Abstract
Purpose:
To compare the histopathologic inflammation and fibrosis of orbital adipose tissue in orbital inflammatory disease (OID) specimens.
Methods:
In this retrospective cohort study, inflammation and fibrosis in orbital adipose tissue from patients with thyroid-associated orbitopathy (TAO), granulomatosis with polyangiitis (GPA), sarcoidosis, nonspecific orbital inflammation (NSOI), and healthy controls were scored by two masked ocular pathologists. Both categories were scored on a scale of 0 to 3 with scoring criteria based on the percentage of specimen containing inflammation or fibrosis, respectively. Tissue specimens were collected from oculoplastic surgeons at 8 international centers representing 4 countries. Seventy-four specimens were included: 25 with TAO, 6 with orbital GPA, 7 with orbital sarcoidosis, 24 with NSOI, and 12 healthy controls.
Results:
The mean inflammation and fibrosis scores for healthy controls were 0.0 and 1.1, respectively. OID groups’ inflammation (I) and fibrosis (F) scores, formatted [I, F] with respective p-values when compared to controls, were: TAO [0.2, 1.4] (p=1, 1), GPA [1.9, 2.6] (p=0.003, 0.009), sarcoidosis [2.4, 1.9] (p=0.001, 0.023), and NSOI [1.3, 1.8] (p=<0.001, 0.018). Sarcoidosis had the highest mean inflammation score. Pairwise analysis demonstrated that sarcoidosis had a significantly higher mean inflammation score than NSOI (p=0.036) and TAO (p<0.0001), but no difference when compared to GPA. GPA had the highest mean fibrosis score, with pairwise analysis demonstrating a significantly higher mean fibrosis score than TAO (p=0.048).
Conclusions:
Mean inflammation and fibrosis scores in TAO orbital adipose tissue samples did not differ from healthy controls. In contrast, the more “intense” inflammatory diseases such as GPA, sarcoidosis, and NSOI did demonstrate higher histopathologic inflammation and fibrosis. This has implications in prognosis, therapeutic selection, and response monitoring in OID.
Précis
To describe histopathologic inflammation and fibrosis of orbital adipose tissue in orbital inflammatory disease specimens.
Introduction:
Orbital inflammatory disease (OID) can have a wide range of underlying etiologies including thyroid-associated orbitopathy (TAO), granulomatosis with polyangiitis (GPA), sarcoidosis, and nonspecific orbital inflammation (NSOI), accounting for up to 16% of orbital diseases (1). While OIDs can affect any part of the orbit, they most commonly involve the adipose tissue, extraocular muscles, and/or lacrimal gland. All OIDs cause acute inflammation during the active phase which can completely resolve without sequela, but persistent inflammation can lead to fibrosis (2). Neutrophils are the main effectors of acute inflammation and release both fibrogenic cytokines, which can perpetuate inflammation, and nonfibrogenic enzymes such as matrix metalloproteinase, elastase, and cathepsins that can remove excessive extracellular matrix (3,4). Chronic inflammation often involves mast cells, which have been found in high numbers in TAO and NSOI (5,6). Fibrosis is a pathologically stereotypical response to tissue injury resulting from excessive deposition of collagenous and noncollagenous extracellular matrix due to proliferation and activation of fibroblasts and myofibroblasts (3). Currently, there is no specific treatment for orbital fibrosis; therefore, management of OID is aimed towards mitigating the inflammatory response.
The pathogenesis and natural history of OIDs are still uncertain. Although systemic corticosteroids are the mainstay for treatment, understanding the inflammatory pathways in OID can better tailor the therapeutics. The U.S. Food and Drug Administration (FDA) has recently approved a fully human monoclonal IGF-1R (insulin like growth factor-1 receptor) antagonist antibody, teprotumumab, for the treatment of patients with TAO (7,8). Further studies will determine the optimal timing of therapy and the potential use in patients with chronic, clinically inactive TAO and orbital fibrosis. Since no treatment has been shown to reverse orbital fibrosis, understanding the natural histopathologic course could aid in prognostic advice, therapeutic selection, and response monitoring. We have previously reported on the likelihood of fibrosis among the OIDs and how transcripts similar to those associated with idiopathic pulmonary fibrosis are upregulated in OIDs (9). We now report on the intensity of inflammation and the correlation between histopathologic inflammation and fibrosis in OID specimens.
Methods:
This study was approved by the Institutional Review Board (IRB) at Oregon Health & Science University (IRB00006301) and at each of the other contributing centers. The study was Health Insurance Portability and Accountability Act compliant with protection of any identifiable information and this report adhered to the ethical principles outlined in the Declaration of Helsinki.
