Abstract
Stereoselective carbon-carbon bond formation via palladium-catalyzed asymmetric allylic alkylation is a crucial strategy to access chiral natural products and active pharmaceutical ingredients. However, catalysts based on the privileged Trost and Pfaltz-Helmchen-Williams PHOX ligands often require high loadings, specific preactivation protocols, and excess chiral ligand. This makes these reactions uneconomical, often unreproducible, and thus unsustainable. Here we report several chiral single-component Pd(0) precatalysts that are active and practically-applicable in a variety of asymmetric allylic alkylation reactions. Despite the decades-long history and widespread use of Trost-type ligands, the precatalysts in this work are the only reported examples of stable, isolable Pd(0) complexes with these ligands. Evaluating these precatalysts across nine asymmetric allylic alkylation reactions reveals high reactivity and selectivity at low Pd loading. Importantly, we also report an unprecedented Pd-catalyzed enantioselective allylation of a hydantoin, achieved on gram scale in high yield and enantioselectivity with only 0.2 mol% catalyst.
Subject terms: Asymmetric catalysis, Homogeneous catalysis, Synthetic chemistry methodology, Stereochemistry
Stereoselective carbon-carbon bond formation via palladium-catalyzed asymmetric allylic alkylation is important to access chiral natural products but commonly used catalyst systems often require high loadings or specific preactivation protocols. Here, the authors report several chiral single-component Pd(0) precatalysts that are active at low loadings for a variety of asymmetric allylic alkylation reactions.
Introduction
Homogeneous catalysis by transition metal complexes is one of the most powerful technologies in synthetic chemistry. Many of the most efficient and selective methods for the construction of organic molecules and materials are based on metal-catalyzed reactions, with applications encompassing bulk chemicals production, natural product synthesis, pharmaceutical manufacturing, and materials preparation. In synthetic organic chemistry, carbon-element bond forming reactions through organopalladium catalysis is without contest the most widely used strategy1–6. Key examples include the well-established Stille7, Suzuki-Miyaura8,9, Sonagashira10, Negishi11, Mizoroki-Heck12,13, and Buchwald-Hartwig reactions14, as well as the Tsuji-Trost reaction2,15–19. This latter example is well represented in natural product synthesis20–24, where it enables stereoselective Csp3–Csp3 and Csp3–Nsp3 couplings through asymmetric allylation of carbon and nitrogen nucleophiles.
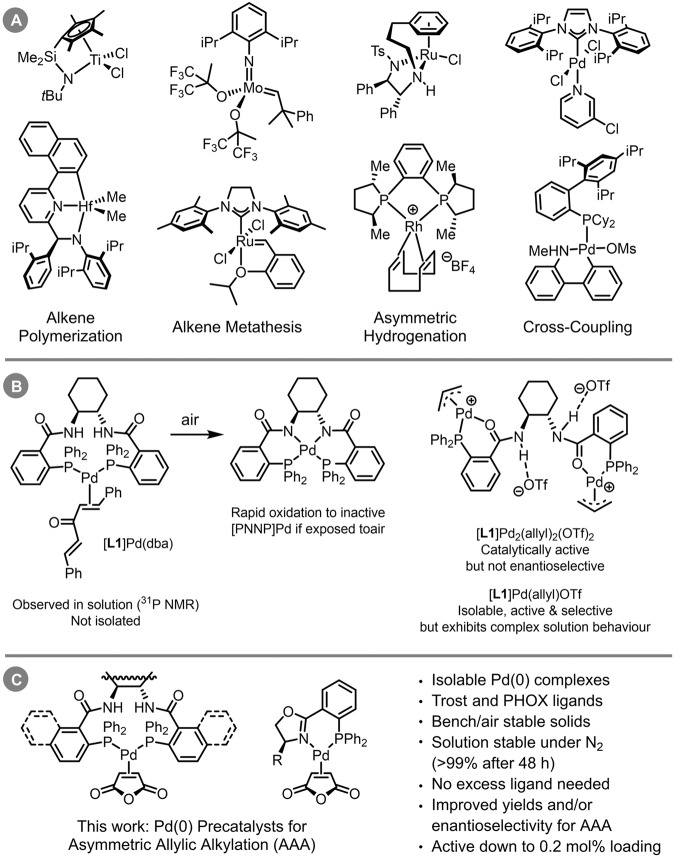
A key aspect of all catalytic reactions is the generation of an active catalyst from stable precursors. In many synthetic applications involving homogeneous organometallic catalysis, this is achieved through in situ combinations of supporting ligands and a metal source that are expected to assemble into an active form. While operationally convenient, this approach often leads to inefficient catalyst generation, negatively impacting activity, reproducibility, and/or selectivity. An alternative strategy is to employ single-component precatalysts. The latter would already contain the required supporting ligands, along with carefully chosen reactive sites that lead to rapid and complete activation. Key examples include group 4 metallocene and post-metallocene systems for alkene polymerization25–28; Mo- and Ru-based complexes for olefin metathesis29–32; Ru-, Rh-, and Ir-based hydrogenation catalysts33–35; and Pd-carbene or phosphine complexes for cross-coupling reactions (Fig. 1A)36–41.
Fig. 1. Homogeneous catalysts in organic chemistry.
A Exemplar organometallic precatalysts for specific transformations from across the periodic table. B Challenges with accessing precatalysts containing Trost-type ligands, including rapid oxidation of Pd(0) species derived from Pd2(dba)3, and complex solution behavior of Pd(II) allyl species. C This work: single-component Pd(0) precatalysts for asymmetric allylic alkylation.
Despite the success and widespread application of Pd-catalyzed asymmetric allylic alkylation, single-component precatalysts are rarely used for this chemistry2,17–19,42–46. Instead, these reactions rely on in situ catalyst generation, often using prolonged pretreatment of an achiral Pd source (e.g. Pd2(dba)3 or [Pd(allyl)Cl]2) with excess chiral ligand to ensure complete metalation. In some cases, this also requires heating for extended periods of time, rendering the process significantly more prone to reproducibility issues. Furthermore, the use of Pd2(dba)3 is itself a reproducibility concern, given the known issues of quality and stability associated with this compound, with (multiple) recrystallization required to ensure high purity47,48. Finally, the stabilizing ligands (e.g. dba) must be removed from the desired product during purification, which can be non-trivial.
While there are examples of L*-Pd(0)-alkene complexes with phosphinooxazoline (PHOX) ligands45,49–55, along with a few other chiral ligand classes56–60, these are not used as precatalysts for asymmetric allylic alkylation (with one specific exception45). In addition, accessing any isolable Pd complexes of the chiral diamide bisphosphine ligand platform – the eponymous Trost ligands – has proven particularly challenging (Fig. 1B). Early work from Trost, Breit, and Organ established that mixing (S,S)-PhDACH [L1, (S,S)−1,2-diaminocyclohexane-N,N’-bis(2-diphenylphosphinobenzoyl)] with Pd2(dba)3 generates a species consistent with [L1]Pd(dba), with a κ2-(P,P) coordination mode; however, this species was not isolated or further characterized61. Furthermore, this species rapidly oxidizes when exposed to air, forming catalytically inactive bis(amidate) [PNNP]PdII. Further studies by Amatore, Jutand, and co-workers revealed this deactivation can happen spontaneously even in the absence of external oxidant62. Thus, Pd(0) complexes of the Trost ligands were considered unstable and therefore unsuitable as precatalysts, as the [PNNP]PdII species is known to be catalytically inactive63.
