Abstract
Background
Which phenotypes are we able to recognize in the optic nerve of patients with primary open angle glaucoma?
Methods
Retrospective interventional case series. 885 eyes from 885 patients at an outpatient tertiary care centre who met specified criteria for POAG were included. Disc photographs were classified by three glaucoma specialists into the following phenotypes according to their predominant characteristics: (1) concentric rim thinning, (2) focal rim thinning, (3) acquired pit of the optic nerve (APON), (4) tilted, (5) extensive peripapillary atrophy (PPA), and (6) broad rim thinning. Demographic, medical, and ocular data were collected. Kruskal–Wallis was used as a non-parametric test and pairwise comparison was performed by using Wilcoxon rank sum test corrected.
Results
Phenotypic distribution was as follows: 398(45%) focal thinning, 153(18%) concentric thinning, 153(17%) broad thinning, 109(12%) tilted, 47(5%) extensive PPA and 25(3%) APON. Phenotypic traits of interest included a higher proportion of female patients with the focal thinning phenotype (p = 0.015); myopia (p = 0.000), Asian race (OR: 8.8, p = 0.000), and younger age (p = 0.000) were associated with the tilted phenotype; the concentric thinning patients had thicker RNFL (p = 0.000), higher MD (p = 0.008) and lower PSD (p = 0.043) than broad thinning, despite no difference in disc sizes (p = 0.849). The focal thinning group had a localized VF pattern with high PSD compared to concentric thinning (p = 0.005).
Conclusion
We report six phenotypic classifications of POAG patients with demographic and ocular differences between phenotypes. Future refinement of phenotypes should allow enhanced identification of genetic associations and improved individualization of patient care.
Subject terms: Education, Optic nerve diseases
Introduction
The definition of primary open-angle glaucoma (POAG) has evolved over recent decades. It is now defined as a progressive optic neuropathy resulting from a complex interaction of different ocular and non-ocular risk factors, with characteristic clinical signs [1]. The occurrence of risk factors in a genetically predisposed eye can lead to the premature senescent death of retinal ganglion cells which manifests as glaucoma [2]. The typical clinical appearance of optic disc rim abnormalities and corresponding pattern of visual deficits is highly suggestive of this disease and is essential to its diagnosis.
Different presentations of optic disc damage have been defined by their clinical appearance: loss of the rim may be diffuse, broad, or focal [3]. Nicolela and Drance proposed a categorization of the optic disc appearance into four phenotypes which they termed focal ischemic, myopic, senile sclerotic, and generalized enlargement [4]. These phenotypes were associated with certain demographic, systemic and ocular conditions with specific visual field patterns and prognosis. In addition, another type of optic disc appearance was first described over 50 years ago, in which a pit-like area of ectasia was detected in the lamina cribrosa in eyes with glaucoma [5]. The clinical appearance of this abnormality has been termed “acquired pit of the optic nerve” (APON) [6–8].
The present study proposes an alternative, expanded framework for the identification of optic disc phenotypes. Our aim is to enlarge and improve prior classifications based on the description of six distinct phenotypic expressions associated with certain demographic, systemic and ocular conditions. This may help researchers discover associations between phenotypes and genetic variants, which is key to future targeted diagnoses and individualized treatment.
Material and methods
This study was approved by the Institutional Review Board of UCLA and all study procedures adhered to the recommendations of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. Records of patients from the UCLA Stein Glaucoma Division between 1997 and 2019 were reviewed. A total of 939 patients were included who had a diagnosis of POAG, stereoscopic optic disc photographs within 2 years of a 24-2 Humphrey visual field (VF) with a Mean Deviation (MD) > −10 dB and a Pattern Standard Deviation (PSD) probability <0.05, both on two successive VFs. VFs with MD worse than −10 dB were excluded to avoid eyes with advanced optic nerve damage and loss of phenotypic features. Eyes with an optic disc area <1.00 and >3.00 mm2 as measured by Cirrus Optical Coherence Tomography (OCT) images were excluded to avoid size extremes affecting phenotypes in this study. One eye per patient was included; if fellow eyes met the inclusion criteria, one eye was chosen randomly for inclusion.
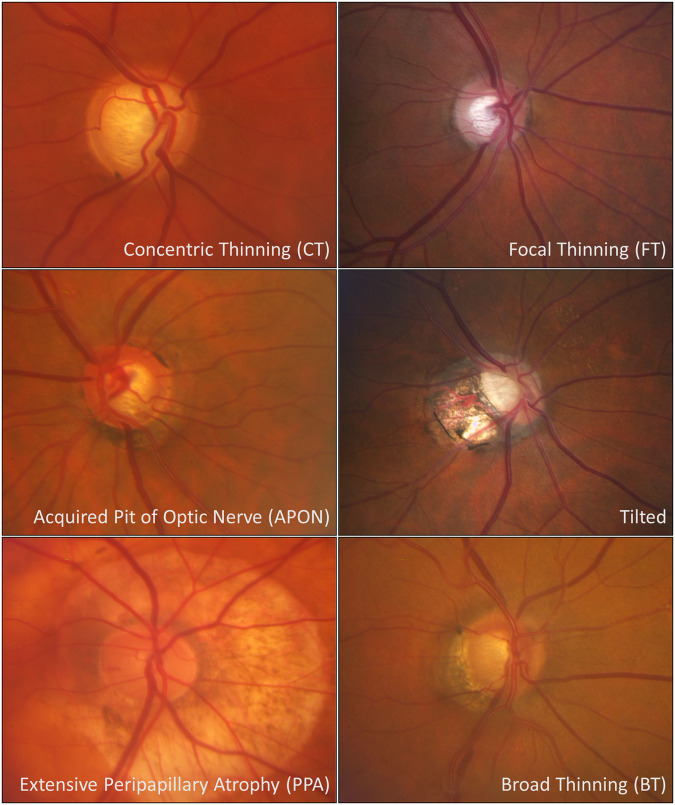
The photos were classified independently by three glaucoma specialists. Prior to grading the images, a standard reference photograph for each was agreed upon by the three graders (Fig. 1). The six phenotypes were defined as follows: concentric thinning was defined as a uniform rim thinning. Focal thinning was described as a disc with inferior or superior notching. APON was defined as a deep, localized excavation of the neural rim with sharply localized depression; the affected area is pale, and little or no laminar tissue remains in its depth [7]. The tilted phenotype was described as vertically tilted which may or may not be associated with peripapillary atrophy (PPA). The extensive PPA phenotype is characterized by a disc with shallow diffuse excavation and 360° of PPA around the disc; PPA is defined by a loss and irregular disruption of the retinal pigment epithelium and of the choriocapillaris in the area surrounding the optic disc [9]. Finally, broad thinning presents with a total loss of disc rim, which is more extensive than a notch, but extends for <180° of the optic disc circumference.
