Abstract
Objective
To report the demographic profile and clinical characteristics of retinopathy of prematurity (ROP) in posterior Zone I.
Methods
In a partly retrospective (ten years) and partly prospective (one year) study, we analysed the demographic profile and clinical characteristics of babies with ROP in posterior Zone I.
Results
The study included 130 eyes of 67 infants with a mean gestational age and birth weight of 29.3 (±2.2) weeks and 1217.3 (±381.9) grams, respectively. All babies had received unblended oxygen. In 47 of 51 (91.1%) babies, the weekly weight gain was <100 g (details were not available in 16 babies). The ROP subtypes included aggressive, threshold, hybrid, stage 4, and atypical types in 78 (60%), 20 (15.4%), 11 (8.5%), 15 (11.5%), and 6 (4.6%) eyes, respectively. Fibrovascular proliferation, when present, was prominent nasally, occasionally overriding the disc margin. Extensive arteriovenous tortuosity was more prominent than vascular dilatation. Atypical observations included bleb-like detachment (6 eyes; 4.6%) and candle wax-like preretinal deposits (23 eyes; 17.7%).
Conclusions
Retinopathy of Prematurity in posterior Zone I in this cohort was strongly associated with 100% unblended oxygen supplementation, poor weight gain, and multiple systemic co-morbidities. ROP in posterior zone 1 has a distinct profile with several atypical characteristics different from ROP in other zones.
Subject terms: Retinal diseases, Diseases
Introduction
With improved neonatal care and greater availability of basic neonatal life-saving measures, even in low-resource regions, smaller and sicker babies are increasingly surviving. It has resulted in a rise in the number of babies with retinopathy of prematurity (ROP) including those with very posterior retinal locations. ROP in Zone I have been described [1–3]. It progresses rapidly, and the outcome is poor following ablative therapy- poor results were reported in 77.8% and 55.2% of eyes in CRYOROP [4] and ETROP [5] studies, respectively. The posterior zone I is confined between the optic disc and the foveal centre; there are only few reports on ROP in this zone [3, 6–8]. and needs further attention. While it is mainly restricted to small and extremely low birth weight infants in western countries, there are reports of similar disease in bigger and older babies from countries like India [1]. These babies tend to have many atypical characteristics, sometimes identical to oxygen-induced retinopathy [1]. The current report describes the demographic and clinical characteristics of ROP in the posterior zone I collected retrospectively over 10 years and prospectively for 1 year at a tertiary eye care facility in India.
Methodology
The present study combines prospective analysis of systematically collected retrospective data (10 years) and prospectively collected data (1 year) conducted at a tertiary centre in India (the Eastern state of Odisha) from January 2011 to December 2021.The study included babies diagnosed with ROP in the posterior zone I from several neonatal care centres with different levels of care. As the study included babies over 11 years, there were some changes in the criteria for screening over time. In the initial years, this was similar to the criteria set up by the National Neonatology Forum of India [9] followed by the operational guidelines of ROP screening recommended by the Rashtriya Bal Swasthya Karyakram (RBSK), India from 2017 onwards [10]. It included babies with GA (gestational age) <34 weeks and/or BW (birth weight) <2000 gm; or BW > 2000 gm and/or GA > 34 wks in babies with systemic co-morbidities, or those whom paediatricians had a high index of suspicion for ROP [1]. The timing for the first examination was within 30 days of birth; 2–3 weeks for birth weight less than 1200 gm and/or GA less than 28 wks. Informed written consent from the parents for sharing the de-identified images were already available from the subjects collected earlier (10 years), and new consent was obtained for the prospectively collected images. ROP was said to be in posterior Zone I or Zone Half when the extent of retinal vascularization had not reached the fovea/ macula centre or was within a four-disc diameter (DD) radius from the disc margin where the foveal anatomy was ill-defined. As per the published reports, we presumed the disc fovea distance to be approximately 4.4 DD ± 0.4 DD [7, 8, 11–13]. We excluded the subjects with incomplete details or lack of consent. The classification of ROP was done as per the International Classification of ROP (ICROP) standards I, II, and III [11, 14–16] and the hybrid ROP described in the literature [17]. Many babies were recruited before the publication of ICROP III. Some of the previous diagnoses were revised as per ICROP III classification based on the available images during the data analysis of the study. For example, babies who were previously termed APROP, we renamed AROP.
