Abstract
Background/Objectives
Cataract surgery with intraocular lens (IOL) implantation is one of the most commonly performed surgeries worldwide. Within the UK, publicly funded cataract surgery is remunerated by two models: (1) “block contract” (BC), which commissions organisations to deliver whole service pathways without considering specific activity items; or (2) “payment by results” (PbR), which pays a tariff price for each procedure. This study aimed to examine the association between remuneration model and the cost and types of IOL used.
Subjects/Methods
Cataract operations recorded on the Royal College of Ophthalmologists’ National Ophthalmology Database were included, with additional data collected for remuneration model from NHS England and cost of IOL from the NHS Spend Comparison Service.
Results
We included 907,052 cataract operations from 87 centres. The majority of operations were performed in PbR centres (456 198, 50.3%), followed by BC centres (240 641, 26.5%) and mixed models centres (210 213, 23.2%). The mean price of hydrophobic (n = 7) and hydrophilic IOLs (n = 5) were £45.72 and £42.86, respectively. Hydrophobic IOLs were predominantly used (650 633, 71.7%) and were significantly more commonly used in centres remunerated by BC (96.5% vs. 3.5%) than those by PbR (65.7% vs. 34.3%) when compared to hydrophilic IOLs (p < 0.001).
Conclusions
This study demonstrated that the IOL choice may be perversely incentivised by the IOL cost and remuneration model. Although hydrophobic IOLs are more expensive at the point of surgery, their potential longer-term cost-effectiveness due to reduced requirement for YAG capsulotomy should be considered.
Subject terms: Health services, Lens diseases, Health care economics
Introduction
Cataract is the leading cause of blindness and visual impairment, with 94 million people affected globally, a number growing rapidly due to ageing poulations [1]. With the possible exception of obstetric procedures, cataract surgery with intraocular lens (IOL) implantation is the most commonly performed operation worldwide. It is estimated that more than 20 million cases are performed annually, including approximately 500,000 cases in the United Kingdom (UK) alone (https://live-nod-audit.pantheonsite.io/healthcare-professionals/publications-news-and-events/national-cataract-audit-annual-report-01) [2, 3].
Cataract surgery has been consistently shown to be an effective and cost-effective operation for improving vision and vision related quality of life [4]. However, the sheer volume of cataract surgeries performed each year places significant financial burden on healthcare services. Apart from the required operating facility, workforce and technical expertise, the cost of cataract surgery is associated with the phacoemulsification machine, surgical instruments and consumables, including IOLs [5].
The value of the IOL business is currently estimated at £4.3 billion ($5.2 billion) per year globally and is projected to rise to £6.8 billion ($8.2 billion) per year in 5 years (https://www.fortunebusinessinsights.com/industry-reports/intraocular-lens-market-101220). This is largely driven by the rising demand for cataract surgery as a result of increased longevity of the population, improvement in surgical techniques, and refinement in the quality and technology of IOLs [3, 6–11]. To meet the wide-ranging demand of the growing population, various types of IOLs have been designed, with some focusing on correcting ametropia (e.g., monofocal and toric IOLs) and some correcting both ametropia and presbyopia (e.g., multifocal, extended depth of focus, and accommodating IOLs). Based on the types of material, IOLs are broadly made of either hydrophilic acrylic, hydrophobic acrylic, polymethylmethacrylate (PMMA), silicone or collamers [11].
The cost of IOLs can vary considerably. The premium IOLs (e.g., presbyopia-correcting IOLs) are significantly more expensive in the UK and are typically used in the private sector only as they are considered refractive surgeries, which are not justified for routine use in publicly funded services. From the material standpoint, hydrophilic and hydrophobic acrylic IOLs are the two main types of IOLs used in cataract surgery, each of which is associated with some inherent strengths and weaknesses. For instance, hydrophobic IOL’s are usually slightly more expensive than hydrophilic IOL’s but are associated with a lower risk of posterior capsular opacification (PCO) [12, 13].
Within the context of the UK National Health Service (NHS), remuneration models are largely divided into payment by results (PbR) or block contract (BC). PbR refers to a remuneration model where the payment is made to the service provider based on the level of activity whereas BC refers to a remuneration model where a regular fixed payment (agreed in advance) is made to the service provider for a broadly defined service without fully taking into account the precise level of activity.
In view of the significant number of cataract operations performed annually and their potential financial impact on healthcare systems, we hypothesised that the choice of IOL may be affected by three factors: (1) the immediate cost of IOL to providers; (2) the overall cost implications of the IOL for the whole NHS cataract pathway; and (3) the payment model. Service providers remunerated by the PbR model may be influenced more by the immediate cost of purchasing IOL compared to those on the BC model. Providers working with a BC arrangement may be more influenced by rates of PCO associated with different IOLs, and the potential cost and workload implications of needing to perform greater numbers of YAG capsulotomies. In this study, we aimed to evaluate the potential influence of remuneration models and IOL pricing on the choice of IOL used for cataract surgery in the UK.