Fig. 1.
Standard reference photos for phenotypic subgroups.
All color photographs were taken with a Zeiss FF 450 plus IR camera after pupil dilation. A web-based interface was created to perform the grading, with one image from each of the 939 identified patients included, and phenotypes for the graders to choose from: (1) concentric rim thinning, (2) focal rim thinning, (3) APON, (4) tilted (with or without PPA), (5) extensive PPA, and (6) broad rim thinning. In addition, there were options for “not glaucoma” if another non-glaucomatous optic neuropathy appeared to exist or co-exist with POAG, and “poor quality” for those images which were not sufficiently clear to grade. All other eyes were categorized into one of the six phenotypes; if an eye appeared to have characteristics of more than one phenotype, it was assigned to the one phenotype that appeared to dominate its appearance. Agreement was defined by the coincident choice for two of the three graders. Instances of disagreement between all three graders were reviewed in a joint meeting to reach a consensus. Images that were classified as “poor quality” or “not glaucoma” by at least two of the three graders were excluded from the analysis.
Data were collected by review of medical records and included demographic, medical, and ocular information collected based on the visit closest to the date of the graded photograph. The variables included: age, race/ethnicity, gender, family history of glaucoma, smoking status, hypertension, cardiovascular disease, diabetes, glaucoma type, visual acuity, IOP, refractive error, number of medications, central corneal thickness (CCT), visual field MD and PSD, disc area and RNFL thickness.
Visual acuity is reported as the logarithm of the minimal angle of resolution (logMAR) [10]. Refractive error was collected as the spherical equivalent of the manifest refraction. If the patient was pseudophakic, the oldest available refraction prior to cataract surgery was recorded. For IOP, five consecutive values for each patient were recorded from five visits closest to the date of the selected photo; the first two were taken from the previous visits, the third from the date of the photo, and the last two from the following visits. Mean IOP was calculated as the mean of these five readings. The highest measured IOP (peak IOP) from all readings were recorded for each eye. Medications were recorded as those used at the time of the photograph. CCT was recorded in microns.
Cardiovascular disease was identified from the medical records according to the ICD-10 codes for the following conditions: hypertension, acute myocardial infarction, aortic aneurysm, angina pectoris, atrial fibrillation, cardiomyopathy, coronary artery disease, dissection of aorta, heart failure, heart transplant status, hypertensive heart disease, paroxysmal tachycardia, and sick sinus syndrome.
All visual fields were obtained with the Humphrey Field Analyzer 630 (Program 30-2 or 24-2, SITA Standard algorithm; Humphrey Instruments Inc., San Leandro, CA). An acceptably reliable visual field defect was defined as one with <30% false-positive responses. Mean MD and PSD were calculated from three consecutive measurements for each eye; the first one from the date of the photo, the second from the previous visit and the third, from the visit after the date of the photo.
Statistical analyses were performed with the open-source programming language R, version 4.1.1 (R Project for Statistical Computing) [11]. The Shapiro–Wilk test was used to test the normality of data. For univariate analysis, continuous data were analyzed using a non-parametric method such as Kruskal–Wallis test (KW), and the median and interquartile ranges (IQL) are reported in every variable per phenotype. Pearson’s Chi-Squared test was used to compare categorical data, shown as frequencies (n = ) and percentages (%). Probability values of <0.05 were considered statistically significant. Because of the exploratory nature of this study, we used Wilcoxon rank sum test for pairwise comparisons adjusted with Bonferroni correction.
Multinomial logistic regression analysis was used to develop the best model to describe the relationship between the dependent variables (covariates) and the independent variable (phenotype) [12], and are reported as odds ratios (OR) with 95% Confidence Intervals (CIs).
Results
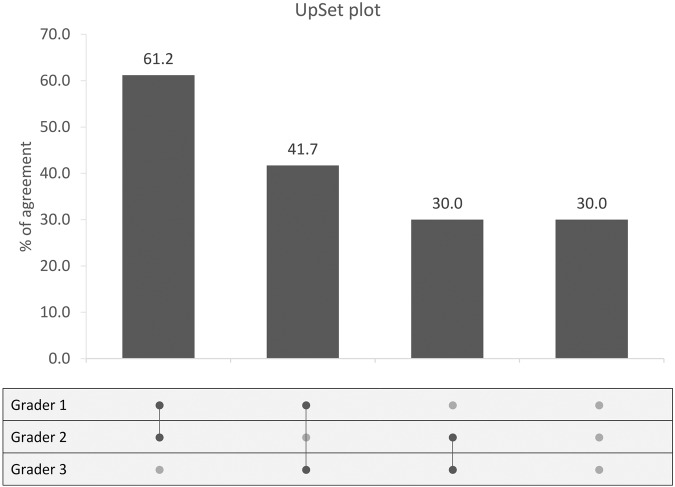
Of the 939 eyes originally identified for the study, 54 were excluded for “poor-quality” (28 eyes) or “not glaucoma” (26 eyes). The assigned phenotypes for the remaining 885 images were: 398 focal thinning (45%), 153 concentric thinning (18%), 153 broad thinning (17%), 109 tilted (12%), 47 extensive PPA (5%) and 25 APON (3%). Inter-grader agreement for disc photographs is shown in an UpSet plot [13] in Fig. 2. For 101 images (30%), there was no initial agreement between the graders; these were reviewed in a joint meeting to reach a consensus about phenotypic assignment.