Study design
Methods for the analysis of orbital biopsies have been described elsewhere (9–11). In brief, formalin-fixed, paraffin-embedded orbital adipose biopsies were obtained from healthy controls (n=12) and patients with TAO (n=25), GPA (n=6), sarcoidosis (n=7) and NSOI (n=24) based on clinical and histopathological information provided by the respective oculoplastic surgeons and ocular pathologists in the Orbital Inflammatory Disease Consortium. Samples were obtained from North America (Oregon, Ohio, California, Wisconsin, North Carolina, New York, Florida, and British Columbia), Australia, and Saudi Arabia. Control orbital tissue was obtained from patients with no history of orbital disease and undergoing routine blepharoplasty or enucleation. Histopathological diagnoses were determined independently by two ocular pathologists (DJW and HEG) masked to the clinical diagnosis. Patterns of inflammation helped distinguish among these OIDs. For sarcoidosis, non-caseating, naked, granulomas with few lymphocytes are classically found. The histologic triad of GPA is vasculitis, granulomatous inflammation, and necrobiosis of collagen. For TAO, there may be relative sparing of orbital fat with inflammation. NSOI often have a nonspecific inflammatory pattern. In all cases, the consensus diagnosis obtained by DJW and JTR based on the histopathology reports and the clinical data agreed with the contributing center’s diagnosis.
Histopathological scoring
The degree of orbital adipose tissue inflammation and fibrosis was scored on an ordinal scale from 0 to 3 based on viewing the whole tissue slides. Both were scored as absent (0, no inflammatory cells or fibrosis), mild (1, up to 1/3 of the specimen containing inflammatory cells or fibrosis), moderate (2, 1/3 to 2/3 of the specimen containing inflammatory cells or fibrosis), or severe (3, greater than 2/3 of the specimen containing inflammatory cells or fibrosis). The inflammatory cells in the tissue sections that were included for grading were neutrophils, lymphocytes, plasma cells, macrophages, eosinophils, histiocytes, and giant cells.
Statistical analyses
Statistical analysis was performed using SPSS version 11.0.1 (SPSS Inc, Chicago, IL). All tests were performed using a 0.05 alpha level with two-tailed significance. Statistical significance was achieved when p≤0.05. The pathologists’ scores were averaged to give a combined score used in further analyses comparing groups using the Mann-Whitney U test. Multiple test correction was performed using Bonferroni correction method with adjusted p-values reported where appropriate. Intraclass correlation coefficient (ICC) was used to compare the agreement between pathologists using a single-measurement agreement, and mixed-effects model. The results were summarized as ICC values with 95% confidence intervals.
Results:
Orbital adipose tissue biopsies were obtained from 62 patients with OID including 25 with TAO, 6 with orbital GPA, 7 with orbital sarcoidosis, and 24 with NSOI, which were compared to tissues from 12 healthy controls who underwent blepharoplasty or enucleation with non-inflamed orbits (Table 1, Figure 1).
