Abstract
Introduction
Trichohepatoenteric syndrome (THES) is caused by pathogenic mutations in TTC37 and SKIV2L genes and characterized by intractable diarrhea, facial dysmorphism, hair abnormality, immunodeficiency, and skin abnormalities. Lipoid proteinosis is caused by pathogenic mutations in ECM1 gene and characterized by deposition of hyaline-like material in various tissues resulting in heterogenous clinical findings.
Case Presentation
Four years after the diagnosis and management of THES, due to new clinical findings, another reason for underlying features of the patient was considered. WES was performed and a homozygous c.507delT (p.Arg171GlyfsTer7) mutation in the ECM1 gene was detected.
Conclusion
This case provides an example of co-existence of multiple genetic defects in a single patient born to consanguineous parents.
Keywords: Lipoid proteinosis, Trichohepatoenteric syndrome, Immunodeficiency
Established Facts
-
•
Trichohepatoenteric syndrome (THES) is a rare autosomal recessive disorder caused by biallelic pathogenic variants in TTC37 and SKIVL2 genes. Clinical features of THES are intractable diarrhea, facial dysmorphism, hair abnormality, immunodeficiency, and skin abnormalities.
-
•
Lipoid proteinosis (LP) is characterized by persistent voice hoarseness and skin changes, such as fragility, infiltrated papules and nodules over the skin, and mucosae. LP is caused by biallelic pathogenic variants in ECM1 gene.
Novel Insights
-
•
This is the first case carrying homozygous pathogenic mutations both in TTC37 and ECM1 genes.
-
•
This case provides an example of the co-existence of multiple genetic defects in a single patient from consanguineous parents and supports consideration of additional molecular diagnoses in a patient with atypical disease symptoms.
Introduction
Trichohepatoenteric syndrome (THES) is characterized by intractable diarrhea, facial dysmorphism, hair abnormality, immunodeficiency, and skin abnormalities [Bourgeois et al., 2018]. It is a rare congenital disorder with an autosomal recessive inheritance pattern, caused by mutations in TTC37 (tetratricopeptide repeat domain-containing protein 37) or SKIV2L (superkiller viralicidic activity 2) genes [Lee et al., 2016; Vardi et al., 2018]. THES has an estimated incidence of 1/300,000–400,000 live births [Zheng et al., 2016]. Other clinical findings are hepatic involvement varying from minor findings to cirrhosis or hepatic hemosiderosis, intrauterine growth retardation, platelet dysfunction, cardiac abnormalities, and developmental delay [Hiejima et al., 2017]. Both causative genes, TTC37 and SKIVL2, have been identified in 2010 and 2012, respectively, and gave the opportunity to understand phenotypic and genotypic heterogeneity of this syndrome [Bourgeois et al., 2018]. These two genes encode two components of human superkiller complex functioning as the cofactor of cytosolic exosome [Bourgeois et al., 2018; Lee et al., 2016].
Lipoid proteinosis (LP) is characterized by deposition of hyaline-like material in various tissues resulting in heterogenous clinical findings. It was first described by Urbach and Wiethe in 1929 under the name “hyalinosis cutis et mucosae” [Agredano et al., 2020]. It is an autosomal recessive disease resulting from a mutation in the extracellular matrix protein 1 (ECM1) gene, located on chromosome 1q21.2 [Agredano et al., 2020; Hamata, 2002; Chan et al., 2007]. LP cases have generalized thickening and scarring of the skin and mucosae developing in the first years of life accompanying a hoarse cry or voice, caused by the infiltration of the vocal cords [Chan et al., 2007; Ravi Prakash et al., 2013].
We would like to present a case carrying both of the mutated genes of TTC37 and ECM1 and showing almost all the clinical signs of these very rare diseases. After 9 years of follow-up, we briefly mention the patient’s detailed laboratory and clinical findings and emphasize the management of these co-existed diseases. Pathognomonic clinical findings for THES and LP were also discussed.