Fig. 2. UpSet plot for inter-grader agreement.
The matrix (bottom) shows correlation between graders. For inter-grader agreement, a dark gray dot is displayed connected by a solid line. The vertical histogram represents the proportion of agreement between the glaucoma specialists. The first bar shows agreement between grader 1 and grader 2. The second bar shows agreement between grader 1 and grader 3. The third bar shows agreement between grader 2 and grader 3. The last bar shows the images with no agreement.
The summary statistics for the different phenotypes and the corresponding p values are shown in Tables 1 and 2. The Shapiro–Wilk test indicated that our data were not generally normally distributed, therefore, we applied KW to all variables studied. The covariates that were found to be statistically significant by KW and Pearson’s Chi-Squared analyses among the groups were as follows: gender, race, age, median IOP, peak IOP, visual acuity in logMAR notation, CCT, refraction, MD, PSD, disc area, and RNFL. Pairwise comparisons with the Wilcoxon rank sum test are shown in Supplementary Table 1. The median age was lowest in the tilted phenotypic group and highest in the extensive PPA group (p < 0.000). In the broad thinning and focal thinning phenotypes, female gender was more common compared to concentric thinning (p = 0.022 and p = 0.008, respectively), which favored male gender. The tilted phenotype was also more highly associated with myopia, compared with concentric thinning (p < 0.000), focal thinning (p < 0.000), APON (p = 0.011) and broad thinning (p < 0.000). The concentric thinning group had the highest median IOP (13.7 mmHg, IQR 4.6) and APON had the lowest IOP (12.3 mmHg, IQR 4.9; KW p = 0.035), with no statistically significant difference in number of medications between the groups (p = 0.133).
Table 1.
Summary statistics.
| All | Gender | Race | HTa | DMa | Cardioa | Family Historya | Smokinga | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = % | Male (%) | Female (%) | Caucasian (%) | Asian (%) | African Descent (%) | Hispanic (%) | Other (%) | Yes (%) | No (%) | Yes (%) | No (%) | Yes (%) | No (%) | Yes (%) | No (%) | Yes (%) | No (%) | |
| All | 885 (100) | 370 (41.8) | 515 (58.2) | 508 (57.4) | 146 (16.5) | 84 (9.5) | 38 (4.3) | 109 (12.3) | 440 (49.7) | 431 (48.7) | 122 (13.8) | 749 (84.6) | 175 (19.8) | 696 (78.6) | 350 (39.5) | 342 (58.8) | 282 (31.9) | 562 (66.6) |
| Concentric Thinning | 153 (18) | 83 (54.2) | 70 (45.8) | 87 (56.9) | 14 (9.2) | 18 (11.8) | 10 (6.5) | 24 (15.7) | 85 (55.6) | 65 (42.5) | 32 (20.9) | 118 (77.1) | 36 (23.5) | 114 (74.5) | 52 (34.0) | 59 (64.1) | 57 (37.3) | 88 (60.8) |
| Focal Thinning | 398 (45) | 151 (37.9) | 247 (62.1) | 238 (59.8) | 56 (14.1) | 38 (9.5) | 16 (4.0) | 50 (12.6) | 185 (46.5) | 206 (51.8) | 45 (11.3) | 346 (86.9) | 80 (20.1) | 311 (78.1) | 164 (41.2) | 147 (57) | 121 (30.4) | 262 (67.8) |
| APON | 25 (3) | 10 (40.0) | 15 (60.0) | 17 (68.0) | 3 (12.0) | 3 (12.0) | 0 (0.0) | 2 (8.0) | 14 (56.0) | 11 (44.0) | 3 (12.0) | 22 (88.0) | 5 (20.0) | 20 (80.0) | 9 (36.0) | 11 (64.0) | 6 (24.0) | 16 (76.0) |
| Tilted | 109 (12) | 47 (43.1) | 62 (56.9) | 43 (39.4) | 46 (42.2) | 7 (6.4) | 3 (2.8) | 10 (9.2) | 57 (52.3) | 52 (47.7) | 18 (16.5) | 91 (83.5) | 13 (11.9) | 96 (88.1) | 45 (41.3) | 50 (58.7) | 32 (29.4) | 75 (70.6) |
| Extensive PPA | 47 (5) | 22 (46.8) | 25 (53.2) | 33 (70.2) | 4 (8.5) | 2 (4.3) | 2 (4.3) | 6 (12.7) | 21 (44.7) | 26 (55.3) | 6 (12.8) | 41 (87.2) | 14 (29.8) | 33 (70.2) | 18 (38.3) | 20 (61.7) | 20 (42.6) | 25 (57.5) |
| Broad Thinning | 153 (17) | 57 (37.3) | 96 (62.7) | 90 (58.8) | 23 (15.0) | 16 (10.5) | 7 (4.6) | 17 (11.2) | 78 (51.0) | 71 (46.4) | 18 (11.8) | 131 (85.6) | 27 (17.6) | 122 (79.7) | 62 (40.5) | 55 (56.8) | 46 (30.1) | 96 (67.3) |
| P value | 0.015 | 0.000 | 0.398 | 0.079 | 0.105 | 0.523 | 0.089 | |||||||||||
Categorical variables. Statistically significant variables (p < 0.05) based on Pearson’s Chi-Squared test are highlighted.
HT Hypertension, DM Diabetes Mellitus, Cardio Cardiovascular disease.
aPatients for whom this data was not available are excluded from this portion of the analysis.
Table 2.