Table 1.
Age and Sex for each OID group
| Overall | |||
|---|---|---|---|
| Diagnosis | N | ||
| at biopsy (sd) | % | ||
| TAO | 25 | 51.6 ± 14.0 | 76% |
| GPA | 6 | 40.2 ± 12.2 | 67% |
| Sarcoidosis | 7 | 45.8 ± 12.0 | 71% |
| NSOI | 25 | 50.4 ± 23.5 | 64% |
| Normal | 20 | 63.6 ± 14.5 | 70% |
tao, thyroid-associated orbitopathy; gpa, granulomatosis with polyangiitis; nsoi, nonspecific orbital inflammation
Figure 1.
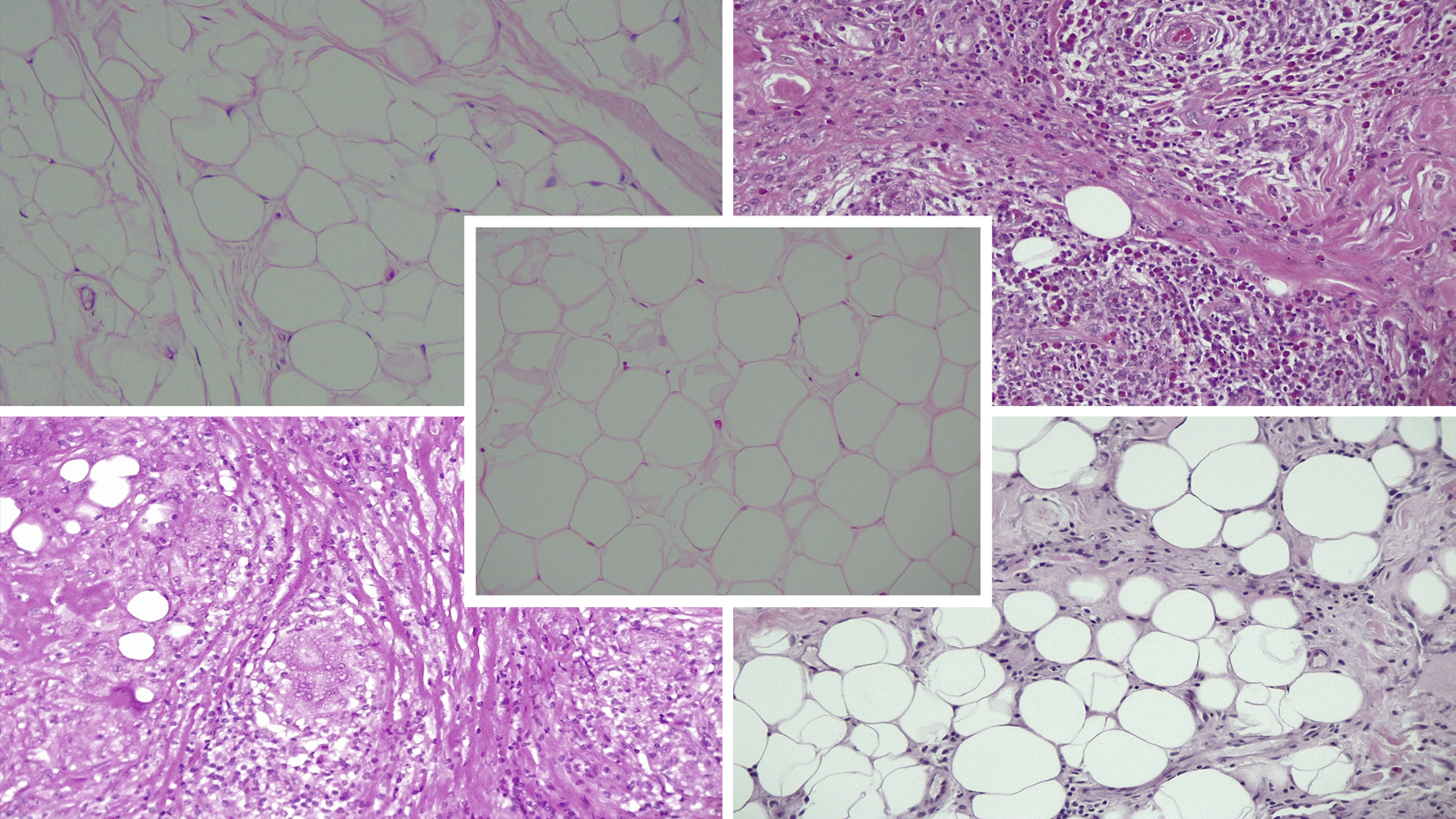
Specimen grading examples.
Representative images demonstrating the histologic variability of orbital fat in subjects diagnosed with TAO, GPA, sarcoidosis, and NSOI compared to heathy controls. Hematoxylin and eosin staining in paraffin embedded sections (20x).
A. Control (center)
Average inflammation score of 0 (DJW 0, HEG 0). Average fibrosis score of 0.5 (DJW 0, HEG 1).
B. TAO (top left)
49-year-old female within 12 months of ocular onset. No history of steroid or steroid sparing agents or radiotherapy.
Average inflammation score of 0 (DJW 0, HEG 0). Average fibrosis score of 2 (DJW 2, HEG 2).
C. GPA (top right)
24-year-old male within 5 months of ocular onset. No history of steroid or steroid sparing agents or radiotherapy.
Average inflammation score of 3 (DJW 3, HEG 3). Average fibrosis score of 2 (DJW 2, HEG 2).
D. Sarcoidosis (bottom left)
60-year-old female within 8 months of ocular onset. On high dose steroids at time of biopsy without any history of steroid sparing agents or radiotherapy.
Average inflammation score of 3 (DJW 3, HEG 3). Average fibrosis score of 2 (DJW 2, HEG 2).
E. NSOI (bottom right)
83-year-old male within 1 month of ocular onset. On high dose steroids at time of biopsy without any history of steroid sparing agents or radiotherapy.
Average inflammation score of 1 (DJW 1, HEG 1). Average fibrosis score of 1.5 (DJW 1, HEG 2).
Of the 62 patients with OID, the mean (SD) age was 49.6 (19.6) years, and 44 (70%) subjects were female. Of the 12 healthy controls, the mean (SD) age was 63.5 (14.5) years, and 20 (70%) subjects were female. All groups comprised of samples from a majority of female subjects, and there was no statistically significant difference in female ratios across the groups (χ2=0.893, p=0.944).
Of the 25 patients with TAO, the majority were white (80%) and the median time from ocular symptoms onset to biopsy was 40 months (range 1–432 months) (Figure 2). Nine subjects were biopsied before 2 years of TAO onset and 10 were biopsied after 2 years from disease onset. The majority had a history of hyperthyroidism (n=19, 76%), 1 had hypothyroidism and 3 were euthyroid. All reported providers stated their reason for surgery as “symptomatic relief” (n=20). Although six patients (24%) had a history of treatment with corticosteroids, only one was actively on high dose at the time of biopsy defined as a daily dose of at least 1mg/kg/day of prednisone. The rest were 7–38 months out from steroids use. No patients had any history of steroid sparing agents or radiotherapy.