Case Report
Our patient was a 22-month-old boy and the third child of consanguineous parents. He was admitted for recurrent skin abscesses, oral lesions, aphthous stomatitis, and ulcerous lesions on his hands. Severe combined immunodeficiency, chronic granulomatous disease, leukocyte adhesion defects, and hyper-IgE syndromes were excluded by detailed immunologic tests. Selective IgA deficiency was observed in his serum immunoglobulin level measurements. In addition, he had coarse hair and sterile erythematous-violaceous pyoderma gangrenosum-like plaques on his neck. Dermatitis herpetiformis was ruled out by normal small bowel histopathology and negative antigliadin and antiendomysial antibody titers.
At age 6 years, a homozygous mutation in the TTC37 gene c.2210T>C (p.Val737Ala) was detected by targeted next-generation sequencing with a comprehensive IOn AMPliSeq primary immunodeficiency panel designed for sequencing 264 genes. SKIVL2 gene was also present and sequenced in this panel, but no mutation was found.
TTC37 mutations cause THES. THES is characterized by early-onset diarrhea, immune deficiency, and trichorrhexis nodosa. The parents were heterozygous for the same mutation. We published this case with a title “A Novel TTC37 Mutation Causing Clinical Symptoms of Trichohepatoenteric Syndrome such as Pyoderma Gangrenosum and Immunodeficiency Without Severe Diarrhea” in 2019 [Karaca Edeer et al., 2019].
After the diagnosis of THES, during follow-up the patient developed IgG deficiency in addition to IgA deficiency. He began to receive intravenous immunoglobulin therapy to control recurrent skin and oral lesions following respiratory tractus infections.
In 2021, this THES patient was 10 years old and he was followed up for four more years in our clinic after his initial diagnosis. During these follow-up years, he experienced minor problems and complications and he continued to receive intravenous immunoglobulin replacements. He had severe episodes of mucositis requiring hospitalization at least twice per year. He also had peg teeth with rapid development of caries and root anomaly. In further evaluation, trichorrhexis nodosa was confirmed in his hair shafts.
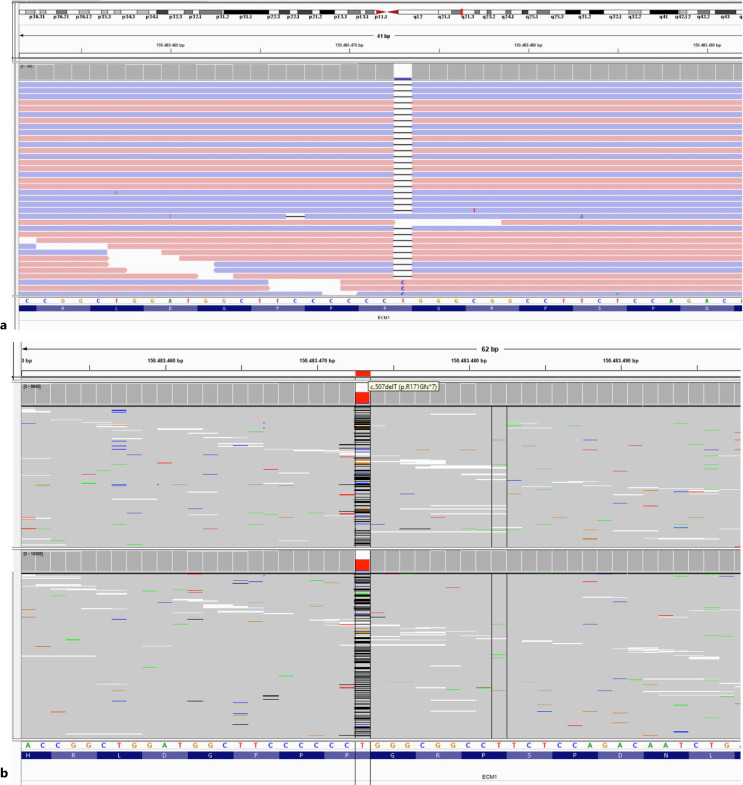
A year ago, skin thickening was observed on the neck and both extremities of the patient. Ophthalmologic examination revealed moniliform blepharosis. Laryngoscopy was performed because of hoarseness in his voice and vocal cord thickening and nodules were observed. Endoscopy revealed multiple polyps in the esophagus and stomach antrum. Bilateral temporomedial calcification was detected in the computerized tomography of the brain taken due to persistent headaches and panic attack complaints during the follow-up of the patient. Due to these new clinical findings that could not be explained only by THES, another reason underlying features of the patient was considered. Whole-exome sequencing (WES) was performed and a homozygous c.507delT (p.Arg171GlyfsTer7) mutation in the ECM1 gene was