Summary Statistics.
| All | Age (yrs) | IOP (mmHg) | Peak IOP | Visual acuity (LogMAR) | CCT (µm) | Refraction (dp) | Medications | MD (dB) | PSD (dB) | Disc Area (mm2) | RNFL (µm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = | Median (IQR) | Median (IQR) | (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| All | 885 (100) | 71.0 (15.0) | 13.2 (4.4) | 15.0 (5.0) | 0.1 (0.2) | 547.0 (51.5) | −1.0 (2.7) | 1.0 (1.0) | −4.4 (3.1) | 5.3 (2.8) | 1.8 (0.5) | 67.0 (17.0) |
| Concentric Thinning | 153 (18) | 72.0 (15.0) | 13.7 (4.6) | 16.0 (6.0) | 0.1 (0.2) | 549.0 (46.5) | −0.7 (1.9) | 1.0 (1.0) | −4.0 (2.8) | 4.8 (2.0) | 1.8 (0.5) | 71.0 (18.0) |
| Focal Thinning | 398 (45) | 72.0 (15.0) | 13.4 (4.2) | 15.0 (5.0) | 0.1 (0.2) | 547.0 (50.0) | −1.0 (2.2) | 2.0 (1.0) | −4.3 (3.0) | 5.4 (2.9) | 1.7 (0.4) | 67.0 (17.1) |
| APON | 25 (3) | 68.0 (8.0) | 12.3 (4.9) | 14.0 (6.0) | 0.0 (0.1) | 557.0 (58.0) | −1.2 (2.2) | 1.0 (1.0) | −3.8 (2.4) | 6.8 (3.9) | 1.9 (0.3) | 66.0 (10.0) |
| Tilted | 109 (12) | 66.0 (13.0) | 12.6 (3.8) | 14.0 (4.0) | 0.1 (0.1) | 554.5 (42.0) | −3.6 (4.6) | 1.0 (1.0) | −4.5 (2.9) | 5.7 (3.1) | 1.6 (0.5) | 69.0 (15.0) |
| Extensive PPA | 47 (5) | 74.0 (15.0) | 13.6 (5.6) | 15.0 (8.0) | 0.1 (0.2) | 545.0 (70.0) | −1.5 (3.2) | 1.0 (1.0) | −5.4 (3.2) | 5.1 (2.2) | 1.6 (0.5) | 68.0 (16.5) |
| Broad Thinning | 153 (17) | 72.0 (13.0) | 12.4 (4.0) | 14.0 (5.0) | 0.1 (0.3) | 534.0 (53.2) | −1.5 (2.3) | 1.0 (2.0) | −5.1 (2.8) | 5.3 (2.8) | 1.8 (0.5) | 62.8 (12.0) |
| P value | 0.000 | 0.035 | 0.022 | 0.003 | 0.014 | 0.000 | 0.133 | 0.001 | 0.001 | 0.000 | 0.000 |
Continuous variables. Statistically significant variables (p < 0.05) based on the Kruskal–Wallis test are highlighted.
IOP Intraocular Pressure, LogMAR Logarithm of the Minimal Angle of Resolution, CCT Central Corneal Thickness, MD Mean Deviation, PSD Pattern Standard Deviation, RNFL Retinal Nerve Fiber Layer.
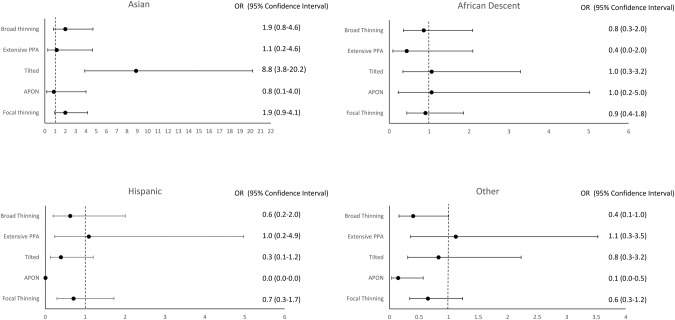
For OCT-based measurements, RNFL was thinner in eyes with broad thinning than in eyes with concentric thinning (p < 0.000), focal thinning (p < 0.000), or tilted phenotype (p < 0.000). The disc area was smaller in tilted discs compared with the concentric thinning and broad thinning (p = 0.002 and p = 0.010, respectively) phenotypes. Visual field median PSD was higher in focal thinning and tilted phenotypes, compared with concentric thinning (p = 0.043 and 0.011, respectively); while median MD did not vary between these three phenotypes (p = 0.793 and 0.519, respectively). Between the concentric thinning and broad thinning phenotypes, broad thinning had a thinner median RNFL (p < 0.000), lower MD (p = 0.008) and higher PSD (p = 0.043), with no difference in disc area (p = 0.849) compared to the concentric thinning phenotype. In the multinomial logistic regression analysis, the odds ratio of having the tilted phenotype was 8.8 times higher in Asians (reference Caucasian (95% CI: 3.9–20.2)) than in eyes with concentric thinning (Fig. 3).
Fig. 3.
Forest plot showing the association between different races and phenotypes, with Caucasian and concentric thinning as references. Odds ratio and confidence interval are listed next to each plot.
Discussion
In this study, we propose a categorization of optic disc phenotypes in patients with POAG. We have applied this classification to a sample of 885 optic disc stereo photographs and found significant associations between demographic and ocular characteristics and certain phenotypes. Eyes with advanced damage or with extremes of disc size (large or small) were excluded. Our results showed that (1) the focal thinning phenotype was more frequently observed in female patients; (2) the tilted phenotype was highly associated with Asian race, myopia, and younger age; (3) the extensive PPA phenotype was associated with older age; (4) the broad thinning phenotype had a thinner median RNFL, lower MD, and higher PSD compared to the concentric thinning phenotype, despite no difference in disc size; and (5) the focal thinning phenotype showed a localized VF pattern reflected by a high PSD, compared to the concentric thinning phenotype.
Previous studies have investigated relationships between different optic disc appearances and demographic and ocular characteristics [4, 14–16]. A retrospective study of patients with APON showed that 92% (34/37) of patients were female, and median age at diagnosis of POAG was 55.1 years (range, 19.9–79.3). Spaeth also reported that female gender was predominant in this type of disc presentation: women are approximately three times more likely to be affected than men [16], a finding which is confirmed in this study.
