Abstract
The diagnosis and management of keratoconus in the paediatric age group presents additional challenges to those encountered in adults. The most significant of these, encountered in some young patients, are delayed presentation of unilateral disease, more advanced disease at diagnosis, difficulty in obtaining reliable corneal imaging, faster rates of disease progression and challenges in contact lens management. The stabilisation effect of corneal cross-linking (CXL), more extensively studied in adults with randomised trials and long-term follow-up, has been much less rigorously examined in children and adolescents. The high heterogeneity of published studies in younger patients, particularly in the choice of tomography parameters designated as primary outcome measures and the definitions of progression, indicates that improved standardisation for future studies on CXL will be necessary. There is no evidence that corneal transplant outcomes in young patients are poorer than those in adults. This review provides a current perspective on the optimal diagnosis and treatment of keratoconus in children and adolescents.
Subject terms: Corneal diseases, Surgery, Diagnosis
Abstract
儿童年龄组圆锥角膜的诊断和治疗相较于成人面临更大的挑战。在一些年轻患者中, 最重要的是单眼患病的延迟表现、诊断时处于晚期的疾病、难以获得可靠的角膜图像、疾病进展速度快以及接触镜管理方面的挑战。通过随机试验和长期随访, 成人角膜交联术 (CXL) 的稳定作用已得到了广泛研究, 但关于儿童和青少年的研究要少得多。已发表的针对年轻患者的研究具有高度异质性, 尤其在选择指定断层扫描参数作为首要结果测定和进展定义方面, 这表明有必要改善未来CXL研究的标准化。没有证据表明年轻患者的角膜移植效果比成人更差。本综述对儿童和青少年圆锥角膜的最佳诊断和治疗提供了目前的观点。
Introduction
Keratoconus onset in young patients presents challenges not seen in the adult age group. These will be reviewed and some recommendations made, where feasible, on the basis of published evidence. For the purpose of this review, the upper age is limited to 16 years as it captures the more particular characteristics of paediatric keratoconus: analysis of keratoconus features and outcomes in paediatric keratoconus is frustrated rather than served by the inclusion of patients up to 18 or 19 years, as in some reports. Moreover, a restriction to 16 years aligns with age-segregated clinic arrangements in most ophthalmology departments internationally.
Epidemiology in young patients
There is considerable variation in the prevalence of keratoconus both geographically and between ethnic groups. Reported prevalence from cohort studies range from 4.8% in Saudi Arabian youths (6–21 years old), 1.2% in Australian 20-year-olds, 0.52% in New Zealand adolescents (13 - 16 years old), and 0.27% in Dutch 10–40 year olds [1–4]. Sub-population analysis has revealed that the disease affects Indians, Pakistanis, Arabs, and Polynesians four times more than Caucasians [5, 6]. The progression of keratoconus appears to be faster in certain populations, such as in Middle-Eastern people, who suffer more rapid changes in corneal tomography than Europeans and East Asians [7]. The true prevalence of keratoconus is difficult to assess as detection rates are highly dependent on access to corneal imaging, the availability of representative populations for screening and the diagnostic criteria applied. Furthermore, technological advances in imaging are likely to have increased the detection rates of keratoconus over time, due to the greater detection of subclinical or ‘forme fruste’ keratoconus [8].
Clinical assessment of suspected keratoconus in children
The disease is usually bilateral, but asymmetry in the severity of keratoconus is a feature more commonly seen in young patients (Fig. 1). It is common for children and adolescents not to report any symptoms unless or until the consequences of keratoconus impair vision in the second affected eye [9]. This is often in the context of significantly asymmetric disease, in which visual function in the better eye compensates and binocular vision appears normal to the child. As a result of a paucity of symptoms, patients are often referred by community optometrists reporting an abnormal retinoscopic reflex or rapidly progressing asymmetric refractive error or high astigmatism, particularly that which can no longer be corrected fully with spectacles or soft contact lenses [5]. Retrospective cohort studies comparing adult and paediatric groups have found that children tend to have a more severe stage of keratoconus at diagnosis, with 28% at stage IV Amsler-Krumeich versus 8% of adults, higher astigmatism, and thinner corneas [10–12]. Paediatric patients can even present for the first occasion with painless hydrops secondary to advanced keratoconus, unlike hydrops in adults, which is invariably painful (Fig. 2) [13]. Symptoms and examination signs of ocular allergy may be also be present due to the strong association between ocular atopy and keratoconus [14, 15]. Key investigations include formal refraction with retinoscopy and corneal imaging using Placido-based or Scheimpflug principle-based tomography [16].
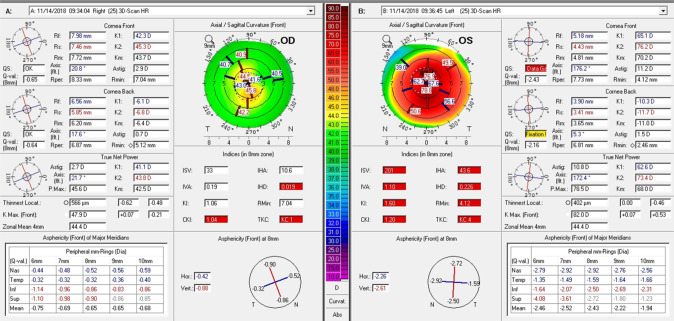
Fig. 1.