As part of extensive studies on the solution behavior of catalytically relevant Pd species in asymmetric allylation reactions, Lloyd-Jones and co-workers reported a dipalladium(II) complex with L1, where each Pd center is coordinated by one phosphine and one carbonyl oxygen ([L1]Pd2(allyl)2(OTf)2, Fig. 1B)42. While this compound is catalytically active, it gives essentially racemic product during an attempted kinetic resolution of (±)-cyclopent-2-en-1-yl pivalate with NaCH(CO2Me)2, likely due to the κ2-(P,O) rather than κ2-(P,P) coordination mode. A monopalladium compound of the empirical formula [L1]Pd(allyl)(OTf) can be isolated when using a 1:1 stoichiometry of L1 to [Pd(allyl)(MeCN)2][OTf]. While this compound is both active and enantioselective, it is not well-defined, exhibiting concentration-dependent solution behavior. Multiple Pd-containing species can be present, including [L1]Pd2(allyl)2(OTf)2 and higher oligomers42,44. To the best of our knowledge, [L1]Pd(allyl)(OTf) is the only example of an isolable single-component precatalyst based on the Trost ligand platform, though the complexities of its synthesis and characterization seem to have precluded its wider use.
Here we report a class of stable and isolable chiral Pd precatalysts for asymmetric allylic alkylations (Fig. 1C). These Pd(0) complexes, which are based on either Trost-type or PHOX-type ligands, are easily prepared, well defined, monomeric, and easily handled without the need for an inert atmosphere or glovebox. Importantly, the oxidative degradation exhibited by [L1]Pd(dba) is not a significant issue with these precatalysts. In addition, they are highly effective as single-component precatalysts for a multitude of asymmetric allylic alkylation reactions, operating with improved yield, selectivity, catalyst loading, and/or operational simplicity relative to established systems. The advantages of these compounds as precatalysts are exemplified not only in known allylation chemistry, but also by their ability to catalyze the unprecedented enantioselective allylation of a hydantoin-derived nucleophile, exhibiting high yield and stereoselectivity with only 0.2 mol% catalyst on gram scale (TON = 465).
Results
Precatalyst synthesis and characterization
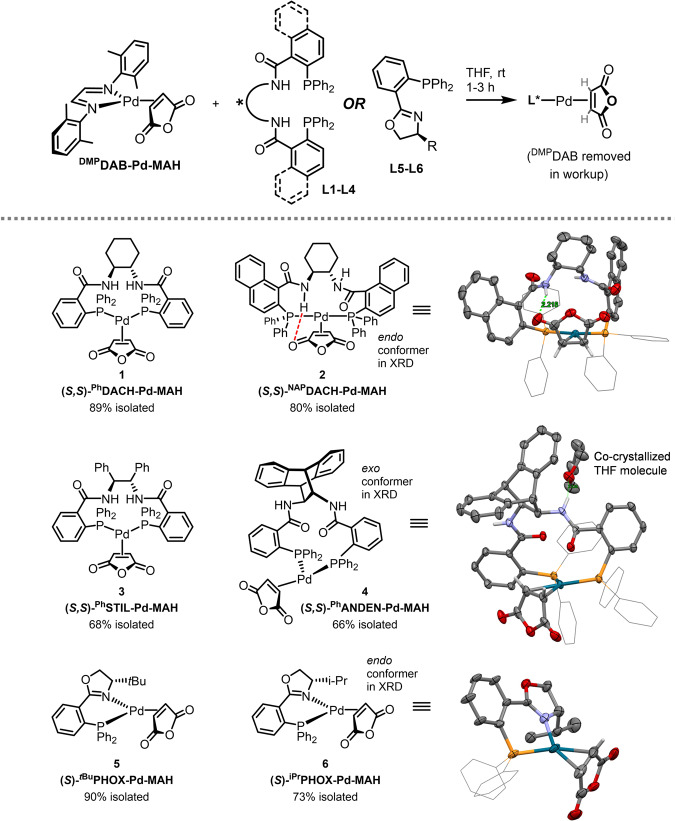
Given the aforementioned challenges with accessing discrete, stable Pd precatalysts with Trost-type ligands, we targeted these Pd(0) species first. Using DMPDAB-Pd-MAH – a Pd(0) source we recently reported64 – we examined ligand substitution reactions with a set of six chiral ligands (L1–L6, Fig. 2). In each case, reaction monitoring by 1H and 31P NMR spectroscopy (with internal standard) revealed complete consumption of DMPDAB-Pd-MAH and quantitative formation of L*-Pd-MAH (1–6) in <20 min at room temperature; this rapid metalation is also evident by a near-immediate color change from red/purple to yellow.
Fig. 2. Synthesis of chiral Pd(0) precatalysts 1–6 via ligand substitution of DMPDAB-Pd-MAH. Solid-state molecular structures (X-ray diffraction, 50% probability ellipsoids, Ph groups on phosphorus shown in wireframe for clarity) shown for compounds 2, 4, and 6; crystal/diffraction data and metrical parameters are in Supplementary Information.
Intramolecular H-bond in 2 indicated with green dashed line, NH---O distance = 2.22 Å. Intermolecular H-bond in 4 between amide N–H and THF molecule indicated with green dashed line, NH---OTHF distance = 2.23 Å.
On preparative-scale, 1-6 can be easily isolated and purified by simple precipitation to remove the soluble DMPDAB, giving the chiral Pd(0) complexes in 66–90% isolated yield after purification. We have prepared up to 340 mg of 1 in a single batch, demonstrating a path to scalable synthesis. These complexes are derived from the most common chiral ligands used in asymmetric allylation: four Trost ligands (L1–L4), and two PHOX ligands (L5 and L6). In addition to full characterization by multinuclear NMR spectroscopy (vide infra and SI) and high-resolution mass spectrometry, we have obtained solid-state molecular structures by X-ray crystallography for three complexes (2, 4, and 6). Surprisingly, complexes 1-4 represent the only characterized examples of isolable Pd complexes containing Trost-type ligands bearing the desired κ2-(P,P) binding mode. This is especially noteworthy given the long and extensive history of these ligands in Pd catalysis.
The solid-state molecular structures of 2 and 4 reveal not only the desired κ2-(P,P) coordination mode, but also two distinct conformations. In complex 2, the MAH binds with the carbonyl groups pointing toward the chiral tether of the NAPDACH ligand (endo conformation). This enables an intramolecular hydrogen bond between an amide N–H and a carbonyl oxygen from MAH. Importantly, this type of hydrogen bond between ligand and substrate is proposed as a critical intramolecular interaction in the mechanism for stereoinduction in many asymmetric allylic alkylation reactions65. Here, we directly observe this interaction in both the solid state and in solution (vide infra). In contrast, the PhANDEN complex 4 has the MAH oxygens pointing away from the chiral tether (exo conformation). Notably, there is an intermolecular hydrogen bond observed between one of the amides N–H and a co-crystallized THF molecule. These alternative geometries are due to the flexibility of the large macrocyclic chelate in these Trost-type complexes.
Solution-phase characterization of complexes 1–6 by multinuclear and multidimensional NMR spectroscopy reveals the presence of two distinct species in every case. For both PHOX-based complexes 5 and 6, these two species are present in an approximately 1:1 ratio, as indicated by 1H and 31P NMR spectroscopy in either d2-DCM or d8-THF. We attribute this solution behavior to the presence of both endo and exo conformers, with no clear energetic preference for either45,53. This conformer assignment is supported by 2D NMR spectroscopy characterization data, including 2D NOESY (see SI). For complex 1, 31P NMR spectra obtained in either d2-DCM or d8-THF also contain two sets of signals, each of which is a matching pair of doublets that is characteristic of bidentate κ2-(P,P) coordination to Pd (Fig. 3A). In DCM, the two sets of signals have a 55:45 peak area ratio; in contrast, in THF there is a 14:1 peak area ratio between the major and minor signals. As for 5 and 6, we attribute this to the presence of two distinct conformers, the ratio of which has a clear solvent dependence.
Fig. 3. Conformational analysis of compound 1.

A 31P NMR spectra of complex 1 in d2-DCM and d8-THF, revealing two conformers (1-exo and 1-endo). B Calculated structures of 1-endo and 1-exo (gas phase geometry shown). C Relative electronic energies of 1-exo and 1-endo in the gas phase, DCM, and THF (implicit solvation models; one explicit THF molecule included in THF-solvation calculations).