We found a difference in median age between the tilted disc (66 years) and extensive PPA (74 years). It has been proposed that extensive PPA is mainly due to a chronic vascular insufficiency of the optic disc blood supply [17] while aging plays an important role in the structural stiffness of the sclera and the lamina cribrosa [18, 19]. Other genetic factors related with race and myopia may be important indicators of laminar remodelling in the tilted phenotype [20].
There is a well-studied relationship between glaucoma and myopia. The Blue Mountain Eye Study showed that glaucoma was almost three times as frequent in eyes with moderate-to-high myopia (4.4%) as it was in eyes without myopia (1.5%) [21]. The clinical diagnosis of optic nerve damage in highly myopic eyes is sometimes hampered by myopia-related changes in the appearance of the optic nerve head [15]. We found a strong association between the tilted phenotype and myopia. The longer axial length and thinner sclera in myopic eyes may cause the optic nerve to be more vulnerable to glaucomatous damage [22]. Asian race, a population known to have a higher prevalence of myopia, [23] was associated with the tilted phenotype.
Past studies investigated the relationship between phenotype and level of intraocular pressure [16, 24, 25]. In our report, even though the differences in median IOP between the six phenotypes were relatively small, these trends may be important in informing clinical decision-making. Moreover, our institution is a referral centre, and most of our patients present already on IOP-lowering treatment.
Variations in characteristic patterns of visual field loss between normal- and high- tension glaucoma have also been studied [26–29]. In 1984, Caprioli and Spaeth reported that scotomas were significantly deeper and closer to fixation in the low-tension glaucoma group than in the high-tension glaucoma groups [30]. We report here that focal thinning, APON, and broad thinning phenotypes have a higher median PSD and low median IOP values, compared with the concentric thinning phenotype. This suggests that the localized VF pattern is typical of these types of optic discs. These results have also been reported by others, who found correlations between different optic disc phenotypes and the VF [31]. The APON phenotype is a particular example of focal damage, in which fast rates of VF decay were identified [32].
OCT measurements of the optic disc size, the neuroretinal rim, and RNFL are important and well-studied tools for glaucoma diagnosis [33–38], and it is well known that glaucomatous eyes have a thinner RNFL [39–41]. In our study, we included optic discs with an area between 1 mm2 and 3 mm2, to avoid the difficulty of misclassification of very small or very large discs. In our study, the broad thinning phenotype had a thinner median RNFL and lower MD, while the concentric thinning phenotype had a thicker median RNFL and higher MD, though both had the same median disc area (1.8 mm2). This could be an important consideration for treatment management among these groups. For example, clinicians treating patients with the phenotypes of broad thinning and focal thinning may aim for a lower IOP with closer follow-up, since these patients have more central perimetric damage and lower retinal ganglion cell thicknesses.
In our framework, we do not include the OCT Bruch’s membrane opening minimum rim width (BMO-MRW) as a variable for distinguishing the rim size within the different optic disc phenotypes. Although the BMO-MRW is a reliable parameter to measure the neuroretinal rim width [42–45], the purpose of our study was identifying different phenotypes based on the observable traits of the optic discs. These traits are not dependent on small differences in the definition of the disc edge. For instance, in phenotypes in which PPA exists, such as the ones in the extensive PPA phenotype or some of the tilted optic disc phenotype, the BMO-MRW would not be helpful, since we use features other than the rim measurement. Similarly, if defining the phenotypes based on BMO-MRW, it would be difficult to differentiate the APON phenotype from the focal thinning phenotype since the actual pit is something visualized in the optic disc photo. Future studies might include the BMO-MRW as a measurement complementary to RNFL thickness.
Primary open-angle glaucoma is a heterogeneous disease and is likely caused by the interaction of many genetic and post-genetic factors. More targeted, individualized treatment based on different clinical phenotypes could provide better outcomes for patients. The APON phenotype serves as an appropriate example. It has been shown that the finding of an APON is associated with low blood pressure, migraines, and Raynaud’s phenomena [30], traits which have been attributed to vascular instability [46, 47]. Topical beta blockers exacerbate vasospasm in susceptible individuals [48], so in this low-IOP phenotype, extremely low IOPs have been targeted [49–51] with the avoidance of topical beta blockers [52–54] to achieve slower rates of progression [7]. The broad thinning phenotype is another example of a low IOP phenotype, for which one may set a low IOP treatment target. The concentric thinning phenotype is associated with higher IOPs compared to other phenotypes. This may be explained by the different pathogenesis of glaucoma in this group; IOP causes stress and strain on the components of the lamina cribrosa and probably plays an important pathogenetic role [55, 56]. In this group, OCT showed a thicker RNFL, and VF showed a lower PSD and higher MD, which indicate more diffuse rather than highly localized VF loss. The IOP targeted for this group may not be as low as for other phenotypes. The tilted phenotype is more commonly seen in myopic, young Asians; the optic disc rims of these patients should be carefully examined for signs of glaucomatous progressive thinning, since the presence of a tilted disc can make detection and diagnosis difficult. This is particularly important considering the younger average age in this group.
POAG is associated with different manifest patterns of optic nerve head damage during the progression of the disease. POAG has been studied with genome-wide associations (GWAS), where a multitude (>120 thus far) of different genetic variants associated with POAG among multi-ethnic populations have been identified [20, 57–61]. The challenge of making the appropriate inferences regarding pathogenesis with diverse and numerous genetic findings in a heterogeneous disease may lie chiefly in the lack of the identification of relatively pure phenotypic subtypes. Ultimately, our goal is the development of a sufficiently large database of glaucoma patients which will help to develop a convolutional neural network (CNN) or other machine learning approaches capable of differentiating between phenotypes based on optic disc characteristics and other cross-sectional data.
