholesterol to open chromatin regions and regulate gene expression [156, 224]. Therefore, a comprehensive ascertainment of cellular and molecular effects of lipids in disease states and health conditions will provide better mechanistic insight on the role of lipids in AD and NPDs. In addition, although glial LDs are known to be largely neuroprotective [150], the cellular and molecular ramifications of LDs in human neurons are less clear [150]. Furthermore, despite the supportive genetic evidence for the involvement of lipid-related genes in AD and NPDs, whether neural LDs, FFA, and cholesterol are correlated with peripheral plasma lipid levels and other clinical outcomes of AD and NPDs during disease progression is unknown. To address these challenging questions, besides the traditionally used postmortem brains and animal models, iPSC-derived neurons, astrocytes, and microglia may provide invaluable in vitro cellular models [184, 187, 225–231] for studying cell type-specific lipids and LDs in the context of different disease states, which will significantly advance our understanding of how cell type-specific abnormal lipids contribute to the risk for AD and some NPDs. Some cellular lipid metrics may serve as biomarkers for early AD diagnosis and progression, and the downstream gene pathways of lipids and LDs may be promising targets for developing more tailored and effective treatments for AD.
Conflict of Interest Statement
The authors declare no conflicts of interests.
Funding Sources
The time effort for preparation of the manuscript is supported by the National Institute of Health (NIH) grants R01AG081374, R01AG063175, R01MH106575, and R01MH116281 (Xiaojie Zhao, Siwei Zhang, Alan R. Sanders, and Jubao Duan).
Author Contributions
X.Z. wrote the manuscript. S.Z. collected the data and prepared the graphs. A.R.S. and J.D. edited and wrote the manuscript. J.D. supervised the work. All authors have approved the final manuscript.
Funding Statement
The time effort for preparation of the manuscript is supported by the National Institute of Health (NIH) grants R01AG081374, R01AG063175, R01MH106575, and R01MH116281 (Xiaojie Zhao, Siwei Zhang, Alan R. Sanders, and Jubao Duan).
References
- 1. Arias D, Saxena S, Verguet S. Quantifying the global burden of mental disorders and their economic value. EClinicalMedicine. 2022;54:101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloom DE, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, et al. The global economic burden of noncommunicable diseases. Program on the Global Demography of Aging; 2012. [Google Scholar]
- 3. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327–406. [DOI] [PubMed] [Google Scholar]
- 4. Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. Lancet. 2016;388(10043):505–17. [DOI] [PubMed] [Google Scholar]
- 5. Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460(7256):753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. International Schizophrenia Consortium; Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schizophrenia Psychiatric Genome-Wide Association Study GWAS Consortium . Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ripke S; Concortium; SGoPG . Psychiatric genomics consortium quadruples schizophrenia GWAS sample size to 35,000 cases and 47,000 controls. Abstract. American Society of Human Genetics Annual Meeting. 2013;63. [Google Scholar]
- 10. Schizophrenia Working Group of the Psychiatric Genomics Consortium . Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53(6):817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ripke S, Walters JT, O’Donovan MC. Mapping genomic loci prioritises genes and implicates synaptic biology in schizophrenia. medRxiv. 2020. [Google Scholar]
- 13. Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604(7906):502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh T, Poterba T, Curtis D, Akil H, Al Eissa M, Barchas JD, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604(7906):509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180(3):568–84.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51(5):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45(12):1452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet. 2019;51(3):404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. 2022;54(4):412–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am J Psychiatry. 1976;133(2):197–202. [DOI] [PubMed] [Google Scholar]
- 25. Hamilton JA, Hillard CJ, Spector AA, Watkins PA. Brain uptake and utilization of fatty acids, lipids and lipoproteins: application to neurological disorders. J Mol Neurosci. 2007;33(1):2–11. [DOI] [PubMed] [Google Scholar]
- 26. Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24(2):69–176. [DOI] [PubMed] [Google Scholar]
- 27. Kao YC, Ho PC, Tu YK, Jou IM, Tsai KJ. Lipids and alzheimer’s disease. Int J Mol Sci. 2020;21(4):1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang AC, Tsai SJ. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int J Mol Sci. 2017;18(8):1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foley P. Lipids in Alzheimer’s disease: a century-old story. Biochim Biophys Acta. 2010;1801(8):750–3. [DOI] [PubMed] [Google Scholar]