detected (Fig. 1) in addition to our previous finding, mutated TTC37 gene. This mutation was previously identified as pathogenic for “LP” in the ClinVar database, and the patient was diagnosed to have LP in addition to THES. The parents were heterozygous also for this mutation. If it was possible to perform WES testing instead of targeted next-generation sequencing 4 years ago, we would learn both of the genetic defects much more earlier. The clinical findings of LP and our patient are listed in Table 1.
Fig. 1.
a Homozygous variant of c.507delT (p.Arg171GlyfsTer7) in the ECM1 gene of the patient. b The same variant was detected as heterozygous in the parents’ segregation analysis.
Table 1.
Clinical findings in LP and in the presented patient
| LP | The presented patient |
|---|---|
| Patchy and diffuse alopecia | Ø |
| Hyperkeratotic and verrucous lesions | ✓ |
| Moniliform blepharosis | ✓ |
| Recurrent aphthous lesions | ✓ |
| Recurrent parotitis | ✓ |
| Poor dental health | ✓ |
| Thickened sublingual frenulum | ✓ |
| Hoarse voice | ✓ |
| Severe dysphonia and/or complete aphonia | ✓ |
| Breathing difficulties | Ø |
| Respiratory tract infection | ✓ |
| Headache | ✓ |
| Panic attack | ✓ |
| Neuropsychiatric disorders | Ø |
| Epilepsy | Ø |
| Intracranial calcifications in brain temporal lobes | ✓ |
| Spontaneous CNS hemorrhage | Ø |
| Gastrointestinal bleeding | Ø |
| Yellowish nodules throughout the GI tract | ✓ |
After the diagnosis of LP, the patient is being followed up one more year under the treatment of 10% subcutaneous immunoglobulin with recombinant hyaluronidase 10/g once a month. He had two times attacks of parotitis in the last year and recovered each time. His symptoms and quality of life and school attendance are much more better than previous years.
Discussion
THES causes pyoderma gangrenosum-like skin lesions, immunodeficiency, and mostly intractable diarrhea. Our patient had typical facial features of THES, wooly and coarse hair, trichorrhexis nodosa, and hypogammaglobulinemia [Karaca Edeer et al., 2019]. He did not have chronic/intractable diarrhea and liver disease. Facial dysmorphism, hair abnormalities, immunodeficiency, and skin lesions are pathognomonic signs of THES. Skin thickening, moniliform blepharosis, hoarse voice, bilateral temporomedial calcification of the brain led us to believe this was more than just THES.
LP, which is characterized by deposition of hyaline-like material in the larynx, oral cavity, skin, and internal organs, should be suspected in individuals with the following clinical manifestations, neuroimaging findings, and family history [Vahidnezhad et al., 2016]. The first manifestation in almost all individuals is a weak cry or hoarse voice (due to infiltration and deposition of hyaline-like material in the vocal cords) appearing in early infancy. This hoarse voice usually persists lifelong and can progress to severe dysphonia [Savage et al., 1988]. In some individuals, infiltration of the laryngeal mucosa may lead to breathing difficulties.
