Abstract
Streptococcus equi subsp. zooepidemicus is an opportunistic pathogen associated with disease in a range of domestic and wild animals. Despite its importance, very limited data is available on its survival and persistence on the environment. The goal of this study was to evaluate survival of S. zooepidemicus under ideal culture conditions and farm-like setting, in various surface types. Rubber, plastic, wood, and concrete samples were sterilized and inoculated with 109 CFU/mL of S. zooepidemicus with or without feces, and cultured under ideal conditions (37 °C, 5% CO2) or farm-like settings (20oC on air) for a maximum period of 25 days (n = 3/material/environment/feces-group/time-point). Under ideal conditions without feces, the bacterium survived for up to 17 days on plastic and rubber surfaces, 4 days on wood and less than 1 day on concrete (P < 0.05 between materials). Samples under ideal conditions with feces and farm-like settings without feces were negative by day 1 post-inoculation, regardless of the surface material used. Wood and concrete allowed S. zooepidemicus persistence for up to 3 days under farm-like settings when feces were present. This data suggests that environmental persistence of S. zooepidemicus is affected by surface type and incubation temperature.
Keywords: Zooepidemicus, swine, sepsis, sudden death, disease, enviroment, persistence, contamination
Introduction
Streptococcus equi subsp. zooepidemicus (S. zooepidemicus) is linked to disease in multiple domestic animal species, often concurrent with severe outbreaks leading to increased mortality (Bade et al. 2009; Cebra et al. 2000; Costa and Lage 2020; FitzGerald et al. 2017; Lamm et al. 2010; Pisoni et al. 2009; Priestnall and Erles 2011; Soedarmanto et al. 1996). It has also been related to zoonotic disease in immunosuppressed or elderly patients (Kerdsin et al. 2021; Kim et al. 2022; Klapa et al. 2021; Matsubayashi et al. 2021; Torres et al. 2018). This Gram-positive, Lancefield group C, facultative anaerobe is commonly identified in the upper respiratory or reproductive tract mucosa of adult domestic animals without disease (Kernaghan et al. 2012; Li et al. 2021). In 2019 S. zooepidemicus emerged in North America as cause of mass mortality and increased abortion rates in pigs (Costa and Lage 2020; Sitthicharoenchai et al. 2020). Clinically, the disease was undistinguishable from African Swine Fever, an exotic disease not yet identifed in the United States of America or Canada with colossal impacts to the pork industry, as evidenced in a recent outbreak in China (Costa et al. 2022; Wang et al. 2018).
S. zooepidemicus is a subspecies of S. equi, alongside S. equi subsp. equi (S. equi), the causative agent of strangles in horses, with greater than 98% DNA homology between the two subspecies (Timoney 2004). The impact of S. equi to the equine industry is remarkable, and it also has zoonotic potential. Contrary to S. zooepidemicus, a commercial vaccine is available for horses in selected countries, aiding in disease control and prevention (Robinson et al. 2020). While published data on S. equi environmental persistence is limited to 2 studies (Durham et al. 2018; Weese et al. 2009), to the best of our knowledge there is no data on the persistence of S. zooepidemicus on the environment other than composted equine litter (Poulin et al. 2015). This evidences an obvious knowledge gap on S. zooepidemicus epidemiology and the potential for spreading, via contaminated surfaces and environment.
The goal of this study was to investigate the survival period of Streptococcus equi subsp. zooepidemicus on surfaces present in commercial swine operations under ideal culture conditions and farm-like settings.
Methods
Preparation of inoculum
A pure culture of S. zooepidemicus ST-194 was used as inoculum (Costa and Lage 2020). This isolate was resistant to lincomycin, neomycin and tetracycline, based on the Kirby-Bauer disk diffusion method. Zones of growth inhibition were interpreted according to Clinical and Laboratory Standards Institute (CLSI) guidelines (Lewis 2022). Culture was performed using columbia colistin nalidixic acid (CNA) blood agar plates with 5% sheep blood (Becton, Dickinson Co., Maryland, USA). The plate was incubated for 24 h at 37 °C in an incubator gassed with 5% CO2. Using a sterile loop, a single colony was scrapped and transferred to a 50 mL conical tube (Corning Life Sciences, New York, USA) containing brain heart infusion broth (BHI) broth. This was incubated for 8 h in the conditions described above. Optical density (OD) of the broth was measured using a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, Massachusetts, USA) at 600 nm (OD 600) to achieve a final concentration of 1.4 × 109 CFU/mL. The inoculum was plated in a CNA agar plate prior to inoculation to confirm viability.