Keratoconus asymmetry: corneal tomography summary (Pentacam) of a 13-year-old patient at his initial presentation showing features of advanced keratoconus in the left eye and only early changes in the right eye. The unaided Snellen visual acuity was 6/6 right and counting fingers left eye. Right-hand panel showing ‘OS’ is the left eye summary and the left-hand panel showing ‘OD’ is the right eye summary.
Fig. 2.
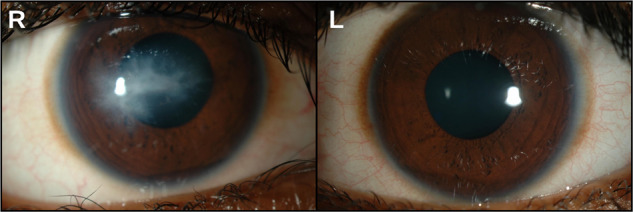
Acute hydrops at presentation: anterior segment photographs of an 11-year-old boy who presented with signs of acute hydrops associated with advanced keratoconus in his right eye. The was no history of acute visual loss or discomfort. Left eye unaided Snellen visual acuity was 6/6, the cornea was normal on slit lamp examination and only early keratoconus signs were evident on corneal tomography. Left-hand panel with ‘R’ for right eye shows central corneal opacity. Right-hand panel with ‘L’ for left eye shows a clear cornea.
Once a diagnosis of keratoconus has been made, a particular challenge in children and adolescents is the parents’ concerns. Time should be made available to fully explain the prognosis and management plans, both in the short and longer terms (Table 1).
Table 1.
Summary of specific challenges encountered in paediatric keratoconus.
| Specific challenges of paediatric keratoconus |
| Delayed presentation of unilateral disease |
| Advanced stage of disease at diagnosis |
| Difficulty in obtaining reliable corneal imaging |
| Rapid rates of disease progression in some patients |
| High rates of rigid gas-permeable contact lens intolerance |
| Parental anxiety at diagnosis |
Corneal tomography – diagnosis
Measurements obtained from corneal tomography are essential for the diagnosis and monitoring of keratoconus [17]. In practice it can be difficult to obtain reliable and accurate measurements in younger children and patients with learning disabilities [9]. Devices with faster image capture such as the CASIA2 (Tomey, Nuremberg, Germany) can be helpful if repeatable tomography is not feasible with slower image capture devices such as Pentacam (OCULUS, Wezlar, Germany) or Orbscan (Bausch + Lomb, Quebec, Canada). As a simple outline, in keratoconus there is usually a paracentral, focal elevation in the anterior corneal curvature with inferior hemi-meridian asymmetry. A keratometric meridional value of over 46 dioptres (D) raises suspicion of keratoconus [16]. Irregularities in the posterior curvature and epithelial thickness are further signs of early and subclinical disease [17].
A recent systematic review and meta-analysis by Ferdi et al. highlighted the current diversity of corneal topographical parameters reported in published studies for diagnosis and monitoring keratoconus, which include: Kmax (steepest single point of anterior corneal curvature), K1 (mean power in the flat corneal meridian), K2 (mean power in the steep corneal meridian), Kmean (the average of K1 and K2), and the corneal thickness at the thinnest point. It is recognised that using single parameters for the clinical evaluation of keratoconus increases the likelihood of erroneous or inaccurate measurements, which may affect management decisions. Kmax, for example, is one of the most commonly used parameters in clinical studies. However, it is limited in that it does not evaluate posterior curvature and may fluctuate due to local thinning and changes in cone morphology, which may paradoxically lead to a reduction in Kmax despite progression of overall corneal ectasia [18]. More generally, to improve diagnostic precision it is best to use a mean of at least three measurements of tomographic parameters at each patient examination [19].
Corneal tomography – monitoring for progression
One of the specific challenges of managing keratoconus in children is the significantly faster progression rate in some compared to adults. Ferdi et al. calculated 0.8 D more Kmax steepening over a twelve month period for every ten-year decrease in age between the ages of 10 and 34 years [7]. These figures were obtained from a heterogeneous group of studies and therefore the precise magnitude by which children, as a whole group, progress faster remains unclear. At the extreme end of the spectrum, very rapidly progressive disease based on tomography is found in some young patients (Fig. 3).
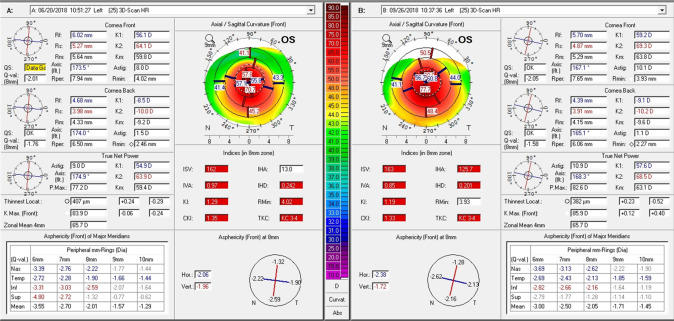
Fig. 3.
Rapidly progressive keratoconus: corneal tomography (Pentacam) of the left eye of a 13-year-old South Asian patient. Baseline examination is shown in the left-hand panel and examination after a 3-month interval on the right-hand panel, indicating an increase in K2 from 64.1 D to 69.3 D and reduction in the minimum corneal thickness (Thinnest locat.) from 407 µm to 382 µm.