The demographic details of the infants, including GA, gender, BW, and postmenstrual age (PMA) at the time of the first screening, were recorded. Details of perinatal risk factors, including oxygen supplementation (mode and duration whenever available), multiple gestations, systemic sepsis, blood transfusion, birth asphyxia, weight on presentation, weight gain/loss at the time of examination (as compared to the weight at birth), etc, were analysed. The demographic and clinical information were gathered by analysing the details mentioned in the electronic medical records, perinatal profile mentioned in the discharge summary, ROP database, and finally, telephonic conversion with the parents in some cases. The retinopathy characteristics were analysed from the images obtained with RetCam Shuttle (Clarity Medical System, Pleasanton, California, USA),3nethra neo (Forus Health, Bangalore, India), and written details of the indirect ophthalmoscopic findings in the case record. We analysed the clinical profile of the disease, such as the retinopathy type (Stage, location and unique characteristics, if any), disease symmetry, rubeosis iridis, and the posterior-most location of the disease measured in disc diameters. The speed of vascular outgrowth was calculated in disc diameter per week as per our earlier publication [18]. We termed Stages 4 and 5 ROP as advanced ROP. The presence or absence of iris new vessels was noted from the external image of the iris obtained with the imaging device or the medical record. In general, new vessels were differentiated from the engorged but normal iris vessels by their location on the surface of the iris, circumferential spread along or close to the pupillary margin in an irregular and meandering pattern [19]. The engorged iris vessels usually lie deep within the iris substance and run radially, while the tunica vasculosa lentis appear as fine capillary plexuses that run on the anterior and posterior surfaces of the lens.
The data were entered into an excel sheet and analysed using descriptive statistics. Patient-based continuous data were described in mean +/− SD or median (inter-quartile range) according to the data distribution.
Results
This cohort included 130 eyes of 67 babies (retrospective: 53 and prospective: 14). The demographic details, perinatal risk factors, and retinopathy characteristics are shown in Tables 1 and 2. In this cohort, 16 babies had GA more than 30 weeks, and 6 had BW greater than 1500 gm (the limits set for ROP screening as per American Academy of Pediatrics guidelines) [20]. In 3 babies, subtle features of AROP, such as multiple tortuous loops of immature retinal vessels, were seen as early as 27 to 28 weeks PMA and about 2–3 weeks post-delivery.
Table 1.
Demographic and perinatal profile.
| Mean | SD | Range | Median | |
|---|---|---|---|---|
| Gestational Age(weeks) | 29.4 | 2.2 | 22–34 | 29.8 |
| Birth weight (grams) | 1217.3 | 381.9 | 650–3500 | 1145 |
| Duration of NICU stay (days) | 35.9 | 20.3 | 7–122 days | 30 |
| Weight gain/ week (grams) | 48.5 | 32.8 | 3–125 | 41.81 |
SD standard deviation, NICU neonatal intensive care unit, PMA post menstrual age.
Table 2.
Disease profile and ocular characteristics, n = 130 eyes, 67 babies.