- 30. Lyssenko NN, Praticò D. ABCA7 and the altered lipidostasis hypothesis of Alzheimer’s disease. Alzheimers Dement. 2021;17(2):164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wingo TS, Cutler DJ, Wingo AP, Le NA, Rabinovici GD, Miller BL, et al. Association of early-onset alzheimer disease with elevated low-density lipoprotein cholesterol levels and rare genetic coding variants of APOB. JAMA Neurol. 2019;76(7):809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y, Thalamuthu A, Mather KA, Crawford J, Ulanova M, Wong MWK, et al. Plasma lipidome is dysregulated in Alzheimer’s disease and is associated with disease risk genes. Transl Psychiatry. 2021;11(1):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piñero J, Bravo À, Queralt-Rosinach N, Gutiérrez-Sacristán A, Deu-Pons J, Centeno E, et al. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2016;45(D1):D833–D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richardson TG, Harrison S, Hemani G, Davey Smith G. An atlas of polygenic risk score associations to highlight putative causal relationships across the human phenome. Elife. 2019;8:e43657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun BB, Kurki MI, Foley CN, Mechakra A, Chen CY, Marshall E, et al. Genetic associations of protein-coding variants in human disease. Nature. 2022;603(7899):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han W, Shepard RD, Lu W. Regulation of GABAARs by transmembrane accessory proteins. Trends Neurosci. 2021;44(2):152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang T, Wei P, Pan W. Integrative analysis of multi-omics data for discovering low-frequency variants associated with low-density lipoprotein cholesterol levels. Bioinformatics. 2021;36(21):5223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luo Y, Eran A, Palmer N, Avillach P, Levy-Moonshine A, Szolovits P, et al. A multidimensional precision medicine approach identifies an autism subtype characterized by dyslipidemia. Nat Med. 2020;26(9):1375–9. [DOI] [PubMed] [Google Scholar]
- 41. Bieberich E. Sphingolipids and lipid rafts: novel concepts and methods of analysis. Chem Phys Lipids. 2018;216:114–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19(3):175–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bouscary A, Quessada C, René F, Spedding M, Turner BJ, Henriques A, et al. Sphingolipids metabolism alteration in the central nervous system: amyotrophic lateral sclerosis (ALS) and other neurodegenerative diseases. Semin Cell Dev Biol. 2021;112:82–91. [DOI] [PubMed] [Google Scholar]
- 44. Farooqui AA, Horrocks LA, Farooqui T. Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem Phys Lipids. 2000;106(1):1–29. [DOI] [PubMed] [Google Scholar]
- 45. Schulze H, Sandhoff K. Lysosomal lipid storage diseases. Cold Spring Harb Perspect Biol. 2011;3(6):a004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Papayannopoulos V, Co C, Prehoda KE, Snapper S, Taunton J, Lim WA. A polybasic motif allows N-WASP to act as a sensor of PIP(2) density. Mol Cell. 2005;17(2):181–91. [DOI] [PubMed] [Google Scholar]
- 47. Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303(5657):495–9. [DOI] [PubMed] [Google Scholar]
- 48. Zeniou-Meyer M, Zabari N, Ashery U, Chasserot-Golaz S, Haeberlé AM, Demais V, et al. Phospholipase D1 production of phosphatidic acid at the plasma membrane promotes exocytosis of large dense-core granules at a late stage. J Biol Chem. 2007;282(30):21746–57. [DOI] [PubMed] [Google Scholar]
- 49. Schaeren-Wiemers N, van der Bijl P, Schwab ME. The UDP-galactose:ceramide galactosyltransferase: expression pattern in oligodendrocytes and Schwann cells during myelination and substrate preference for hydroxyceramide. J Neurochem. 1995;65(5):2267–78. [DOI] [PubMed] [Google Scholar]
- 50. West RJH, Briggs L, Perona Fjeldstad M, Ribchester RR, Sweeney ST. Sphingolipids regulate neuromuscular synapse structure and function in Drosophila. J Comp Neurol. 2018;526(13):1995–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu RK, Tsai YT, Ariga T. Functional roles of gangliosides in neurodevelopment: an overview of recent advances. Neurochem Res. 2012;37(6):1230–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang FL, Wang J, Itokazu Y, Yu RK. Ganglioside GD3 regulates dendritic growth in newborn neurons in adult mouse hippocampus via modulation of mitochondrial dynamics. J Neurochem. 2021;156(6):819–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cuello AC. Gangliosides, NGF, brain aging and disease: a mini-review with personal reflections. Neurochem Res. 2012;37(6):1256–60. [DOI] [PubMed] [Google Scholar]
- 54. Ferrari G, Greene LA. Promotion of neuronal survival by GM1 ganglioside. Phenomenology and mechanism of action. Ann N Y Acad Sci. 1998;845:263–73. [DOI] [PubMed] [Google Scholar]
- 55. Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4(9):a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Won JS, Singh AK, Singh I. Lactosylceramide: a lipid second messenger in neuroinflammatory disease. J Neurochem. 2007;103(Suppl 1):180–91. [DOI] [PubMed] [Google Scholar]
- 57. Desale SE, Chinnathambi S. Phosphoinositides signaling modulates microglial actin remodeling and phagocytosis in Alzheimer's disease. Cell Commun Signal. 2021;19(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Olsen ASB, Færgeman NJ. Sphingolipids: membrane microdomains in brain development, function and neurological diseases. Open Biol. 2017;7(5):170069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fabiani C, Antollini SS. Alzheimer’s disease as a membrane disorder: spatial cross-talk among beta-amyloid peptides, nicotinic acetylcholine receptors and lipid rafts. Front Cell Neurosci. 2019;13:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Valappil DK, Mini NJ, Dilna A, Nath S. Membrane interaction to intercellular spread of pathology in Alzheimer’s disease. Front Neurosci. 2022;16:936897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Drolle E, Negoda A, Hammond K, Pavlov E, Leonenko Z. Changes in lipid membranes may trigger amyloid toxicity in Alzheimer's disease. PLoS One. 2017;12(8):e0182194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Frisardi V, Panza F, Seripa D, Farooqui T, Farooqui AA. Glycerophospholipids and glycerophospholipid-derived lipid mediators: a complex meshwork in Alzheimer’s disease pathology. Prog Lipid Res. 2011;50(4):313–30. [DOI] [PubMed] [Google Scholar]