Preparation of surface samples
The surface area of all materials tested was 1 cm2. Rubber samples were melamine free (VWR, Pennsylvania, USA, Cat. # 59580-069). Polyethylene terephthalate (PET) plastic from household bottles were used as plastic surface. Northern white birch plank (Fisher Scientific, New Hampshire, USA, Cat. # S80332) was used to simulate wood surface. Commercial ready-mixed concrete (DAP, Maryland, USA) was used by setting it on a silicon tray, then removing the solidified material prior to use. All surface samples were autoclaved (121oC at 15 psi for 30 min) prior to inoculation.
Preparation of fecal samples
To simulate farm-like settings where organic matter is often present, a fresh fecal sample was collected from an 8 weeks-old, commercial crossbred castrated barrow free of any clinical signs suggestive of disease, including S. zooepidemicus. The sample was obtained following digital stimulation, and did not come into contact with the floor or any other surface. It was stored at -20oC until autoclaved at 121oC for 30 min and 15 psi. Autoclaved samples were stored at -20oC until use.
Inoculation and incubation of surfaces
Samples were either made of rubber, wood, plastic or concrete (material), and incubated at 37 °C, 5% CO2 (ideal conditions) or 20oC on air, 70% humidity (farm-like setting, environment), with or without feces (feces-group) over 10 time points (day 0 – within 1 h of inoculation and 1, 2, 3, 4, 7, 9, 13, 17, 20 and 25 days post-inoculation, time-point). A total of 480 surface samples were prepared for inoculation (n = 3/material/environment/feces-group/time-point).
Samples without feces were inoculated using 20 µL of inoculum and spread around the flat surface using sterile pipette tips, in order to cover the entire surface. Autoclaved feces were mixed at a ratio of 2:1 v/v with the inoculum (20 µL) to achieve a pasty consistency. This mixture was gently spread on top of the samples using a sterile 10 µL loop to cover the entire surface. Each sample was kept in a single well of 6-well tissue culture plate (Fisher Scientific, New Hampshire, USA,).
Bacterial viability assessment
At any given time point, 3 samples/material/environment/feces-group were assessed for viability of the inoculum. First, 8 mL of BHI broth was added to each well, covering the entire inoculated material. Broth-immersed surface samples were incubated for 12 h at 37 °C, 5% CO2, and a 10 µL aliquot was plated in CNA blood agar for further incubation at 37 °C, 5% CO2 for 24 h. Samples were considered positive if turbidity was observed in the broth sample and beta-haemolytic, mucoid, transparent colonies were visible in the agar plates following incubation. Sampling stopped for a given group if two consecutive samplings resulted in no growth in both broth and agar plates.
Statistical analyses
Differences between bacterial survival period on different materials within culture conditions and feces group was evaluated using the Mantel-Cox test (SPSS v23, IBM, Waltham, MA).
Results
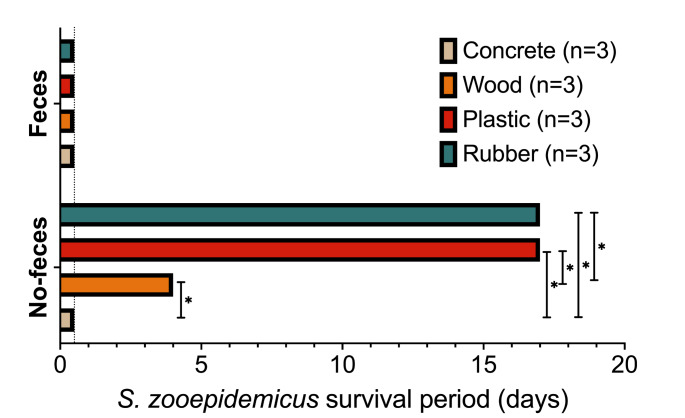
Viable bacteria were present in all groups immediately after inoculation. Plastic and rubber samples incubated at ideal conditions for S. zooepidemicus growth without feces enabled bacterial survival for up to 17 days, inclusive. This survival period was significantly different from wood and concrete surfaces (P = 0.004 and P = 0.001, respectively). Survival on wood was also significantly increased when compared to concrete (4 days and less than 1 day, respectively, P = 0.02). The addition of feces resulted in bacterial inactivation within 1 day of inoculation. A summary of these data is shown in Fig. 1.