Based on these data and the solid-state molecular structures of 2 and 4, we propose that the two species in solution are conformers 1-endo and 1-exo (Fig. 3B), with 1-exo being the major (based on extensive NMR spectroscopy, Supplementary Figs. 1–19). To explain these observations, we propose that in a relatively non-polar solvent (e.g. DCM), 1-endo and 1-exo are similar in energy; however, with the addition of a hydrogen-bond-accepting solvent (e.g. THF), hydrogen bonding between an N–H on the ligand and the solvent will stabilize the 1-exo conformer. Notably, complexes 2–4 exhibit similar NMR spectroscopic characteristics, consistent with endo and exo conformers (see SI for further details).
Finally, to support our conformer assignments, we calculated relative electronic energies of the 1-exo and the 1-endo conformers (RI-B2PLYP-D3BJ/def2-TZVP//RI-BP86-D3BJ/def2-SVP, with def2-TZVP/C and def2/J auxiliary basis sets for the RI part, respectively) using conductor-like polarizable continuum (CPCM) implicit solvation models (Fig. 3C)66. In gas phase calculations, both conformers exhibit an intramolecular hydrogen bond between the amides, and the 1-endo conformer has an additional hydrogen bond between an amide N–H and the MAH (as observed in the solid-state structure of 2). This leads to the 1-endo conformer being 10.5 kJ mol−1 more stable than 1-exo. However, the 1-exo and 1-endo energies calculated in DCM solvent are very close, with 1-exo only slightly more stable (0.5 kJ mol−1 difference). To compare energies in THF, we used both the CPCM implicit model and included one explicit THF molecule hydrogen-bonding to a ligand N–H in 1-exo (as observed in the solid-state structure of 4). This results in 1-exo being 6.3 kJ mol−1 more stable. These calculations support not only the assignment of 1-exo as the major conformer in THF, but also exhibit the same solvent effect trend as our spectroscopic observations.
To assess whether complexes 1–6 could be robust and user-friendly precatalysts, we examined their solution stability in THF (15–50 mg/mL) over 48 h by 31P NMR spectroscopy (Fig. 4). With solutions prepared under N2, the concentrations of all six complexes remain unchanged over this period. Given the aforementioned rapid oxidation of [L1]Pd(dba) to the tetradentate [PNNP]PdII species, we also assessed the stability of a THF solution of complex 1 after exposure to air. After 48 h, there is still >80% of 1 intact, with the mass balance comprised of [PNNP]PdII. In stark contrast, a mixture of L1 and Pd2(dba)3·CHCl3 is 50% oxidized after only 30 min, and is completely converted to [PNNP]PdII within 18 h. As a solid, complex 1 is very stable when stored under N2 at room temperature (2 years thus far). Complex 1 is even stable for weeks as a solid under air (<6% area of new signals observed in 31P NMR spectra after one month). Thus, precatalysts 1–6 can be handled and used without the need for a glovebox. In fact, all catalytic evaluations were carried out by weighing the precatalysts and preparing solutions under air (vide infra).
Fig. 4. Concentration versus time plot for complexes 1–6 in THF under N2, showing no decomposition over at least 48 h, as well as complex 1 and L1 + Pd2(dba)3•CHCl3 under air.
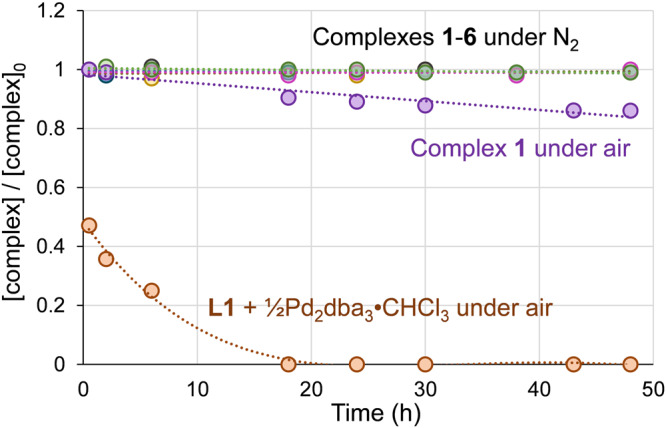
These latter experiments show slow decomposition of 1 (>80% intact after 48 h) and rapid decomposition of [L1]Pd(dba) (<50% remaining after 30 min).
Deploying single component precatalysts in asymmetric allylic alkylations
To conduct an initial assessment of the performance of precatalysts 1–6 in diverse asymmetric allylic alkylations, we compared them against standard catalyst systems for a series of benchmark reactions. Representative comparisons at identical Pd loading are given in Fig. 5; additional comparator data under alternative reaction conditions is available in Supplementary Tables 5–8. We chose five criteria to evaluate each catalyst system, visualized in a quantitative radar plot analysis, including isolated yield, enantioselectivity, reaction time, the Pd to chiral ligand ratio, and the Pd / ligand premixing time. In every case, catalytic reactions were set up and executed without the use of a glovebox.
Fig. 5. Comparison of established catalyst systems with single-component precatalysts for benchmark AAA reactions.
A Malonation of cyclohexenyl carbonate. B Desymmetrization of meso bis(acetate). C Dynamic kinetic asymmetric transformation (DYKAT) of racemic epoxide 12 with phthalimide (11). D Intramolecular decarboxylative asymmetric allylic alkylation (DAAA).
We initially evaluated the extensively studied malonate allylation with racemic cyclohex-2-en-1-yl methyl carbonate (7) (Fig. 5A, Supplementary Table 5)65,67. Under standard reaction conditions (i.e. 5 mol% Pd, a Pd/L ratio of 1:1.5, and a 30 min pretreatment of Pd precursor and ligand), the allylation proceeds efficiently and selectively using Pd2(dba)3·CHCl3 to give 8 (96% yield, 95% ee). However, simply reducing the Pd/L ratio to 1:1 results in no observable reaction. This somewhat shocking result is not only undesirable from a cost perspective (the additional wasted chiral ligand), it indicates a potential and severe failure mode if insufficient L1 is used. In sharp contrast, the use of PhDACH-Pd-MAH (1) as a single-component precatalyst, which has a 1:1 Pd/L ratio and does not require any pretreatment to ensure complete metalation, delivers 8 in 86% isolated yield and 96% ee.
A classic example in asymmetric allylation is the desymmetrisation of meso−2-en-1,4-diol diester 9 using the Pd/L1 system (Fig. 5B, Supplementary Table 6). We employed the bis(acetate) substrate 9 to accentuate reactivity differences between the systems, as it is known to be less reactive than the more commonly used bis(benzoate) derivative68,69. At a Pd loading of 2 mol% and a Pd/L1 ratio of 1:1, Pd2dba3·CHCl3 performs poorly (15% yield, 92% ee) while [Pd(allyl)Cl]2 generates 10 in high enantiopurity but only modest yield (46% yield, 99% ee). PhDACH-Pd-MAH (1) is superior to these known systems, exhibiting excellent enantioselectivity with improved yield, all without catalyst preactivation (68% yield, 98% ee).
Next, we examined the Pd-catalyzed asymmetric allylation of phthalimide (11) as a nitrogen-based nucleophile using racemic epoxide 12 (Fig. 5C, Supplementary Table 7); this transformation has been utilized in several total syntheses70–72. Using [Pd(allyl)Cl]2 or Pd2(dba)3·CHCl3 as the Pd source with NAPDACH (L2) as the ligand (0.8 mol% Pd, 1:1.5 ratio of Pd/L2), we obtained good yields of the homoallyl alcohol 13 (99% and 82% respectively); however, we were not able to achieve the reported level of enantioselectivity even after rigorous purification of every component of the reaction mixture (72% and 64% versus the reported 96%). The reduced enantioselectivites observed for these Pd sources, especially when using Pd2dba3•CHCl3, may be due to a lower effective catalyst concentration due to oxidative decomposition to [PNNP]PdII. Trost et al. reported that the enantioselectivity of this reaction is sensitive to catalyst loading, such that “lowering the catalyst further [below 0.4 mol%] significantly decreased the ee.”70,72 Amatore et al. reported that L2–Pd–dba converts to the corresponding [PNNP]PdII even in the absence of dioxygen. Notably, the single-component precatalyst NAPDACH-Pd-MAH (2) achieves excellent yield while attaining higher enantioselectivity than the in situ systems, without the need for excess L2 or preactivation (99% yield, 81% ee). This result highlights the robustness of our Pd(0) precatalysts, which are effective even for reactions that are clearly sensitive to the specific reaction conditions.