- 63. Williams TL, Serpell LC. Membrane and surface interactions of Alzheimer’s Aβ peptide-insights into the mechanism of cytotoxicity. FEBS J. 2011;278(20):3905–17. [DOI] [PubMed] [Google Scholar]
- 64. Niu Z, Zhang Z, Zhao W, Yang J. Interactions between amyloid beta peptide and lipid membranes. Biochim Biophys Acta Biomembr. 2018;1860(9):1663–9. [DOI] [PubMed] [Google Scholar]
- 65. Cazzaniga E, Bulbarelli A, Lonati E, Orlando A, Re F, Gregori M, et al. Abeta peptide toxicity is reduced after treatments decreasing phosphatidylethanolamine content in differentiated neuroblastoma cells. Neurochem Res. 2011;36(5):863–9. [DOI] [PubMed] [Google Scholar]
- 66. Sanchez-Mejia RO, Mucke L. Phospholipase A2 and arachidonic acid in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801(8):784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jesko H, Stepien A, Lukiw WJ, Strosznajder RP. The cross-talk between sphingolipids and insulin-like growth factor signaling: significance for aging and neurodegeneration. Mol Neurobiol. 2019;56(5):3501–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mencarelli C, Martinez-Martinez P. Ceramide function in the brain: when a slight tilt is enough. Cell Mol Life Sci. 2013;70(2):181–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochem Res. 2007;32(4–5):845–56. [DOI] [PubMed] [Google Scholar]
- 70. Czubowicz K, Jesko H, Wencel P, Lukiw WJ, Strosznajder RP. The role of ceramide and sphingosine-1-phosphate in alzheimer’s disease and other neurodegenerative disorders. Mol Neurobiol. 2019;56(8):5436–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Custodia A, Romaus-Sanjurjo D, Aramburu-Núñez M, Alvarez-Rafael D, Vázquez-Vázquez L, Camino-Castiñeiras J, et al. Ceramide/sphingosine 1-phosphate Axis as a key target for diagnosis and treatment in alzheimer’s disease and other neurodegenerative diseases. Int J Mol Sci. 2022;23(15):8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hur JY. γ-Secretase in Alzheimer’s disease. Exp Mol Med. 2022;54(4):433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hicks DA, Nalivaeva NN, Turner AJ. Lipid rafts and Alzheimer’s disease: protein-lipid interactions and perturbation of signaling. Front Physiol. 2012;3:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hensley K. Neuroinflammation in Alzheimer’s disease: mechanisms, pathologic consequences, and potential for therapeutic manipulation. J Alzheimers Dis. 2010;21(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ghosh S, Dyer RA, Beasley CL. Evidence for altered cell membrane lipid composition in postmortem prefrontal white matter in bipolar disorder and schizophrenia. J Psychiatr Res. 2017;95:135–42. [DOI] [PubMed] [Google Scholar]
- 76. Schwarz E, Prabakaran S, Whitfield P, Major H, Leweke FM, Koethe D, et al. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J Proteome Res. 2008;7(10):4266–77. [DOI] [PubMed] [Google Scholar]
- 77. Shimamoto-Mitsuyama C, Nakaya A, Esaki K, Balan S, Iwayama Y, Ohnishi T, et al. Lipid pathology of the corpus callosum in schizophrenia and the potential role of abnormal gene regulatory networks with reduced microglial marker expression. Cereb Cortex. 2021;31(1):448–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Berger GE, Smesny S, Amminger GP. Bioactive lipids in schizophrenia. Int Rev Psychiatry. 2006;18(2):85–98. [DOI] [PubMed] [Google Scholar]
- 79. Noponen M, Sanfilipo M, Samanich K, Ryer H, Ko G, Angrist B, et al. Elevated PLA2 activity in schizophrenics and other psychiatric patients. Biol Psychiatry. 1993;34(9):641–9. [DOI] [PubMed] [Google Scholar]
- 80. Yu Q, He Z, Zubkov D, Huang S, Kurochkin I, Yang X, et al. Lipidome alterations in human prefrontal cortex during development, aging, and cognitive disorders. Mol Psychiatry. 2020;25(11):2952–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Karege F, Perroud N, Burkhardt S, Fernandez R, Ballmann E, La Harpe R, et al. Alterations in phosphatidylinositol 3-kinase activity and PTEN phosphatase in the prefrontal cortex of depressed suicide victims. Neuropsychobiology. 2011;63(4):224–31. [DOI] [PubMed] [Google Scholar]
- 82. Dwivedi Y, Rizavi HS, Teppen T, Zhang H, Mondal A, Roberts RC, et al. Lower phosphoinositide 3-kinase (PI 3-kinase) activity and differential expression levels of selective catalytic and regulatory PI 3-kinase subunit isoforms in prefrontal cortex and hippocampus of suicide subjects. Neuropsychopharmacology. 2008;33(10):2324–40. [DOI] [PubMed] [Google Scholar]
- 83. Walther A, Cannistraci CV, Simons K, Durán C, Gerl MJ, Wehrli S, et al. Lipidomics in major depressive disorder. Front Psychiatry. 2018;9:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gracia-Garcia P, Rao V, Haughey NJ, Bandaru VV, Smith G, Rosenberg PB, et al. Elevated plasma ceramides in depression. J Neuropsychiatry Clin Neurosci. 2011;23(2):215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Turri M, Marchi C, Adorni MP, Calabresi L, Zimetti F. Emerging role of HDL in brain cholesterol metabolism and neurodegenerative disorders. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(5):159123. [DOI] [PubMed] [Google Scholar]
- 86. Grundy SM. Absorption and metabolism of dietary cholesterol. Annu Rev Nutr. 1983;3:71–96. [DOI] [PubMed] [Google Scholar]
- 87. Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, Bao J, et al. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci. 2010;30(50):17068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21(4):225–45. [DOI] [PubMed] [Google Scholar]
