Abstract
The advent of intravitreal anti-VEGF injections has revolutionised the treatment of both neovascular age-related macular degeneration (nAMD or wet AMD) and diabetic macular oedema (DMO). Despite their efficacy, anti-VEGF injections precipitate significant treatment burden for patients, caregivers and healthcare systems due to the high frequency of injections required to sustain treatment benefit. Therefore, there remains an unmet need for lower-burden therapies. Tyrosine kinase inhibitors (TKI) are a novel class of drugs that may have considerable potential in addressing this issue. This review will summarise and discuss the results of various pilot studies and clinical trials exploring the role of TKIs in treatment of nAMD and DMO, highlighting promising candidates and possible challenges in developments.
Subject terms: Pharmaceutics, Drug therapy
Abstract
玻璃体内注射抗VEGF药物的出现改变了新生血管性年龄相关性黄斑变性 (nAMD或湿性AMD) 和糖尿病性黄斑水肿 (DMO) 的治疗。尽管抗VEGF治疗有效, 但由于维持治疗效果需要高频次注射, 给患者、护理人员和医保系统带来了巨大的治疗负担。因此, 对低负担治疗的需求仍未得到满足。酪氨酸激酶抑制剂 (TKI)为一种新型药物, 在解决这一问题方面可能具有很大的潜力。本综述将总结和讨论TKI在nAMD和DMO治疗中作用的各种探索性研究和临床试验的结果, 强调有前景的备选药物和开发中可能面临的挑战。
Introduction
The two most common treatable retinal causes of blindness are neovascular age-related macular degeneration (nAMD) and diabetic macular oedema (DMO). If left untreated, these conditions can lead to irreversible visual loss. Both conditions are mainly triggered by an increase in intraocular vascular endothelial growth factor (VEGF) that in turn drives vascular hyperpermeability and retinal and choroidal angiogenesis [1–3]. The VEGF family include VEGF-A, B, C and D, placenta growth factor (PIGF) and these bind to receptor tyrosine kinases (RTK). Whilst VEGF-A promotes vascular permeability and angiogenesis, VEGF-B is implicated in non-angiogenic tumour growth and VEGF-C and D have a role in angiogenesis and lymphangiogenesis. PIGF also promotes angiogenesis, vascular permeability, proliferation and chemotaxis. In terms of VEGF receptors, VEGFR-1, 2 and 3 are all RTKs [4].
Anti-VEGF therapies have revolutionised the treatment of these retinal conditions. Most are monoclonal antibodies to all isomers of VEGF-A and these include ranibizumab, bevacizumab and brolucizumab [5–8]. In contrast, aflibercept is a decoy receptor that binds to VEGF-A, VEGF-B and PIGF [8]. Faricimab is a bi-specific antibody that binds to both VEGF-A and angiopoietin-2 (ANG-2) and these growth factors interact with different RTKs [9]. These drugs are given as intravitreal injections. On average, about 50% of patients treated regularly in the first year with an average of 7-8 intravitreal anti-VEGF injections gain 2 lines of vision and 30% may improve even more and less than 5% lose three lines or more. However, sustaining these treatment frequencies for many years is a burden to the patients, caregivers and the healthcare system [2, 10]. Therefore, other options for increasing durability are being evaluated. This review will focus on tyrosine kinase inhibitors (TKIs), a novel class of drug developed to address the issues described above.
Tyrosine kinase Inhibitors
Mechanism of action
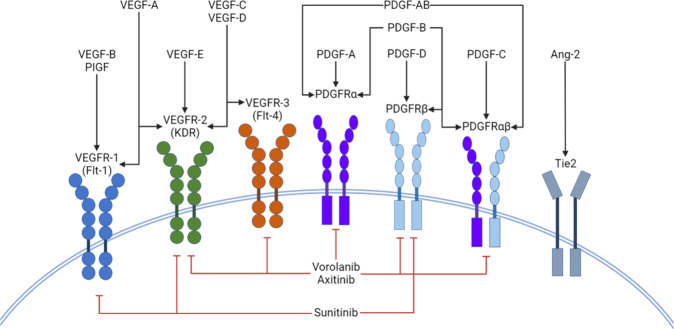
Tyrosine kinases are signal transduction enzymes that are found in many different metabolic pathways. They consist of three categories: RTKs, non-receptor tyrosine kinases and dual-specificity kinases. RTKs consist of an extracellular domain that binds to specific ligands and an intracellular domain that is involved in phosphorylation of specific amino acids on proteins. This phosphorylation results in downstream signalling and activation or inhibition of metabolic pathways. There are approximately 20 different RTK classes that have been identified [11]. Apart from VEGFRs, RTKs also include platelet-derived growth factor receptors (PDGFR), TIE receptors (TIE1 and 2) [12], stem-cell factor receptor (e.g. KIT), and the ErbB receptor family which includes epidermal growth factor receptors (EGFR) and human epidermal growth factor receptor-2 (HER2) [13]. The 2 RTKs implicated in choroidal neovascularisation (CNV) are VEGFR and PDGFR [14]. In comparison to monoclonal antibodies which act on the extracellular components of receptors, TKIs act on the intracellular domains to inhibit phosphorylation activity [15]. Figure 1 shows the schematic representation of tyrosine kinase inhibitors (TKIs) and the receptors they target. TKIs can also be divided into selective or non-selective groups based on in vitro potency against target receptors. Examples of non-selective VEGFR inhibitors are sorafenib (relatively low potency towards VEGFR), sunitinib (moderate potency) and cabozantinib (high potency). Selective inhibitors of VEGFR include pazopanib (moderate potency) and axitinib (high potency) [16].
Fig. 1. Schematic representation of tyrosine kinase inhibitors (TKIs) and the receptors they target.
Ang 2- Angiopoietin 2; Flt- fms like tyrosine kinase; KDR-Kinase insert domain receptor;PDGF-Platelet derived growth factor; PDGFR- Platelet derived growth factor receptor; PIGF- Placenta growth factor;VEGF- Vascular endothelial growth factor; VEGFR- Vascular endothelial growth factor receptor.
Current uses
As TKIs are involved in multiple cellular signalling pathways, they have mainly been evaluated to treat cancer. First-generation reversible TKIs such as erlotinib and gefitinib have been evaluated in non-small cell lung cancers possessing EGFR-sensitising mutations [17, 18]. Osimertinib is a third-generation TKI currently used to treat non-small cell lung cancers that are resistant to first- and second-generation EGFR-specific TKIs [18]. The HER-2-specific TKI tucatinib is a drug approved for treating HER-2-positive metastatic breast cancer [19]. The treatment of renal cell carcinomas has also improved substantially with the use of VEGFR-specific TKIs. Sunitinib and pazopanib are two TKIs that are approved for the first-line treatment of metastatic renal cell carcinomas [18, 20].