The results of this study should be interpreted with caution: an inherent flaw of a retrospective study design is the lack of complete and standardized data. Another limitation is that the phenotypic classifications proposed were based on subjective observations of patients at a referral institution, and the findings may not be generalizable to the population at large. VFs with MD worse than −10 dB were excluded since the extensive loss of neural tissue makes it difficult to differentiate the appearance of the optic nerve into different phenotypes. The ability to detect statistically significant associations with the covariates is limited because of the lack of statistical power due to the small number of patients in some of the phenotypic groups, particularly for APON and extensive PPA.
This study reports six phenotypic classifications of POAG patients, with certain demographic, ocular and systemic associations. The focal thinning phenotype was more frequently observed in female patients and was associated with higher VF median PSD. The tilted phenotype was associated with Asian race, myopia, and younger age. Extensive PPA was associated with older age. The broad thinning phenotype had a thinner median RNFL, while the concentric thinning eyes had a thicker RNFL. Even in an era of emerging technologies and increased access to a variety of devices to aid our diagnostic ability, we believe medical semiology remains an important tool for glaucoma specialists. Further refinement and elucidation of phenotypes should allow improved individualization of patient care and enhance the ability to understand an increasingly complex set of genetic associations with POAG.
Summary
What was known before
Nicolela and Drance identified four different phenotypic expressions of the optic disc in primary open angle glaucoma.
Other investigators identified an additional phenotype with a pit-like area of ectasia in the lamina cribrosa called APON (acquired pit of the optic nerve).
What this study adds
This study expands and improves phenotypic categorization of the optic disc in primary open angle glaucoma.
The authors show demographic, ocular and systemic associations with different phenotypes.
This will help future targeted diagnosis and individualized treatment.
Supplementary information
Acknowledgements
The authors thank Fei Yu Ph.D., for his expertize and assistance with statistical analyses.
Author contributions
All authors contributed to this study. Conception and design: JC, LG, ADG, DSV; Data collection: LG, EB; Formal analysis and interpretation of data: JC, LG, EM; Methodology: JC, LG, ADG, DSV; Project administration: JC, LG, EB; Supervision: JC, EB; Validation: JC; Writing paper: JC, LG, ADG, EB; Writing—review & editing: JC, LG, ADG, EB, SD, EM.
Funding
Research to Prevent Blindness, New York, NY; The Payden Glaucoma Research Fund; The Simms/Mann Family Foundation. The funding organizations had no role in the design or conduct of this research.
Data availability
All data are available from the corresponding author upon a reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02627-4.
References
- 1.Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Investig Ophthalmol Vis Sci. 1997;38:83–91. [PubMed] [Google Scholar]
- 2.Weinreb RN, Tee Khaw P. Primary open-angle glaucoma. Lancet (Lond, Engl) 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 3.Caprioli, J.: Clinical evaluation of the optic nerve in glaucoma. Tr. Am Ophth. Soc. 1994;92:589–641. [PMC free article] [PubMed]
- 4.Nicolela MT, Drance SM. Various glaucomatous optic nerve appearances: Clinical correlations. Ophthalmology. 1996;103:640–9. doi: 10.1016/S0161-6420(96)30640-4. [DOI] [PubMed] [Google Scholar]
- 5.Radius RL, Maumenee AE, Green WR. Pit-like changes of the optic nerve head in open-angle glaucoma. Br J Ophthalmol. 1978;62:389–93. doi: 10.1136/BJO.62.6.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javitt JC, Spaeth GL, Katz LJ, Poryzees E, Addiego R. Acquired Pits of the Optic Nerve: Increased Prevalence in Patients with Low-tension Glaucoma. Ophthalmology. 1990;97:1038–44. doi: 10.1016/S0161-6420(90)32466-1. [DOI] [PubMed] [Google Scholar]
- 7.Ugurlu S, Weitzman M, Nduaguba C, Caprioli J. Acquired pit of the optic nerve: a risk factor for progression of glaucoma. Am J Ophthalmol. 1998;125:457–64. doi: 10.1016/S0002-9394(99)80185-8. [DOI] [PubMed] [Google Scholar]
- 8.Nduaguba C, Ugurlu S, Caprioli J. Acquired pits of the optic nerve in glaucoma: Prevalence and associated visual field loss. Acta Ophthalmol Scand. 1998;76:273–7. doi: 10.1034/J.1600-0420.1998.760304.X. [DOI] [PubMed] [Google Scholar]
- 9.Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989;30:908–18. [PubMed]
- 10.Dandona L, Dandona R. What is the global burden of visual impairment? BMC Med. 2006;4. 10.1186/1741-7015-4-6 [DOI] [PMC free article] [PubMed]
- 11.R: The R Project for Statistical Computing. Accessed June 1, 2022. https://www.r-project.org/
- 12.Abdillah A, Sutisna A, Tarjiah I, Fitria D, Widiyarto T. Application of Multinomial Logistic Regression to analyze learning difficulties in statistics courses. In: Journal of Physics: Conference Series. 1490. Institute of Physics Publishing; 2020. 10.1088/1742-6596/1490/1/012012