Decarboxylative asymmetric allylic alkylation (DAAA) reactions represent a broad class of Pd-catalyzed allylation chemistry, and hence an important testing ground for our precatalysts73,74. We therefore examined Pd-catalyzed DAAA of allyl (2-phenyl-cyclohexyl)carbonate (14, Fig. 5D, Supplementary Table 8) using the (S,S)-PhANDEN (L4) based catalyst. At 2 mol% Pd loading and 3 mol% of L4, Pd2(dba)3·CHCl3 is able to achieve complete conversion in 30 min, giving 15 in 77% yield with good enantioselectivity (83% ee). However, this system fails to reach completion even after 24 h when the Pd/L4 ratio reduced to 1:1 (39% yield). In sharp contrast, the single-component PhANDEN-Pd-MAH (4) complex achieves complete conversion and good enantioselectivity without requiring an excess of L4 (87% yield, 81% ee).
To assess PHOX-type precatalysts 5 and 6, we used the reaction of racemic allyl acetate 16 with dimethyl malonate, which is often used as a model reaction when developing Pd catalysts for asymmetric allylation chemistry. Hence, we compared [Pd(allyl)Cl]2 and Pd2(dba)3·CHCl3 with complexes 5 and 6 for the synthesis of 17 (Fig. 6, Supplementary Table 9)51,75. Using conditions for mild enolate generation with bis(trimethylsilyl)acetamide (BSA) and catalytic KOAc, and the established method of premixing the Pd source and PHOX ligand (1.25 equiv. per Pd) at 50 °C for 1 h to ensure complete metalation, [Pd(allyl)Cl]2 delivered 17 in 93% yield and 95% ee after 1 h (2 mol% Pd). However, if the 1 h preactivation step is conducted at room temperature, [Pd(allyl)Cl]2 suffers from diminished yield even after 24 h (67%). Interestingly, the single-component chiral precatalyst (S)-iPrPHOX-Pd-MAH (6), which can simply be added as a solid to the reaction mixture, requires no preactivation or excess chiral ligand and provides 17 in 84% isolated yield and 96% ee (opposite enantiomer to that obtained with (R)-iPrPHOX), though the reaction requires 24 h to reach completion. Use of the tBuPHOX ligand resulted in similar outcomes, where [Pd(allyl)Cl]2 outperforms the MAH-based catalyst 5 in terms of rate, but the in situ system again requires preactivation at 50 °C.
Fig. 6. Comparison of established PHOX-type catalyst systems with single component precatalysts for benchmark AAA reactions.
A Malonation of allyl acetate derivatives using weakly basic conditions for soft enolization. B Malonation of allyl acetate derivatives using strongly basic conditions to generate a potassium enolate nucleophile.
In contrast to the weakly basic BSA/KOAc reaction conditions, use of KH to generate a harder potassium enolate results in excellent reaction rates for all three catalyst systems (Fig. 6B), with the single-component precatalysts 5 and 6 matching the reactivity of the [Pd(allyl)Cl]2 based in situ system (1 h reaction time for complete conversion in each case). In addition, both 5 and 6 exhibit excellent enantioselectivity. The increase in reaction rate for the MAH-based systems under strongly basic conditions indicates that the catalyst activation processes are likely dependent on the reaction conditions.
Single component precatalysts enable asymmetric allylation of butenolide nucleophiles
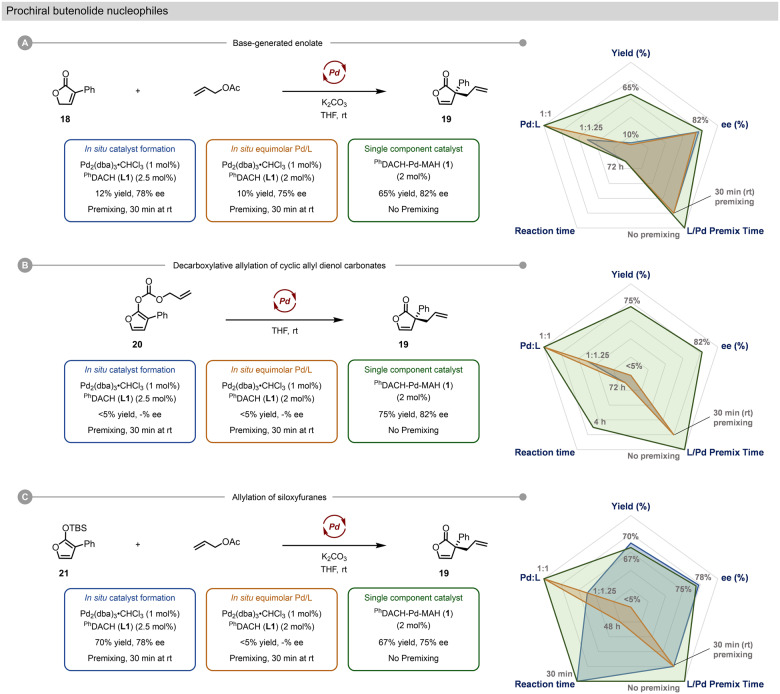
To explore the suitability of our precatalysts beyond well-known allylation chemistry, we turned toward the preparation of enantioenriched heterocycles relevant to the synthesis of natural products and commodity chemicals. Specifically, the enantioselective functionalization of prochiral heterocycles is a vast field with scores of possible methodologies76–79. In Pd-catalyzed asymmetric allylation, there are three predominant methods: a) the direct allylation of a prochiral heterocycle (e.g. 18, Fig. 7A); b) the decarboxylative allylation (e.g. 20, Fig. 7B); or c) the allylation of the corresponding enol silane (e.g. 21, Fig. 7C). We explored the formation of chiral butenolide 19 by each of these methods, comparing in situ catalyst formation using Pd2(dba)3·CHCl3 to the use of single-component chiral precatalyst 180,81.
Fig. 7. Comparison of precatalyst 1 to in situ systems for challenging asymmetric allylic alkylation reactions in the synthesis of chiral butenolide 19.
A Direct allylation of 18. B Decarboxylative allylation of 20. C Allylation of siloxyfuran 21.
The direct allylation of 18 and the decarboxylative allylation of 20 did proceed using high Pd2(dba)3·CHCl3 loading (Supplementary Tables 10 and 11); however, both approaches were ineffective when lowering the Pd loading from 10 to 2 mol%, resulting in little to no reactivity even after extended reaction times (Fig. 7A, B). In sharp contrast, PhDACH-Pd-MAH (1) operates at 2 mol% loading to give 21 in good yield (65-75%) and enantiopurity (82% ee) under both conditions. While the direct allylation reaction time is long (72 h), 1 is able to catalyze complete decarboxylative allylation to form 19 within 4 h.
In the third approach, involving allylation of the enol silane 21, Pd2(dba)3·CHCl3 is able to operate at the lower 2 mol% Pd loading, giving full conversion and good yields after only 15 min; however, this system failed when the Pd/L ratio was reduced to 1:1 (Fig. 7C). This was circumvented by using 2 mol% of PhDACH-Pd-MAH (1) under otherwise identical conditions, giving comparable yield and selectivity without the need for excess chiral ligand. Overall, the ability to reliably reduce both Pd and L loading in these transformations, and avoid pretreatment steps, are significant advantages in cost, robustness, and operational simplicity.