Subjects and methods
This was a retrospective study of cataract surgery data submitted to the Royal College of Ophthalmologists’ National Ophthalmology Database (RCOphth NOD) for operations performed between April 2015 and March 2020 (a 5-year period based on five UK financial / NHS years). As previously described, the RCOphth NOD is a national database that receives anonymised data from around 70% of ophthalmic institutes providing publicly funded cataract surgery in England, Wales and Guernsey, including both NHS Trusts and independent sector treatment centres (ISTCs) [12].
Clinical data were collected from participating RCOphth NOD centres for eligible cataract operations that included implantation of a monofocal single piece hydrophilic or hydrophobic IOL. Only operations where the IOL was known and had been used in at least 200 eligible operations were included. For each NHS year, data from centres were excluded if they had data for <50 eligible operations. Only centres with sufficient data for at least three (≥60%) of the five NHS years were included. Data from participating RCOphth NOD centres that were not located in England were not included as the remuneration and IOL sales data only concerned cataract surgery centres in England.
The clinical data supplied to the RCOphth NOD was recorded on the Medisoft EMR system (Medisoft Ophthalmology, Medisoft Limited, Leeds, UK, www.medisoft.co.uk), the Open Eyes EMR system (www.openeyes.org.uk) or bespoke in-house databases compliant with the RCOphth minimum national cataract dataset [12].
Remuneration model
Data for the remuneration structure was supplied by NHS England & NHS Improvement for English NHS Trusts remuneration structure for the 2015–2019 NHS years. Each centre might have more than one Clinical Commissioning Group (CCG) commissioning the same service (including cataract surgery), with different remuneration models, within the same centre. As it was not possible to determine the exact proportion of operations each CCG commissioned or which CCG was the main CCG for any individual centre, we had taken the following approach to determine the remuneration model for each centre. In each NHS year, a centre’s payment structure was allocated as BC if they had any Clinical Commissioning Group (CCG) commissioning surgery via BC funding. If all CCGs commissioned surgery via PbR then the centre’s payment structure was PbR. In addition, all ISTC sites were allocated to PbR in each NHS year they had data for.
Types and cost of IOL
IOL purchasing data, which included the IOL cost, was supplied for the time period of May 2021 to April 2022 from NHS England Spend Comparison Service. The data for purchasing of IOL was used for monofocal single piece IOL with hydrophilic or hydrophobic material. Any individual lens purchased for >£100 was removed due to the outlying nature of these purchases. For each centre, purchasing information was removed for individual IOL if the centre had purchased <50 of that particular type of IOL during the study period. From the curated purchasing data (based on the above eligibility criteria), the minimum, mean and maximum price for a hydrophilic and a hydrophobic lens were estimated.
Statistical methods
Statistical analyses were conducted using STATA 17 (StataCorp. 2021. College Station, TX: StataCorp LLC). Statistical comparisons were conducted using Pearson’s Chi square test with a significance threshold of 5%. PCO rates at 1, 3 and 5 years post-cataract surgery for hydrophilic and hydrophobic IOLs were used from the RCOphth NOD PCO paper [12] whereas information on the IOL models were retrieved from The RCOphth NOD PCO report.
Ethical approval was not required as this study was considered a service evaluation clinical audit. The study was conducted in accordance with the tenets of Declaration of Helsinki and the UK Data Protection Act 2018.
Results
Overall description
The clinical data sample comprised 907,052 cataract operations performed by 2768 surgeons from 87 centres, where 63 (72.4%) centres were traditional NHS Trusts and 24 (27.6%) centres are ISTC sites. The 63 traditional NHS Trusts performed 719 288 (79.3%) operations and the 24 ISTC sites performed 187 764 (20.7%) operations. The median number of operations per centre was 8 475 (IQR: 5629–12,753; Range: 602–92,966). Sixty-four (73.6%) centres had data for the 2015 NHS year, 79 (90.8%) for the 2016 NHS year, 87 (100%) for the 2017 NHS year, 87 (100%) for the 2018 NHS year, and 83 (95.4%) for the 2019 NHS year. Over the five years, 62 (71.3%) had data for all five NHS years, 15 (17.2%) for four NHS years, and 10 (11.5%) centres for three NHS years.