| Number | |
|---|---|
| Bilateral disease (n = 67 babies) | 63 (94%) |
| Disease symmetry (n = 62 babies) | 50 (74.6%) |
| Circumferential extent of the disease: 360 degree (n = 114 eyes) | 30 (26.3%) |
| Posterior most extent of retinopathy (n = 130 eyes) | Avg: 1.8 DD |
| New Vessels Iris (n = 130 eyes) | 65 (50%) |
| Retinal vascular tortuosity (n = 130 eyes) | 121 (93%) |
| Long Arteriovenous shunts (n = 130 eyes) | 9 (6.9%) |
| Shunting (n = 130 eyes) | 105 (80.7%) |
| Presence of ridge tissue (n = 130 eyes) | 79 (60.7%) |
| Retinal/Pre retinal hemorrhages (n = 65 eyes, 49.2%) | |
| a) Edge hemorrhages (at or within 1 disc diameter from the vascular- avascular junction) | 40 |
| b) Haemorrhages within the vascularized retina: | 19 |
| c) Hemorrhage overlying the optic disc | 6 |
| Central Vascular trunks near optic disc: poorly formed (n = 130 eyes) | 28 (21.5%) |
| Candle wax like pre retinal deposits (n = 130 eyes) | 23 (17.6%) |
One common factor in all babies was using 100% unblended oxygen either by oxygen hood or nasal prongs; it was when required (assessed by the paediatrician) in a few babies, and in others, it was maintained at least for a few days in addition to mechanical ventilation or CPAP (continuous positive airway pressure). The babies were generally smaller, sicker, and stayed in the NICU for more than one month (Average 35.9 + /− 20.3, Median 30 days). The average weight gain (Mean 48.5 + /− 32.8, Median 41.81 grams) was poor (less than 100 gm) in 34 of 67 (50.7%) babies. In others, it was of more than 100 g/week (4 babies), no gain (n = 3 babies) or weight loss (n = 10 babies), and unsure in the remaining 16 babies. All the babies (100%) had respiratory distress, and 45 (69.2%) had additional systemic co- morbidities like anaemia, sepsis, pneumonia, hydrocephalus, and intracranial haemorrhage.
Aggressive ROP was the most prevalent type (60%; n = 78 eyes), followed by threshold (15.3%; n = 20 eyes), hybrid (8.4%, n = 11 eyes), stage 5 (7.6 %; n = 10 eyes), atypical (4.6%; n = 6 eyes) and stage 4 (3.8 %; n = 5 eyes) ROP. (Figs. 1, 2). None of the babies had stage 1 disease.
Fig. 1. Composite Fundus picture of eyes with major subtypes of retinopathy of prematurity (ROP) imaged with RetCam shuttle (Clarity Medical Systems, Inc. CA, USA).
a Left eye of a baby with ROP in Zone I Posterior. Note arteriovenous shunts at multiple levels with marked vascular tortuosity but no dilatation. Nasally the end of blood vessels are 1 disc diameter from the disc margin. b Left eye of a baby with threshold ROP. Note broad nasal ridge and fibrovascular proliferation (FVP) prominent on the nasal side of the disc. c Right eye of a baby with hybrid ROP. Mat like proliferation nasal to disc along with arteriovenous shunts. d Left eye of a baby with Stage 4 A ROP. Note prominent FVP overriding on the nasal margin of optic disc with tractional retinal detachment.
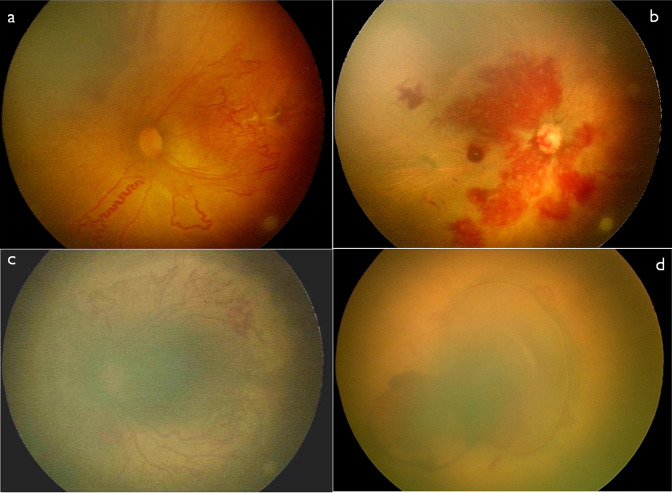
Fig. 2. Composite image depicting few atypical observations in eyes with posterior Zone I ROP.