Precatalyst-enabled discovery and optimization of the enantioselective allylation of hydantoins
The rapid in situ metalation of Trost-type and PHOX-type ligands to the DMPDAB-Pd-MAH precursor, and the stability and ease-of-use for the single-component precatalysts 1–6, render these systems particularly suited to reaction discovery and optimization via parallel experimentation techniques. Indeed, the remarkable air stability of the L*-Pd-MAH complexes both in the solid state and in solution makes setting up array-based experiments operationally simple without the need for a glovebox. To demonstrate this feature of our precatalysts, we employed them in the rapid optimization of a Pd-catalyzed asymmetric allylation of hydantoin 22 (Fig. 8). Hydantoin heterocycles are common motifs in FDA approved drugs; however, there are a striking lack of methods for their asymmetric functionalisation82–85. To the best of our knowledge, there are no prior reports of enantioselective hydantoin allylation using Pd catalysis. If realized, this would be a powerful method to install a stereogenic tetrasubstituted carbon onto a protected amino acid motif.
Fig. 8. Application of MAH-based Pd precatalysts in reaction discovery and optimization for the asymmetric allylation of hydantoin derivative 22.
At low Pd loading. A Ligand selection achieved using in situ catalyst formation with DMPDAB–Pd–MAH. B 24 reaction solvent/base array using precatalyst 1. C Optimization of continuous variable setpoints to maximize yield, enantioselectivity, and catalyst TON. D Gram-scale synthesis of 23 using optimized conditions.
Our initial set of experiments (Round 1) used DMPDAB-Pd-MAH (2 mol%) as the source of Pd(0) to screen chiral ligands for the proposed reaction using in situ catalyst formation (Pd/L, 1:1.5) Notably, only (S,S)-PhDACH gave a good enantioselectivity (70% ee), with all other ligands providing poor selectivity. Round 2 screening utilized chiral complex (S,S)-PhDACH-Pd-MAH (1, 2 mol%) in a solvent/base array to rapidly optimize the reaction conditions at room temperature. While the use of the single-component chiral precatalyst did increase the yield to 90% using NaHMDS/THF, all other solvent/base combinations either failed to give product in >40% yield and/or failed to improve the enantioselectivity. To further optimize the enantioselectivity and catalytic efficiency of this reaction, we reduced the catalyst loading, lowered the reaction temperature, and increased the reactant concentration (Round 3). Consistent with previous reports showing that higher catalyst concentrations can decrease stereoselectivity, we observe improved % ee when lowering the Pd loading from 1 mol% (76% ee) to 0.2 mol% (86% ee)42–44,76. We did not observe any improvement of the enantioselectivity when carrying out the reaction at −40 °C, nor when lowering further the Pd loading to 0.1 mol%. Finally, increasing the overall reaction concentration to 0.2 M and maintaining a Pd catalyst loading of 0.2 mol% led to 90% yield and 88% ee. Translating these reaction conditions to a gram scale (4 mmol) preparation gave 93% isolated yield of 23 with 88% ee, corresponding to a catalyst TON of 465. Notably, only 7.2 mg of precatalyst 1 was required to produce 1.34 g of the desired product 23. Indeed, such low chiral catalyst loading is rare for Pd-catalyzed asymmetric allylation chemistry, and clearly demonstrates the power of using a single-component catalyst system for both screening and preparative-scale synthesis.
Discussion
We have developed a set of bench stable chiral Pd(0) precatalysts based on the two key ligand classes used in Pd-catalyzed asymmetric allylation chemistry. By exploiting the rapid ligand substitution chemistry exhibited by the DMPDAB-Pd-MAH precursor, Pd complexes with Trost-type and PHOX-type ligands can either be generated in situ, or isolated as single-component precatalysts. These complexes have been fully characterized, including X-ray diffraction studies on three examples, enabling interrogation of their conformations in solution and the solid-state. This includes direct observation of an intramolecular hydrogen bond between an amide N–H and a C=O of the coordinated maleic anhydride, which models a key interaction in the proposed mechanism for stereoinduction with the Trost ligand class. Importantly for their application in catalysis, the Trost-type complexes 1–4 do not suffer from rapid decomposition via oxidation, and can be handled as solids or even in solution under air.
To demonstrate the potential of these precatalysts, we have applied them to nine distinct Pd-catalyzed asymmetric allylation reactions. In all cases, these precatalysts compared favorably with traditional catalytic systems, maintaining enantioselectivity while enabling lower catalyst loadings. In addition, the single-component nature of 1–6 gives a perfectly controlled Pd/L stoichiometry, and circumvents the need to ensure ligand metalation is complete prior to substrate addition.
To exemplify the power of these precatalysts, we applied them to realize the heretofore unprecedented enantioselective allylation of a hydantoin derivative at only 0.2 mol% catalyst loading. The discovery and optimization of this reaction was enabled by microscale array-based experiments, to which our precatalysts are ideally suited. Overall, the selectivity, activity, stability, and practicality of these single-component precatalysts make them powerful and attractive alternatives to established in situ catalyst generation procedures. Complexes 1–6 and analogs thereof should therefore be evaluated as standard systems for Pd-catalyzed asymmetric allylations in academic and industrial applications alike.
Methods
General procedure for the synthesis of the single component chiral catalysts
These reactions were set up in a glovebox under an inert nitrogen atmosphere due to the potential oxygen-sensitivity of the phosphine ligands. A representative procedure for the synthesis of 1 is given here, with specific procedures for all precatalysts given in Supplementary Information. A 4 dram (~15 mL) vial was charged with DMPDAB-Pd-MAH (200.1 mg, 0.43 mmol), L1 (294.8 mg, 0.43 mmol, 1.0 equiv), anhydrous inhibitor-free THF (7 mL) and a cross-shaped magnetic stirbar. The reaction mixture was stirred at room temperature for 2 h. During this time, the solution changes color from an initial dark red/purple to yellow. The solvent was then removed in vacuo to give a yellow solid residue. This residue was mixed thoroughly with hexanes/diethyl ether (1:1), followed by decantation of the liquid phase (with or without centrifugation as required). This trituration/decantation process was repeated 5 more times to remove the DMPDAB byproduct as well as any excess phosphine ligand. The solid was then dried in vacuo to give the corresponding single component chiral catalyst 1 as a pale yellow solid (339.0 mg, 89% yield).
Procedure for the gram-scale synthesis of 23
This reaction was set up without use of a glovebox using standard air-free techniques involving nitrogen streams and balloons. An oven-dried 50 mL round-bottom flask was charged with 22 (1.29 g, 4.00 mmol) and a teflon-coated stirbar. The flask was sealed with a rubber septum and placed under nitrogen atmosphere. THF (14 mL) was added, and the contents cooled to 0 °C. NaHMDS (1 M in THF, 6.00 mL, 6.00 mmol, 1.5 equiv) was added via syringe with stirring, and the mixture was stirred for 1 h at 0 °C. PhDACH-Pd-MAH (1) was then added as a solid (7.2 mg, 0.0080 mmol, 0.2 mol%), followed by allyl acetate via syringe (0.860 mL, 8.00 mmol, 2 equiv). The mixture was stirred at 0 °C for 24 h, after which time TLC analysis indicated complete conversion. The reaction was quenched with the addition of H2O (50 mL), followed by extraction with CH2Cl2 (3 ×50 mL). The combined organic layers were then washed with 10% wt/wt aqueous citric acid (2 ×50 mL) and brine (50 mL). The organic phase was dried over MgSO4, filtered, and the solvent removed under reduced pressure. The crude product was purified by column chromatography using silica gel and hexane/EtOAc eluent (100:0 to 80:20 v/v) to yield 23 (1.35 g, 89% ee). Enantiopurity was measured by HPLC using a hexane/isopropanol mobile phase (isocratic 9:1 v/v, 1 mL/min) and a Daicel CHIRALPAKTM IC column (250 ×4.6 mm; 5 μm; 35 °C).
Specific procedures for all catalytic reactions are given in the Supplementary Information.
