Abstract
Escherichia coli strains overproducing the response regulator CheY respond to acetate by increasing their clockwise bias of flagellar rotation, even when they lack other chemotaxis proteins. With acetate metabolism mutants, we demonstrate that both acetate kinase and acetyl coenzyme A synthetase are involved in this response. Thus, a response was observed when one of these enzymes was missing but not when both were absent.
Bacteria such as Escherichia coli approach attractants and avoid repellents by modulating the direction of their flagellar rotation: attractants shift the rotation bias to counterclockwise (CCW), whereas repellents increase the clockwise (CW) bias (13). This is done by modulating the phosphorylation level of the response regulator CheY. The phosphorylated form of CheY (CheY∼P) can bind to the switch at the base of the flagellar motor and change the direction of its rotation from the CCW default direction to CW (for recent reviews, see references 5, 11, and 22). One of the puzzles in bacterial chemotaxis is the observation that the repellent acetate causes a prolonged CW bias even in E. coli strains from which the genes that encode some of the chemotaxis receptors and all of the cytoplasmic chemotaxis proteins are deleted (gutted strains) (25), provided that CheY is produced intracellularly. This acetate effect was attributed to the effect of an intermediate of acetate metabolism on CheY (25).
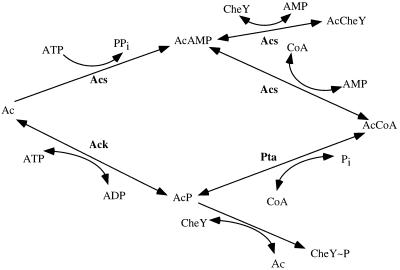
As shown in Fig. 1, acetate can be metabolized to acetyl coenzyme A by two distinct pathways involving the enzymes acetyl coenzyme A synthetase (Acs) (8) or acetate kinase (Ack) and phosphotransacetylase (Pta) (20). The intermediates in both pathways have been shown to chemically modify CheY in vitro: acetyladenylate (AcAMP) acetylates CheY and thereby increases its CW-promoting activity by orders of magnitude (demonstrated in cytoplasm-free envelopes) (6), whereas acetyl phosphate (AcP) phosphorylates CheY (14). It was first believed that the intermediate responsible for the acetate effect was AcAMP (25), but later it was suggested—on the basis of studies with ack mutants and pta mutants—that AcP, not AcAMP, was the intermediate responsible for this effect (10). If AcP is the intermediate, this implies that, in the acetate effect, CheY is activated by the well-known mechanism of CheY phosphorylation. If, however, AcAMP is the intermediate, this implies that another mechanism, different from the known mechanism of signal transduction, is responsible for CheY activation in the acetate effect. Therefore, distinguishing between these possibilities and resolving the molecular mechanism of the acetate effect are of great importance for understanding signal transduction in bacterial chemotaxis. Earlier studies could not distinguish unequivocally between the alternatives, because the acs gene of E. coli was unidentified and therefore Δacs mutants could not be isolated. The subsequent cloning of E. coli acs and the isolation of acs mutants (12) allowed us to identify in this study the intermediate that activates CheY in response to acetate.
FIG. 1.
Two pathways of acetate metabolism to acetyl coenzyme A and their links with CheY. Abbreviations: Ac, acetate; AcAMP, acetyladenylate; AcCheY, acetylated CheY; AcCoA, acetyl coenzyme A; Ack, acetate kinase; AcP, acetyl phosphate; Acs, acetyl coenzyme A synthetase; CheY∼P, phosphorylated CheY; CoA, coenzyme A; Pta, phosphotransacetylase.