Composite Fundus picture imaged (a, b, c and d) on RetCam shuttle and on 3netra neo (c) showing some of the atypical features associated with ROP in posterior zone I. a Left Eye Fundus showing Aggressive ROP (in zone I posterior with corkscrew like tortuosity inferonasally. b Right eye of a case of AROP in zone I posterior showing poorly defined central vascular trunk over disc and multiple superficial retinal hemorrhages. c Right eye fundus of a case of AROP in zone I posterior showing extensive pre retinal deposits from disc to vascular-avascular junction. d Right eye of a case of atypical ROP with a bleb like detachment over the posterior pole with preretinal haemorrhage. The blood vessels, as well as optic disc both, could not be clearly differentiated. The left eye also had mirror image like right eye.
The disease was bilateral in the majority (94% of subjects), with 50% of the eyes having rubeosis iridis. Engorgement of iris vessels, a marker of the disease severity, was included under the plus component. There were many unique findings (Figs. 1, 2), such as very long arterio-venous shunts in 9 eyes (6.9%), bridging the ends of multiple arterial and venous trunks, corkscrew-like retinal vascular tortuosity (Fig. 2a) in 16 eyes (12.3%), candle-wax preretinal deposits (Fig. 2c) in 23 eyes (17.6%), and absence of vascular dilatation despite tortuosity in 23 eyes (17.6%). Some of these morphological changes were difficult to classify by the current ICROP nomenclature.
Discussion
There are limited reports on ROP in posterior Zone I.
We noticed several unique features in our cohort of ROP in posterior zone I. All babies had received 100% unblended oxygen by a hood or nasal prongs either period or at least during some part of their hospital stay in addition to other modes of respiratory support like mechanical ventilation or CPAP. Also, few babies had AROP despite a GA and BW as high as 34 weeks and 3500 grams, respectively. This has been reported from India earlier by Shah et al. [1] and Sanghi et al. [21] unlike from the west [20]; it has been attributed to severe dieback of the retinal vessels or capillary bed loss in the vascularized retina due to oxygen-induced retinal vascular changes. This could have been one of the important reasons for the disease severity and very posterior location noticed in this zone. Few babies had very early changes of AROP as early as 2–3 weeks after birth. We could identify them because of our screening protocol of 1st examination after 2 weeks for babies born with GA less than 28 weeks and/or BW less than 1200 gm [10]. Most babies had an extended NICU stay, poor weight gain, and multiple systemic co-morbidities. A higher incidence of ROP has been reported in babies staying longer in the hospital [22]. These additional risk factors could have contributed to the severity of the disease observed in this zone.
Longer hospital stays could indirectly mean a poor systemic status and susceptibility to multiple systemic abnormalities, which in turn can increase the ROP severity. Most babies in this cohort had an average NICU stay of more than a month. A poor weight gain at an average of 48.5 grams/week (against the normal of approximately 100 grams per week) probably influenced the stunted vascular growth and disease development in this zone [23–25]. In this cohort, 21 eyes (16.1%) of 11 babies had advanced disease (Stage 4/5 or atypical bleb-like detachment) at the time of presentation.
Retinal vascularization starts at GA 13–14 weeks [26, 27].
Considering the rate of vascular growth as 0.7 DD or 0.7 mm/week as per published reports [18] and taking the disc-fovea distance as approximately 4DD in neonates, the retinal vessels should reach fovea approximately by 21–23 weeks.
In this series, 51 babies (76%) had GA of more than 23 weeks, but their retinal vascular growth was still posterior to the fovea. The reasons could be either decelerated growth antenatally [4], or supplemental oxygen causing massive vessel to dieback or severe obliteration of the peripheral segment of the blood vessels, a condition similar to oxygen-induced retinopathy [1].