Apart from cancer, TKIs have also been used in treatment of idiopathic pulmonary fibrosis. Nintendanib is a potent inhibitor of FGFR, PDGFR and VEGFR. A phase 3 trial showed nintendanib significantly slowed the rate of decline of forced vital capacity, which was analogous to a reduction in rate of disease progression, compared to a placebo [21].
Exploratory studies in nAMD and DMO
Oral Sorafenib
Sorafenib, a VEGFR inhibitor, is a TKI that is approved as a therapy for cancer. When administered orally, 200 mg sorafenib has a high potency in inhibition of both the VEGFR1 (65 times higher than 50% inhibitory concentration, IC50) and VEGFR2 (18 times higher than IC50) with a serum half-life of 29.5 hours. The IC50 is a measure of the potency of a substance in inhibiting a specific biological or biochemical function [22]. The lower the value of IC50 the higher the affinity of the agent and inhibitory potential. A case report by Diago et al. followed two patients who chose to receive off-label treatment with sorafenib alongside ranbizumab. The first patient, an 83-year-old male, was followed up for chronic nAMD in the right eye. This patient elected to undertake combination therapy to reduce the frequency of intravitreal injections. Initial visual acuity was Snellen 20/70, optical coherence tomography (OCT) showed macular fluid. The treatment protocol was an initial injection of ranibizumab followed by 200 mg sorafenib 3 times a week for 5 weeks. Visual acuity improved to Snellen 20/60 and OCT showed improvement in macular fluid. On discontinuation of sorafenib, visual acuity decreased and macular fluid increased. Re-starting oral sorafenib resulted in further improvement in vision and macular fluid. The only self-reported adverse effect was mild acral dermatitis after initial dose of sorafenib which resolved spontaneously [23]. The second patient, an 81-year-old male, also elected for oral sorafenib monotherapy to reduce number of ranibizumab injections. The patient received 200 mg oral sorafenib 3 times a week for a month after one month of discontinuing ranibizumab. At follow up, visual acuity remained stable and OCT showed a reduction in intraretinal fluid and decrease in pigment epithelial detachment. The results of this study suggest that sorafenib could potentially be used as an adjuvant or an alternative to regular ranibizumab injections, reducing treatment burden on patients and carers. However, the study is limited by a very small sample size of cases and a lack of long-term follow-up for safety and efficacy. No further studies have been conducted on sorafenib thus far.
Oral and topical Pazopanib
Pazopanib is a non-selective TKI of VEGFR1, 2 and 3, PDGFR α and β and C-Kit. An exploratory study evaluated oral pazopanib in patients with subfoveal CNV secondary to nAMD. The objectives included determination of safety and preliminary efficacy of 15 mg oral pazopanib administered daily for 28 days. Fifteen patients, 50 years or older, with treatment naïve minimally classic or occult sub-foveal CNV due to nAMD were enroled in this study. At follow up, 9 out of 15 patients showed improvements in mean BVCA of 8 letters, −50.28 μm in central retinal thickness and −50.94 μm in central retinal lesion thickness. In contrast, last observation carried forward analysis of all 15 patients (including the remaining 6 patients who received rescue therapy) showed no significant improvement in the 3 previous outcomes and 7 of 15 patients reported ocular adverse effects (AE). One patient experienced hypertension and an increase in urine protein: creatinine ratio. No deaths, severe AEs or withdrawals from the study were reported. The study also identified the 6 patients who required rescue therapy had the high risk CFHY402H CC genotype for AMD. There was a trend for improvement in eyes with the low risk CFHY402H T allele. This study highlights that pazopanib may be unsuitable for monotherapy in patients with the CC genotype but may show potential for those with the T allele. However, a statistical analysis was not performed on this data due to the small sample size [24].
Topical pazopanib was also investigated in patients with active subfoveal CNV secondary to nAMD. Casky et al. performed a multi-country, double blind, active and placebo-controlled, dose-ranging study on 510 participants who had nAMD previously managed on anti-VEGF injections. Patients were divided into 7 equal groups and the following treatment was administered per group for 52 weeks: placebo eye drops 4 times daily, pazopanib 5 mg/ml 3 or 4 times daily, pazopanib 10 mg/ml 2, 3 or 4 times daily, or ranibizumab injection once every 4 weeks. Ranibizumab was given as needed to all 7 treatment groups. Primary outcome was best corrected visual acuity (BCVA) and the need for as-needed injection frequency. At the end of the study, no significant difference in BCVA change between patients in the pazopanib, ranibizumab and placebo groups was found (0.3-1.8 vs. 1.4 vs. 0.2 letters) when accounting for as-needed injections. Pazopanib also did not reduce the frequency of as-needed ranibizumab injections below 50%, the set threshold for a significant reduction. The 2 most common AE reported for pazopanib was pain at application site. However, no treatment-related severe AEs were reported. It was concluded that topical pazopanib/ranibizumab combined therapy or topical pazopanib monotherapy did not offer any therapeutic advantage or reduce treatment burden compared to intravitreal ranibizumab alone [25].
PAN90806
PAN90806 (ClinicalTrials.gov NCT03479372) is a once-daily topical selective TKI targeting VEGFR2 developed to treat nAMD by PanOptica. Previous phase 1/2 trials showed potential for a 4 times daily topical PAN90806 solution monotherapy for nAMD, maintenance therapy following ranibizumab injection for nAMD and monotherapy for DMO. However, the solution also caused reversible punctate keratopathy due to off-target effects. The latest study on PAN90806 investigated a suspension instead of solution formulation and a once-daily dosing regimen for improved safety. A randomised, double blind, expanding dose, phase 1/2 trial was conducted on 51 treatment naïve patients with nAMD. All patients were aged 50 years or older and had newly diagnosed, previously untreated CNV secondary to nAMD [26]. A total of 3 equal groups were given 2, 6 and 10 mg/ml drops over 12 weeks with a 16-week follow-up. Primary outcomes were safety and tolerability of PAN90806 at these concentrations. Secondary outcomes investigated efficacy by measuring changes in central subfield thickness (CST), BCVA and number of potential injections avoided compared to current standard treatment at day 1, week 2, 4 8, 12 and 16 follow-ups. The Data and Safety Monitoring Committee confirmed no major or serious AEs for doses at all concentrations but 2 patients withdrew from the study prior to 12 weeks due to AEs related to treatment [27]. A total of 26 of 51 patients across all doses did not require rescue injections by week 16. In addition, 23 of the 26 patients showed stability or clinical improvement. All 3 groups showed neither a significant decrease or increase in CST from baseline. At weeks 2 and 4, both 6 and 10 mg/ml groups showed a significant improvement in BCVA over the 2 mg/ml group. However, there were no significant differences over the remaining weeks between all groups. By week 16, only the 6 and 10 mg/ml group showed a significant improvement in BCVA from baseline. Patients who required rescue injections had baseline higher mean CST and worse BCVA than patients who did not. This agree with the study on oral pazopanib described previously where patients who had the high-risk CC phenotype required more rescue injections. This study highlights that although there are some biological effects, about 50% of patients needed rescue treatment, questioning its efficacy as a maintenance therapy for nAMD [28].