Remuneration model
During the 5-year study period, 49 (56.3%) centres were funded via PbR in all years, 15 (17.2%) via BC in all years, and 23 (26.4%) changed from PbR to BC or vice versa. For the 23 centres that changed remuneration method during the study period, two were BC in 2015 and 21 were PbR, while in 2019, 17 were BC and six were PbR. Fourteen centres started under one method and remained under the other method after switching, while nine centres switched between remuneration methods across the five NHS years. The PbR centres performed 456 198 (50.3%) operations, the BC centres performed 240,641 (26.5%) operations, and both remuneration methods centres performed 210,213 (23.2%) operations. The median number of operations per centre was 7 894 (IQR: 5 703–11,550; Range: 602–30,172) for the 49 PbR centres, 10,910 (IQR: 6864–14,155; Range: 1 455–92,966) for the 15 BC centres, and 8 475 (IQR: 4924–13,219; Range: 779–21,000) for the 23 centres under both remuneration methods.
Details of the IOL
Twelve different IOL models were included in this study, where 7 (58.3%) were hydrophobic and 5 (41.7%) were hydrophilic. The mean price of hydrophobic and hydrophilic IOL’s were £45.72 (range, £29.32–£60.00) and £42.86 (range, £39.20–£45.00), respectively. Hydrophobic and hydrophilic IOLs were used in 650 633 (71.7%) operations and 256 419 (28.3%) operations, respectively. Hydrophobic IOLs were used in 78 (89.7%) centres, where 48 (55.2%) centres used a hydrophobic IOL in ≥95% of operations. Hydrophilic IOLs were used in 64 (73.6%) centres, where 21 (24.1%) centres used a hydrophilic IOL in ≥95% operations. In the traditional NHS Trusts, a hydrophobic lens was used in 554 092 (77.0%) operations and a hydrophilic lens in 165 196 (23.0%) operations. In the ISTC sites a hydrophobic lens was used in 96 541 (51.4%) operations and a hydrophilic lens in 91 223 (48.6%) operations. The three most commonly used IOLs accounted for 560 741 (61.8%) cases whereas two least commonly used IOLs accounted for <10 000 operations.
Association between remuneration method and choice of IOL
The proportions of operations where a hydrophobic IOL was used were 65.7%, 96.5% and 56.4% in PbR, BC and both remuneration method centres respectively compared to hydrophilic IOLs which were used in 34.3%, 3.5% and 43.6% (p < 0.001). A hydrophobic lens was used in >95% of operations in 28 (57.1%) PbR centres, 12 (80.0%) BC centres and 8 (34.8%) both remuneration method centres. A hydrophilic lens was used in >95% operations in 14 (28.6%) PbR centres, none of the BC centres and 7 (30.4%) both remunerations method centres.
Across the five NHS years, the range in the percentage of operations performed in centres funded via BC that used a hydrophobic IOL was 76.5–89.0% compared to 11.0–23.5% that used a hydrophilic IOL. For centres funded via PbR, the percentage of operations that used a hydrophobic IOL was 68.9% in the 2015 NHS year and decreased in each NHS year to 57.2% in the 2019 NHS year, with the corollary that the percentage of operations that used hydrophilic IOLs was 31.1% in the 2015 NHS year and increased in each NHS year to 42.8% in the 2019 NHS year (Table 1).
Table 1.
The number of centres and operations for each NHS year according to the remuneration method with the percentage of operations that used hydrophobic and hydrophilic IOLs.
| NHS year and remuneration method | Number of centres | Number of operations | Percentage of operations using a hydrophobic lens | Percentage of operations using a hydrophilic lens |
|---|---|---|---|---|
| 2015 | ||||
| BC | 12 | 36,925 | 76.5 | 23.5 |
| PbR | 52 | 97,083 | 68.9 | 31.1 |
| 2016 | ||||
| BC | 23 | 56,062 | 89.0 | 11.0 |
| PbR | 56 | 110,815 | 66.6 | 33.4 |
| 2017 | ||||
| BC | 30 | 77,105 | 79.7 | 20.3 |
| PbR | 57 | 112,732 | 66.2 | 33.8 |
| 2018 | ||||
| BC | 30 | 92,034 | 78.6 | 21.4 |
| PbR | 57 | 120,837 | 65.5 | 34.5 |
| 2019 | ||||
| BC | 36 | 100,911 | 84.7 | 15.3 |
| PbR | 47 | 102,548 | 57.2 | 42.8 |
| Overall | ||||
| BC | 15 | 240,641 | 96.5 | 3.5 |
| PbR | 49 | 456,198 | 65.7 | 34.3 |
| Both | 23 | 210,213 | 56.4 | 43.6 |
BC block contract, PbR payment by results.