Scoring systems based on multivariate analysis may aid clinicians in interpreting data from serial visits to identify those patients with eyes in which there is progressive disease. One example is the Belin ABCD classification, which takes into account anterior curvature, back curvature of the 3 mm area centred on the thinnest point, minimal corneal thickness, and best spectacle-corrected distance acuity. The aim of this approach is to identify any cone and focus analysis in the immediate area, as opposed to centring on the corneal apex [20]. A further example is the Dutch cross-linking for keratoconus (DUCK) score, which incorporates a combination of age, objective clinical measurements and subjective visual impact on daily life [21]. A notable limitation of progression indices is the reliance on sets of normative values obtained from a full range of ages. There is therefore an assumption that normal corneas in the young are identical to adult corneas when determining if there are any abnormalities. However, a recent study by Hashem et al. comparing Pentacam images of normal corneas within both adult and paediatric cohorts reported significant differences in measurements of corneal curvature, elevation and pachymetry between the two groups. The authors suggested that devising separate paediatric normative datasets may further improve diagnostic accuracy of progression indices in this age group [22].
In summary, the key to successful monitoring in keratoconus is obtaining good quality and reproducible tomographic measurements. In a routine clinical setting, rapid analysis of a single parameter such as K2, which is itself a mean value, may be sufficient to effectively monitor keratoconus in the majority of cases.
Screening
Advances in the understanding of the diagnostic features of subclinical keratoconus, coupled with the absence of symptoms in early disease in most, supports active screening for the disease [23, 24]. Furthermore, there is clinical value in detecting keratoconus early as corneal cross-linking appears to be effective in stabilising disease progression. Conversely, there is the potential for harm in screening, as the balance between optimising both sensitivity and specificity invariably leads to a proportion of false positives and false negatives. In the case of false positives, this may lead to avoidable anxiety for patients and further unnecessary investigations. The overall benefit of keratoconus screening has yet to be determined. There are hurdles to be overcome including: proving clinical benefit of screening, selecting the appropriate population to be screened, selecting screening criteria, logistical difficulties in delivering widespread screening and demonstrating economic viability [25, 26].
Corneal cross-linking
First described as the ‘Dresden protocol’ by Wollensak et al. in 2003, corneal cross-linking (CXL) has rapidly become the new standard care for Amsler-Krumeich stage I and stage II keratoconus with evidence of progression [27]. Over the past two decades, there have been several new variations of the protocol, but all involve the use of topical riboflavin with the subsequent application of ultraviolet-A (UVA) to the cornea. It appears that additional biomechanical strength is induced in the cornea; however the precise mechanism of effect is unknown, as an effect on cross-linking of stromal collagen fibres has not been identified in ultrastructural studies [28]. The treatment depth is thought to be between 250 and 280 µm using the Dresden protocol, which necessitates a minimum corneal thickness in order to avoid total penetrance of the cornea by UVA and potentially damage to the retina [29]. Post-operative complications are uncommon, but include corneal haze, sterile infiltrates, microbial keratitis, corneal oedema/endothelial cell loss and persistent epithelial defects (Figs. 4 and 5) [30]. For this reason CXL should be reserved for patients with proven progression of keratoconus, typically determined by longitudinal corneal tomography measurements and change in best-corrected visual acuity (BCVA) [25]. The reported rates of untreated progression are variable, seeming to depend largely on differences in study design and population. One study, albeit retrospective, reported the rate of untreated progression in a paediatric cohort as 77% over a mean follow-up of three years [31]. This rate may be exaggerated due to referral bias and high re-test variability of tomography in advanced keratoconus. One further study, a prospective trial, found an untreated progression rate of 43% over a period of 18 months, based on a definition of increase in K2 of at least 1.5 D from baseline [23]. The lack of data regarding prevalence of keratoconus progression in untreated keratoconus in young patients is a significant limitation in the current literature.
Fig. 4.
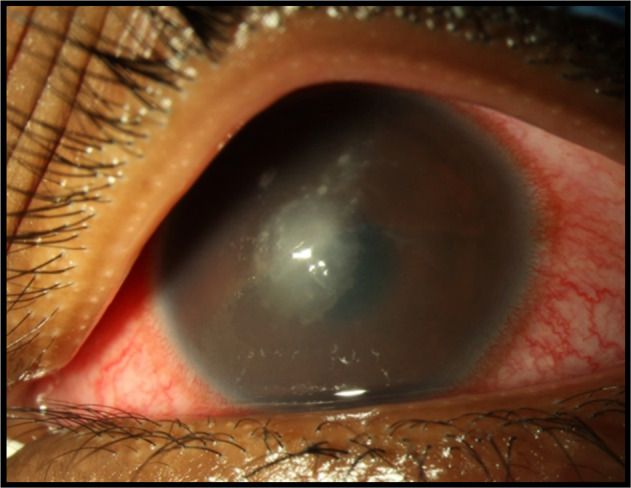
Microbial keratitis following CXL treatment: photograph of a corneal stromal opacity resulting from acute bacterial keratitis in a 13-year-old patient following CXL. S. aureus was isolated on culture.
Fig. 5.