Fig. 1.
S. zooepidemicus survival period under ideal culture conditions (37 °C, 5% CO2) on different surface materials with or without the presence of feces. Survival period is reported as the last day where a positive bacterial culture was obtained. Dotted line depicts post-inoculation control (1-hour). *- Significant difference between materials within feces group, as per Mantel-Cox test
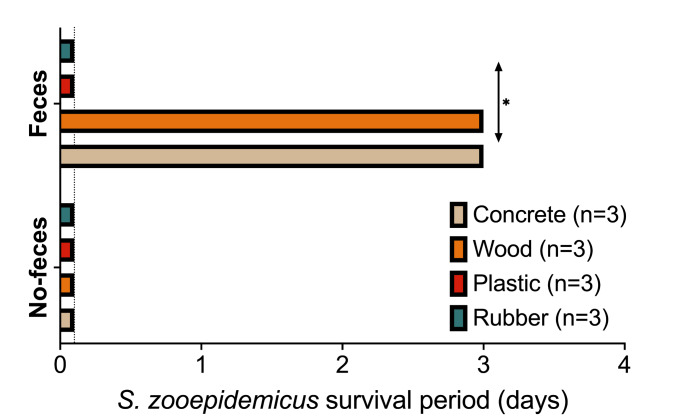
When samples were kept at 20oC the absence of feces resulted in no bacterial survival for longer than 24 h (Fig. 2). In contrast, the presence of feces extended S. zooepidemicus survival for up to 3 days in concrete and surface samples, when compared to rubber or plastic (P = 0.04). Plastic and rubber surfaces inoculated with feces were negative by 1 day post-inoculation.
Fig. 2.
S. zooepidemicus survival period under farm-like conditions (20oC on air, 30% humidity) on different surface materials with or without the presence of feces. Survival period is reported as the last day where a positive bacterial culture was obtained. Dotted line depicts post-inoculation control (1-hour). *- Significant difference between materials within feces group, as per Mantel-Cox test
Discussion
The data presented here evidenced that the survival of S. zooepidemicus in the environment is affected by the culture conditions, the presence of feces and the material type of the inoculated surface. Interestingly, under ideal culture conditions (37 °C, 5% CO2) the presence of feces inhibited bacterial growth, regardless of the surface material. The opposite was observed at room temperature, where surfaces inoculated with feces and S. zooepidemicus resulted in longer bacterial survival period. This data may guide the development of future biosecurity and disease elimination protocols, particularly in swine operations.
S. zooepidemicus survival in the environment is very poorly studied. A single report investigated its survival in compost of horse litter (Poulin et al. 2015). In that study, autoclaved litter at room temperature (21oC-23oC) enabled the survival of S. zooepidemicus for 5 days. This is similar what we found when inoculated wood and concrete surfaces were incubated with autoclaved swine feces (3 days) at 20oC. In parallel, Streptococcus equi subsp. equi (S. equi) is suggested to persist in wet sites for 9 days, and dry sites for 2 days outdoor in the summer. Persistence increased in the winter, for up to 34 days (Durham et al. 2018). A second study reported that S. equi could not survive for longer than 3 days on bare wood, painted wood, metal or rubber surfaces during summer and under the exposure to sunlight (which is not present in modern swine barns, that are fully enclosed) (Weese et al. 2009). In contrast to our findings, Weese et al., 2009 did not identify an effect associated with surface type, but equine respiratory mucus showed a degree of protective effect on bacterial survival. It is also noteworthy that both studies using S. equi did not use autoclaved surfaces, which may have a potential positive or negative impact on survival of the bacterium of interest. While autoclaving the surfaces does not simulate farm-like settings, it removes any live competitors or inhibitors produced by other microbes, allowing the direct evaluation of S. zooepidemicus survival in a best-case scenario. Based on this, our data could be cautiously extrapolated to infer that S. zooepidemicus survival for longer than 17 days in a barn is unlikely.