Our aim was to determine whether the acetate effect occurs in mutants lacking one of the pathways for acetate metabolism. To this end we prepared an acs mutant, an ack pta double mutant, and an acs ack pta triple mutant (Fig. 1) in a gutted background. The construction of the gutted strain RBB1041 (Table 1), which is RP437 carrying Δ(cheA-cheZ)::Zeor, is described elsewhere (1). Bacteriophage P1 grown on RBB1041 was used to transduce CP875, AJW803, and CP911 to Zeor, yielding RBB1106, RBB1094, and RBB1097, respectively. P1 grown on AJW803 cells was used to transduce RBB1097 to Kanr, yielding RBB1109. Into each of these mutants we transformed the plasmid pRL22ΔPvuII (received from P. Matsumura), which encodes overproduction of CheY under the control of the tryptophan promoter (15). Unless indicated otherwise, the strains (Table 1) were grown to an optical density at 590 nm (OD590) of 0.4 in tryptone broth (TB) containing, when required, ampicillin (0.1 mg/ml) and, when indicated, acetate (5 mM). The cells were washed with motility medium containing 10 mM potassium phosphate buffer (pH 7.0) and 0.1 mM EDTA. The cells were tethered as described previously (19) in a flow chamber (7). The rotation of the tethered cells was recorded on video tape before and after the addition of 10 mM acetate (in motility medium). The recordings were analyzed manually and by a computerized motion analysis system (Galai, Migdal Haemek, Israel).
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| CP875 | thi-1 thr(Am)-1 leuB6 hisF(Am)159 rpsL136 lacY1 ΔlacX74 λlacY | 16 |
| AJW803 | CP875 Φ(Δacs::Km-1) | 12 |
| CP911 | CP875 Δ(ackA pta hisJ hisP dhu) zej-223::Tn10 | 16 |
| RBB1041 | RP437 Δ(cheA-cheZ)::Zeor | 1 |
| RBB1106 | CP875 Δ(cheA-cheZ)::Zeor | This study |
| RBB1094 | CP875 Φ(Δacs::Km-1) Δ(cheA-cheZ)::Zeor | This study |
| RBB1097 | CP875 Δ(ackA pta hisJ hisP dhu) zej-223::Tn10 Δ(cheA-cheZ)::Zeor | This study |
| RBB1109 | CP875 Δ(ackA pta hisJ hisP dhu) zej-223::Tn10 Φ(Δacs::Km-1) Δ(cheA-cheZ)::Zeor | This study |
| EW128 | RBB1106 (pRL22ΔPvuII) | This study |
| EW129 | RBB1094 (pRL22ΔPvuII) | This study |
| EW130 | RBB1097 (pRL22ΔPvuII) | This study |
| EW131 | RBB1109 (pRL22ΔPvuII) | This study |
| AJW524 | Δ(cheA-cheY)1590::Xhol (Tn5) Δ(lac)X74 | A. J. Wolfe |
| EW66 | AJW524 (pRL22ΔPvuII) | This study |
| AJW145 | AJW524 Δ(dhuA pta ackA hisQ hisP) zej-223::Tn10 | A. J. Wolfe |
| EW60 | AJW145 (pRL22ΔPvuII) | This study |
| AJW149 | AJW145 (λDFB19) | A. J. Wolfe |
| AJW538 | AJW524 (λDFB19) | A. J. Wolfe |
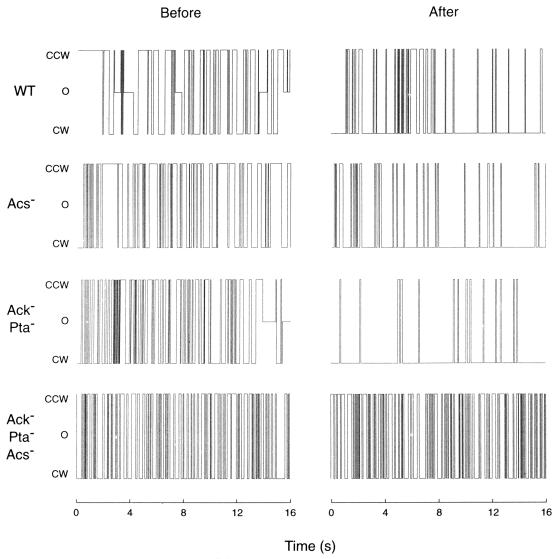
Figure 2 includes representative recordings demonstrating that all of the mutants, except the acs ack pta triple mutant, responded to acetate by increasing their CW bias. There were no signs of adaptation within the 20-min observation period. Table 2 summarizes the results for all of the cells examined. The response of the mutants lacking either the AcAMP pathway or the AcP pathway (strains EW129 and EW130, respectively) was less pronounced than that of the strain containing both pathways (strain EW128). This quantitative difference was reflected in the smaller fraction of responding cells and in the longer response delay time of the mutants. It should be noted that some of the mutant cells responded to acetate by stopping.