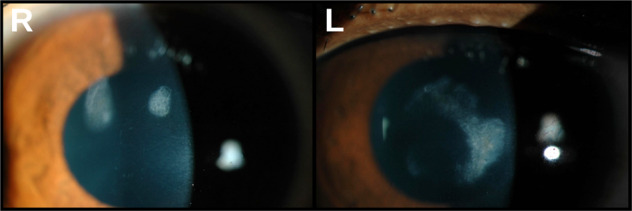
Sterile stromal infiltrates following CXL treatment: persisting sterile stromal infiltrates in the central corneal stroma of a paediatric patient following CXL. These resulted in long term reduction in best-corrected visual acuity Left-hand panel with ‘R’ for right eye and right-hand panel with ‘L’ for left eye, both showing central faint corneal opacities.
Accelerated corneal cross-linking
Current trial protocols more frequently report use of the accelerated corneal cross-linking (A-CXL) technique, which reduces the UV treatment time from 30 minutes to 8–10 minutes by increasing the use of pulsed, high-fluence UVA. A retrospective study by Baenninger et al. compared 78 eyes of patients aged 18 or under with keratoconus, half of which underwent Dresden protocol CXL and the other A-CXL. At 12 months there was no significant difference in BCVA, Kmax, or rate of treatment failure between the two groups [32]. This study is limited by the lack of randomisation and relatively short follow-up duration but it provides the best available evidence for the routine use of A-CXL over Dresden protocol CXL in children. Furthermore, a meta-analysis of six randomised controlled trials involving patients with a range of ages found no difference between conventional CXL and A-CXL [33].
Epithelium-on cross-linking
In the two above protocols, the corneal epithelium is removed at the beginning of the procedure to allow more riboflavin penetration and UVA energy to reach the stroma. The drawbacks of this approach are two or more days of post-operative pain until the epithelial defect heals and increased infection risk. The concept of epithelium-on (epi-on) CXL was devised; however, the best available evidence from long-term prospective cohort studies suggests that epi-on CXL is inferior in efficacy compared with conventional epithelium-off CXL [34].
Efficacy and safety of cross-linking for paediatric keratoconus
The reported rates of progression post-CXL are also heavily influenced by variability in study design and population. In particular, the shortage of comparator groups in reports of CXL efficacy is a significant limitation as there is an unjustified assumption that progression is inevitable and spontaneous stabilisation of keratoconus does not occur. In paediatric keratoconus, a 2021 systematic review and meta-analysis by Kobashi et al. included a total of 26 prospective and retrospective cohort studies, with analysis of four distinct patient groups based on both accelerated versus conventional and epi-on versus epi-off CXL protocols. In the epi-off, non-accelerated CXL group, there appeared to be significant reduction of Kmax and increase in BCVA at 1 year post-procedure. The remaining treatment groups tended towards significance in these measures, but had insufficient statistical power, including the epi-off, accelerated group, which comprised of only 4 studies [30]. Another systematic review of CXL in paediatric keratoconus by Achiron et al. focussed on comparison of methodology used between studies. The mean pooled progression rate after epi-off CXL was 9.9% (95% confidence interval (CI), 6.1% to 14.6%, total pooled sample size: 1 508 eyes). In terms of methodology, the most common measurement chosen to define progression was Kmax in two-thirds of studies, with the remaining studies using K2 as the primary measure, usually requiring an increase of at least 1 D [35]. Both of these systematic reviews have significant limitations. Firstly, the authors were limited by the availability of good quality evidence. Neither review included a randomised controlled trial, instead being comprised of retrospective or prospective studies, or unclear study designs with various definitions of progression and numerous therapeutic protocols, which could lead to unintentional bias. There were high heterogeneity measures in the majority of outcomes due to variability in study design and weak statistical power. Kobashi et al. limited their methodology to studies reporting Kmax as the primary outcome measure, which may reduce heterogeneity, but excludes substantial evidence from publications using alternative outcome measures. The primary outcomes were reported at one year by Kobashi et al., or at variable end points depending on each study design in the case of Achiron et al. Accordingly, these reviews do not provide clear evidence that the apparent effects of CXL persist in the long-term in children and adolescents.
In 2021, the first randomised controlled trial investigating the efficacy and safety of CXL in paediatric keratoconus was published. The KERALINK trial compared A-CXL with standard care, including provision of glasses or contact lenses, in two groups of 30 patients with documented progressive keratoconus, each with one study eye, aged between 10 to 16 years and in the United Kingdom. The primary outcome was change in K2, with a 1.5 D increase defined as progression, this being estimated to correlate with a clinically meaningful change in refraction. Patients were eligible if there was an increase in K2 over a period of at least three months. At 18 months, the adjusted mean difference in K2 in the study eye was 3.0 D lower in the CXL group versus standard care (95% CI, −4.9 to −1.1 D; p = 0.002). A difference in BCVA of −0.51 LogMAR (95% CI, −1.37 to 0.35 LogMAR; p = 0.002) also favoured the CXL group. The defined progression rate was 7% in the CXL group versus 43% in the standard care group, equivalent to a 90% lower chance of experiencing progression after CXL. There were no adverse events reported during the trial [23]. Of note, the difference in Kmax trended towards favouring CXL but was not statistically significant. For this reason, K2 may be a more reliable indicator of keratoconus progression as it takes into account changes along the central cornea steep meridian rather than a single point. The treated progression rate of 7% reported in this trial at 18 months is higher than the rate reported in adults, reflecting a more aggressive disease course seen in younger age groups [7]. Overall and at 18 months, the results from this trial provide high-level evidence that CXL in the paediatric population is a safe and highly effective treatment option when applied to progressive, stage I to II keratoconus. Future prospective trials of CXL in non-European populations and more evidence on the long-term effects of CXL in young patients would be of particular value. At present, the longest term evidence is from a retrospective cohort study by Simantov et al., with seven years of follow-up data. Of the 30 eyes undergoing CXL, only one eye has shown evidence of progression thus far, compared with eight of the fellow untreated control eyes [36]. This data gives reason for optimism that other methodologically stronger studies will also show lasting stability in due course.