S. zooepidemicus is an opportunistic pathogen capable of infecting a wide range of warm and cold-blooded hosts(Abbott et al. 2010; Bade et al. 2009; Cebra et al. 2000; Corpa et al. 2018; Garmyn et al. 2020; Pisoni et al. 2009; Priestnall and Erles 2011; Soedarmanto et al. 1996; Stoughton and Gold 2015). Its importance to livestock is often reported as sporadic outbreaks, controlled following antimicrobial therapy (Pelkonen et al. 2013; Pisoni et al. 2009). However, since 2019, S. zooepidemicus emerged as a significant cause of mortality and abortions in swine reared in commercial settings, leading to significant economic loss to producers in North America (Costa and Lage 2020). These outbreaks, described by Costa and Lage, 2020, were associated with a specific sequence type – ST-194, the strain used in this study. Many questions surround this emergence in North American swine farms in the past 3 years, including the missing epidemiological link between the multiple sites affected across North America (Kuchipudi et al. 2021; M. Houben, 2021; Sitthicharoenchai et al., 2020). While direct contact seems to be required for infection of pigs, it is still unclear if S. zooepidemicus is able to persist in the environment and potentially become a source for re(infection) of pigs (Costa et al. 2022). Since no efficacious vaccine is available for use in this species, efforts to control this pathogen are largely based on biosecurity and antimicrobial therapy. A recent attempt to eliminate the pathogen by herd depopulation in a commercial swine operation was unsuccessful. Despite vigorous washing, disinfection and 45 days down time prior to repopulating the site with pigs from a herd free S. zooepidemicus, naïve animals were diagnosed with the disease weeks following reintroduction (White 2022). In this case, environmental persistence was suggested as one of the potential routes leading to the re-break. While this remains to be clarified in this specific case, our data shows that environmental contamination may not be a key factor here, whereas the presence of pests or other potential live carriers may have played a bigger role.
We understand that the data presented here is not definitive, and further studies looking at S. zooepidemicus survival under other conditions are required. However, it provides an initial look at the potential for persistence of this pathogen in areas where active shedding happened. It is difficult to make specific guidelines for the management of cleaning and decontamination protocols of potentially contaminated premises. Nevertheless, under the conditions of modern commercial swine farms, it is reasonable to expect that the environment should not be a source of infection for animals following cleaning and disinfection procedures and if pigs are reared in batches. This is radically different for backyard or small scale, organic premises where sunlight and the weather may directly impact pathogen survival, as well as wildlife may be in contact with infected pigs.
Conclusion
S. zooepidemicus persists for a limited period in an abiotic surface. Under the conditions of this study, the longest period where viable bacterium was detected was for 17 days at 37oC on plastic and rubber surfaces without feces. Concrete and wood supported bacterial viability for 3 days at 20oC if feces were present, the longest range detected under farm-like settings. Environmental contamination is an unlikely route of pathogen dissemination if biosecurity protocols are in place, but it may happen in unsupervised conditions. Future research on S. zooepidemicus viability on colder, winter-like temperatures is warranted to help further understand its epidemiology.
Author contributions
MC and SK designed and planned the experiment. SK and CR performed the experiments, collected the data. MC, CR and SK analyzed the data. MC and SK drafted the manuscript. All authors reviewed the manuscript.
Funding statement
This study was funded by: Saskatchewan Agriculture Ministry – Agriculture Development Fund (#20200280). Results Driven Agriculture Research (2021F122R). Natural Sciences and Engineering Research Council of Canada – Alliance Program (561369-20).
Data availability
All the data from this study is available in the manuscript.
Declarations
Conflict of interest disclosure
The authors declare no conflict of interest.
Consent to participate and publish
This study did not make use of human research participants.