We also observed several vascular abnormalities. These were arterio-venous shunting at multiple levels, large vessel-free pockets within the vascular loops, retinal vascular tortuosity disproportionately more than dilatation, and absence of dilatation despite tortuosity in some vessels. Arteriovenous shunting is a phenomenon to compensate for peripheral ischemia. In our cohort, around 80% of eyes had arterio-venous shunting, indicating a greater impact of peripheral non-perfusion in the pathogenesis of ROP in this zone. Unlike classic arterio-venous shunting, we observed that around 14% of eyes had long arterio-venous shunts bridging multiple arteries and veins. We have earlier reported similar large arterio- venous loops in ROP eyes treated with intravitreal bevacizumab, though the dilatation and tortuosity were less severe than that noted in the treatment naïve posterior zone I ROP eyes in the present series. This possibly is indicative of extensive hypoxia and a higher demand to compensate in this zone. Also, 12% of eyes in this cohort had a characteristic “corkscrew” tortuosity. This could probably be secondary to severe backlogging of the blood column within a small segment of blood vessels. The central retinal vascular trunks were well formed in most eyes, though it was poorly developed in approximately 18% of eyes and was difficult to identify even in relatively clear media (Fig. 2b). We are unsure if it influences the treatment outcomes.
We have also earlier described atypical patterns such as bleb-like detachments and large candle wax-like deposits [28, 29]. In this series, we observed this abnormality in 4.6% and 17.6% of eyes, respectively.
These appearances in human eyes are close to oxygen-induced retinopathy in animal models [1]. The candle wax deposits have been hypothesized to occur due to the poorly formed retinal vessel and blood-retinal barrier break secondary to oxygen toxicity [29]. We need further studies to understand their origin, nature, pathogenesis, and clinical significance. The hyaloid vascular system, expected to disappear by 28 to 34 weeks of GA [27], was persistent in 31% of eyes in the current cohort of babies. The persistence of the hyaloid plays an important role in the vitreous condensing around it and can be one of the factors responsible for the closing of the retina over the optic disc, causing a posteriorly located closed funnel retinal detachment.
Study limitations
Fundus fluorescein angiogram and optical coherence tomography were not performed on these babies. Also, we did not have all details of oxygen supplementation.
Study strengths
The present series on the posterior zone I is the largest in the literature (Medline search). Association with oxygen supplementation (100% and unblended) in all cases and retinopathy occurring even in some heavier and older infants require further studies. The detailed clinical description provides the distinctive features that help differentiate these from retinopathy in other zones. The disease tends to be advanced in the majority, and the stage 1 disease is usually non- existent in this zone. Further research should address the atypical patterns, timing of screening, the chronology of events resulting in restricted vascular outgrowth to this zone, the rapidity of disease progression, and the standard of care for ROP in posterior Zone I.
Summary
What was known before
ROP in posterior Zone I affects smallest and sickest babies with many collateral health issues.
What this study adds
Unblended 100% oxygen use was seen in all subjects with ROP in posterior zone I in this series. Many babies had GA and BW beyond the screening limit set in western guidelines. Some of the babies showed early features of AROP as early as 2–3 weeks of life.
The Retinopathy in this zone has many atypical characteristics including bleb like combined detachments, excessive candle wax like pre retinal deposits and absence of dilatation despite tortuosity of retinal vessels in some.
Acknowledgements
We acknowledge Mr. Abhinav Sekar for his help in editing and language check in the manuscript.
Author contributions
TRP: Concept, data acquisition, analysis, Interpretation, preparation of the draft. Critical revision, final approval, and agree to be accountable for all aspects of the work. MS: Data acquisition, analysis, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. SUS: Data acquisition, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. TD: Data analysis, Critical revision, final approval. UTB: Data acquisition, preparation of the draft, final approval, and agree to be accountable for all aspects of the work. AS: Data acquisition, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. SBN: Data acquisition, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. SN: Data acquisition, critical revision, final approval, and agree to be accountable for all aspects of the work. SAN: Concept and design, analysis, interpretation, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. BVP: Concept and design, analysis, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. KA: Concept and design, analysis, interpretation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. BKS: Data collection, interpretation, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. SD: Data collection, interpretation, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. KSR: Data collection, interpretation, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. LP: Data collection, interpretation, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work. SJ: Concept, design, analysis, interpretation, preparation of the draft, critical revision, final approval, and agree to be accountable for all aspects of the work.