Patients with Down syndrome are usually excluded from CXL studies due to the often reduced reliability of obtainable tomography measurements. A retrospective case series of nine children with Down syndrome who underwent CXL under general anaesthesia for progressive keratoconus suggests that CXL is still a good option, with results of stable keratometry and BCVA at six months with no adverse treatment or anaesthetic events [37].
Topography-guided photorefractive keratectomy combined with CXL
There is little published data on treatment using simultaneous topography-guided partial photorefractive keratectomy (PRK) and CXL in children. The aim of treatment being to both stabilise ectasia and normalise the corneal surface to reduce refractive error. A prospective study has reported outcomes in 39 eyes of 21 patients under the age of 18 with proven progressive keratoconus, absent central corneal scarring and thinnest pachymetry of at least 400 µm. The four-year results showed a significant improvement in both unaided and best-corrected visual acuity, as well as significant flattening of K1, K2 and Kmax. The main adverse event was two cases of late-onset deep corneal haze which resolved with long courses of topical steroids. Endothelial cell count was not affected [38]. Despite the promising outcomes, the methodology of this study limits the strength of any conclusions that can be drawn from the results: firstly the authors correctly highlight that lack of a CXL-only control group removes the ability to determine any additive effect of the PRK treatment, as CXL alone could explain the above results, and secondly there may be an element of inclusion bias as the nature of the case selection is not entirely clear. In summary, combined partial PRK and CXL is an emerging treatment area and the safety and efficacy will need to be proven before it can be adopted in routine practice for children with keratoconus.
Visual rehabilitation
In cases of mild keratoconus, many children with both eyes affected are able to achieve good functional vision with spectacles or soft contact lenses. As the severity of keratoconus increases, spectacles become less effective due to ectasia and scarring of the cornea, at which stage rigid gas-permeable (RGP) contact lenses provide better visual outcomes [39]. However, children often have reduced tolerability to RGP lenses, with reduced daily wear duration and higher symptoms of discomfort when compared with soft lenses [40]. Mini-scleral and scleral contact lenses are a further option for children with keratoconus, but there may still be tolerance problems. Patient selection is key as the child and parents must be motivated for success in lens wear and management [41, 42].
Intracorneal ring segments
Surgical implantation of intracorneal ring segments (ICRS) into the deep stroma of patients with keratoconus has the effect of flattening the central cornea with the aim of reducing refractive error. There are two notable studies which have investigated the effect of this treatment in paediatric patients. Alfonso et al. published a series of a Ferrara-type ICRS implantation in 118 eyes of 88 patients under the age of eighteen with keratoconus. The minimum follow-up duration was six months, with a cohort of 23 patients extending to 60 months. The results demonstrated an improvement in both unaided distance visual acuity (UCVA) and corrected distance visual acuity (CDVA). Eight eyes had an increase in Kmax of >1.5 D beyond six months of follow-up, but CDVA remained stable [43]. This data should be interpreted with caution as this was a non-randomised trial with no control arm; there was a large proportion of patients lost to follow-up; the trial design was a single-centre, single device; and progression of keratoconus was not confirmed before intervention. The eight patients who experienced a significant increase in Kmax suggests that ICRS alone does not treat the underlying processive ectasia. Mendez et al. attempted to address this particular question by publishing a retrospective observational study involving 26 eyes of 19 patients with keratoconus under the age of eighteen, with sixteen eyes receiving Ferrara-type ICRS implantation with A-CXL and ten eyes receiving ICRS implantation alone. The combined results of both treatment arms appear to show a stable or improved UCVA and BCVA, and a reduction in K2 with no adverse complications reported; however no statistical significance calculations were included [44]. The lack of specific treatment criteria, variable timing of CXL treatment post-ICRS implantation and the limited statistical power of this study limit the conclusions that can be drawn from this study and further trials are clearly needed to determine the role of ISCRS in the management paediatric keratoconus.
Corneal transplantation
Progressive keratoconus can lead to extreme corneal thinning and stromal scarring. When rigid gas-permeable contact lenses are not tolerated or fail to improve BCVA, corneal transplantation can be the only remaining treatment option to restore visual potential [4]. Better transplant survival rates are found in keratoconus than other indications, but the commonly held assumption that keratoplasty outcomes for keratoconus are inferior [45] in children compared with adults was called into question in two recent reports. A national registry study of keratoplasty data from the United Kingdom reported that outcomes in children up to 16 years were comparable to adults in respect of (i) interval from surgery to first immunological rejection episode, if any, and (ii) functioning transplant survival rates at 2 years. There was a detectable small hazard of rejection with decreasing age, but this did not lead to a statistically significant difference in rejection rates [46]. In another report, outcomes in penetrating or deep anterior lamellar keratoplasty, the latter sparing the host’s Descemet’s membrane which is normal in eyes with keratoconus in which hydrops has not occurred, were compared in children and adolescents aged 18 or under. This comparison case series showed no significant difference in terms of visual and survival outcomes after six years of follow-up [47]. It should be added that long-term superiority of deep anterior lamellar keratoplasty may become apparent with a longer follow-up duration than in these two studies, due to avoidance of allogeneic endothelial rejection and preservation of the host endothelial density conferring increased transplant longevity. For this reason anterior lamellar transplantation may be, subject to technical feasibility, the surgical procedure of choice in young patients with keratoconus.