Recurrent episodes of parotitis caused by stenosis of the parotid duct and submandibular gland inflammation have been reported. Infiltration of the tongue may destroy the dorsal papillae, causing the tongue to have a smooth surface. Dental health is often poor [Chan et al., 2007; Ravi Parakash et al., 2013]. Moniliform blepharosis is a pathognomonic sign. The presence of multiple beaded papules along the eyelid margins and inner canthus and papular infiltration can be quite subtle in some individuals [Belliveau et al., 2015]. Vesicles can be one of the earliest clinical manifestations in up to 50% of affected neonates. Later, with increasing hyaline deposition in the dermis, the skin becomes diffusely thickened and appears waxy with a yellowish discoloration; papules, nodules, and plaques appear on the face and lips. Verrucous and keratotic cutaneous lesions may develop on extensor surfaces, especially on the elbows [Hamada, 2002].
Central nervous system and neuropsychiatric manifestations commonly include epilepsy and behavioral manifestations (memory impairment, paranoia, aggressive behavior, hallucinations, and panic attack) in association with calcification of the temporal lobes or hippocampi [Siebert et al., 2003; Thornton et al., 2008]. Computerized tomography of the brain detects mineral deposits and calcification. In some patients, it reveals circumscribed, horn-shaped bilateral symmetric calcification [Vahidnezhad et al., 2016; Siebert et al., 2003]. Spontaneous central nervous system hemorrhage including small deep brain hemorrhages and large brain hematomata has been reported and can lead to hemiparesis and hemiplegia [Siebert et al., 2003]. Multiple yellowish nodules can be found throughout the esophagus, stomach, duodenum, and colon and are usually asymptomatic; however, small intestinal bleeding has been observed in one individual [Siebert et al., 2003; Gonçalves et al., 2010; Agredano et al., 2020].
Weak cry and hoarse voice, recurrent parotitis, moniliform blepharosis, and calcification of brain temporal lobes are some specific characteristics that are together unique to LP. As listed in Table 1, this patient presented most of the typical features of LP. The first sign of the disease was hoarseness in the voice that started in the early childhood. Later, he developed classical skin lesions and cranial calcifications which are frequently seen in LP. Additionally, the homozygous mutation observed in our patient had been reported previously in a LP patient and listed as disease causing in database.
This case is very important because it provides an example of co-existence of multiple genetic defects aggregating in a single patient born to consanguineous parents and supports consideration of additional molecular diagnoses in a patient with atypical disease symptoms. This is the first case carrying homozygous pathogenic mutations both in TTC37 and ECM1 genes. Second, it serves to emphasize LP as a cause of voice changes and hoarseness in the infant and young children. Additionally, brain parenchymal calcifications are the hallmark of LP and this diagnosis has to be considered especially if they are recognized in amygdalae, hippocampus, and the striatum. It is also worth to mention that the prognosis of LP is generally good, although the disease is progressive until early adult life. Lastly, we have to mention that WES found both genetic defects and if possible, we recommend WES as first-line testing in complicated genetic disorders.
Acknowledgments
We are deeply grateful to the patient and his family for participating in this study.
Statement of Ethics
Ethical approval was not required for this study in accordance with local/national guidelines. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Funding Sources
This study did not receive any funding in any form.
Author Contributions
Necil Kutukculer and Hatice Ceren Eser designed and wrote the article. Ezgi Topyildiz and Durdugul Ayyildiz Emecen provided patient clinical information. Esra Isik, Neslihan Edeer Karaca, Guzide Aksu, and Tahir Atik performed clinical follow-up of the patients. Necil Kutukculer and Ferda Ozkınay edited the final version.