The two most frequently used IOLs were both hydrophobic lenses that were used in 76.1% of BC operations compared to 39.1% of PbR centres and 23.6% of both remuneration methods centres operations. Both of these hydrophobic IOLs had lower PCO rates than the most frequently used hydrophilic IOL (16.6% and 26.5% vs. 56.3% at 5 years’ postoperative) as reported in the recent RCOphth NOD study [12]. This hydrophilic IOL was the third most commonly used IOL in this study and was used in 0.8% of BC operations compared to 22.4 and 21.6% of PbR and both remuneration method centres, respectively. Each of the five hydrophilic IOLs were used in <3.0% of BC operations, and for each of these they were used in a higher proportion of PbR centres operations than BC centres operations. There was a significant difference in the use of IOL between the three remuneration methods (p < 0.001; Table 2).
Table 2.
The proportion of operations under each remuneration method that used each IOL with individual IOL posterior capsular opacification (PCO) rates.
| Remuneration method – percentage of operations for each IOL (column %) | PCO rates at (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IOL model | IOL material | Number of operations for each IOL | Number of centres that used the IOL | BC (N = 240,641) | PbR (N = 456,198) | Both (N = 210,213) | 1 year | 3 years | 5 years |
| Tecnis ZCB00 | Hydrophobic | 240,691 | 52 | 33.6 | 29.7 | 11.6 | 3.7 | 13.5 | 26.5 |
| AcrySof IQ SN60WF | Hydrophobic | 170,590 | 39 | 42.5 | 9.4 | 12.0 | 2.3 | 8.2 | 16.6 |
| Akreos Adapt | Hydrophilic | 149,460 | 40 | 0.8 | 22.4 | 21.6 | 6.2 | 38.7 | 56.3 |
| Hoya ISERT | Hydrophobic | 77,242 | 20 | 3.6 | 12.1 | 6.4 | 3.8 | 13.9 | 30.4 |
| Rayner Hydrophilic | Hydrophilic | 69,176 | 41 | 2.7 | 4.8 | 19.4 | 6.0 | 26.8 | 44.5 |
| EYECEE ONE | Hydrophobic | 67,954 | 29 | 11.6 | 1.9 | 15.0 | 4.7 | 15.4 | 26.0 |
| AcrySof SA60AT | Hydrophobic | 54,765 | 39 | 1.7 | 10.1 | 2.1 | 3.3 | 9.7 | 19.4 |
| Lenstac Softec | Hydrophilic | 29,466 | 17 | <0.1 | 5.4 | 2.4 | 5.8 | 27.7 | 46.7 |
| Zeiss CT Lucia | Hydrophobic | 28,013 | 22 | 1.6 | 2.4 | 6.4 | 2.2 | 17.0 | 46.6 |
| B & L Envista MX60 | Hydrophobic | 11,378 | 13 | 1.8 | 0.2 | 3.0 | 4.1 | 21.5 | 45.4 |
| B & L Incise | Hydrophilic | 7552 | 4 | <0.1 | 1.6 | 0.1 | 5.1 | 18.6 | 31.3 |
| Physiol A123 | Hydrophilic | 765 | 4 | 0.0 | 0.1 | <0.1 | 6.0 | 45.5 | 64.8 |
BC block contract, PbR payment by results.
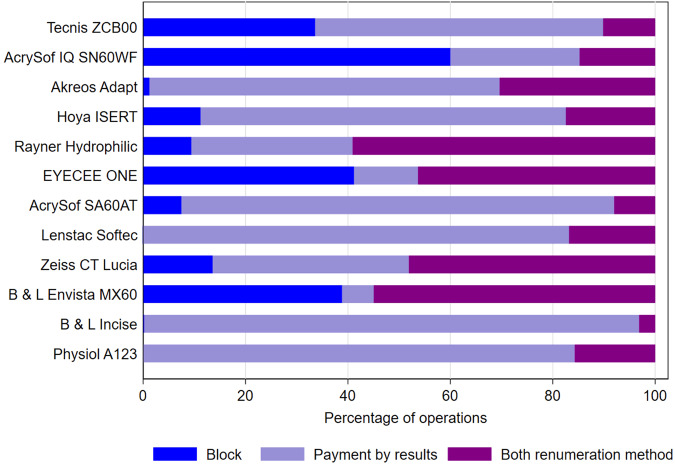
When the most frequently used hydrophobic IOL was used, 56.2% of operations were performed in centres funded via PbR, 33.6% via BC, and 10.2% via both remuneration methods. When the second most frequently used hydrophobic IOL was used, 25.3% of operations were performed in centres funded via PbR, 60.0% via BC, and 14.7% via both remuneration methods. In contrast, when the most frequently used hydrophilic lens was used, 68.4% of operations were performed in centres funded via PbR, 1.2% via BC, and 30.4% via both remuneration methods, (Fig. 1).