- 89. Fünfschilling U, Saher G, Xiao L, Möbius W, Nave KA. Survival of adult neurons lacking cholesterol synthesis in vivo. BMC Neurosci. 2007;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nieweg K, Schaller H, Pfrieger FW. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem. 2009;109(1):125–34. [DOI] [PubMed] [Google Scholar]
- 91. Essayan-Perez S, Südhof TC. Neuronal γ-secretase regulates lipid metabolism, linking cholesterol to synaptic dysfunction in Alzheimer’s disease. Neuron. 2023;111(20):3176–94.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Berghoff SA, Spieth L, Sun T, Hosang L, Depp C, Sasmita AO, et al. Neuronal cholesterol synthesis is essential for repair of chronically demyelinated lesions in mice. Cell Rep. 2021;37(4):109889. [DOI] [PubMed] [Google Scholar]
- 93. Lin JP, Mironova YA, Shrager P, Giger RJ. LRP1 regulates peroxisome biogenesis and cholesterol homeostasis in oligodendrocytes and is required for proper CNS myelin development and repair. Elife. 2017;6:e30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xie Y, Zhang X, Xu P, Zhao N, Zhao Y, Li Y, et al. Aberrant oligodendroglial LDL receptor orchestrates demyelination in chronic cerebral ischemia. J Clin Invest. 2021;131(1):e128114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gamba P, Giannelli S, Staurenghi E, Testa G, Sottero B, Biasi F, et al. The controversial role of 24-S-hydroxycholesterol in alzheimer’s disease. Antioxidants. 2021;10(5):740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hughes TM, Rosano C, Evans RW, Kuller LH. Brain cholesterol metabolism, oxysterols, and dementia. J Alzheimers Dis. 2013;33(4):891–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sodero AO. 24S-hydroxycholesterol: cellular effects and variations in brain diseases. J Neurochem. 2021;157(4):899–918. [DOI] [PubMed] [Google Scholar]
- 98. Corraliza-Gomez M, Sanchez D, Ganfornina MD. Lipid-binding proteins in brain health and disease. Front Neurol. 2019;10:1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Qi G, Mi Y, Shi X, Gu H, Brinton RD, Yin F. ApoE4 impairs neuron-astrocyte coupling of fatty acid metabolism. Cell Rep. 2021;34(1):108572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Raulin AC, Martens YA, Bu G. Lipoproteins in the central nervous system: from biology to pathobiology. Annu Rev Biochem. 2022;91:731–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 2006;17(9):891–6. [DOI] [PubMed] [Google Scholar]
- 102. Koldamova RP, Lefterov IM, Ikonomovic MD, Skoko J, Lefterov PI, Isanski BA, et al. 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J Biol Chem. 2003;278(15):13244–56. [DOI] [PubMed] [Google Scholar]
- 103. Jin U, Park SJ, Park SM. Cholesterol metabolism in the brain and its association with Parkinson’s disease. Exp Neurobiol. 2019;28(5):554–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Borràs C, Mercer A, Sirisi S, Alcolea D, Escolà-Gil JC, Blanco-Vaca F, et al. HDL-like-mediated cell cholesterol trafficking in the central nervous system and alzheimer’s disease pathogenesis. Int J Mol Sci. 2022;23(16):9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Vitali C, Wellington CL, Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc Res. 2014;103(3):405–13. [DOI] [PubMed] [Google Scholar]
- 106. Berghoff SA, Spieth L, Sun T, Hosang L, Schlaphoff L, Depp C, et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat Neurosci. 2021;24(1):47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Saher G, Brügger B, Lappe-Siefke C, Möbius W, Tozawa R, Wehr MC, et al. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8(4):468–75. [DOI] [PubMed] [Google Scholar]
- 108. Camargo N, Goudriaan A, van Deijk ALF, Otte WM, Brouwers JF, Lodder H, et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017;15(5):e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Serrano-Pozo A, Qian J, Monsell SE, Betensky RA, Hyman BT. APOEε2 is associated with milder clinical and pathological Alzheimer disease. Ann Neurol. 2015;77(6):917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Reiman EM, Arboleda-Velasquez JF, Quiroz YT, Huentelman MJ, Beach TG, Caselli RJ, et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun. 2020;11(1):667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wennberg AM, Tosakulwong N, Lesnick TG, Murray ME, Whitwell JL, Liesinger AM, et al. Association of apolipoprotein E ε4 with transactive response DNA-binding protein 43. JAMA Neurol. 2018;75(11):1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Blanchard JW, Akay LA, Davila-Velderrain J, von Maydell D, Mathys H, Davidson SM, et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature. 2022;611(7937):769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tcw J, Qian L, Pipalia NH, Chao MJ, Liang SA, Shi Y, et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell. 2022;185(13):2213–33.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc Natl Acad Sci USA. 2021;118(33):e2102191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cashikar AG, Toral-Rios D, Timm D, Romero J, Strickland M, Long JM, et al. Regulation of astrocyte lipid metabolism and ApoE secretionby the microglial oxysterol, 25-hydroxycholesterol. J Lipid Res. 2023;64(4):100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Victor MB, Leary N, Luna X, Meharena HS, Scannail AN, Bozzelli PL, et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell. 2022;29(8):1197–212.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lee SI, Jeong W, Lim H, Cho S, Lee H, Jang Y, et al. APOE4-carrying human astrocytes oversupply cholesterol to promote neuronal lipid raft expansion and Aβ generation. Stem Cell Rep. 2021;16(9):2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bandaru VV, Troncoso J, Wheeler D, Pletnikova O, Wang J, Conant K, et al. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer’s but not normal brain. Neurobiol Aging. 