FIG. 2.
Representative plots of the rotational states of acetate metabolism mutants in a gutted background before and after acetate addition. The acetate-metabolizing wild-type strain was EW128, the Acs− strain was EW129, the Ack− Pta− strain was EW130, and the Acs− Ack− Pta− strain was EW131. Plots on the left represent the time 15 s before the addition of acetate; those on the right represent the time after the addition of 10 mM acetate (from top to bottom, 315, 415, 415, and 400 s).
TABLE 2.
Response of acetate metabolism mutants in a gutted background to acetate
| Gutted strain | Growth on acetatea | No. of cells analyzed | % Cells responding by CWb | % Cells responding by stoppingc | Response delay time (s)d |
|---|---|---|---|---|---|
| EW128 (wte) | − | 36 | 92 | 0 | 50 ± 35 |
| + | 32 | 91 | 9 | 93 ± 36 | |
| EW129 (Δacs) | − | 67 | 67 | 24 | 138 ± 65 |
| + | 34 | 65 | 24 | 106 ± 29 | |
| EW130 [Δ(ack pta)] | − | 34 | 27 | 44 | 178 ± 86 |
| + | 21 | 43 | 6 | 119 ± 32 | |
| +f | 46 | 70 | 17 | 67 ± 31 | |
| EW131 [Δ(acs ack pta)] | − | 36 | 0 | 0 | 0 |
| + | 29 | 0 | 0 | 0 |
Growth on TB containing 5 mM acetate.
Cells were considered to be CW responders if their CCW bias decreased by at least 50% in response to acetate addition.
Only cells which came to a complete stop and did not resume the rotation until the end of the observation period (10 min after acetate addition) were considered to have responded by stopping.
Mean ± standard deviation. The response delay time was measured from the time that acetate entered the cell (measured in a separate experiment, with nontethered dead cells as markers) and the first response.
Wild type with respect to acetate metabolism.
In this experiment, the cells were grown to an OD590 of 0.85 instead of 0.4.
Since Acs is inducible (9), we repeated the experiment with cells grown in TB in the presence of the inducer sodium acetate (5 mM). The consequence of this pretreatment was an increased response of the strain lacking the AcP pathway (EW130). The other strains were not affected. An acetate effect and an effect of Acs induction similar to those observed in EW130 were also observed in strain EW60 (Table 1), which is an ack pta mutant in another gutted background (data not shown). Here, too, the response was smaller than that of the acetate-metabolizing wild-type parent in this gutted background (strain EW66).
To examine further the involvement of Acs in the acetate effect, we took advantage of the fact that, with the progression of growth during the exponential phase, the Acs level and activity increase (12, 21, 24) and the AcP level decreases (17). All of the experiments reported above were carried out with strains grown to an OD590 of 0.4. To examine the effect of increased Acs activity on the acetate effect, we repeated the experiment with cells grown to an OD590 of 0.85, at which most of the acetate metabolism is carried out by Acs (12, 24). As shown in Table 2, ack pta mutants grown to such an OD responded to acetate more vigorously than cells grown to an OD590 of 0.4. The more vigorous acetate effect was evident from the higher fraction of responding cells and the shorter response delay time.