Regorafenib
The DREAM study was an open-label phase IIa/b trial that investigated topical regorafenib, a multikinase inhibitor, in patients with nAMD [29]. Patients with CNV secondary to AMD received regorafenib (25 μl, 30 mg ml–1) three times a day for 12 weeks. The primary endpoint was mean change in BCVA from baseline to weeks 4 and 12. In nAMD patients (N = 51), mean changes in BCVA were +1.2 [90% confidence interval (CI) –0.61, 2.97] and −2.4 (90% CI − 4.18, −0.54) letters at weeks 4 and 12, respectively. Ocular treatment‐emergent adverse events (TEAEs) in the study eye were reported in 21 patients by week 12. There was one serious ocular TEAE (reduced visual acuity) that was not drug related. Twenty patients required rescue intravitreal ranibizumab. As the efficacy was lower than with available anti-VEGF therapies this programme was terminated. Elaborate post hoc analyses indicate that the lower efficacy was likely due to insufficient drug exposure to the target in the posterior segment.
TKIs in development for nAMD and DMO
GB102 (Sunitinib)
GB102 is an injectable, self-aggregating intravitreal sunitinib maleate depot developed by GrayBug Vision. Sunitinib is a non-selective TKI that inhibits the RTKs - VEGFR1, VEGFR2 and PDGFR α and β isoforms [30]. It also has activity against other RTKs. GB102 has been investigated in phase 1 (NCT03249740) and phase 2 (NCT03953079) clinical trials. Phase 2 was a randomised, single blind, aflibercept-controlled study on 56 patients over 12 months with follows up every 2 months. Patients were 50 years or older with CNV secondary to nAMD with BCVA 35 letters or better, treated with anti-VEGF injections at least 3 times prior to study onset and demonstrated response to prior anti-VEGF treatment. Patients were divided into 3 groups. The first group (n = 21) received intravitreal injections of GB102 at dose of 1 mg/mg at baseline and month 6 with sham injections at months 2, 4, 8 and 10. The second group (n = 22) received 2 mg/mg at baseline, 1 mg/mg at month 6 and sham injections at months 2, 4, 8 and 10. The final group (n = 13) received aflibercept 2 mg at baseline and months 2, 4, 6, 8 and 10. Primary outcome was median time to first rescue treatment. Secondary outcomes were based on changes to BCVA, CST and presence of exudation across the study period. It was initially intended for group 2 to receive 2 mg/mg every 2 months but this was changed to 1 mg due to AEs. Primary outcomes were 5 months for the first group and 4 months for the second group, with no significant difference between the 2 groups. Both groups also showed no significant difference in number of times at least 1 rescue criterion was met. These results suggest doses above 1 mg/mg may be unnecessary for maintenance therapy, in keeping with the initial lowering of dosage in group 2. The second group demonstrated higher efficacy in reducing exudation over the first but more patients also experienced serious AEs (total serious side effects was 2 in the first group and 4 in the second group). All 3 groups showed reduction in CST but no significant difference between each group. GB102 groups showed a significant decrease from baseline BCVA compared to the aflibercept group. Frequency of AEs not including serious AEs was much higher in both GB-102 groups compared to aflibercept. Only 1 patient in the first group did not experience an AE and all patients in the second group experienced at least 1 AE. In comparison, 8 of 13 patients in the aflibercept group experienced AEs [31]. From these results, GB-102 is worse than aflibercept at maintaining BCVA but non-inferior for maintaining CST. However, the higher frequency of side effects adds to the treatment burden and therefore, unlikely to offer a relief to the high burden of frequent intravitreal injections in the current standard of care. The AEs may be explained by the non-selective nature of sunitinib. A third formulation of GB102 is being designed to reduce time of microparticle aggregation and increase stability [32].
EYP-1901(Vorolanib)
Vorolanib is a non-selective TKI that inhibits all isoforms of VEGFR and PDGFR, FLT3, C-Kit, RET RTKs [16] and AMPKα1 which is a serine/threonine kinase receptor [33]. Vorolanib was developed on the same chemical structure as sunitinib [34]. Compared to sunitinib, IC50 values of vorolanib are lower for VEGFR2, FLT3 and C-Kit, higher for RET and AMPKα1, and the same for PDGFRβ [16]. Previous trials on oral vorolanib (X-82) for the treatment of nAMD were discontinued due to systemic toxicity and AEs [35]
EYP-1901 (EyePoint Pharma) utilizes a bioerodible formulation of Durasert®. Durasert® is a miniaturized injectable drug delivery system for small molecules that can be administered safely in in-office setting. It is a sustained release vorolanib intravitreal injection developed by Eyepoint Pharmaceuticals. The Durasert® technology is highly efficient as 90% of the insert can be the drug itself, translating to a high loading dose. There is no polyimide covering, instead a polyvinyl alcohol coating covers the entire surface of the cylinder with the aim to deliver a consistent rate of diffusion of vorolanib out of the insert [36]. The initial drug burst from the surface of insert allows for rapid attainment of therapeutic levels in ocular tissues. Sustained, zero-order kinetics drug release over 6–9 months in bioerodible formulation is expected to provide consistent drug levels throughout treatment course. It is administered as single intravitreal injection of up to 3 inserts. Single insert is ~1/5,000 the volume of the vitreous.
The phase 1 clinical trials have been completed. Table 1 shows a summary of clinical trial data for EYP-1901. In-vivo studies have also demonstrated encouraging neuroprotection and anti-fibrosis data [35, 37]. As data from APEX study on X-82 showed significant systemic toxicity, EYP-1901 was developed to have reduced off-target binding of receptors thereby reducing the incidence of TKI-related systemic side effects.
Table 1.