- 13.Lex A, Gehlenborg N, Strobelt H, Vuillemot R, Pfister H. UpSet: Visualization of intersecting sets. IEEE Trans Vis Comput Graph. 2014;20:1983–92. doi: 10.1109/TVCG.2014.2346248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broadway DC, Nicolela MT, Drance SM. Optic disk appearances in primary open-angle glaucoma. Survey Ophthalmol. 1999;43. 10.1016/S0039-6257(99)00007-7 [DOI] [PubMed]
- 15.Tan NYQ, Sng CCA, Jonas JB, Wong TY, Jansonius NM, Ang M. Glaucoma in myopia: diagnostic dilemmas. Br J Ophthalmol. 2019;103:1347–55. doi: 10.1136/BJOPHTHALMOL-2018-313530. [DOI] [PubMed] [Google Scholar]
- 16.Spaeth GL. A new classification of glaucoma including focal glaucoma. Survey Ophthalmol. 1994;38. 10.1016/0039-6257(94)90042-6 [DOI] [PubMed]
- 17.Geijssen HC, Greve EL. The spectrum of primary open angle glaucoma I: Senile sclerotic glaucoma versus high tension glaucoma. Ophthalmic Surg. 1987;18:207–13. doi: 10.3928/1542-8877-19870301-11. [DOI] [PubMed] [Google Scholar]
- 18.Downs JC, Girkin CA. Lamina Cribrosa in Glaucoma. 10.1097/ICU.0000000000000354 [DOI] [PMC free article] [PubMed]
- 19.Albon J, Purslow PP, Karwatowski WSS, Easty DL. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;84:318–23. doi: 10.1136/BJO.84.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zukerman R, Harris A, Vercellin AV, Siesky B, Pasquale LR, Ciulla TA. Molecular Genetics of Glaucoma: Subtype and Ethnicity Considerations. Genes. 2020;12:1–36. doi: 10.3390/GENES12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–5. doi: 10.1016/S0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 22.Jonas JB, Wang YX, Dong L, Panda-Jonas S. High Myopia and Glaucoma-Like Optic Neuropathy. Asia-Pac J Ophthalmol (Phila, Pa) 2020;9:234–8. doi: 10.1097/APO.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yo C. Asian Americans: myopia and refractive surgery. Int Ophthalmol Clin. 2003;43:173–87. doi: 10.1097/00004397-200343040-00015. [DOI] [PubMed] [Google Scholar]
- 24.Geijssen HC, Greve EL. Focal ischaemic normal pressure glaucoma versus high pressure glaucoma. Doc Ophthalmol Adv Ophthalmol. 1990;75:291–302. doi: 10.1007/BF00164843. [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa T, Fuse N, Omodaka K, Aizawa N, Kuwahara S, Nishida K. Different types of optic disc shape in patients with advanced open-angle glaucoma. Jpn J Ophthalmol. 2010;54:291–5. doi: 10.1007/S10384-010-0816-Y. [DOI] [PubMed] [Google Scholar]
- 26.Park IK, Kim KW, Moon NJ, Shin JH, Chun YS. Comparison of superior and inferior visual field asymmetry between normal-tension and high-tension glaucoma. J Glaucoma. 2021;30:648–55. doi: 10.1097/IJG.0000000000001872. [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Yoo C, Park J, Kim YY. Visual Field Defects in Young Patients With Open-angle Glaucoma: Comparison Between High-tension and Normal-tension Glaucoma. J Glaucoma. 2017;26:541–7. doi: 10.1097/IJG.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 28.Ganeshrao SB, Senthil S, Choudhari N, Durga SS, Garudadri CS. Comparison of visual field progression rates among the high tension glaucoma, primary angle closure glaucoma, and normal tension glaucoma. Investig Ophthalmol Vis Sci. 2019;60:889–900. doi: 10.1167/iovs.18-25421. [DOI] [PubMed] [Google Scholar]
- 29.Häntzschel J, Terai N, Sorgenfrei F, Haustein M, Pillunat K, Pillunat LE. Morphological and functional differences between normal-tension and high-tension glaucoma. Acta Ophthalmol. 2013;91. 10.1111/aos.12061 [DOI] [PubMed]
- 30.Caprioli J, Spaeth GL. Comparison of visual field defects in the low-tension glaucomas with those in the high-tension glaucomas. Am J Ophthalmol. 1984;97:730–7. doi: 10.1016/0002-9394(84)90505-1. [DOI] [PubMed] [Google Scholar]
- 31.Ekici E, Moghimi S, Hou H, Proudfoot J, Zangwill LM, Do JL, et al. Central visual field defects in patients with distinct glaucomatous optic disc phenotypes. Am J Ophthalmol. 2021;223:229–40. 10.1016/j.ajo.2020.10.015 [DOI] [PMC free article] [PubMed]
- 32.Mahmoudinezhad G, Lin M, Rabiolo A, Morales E, Hirunpatravong P, Sharifipour F, et al. Rate of visual field decay in glaucomatous eyes with acquired pits of the optic nerve. Br J Ophthalmol. 2021;105:381–6. 10.1136/bjophthalmol-2020-315980 [DOI] [PubMed]
- 33.Moghimi S, Hosseini H, Riddle J, et al. Measurement of optic disc size and rim area with spectral-domain OCT and scanning laser ophthalmoscopy. Investig Ophthalmol Vis Sci. 2012;53:4519–30. doi: 10.1167/iovs.11-8362. [DOI] [PubMed] [Google Scholar]
- 34.Caprioli J, Miller JM. Optic disc rim area is related to disc size in normal subjects. Arch Ophthalmol. 1987;105:1683–5. 10.1001/archopht.1987.01060120081030 [DOI] [PubMed]
- 35.Schuman JS, Hee MR, Puliafito CA, Wong C, Pedut-Kloizman T, Lin CP, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–96. 10.1001/archopht.1995.01100050054031 [DOI] [PubMed]
- 36.Swaminathan SS, Jammal AA, Berchuck SI, Medeiros FA. Rapid initial OCT RNFL thinning is predictive of faster visual field loss during extended follow-up in glaucoma. Am J Ophthalmol. 2021;229:100. doi: 10.1016/J.AJO.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miki A, Medeiros FA, Weinreb RN, Jain S, He F, Sharpsten L, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121:1350–8. 10.1016/j.ophtha.2014.01.017 [DOI] [PMC free article] [PubMed]
- 38.Sung KR, Kim S, Lee Y, Yun SC, Na JH. Retinal nerve fiber layer normative classification by optical coherence tomography for prediction of future visual field loss. Investig Ophthalmol Vis Sci. 2011;52:2634–9. doi: 10.1167/IOVS.10-6246. [DOI] [PubMed] [Google Scholar]