To determine whether the response to acetate also occurs in gutted cells that express wild-type levels of CheY, we tested strain AJW538 and its Δ(ack-pta) derivative, strain AJW149 (Table 1). These strains are single lysogens of a phage, λDFB19, that carries cheY under the variable control of the lactose promoter and its inducer isopropyl-β-d-thiogalactopyranoside (IPTG) (25). In the absence of IPTG, these cells express approximately wild-type levels of CheY (25). In accordance with the findings of Wolfe et al. (25), all of the AJW538 cells, in the absence of IPTG, responded to acetate. Cells of the Δ(ack-pta) mutant strain, AJW149, also responded to acetate in the absence of IPTG, though to a lesser degree (data not shown). As was true of cells that overexpressed CheY, the response by AJW149 cells was increased by growing them on acetate first. In either case, the response was greater in cells with overexpressed CheY.
To conclude, this study demonstrates that both routes of acetate metabolism, the Acs route via AcAMP and the Ack Pta route via AcP, contribute to the acetate effect. Neither one of the routes alone can achieve the complete acetate effect. The conclusion that Acs is involved in the acetate effect is based on the occurrence of the effect in ack pta double mutants (which completely lack AcP [17]) and on the observation that, in these mutants, the effect is increased with Acs induction and with increased Acs activity during the progression of growth. The involvement of Ack in the acetate effect was demonstrated earlier by Dailey and Berg (10). While endorsing their conclusion, the current study demonstrates that Ack is not the only player and that Acs is also involved.
It was shown in vitro that AcAMP acetylates CheY (6) and that AcP phosphorylates CheY, as does CheA (14), and that the consequence of both is CW rotation (3, 6). These observations, taken together with the fact that the acetate effect can only be observed in the presence of intracellular CheY (4, 25), suggest that the effect is caused by both Acs-mediated CheY acetylation and Ack-mediated CheY phosphorylation. Acs-mediated CheY acetylation was demonstrated both in vitro (6) and in vivo (2), and recently the acetylation sites were identified (18).
The acetate effect occurs also in cells expressing wild-type levels of CheY (reference 25 and this study). Nevertheless, it appears to be an effect that is not directly involved in the known mechanism of bacterial chemotaxis. This is because of its relatively long response delay time and because of the observation that acetate metabolism mutants appear to respond normally to classical chemotactic stimuli (10, 23). The acetate effect could modulate the chemotactic response or sensitivity according to the metabolic state of the cell. If so, the acetate effect via any of the two pathways might be one of the connecting links between central metabolism and chemotaxis. Even though the physiological role of the acetate effect is not yet known, the results described herein are important in the sense that they demonstrate unequivocally that CheY can be activated in vivo not only by phosphorylation but also by Acs, probably by acetylation.
Acknowledgments
We thank Alan J. Wolfe for helpful discussions, encouragement, and strains and Robert B. Bourret for helpful comments.
M.E. is an incumbent of the Jack and Simon Djanogly Professorial Chair in Biochemistry. This study was supported in part by grant no. 93-00211 from the United States-Israel Binational Science Foundation, Jerusalem, Israel, and by grant no. 449/96 from the Israel Science Foundation (both to M.E.). W.N.A. was supported by grant GM50860 from the National Institutes of Health to Robert B. Bourret.
REFERENCES
- 1.Abouhamad, W. N., D. Bray, and R. B. Bourret. Unpublished data.
- 2.Barak, R., and M. Eisenbach. Unpublished data.
- 3.Barak R, Eisenbach M. Correlation between phosphorylation of the chemotaxis protein CheY and its activity at the flagellar motor. Biochemistry. 1992;31:1821–1826. doi: 10.1021/bi00121a034. [DOI] [PubMed] [Google Scholar]
- 4.Barak, R., and M. Eisenbach. 1995. Unpublished data.