Supplementary information
Source data
Acknowledgements
J.H., J.L. and D.C.L. acknowledge with respect the Lekwungen peoples, on whose traditional territory the University of Victoria (UVic) stands, and the Songhees, Esquimalt, and WSÁNEĆ peoples whose historical relationships with the land continue today. They also thank Prof. Irina Paci at UVic for fruitful discussions regarding computational studies. D.C.L. thanks UVic (start-up funding), NSERC (RGPIN-2019-04985 and I2IPJ 561560 – 21), and CFI / BCKDF (JELF 38750) for general operating and equipment funds. S.A. and T.K. thank Dr. Rodolphe Tamion and Dr. Jean Fournier for fruitful discussions. S.A. thanks Dr. Lucile Vaysse-Ludot from Oril Industrie affiliated to Les Laboratoires Servier and Queen Mary University of London for financial support. S.E.J. thanks Dr. William A. Sabbers, Dr. Taylor M. Keller, and Prof. Michael J. Zdilla for fruitful discussions regarding X-ray crystallography.
Author contributions
J.H., T.K., D.C.L. and S.A. conceived and designed the study. J.H., T.K. and F.R. performed the synthetic experiments and analyzed the data for all compounds. J.L. performed computational analysis. S.E.J. conducted single crystal X-ray diffraction experiments and analysis. A.J., S.A. and D.C.L. supervised the research. J.H., T.K., F.R., S.A. and D.C.L. co-wrote the paper with input from all authors.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
Source data are present. All processed spectroscopic and chromatographic data from the present study is contained in the Supplementary Information. The atomic coordinates for structures generated by DFT calculations of 1-exo and 1-endo are provided in xlsx format as a Source Data File. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2258957-2258960. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
Competing interests
The authors declare the following competing financial interest(s): PCT international patent applications have been filed based partly on this work (WO2022153180A1, WO2023156972A1). DMPDAB-Pd-MAH is commercially available from MilliporeSigma (product number 922889).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jingjun Huang, Thomas Keenan.
Contributor Information
Stellios Arseniyadis, Email: s.arseniyadis@qmul.ac.uk.
David C. Leitch, Email: dcleitch@uvic.ca
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-43512-8.
References
- 1.Negishi E. A genealogy of Pd-catalyzed cross-coupling. J. Organomet. Chem. 2002;653:34–40. doi: 10.1016/S0022-328X(02)01273-1. [DOI] [Google Scholar]
- 2.Trost BM, Crawley ML. Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem. Rev. 2003;103:2921–2944. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]
- 3.Nicolaou KC, Bulger PG, Sarlah D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005;44:4442–4489. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- 4.Magano J, Dunetz JR. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011;111:2177–2250. doi: 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- 5.Brown DG, Boström J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 2016;59:4443–4458. doi: 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Castillo P, Buchwald SL. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 2016;116:12564–12649. doi: 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordovilla C, Bartolomé C, Martínez-Ilarduya JM, Espinet P. The Stille reaction, 38 years later. ACS Catal. 2015;5:3040–3053. doi: 10.1021/acscatal.5b00448. [DOI] [Google Scholar]
- 8.Miyaura Norio, Suzuki Akira. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]
- 9.Martin R, Buchwald SL. Palladium-catalyzed Suzuki−Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 2008;41:1461–1473. doi: 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinchilla R, Nájera C. The Sonogashira reaction: a booming methodology in synthetic organic chemistry. Chem. Rev. 2007;107:874–922. doi: 10.1021/cr050992x. [DOI] [PubMed] [Google Scholar]
- 11.Haas D, Hammann JM, Greiner R, Knochel P. Recent developments in Negishi cross-coupling reactions. ACS Catal. 2016;6:1540–1552. doi: 10.1021/acscatal.5b02718. [DOI] [Google Scholar]
- 12.Heck RF. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979;12:146–151. doi: 10.1021/ar50136a006. [DOI] [Google Scholar]
- 13.Beletskaya IP, Cheprakov AV. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- 14.Buchwald SL, Hartwig JF. In praise of casic research as a vehicle to practical applications: palladium-catalyzed coupling to form carbon-nitrogen bonds. Isr. J. Chem. 2020;60:177–179. doi: 10.1002/ijch.201900167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuji J, Takahashi H, Morikawa M. Organic syntheses by means of noble metal compounds XVII. Reaction of π-allylpalladium chloride with nucleophiles. Tet. Lett. 1965;6:4387–4388. doi: 10.1016/S0040-4039(00)71674-1. [DOI] [Google Scholar]
- 16.Trost BM, Fullerton TJ. New synthetic reactions. Allylic alkylation. J. Am. Chem. Soc. 1973;95:292–294. doi: 10.1021/ja00782a080. [DOI] [Google Scholar]
- 17.Trost BM, Van Vranken DL. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 1996;96:395–422. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]
- 18.Butt NA, Zhang W. Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates. Chem. Soc. Rev. 2015;44:7929–7967. doi: 10.1039/C5CS00144G. [DOI] [PubMed] [Google Scholar]
- 19.Pàmies O, et al. Recent advances in enantioselective Pd-catalyzed allylic substitution: from design to applications. Chem. Rev. 2021;121:4373–4505. doi: 10.1021/acs.chemrev.0c00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trost BM, Bai Y, Bai W-J, Schultz JE. Enantioselective divergent synthesis of C19-oxo eburnane alkaloids via palladium-catalyzed asymmetric allylic alkylation of an N-alkyl-α,β-unsaturated lactam. J. Am. Chem. Soc. 2019;141:4811–4814. doi: 10.1021/jacs.9b00788. [DOI] [PubMed] [Google Scholar]
- 21.Richard F, et al. Enantioselective synthesis of γ-butenolides through Pd-catalysed C5-selective allylation of siloxyfurans. Nat. Synth. 2022;1:641–648. doi: 10.1038/s44160-022-00109-1. [DOI] [Google Scholar]
- 22.Robert A, et al. Enantioselective, convergent synthesis of the ineleganolide core by a tandem annulation cascade. Chem. Sci. 2016;8:507–514. doi: 10.1039/c6sc03347d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White DE, Stewart IC, Seashore-Ludlow BA, Grubbs RH, Stoltz BM. A general enantioselective route to the chamigrene natural product family. Tetrahedron. 2010;66:4668–4686. doi: 10.1016/j.tet.2010.04.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trost BM, Horne DB, Woltering MJ. Palladium-catalyzed DYKAT of butadiene monoepoxide: enantioselective total synthesis of (+)-DMDP, (−)-bulgecinine, and (+)-broussonetine G. Chem. Eur. J. 2006;12:6607–6620. doi: 10.1002/chem.200600202. [DOI] [PubMed] [Google Scholar]
- 25.Kaminsky W. Highly active metallocene catalysts for olefin polymerization. J. Chem. Soc. Dalton Trans. 1998;1998:1413–1418. doi: 10.1039/a800056e. [DOI] [PubMed] [Google Scholar]
- 26.McKnight AL, Waymouth RM. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998;98:2587–2598. doi: 10.1021/cr940442r. [DOI] [PubMed] [Google Scholar]