Funding
Hyderabad Eye Research Foundation, Hyderabad, India. Though not directly related to this project, the institute’s ROP program as a whole has been supported by the Miriam Hyman Memorial Trust, London; Queen Elizabeth Diamond Jubilee Trust, London; Public Health Foundation of India, Rashtriya Bal Swasthya Karyakram, India; Dalmia Holdings, New Delhi; and Cognizant Foundation at different point of time.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shah PK, Narendran V, Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child Fetal Neonatal Ed. 2012;97:F371–375. doi: 10.1136/fetalneonatal-2011.. [DOI] [PubMed] [Google Scholar]
- 2.Jalali S, Kesarwani S, Hussain A. Outcomes of a protocol-based management for zone 1 retinopathy of prematurity: the Indian Twin Cities ROP Screening Program report number 2. Am J Ophthalmol. 2011;151:719–24. doi: 10.1016/j.ajo.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Kychenthal A, Dorta P, Katz X. Zone I retinopathy of prematurity: clinical characteristics and treatment outcomes. Retina. 2006;26:S11–5. doi: 10.1097/01.iae.0000244285.79004.e6. [DOI] [PubMed] [Google Scholar]
- 4.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicentre for trial for cryotherapy of retinopathy of prematurity: three-month outcome. Arch Ophthalmol. 1990;108:195–204. doi: 10.1001/archopht.1990.01070040047029. [DOI] [PubMed] [Google Scholar]
- 5.WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. The early treatment for retinopathy of prematurity study: structural findings at age 2 years. Br J Ophthalmol. 2006;90:1378–82. doi: 10.1136/bjo.2006.098582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katoch D, Dogra MR, Aggarwal K, Sanghi G, Samanta R, Handa S, et al. Posterior zone I retinopathy of prematurity: spectrum of disease and outcome after laser treatment. Can J Ophthalmol. 2019;54:87–93. doi: 10.1016/j.jcjo.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Parchand SM, Agrawal D, Gangwe A, Saraogi T, Agrawal D. Combined intravitreal ranibizumab and zone I sparing laser ablation in infants with posterior zone I retinopathy of prematurity. Indian J Ophthalmol. 2021;69:2164–70. doi: 10.4103/ijo.IJO_2581_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachan A, Chandra P, Chandra Lakshmi C, Chawla R, Shah PR, Kumar A. Clinical profile and management outcomes of posterior zone 1 retinopathy of prematurity. Int Ophthalmol. 2022;42:3303–9. doi: 10.1007/s10792-022-02329-y.. [DOI] [PubMed] [Google Scholar]
- 9.Pjaver RK, Bilagi AP, Vinekar A, Deorari AK, Jalali S. Evidence based ROP guidelines in the NNF evidence based guidelines. (2010). P. 253-63. Available from http:aimaonline.org/iap-neochap-2013/uploads/acdcorner/nnf_guidelines-2011.pdf. Last accessed Feb 17, 2023.
- 10.Guidelines for Universal Eye Screening in Newborns Including Retinopathy of Prematurity [Internet]. Rashtriyabalswasthya karyakram; 2017. Available karyakram; 2017. Available from: https://www.nhm.gov.in/images/pdf/programmes/RBSK/Resource_Documents/Revised_ROP_Guidelines-Web_Optimized.pdf-Google Search [Internet]. Last accessed Feb 17, 2023.