- 5.Barak R, Eisenbach M. Regulation of interaction between signaling protein CheY and flagellar motor during bacterial chemotaxis. Curr Top Cell Regul. 1996;34:137–158. doi: 10.1016/s0070-2137(96)80005-7. [DOI] [PubMed] [Google Scholar]
- 6.Barak R, Welch M, Yanovsky A, Oosawa K, Eisenbach M. Acetyladenylate or its derivative acetylates the chemotaxis protein CheY in vitro and increases its activity at the flagellar switch. Biochemistry. 1992;31:10099–10107. doi: 10.1021/bi00156a033. [DOI] [PubMed] [Google Scholar]
- 7.Berg H C, Block S M. A miniature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984;130:2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- 8.Berg P. Acyl adenylates: an enzymatic mechanism of acetate activation. J Biol Chem. 1956;222:991–1013. [PubMed] [Google Scholar]
- 9.Brown T D K, Jones-Mortimer M C, Kornberg H L. The enzymatic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977;102:327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- 10.Dailey F E, Berg H C. Change in direction of flagellar rotation in Escherichia coli mediated by acetate kinase. J Bacteriol. 1993;175:3236–3239. doi: 10.1128/jb.175.10.3236-3239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenbach M. Control of bacterial chemotaxis. Mol Microbiol. 1996;20:903–910. doi: 10.1111/j.1365-2958.1996.tb02531.x. [DOI] [PubMed] [Google Scholar]
- 12.Kumari S, Tishel R, Eisenbach M, Wolfe A J. Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol. 1995;177:2878–2886. doi: 10.1128/jb.177.10.2878-2886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen S H, Reader R W, Kort E N, Tso W-W, Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974;249:74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- 14.Lukat G S, McCleary W R, Stock A M, Stock J B. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc Natl Acad Sci USA. 1992;89:718–722. doi: 10.1073/pnas.89.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumura P, Rydel J J, Linzmeier R, Vacante D. Overexpression and sequence of the Escherichia coli cheY gene and biochemical activities of the CheY protein. J Bacteriol. 1984;160:36–41. doi: 10.1128/jb.160.1.36-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prüss B M, Nelms J M, Park C, Wolfe A J. Mutations in NADH:ubiquinone oxidoreductase of Escherichia coli affect growth on mixed amino acids. J Bacteriol. 1994;176:2143–2150. doi: 10.1128/jb.176.8.2143-2150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prüss B M, Wolfe A J. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol Microbiol. 1994;12:973–984. doi: 10.1111/j.1365-2958.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramakrishnan, R., and R. B. Bourret. Personal communication.
- 19.Ravid S, Eisenbach M. Correlation between bacteriophage chi adsorption and mode of flagellar rotation of Escherichia coli chemotaxis mutants. J Bacteriol. 1983;154:604–611. doi: 10.1128/jb.154.2.604-611.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose I A, Grunberg-Manago M, Korey S R, Ochoa S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954;211:737–756. [PubMed] [Google Scholar]
- 21.Shin S, Song S G, Lee D S, Pan J G, Park C. Involvement of iclR and rpoS in the induction of acs, the gene for acetyl coenzyme A synthetase of Escherichia coli K-12. FEMS Microbiol Lett. 1997;146:103–108. doi: 10.1111/j.1574-6968.1997.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 22.Stock J B, Surette M G. Chemotaxis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 1103–1129. [Google Scholar]
- 23.Tishel R. M.Sc. thesis. Rehovot, Israel: Weizmann Institute of Science; 1995. [Google Scholar]
- 24.Wolfe, A. J. Personal communication.
- 25.Wolfe A J, Conley M P, Berg H C. Acetyladenylate plays a role in controlling the direction of flagellar rotation. Proc Natl Acad Sci USA. 1988;85:6711–6715. doi: 10.1073/pnas.85.18.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]