Figure 2.
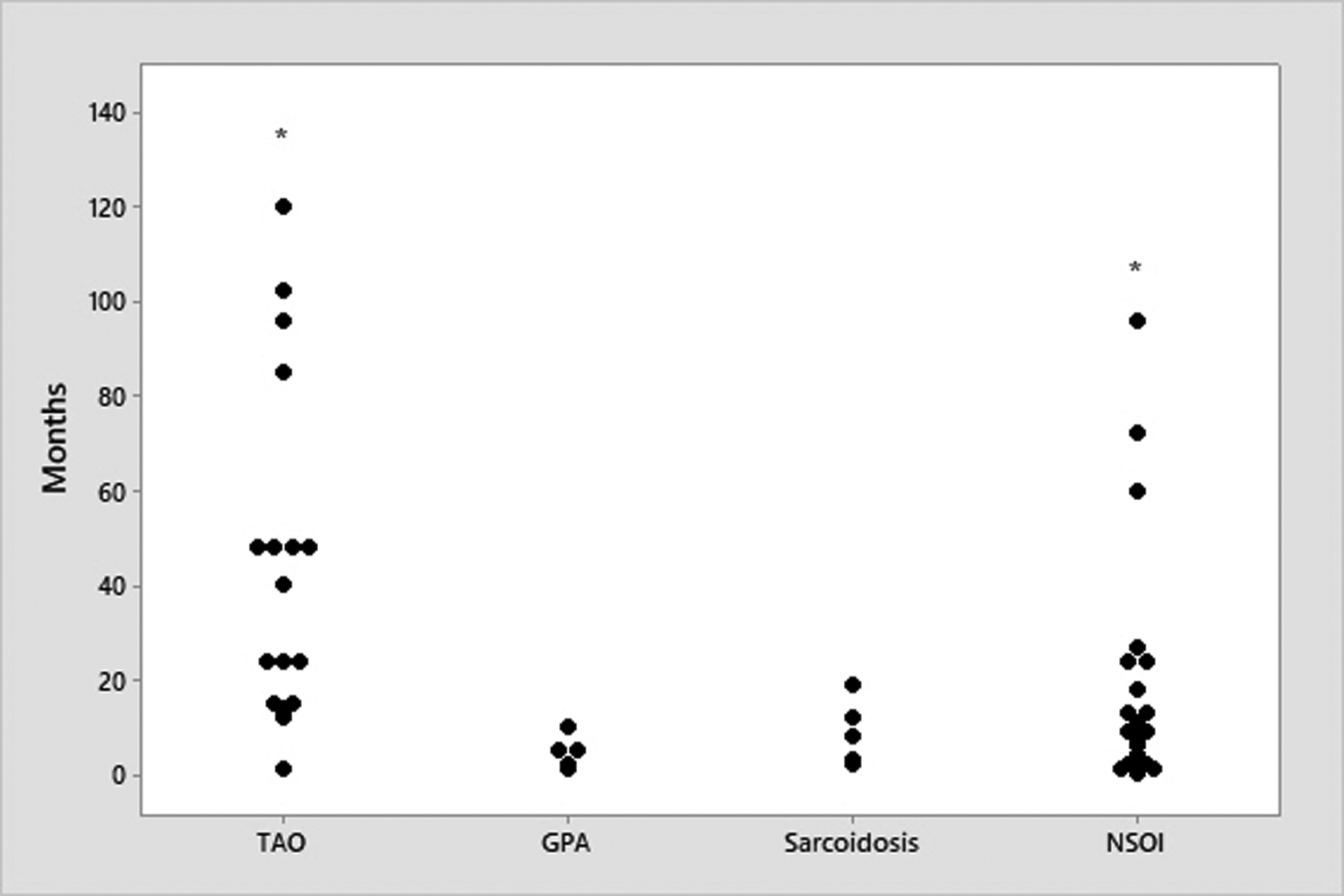
Time in months from ocular symptom onset to biopsy in the OID groups.
Asterisks indicate outlying data points: 1 sample in TAO: 432 months; 1 sample in NSOI: 444. All pairwise differences of median time of biopsy were statistically significant by the Mann-Whitney U test except between GPA and sarcoidosis. TAO = thyroid-associated orbitopathy; GPA = granulomatosis with polyangiitis; NSOI = nonspecific orbital inflammation.