2009;30(4):591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sienski G, Narayan P, Bonner JM, Kory N, Boland S, Arczewska AA, et al. APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci Transl Med. 2021;13(583):eaaz4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Moulton MJ, Barish S, Ralhan I, Chang J, Goodman LD, Harland JG, et al. Neuronal ROS-induced glial lipid droplet formation is altered by loss of Alzheimer’s disease-associated genes. Proc Natl Acad Sci USA. 2021;118(52):e2112095118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wang N, Wang M, Jeevaratnam S, Rosenberg C, Ikezu TC, Shue F, et al. Opposing effects of apoE2 and apoE4 on microglial activation and lipid metabolism in response to demyelination. Mol Neurodegener. 2022;17(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Windham IA, Ragusa JV, Wallace ED, Wagner CH, White KK, Cohen S. APOE traffics to astrocyte lipid droplets and modulates triglyceride saturation and droplet size. bioRxiv. 2023:2023.04.28.538740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yerbury JJ, Poon S, Meehan S, Thompson B, Kumita JR, Dobson CM, et al. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007;21(10):2312–22. [DOI] [PubMed] [Google Scholar]
- 124. Yuste-Checa P, Trinkaus VA, Riera-Tur I, Imamoglu R, Schaller TF, Wang H, et al. The extracellular chaperone Clusterin enhances Tau aggregate seeding in a cellular model. Nat Commun. 2021;12(1):4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. De Roeck A, Van Broeckhoven C, Sleegers K. The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol. 2019;138(2):201–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dib S, Pahnke J, Gosselet F. Role of ABCA7 in human health and in alzheimer’s disease. Int J Mol Sci. 2021;22(9):4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tanaka N, Abe-Dohmae S, Iwamoto N, Fitzgerald ML, Yokoyama S. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J Lipid Res. 2010;51(9):2591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol. 2011;68(10):1239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Iwagami M, Qizilbash N, Gregson J, Douglas I, Johnson M, Pearce N, et al. Blood cholesterol and risk of dementia in more than 1·8 million people over two decades: a retrospective cohort study. Lancet Healthy Longev. 2021;2(8):e498–506. [DOI] [PubMed] [Google Scholar]
- 130. Walker AJ, Kim Y, Borissiouk I, Rehder R, Dodd S, Morris G, et al. Statins: neurobiological underpinnings and mechanisms in mood disorders. Neurosci Biobehav Rev. 2021;128:693–708. [DOI] [PubMed] [Google Scholar]
- 131. Markovic VM, Cupic Z, Macesic S, Stanojevic A, Vukojevic V, Kolar-Anic L. Modelling cholesterol effects on the dynamics of the hypothalamic-pituitary-adrenal (HPA) axis. Math Med Biol. 2016;33(1):1–28. [DOI] [PubMed] [Google Scholar]
- 132. Martins-De-Souza D, Wobrock T, Zerr I, Schmitt A, Gawinecka J, Schneider-Axmann T, et al. Different apolipoprotein E, apolipoprotein A1 and prostaglandin-H2 D-isomerase levels in cerebrospinal fluid of schizophrenia patients and healthy controls. World J Biol Psychiatry. 2010;11(5):719–28. [DOI] [PubMed] [Google Scholar]
- 133. Dean B, Digney A, Sundram S, Thomas E, Scarr E. Plasma apolipoprotein E is decreased in schizophrenia spectrum and bipolar disorder. Psychiatry Res. 2008;158(1):75–8. [DOI] [PubMed] [Google Scholar]
- 134. Jonas K, Clouston S, Li K, Fochtmann LJ, Lencz T, Malhotra AK, et al. Apolipoprotein E-ε4 allele predicts escalation of psychotic symptoms in late adulthood. Schizophr Res. 2019;206:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. González-Castro TB, Tovilla-Zárate CA, Hernández-Díaz Y, Fresán A, Juárez-Rojop IE, Ble-Castillo JL, et al. No association between ApoE and schizophrenia: evidence of systematic review and updated meta-analysis. Schizophr Res. 2015;169(1–3):355–68. [DOI] [PubMed] [Google Scholar]
- 136. Yang Y, Wan C, Li H, Zhu H, La Y, Xi Z, et al. Altered levels of acute phase proteins in the plasma of patients with schizophrenia. Anal Chem. 2006;78(11):3571–6. [DOI] [PubMed] [Google Scholar]
- 137. Huang JT, Wang L, Prabakaran S, Wengenroth M, Lockstone HE, Koethe D, et al. Independent protein-profiling studies show a decrease in apolipoprotein A1 levels in schizophrenia CSF, brain and peripheral tissues. Mol Psychiatry. 2008;13(12):1118–28. [DOI] [PubMed] [Google Scholar]
- 138. Saari K, Jokelainen J, Veijola J, Koponen H, Jones PB, Savolainen M, et al. Serum lipids in schizophrenia and other functional psychoses: a general population northern Finland 1966 birth cohort survey. Acta Psychiatr Scand. 2004;110(4):279–85. [DOI] [PubMed] [Google Scholar]
- 139. Kim DD, Barr AM, Fredrikson DH, Honer WG, Procyshyn RM. Association between serum lipids and antipsychotic response in schizophrenia. Curr Neuropharmacol. 2019;17(9):852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pillinger T, McCutcheon RA, Howes OD. Variability of glucose, insulin, and lipid disturbances in first-episode psychosis: a meta-analysis. Psychol Med. 2023;53(7):3150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Qiu Y, Li S, Teng Z, Tan Y, Xu X, Yang M, et al. Association between abnormal glycolipid level and cognitive dysfunction in drug-naïve patients with bipolar disorder. J Affect Disord. 2022;297:477–85. [DOI] [PubMed] [Google Scholar]