Clinical trial data on EYP-1901 (Vorolanib implant).
| wet AMD | wet AMD | Non-proliferative diabetic retinopathy (NPDR) | |
|---|---|---|---|
| Phase |
I (DAVIO) NCT04747197 |
II (DAVIO 2) NCT05381948 |
II (PAVIA) NCT05383209 |
| Geography | US | US | US |
| Status | Completed | Ongoing | Ongoing |
| No. of Participants | 17 | 150 | 105 |
| Design | Non-randomized, Sequential Assignment, Multicenter, Prospective, Open-Label, Dose Escalation | Randomized, Multi-centre, Prospective, Randomized, Double-blind, Parallel Assignment | Multi-centre, Prospective, Double-blind, Parallel |
| Inclusion criteria |
• Diagnosed with wet AMD in the study eye within 18 months before the screening visit. • ≥ 3 prior injections of any anti-VEGF • Response to anti-VEGF in the study eye • BCVA using ETDRS charts of 25 to 75 letters |
• Diagnosed within the past 9 months • History of response to anti-VEGF • History of at least 2 injections in the last 6 months • BCVA ETDRS letter score: 35 letters (20/200 Snellen equivalent) - 80 letters (20/25 Snellen equivalent) in the study eye at the screening visit and on Day 1 |
• Moderately severe to severe NPDR (DRSS 47 or 53) • BCVA: ETDRS letter score ≥69 letters ( ~ 20/40 or better) • HbA1c%: ≤12% • No anti-VEGF injections in the past 12 months |
| Dose & Frequency |
• EYP-1901: 440 μg, low dose (n = 3) • EYP-1901: 1030 μg, low mid-dose (n = 1) • EYP-1901: 2060 μg, mid dose (n = 8) • EYP-1901 3090 μg, high dose (n = 5) |
• EYP-1901: 2 mg low dose (n = 48) • EYP-1901: 3 mg high dose (n = 48) • Aflibercept: 2 mg (0.05 mL) Q8W (n = 48) |
• EYP-1901: 2 mg low dose (n = 35) • EYP-1901: 3 mg high dose (n = 35) • Day 1 single injection sham comparator (n = 35) |
| Primary endpoint | Incidence of ocular (study eye) and systemic treatment-emergent adverse events (TEAEs) – week 48 | Change in (BCVA) – week 28 and week 32 | % of subjects improving ≥2 steps in the DRSS score each EYP-1901 dose level versus the sham group. |
| Secondary endpoints |
• Mean change in central retinal thickness on OCT (week 48) • Mean change in BCVA (week 48) |
• Change in BCVA (week 56) • Mean change in CRT on OCT (week 56) • Number of rescue injections (week 56) |
• % of subjects improving ≥2 steps in the DRSS score in each dose level vs. sham (at weeks 48 & 96) • % of subjects who developed a vision-threatening complication due to DR (at weeks 48 & 96) • Rates of ocular (study eye & fellow eye) and non-ocular TEAEs (at weeks 24, 48, & 96) |
| Results Efficacy |
6- & 12-month data: • Significant reduction in the treatment burden of 75% at 6 months supporting treatment to maintain positioning & 73% reduction at 12 months • 53% supplemental anti-VEGF supplement injection free for up to 6 months Supplemental anti-VEGF therapy through 6 months: • Met all objectives (positive efficacy & durability) • Stabilization of mean BCVA and OCT throughout 6 months was achieved • 79% reduction in the treatment burden at 6 months Subjects 9/17 (53%) with no “excess fluid” at screening: • Mean change in BCVA from screening visit (n = 9) ○ +1.2 letters at 5 months ○ -0.4 letters at 6 months • Mean change in CST from screening visit (n = 9) ○ +20.8 microns at 5 months ○ -1.0 microns at 6 months • 92% reduction in treatment burden at 6 months among subjects with no “excess fluid” at screening (n = 9) |
NA | NA |
| Safety |
12-month data: • Phase 1 DAVIO clinical trial demonstrated favourable overall safety data at 12 months meeting the primary endpoint: No ocular SAEs, drug-related systemic SAEs, Durasert-related toxicity or tolerance issues & dose-limiting toxicity. • Supplemental anti-VEGF therapy through 6 months: • Met all objectives • No ocular SAEs, or drug-related systemic SAEs • Ocular AEs – the majority were mild and expected |
NA | NA |
AE adverse event, anti-VEGF anti-vascular endothelial growth factor, BCVA best corrected visual acuity, CRT central retinal thickness, CST central subfield thickness, DR Diabetic retinopathy, DRSS Diabetic Retinopathy Severity Score, ETDRS Early treatment diabetic retinopathy study, NPDR Non-proliferative diabetic retinopathy, OCT optical coherence tomography, SAE serious adverse event, TEAE treatment emergent adverse event, wet AMD wet age-related macular degeneration.
EYP-1901 positioned as a potential “Treat-to-Maintain” therapy in wet AMD
EYP-1901 demonstrated clinically significant reduction in treatment burden of 75% at 6 Months supporting treat to maintain positioning. About half of eyes in DAVIO study could go up to 6 months on EYP-1901 alone. Another ~30% received only a single supplemental anti-VEGF during 6-months. About 15% failed both standard of care and EYP-1901 and required multiple supplements. Therefore, a potential treatment plan for treatment naïve wet AMD patients could be to treat initially with current anti-VEGF standard of care until maximal visual acuity is achieved and retina is as dry as possible (induction phase). Then, maintaining a dry and stable macula with EYP-1901 every six months, supplementing, if needed with current anti-VEGF biologics may be a future treatment pathway.
OTX-TKI (Axitinib)
OTX-TKI is an axitinib intravitreous, bioerodible, hydrogel implant developed by Ocular Therapeutix Inc. Axitinib is a highly selective inhibitor of all VEGF and PDGF receptors with high affinity and low solubility compared to other ocular TKIs [38]. Compared to vorolanib and sunitinib, axitinib has a much lower IC50 (in nM) for VEGFR2 and kinase insert domain receptor (KDR) (IC50 0.2 for axitinib, 43 for sunitinib and 52 for vorolanib) which indicates higher affinity [39, 40].
Axitinib intravitreal implant is designed on a hydrogel delivery platform. Ocular Therapeutix’s proprietary bioresorbable polymer matrix is hydrogel-based versatile, biocompatible platform for localized and sustained drug delivery. Polyethylene glycol (PEG) is the most used polymer for drug delivery and the OTX-TKI uses PEG hydrogel [41]. The high solubility of PEG hydrogel can be attributed to the change in its physical state from a cross linked polymer hydrogel network with hydrolysable linkages, swollen with water, to a soluble low molecular weight polymer that facilitates clearance from vitreous [34]. The implant is completely bioresorbable over 6 to 12 months. Table 2 summarizes the salient points from ongoing, completed and planned clinical trials for OTX-TKI.