The median time from onset of ocular symptoms to biopsy in GPA was 5 months (range 1–10 months), with sarcoidosis at 8 months (range 2–19), and NSOI at 9 months (range 0.3–444 months) (Figure 2). 14 patients with NSOI had been on steroids (n=13) or steroid sparing agents (n=1, methotrexate) prior to biopsy with 7 patients were actively on high dose steroids at the time of biopsy. Two patients had a history of radiation more than 12 months prior to the biopsy. Two patients with sarcoidosis had a history of steroid use prior to biopsy with one concurrently on steroids at the time of surgery. No patients with GPA had a history of steroid or radiotherapy prior to biopsy.
For the 74 adipose tissue biopsies scored by both pathologists for inflammation, 51 (68.9%) had identical scores, 17 times (23.0%) the scores differed by one, and 6 times (8.1%) the scores differed by two. For fibrosis grading, 33 (44.6%) had identical scores, 37 times (50%) the scores differed by one, and 4 times (5.4%) the scores differed by two or more.
The mean inflammatory and fibrosis scores for healthy control, TAO, GPA, sarcoidosis, and NSOI are listed in Tables 2 and 3. Sarcoidosis has the highest mean inflammatory score, and this was significantly more than NSOI (p=0.036), but not significantly different from GPA. GPA has the highest mean fibrosis score, but this was not significantly more than sarcoidosis or NSOI.
Table 2.
mean inflammatory score in each OID group
| Mean inflammatory score | Adjusted P-value vs Control* | vs Tao | vs GPA | vs Sarcoidosis | vs NSOI | |
|---|---|---|---|---|---|---|
| TAO | 0.22 | 1 | - | - | - | - |
| GPA | 1.92 | 0.003 | 0.002 | - | - | - |
| Sarcoidosis | 2.35 | 0.001 | <0.001 | 1 | - | - |
| NSOI | 1.33 | <0.001 | <0.001 | 1 | 0.036 | - |
Mann-Whitney U test with Bonferroni’s correction for 10 tests
tao, thyroid-associated orbitopathy; gpa, granulomatosis with polyangiitis; nsoi, nonspecific orbital inflammation
Table 3.
mean fibrosis score in each OID group
| Mean fibrosis score | Adjusted P-value vs Control* | vs Tao | vs GPA | vs Sarcoidosis | vs NSOI | |
|---|---|---|---|---|---|---|
| TAO | 1.48 | 1 | - | - | - | - |
| GPA | 2.66 | 0.009 | 0.048 | - | - | - |
| Sarcoidosis | 1.92 | 0.023 | 1 | 0.536 | - | - |
| NSOI | 1.77 | 0.018 | 1 | 0.278 | 1 | - |
Mann-Whitney U test with Bonferroni’s correction for 10 tests
tao, thyroid-associated orbitopathy; gpa, granulomatosis with polyangiitis; nsoi, nonspecific orbital inflammation
TAO samples did not have significantly more inflammation or fibrosis than healthy controls. A subgroup analysis, comparing early TAO samples (time from ocular symptoms onset to biopsy <2 years, n=9) to late TAO samples (>2 years, n=10) were not statistically significant for mean inflammatory or fibrosis score (p=0.139 and 0.218 respectively using a two-sample t test assuming equal variances). There appears to be more fibrosis than inflammation along the entire disease course of TAO (Figure 3a). The amount of inflammation or fibrosis, however, does not appear to change along the disease timeline.
Figure 3.
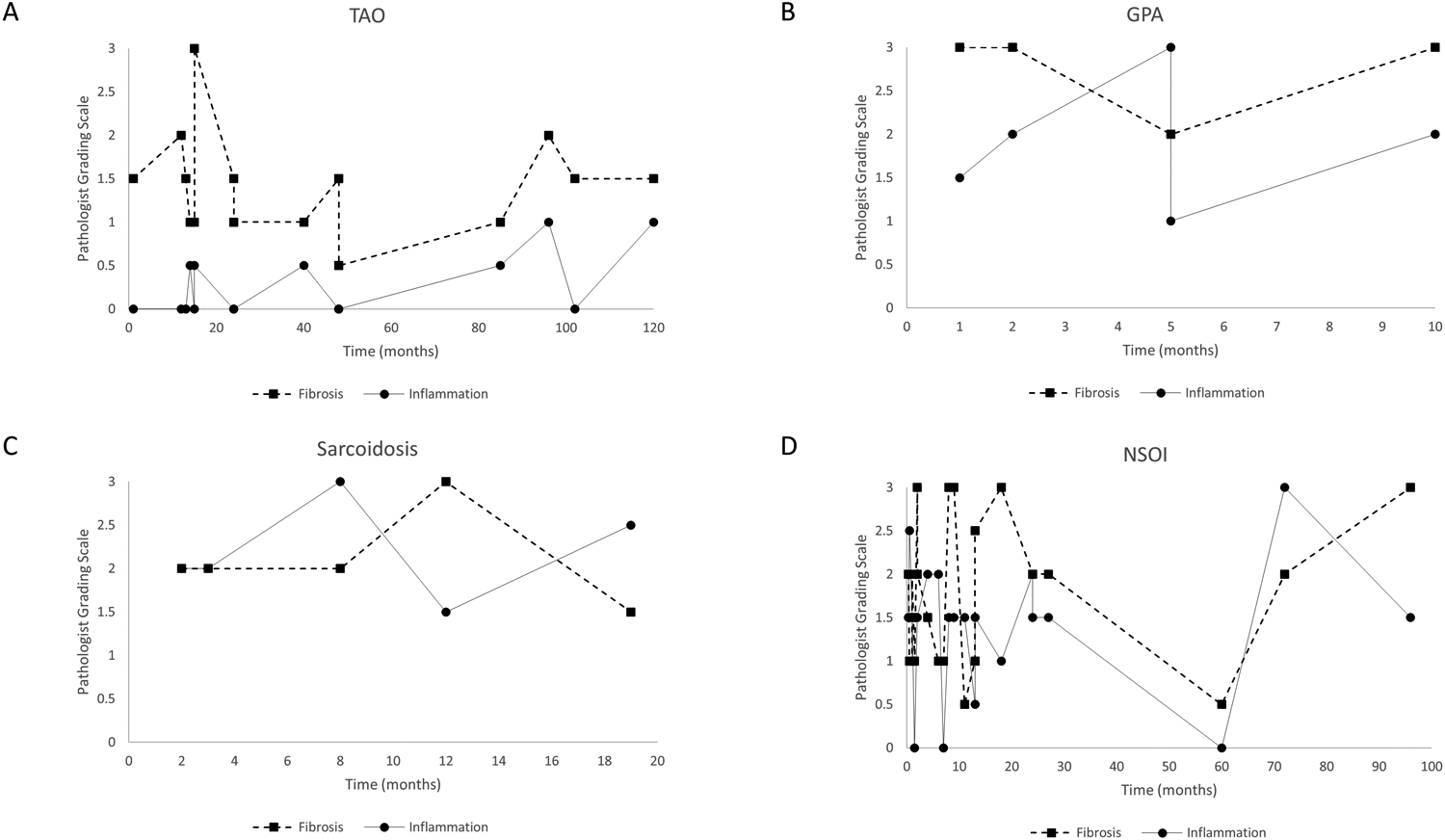
Pathologist Grading Scale of inflammation and fibrosis in the OID groups with respect to time in months from ocular symptoms onset to biopsy. A. TAO. B. GPA. C. Sarcoidosis. D. NSOI. TAO = thyroid-associated orbitopathy; GPA = granulomatosis with polyangiitis; NSOI = nonspecific orbital inflammation.