Fig. 1. IOL model usage broken down by remuneration method.
The proportion of each IOL’s usage by centres remunerated by Block Contract, Payment by Results and a mixture of these methods is presented. (N = 907,052 cataract operations performed by 2768 surgeons from 87 centres).
Discussion
To the best of our knowledge, this is the first UK study evaluating the influence of remuneration model on the choice of IOLs. Based on the data of >900 000 cataract operations, we demonstrated that cataract service providers in the UK who were on the BC model were significantly more likely to use hydrophobic IOLs than those on the PbR model, which favoured the use of hydrophilic IOLs. The preferential selection of certain IOL material by the service providers are likely driven by several factors, including the cost of IOL, the properties of IOL (which can influence the risk of certain postoperative complications), the short- and long-term impact on patients and healthcare services (for instance, need for YAG capsulotomy and risk of IOL opacification), and surgeons’/centre’s preference.
Currently PbR and BC are the two main remuneration models in the UK, each of which has its advantages and disadvantages. The BC model offers the advantages of timely and predictable payment and potentially lower costs for the commissioners (particularly for high-volume activities) but is disadvantaged by the lack of transparency and incentives for improving clinical care (as a regular fixed amount of payment is already made in advance) (https://www.england.nhs.uk/wp-content/uploads/2020/11/22-23NT_Introduction-to-the-national-tariff.pdf). On the other hand, the PbR model incentivises the providers to manage and treat more patients as the remuneration is proportionately linked to the level of activity [14]. It also provides greater transparency for activity transaction, which in turn facilitates analyses of the number and/or changing trends in the service activity, planning of hospital resources and workforce, and evaluation of the impact on health economies. Nonetheless, as the providers are remunerated by the level of activity, they may be less incentivised to manage complex or highly specialised cases (which will require considerably more resources which are disproportionately higher than the value of remuneration) or to adopt a more holistic approach to reduce the need for subsequent treatment or interventions after the initial treatment (as the providers will be remunerated for each treatment episode).
This study identified PbR as the main remuneration model (56%) adopted by the cataract service providers in 2015-2019 as opposed to BC model (17%). Centres remunerated by the PbR model were shown to be more likely to opt for a hydrophilic IOL instead of a hydrophobic IOL. The cost of IOLs can vary considerably by the IOL material, design and optical properties [15], but on average, the hydrophobic IOLs are more expensive than hydrophilic IOLs, though the cost of the same IOL may be different for different centres and is influenced by the volume of surgery and the local discussion and agreement held between the centre and the IOL manufacturer / distributor. Based on the mean cost, the hydrophobic IOLs were shown to be ≈£3 (or ≈7%) more expensive than the hydrophilic IOLs. In high-volume activities such as cataract operations, a small difference in the price per case can translate to a significant figure. Therefore, this could explain why providers remunerated by the PbR model were more likely to choose hydrophilic IOLs over hydrophobic IOLs for cost-saving purposes.
However, hydrophobic IOLs, initially more expensive for the service providers, may have a positive impact on the healthcare services and health economics in the longer term. A recent RCOphth NOD study [12] demonstrated that the risk of PCO is significantly lower in cases with hydrophobic IOLs than those with hydrophilic IOLs, consistent with the results of a recent meta-analysis by Zhao et al. [13], who reported a 62% significantly lesser risk of PCO with hydrophobic IOLs than with hydrophilic IOLs after cataract surgery. The RCOphth NOD data on the UK’s three most commonly used IOLs showed that the two hydrophobic IOLs had a 2–3 times lower risk of developing PCO than the hydrophilic IOL (16.6 and 26.5% vs. 56.3%) at 5 years postoperative and therefore less need for YAG capsulotomy. Other lens design factors, including posterior optic square-edged design, have also been shown to reduce the risk of PCO postoperatively [16–19]. Reassuringly, all 12 commonly used IOLs in the UK that were included in our study (see Table 2) adopt a posterior optic square-edged design, reflecting the continuous advancement in the IOL design and persistent effort in reducing postoperative complication following cataract surgery. Hydrophilic IOLs have also been reported to be associated with an increased risk of IOL opacification, which is more commonly observed after vitrectomy and endothelial keratoplasty with intraocular air or gas injection [20–22]. Interestingly, we observed that ISTCs (which were all remunerated by the PbR model) had an almost equal preference for hydrophobic and hydrophilic IOLs, suggesting that the selection of IOL material is influenced by more than a single factor (i.e., more than cost or remuneration model alone). Recently, the UK Ophthalmology Alliance (UKOA) has published a comprehensive guideline for guiding IOL selection within the NHS departments, taking into account the cost, safety, outcomes, and usability of IOLs (https://uk-oa.co.uk/wp-content/uploads/2019/02/Procuring-IOLs-1-December-2018.pdf).