Future research requirements to inform clinical practice Keratoconus is a disorder with twofold challenges in young patients: rapidly increasing referral numbers due to earlier case detection using new imaging methods and availability of a new management option, the indications for which have not been agreed. Several lines of investigations now have priority. (i) There is limited information available on rates of progression of untreated early keratoconus in young patients, and indeed young patients in different ethnic groups or geographic regions. Without more published data on this, it is difficult to confidently recommend a CXL procedure to parents of those patients with mild ectasia and relatively good visual acuity. (ii) Agreed definitions of keratoconus for diagnosis and progression are urgently needed, not least to inform future clinical trials. Within the published literature, there are various criteria chosen to define progression, which makes comparison and pooled analysis of studies difficult [7]. If a hallmark of good scientific research is reproducible specification of the material under investigation, this is absent in keratoconus studies including trials, in which the definitions used have been single measurements such as Kmax or K2, or a combination of factors such as a scoring system or progression index. Any criteria adopted to define progression would need to take into account variation in measurements between tomography devices from different manufacturers and ideally use specific paediatric normative datasets [7, 18, 20, 22, 35]. (iii) Detailed CXL outcome studies, including long-term follow-up and, in particular, keratoconus stage-specific CXL outcome analysis are needed. It seems simplistic that a single CXL protocol should be advised in all patients and such studies will allow development of a range of CXL treatment protocols for trial. A further benefit of such outcome studies would be to inform management of those young patients in whom progression continues despite CXL or following an interval of apparent stabilisation. The available information that high pre-CXL Kmax and corneal thickness less than 450 µm are predictive factors for CXL treatment failure is likely to be simplistic [46]. An understanding of the reasons behind CXL failure would allow better management of all patients and may advance our understanding of the mechanism of CXL, for example if there are demonstrable differences in corneal ultrastructure in corneas in which tomographic stabilisation is achieved compared to those in which it is not.
Acknowledgements
Supported in part by the National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation Programme (reference. [14, 18, 23]), an MRC and NIHR partnership. The trial was otherwise supported in part by the NIHR Moorfields Biomedical Research Centre and NIHR Moorfields Clinical Research Facility. The funding organisation had no role in design or conduct of this research.
Funding
This review article received no specific funding.
Author contributions
L.P. and D.L. had equal involvement in producing this manuscript in all of the following criteria: 1. Conceived and/or designed the work that led to the submission, acquired data, and/or played an important role in interpreting the results. 2. Drafted or revised the manuscript. 3. Approved the final version. 4. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability
Liam Price, as the corresponding author have had full access to all the data in this review article and had final decision to submit for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torres Netto EA, Al-Otaibi WM, Hafezi NL, Kling S, Al-Farhan HM, Randleman JB, et al. Prevalence of keratoconus in paediatric patients in Riyadh, Saudi Arabia. Br J Ophthalmol. 2018;102:1436–41. doi: 10.1136/bjophthalmol-2017-311391. [DOI] [PubMed] [Google Scholar]
- 2.Chan E, Chong EW, Lingham G, Stevenson LJ, Sanfilippo PG, Hewitt AW, et al. Prevalence of keratoconus based on scheimpflug imaging: The raine study. Ophthalmology. 2021;128:515–21. doi: 10.1016/j.ophtha.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Papali’i-Curtin AT, Cox R, Ma T, Woods L, Covello A, Hall RC. Keratoconus prevalence among high school Students in New Zealand. Cornea. 2019;38:1382–9. doi: 10.1097/ICO.0000000000002054. [DOI] [PubMed] [Google Scholar]
- 4.Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RP. Age-specific incidence and prevalence of keratoconus: A nationwide registration study. Am J Ophthalmol. 2017;175:169–72. doi: 10.1016/j.ajo.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Anitha V, Vanathi M, Raghavan A, Rajaraman R, Ravindran M, Tandon R. Pediatric keratoconus - Current perspectives and clinical challenges. Indian J Ophthalmol. 2021;69:214–25. doi: 10.4103/ijo.IJO_1263_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson AR, Soneji B, Sarvananthan N, Sandford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus? Eye. 2000;14:625–8. doi: 10.1038/eye.2000.154. [DOI] [PubMed] [Google Scholar]