- 27.Resconi L, Cavallo L, Fait A, Piemontesi F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000;100:1253–1346. doi: 10.1021/cr9804691. [DOI] [PubMed] [Google Scholar]
- 28.Baier MC, Zuideveld MA, Mecking S. Post-metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Ed. 2014;53:9722–9744. doi: 10.1002/anie.201400799. [DOI] [PubMed] [Google Scholar]
- 29.Schrock RR. High-oxidation-state molybdenum and tungsten alkylidyne complexes. Acc. Chem. Res. 1986;19:342–348. doi: 10.1021/ar00131a003. [DOI] [Google Scholar]
- 30.Trnka TM, Grubbs RH. The development of L2X2RuCHR olefin metathesis catalysts: an organometallic success story. Acc. Chem. Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 31.Schrock RR. Recent advances in high oxidation state Mo and W imido alkylidene chemistry. Chem. Rev. 2009;109:3211–3226. doi: 10.1021/cr800502p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vougioukalakis GC, Grubbs RH. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 2010;110:1746–1787. doi: 10.1021/cr9002424. [DOI] [PubMed] [Google Scholar]
- 33.Noyori R, Hashiguchi S. Asymmetric transfer hydrogenation catalyzed by chiral ruthenium complexes. Acc. Chem. Res. 1997;30:97–102. doi: 10.1021/ar9502341. [DOI] [Google Scholar]
- 34.Xie J-H, Zhu S-F, Zhou Q-L. Transition metal-catalyzed enantioselective hydrogenation of enamines and imines. Chem. Rev. 2011;111:1713–1760. doi: 10.1021/cr100218m. [DOI] [PubMed] [Google Scholar]
- 35.Dub PA, Gordon JC. The role of the metal-bound N–H functionality in Noyori-type molecular catalysts. Nat. Rev. Chem. 2018;2:396–408. doi: 10.1038/s41570-018-0049-z. [DOI] [Google Scholar]
- 36.Marion N, Nolan SP. Well-defined N-heterocyclic carbenes−palladium(II) precatalysts for cross-coupling reactions. Acc. Chem. Res. 2008;41:1440–1449. doi: 10.1021/ar800020y. [DOI] [PubMed] [Google Scholar]
- 37.Valente C, Belowich ME, Hadei N, Organ MG. Pd-PEPPSI complexes and the Negishi reaction. Eur. J. Org. Chem. 2010;2010:4343–4354. doi: 10.1002/ejoc.201000359. [DOI] [Google Scholar]
- 38.Bruno NC, Tudge MT, Buchwald SL. Design and preparation of new palladium precatalysts for C–C and C–N cross-coupling reactions. Chem. Sci. 2013;4:916–920. doi: 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeAngelis AJ, Gildner PG, Chow R, Colacot TJ. Generating active “L-Pd(0)” via neutral or cationic π-allylpalladium complexes featuring biaryl/bipyrazolylphosphines: synthetic, mechanistic, and structure–activity studies in challenging cross-coupling reactions. J. Org. Chem. 2015;80:6794–6813. doi: 10.1021/acs.joc.5b01005. [DOI] [PubMed] [Google Scholar]
- 40.Hazari N, Melvin PR, Beromi MM. Well-defined nickel and palladium precatalysts for cross-coupling. Nat. Rev. Chem. 2017;1:1–16. doi: 10.1038/s41570-017-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaughnessy KH. Development of palladium precatalysts that efficiently generate LPd(0) active species. Isr. J. Chem. 2019;59:1–16. [Google Scholar]
- 42.Butts CP, Crosby J, Lloyd-Jones GC, Stephen SC. Robust and catalytically active mono- and bis-Pd-complexes of the ‘Trost modular ligand’. Chem. Commun. 2019;1999:1707–1708. [Google Scholar]
- 43.Fairlamb IJS, Lloyd-Jones GC. On the effect of catalyst loading in Pd-catalysed allylic alkylation. Chem. Commun. 2000;2000:2447–2448. doi: 10.1039/b007785m. [DOI] [Google Scholar]
- 44.Racys DT, et al. Pd-η3-C6H9 complexes of the Trost modular ligand: high nuclearity columnar aggregation controlled by concentration, solvent and counterion. Chem. Sci. 2015;6:5793–5801. doi: 10.1039/C5SC01181G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arthurs RA, Hughes DL, Richards CJ. Planar chiral palladacycle precatalysts for asymmetric synthesis. Org. Biomol. Chem. 2020;18:5466–5472. doi: 10.1039/D0OB01331E. [DOI] [PubMed] [Google Scholar]
- 46.Masson-Makdissi J, Prieto L, Abel-Snape X, Lautens M. Enantio- and diastereodivergent sequential catalysis featuring two transition-metal-catalyzed asymmetric reactions. Angew. Chem. Int. Ed. 2021;60:16932–16936. doi: 10.1002/anie.202105800. [DOI] [PubMed] [Google Scholar]
- 47.Zalesskiy SS, Ananikov VP. Pd2(dba)3 as a precursor of soluble metal complexes and nanoparticles: determination of palladium active species for catalysis and synthesis. Organometallics. 2012;31:2302–2309. doi: 10.1021/om201217r. [DOI] [Google Scholar]
- 48.Weber P, et al. A comparative study of dibenzylideneacetone palladium complexes in catalysis. Org. Process Res. Dev. 2019;23:1462–1470. doi: 10.1021/acs.oprd.9b00214. [DOI] [Google Scholar]
- 49.Steinhagen H, Reggelin M, Helmchen G. Palladium-catalyzed allylic alkylation with phosphinoaryldihydrooxazole ligands: first evidence and NMR spectroscopic structure determination of a primary olefin–Pd0 complex. Angew. Chem. Int. Ed. Engl. 1997;36:2108–2110. doi: 10.1002/anie.199721081. [DOI] [Google Scholar]
- 50.Selvakumar K, Valentini M, Wörle M, Pregosin PS, Albinati A. Palladium(0) olefin complexes and enantioselective allylic amination/alkylation with a P,N-auxiliary. Organometallics. 1999;18:1207–1215. doi: 10.1021/om980920e. [DOI] [Google Scholar]
- 51.Helmchen G, Pfaltz A. Phosphinooxazolines: a new class of versatile, modular P,N-ligands for asymmetric catalysis. Acc. Chem. Res. 2000;33:336–345. doi: 10.1021/ar9900865. [DOI] [PubMed] [Google Scholar]
- 52.Zehnder M, Neuburger M, Schaffner S, Jufer M, Plattner DA. Synthesis, X-ray structures, NMR studies and density functional calculations of (η2-fumarodinitrile)palladium(0) complexes containing dihydro(phosphanylphenyl)oxazole ligands. Eur. J. Inorg. Chem. 2002;2002:1511–1517. doi: 10.1002/1099-0682(200206)2002:6<1511::AID-EJIC1511>3.0.CO;2-C. [DOI] [Google Scholar]
- 53.Dotta P, Magistrato A, Rothlisberger U, Pregosin PS, Albinati A. Dialkyl effect on enantioselectivity: π-stacking as a structural feature in P,N Complexes of palladium(II) Organometallics. 2002;21:3033–3041. doi: 10.1021/om020314q. [DOI] [Google Scholar]
- 54.Zehnder M, Schaffner S, Neuburger M, Plattner DA. X-ray crystallographic and NMR spectroscopical characterization of intermediates in the Pd-catalyzed allylic substitution reaction with 4-substituted phosphinooxazolines. Correlation between intermediate structure and product configuration. Inorg. Chim. Acta. 2002;337:287–298. doi: 10.1016/S0020-1693(02)00998-2. [DOI] [Google Scholar]
- 55.Sherden NH, Behenna DC, Virgil SC, Stoltz BM. Unusual allylpalladium carboxylate complexes: identification of the resting state of catalytic enantioselective decarboxylative allylic alkylation reactions of ketones. Angew. Chem. Int. Ed. 2009;48:6840–6843. doi: 10.1002/anie.200902575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernández-Galán R, et al. New chiral palladium(0) and -(II) complexes of (aminoferrocenyl)phosphine Ligands PPFA and PTFA. X-ray crystal structure analysis and fluxional Behavior involving alkene rotation, Pd−N bond rupture, and selective η3−η1−η3 allyl isomerization. Organometallics. 1997;16:3758–3768. doi: 10.1021/om9610354. [DOI] [Google Scholar]