Table 2.
Clinical trial data for OTX-TKI (Axitinib implant).
| wet AMD | wet AMD | NPDR | |
|---|---|---|---|
| Phase |
I NCT04989699 |
I NCT03630315 |
I (HELIOS) |
| Geography | US | Australia | US |
| Status | Ongoing (positive interim results to date) | Completed (positive interim results, follow-up ongoing) | Initiated in December 2022^ |
| No of Patients | 21 (recruiting) | 29 | 21 (Jan 2023) |
| Design | Randomized, Prospective, Multi-centre, Double-blind, Open-label, Parallel Assignment | Randomized, Multi-centre, Open-label, Dose-escalation, Sequential Assignment | Randomized, multicenter, double asked, parallel assignment |
| Inclusion criteria |
• Previously treated subfoveal neovascularization (SFNV) secondary to nAMD with leakage involving the fovea • Previously treated with documented evidence of an initially favourable clinical response to anti-VEGF therapy • The macular appearance on OCT was free of excess intraretinal and/or subretinal fluid as judged by the investigator. • Must have received at least 3 anti-VEGF injections in the past year. • Have received the most recent anti-VEGF injection within the past 1 to 4 weeks prior to the screening visit. • BCVA ETDRS score between 24 & 83 letters approximately |
• Eligible for standard therapy • Have active primary CNVM secondary to AMD - either newly diagnosed or previously treated with documented response to the anti-VEGF therapy in study eye • Post-menopausal women for at least 12 months prior to screening or surgically sterile • Male or female of childbearing potential willing to use two forms of adequate contraception |
• Treatment naïve adults with NPDR secondary to diabetes mellitus type 1 or 2 • Moderately severe to severe NPDR (DRSS level 47 or 53, without DME)) in the study eye • BCVA ≥ 69 ETDRS letters (Snellen equivalent approximately 20/40 or better) in the study eye |
| Dose & Frequency |
• One dose of OTX-TKI 600 µg. OTX-TKI is one dose so subsequent visits will be sham to maintain the mask • Aflibercept administered every 8 weeks |
• Cohort 1: OTX-TKI, low dose (200 µg) • Cohort 2: OTX-TKI, Mid dose (400 µg) • Cohort 3a: OTX-TKI, High dose (600 µg) • Cohort 3b: Anti-VEGF + OTX-TKI (400 µg) • Cohort 4a: OTX-TKI, High dose (600 µg) single implant • Cohort 4: Anti-VEGF + OTX-TKI (600 µg) single implant |
• 600 µg single implants (n = 14) • Sham comparator (n = 7) |
| Primary endpoint | Safety and Tolerability (12 months) | Incidence of treatment-emergent adverse events for each subject (9 months) | Safety, tolerability & biological activity (12 months) |
| Secondary endpoints | Change in BCVA & CST, Rescue Therapy, Absence of Fluid, Number of injections (an average of 1 year) | Determine the Maximum Tolerated Dose of the OTX-TKI injection (9 months) | NA |
|
Baseline Mean (SD) BCVA Mean (SD) CSFT |
• OTX-TKI: 73.7(14.4) letters • Aflibercept: 73.8(9.0) letters • OTX-TKI: 269(40.3) µm • Aflibercept: 240.6(29.6) µm |
Interim data(n = 23) (41) • Cohort 1: 48(12.0) letters • Cohort 2: 62(8.5) letters • Cohort 3a: 46(6.4) letters • Cohort 3b: 47(11.8) letters • All patients: 51(4.7) letters • Cohort 1: 680(159) µm • Cohort 2: 450(29) µm • Cohort 3a: 521(68) µm • Cohort 3b: 435(58) µm • All patients: 526(49) µm |
NA |
| Combined Results |
OTX-TKI met its primary endpoint in both US & Australia Phase I trials with potential best-in-class durability observed. US study • Interim results demonstrated potential as a durable sustained release product AUS study • Interim 7-month results show potential as a durable sustained-release maintenance therapy for 6-12 months in subjects with controlled retinal fluid demonstrating biological activity in subjects with pre-existing fluid Proof of biological activity & maintenance therapy with SOC • Demonstrated a reduction in the retinal fluid in treatment naïve wet AMD subjects with active retinal fluid • Showed a sustained & stable maintenance of fluid and vision for 7 months in previously treated wet AMD subjects with controlled fluid Potential best-in-class durability & treatment burden reduction • Over 80% of subjects treated with OTX-TKI 600 µg were rescue-free at 6 months in both studies • A clinically meaningful reduction in the treatment burden of up to 93% Generally well-tolerated with a favourable safety profile • Over 850 subject visits from OTX-TKI clinical studies without any ocular SAEs • No drug-related ocular or systemic SAEs • No elevated intraocular pressure, retinal detachment, or implant dislocation into the anterior chamber events in the OTX-TKI cohort. AEs were mild & transient |
NA | |
| Separate results |
Demonstrated stable & sustained BCVA and CST maintenance through 7 months in subjects with controlled fluid Mean (SD) change in BCVA from screening visit • -1.3(5.2) letters at 7 months OTX-TKI • -1.0(5.3) letters at 7 months aflibercept Mean (SD) change in CST from screening visit • + 9.2(38.6) microns at 7 months OTX-TKI • + 0.4(9.1) microns at 7 months Aflibercept • 80% of subjects were rescue-free for up to 6-months • 93% treatment burden reduction had shown at 7 months following a single OTX-TKI implant |
OTX-TKI monotherapy demonstrated a clinically meaningful reduction in retinal fluid in treatment naïve subjects | NA |
AE adverse event, anti-VEGF anti vascular endothelial growth factor, BCVA best corrected visual acuity, CNVM choroidal neovascular membrane, CST central subfield thickness, DR Diabetic retinopathy, DRSS Diabetic Retinopathy Severity Score, ETDRS Early treatment diabetic retinopathy study, nAMD neovascular age-related macular degeneration, NPDR Non-proliferative diabetic retinopathy, OCT optical coherence tomography, SAE serious adverse event; wet AMD wet age-related macular degeneration.