This study represents the first study in the UK evaluating the potential perverse incentives of cost and remuneration model on the choice of IOL. Although not all English centres were included in this study, we captured data from many major cataract service providers in England (see “acknowledgement” section) and the findings are likely representative of the practice pattern in England. One of the limitations relates to the fact that we could not determine the exact proportion of operations each CCG commissioned or which CCG was the main CCG for any individual centre to decide on the remuneration models. BC model was assumed when it was employed by one of the CCGs for that individual centre as BC model is usually commissioned for treatment episodes with high levels of activity and is normally adopted by the main CCG of a particular centre.
In summary, we demonstrated that service providers remunerated by BC were more likely to select hydrophobic IOLs over hydrophilic IOLs. Although hydrophobic IOLs appear to be more expensive than hydrophobic IOLs at the point of surgical intervention, the potential favourable longer-term cost-effectiveness (primarily driven by reduced need for YAG capsulotomy postoperatively) and economic impact need to be taken into consideration. A future study modelling the longer-term health economics of the choice and cost of IOLs and the need for YAG capsulotomy would be beneficial.
Summary
What was known before
Cataract surgery with intraocular lens (IOL) implantation is one of the most commonly performed surgeries worldwide, and it places significant clinical and economic burden on the healthcare services.
Currently in the UK National Health Service, cataract service providers are mainly remunerated by two models; payment by results and block contract.
Hydrophobic IOLs are associated with less risk of posterior capsular opacification and reduced need for YAG capsulotomy.
What this study adds
This is the first UK study evaluating the potential influence of remuneration model and cost of IOL on the choice of IOL.
Cataract service providers remunerated by block contract, commissioned to deliver whole pathways of care, were more likely to select hydrophobic IOLs over hydrophilic IOLs.
Hydrophobic IOLs, although more expensive in the short term, can be expected to be economically favourable when whole-pathway costs are considered hence are attractive to providers on block contract.
Acknowledgements
It is with gratitude that we remember our friend and colleague Robert Johnston, who sadly died in September 2016. Without his inspirational vision, determination and career long commitment to quality improvement in ophthalmology this work would not have been possible. We acknowledge the support of the hospitals that participated in this National Ophthalmology Database Audit study and thank our medical and non-medical colleagues for the considerable time and effort devoted to data collection. The 87 centres with data in this analysis are listed in alphabetical order below separated into NHS Trusts and ISTC sites.
NHS Trusts:
Barking, Havering and Redbridge University Hospitals NHS Trust; Barts Health NHS Trust; Bolton NHS Foundation Trust; Bradford Teaching Hospitals NHS Foundation Trust; Calderdale and Huddersfield NHS Foundation Trust; Chesterfield Royal Hospital NHS Foundation Trust; County Durham and Darlington NHS Foundation Trust; East Kent Hospitals University NHS Foundation Trust; East Suffolk and North Essex NHS Foundation Trust; East Sussex Healthcare NHS Trust; Epsom and St Helier University Hospitals NHS Trust; Frimley Health NHS Foundation Trust; Gloucestershire Hospitals NHS Foundation Trust; Great Western Hospitals NHS Foundation Trust; Hampshire Hospitals NHS Foundation Trust; Harrogate and District NHS Foundation Trust; Imperial College Healthcare NHS Trust; Isle of Wight NHS Trust; James Paget University Hospitals NHS Foundation Trust; King’s College Hospital NHS Foundation Trust; Kingston Hospital NHS Foundation Trust; Leeds Teaching Hospitals NHS Trust; Liverpool University Hospitals NHS Foundation Trust; Manchester University NHS Foundation Trust; Mid Cheshire Hospitals NHS Foundation Trust; Mid and South Essex NHS Foundation Trust; Moorfields Eye Hospital NHS Foundation Trust*; Norfolk and Norwich University Hospitals NHS Foundation Trust; North Cumbria Integrated Care NHS Foundation Trust; North Middlesex University Hospital NHS Trust; North West Anglia NHS Foundation Trust; Nottingham University Hospitals NHS Trust; Oxford University Hospitals NHS Foundation Trust; Portsmouth Hospitals University NHS Trust; Royal Berkshire NHS Foundation Trust; Royal Cornwall Hospitals NHS Trust; Royal Free London NHS Foundation