- 11.Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International classification of retinopathy of prematurity, third edition. Ophthalmology. 2021;128:e51–68. doi: 10.1016/j.ophtha.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Silva DJ, Cocker KD, Lau G, Clay ST, Fielder AR, Moseley MJ. Optic disk size and optic disk-to-fovea distance in preterm and full-term infants. Invest Ophthalmol Vis Sci. 2006;47:4683–6. doi: 10.1167/iovs.06-0152. [DOI] [PubMed] [Google Scholar]
- 13.Vinekar A. “Timing of laser following intravitreal anti-vascular endothelial growth factor injections for aggressive posterior zone 1 retinopathy of prematurity.”. Indian J Ophthalmol. 2021;69:1988–9. doi: 10.4103/ijo.IJO_373_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Committee for the Classification of Retinopathy of Pre-maturity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–4. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 15.An international classification of retinopathy of prematurity. II. The classification of retinal detachment. The international committee for the classification of the late stages of retinopathy of prematurity. Arch Ophthalmol. 1987;105:906–12. [PubMed] [Google Scholar]
- 16.Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of pre-maturity revisited. Arch Ophthalmol. 2005;123:991e999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 17.Sanghi G, Dogra MR, Dogra M, Katoch D, Gupta A. A hybrid form of retinopathy of prematurity. Br J Ophthalmol. 2012;96:519–22. doi: 10.1136/bjophthalmol-2011-300321. [DOI] [PubMed] [Google Scholar]
- 18.Padhi TR, Bhusal U, Padhy SK, Patel A, Kelgaonker A, Khalsa A, et al. The retinal vascular growth rate in babies with retinopathy of prematurity could indicate treatment need. Indian J Ophthalmol. 2022;70:1270–7. doi: 10.4103/ijo.IJO_1484_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritch R, Shields MB, Krupin T. The Glucomas. St. Louis, MO: Mosby; 1989. [Google Scholar]
- 20.Section on Ophthalmology American Academy of Pediatrics; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–6. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 21.Sanghi G, Dogra MR, Katoch D, Gupta A. Aggressive posterior retinopathy of prematurity in infants ≥1500 g birth weight. Indian J Ophthalmol. 2014;62:254–7. doi: 10.4103/0301-4738.128639.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. SurvOphthalmol. 2018;63:618–37. doi: 10.1016/j.survophthal.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace DK, Kylstra JA, Phillips SJ, Hall JG. Poor postnatal weight gain: a risk factor for severe retinopathy of prematurity. J AAPOS. 2000;4:343–7. doi: 10.1067/mpa.2000.110342. [DOI] [PubMed] [Google Scholar]
- 24.Allegaert K, Vanhole C, Casteels I, Naulaers G, Debeer A, Cossey V, et al. Perinatal growth characteristics and associated risk of developing threshold retinopathy of prematurity. J AAPOS. 2003;7:34–37. doi: 10.1067/mpa.2003.S1091853102420150.. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Jin JY, Kim SS. Postnatal weight gain in the first two weeks as a predicting factor of severe retinopathy of prematurity requiring treatment.”. Korean J Pediatr. 2015;58:52–9. 10.3345/kjp.2015.58.2.52. [DOI] [PMC free article] [PubMed]
- 26.Provis JM. Development of the primate retinal vasculature. Prog Retin Eye Res. 2001;20:799–821. doi: 10.1016/s1350-9462(01)00012-x. [DOI] [PubMed] [Google Scholar]
- 27.Pau H. Hypothesis on the pathogenesis of retinopathy of prematurity–it is not VEGF alone but anatomical structures that are crucial. Graefes Arch Clin Exp Ophthalmol. 2010;248:1–3. doi: 10.1007/s00417-009-1190-7. [DOI] [PubMed] [Google Scholar]
- 28.Patel A, Padhy SK, Saoji K, Saldna M, Multani PK, Khalsa A, Kelgaonkar A, Bhusal U. Bleb-like posterior combined retinal detachment in severe retinopathy of prematurity: clinical characteristics, management challenges and outcome. Eye. 2021;35:3152–5. doi: 10.1038/s41433-020-01223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah MS, Padhy SK, Sahu S, Padhi TR. Atypical case of retinopathy of prematurity with candle wax-like preretinal deposits and its surprising response to intravitreal anti-vascular endothelial growth factor. BMJ Case Rep. 2022;15:e244998. doi: 10.1136/bcr-2021-244998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.