CLS-AX (Suprachoridal delivery of axitinib)
Clearside Biomedical developed the CLS-AX (axitinib injectable suspension) for suprachoroidal use. This is performed using the Clearside Biomedical proprietary SCS (suprachoroidal space) Microinjector®. The core advantages of drug delivery to the SCS include a) targeted delivery for higher efficacy of the drug nearer to the area of pathology; b) compartmentalization of the drug in in the suprachoroidal space, which helps keep it away from non-diseased tissues thereby increasing safety profile; and c) increased bioavailability and sustained drug levels for durability [42–44]. Preclinical data also supports durability potential of small molecule suspensions delivered into the suprachoroidal space and has shown that drug levels across central/peripheral retina and SCS are similar. A natural pressure gradient exists such that intraocular pressure (IOP) is more than the anterior SCS pressure which in turn is more than the posterior SCS pressure [45–47]. This gradient drives injectate towards the lower pressured posterior SCS. OCT images have demonstrated expansion of the SCS after injection, including the macula SCS in humans. OASIS Phase I/IIa (NCT04626128) and OASIS extension (NCT05131646) clinical trial data is summarized in Table 3.
Table 3.
Clinical trial data on CLS-AX (Suprachoroidal delivery of Axitinib).
| wet AMD | wet AMD | |
|---|---|---|
| Phase |
I/IIa (OASIS) NCT04626128 |
OASIS Extension study NCT05131646 |
| Geography | US | US |
| Status | Completed | Ongoing |
| No of Patients | 27 (recruiting) | 19 (enrolment completed) |
| Design | Non-randomized, Multi-centre, Open-label, Dose-escalation, Sequential Assignment | Non-interventional, Open-label, Dose-extension |
| Inclusion criteria |
• Diagnosis of nAMD in the study eye • Active subfoveal choroidal neovascularization secondary to AMD • 2 or more prior anti-VEGF intravitreal injections • EDTRS BCVA score ≤ 75 & ≥ 20 letters |
Enrolled in & completed the Parent study (OASIS), as part of cohort 2 or cohort 3, or cohort 4. |
| Dose & Frequency |
Dosing: 0.03, 0.10, 0.50, or 1 mg of CLS-AX; Suprachoroidal injection • Cohort1: 0.03 mg (low dose) (n = 6) • Cohort 2: 0.10 mg low-mid dose (n = 5) • Cohort 3: 0.50 mg high-mid dose (n = 8) • Cohort 4: 1.0 mg high dose (n = 8) |
Participants who completed cohorts 2 or 3 or 4, were followed for an additional 12 weeks exit from the parent OASIS study. • Cohort 2: 0.10 mg low-mid dose (n = 5) • Cohort 3: 0.50 mg high-mid dose (n = 8) • Cohort 4: 1.0 mg high dose (n = 8) |
| Primary endpoint |
• Incidence of TEAEs (week 12) • Incidence of serious AEs (week 12) |
• The number of subjects experiencing TEAEs (24 weeks) • The number of subjects experiencing serious AEs (24 weeks) |
| Secondary endpoint |
• Change from baseline in pre-injection Intraocular Pressure (week 12) • Change from Baseline (Visit 2) in central subfield retinal thickness & BCVA • Incidence of subjects receiving & qualifying to receive additional IVT aflibercept injections (week 12) |
• Change from baseline in pre-injection Intraocular Pressure (24 weeks) • Change from Baseline in central subfield retinal thickness & BCVA (24 weeks) • Incidence of subjects receiving additional therapy in the study eye (8 weeks) |
|
Results Safety |
• Excellent safety profile at all doses and timepoints • No Serious Adverse Events No dose limiting toxicities • No Adverse Events from inflammation |
|
|
Results Durability |
• In OASIS, to 3 months: ≥73% reduction in treatment burden 69% of patients did not receive additional therapy 92% of patients did not receive additional therapy as per protocol • In Extension Study, to 6 months (interim data: 77% reduction in treatment burden. |
|
|
Results Biologic Effect |
• Stable mean Best Corrected Visual Acuity (BCVA) • Stable mean Central Subfield Thickness (CST) • On optical coherence tomography (OCT), anatomical signs of tyrosine kinase inhibitor (TKI) biologic effect were observed in anti-VEGF treatment-experienced sub-responders |
|
AE adverse event, anti-VEGF anti vascular endothelial growth factor, BCVA best corrected visual acuity, CST central subfield thickness, ETDRS Early treatment diabetic retinopathy study, IVT intravitreal therapy, nAMD neovascular age-related macular degeneration, NPDR Nonproliferative diabetic retinopathy, OCT optical coherence tomography, SAE serious adverse event, TEAE treatment emergent adverse event, wet-AMD wet age-related macular degeneration.
Out of all the drugs explored in this section, thus far, EYP 1901, OTX-TKI and CLS-AX have shown good safety but moderate durability signals on PhI/IIa studies on nAMD patients who are sub-optimal responders to standard of care. Better durability may be anticipated if evaluated on treatment naïve nAMD. A 6-monthly treatment interval for most of the patients with nAMD will be revolutionary in this field. Expanding the indication of TKI to diabetic retinopathy may allow a wider use of these agents in Ophthalmology. Investigating the efficacy of PDFGR-specific and FGR-specific TKIs may provide novel treatments for patients with disease resistant to current anti-VEGF therapy. In nAMD, pericyte envelope the macular neovascularisation over time that leads to maturation However, the role of PDGF in the pathology of DR and DMO is more complex. For example, there is evidence that PDGFR engagement contributes to both pericyte survival as well as upregulation of VEGF and neovascularisation [48]. In diabetic eyes, where pericytes are important and vulnerable, PDGF blockage may lead to collateral damage and therefore requires a guarded approach.
Future directions
Topical cediranib
Recently, Lorenzo-Soler et al. investigated the efficacy of cediranib maleate nanoparticle eye drops. Cediranib is a TKI targeting all forms of VEGFR. Cediranib maleate with γ cyclodextrin nanoparticles was formulated and tested as topical 3% eye drops in live rabbit eyes. Cyclodextrins increase aqueous solubility of lipophilic drugs without compromising the ability of the drug to cross lipophilic membranes. The results showed that the eyedrops achieved intraocular penetration. In particular, the peak concentrations of the drug in the vitreous was 10 times higher than the IC50 of both VEGFR2 phosphorylation and VEGF-induced proliferation and 100 times higher in the retina, indicating the potential for a highly potent anti-VEGF effect. However, drug clearance was only investigated up to 6 hours, so the half-life and treatment effect of the drug needs to be explored. Additionally, while the drug concentration in the blood samples were deemed to be significantly lower than in the ocular tissues, frequency and systemic and ocular AEs resulting from both the nanoparticles and cediranib maleate will not be known until human trials have been conducted. Due to the impressive topical absorption of these cyclodextrin nanoparticles, it would be worth exploring this formulation or other formulations of TKIs with γ cyclodextrin nanoparticles in phase 1 trials [2].