- 7.Ferdi AC, Nguyen V, Gore DM, Allan BD, Rozema JJ, Watson SL. Keratoconus natural progression: A systematic review and meta-analysis of 11 529 eyes. Ophthalmology. 2019;126:935–45. doi: 10.1016/j.ophtha.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Consejo A, Jiménez-García M, Issarti I, Rozema JJ. Detection of subclinical keratoconus with a validated alternative method to corneal densitometry. Transl Vis Sci Technol. 2021;10:32. doi: 10.1167/tvst.10.9.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivo-Payne A, Abdala-Figuerola A, Hernandez-Bogantes E, Pedro-Aguilar L, Chan E, Godefrooij D. Optimal management of pediatric keratoconus: challenges and solutions. Clin Ophthalmol. 2019;13:1183–91. doi: 10.2147/OPTH.S183347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Léoni-Mesplié S, Mortemousque B, Touboul D, Malet F, Praud D, Mesplié N, et al. Scalability and severity of keratoconus in children. Am J Ophthalmol. 2012;154:56–62. doi: 10.1016/j.ajo.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Chatzis N, Hafezi F. Progression of keratoconus and efficacy of pediatric [corrected] corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28:753–8. doi: 10.3928/1081597X-20121011-01. [DOI] [PubMed] [Google Scholar]
- 12.Naderan M, Rajabi MT, Zarrinbakhsh P, Farjadnia M. Is keratoconus more severe in pediatric population? Int Ophthalmol. 2017;37:1169–73. doi: 10.1007/s10792-016-0382-5. [DOI] [PubMed] [Google Scholar]
- 13.Maharana PK, Sharma N, Vajpayee RB. Acute corneal hydrops in keratoconus. Indian J Ophthalmol. 2013;61:461–4. doi: 10.4103/0301-4738.116062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahuja P, Dadachanji Z, Shetty R, Nagarajan SA, Khamar P, Sethu S, et al. Relevance of IgE, allergy and eye rubbing in the pathogenesis and management of Keratoconus. Indian J Ophthalmol. 2020;68:2067–74. doi: 10.4103/ijo.IJO_1191_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng SF, Jan RL, Wang JJ, Tseng SH, Chang YS. Association between atopic keratoconjunctivitis and the risk of keratoconus. Acta Ophthalmol. 2021;99:e54–e61. doi: 10.1111/aos.14509. [DOI] [PubMed] [Google Scholar]
- 16.Cavas-Martínez F, De La Cruz Sánchez E, Nieto Martínez J, Fernández Cañavate FJ, Fernández-Pacheco DG. Corneal topography in keratoconus: state of the art. Eye Vis. 2016;3:5. doi: 10.1186/s40662-016-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R, Jr, Guell JL, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34:359–69. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 18.Belin MW, Alizadeh R, Torres-Netto EA, Hafezi F, Ambrósio R, Jr, Pajic B. Determining progression in ectatic corneal disease. Asia Pac J Ophthalmol (Philos) 2020;9:541–8. doi: 10.1097/APO.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 19.Guber I, McAlinden C, Majo F, Bergin C. Identifying more reliable parameters for the detection of change during the follow-up of mild to moderate keratoconus patients. Eye Vis. 2017;4:24. doi: 10.1186/s40662-017-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosekahya P, Caglayan M, Koc M, Kiziltoprak H, Tekin K, Atilgan CU. Longitudinal evaluation of the progression of keratoconus using a novel progression display. Eye Contact Lens. 2019;45:324–30. doi: 10.1097/ICL.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 21.Wisse RPL, Simons RWP, Van Der Vossen MJB, Muijzer MB, Soeters N, Nuijts RMMA, et al. Clinical evaluation and validation of the dutch crosslinking for keratoconus score. JAMA Ophthalmol. 2019;137:610. doi: 10.1001/jamaophthalmol.2019.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashem AO, Roshdy MM, Wahba SS, Saleh MI, Elkitkat RS. Normative values of various pentacam HR parameters for pediatric corneas. Cornea. 2020;39:1394–400. doi: 10.1097/ICO.0000000000002481. [DOI] [PubMed] [Google Scholar]
- 23.Larkin DFP, Chowdhury K, Burr JM, Raynor M, Edwards M, Tuft SJ, et al. Effect of corneal cross-linking versus standard care on keratoconus progression in young patients: The KERALINK randomized controlled trial. Ophthalmology. 2021;128:1516–26. doi: 10.1016/j.ophtha.2021.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Gore DM, Leucci MT, Koay SY, Kopsachilis N, Nicolae MN, Malandrakis MI, et al. Accelerated pulsed high-fluence corneal cross-linking for progressive keratoconus. Am J Ophthalmol. 2021;221:9–16. doi: 10.1016/j.ajo.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 25.O’Brart D, Zarei-Ghanavati M, Vasquez-Perez A, Liu C. Comment on: “What are the costs, capacity, and clinical implications of “waiting for documented progression” in young West of Scotland patients prior to collagen cross linking?”. Eye. 2022;36:1512. doi: 10.1038/s41433-021-01704-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maile H, Li J-PO, Gore D, Leucci M, Mulholland P, Hau S, et al. Machine learning algorithms to detect subclinical keratoconus: systematic review. JMIR Med Inform. 2021;9:e27363. doi: 10.2196/27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 28.Hayes S, Boote C, Kamma-Lorger CS, Rajan MS, Harris J, Dooley E, et al. Riboflavin/UVA collagen cross-linking-induced changes in normal and keratoconus corneal stroma. PLoS ONE. 2011;6:e22405. doi: 10.1371/journal.pone.0022405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wollensak G. Histological changes in human cornea after cross-linking with riboflavin and ultraviolet A. Acta Ophthalmologica. 2010;88:e17–e8. doi: 10.1111/j.1755-3768.2008.01474.x. [DOI] [PubMed] [Google Scholar]