- 142. Torsvik A, Brattbakk HR, Trentani A, Holdhus R, Stansberg C, Bartz-Johannessen CA, et al. Patients with schizophrenia and bipolar disorder display a similar global gene expression signature in whole blood that reflects elevated proportion of immature neutrophil cells with association to lipid changes. Transl Psychiatry. 2023;13(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Das D, Peng X, Lam ATN, Bader JS, Avramopoulos D. Transcriptome analysis of human induced excitatory neurons supports a strong effect of clozapine on cholesterol biosynthesis. Schizophr Res. 2021;228:324–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20(3):137–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gluchowski NL, Becuwe M, Walther TC, Farese RV Jr. Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2017;14(6):343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cohen S. Lipid droplets as organelles. Int Rev Cell Mol Biol. 2018;337:83–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Walther TC, Farese RV Jr. Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585(2–3):202–12. [DOI] [PubMed] [Google Scholar]
- 149. Farmer BC, Walsh AE, Kluemper JC, Johnson LA. Lipid droplets in neurodegenerative disorders. Front Neurosci. 2020;14:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ralhan I, Chang CL, Lippincott-Schwartz J, Ioannou MS. Lipid droplets in the nervous system. J Cell Biol. 2021;220(7):e202102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Guttenplan KA, Weigel MK, Prakash P, Wijewardhane PR, Hasel P, Rufen-Blanchette U, et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature. 2021;599(7883):102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Fader Kaiser CM, Romano PS, Vanrell MC, Pocognoni CA, Jacob J, Caruso B, et al. Biogenesis and breakdown of lipid droplets in pathological conditions. Front Cell Dev Biol. 2021;9:826248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yang C, Wang X, Wang J, Wang X, Chen W, Lu N, et al. Rewiring neuronal glycerolipid metabolism determines the extent of axon regeneration. Neuron. 2020;105(2):276–92.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177(6):1522–35.e14. [DOI] [PubMed] [Google Scholar]
- 155. Loving BA, Bruce KD. Lipid and lipoprotein metabolism in microglia. Front Physiol. 2020;11:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Loats AE, Carrera S, Fleming AF, Roberts ARE, Sherrard A, Toska E, et al. Cholesterol is required for transcriptional repression by BASP1. Proc Natl Acad Sci USA. 2021;118(29):e2101671118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Islimye E, Girard V, Gould AP. Functions of stress-induced lipid droplets in the nervous system. Front Cell Dev Biol. 2022;10:863907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bruce KD, Zsombok A, Eckel RH. Lipid processing in the brain: a key regulator of systemic metabolism. Front Endocrinol. 2017;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Weightman Potter PG, Vlachaki Walker JM, Robb JL, Chilton JK, Williamson R, Randall AD, et al. Basal fatty acid oxidation increases after recurrent low glucose in human primary astrocytes. Diabetologia. 2019;62(1):187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. He M, Pei Z, Mohsen AW, Watkins P, Murdoch G, Van Veldhoven PP, et al. Identification and characterization of new long chain acyl-CoA dehydrogenases. Mol Genet Metab. 2011;102(4):418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003;23(13):5928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shimabukuro MK, Langhi LG, Cordeiro I, Brito JM, Batista CM, Mattson MP, et al. Lipid-laden cells differentially distributed in the aging brain are functionally active and correspond to distinct phenotypes. Sci Rep. 2016;6:23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Harkins D, Cooper HM, Piper M. The role of lipids in ependymal development and the modulation of adult neural stem cell function during aging and disease. Semin Cell Dev Biol. 2021;112:61–8. [DOI] [PubMed] [Google Scholar]
- 164. Capilla-Gonzalez V, Cebrian-Silla A, Guerrero-Cazares H, Garcia-Verdugo JM, Quiñones-Hinojosa A. Age-related changes in astrocytic and ependymal cells of the subventricular zone. Glia. 2014;62(5):790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hamilton LK, Dufresne M, Joppé SE, Petryszyn S, Aumont A, Calon F, et al. Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of alzheimer’s disease. Cell Stem Cell. 2015;17(4):397–411. [DOI] [PubMed] [Google Scholar]
- 166. Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020;23(2):194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bailey AP, Koster G, Guillermier C, Hirst EM, MacRae JI, Lechene CP, et al. Antioxidant role for lipid droplets in a stem cell niche of Drosophila. Cell. 2015;163(2):340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kis V, Barti B, Lippai M, Sass M. Specialized cortex glial cells accumulate lipid droplets in Drosophila melanogaster. PLoS One. 2015;10(7):e0131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160(1–2):177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Smolic T, Tavcar P, Horvat A, Cerne U, Haluzan Vasle A, Tratnjek L, et al. Astrocytes in stress accumulate lipid droplets. Glia. 2021;69(6):1540–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Lee LL, Aung HH, Wilson DW, Anderson SE, Rutledge JC, Rutkowsky JM. Triglyceride-rich lipoprotein lipolysis products increase blood-brain barrier transfer coefficient and induce astrocyte lipid droplets and cell stress. Am J Physiol Cell Physiol. 2017;312(4):C500–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Etschmaier K, Becker T, Eichmann TO, Schweinzer C, Scholler M, Tam-Amersdorfer C, et al. Adipose triglyceride lipase affects triacylglycerol metabolism at brain barriers. J Neurochem. 2011;119(5):1016–28. [DOI] [PubMed] [Google Scholar]
- 173. Chausse B, Kakimoto PA, Caldeira-da-Silva CC, Chaves-Filho AB, Yoshinaga MY, da Silva RP, et al. Distinct metabolic patterns during microglial remodeling by oleate and palmitate. Biosci Rep. 2019;39(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of alzheimer’s 1907 paper, “über eine eigenartige erkankung der hirnrinde”. Clin Anat. 1995;8(6):429–31. [DOI] [PubMed] [Google Scholar]