Funding Statement
This study did not receive any funding in any form.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- Agredano PM, Del Barrio CM, Martinez MC, Cabrera CA. Intracranial calcifications associated with epilepsy: a case report of lipoid proteinosis. Seizure. 2020;83:172–4. 10.1016/j.seizure.2020.10.027. [DOI] [PubMed] [Google Scholar]
- Belliveau MJ, Alkhotani A, Ali A. Moniliform blepharosis of lipoid proteinosis. JAMA Ophthalmol. 2015;133(7):e150688. 10.1001/jamaophthalmol.2015.0688. [DOI] [PubMed] [Google Scholar]
- Bourgeois P, Esteve C, Chaix C, Beroud C, Levy N; THES clinical consortium, et al. Tricho-Hepato-Enteric Syndrome mutation update: mutations spectrum of TTC37 and SKIV2L, clinical analysis and future prospects. Hum Mutat. 2018;39(6):774–89. 10.1002/humu.23418. [DOI] [PubMed] [Google Scholar]
- Chan I, Liu L, Hamada T, Sethuraman G, McGrath JA. The molecular basis of lipoid proteinosis: mutations in extracellular matrix protein 1. Exp Dermatol. 2007;16(11):881–90. 10.1111/j.1600-0625.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- Gonçalves FG, de Melo MB, de L Matos V, Barra FR, Figueroa RE. Amygdalae and striatum calcification in lipoid proteinosis. AJNR Am J Neuroradiol. 2010;31(1):88–90. 10.3174/ajnr.A1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T. Lipoid proteinosis. Clin Exp Dermatol. 2002;27(8):624–9. 10.1046/j.1365-2230.2002.01143.x. [DOI] [PubMed] [Google Scholar]
- Hiejima E, Yasumi T, Nakase H, Matssura M, Honzawa Y, Higuchi H, et al. Tricho-hepato-enteric syndrome with novel SKIVL2 gene mutations. Medicine. 2017;96:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca Edeer N, Aykut A, Parıltay E, Aksu G, Cogulu O, Kutukculer N. A novel TTC37 mutation causing clinical symptoms of trichohepatoenteric syndrome such as pyoderma gangrenosum and immunodeficiency without severe diarrhea. J Investig Allergol Clin Immunol. 2019;29(5):396–8. 10.18176/jiaci.0418. [DOI] [PubMed] [Google Scholar]
- Lee WS, Teo KM, Ng RT, Chong SY, Kee BP, Chua KH. Novel mutations in SKIV2L and TTC37 genes in Malaysian children with trichohepatoenteric syndrome. Gene. 2016;586:1–6. 10.1016/j.gene.2016.03.049. [DOI] [PubMed] [Google Scholar]
- Ravi Prakash SM, Verma S, Sumalatha MN, Chattopadhyay S. Oral manifestations of lipoid proteinosis: a case report and literature review. Saudi Dent J. 2013;25(2):91–4. 10.1016/j.sdentj.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage MM, Crockett DM, McCabe BF. Lipoid proteinosis of the larynx: a cause of voice change in the infant and young child. Int J Pediatr Otorhinolaryngol. 1988;15(1):33–8. 10.1016/0165-5876(88)90048-1. [DOI] [PubMed] [Google Scholar]
- Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126(Pt 12):2627–37. 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- Thornton HB, Nel D, Thornton D, van Honk J, Baker GA, Stein DJ. The neuropsychiatry and neuropsychology of lipoid proteinosis. J Neuropsychiatry Clin Neurosci. 2008;20(1):86–92. 10.1176/jnp.2008.20.1.86. [DOI] [PubMed] [Google Scholar]
- Vahidnezhad H, Youssefian L, Uitto J. Lipoid proteinosis. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 2016 Jan 21. [Google Scholar]
- Vardi I, Barel O, Sperber M, Schvimer M, Nunberg M, Field M, et al. Genetic and structural analysis of a SKIV2L mutation causing tricho-hepato-enteric syndrome. Dig Dis Sci. 2018;63(5):1192–9. 10.1007/s10620-018-4983-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Pan J, Jin Y, Wang C, Liu Z. Targeted next-generation sequencing identification of a novel missense mutation of the SKIV2L gene in a patient with trichohepatoenteric syndrome. Mol Med Rep. 2016;14(3):2107–10. 10.3892/mmr.2016.5503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.