Animal ethics
This study did not make use of live sentient animals. Ethics approval was not required as per the Animal Research Ethics Board – University of Saskatchewan.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbott Y, Acke E, Khan S, Muldoon EG, Markey BK, Pinilla M, Leonard FC, Steward K, Waller A. Zoonotic transmission of Streptococcus equi subsp. zooepidemicus from a dog to a handler. J Med Microbiol. 2010;59:120–123. doi: 10.1099/jmm.0.012930-0. [DOI] [PubMed] [Google Scholar]
- Bade D, Sibert G, Hallberg J, Portis E, Boucher J, Bryson L. Ceftiofur susceptibility of Streptococcus equi subsp zooepidemicus isolated from horses in North America between 1989 and 2008. Vet Ther. 2009;10:E1–7. [PubMed] [Google Scholar]
- Cebra CK, Heidel JR, Cebra ML, Tornquist SJ, Smith BB. Pathogenesis of Streptococcus zooepidemicus infection after intratracheal inoculation in llamas. Am J Vet Res. 2000;61:1525–1529. doi: 10.2460/ajvr.2000.61.1525. [DOI] [PubMed] [Google Scholar]
- Corpa JM, Carvallo F, Anderson ML, Nyaoke AC, Moore JD, Uzal FA. Streptococcus equi subspecies zooepidemicus septicemia in alpacas: three cases and review of the literature. J Vet Diagnost Invest. 2018;30:598–602. doi: 10.1177/1040638718772071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MO, Harding JCS, Huang Y, Nosach R (2022) Streptococcus equi subsp. zooepidemicus infection of pigs leads to shedding in feces and a carrier state. Transbound Emerg Dis [DOI] [PMC free article] [PubMed]
- Costa MO, Lage B. Streptococcus equi subsp. zooepidemicus–Associated Sudden deaths in Swine, Canada Emerg. Infect Dis. 2020;26:812636. doi: 10.3201/eid2610.191485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham AE, Hall YS, Kulp L, Underwood C. A study of the environmental survival of Streptococcus equi subspecies equi. Equine Vet J. 2018;50:861–864. doi: 10.1111/evj.12840. [DOI] [PubMed] [Google Scholar]
- FitzGerald W, Crowe B, Brennan P, Cassidy JP, Leahy M, McElroy MC, Casey M, Waller A, Mitchell C. Acute fatal haemorrhagic pneumonia caused by Streptococcus equi zooepidemicus in greyhounds in Ireland with subsequent typing of the isolates. Vet Rec. 2017;181:119. doi: 10.1136/vr.104275. [DOI] [PubMed] [Google Scholar]
- Garmyn A, Van de Velde N, Braeckmans D, Ronsmans S, Boyen F, Verlinden M. An Outbreak Associated with Streptococcus equi Subsp. zooepidemicus in Layers: evidence of Fecal Transmission. Avian Dis. 2020;64:343–346. doi: 10.1637/aviandiseases-D-19-00191. [DOI] [PubMed] [Google Scholar]
- Kerdsin A, Chopjitt P, Hatrongjit R, Boueroy P, Gottschalk M (2021) Zoonotic infection and clonal dissemination of Streptococcus equi subspecies zooepidemicus sequence type 194 isolated from humans in Thailand. Transbound Emerg Dis [DOI] [PubMed]
- Kernaghan S, Bujold AR, MacInnes JI. The microbiome of the soft palate of swine. Anim Health Res Rev. 2012;13:110–120. doi: 10.1017/S1466252312000102. [DOI] [PubMed] [Google Scholar]
- Kim M, Heo ST, Oh H, Kim M, Jo J. Human zoonotic infectious disease caused by Streptococcus equi subsp. zooepidemicus. Zoonoses Public Health. 2022;69:136–142. doi: 10.1111/zph.12895. [DOI] [PubMed] [Google Scholar]
- Klapa S, Grefer J, Sobottka I, Kurowski V (2021) A 56-Year-Old Woman with Chronic Hepatitis C Liver Disease and Meningitis due to Streptococcus equi subsp. Zooepidemicus. Case Rep Crit Care 2021, 7227054 [DOI] [PMC free article] [PubMed]
- Kuchipudi SV, Surendran Nair M, Yon M, Gontu A, Nissly RH, Barry R, Greenawalt D, Pierre T, Li L, Thirumalapura N, Tewari D, Jayarao B. A Novel Real-Time PCR assay for the Rapid detection of virulent Streptococcus equi Subspecies zooepidemicus-An Emerging Pathogen of Swine. Front Vet Sci. 2021;8:604675. doi: 10.3389/fvets.2021.604675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm CG, Ferguson AC, Lehenbauer TW, Love BC. Streptococcal infection in dogs: a retrospective study of 393 cases. Vet Pathol. 2010;47:387–395. doi: 10.1177/0300985809359601. [DOI] [PubMed] [Google Scholar]
- Lewis JS (2022) Performance standards for antimicrobial susceptibility testing, 32nd Edition. Clinical and Laboratory Standards Institute