- 57.Selvakumar K, Valentini M, Pregosin PS, Albinati A. Chiral phosphito−thioether complexes of palladium(0). Comments on the Pd, Rh, and Ir regio- and-enantioselective allylic alkylations of PhCHCHCH(OAc)R, R = H, Me, Et. Organometallics. 1999;18:4591–4597. doi: 10.1021/om990452o. [DOI] [Google Scholar]
- 58.Foltz C, Enders M, Bellemin-Laponnaz S, Wadepohl H, Gade LH. Using a tripod as a chiral chelating ligand: chemical exchange between equivalent molecular structures in palladium catalysis with 1,1,1-tris(oxazolinyl)ethane (“Trisox”) Chem. Eur. J. 2007;13:5994–6008. doi: 10.1002/chem.200700307. [DOI] [PubMed] [Google Scholar]
- 59.Zalubovskis R, et al. Self-adaptable catalysts: substrate-dependent ligand configuration. J. Am. Chem. Soc. 2008;130:1845–1855. doi: 10.1021/ja074044k. [DOI] [PubMed] [Google Scholar]
- 60.Théveau L, et al. Cofactor-controlled chirality of tropoisomeric ligand. Organometallics. 2016;35:1956–1963. doi: 10.1021/acs.organomet.6b00265. [DOI] [Google Scholar]
- 61.Trost BM, Breit B, Organ MG. On the nature of the asymmetric induction in a palladium catalyzed allylic alkylation. Tet. Lett. 1994;35:5817–5820. doi: 10.1016/S0040-4039(00)78192-5. [DOI] [Google Scholar]
- 62.Amatore C, Jutand A, Mensah L, Ricard L. On the formation of Pd(II) complexes of Trost modular ligand involving N–H activation or P,O coordination in Pd-catalyzed allylic alkylations. J. Organomet. Chem. 2007;692:1457–1464. doi: 10.1016/j.jorganchem.2006.11.039. [DOI] [Google Scholar]
- 63.Tsarev VN, Wolters D, Gais H-J. Redox reaction of the Pd0 complex bearing the Trost ligand with meso-cycloalkene-1,4-biscarbonates leading to a diamidato PdII complex and 1,3-cycloalkadienes: enantioselective desymmetrization versus catalyst deactivation. Chem. Eur. J. 2010;16:2904–2915. doi: 10.1002/chem.200902739. [DOI] [PubMed] [Google Scholar]
- 64.Huang J, et al. DMPDAB–Pd–MAH: a versatile Pd(0) source for precatalyst formation, reaction screening, and preparative-scale synthesis. ACS Catal. 2021;11:5636–5646. doi: 10.1021/acscatal.1c00288. [DOI] [Google Scholar]
- 65.Butts, C. P. et al. Structure-based rationale for selectivity in the asymmetric allylic alkylation of cycloalkenyl esters employing the Trost ‘Standard Ligand’ (TSL): isolation, analysis and alkylation of the monomeric form of the cationic η3-cyclohexenyl complex [(η3-c-C6H9)Pd(TSL)]+. J. Am. Chem. Soc.131, 9945–9957 (2009). [DOI] [PubMed]
- 66.Barone V, Cossi M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A. 1998;102:1995–2001. doi: 10.1021/jp9716997. [DOI] [Google Scholar]
- 67.Trost BM, Bunt RC. Asymmetric induction in allylic alkylations of 3-(acyloxy)cycloalkenes. J. Am. Chem. Soc. 1994;116:4089–4090. doi: 10.1021/ja00088a059. [DOI] [Google Scholar]
- 68.González-Bobes F, et al. Scale-up of azide chemistry: a case study. Org. Process Res. Dev. 2012;16:2051–2057. doi: 10.1021/op3002646. [DOI] [Google Scholar]
- 69.Trost BM, Pulley SR. On the flexibility of allylic azides as synthetic intermediates. Tet. Lett. 1995;36:8737–8740. doi: 10.1016/0040-4039(95)01898-R. [DOI] [Google Scholar]
- 70.Trost BM, Lemoine RC. An asymmetric synthesis of vigabatrin. Tet. Lett. 1996;37:9161–9164. doi: 10.1016/S0040-4039(96)02148-X. [DOI] [Google Scholar]
- 71.Xiong H, et al. Enantioselective synthesis and profiling of two novel diazabicyclooctanone β-lactamase inhibitors. ACS Med. Chem. Lett. 2014;5:1143–1147. doi: 10.1021/ml500284k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trost BM, Bunt RC, Lemoine RC, Calkins TL. Dynamic kinetic asymmetric transformation of diene monoepoxides: a practical asymmetric synthesis of vinylglycinol, vigabatrin, and ethambutol. J. Am. Chem. Soc. 2000;122:5968–5976. doi: 10.1021/ja000547d. [DOI] [Google Scholar]
- 73.Trost BM, Xu J, Schmidt T. Palladium-catalyzed decarboxylative asymmetric allylic alkylation of enol carbonates. J. Am. Chem. Soc. 2009;131:18343–18357. doi: 10.1021/ja9053948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.James J, Jackson M, Guiry PJ. Palladium-catalyzed decarboxylative asymmetric allylic alkylation: development, mechanistic understanding and recent advances. Adv. Synth. Catal. 2019;361:3016–3049. doi: 10.1002/adsc.201801575. [DOI] [Google Scholar]
- 75.von Matt P, Pfaltz A. Chiral phosphinoaryldihydrooxazoles as ligands in asymmetric catalysis: Pd-catalyzed allylic substitution. Angew. Chem. Int. Ed. Engl. 1993;32:566–568. doi: 10.1002/anie.199305661. [DOI] [Google Scholar]
- 76.Nascimento de Oliveira M, Fournier J, Arseniyadis S, Cossy J. A Palladium-catalyzed asymmetric allylic alkylation approach to α-quaternary γ-butyrolactones. Org. Lett. 2017;19:14–17. doi: 10.1021/acs.orglett.6b02971. [DOI] [PubMed] [Google Scholar]
- 77.Nascimento de Oliveira M, Arseniyadis S, Cossy J. Palladium-catalyzed asymmetric allylic alkylation of 4-substituted isoxazolidin-5-ones: straightforward access to β2,2-amino acids. Chem. Eur. J. 2018;24:4810–4814. doi: 10.1002/chem.201800641. [DOI] [PubMed] [Google Scholar]
- 78.Song T, Arseniyadis S, Cossy J. Highly enantioselective, base-free synthesis of α-quaternary succinimides through catalytic asymmetric allylic alkylation. Chem. Eur. J. 2018;24:8076–8080. doi: 10.1002/chem.201800920. [DOI] [PubMed] [Google Scholar]
- 79.Song T, Arseniyadis S, Cossy J. Asymmetric synthesis of α-quaternary γ-lactams through palladium-catalyzed asymmetric allylic alkylation. Org. Lett. 2019;21:603–607. doi: 10.1021/acs.orglett.8b03613. [DOI] [PubMed] [Google Scholar]
- 80.Aubert S, Katsina T, Arseniyadis S. A sequential Pd-AAA/cross-metathesis/Cope rearrangement strategy for the stereoselective synthesis of chiral butenolides. Org. Lett. 2019;21:2231–2235. doi: 10.1021/acs.orglett.9b00521. [DOI] [PubMed] [Google Scholar]
- 81.Fournier J, Lozano O, Menozzi C, Arseniyadis S, Cossy J. Palladium-catalyzed asymmetric allylic alkylation of cyclic dienol carbonates: efficient route to enantioenriched γ-butenolides bearing an all-carbon α-quaternary stereogenic center. Angew. Chem. Int. Ed. 2013;52:1257–1261. doi: 10.1002/anie.201206368. [DOI] [PubMed] [Google Scholar]
- 82.Konnert L, Lamaty F, Martinez J, Colacino E. Recent advances in the synthesis of hydantoins: the state of the art of a valuable scaffold. Chem. Rev. 2017;117:13757–13809. doi: 10.1021/acs.chemrev.7b00067. [DOI] [PubMed] [Google Scholar]
- 83.Taylor RD, MacCoss M, Lawson ADG. Rings in drugs. J. Med. Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 84.Shearer J, Castro JL, Lawson ADG, MacCoss M, Taylor RD. Rings in clinical trials and drugs: present and future. J. Med. Chem. 2022;65:8699–8712. doi: 10.1021/acs.jmedchem.2c00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keenan T, Jean A, Arseniyadis S. Phase-transfer-catalyzed alkylation of hydantoins. ACS Org. Inorg. Au. 2022;2:312–317. doi: 10.1021/acsorginorgau.1c00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are present. All processed spectroscopic and chromatographic data from the present study is contained in the Supplementary Information. The atomic coordinates for structures generated by DFT calculations of 1-exo and 1-endo are provided in xlsx format as a Source Data File. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2258957-2258960. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.