- 39.Sehi M, Zhang X, Greenfield DS, Chung Y, Wollstein G, Francis BA, et al; Advanced Imaging for Glaucoma Study Group. Retinal nerve fiber layer atrophy is associated with visual field loss over time in glaucoma suspect and glaucomatous eyes. Am J Ophthalmol. 2013;155:73–82.e1. 10.1016/j.ajo.2012.07.005 [DOI] [PMC free article] [PubMed]
- 40.David RCC, Moghimi S, Ekici E, Do JL, Hou H, Proudfoot JA, et al. Rates of retinal nerve fiber layer thinning in distinct glaucomatous optic disc phenotypes in early glaucoma. Am J Ophthalmol. 2021;229:8–17. 10.1016/j.ajo.2021.04.010 [DOI] [PMC free article] [PubMed]
- 41.Lalezary M, Medeiros FA, Weinreb RN, Bowd C, Sample PA, Tavares IM, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol. 2006;142:576–82. 10.1016/j.ajo.2006.05.004 [DOI] [PubMed]
- 42.Enders P, Adler W, Schaub F, Hermann MM, Dietlein T, Cursiefen C, et al. Novel Bruch’s membrane opening minimum rim area equalizes disc size dependency and offers high diagnostic power for glaucoma. Invest Ophthalmol Vis Sci. 2016;57:6596–603. 10.1167/iovs.16-20561 [DOI] [PubMed]
- 43.Park DY, Lee EJ, Han JC, Kee C. Applicability of ISNT Rule Using BMO-MRW to Differentiate Between Healthy and Glaucomatous Eyes. J Glaucoma. 2018;27:610–6. doi: 10.1097/IJG.0000000000000970. [DOI] [PubMed] [Google Scholar]
- 44.Kabbara SW, Zangwill LM, Mundae R, Hammel N, Bowd C, Medeiros FA, et al. Comparing optical coherence tomography radial and cube scan patterns for measuring Bruch’s membrane opening minimum rim width (BMO-MRW) in glaucoma and healthy eyes: cross-sectional and longitudinal analysis. Br J Ophthalmol. 2018;102:344–51. 10.1136/bjophthalmol-2016-310111 [DOI] [PubMed]
- 45.Gmeiner JMD, Schrems WA, Mardin CY, Laemmer R, Kruse FE, Schrems-Hoesl LM. Comparison of Bruch’s Membrane Opening Minimum Rim Width and Peripapillary Retinal Nerve Fiber Layer Thickness in Early Glaucoma Assessment. Investig Ophthalmol Vis Sci. 2016;57:OCT575–OCT584. doi: 10.1167/IOVS.15-18906. [DOI] [PubMed] [Google Scholar]
- 46.Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013;4:14. doi: 10.1186/1878-5085-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. 10.1097/ICU.0b013e32831eef82 [DOI] [PMC free article] [PubMed]
- 48.Graf-Grauwiller T, Stiimpfig D, Flammer J, Words K. Original Paper • Travail original Originalarbeit Do Beta-Blockers Cause Vasospasm? Beta-blockers Timolol Betaxolol Laser-Doppler velocimetry. Ophthalmologica. 1993;206:45–50. doi: 10.1159/000310362. [DOI] [PubMed] [Google Scholar]
- 49.Anderson DR, Drance SM, Schulzer M; Collaborative Normal-Tension Glaucoma Study Group. Natural history of normal-tension glaucoma. Ophthalmology. 2001;108:247–53. 10.1016/s0161-6420(00)00518-2 [DOI] [PubMed]
- 50.Jonas JB, Papastathopoulos KI. Pressure-dependent changes of the optic disk in primary open-angle glaucoma. Am J Ophthalmol. 1995;119:313–7. doi: 10.1016/S0002-9394(14)71173-0. [DOI] [PubMed] [Google Scholar]
- 51.Anderson DR, Drance SM, Schulzer M. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/S0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 52.Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the low-pressure glaucoma treatment study. Am J Ophthalmol. 2011;151:671–81. doi: 10.1016/J.AJO.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 53.Hayreh SS, Podhajsky P, Zimmerman MB. Beta-blocker eyedrops and nocturnal arterial hypotension. Am J Ophthalmol. 1999;128:301–9. doi: 10.1016/S0002-9394(99)00160-9. [DOI] [PubMed] [Google Scholar]
- 54.De Moraes CG, Liebmann JM, Greenfield DS, Gardiner SK, Ritch R, Krupin T. Risk factors for visual field progression in the low-pressure glaucoma treatment study. Am J Ophthalmol. 2012;154:702–11. doi: 10.1016/j.ajo.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Burgoyne CF, Crawford Downs J, Bellezza AJ, Francis Suh JK, Hart RT. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retinal Eye Res. 2005;24:39–73. doi: 10.1016/J.PRETEYERES.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic Nerve Damage in Human Glaucoma: II. The Site of Injury and Susceptibility to Damage. Arch Ophthalmol. 1981;99:635–49. doi: 10.1001/ARCHOPHT.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 57.Gharahkhani P, Jorgenson E, Hysi P, Khawaja AP, Pendergrass S, Han X, O, et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat Commun. 2021;12:1258. 10.1038/s41467-020-20851-4 [DOI] [PMC free article] [PubMed]
- 58.Hauser MA, Allingham RR, Aung T, et al. Association of genetic variants with primary open-angle glaucoma among individuals with african ancestry. JAMA. 2019;322:1682–91. doi: 10.1001/JAMA.2019.16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo X, Rotter JI. Genome-Wide Association Studies. J Am Med Assoc. 2019;322:1705–6. doi: 10.1001/jama.2019.16479. [DOI] [PubMed] [Google Scholar]
- 60.Osman W, Low SK, Takahashi A, Kubo M, Nakamura Y. A genome-wide association study in the Japanese population confirms 9p21 and 14q23 as susceptibility loci for primary open angle glaucoma. Hum Mol Genet. 2012;21:2836–42. doi: 10.1093/HMG/DDS103. [DOI] [PubMed] [Google Scholar]
- 61.Taylor KD, Guo X, Zangwill LM, Liebmann JM, Girkin CA, Feldman RM, et al. Genetic architecture of primary open-angle glaucoma in individuals of African descent: the African descent and glaucoma evaluation study III. Ophthalmology. 2019;126:38–48. 10.1016/j.ophtha.2018.10.031 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding author upon a reasonable request.