Potential role in diabetic retinopathy and DMO
Diabetic retinopathy is a microvascular complication of diabetes and is a leading cause of blindness in the working-age population globally. The mechanistic trigger of DR is unclear bit it is underpinned by oxidative stress, inflammation and angiogenesis. All these pathways drive the release of VEGF and many other cytokines in DR [49]. Both these pathways are responsible for progression of non-proliferative DR (NPDR) to proliferative DR (PDR). As high as ~52–75% patients with severe NPDR progress to PDR in 1 year. Anti-VEGF treatment in NPDR has demonstrated clinical benefit. A significant improvement in Diabetic Retinopathy Severity Scale (DRSS) and reduced vision-threatening complications of DR were found in the PANORAMA and Protocol W trials in patients treated with intravitreal aflibercept [50]. However, utilization of anti-VEGFs to treat severe NPDR is low, due to high treatment burden [51, 52]. Therefore, there is a need for early intervention with a longer lasting therapy in DR. Preclinical and clinical evidence show that TKIs can effectively suppress exudation. In clinical trials, OTX-TKI showed sustained and stable maintenance of fluid over 6 months. OTX-TKI prevented neovascularization for 12 months in rabbits following VEGF challenge [53]. In conclusion, as OTX-TKI clinical outcomes were comparable to intravitreal aflibercept in patients with wet AMD, there is also potential for use in DR, but with caution [54].
Acknowledgements
The research was supported by the NIHR Biomedical Research Centre and the Clinical Research Facility at Moorfields Eye Hospital NHS Foundation trust and UCL Institute of Technology.
Author contributions
Conceptualization, T.E. and S.S.; methodology, S.C., T.E., and S.S.; data curation, S.C. and E.Y.T.; writing—original draught preparation, S.C., E.Y.T., and S.S.; writing—review and editing, T.E., and S.S.; supervision, S.S.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Competing interests
S.S. has received funding/fees from Bayer, Novartis, Abbvie, Roche, Boehringer Ingelheim, Optos, EyeBiotech, Biogen, and Apellis. T.E. is an employee of Boehringer Ingelheim Ltd, Germany. S.S. and S.C. are members of the Eye editorial board.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shruti Chandra, Emanuel Yuquan Tan.
References
- 1.Khan M, Aziz AA, Shafi NA, Abbas T, Kha mab. Cells NLM (Medlin) 2020;9:1869. doi: 10.3390/cells9081869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorenzo-Soler L, Praphanwittaya P, Olafsdottir OB, Kristinsdottir IM, Asgrimsdottir GM, Loftsson T, et al. Topical noninvasive retinal drug delivery of a tyrosine kinase inhibitor: 3% cediranib maleate cyclodextrin nanoparticle eye drops in the rabbit eye. Acta Ophthalmol. 2022;100:788–96. doi: 10.1111/aos.15101. [DOI] [PubMed] [Google Scholar]
- 3.Mirando AC, Shen J, Silva RLE, Chu Z, Sass NC, Lorenc VE, et al. A collagen IV-derived peptide disrupts α5β1 integrin and potentiates Ang2/Tie2 signaling. JCI Insight. 2019;4:e122043. doi: 10.1172/jci.insight.122043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witmer AN, Vrensen GFJM, van Noorden CJF, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retinal Eye Res. 2003;22:1–29. doi: 10.1016/S1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 5.Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Vol. 86, Cancer Treatment Reviews. W.B. Saunders Ltd; 2020. [DOI] [PubMed]
- 6.Paling T, Hewitt C, Hay N, Beggs L Fast Track Appraisal: Brolucizumab for treating wet age-related macular degeneration [ID1254]. 2020.
- 7.National Institute for Health and Care Excellence. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration. TA155. London: National Institute for Health and Care Excellence. 2012.
- 8.Joint Formulary Committee. Aflibercept for treating diabetic macular oedema. British National Formulary. 2015.
- 9.O’Brien S, Stevenson M, Renehan A, Nwulu U Fast Track Appraisal: Faricimab for treating diabetic macular oedema and wet age-related macular degeneration [ID3898]. 2022.
- 10.Apte RS. Tyrosine kinase inhibitors in age-related macular degeneration. JAMA Ophthalmol Am Med Assoc. 2017;135:767–8. doi: 10.1001/jamaophthalmol.2017.1600. [DOI] [PubMed] [Google Scholar]
- 11.Ségaliny AI, Tellez-Gabriel M, Heymann MF, Heymann D. Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers. J Bone Oncol. 2015;4:1–12. doi: 10.1016/j.jbo.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saharinen P, Jeltsch M, Santoyo MM, Leppänen VM, Alitalo K. The TIE Receptor Family. Receptor Tyrosine Kinases: Family and Subfamilies. 2015;743. Available from: /pmc/articles/PMC7123982/
- 13.Thomson RJ, Moshirfar M, Ronquillo Y. Tyrosine Kinase Inhibitors. StatPearls. 2022. https://www.ncbi.nlm.nih.gov/books/NBK563322/ [PubMed]
- 14.Esteban-Villarrubia J, Soto-Castillo JJ, Pozas J, Román-Gil MS, Orejana-Martín I, Torres-Jiménez J, et al. Tyrosine kinase receptors in oncology. Int J Mol Sci. 2020;21:1–48. doi: 10.3390/ijms21228529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujinaka H, Fu J, Shen J, Yu Y, Hafiz Z, Kays J, et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat Commun. 2020;11:694. doi: 10.1038/s41467-020-14340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang C, Yuan X, Shen Z, Wang Y, Ding L. Vorolanib, a novel tyrosine receptor kinase receptor inhibitor with potent preclinical anti-angiogenic and anti-tumor activity. Mol Ther Oncolytics. 2022;24:577–84. doi: 10.1016/j.omto.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16:1307–14. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Jiang S, Shi Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020) J Hematol Oncol. 2020;13:143. doi: 10.1186/s13045-020-00977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 21.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 22.Aykul S, Martinez-Hackert E. Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal Biochem. 2016;508:97–103. doi: 10.1016/j.ab.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diago T, Pulido JS, Molina JR, Collet LC, Link TP, Ryan EH. Ranibizumab combined with low-dose sorafenib for exudative age-related macular degeneration. Mayo Clin Proc. 2008;83:231–4. doi: 10.1016/S0025-6196(11)60847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin MM, Paglione MG, Slakter J, Tolentino M, Ye L, Xu CF, et al. Initial exploration of oral pazopanib in healthy participants and patients with age-related macular degeneration. JAMA Ophthalmol. 2013;131:1595–601. doi: 10.1001/jamaophthalmol.2013.5002. [DOI] [PubMed] [Google Scholar]
- 25.Csaky KG, Dugel PU, Pierce AJ, Fries MA, Kelly DS, Danis RP, et al. Clinical evaluation of pazopanib eye drops versus ranibizumab intravitreal injections in subjects with neovascular age-related macular degeneration. Ophthalmology. 2015;122:579–88. doi: 10.1016/j.ophtha.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 26.PanOptica Inc.A Randomized, Double Masked, Uncontrolled, Multicenter Phase I/II Study to Evaluate Safety and Tolerability of PAN-90806 Eye Drops, Suspension in Treatment-Naïve Participants With Neovascular Age-Related Macular Degeneration (AMD). ClinicalTrials.gov NCT03479372. 2018.