Trust; Royal United Hospitals Bath NHS Foundation Trust; Salisbury NHS Foundation Trust; Sandwell and West Birmingham Hospitals NHS Trust; Sheffield Teaching Hospitals NHS Foundation Trust; Sherwood Forest Hospitals NHS Foundation Trust; South Tees Hospitals NHS Foundation Trust; South Warwickshire University NHS Foundation Trust; Southport and Ormskirk Hospital NHS Trust; St Helens and Knowsley Teaching Hospitals NHS Trust; Surrey and Sussex Healthcare NHS Trust; The Hillingdon Hospitals NHS Foundation Trust; The Mid Yorkshire Hospitals NHS Trust; The Newcastle upon Tyne Hospitals NHS Foundation Trust; The Princess Alexandra Hospital NHS Trust; The Shrewsbury and Telford Hospital NHS Trust; Torbay and South Devon NHS Foundation Trust; United Lincolnshire Hospitals NHS Trust; University Hospital Southampton NHS Foundation Trust; University Hospitals Birmingham NHS Foundation Trust; University Hospitals Bristol and Weston NHS Foundation Trust; University Hospitals Coventry and Warwickshire NHS Trust; University Hospitals Dorset NHS Foundation Trust; University Hospitals Plymouth NHS Trust; Warrington and Halton Teaching Hospitals NHS Foundation Trust; Wirral University Teaching Hospital NHS Foundation Trust; Yeovil District Hospital NHS Foundation Trust;
ISTC sites:
St. Stephens Gate Medical Practice;
The following sites from Optegra Eye Health Care: Birmingham Eye Hospital; Central London Eye Hospital; Hampshire Eye Hospital; Manchester Eye Hospital; North London Eye Hospital; Surrey Eye Hospital; Yorkshire Eye Hospital;
The following sites from Practice Plus Group: Emersons Green; Ilford; Plymouth; Shepton Mallet; Southampton; Rochdale; Devizes; Gillingham; St. Mary’s Portsmouth
The following sites from SpaMedica: Birkenhead; Bolton; Liverpool; Manchester; Newton-le-Willows; Sheffield; Wakefield;
*Includes data from Bedford Hospital within Bedfordshire Hospitals NHS Foundation Trust and Croydon Health Services NHS Trust as the ophthalmology services in these places are part of Moorfields Eye Hospital NHS Foundation Trust.
Author contributions
All authors participated in initial discussions regarding study design and definitions. All authors reviewed initial drafts and approved final manuscript. DSJT and PHJD prepared the first draft (PHJD – “Methods” and “Results”; DSJT – “Introduction” and “Discussion”).
Funding
This analysis was funded by an unconditional grant from Alcon (Geneva, Switzerland) in support of the Royal College of Ophthalmologists’ National Ophthalmology Database cataract audit. The funders did not have any editorial oversight, right of veto or academic input into the analysis or write up of this work. The National Cataract Audit is currently funded through participation fees from centres as well as unrestricted financial contributions from Bausch + Lomb and Alcon.
Data availability
The NHS England Spend Comparison Service (SCS) data utilised for this study is available to appropriate NHS bodies to the SCS by application, but cannot be made publicly available by those accessing it for secondary purposes. Access to the data from Royal College of Ophthalmologists’ National Ophthalmology Database cataract audit can be accessed by application from appropriate academic or clinical organisations to noa.project@rcophth.ac.uk, however, preparation of the data would require a fee to cover full costs of extracting and preparing the necessary data for the intended purpose from the National Ophthalmology Database.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cicinelli MV, Buchan JC, Nicholson M, Varadaraj V, Khanna RC. Cataracts. Lancet. 2023;401:377–89. doi: 10.1016/S0140-6736(22)01839-6. [DOI] [PubMed] [Google Scholar]
- 2.Ting DSJ, Deshmukh R, Ting DSW, Ang M. Big data in corneal diseases and cataract: current applications and future directions. Front Big Data. 2023;6:1017420. doi: 10.3389/fdata.2023.1017420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Xu J, Chen X, Yao K. Cataract: advances in surgery and whether surgery remains the only treatment in future. Adv Ophthalmol Pract Res. 2021;1:100008. doi: 10.1016/j.aopr.2021.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamoureux EL, Fenwick E, Pesudovs K, Tan D. The impact of cataract surgery on quality of life. Curr Opin Ophthalmol. 2011;22:19–27. doi: 10.1097/ICU.0b013e3283414284. [DOI] [PubMed] [Google Scholar]