Both GPA and sarcoidosis had high amount of fibrosis and inflammation which did not fluctuate much with time. Sarcoidosis had higher inflammation than fibrosis score, whereas GPA was the opposite. NSOI had the most fluctuation of inflammation and fibrosis among the OIDs.
The two pathologists were in moderate to good agreement in their results as assessed by ICC (inflammation ICC = 0.69 with 95% confidence interval = 0.53–0.80; fibrosis ICC = 0.65 with 95% confidence interval = 0.47–0.77).
Discussion:
Adipose tissue fills approximately half of the orbital cavity protecting the globe, extraocular muscles, vessels, and nerves (12). The role of orbital fat beyond a space-occupying tissue is expanding with its contribution to endocrine and immune pathways as well as a reservoir of stem cells (12,13). Despite its importance, relatively little is known about its role in orbital diseases. Dysregulation of orbital fat is seen with TAO where de novo adipogenesis is enhanced which may result in corneal exposure, diplopia, and permanent vision loss (14). In early, active TAO there is thought to be infiltration of the orbital fat, extraocular muscles, and connective tissue with lymphocytes and aberrant cytokine expression activating fibroblasts driving inflammation and tissue remodeling (15). In late stage TAO, there appears to be heterogeneous, preferential fibrosis of the extraocular muscles with resultant diplopia (16). OID, in addition to TAO, may also have an active inflammatory phase followed either by irreversible fibrosis or complete resolution.
Orbital adipose tissues from patients with TAO, however, were not significantly different from healthy controls in terms of inflammation or fibrosis as was previously reported (11). The paucity of inflammation and fibrosis in TAO is consistent with prior studies (5, 11, 17–20). Although 9 TAO samples were within two years of ocular symptom onset, the majority were from patients undergoing decompression more than three years after the onset of orbital symptoms and when the disease has stabilized. While it is possible there might have been more active reaction at early stages, we did analyze the 9 early disease specimens separately and found no difference from healthy controls. Four of these patients had a history of steroid use prior to biopsy and one had ongoing therapy, which may have diminished the inflammatory response. Moreover, the inflammatory and fibrotic process may be focal and not diffusely distributed throughout the whole orbital adipose tissue. While other OID may present with an orbital pseudotumor on imaging guiding biopsy, TAO rarely causes a focal mass. This might increase the risk of biopsy sampling error. Although TAO is a clinically heterogenous disease, there may be two non-mutually exclusive subsets which might provide some prognostic and therapeutic benefit. Type I is associated with younger patients and predominantly fat compartment enlargement. Type II is associated with older patients and predominantly extraocular muscle enlargement (21,22). It is possible that the samples were mainly from type II TAO patients and the orbital adipose tissue was not as involved. In fact, our mean age of biopsy for TAO patients (table 1, mean (SD) age was 51.6 (14.0) years) was similar to a prior study where extraocular muscle involvement was more predominant (21).