- Li J, Zhao Y, Gao Y, Zhu Y, Holyoak GR, Zeng S. Treatments for Endometritis in Mares caused by Streptococcus equi Subspecies zooepidemicus: a structured literature review. J Equine Vet Sci. 2021;102:103430. doi: 10.1016/j.jevs.2021.103430. [DOI] [PubMed] [Google Scholar]
- Houben M, Peeters MOCL, Geudeke T, van Helmond J, Kwinten J, van Engelen E, Junker K, van der Putten B (2021) Streptococcus equi subsp. zooepidemicus, an emerging pig pathogen? In: European Symposium of Swine Health Management, Online, April 14th-17th
- Matsubayashi Y, Takashima N, Kondo Y, Wakisaka H, Suzuki T. Infected aortic aneurysm caused by Streptococcus zooepidemicus: a Case Report and Literature Review. Ann Vasc Dis. 2021;14:71–74. doi: 10.3400/avd.cr.20-00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen S, Lindahl SB, Suomala P, Karhukorpi J, Vuorinen S, Koivula I, Vaisanen T, Pentikainen J, Autio T, Tuuminen T. Transmission of Streptococcus equi subspecies zooepidemicus infection from horses to humans. Emerg Infect Dis. 2013;19:1041–1048. doi: 10.3201/eid1907.121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni G, Zadoks RN, Vimercati C, Locatelli C, Zanoni MG, Moroni P. Epidemiological investigation of Streptococcus equi subspecies zooepidemicus involved in clinical mastitis in dairy goats. J Dairy Sci. 2009;92:943–951. doi: 10.3168/jds.2008-1548. [DOI] [PubMed] [Google Scholar]
- Poulin A, Mitchell SD, Myer A, Harvey K, Hutchinson M, Causey R. A sustainable approach to the control of pathogens: the fate of streptococci in equine compost. Eur Sci Journ. 2015;11:3. [Google Scholar]
- Priestnall S, Erles K. Streptococcus zooepidemicus: an emerging canine pathogen. Vet J. 2011;188:142–148. doi: 10.1016/j.tvjl.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Waller AS, Frykberg L, Flock M, Zachrisson O, Guss B, Flock JI. Intramuscular vaccination with Strangvac is safe and induces protection against equine strangles caused by Streptococcus equi. Vaccine. 2020;38:4861–4868. doi: 10.1016/j.vaccine.2020.05.046. [DOI] [PubMed] [Google Scholar]
- Sitthicharoenchai P, Derscheid R, Schwartz K, Macedo N, Sahin O, Chen X, Li G, Main R, Burrough E. Cases of high mortality in cull sows and feeder pigs associated with Streptococcus equi subsp. zooepidemicus septicemia. J Vet Diagnost Invest. 2020;32:565–571. doi: 10.1177/1040638720927669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soedarmanto I, Pasaribu FH, Wibawan IW, Lammler C. Identification and molecular characterization of serological group C streptococci isolated from diseased pigs and monkeys in Indonesia. J Clin Microbiol. 1996;34:2201–2204. doi: 10.1128/jcm.34.9.2201-2204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoughton WB, Gold J. Streptococcus equi subsp zooepidemicus pleuropneumonia and peritonitis in a dromedary camel (Camelus dromedarius) calf in North America. J Am Vet Med Assoc. 2015;247:300–303. doi: 10.2460/javma.247.3.300. [DOI] [PubMed] [Google Scholar]
- Timoney JF. The pathogenic equine streptococci. Vet Res. 2004;35:397–409. doi: 10.1051/vetres:2004025. [DOI] [PubMed] [Google Scholar]
- Torres R, Santos TZ, Bernardes AFL, Soares PA, Soares ACC, Dias RS (2018) Outbreak of Glomerulonephritis caused by Streptococcus zooepidemicus SzPHV5 type in Monte Santo de Minas, Minas Gerais, Brazil. J. Clin. Microbiol. 56. [DOI] [PMC free article] [PubMed]
- Wang T, Sun Y, Qiu HJ. African swine fever: an unprecedented disaster and challenge to China. Infect Dis Poverty. 2018;7:111. doi: 10.1186/s40249-018-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese JS, Jarlot C, Morley PS. Survival of Streptococcus equi on surfaces in an outdoor environment. Can Vet J. 2009;50:968–970. [PMC free article] [PubMed] [Google Scholar]
- White J (2022) Experiences with Depopulation for Strep Zoo. In: Western Canadian Association of Swine Veterinarins Conference, Saskatoon, Canada, October 20th, 2022, 34
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data from this study is available in the manuscript.