- 5.Muralikrishnan R, Venkatesh R, Prajna NV, Frick KD. Economic cost of cataract surgery procedures in an established eye care centre in Southern India. Ophthalmic Epidemiol. 2004;11:369–80. doi: 10.1080/09286580490888762. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan S, Ting DS, Lyall DA. Implantation of a customized toric intraocular lens for correction of post-keratoplasty astigmatism. Eye. 2013;27:531–7. doi: 10.1038/eye.2012.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erie JC. Rising cataract surgery rates: demand and supply. Ophthalmology. 2014;121:2–4. doi: 10.1016/j.ophtha.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Salerno LC, Tiveron MC, Jr, Alió JL. Multifocal intraocular lenses: types, outcomes, complications and how to solve them. Taiwan J Ophthalmol. 2017;7:179–84. doi: 10.4103/tjo.tjo_19_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ting DSJ, Rees J, Ng JY, Allen D, Steel DHW. Effect of high-vacuum setting on phacoemulsification efficiency. J Cataract Refract Surg. 2017;43:1135–9. doi: 10.1016/j.jcrs.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Day AC, Burr JM, Bennett K, Bunce C, Doré CJ, Rubin GS, et al. Femtosecond laser-assisted cataract surgery versus phacoemulsification cataract surgery (FACT): a randomized noninferiority trial. Ophthalmology. 2020;127:1012–9. doi: 10.1016/j.ophtha.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo C, Wang H, Chen X, Xu J, Yin H, Yao K. Recent advances of intraocular lens materials and surface modification in cataract surgery. Front Bioeng Biotechnol. 2022;10:913383. doi: 10.3389/fbioe.2022.913383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donachie PHJ, Barnes BL, Olaitan M, Sparrow JM, Buchan JC. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: Report 9, Risk factors for posterior capsule opacification. Eye. 2023;37:1633–9. [DOI] [PMC free article] [PubMed]
- 13.Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: an updated meta-analysis. Medicine. 2017;96:e8301. doi: 10.1097/MD.0000000000008301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon J. Payment by results-new financial flows in the NHS. BMJ. 2004;328:969–70. doi: 10.1136/bmj.328.7446.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berdahl J, Bala C, Dhariwal M, Rathi H, Gupta R. Cost-benefit analysis of a trifocal intraocular lens versus a monofocal intraocular lens from the patient’s perspective in the United States. PLoS ONE. 2022;17:e0277093. doi: 10.1371/journal.pone.0277093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanavaty MA, Spalton DJ, Boyce J, Brain A, Marshall J. Edge profile of commercially available square-edged intraocular lenses. J Cataract Refract Surg. 2008;34:677–86. doi: 10.1016/j.jcrs.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Nanavaty MA, Zukaite I, Salvage J. Edge profile of commercially available square-edged intraocular lenses: Part 2. J Cataract Refract Surg. 2019;45:847–53. doi: 10.1016/j.jcrs.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Ursell PG, Dhariwal M, Majirska K, Ender F, Kalson-Ray S, Venerus A, et al. Three-year incidence of Nd:YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: a UK Real World Evidence study. Eye. 2018;32:1579–89. doi: 10.1038/s41433-018-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ursell PG, Dhariwal M, O'Boyle D, Khan J, Venerus A. 5 year incidence of YAG capsulotomy and PCO after cataract surgery with single-piece monofocal intraocular lenses: a real-world evidence study of 20,763 eyes. Eye. 2020;34:960–8. doi: 10.1038/s41433-019-0630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giers BC, Tandogan T, Auffarth GU, Choi CY, Auerbach FN, Sel S, et al. Hydrophilic intraocular lens opacification after posterior lamellar keratoplasty - a material analysis with special reference to optical quality assessment. BMC Ophthalmol. 2017;17:150. doi: 10.1186/s12886-017-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng JY, Ting DSJ, Thomas S, Auffarth GU, Merz P. Opacification of hydrophilic acrylic intraocular lens following vitreoretinal surgery: a clinicopathological report. Can J Ophthalmol. 2021;56:e9–e11. doi: 10.1016/j.jcjo.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Grzybowski A, Zemaitiene R, Markeviciute A, Tuuminen R. Should we abandon hydrophilic intraocular lenses? Am J Ophthalmol. 2022;237:139–45. doi: 10.1016/j.ajo.2021.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The NHS England Spend Comparison Service (SCS) data utilised for this study is available to appropriate NHS bodies to the SCS by application, but cannot be made publicly available by those accessing it for secondary purposes. Access to the data from Royal College of Ophthalmologists’ National Ophthalmology Database cataract audit can be accessed by application from appropriate academic or clinical organisations to noa.project@rcophth.ac.uk, however, preparation of the data would require a fee to cover full costs of extracting and preparing the necessary data for the intended purpose from the National Ophthalmology Database.