While the pathogenesis of other OID such as GPA, sarcoidosis, and NSOI is less well studied than TAO, they appear to have a more severe inflammatory and fibrotic disease process. GPA is associated with vasculitis of the small to medium-sized vessels resulting in granulomatous inflammation and eventual tissue necrosis which is followed by progressive fibrosis (23). In fact, GPA had the highest mean fibrosis score as previously reported which may be of diagnostic and therapeutic value (11). Although none of these patients were on any immunosuppressive agents, biopsies as early as 1 month from ocular symptom onset, demonstrated grade 3 fibrosis. Sarcoidosis involving the adipose tissue is thought to be rare, with the lacrimal gland being the most common orbital location (24). Our data demonstrate that when sarcoidosis does involve the orbital adipose tissue, it has a strong inflammatory response. While NSOI had more histological inflammation than TAO, its inflammation scores were lower than for sarcoidosis. This may be the case if NSOI is not a single entity. We have previous reported that forms of NSOI may have a limited form of GPA or sarcoidosis based on gene expression profile while others cannot be distinguished from healthy controls (25,26). Most patients with NSOI had a history of steroid use and many were on high dose at the time of biopsy which may have reduced the inflammatory response. Early diagnosis with aggressive immunosuppressive therapy may help prevent orbital fibrosis in OID.
While the acute inflammatory phase in OID may be modified by steroids, steroid sparing agents, and radiation, there still is histological evidence of inflammation and fibrosis based on our study (27,28). This has implications for the timing, dosage, and choice of immunosuppressive therapy. In orbital adipose tissue from TAO, however, there appears to be minimal inflammation and fibrosis. It is possible that there is a threshold level of inflammation that results in fibrosis and that TAO does not meet this. With the lack of significant fibrosis of the orbital adipose tissue, it is possible that immunomodulators would be able to benefit inactive, clinically stable patients with TAO. Anecdotal reports suggest that this might be the case with teprotumumab, but further randomized study is required for a more definitive test of this hypothesis (29,30).
Limitations of our study include the small sample size of rarer causes of OID and the possible biopsy bias in TAO samples which may be responsible for the statistical insignificance of the results. While longitudinal data on patients at different stages of their OID course would yield more granular data, repeat biopsies are not often feasible or justifiable. Despite the fact we only had two ocular pathologists, the inter-observer agreement on histological grading was good, thus arguing that additional independent score of inflammation or fibrosis would not have impacted our conclusions.
Funding:
This research was supported by funding from National Institutes of Health (NIH) USA Grants (www.nih.gov) EY020249 (JTR), an NIH/NEI Core Grant P30 EY010572, and an unrestricted grant from Research to Prevent Blindness. The sponsors did not have any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication. JTR receives financial support from the Grandmaison Fund for Autoimmunity Research, the William and Mary Bauman Family Foundation, and the Stan and Madelle Rosenfeld Family Trust.
JTR has in the past consulted for Genentech/Roche and was a co-investigator on a study funded by Genentech to evaluate the use of rituximab for orbital inflammatory diseases. JTR, RAD, and DOK are consultants while GJH and BSK have in the past consulted for Horizon Pharmaceuticals which manufactures teprotumumab.