- 30.Kobashi H, Hieda O, Itoi M, Kamiya K, Kato N, Shimazaki J, et al. Corneal cross-linking for paediatric keratoconus: A systematic review and meta-analysis. J Clin Med. 2021;10:2626. doi: 10.3390/jcm10122626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer JJ, Gokul A, Vellara HR, McGhee CNJ. Progression of keratoconus in children and adolescents. Br J Ophthalmol. 2023;107:176–80. doi: 10.1136/bjophthalmol-2020-316481. [DOI] [PubMed] [Google Scholar]
- 32.Baenninger PB, Bachmann LM, Wienecke L, Thiel MA, Kaufmann C. Pediatric corneal cross-linking: Comparison of visual and topographic outcomes between conventional and accelerated treatment. Am J Ophthalmol. 2017;183:11–6. doi: 10.1016/j.ajo.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Kobashi H, Tsubota K. Accelerated versus standard corneal cross-linking for progressive keratoconus: A meta-analysis of randomized controlled trials. Cornea. 2020;39:172–80. doi: 10.1097/ICO.0000000000002092. [DOI] [PubMed] [Google Scholar]
- 34.Henriquez MA, Hernandez-Sahagun G, Camargo J, Izquierdo L., Jr Accelerated epi-on versus standard Epi-Off corneal collagen cross-linking for progressive keratoconus in pediatric patients: Five years of follow-up. Cornea. 2020;39:1493–8. doi: 10.1097/ICO.0000000000002463. [DOI] [PubMed] [Google Scholar]
- 35.Achiron A, El-Hadad O, Leadbetter D, Hecht I, Hamiel U, Avadhanam V, et al. Progression of Pediatric Keratoconus After Corneal Cross-Linking: A Systematic Review and Pooled Analysis. Cornea. 2022;41:874–8. doi: 10.1097/ICO.0000000000002808. [DOI] [PubMed] [Google Scholar]
- 36.Simantov I, Or L, Gazit I, Dubinsky-Pertzov B, Zadok D, Pras E, et al. Seven years follow-up of corneal cross-linking (CXL) in pediatric patients: Evaluation of treated and untreated eye. Eur J Ophthalmol. 2022;32:1482–90. doi: 10.1177/11206721211020632. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad TR, Pasricha ND, Rose-Nussbaumer J, J TO, J MS, Indaram M. Corneal collagen cross-linking under general anesthesia for pediatric patients with keratoconus and developmental delay. Cornea. 2020;39:546–51. doi: 10.1097/ICO.0000000000002197. [DOI] [PubMed] [Google Scholar]
- 38.Kanellopoulos AJ, Vingopoulos F, Sideri AM. Long-term stability with the athens protocol (Topography-Guided Partial PRK Combined With Cross-Linking) in pediatric patients with keratoconus. Cornea. 2019;38:1049–57. doi: 10.1097/ICO.0000000000001996. [DOI] [PubMed] [Google Scholar]
- 39.Downie LE, Lindsay RG. Contact lens management of keratoconus. Clin Exp Optom. 2015;98:299–311. doi: 10.1111/cxo.12300. [DOI] [PubMed] [Google Scholar]
- 40.Jones-Jordan LA, Walline JJ, Mutti DO, Rah MJ, Nichols KK, Nichols JJ, et al. Gas permeable and soft contact lens wear in children. Optom Vis Sci. 2010;87:414–20. doi: 10.1097/OPX.0b013e3181dc9a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alipour F, Jamshidi Gohari S, Azad N, Mehrdad R. Miniscleral contact lens in pediatric age group: indications, safety, and efficacy. Eye Contact Lens. 2021;47:408–12. doi: 10.1097/ICL.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 42.Severinsky B, Lenhart p. Scleral contact lenses in the pediatric population-Indications and outcomes. Cont Lens Anterior Eye. 2022;45:101452. doi: 10.1016/j.clae.2021.101452. [DOI] [PubMed] [Google Scholar]
- 43.Alfonso JF, Fernández-Vega-Cueto L, Lisa C, Monteiro T, Madrid-Costa D. Long-term follow-up of intrastromal corneal ring segment implantation in pediatric keratoconus. Cornea. 2019;38:840–6. doi: 10.1097/ICO.0000000000001945. [DOI] [PubMed] [Google Scholar]
- 44.Mendez EA, Roys N, Mejia ME, Plata MC, Rosenstiehl SM. Results of follow-up in pediatric keratoconus treated with intracorneal ring segments implantation alone or in combination with corneal cross-linking. J Pediatr Ophthalmol Strabismus. 2022;59:118–27. doi: 10.3928/01913913-20210719-02. [DOI] [PubMed] [Google Scholar]
- 45.Kelly T-L. Corneal transplantation for keratoconus. Arch Ophthalmol. 2011;129:691. doi: 10.1001/archophthalmol.2011.7. [DOI] [PubMed] [Google Scholar]
- 46.Wajnsztajn D, Hopkinson CL, Larkin DFP. National health service b, transplant ocular tissue advisory g, contributing o. keratoplasty for keratoconus in young patients: demographics, clinical features, and post-transplant outcomes. Am J Ophthalmol. 2021;226:68–75. doi: 10.1016/j.ajo.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Feizi S, Javadi MA, Karimian F, Abolhosseini M, Moshtaghion SM, Naderi A, et al. Penetrating keratoplasty versus deep anterior lamellar keratoplasty in children and adolescents with keratoconus. Am J Ophthalmol. 2021;226:13–21. doi: 10.1016/j.ajo.2021.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Liam Price, as the corresponding author have had full access to all the data in this review article and had final decision to submit for publication.