- 175. van der Kant R, Langness VF, Herrera CM, Williams DA, Fong LK, Leestemaker Y, et al. Cholesterol metabolism is a druggable Axis that independently regulates tau and amyloid-beta in iPSC-derived alzheimer's disease neurons. Cell Stem Cell. 2019;24(3):363–75.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399(10323):473–86. [DOI] [PubMed] [Google Scholar]
- 177. Hussain G, Wang J, Rasul A, Anwar H, Imran A, Qasim M, et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019;18(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–76. [DOI] [PubMed] [Google Scholar]
- 179. Peruzzolo TL, Pinto JV, Roza TH, Shintani AO, Anzolin AP, Gnielka V, et al. Inflammatory and oxidative stress markers in post-traumatic stress disorder: a systematic review and meta-analysis. Mol Psychiatry. 2022;27(8):3150–63. [DOI] [PubMed] [Google Scholar]
- 180. Xie Z, Jones A, Deeney JT, Hur SK, Bankaitis VA. Inborn errors of long-chain fatty acid β-oxidation link neural stem cell self-renewal to autism. Cell Rep. 2016;14(5):991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ramosaj M, Madsen S, Maillard V, Scandella V, Sudria-Lopez D, Yuizumi N, et al. Lipid droplet availability affects neural stem/progenitor cell metabolism and proliferation. Nat Commun. 2021;12(1):7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Clark D, Dedova I, Cordwell S, Matsumoto I. A proteome analysis of the anterior cingulate cortex gray matter in schizophrenia. Mol Psychiatry. 2006;11(5):459–70, 423. [DOI] [PubMed] [Google Scholar]
- 183. Krohn J, Domart F, Do TT, Dresbach T. The synaptic vesicle protein Mover/TPRG1L is associated with lipid droplets in astrocytes. Glia. 2023;71(12):2799–814. [DOI] [PubMed] [Google Scholar]
- 184. Muhtaseb AW, Duan J. Modeling common and rare genetic risk factors of neuropsychiatric disorders in human induced pluripotent stem cells. Schizophr Res. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Lee SJ, Zhang J, Choi AM, Kim HP. Mitochondrial dysfunction induces formation of lipid droplets as a generalized response to stress. Oxid Med Cell Longev. 2013;2013:327167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Benador IY, Veliova M, Liesa M, Shirihai OS. Mitochondria bound to lipid droplets: where mitochondrial dynamics regulate lipid storage and utilization. Cell Metab. 2019;29(4):827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Zhang S, Zhang H, Forrest MP, Zhou Y, Sun X, Bagchi VA, et al. Multiple genes in a single GWAS risk locus synergistically mediate aberrant synaptic development and function in human neurons. Cell Genom. 2023;3(9):100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr. 2017;37(1):225–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kanoni S, Graham SE, Wang Y, Surakka I, Ramdas S, Zhu X, et al. Implicating genes, pleiotropy, and sexual dimorphism at blood lipid loci through multi-ancestry meta-analysis. Genome Biol. 2022;23(1):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020. [DOI] [PubMed] [Google Scholar]
- 191. Guo L, Zhong MB, Zhang L, Zhang B, Cai D. Sex differences in Alzheimer’s disease: insights from the multiomics landscape. Biol Psychiatry. 2022;91(1):61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wang JM, Irwin RW, Brinton RD. Activation of estrogen receptor alpha increases and estrogen receptor beta decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Natl Acad Sci USA. 2006;103(45):16983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ratnakumar A, Zimmerman SE, Jordan BA, Mar JC. Estrogen activates Alzheimer's disease genes. Alzheimers Dement. 2019;5:906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Nathan BP, Barsukova AG, Shen F, McAsey M, Struble RG. Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons. Endocrinology. 2004;145(7):3065–73. [DOI] [PubMed] [Google Scholar]
- 195. Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. 2004;1019:24–8. [DOI] [PubMed] [Google Scholar]
- 196. Fisher DW, Bennett DA, Dong H. Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiol Aging. 2018;70:308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, et al. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33(4):720–31.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Fleisher A, Grundman M, Jack CR Jr, Petersen RC, Taylor C, Kim HT, et al. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62(6):953–7. [DOI] [PubMed] [Google Scholar]
- 199. Brown CM, Choi E, Xu Q, Vitek MP, Colton CA. The APOE4 genotype alters the response of microglia and macrophages to 17beta-estradiol. Neurobiol Aging. 2008;29(12):1783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Hsu M, Dedhia M, Crusio WE, Delprato A. Sex differences in gene expression patterns associated with the APOE4 allele. F1000Res. 2019;8:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Prakash P, Manchanda P, Paouri E, Bisht K, Sharma K, Wijewardhane PR, et al. Amyloid β induces lipid droplet-mediated microglial dysfunction in Alzheimer’s disease. bioRxiv. 2023:2023.06.04.543525. [Google Scholar]
- 202. Green T, Flash S, Reiss AL. Sex differences in psychiatric disorders: what we can learn from sex chromosome aneuploidies. Neuropsychopharmacology. 2019;44(1):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Stoney CM, Matthews KA, Mcdonald RH, Johnson CA. Sex differences in lipid, lipoprotein, cardiovascular, and neuroendocrine responses to acute stress. Psychophysiology. 1988;25(6):645–56. [DOI] [PubMed] [Google Scholar]
- 204. Ramos-Loyo J, Medina-Hernández V, Estarrón-Espinosa M, Canales-Aguirre A, Gómez-Pinedo U, Cerdán-Sánchez LF. Sex differences in lipid peroxidation and fatty acid levels in recent onset schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;44:154–61. [DOI] [PubMed] [Google Scholar]