- 27.PanOptica Inc. PanOptica: Anti-VEGF Eye Drop Shows Promise in Treatment of Wet AMD. Eyewire. 2019. https://eyewire.news/articles/panoptica-anti-vegf-eye-drop-shows-promise-in-treatment-of-wet-amd/?c4src=article:infinite-scroll
- 28.PanOptica Inc. PAN-90806: Once-daily topical anti-VEGF eye drop for wet AMD and other neovascular eye disease. Ophthalmology Innovation Source (OIS) Summit. 2019 [cited 2023 Jan 14]. Available from: https://www.panopticapharma.com/wp-content/uploads/2019/10/PAN-90806-Data-at-OIS@AAO.pdf
- 29.Joussen AM, Wolf S, Kaiser PK, Boyer D, Schmelter T, Sandbrink R, et al. The Developing Regorafenib Eye drops for neovascular Age‐related Macular degeneration (DREAM) study: an open‐label phase II trial. Br J Clin Pharm. 2019;85:347–55. doi: 10.1111/bcp.13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papaetis GS, Syrigos KN. Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs. 2009;23:377–89. doi: 10.2165/11318860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Graybug Vision Inc. A Phase 2b Multicenter Dose-Ranging Study Evaluating the Safety and Efficacy of Sunitinib Malate Depot Formulation (GB-102) Compared to Aflibercept in Subjects With Neovascular (Wet) Age-related Macular Degeneration (ALTISSIMO Study). ClinicalTrials.gov NCT03953079. 2022;
- 32.Graybug Vision Inc. ALTISSIMO full-data analysis 12-month treatment phase. 2021.
- 33.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the α1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–54. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 34.Khanani AM, Regillo CD, Wykoff CC, Moshfeghi A, Weng CY, Bakri SJ, et al. Sustained-release Tyrosine Kinase Inhibitors for the Treatment of nAMD. Retin Physician. 2022;19:23–25. [Google Scholar]
- 35.Cohen MN, O’Shaughnessy D, Fisher K, Cerami J, Awh CC, Salazar DE, et al. APEX: a phase II randomised clinical trial evaluating the safety and preliminary efficacy of oral X-82 to treat exudative age-related macular degeneration. Br J Ophthalmol. 2021;105:716–22. doi: 10.1136/bjophthalmol-2020-316511. [DOI] [PubMed] [Google Scholar]
- 36.Saim S, Sparks M, Paggiarino D, Karzoun B. Bioerodible Ocular Drug Delivery Insert And Therapeutic Method. United States: The U.S. Patent and Trademark Office; US 2022/0168142 A1, 2022.
- 37.Jackson TL, Boyer D, Brown DM, Chaudhry N, Elman M, Liang C, et al. Oral tyrosine kinase inhibitor for neovascular age-related macular degeneration: A phase 1 dose-escalation study. JAMA Ophthalmol. 2017;135:761–7. doi: 10.1001/jamaophthalmol.2017.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist. 2015;20:660–73. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross-Goupil M, Françlois L, Quivy A, Ravaud A. Axitinib: A review of its safety and efficacy in the treatment of adults with advanced renal cell carcinoma. Clin Med Insights Oncol. 2013;7:CMO.S10594. doi: 10.4137/CMO.S10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UNITED STATES SECURITIES AND EXCHANGE COMMISSION. Form 8-K, Current Report for EyePoint Pharmaceuticals, Inc. Washington, D.C.; 2023.
- 41.Andrew A Moshfeghi, Ocular Therapeutix Inc. Intravitreal Hydrogel-Based Axitinib Implant (OTX-TKI) for the Treatment of Neovascular AMD: A Phase 1 Trial Update. Angiogenesis, Exudation and Degeneration Symposium. 2021.
- 42.Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev. 2018;126:58–66. doi: 10.1016/j.addr.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moisseiev E, Loewenstein A, Yiu G. The suprachoroidal space: from potential space to a space with potential. Clin Ophthalmol. 2016;10:173–8. doi: 10.2147/OPTH.S89784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rai UDJP, Young SA, Thrimawithana TR, Abdelkader H, Alani AWG, Pierscionek B, et al. The suprachoroidal pathway: a new drug delivery route to the back of the eye. Drug Discov Today. 2015;20:491–5. doi: 10.1016/j.drudis.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Lampen SIR, Khurana RN, Noronha G, Brown DM, Wykoff CC. Suprachoroidal Space alterations following delivery of triamcinolone acetonide: post-hoc analysis of the phase ½ HULK study of patients with diabetic macular edema. Ophthalmic Surg Lasers Imaging Retin. 2018;49:692–7. doi: 10.3928/23258160-20180831-07. [DOI] [PubMed] [Google Scholar]
- 46.Muya L, Kansara V, Ciulla T. Pharmacokinetics and Ocular Tolerability of Suprachoroidal CLS-AX (axitinib injectable suspension) in rabbits. Invest Ophthalmol Vis Sci. 2020;61:4925. [Google Scholar]
- 47.Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci. 1989;30:233–8. [PubMed] [Google Scholar]
- 48.Thakur A, Scheinman RI, Rao VR, Kompella UB. Pazopanib, a multitargeted tyrosine kinase inhibitor, reduces diabetic retinal vascular leukostasis and leakage. Microvasc Res. 2011;82:346–50. doi: 10.1016/j.mvr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semeraro F, Cancarini A, dell’Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res. 2015;2015:1–16. doi: 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown DM, Wykoff CC, Boyer D, Heier JS, Clark WL, Emanuelli A, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy. JAMA Ophthalmol. 2021;139:946. doi: 10.1001/jamaophthalmol.2021.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Market Scope. US Retina Quarterly Update Q2 - 2022.
- 52.Downs P 2022 Retinal Pharmaceuticals Market Report: Global Analysis for 2021 to 2027, August, 2022. 2022.
- 53.Jarrett PK, et al. ARVO Annual Meeting. ARVO. 2018.
- 54.Kaiser PK, Et al. Eyecelerator. American Academy of Ophthalmology Meeting, Chicago, US. Retina Showcase. September 29, 2022.