References
- 1.Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111(5):997–1008 [DOI] [PubMed] [Google Scholar]
- 2.Chen CZ, Raghunath M. Focus on collagen: in vitro systems to study fibrogenesis and antifibrosis—state of the art. Fibrogenesis Tissue Repair. 2009;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wick G, Grundtman C, Mayerl C, et al. The immunology of fibrosis. Annu Rev Immunol. 2013;31:107–135 [DOI] [PubMed] [Google Scholar]
- 4.Lefkowitz DL, Lefkowitz SS. Macrophage-neutrophil interaction: a paradigm for chronic inflammation revisited. Immunol. Cell Biol 2001;79:502–6 [DOI] [PubMed] [Google Scholar]
- 5.Matos K, Nose V, Manso PG, et al. Correlation between clinical and histological analyses in retroocular connective tissues and extraocular muscles from patients with Graves’ ophthalmopathy. Endocr Pathol. 2000;11:185–194 [DOI] [PubMed] [Google Scholar]
- 6.Yan J, Li Y, Qiu H, et al. Immunohistochemical study of the presence of mast cells in idiopathic orbital inflammatory pseudotumor: possible role of mast cells in the course of its pathogenesis. Int Ophthalmol. 2007;27(4):235–9 [DOI] [PubMed] [Google Scholar]
- 7.Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas RS, Kahaly GJ, Patel A, Sile S, et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N Engl J Med. 2020;382:341–352 [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum JT, Choi D, Wilson DJ, et al. Fibrosis, gene expression and orbital inflammatory disease. Br J Ophthalmol. 2015;99(10):1424–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenbaum JT, Choi D, Wilson DJ, Grossniklaus HE, Sibley CH, Harrington CA, Planck SR, Orbital Disease Consortium. Molecular diagnosis of orbital inflammatory disease. Exp Mol Pathol. 2015;98:225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenbaum JT, Choi D, Wong A, et al. The role of the immune response in the pathogenesis of thyroid eye disease. PLoS One. 2015;10(9):e0137654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wester ST. Orbital Stem Cells. Curr Ophthalmol Rep. 2014;2:107–115 [Google Scholar]
- 13.Borzenok SA, Afanasyeva DS, Gushchina MB. Orbital Adipose Tissue: Just a Fat Pad or Terra Incognita in Ophthalmology. Vestn Ross Akad Med Nauk. 2015;4:464–7 [PubMed] [Google Scholar]
- 14.Khong JJ, McNab AA, Ebeling PR, et al. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. British Journal of Ophthalmology. 2016;100:142–150 [DOI] [PubMed] [Google Scholar]
- 15.Regensburg NI, Wiersinga WM, van Velthoven MEJ, et al. Age and gender-specific reference values of orbital fat and muscle volumes in Caucasians. British Journal of Ophthalmology. 2011;95:1660–1663 [DOI] [PubMed] [Google Scholar]
- 16.Pappa A, Lawson JM, Calder V, et al. T cells and fibroblasts in affected extraocular muscles in early and late thyroid associated ophthalmopathy. British Journal of Ophthalmology. 2000;84:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tallstedt L, Norberg R. Immunohistochemical staining of normal and Graves’ extraocular muscle. Invest Ophtalmol Vis Sci. 1988;29:175–184 [PubMed] [Google Scholar]
- 18.van der Gaag Ruth, Schmidt E. Donné, Zonneveld Frans W. & Koornneef Leo. Orbital pathology in thyroid-associated ophthalmopathy. Orbit. 1996;15(3):109–117 [Google Scholar]
- 19.Heufelder AE. Pathogenesis of ophthalmopathy in autoimmune thyroid disease. Reviews in Endocrine and Metabolic Disorders. 2000;1:87–95 [DOI] [PubMed] [Google Scholar]
- 20.Bahn RS. Graves’ ophthalmopathy. The New England Journal of Medicine. 2010;362(8):726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2000;85:1194–1199 [DOI] [PubMed] [Google Scholar]
- 22.Wiersinga WM, Regensburg NI, Mourits MP. Differential involvement of orbital fat and extraocular muscles in graves’ ophthalmopathy. Eur Thyroid J. 2013;2(1):14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalina PH, Lie JT, Campbell RJ, Garrity JA. Diagnostic value and limitations of orbital biopsy in Wegener’s granulomatosis. Ophthalmology. 1992; 99(1):120–4 [DOI] [PubMed] [Google Scholar]
- 24.Bradley D, Baughman RP, Raymond L, Kaufman AH. Ocular manifestations of sarcoidosis. Semin Respir Crit Care Med. 2002;23(6):543–548 [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum JT, Choi D, Wilson DJ, et al. Orbital pseudotumor can be a localized form of granulomatosis with polyangiitis as revealed by gene expression profiling. Exp Mol Pathol. 2015;99(2):271–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum JT, Choi D, Harrington CA, et al. Gene Expression Profiling and Heterogeneity of Nonspecific Orbital Inflammation Affecting the Lacrimal Gland. JAMA Ophthalmol. 2017; 135(11):1156–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronchetti S, Ricci E, Migliorati G, Gentili M, Riccardi C. How Glucocorticoids Affect the Neutrophil Life. Int J Mol Sci. 2018;19(12):4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arenas M, Sabater S, Hernández V, Rovirosa A, Lara PC, Biete A, Panés J. Anti-inflammatory effects of low-dose radiotherapy. Indications, dose, and radiobiological mechanisms involved. Strahlenther Onkol. 2012. Nov;188(11):975–81 [DOI] [PubMed] [Google Scholar]
- 29.Ozzello DJ, Dallalzadeh LO, Liu CY. Teprotumumab for chronic thyroid eye disease. Orbit. 2021;1:1–8 [DOI] [PubMed] [Google Scholar]
- 30.Ugradar S, Kang J, Kossler AL et al. Teprotumumab for the treatment of chronic thyroid eye disease. Eye. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]