- 205. Brivio E, Kos A, Ulivi AF, Karamihalev S, Ressle A, Stoffel R, et al. Sex shapes cell-type-specific transcriptional signatures of stress exposure in the mouse hypothalamus. Cell Rep. 2023;42(8):112874. [DOI] [PubMed] [Google Scholar]
- 206. Millett CE, Phillips BE, Saunders EFH. The sex-specific effects of LPS on depressive-like behavior and oxidative stress in the Hippocampus of the mouse. Neuroscience. 2019;399:77–88. [DOI] [PubMed] [Google Scholar]
- 207. McNamara RK, Jandacek R, Rider T, Tso P, Hahn C-G, Richtand NM, et al. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr Res. 2007;91(1–3):37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175(1–2):47–53. [DOI] [PubMed] [Google Scholar]
- 209. Yin F. Lipid metabolism and Alzheimer’s disease: clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2023;290(6):1420–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, et al. Simvastatin strongly reduces levels of Alzheimer’s disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98(10):5856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, et al. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7(4):321–31. [DOI] [PubMed] [Google Scholar]
- 212. Daneschvar HL, Aronson MD, Smetana GW. Do statins prevent Alzheimer’s disease? A narrative review. Eur J Intern Med. 2015;26(9):666–9. [DOI] [PubMed] [Google Scholar]
- 213. Power MC, Weuve J, Sharrett AR, Blacker D, Gottesman RF. Statins, cognition, and dementia-systematic review and methodological commentary. Nat Rev Neurol. 2015;11(4):220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Dagliati A, Peek N, Brinton RD, Geifman N. Sex and APOE genotype differences related to statin use in the aging population. Alzheimers Dement. 2021;7(1):e12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kim JM, Stewart R, Kang HJ, Bae KY, Kim SW, Shin IS, et al. A prospective study of statin use and poststroke depression. J Clin Psychopharmacol. 2014;34(1):72–9. [DOI] [PubMed] [Google Scholar]
- 216. Kim SW, Kang HJ, Bae KY, Shin IS, Hong YJ, Ahn YK, et al. Interactions between pro-inflammatory cytokines and statins on depression in patients with acute coronary syndrome. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;80(Pt C):250–4. [DOI] [PubMed] [Google Scholar]
- 217. Rej S, Schulte SW, Rajji TK, Gildengers AG, Miranda D, Menon M, et al. Statins and cognition in late-life bipolar disorder. Int J Geriatr Psychiatry. 2018;33(10):1355–60. [DOI] [PubMed] [Google Scholar]
- 218. Nomura I, Kishi T, Ikuta T, Iwata N. Statin add-on therapy in the antipsychotic treatment of schizophrenia: a meta-analysis. Psychiatry Res. 2018;260:41–7. [DOI] [PubMed] [Google Scholar]
- 219. Sommer IE, Gangadin SS, de Witte LD, Koops S, van Baal C, Bahn S, et al. Simvastatin augmentation for patients with early-phase schizophrenia-spectrum disorders: a double-blind, randomized placebo-controlled trial. Schizophr Bull. 2021;47(4):1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Fotso Soh J, Beaulieu S, Trepiccione F, Linnaranta O, Torres-Platas G, Platt RW, et al. A double-blind, randomized, placebo-controlled pilot trial of atorvastatin for nephrogenic diabetes insipidus in lithium users. Bipolar Disord. 2021;23(1):66–75. [DOI] [PubMed] [Google Scholar]
- 221. Köhler-Forsberg O, Gasse C, Petersen L, Nierenberg AA, Mors O, Østergaard SD. Statin treatment and the risk of depression. J Affect Disord. 2019;246:706–15. [DOI] [PubMed] [Google Scholar]
- 222. Okereke OI, Vyas CM, Mischoulon D, Chang G, Cook NR, Weinberg A, et al. Effect of long-term supplementation with marine omega-3 fatty acids vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2021;326(23):2385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Liang CS, Tseng PT, Su KP. Effect of long-term supplementation with marine omega-3 fatty acids vs placebo on risk of depression. JAMA. 2022;327(13):1290–1. [DOI] [PubMed] [Google Scholar]
- 224. Papsdorf K, Brunet A. Linking lipid metabolism to chromatin regulation in aging. Trends Cell Biol. 2019;29(2):97–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Parnell E, Culotta L, Forrest MP, Jalloul HA, Eckman BL, Loizzo DD, et al. Excitatory dysfunction drives network and calcium handling deficits in 16p11.2 duplication schizophrenia induced pluripotent stem cell-derived neurons. Biol Psychiatry. 2023;94(2):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zhang S, Sanders AR, Duan J. Modeling PTSD neuronal stress responses in a dish. Nat Neurosci. 2022;25(11):1402–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kozlova A, Zhang S, Kotlar AV, Jamison B, Zhang H, Shi S, et al. Loss of function of OTUD7A in the schizophrenia- associated 15q13.3 deletion impairs synapse development and function in human neurons. Am J Hum Genet. 2022;109(8):1500–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Yue W, Huang H, Duan J. Potential diagnostic biomarkers for schizophrenia. Med Rev Berl. 2022;2(4):385–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Zhang S, Zhang H, Zhou Y, Qiao M, Zhao S, Kozlova A, et al. Allele-specific open chromatin in human iPSC neurons elucidates functional disease variants. Science. 2020;369(6503):561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Butler Iii RR, Kozlova A, Zhang H, Zhang S, Streit M, Sanders AR, et al. The genetic relevance of human induced pluripotent stem cell-derived microglia to alzheimer’s disease and major neuropsychiatric disorders. Mol Neuropsychiatry. 2020;5(Suppl 1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Barretto N, Zhang H, Powell SK, Fernando MB, Zhang S, Flaherty EK, et al. ASCL1- and DLX2-induced GABAergic neurons from hiPSC-derived NPCs. J Neurosci Methods. 2020;334:108548. [DOI] [PMC free article] [PubMed] [Google Scholar]