Abstract
OBJECTIVE
Observational studies have indicated that cocoa flavanol supplementation may be a promising strategy for type 2 diabetes (T2D) prevention. We aimed to directly evaluate its clinical efficacy in a large randomized clinical trial (RCT).
RESEARCH DESIGN AND METHOD
The Cocoa Supplement and Multivitamin Outcomes Study (COMSOS) was a 2 × 2 factorial RCT performed from June 2015 to December 2020 that tested cocoa extract and a multivitamin for the prevention of cardiovascular disease (CVD) and cancer. A total of 21,442 U.S. adults free of CVD and recent cancer, including 12,666 women aged ≥65 years and 8,776 men aged ≥60 years, were randomly assigned to receive cocoa extract [500 mg/day cocoa flavanols, including 80 mg (−)-epicatechin] or placebo. In this study, we included 18,381 participants without diabetes at enrollment and examined the effect of cocoa extract supplementation on incident self-reported T2D in intention-to-treat analyses.
RESULTS
During a median follow-up of 3.5 years, 801 incident T2D cases were reported. Compared with placebo, taking a cocoa extract supplement did not reduce T2D (adjusted hazard ratio 1.04, 95% CI 0.91–1.20, P = 0.58). Stratification analyses showed that the effect of cocoa extract supplementation was not significantly modified by sex, race, BMI, smoking, physical activity, dietary quality, flavanol status at baseline, or randomized multivitamin assignment.
CONCLUSIONS
Middle-aged and older adults taking a cocoa extract supplement for a median of 3.5 years did not reduce their risk of incident T2D. Further studies of cocoa extract supplementation beginning earlier in adulthood and in populations with different background diets are warranted.
Graphical Abstract
Introduction
Type 2 diabetes (T2D) continues to be a global epidemic, and simple, safe, and effective strategies for primary prevention are urgently needed (1). Cocoa, a fermented product made from the bean of the cacao tree, has historically been considered to have strong medicinal properties (2). Observational studies suggested that intake of cocoa products, such as dark chocolate and cocoa beverages, may be associated with lower T2D risk and more favorable glycemic measures (3–5). Purported health benefits of cocoa products have been mainly attributed to its high flavanol content, including catechins and epicatechins (6,7). Potential mechanisms for beneficial effects of cocoa on glycemia include enhancing endothelial function (8), antioxidant (6) and anti-inflammatory (9) activity, and modulation of insulin resistance (10). Therefore, cocoa supplementation may represent a promising intervention strategy for T2D prevention and control.
Although some small-scale clinical trials have shown beneficial short-term effects of cocoa on glucose and insulin metabolism (10–13), these have been limited by small sample sizes (10,14), short durations (15,16), cross-over designs (14,17), wide ranges of flavanol doses (10,18), type of intervention (chocolate, cocoa, cocoa extracts, or refined cocoa flavan-3-ols) (15–22), and reliance on intermediate biomarkers rather than clinical outcomes (23). Despite the decreased risk of cardiometabolic diseases associated with cocoa flavanols found in observational studies and some small trials, the effects of flavanol-rich cocoa extract in combination with other bioactive components of the cocoa bean on T2D prevention have not been examined in a large, randomized, placebo-controlled trial with long-term follow-up for clinical outcomes. We therefore addressed this question in the Cocoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial (RCT) that tested the effects of a cocoa extract supplement on the prevention of cardiovascular disease (CVD) and a multivitamin on the prevention of cancer among 21,442 older men and women (24–26).
Research Design and Methods
Study Design and Participant Recruitment
COSMOS is a recently completed, randomized, double-blind, placebo-controlled, 2 × 2 factorial trial designed to simultaneously test the effects of a cocoa extract supplement [2 capsules/day containing 500 mg flavanols/day, including 80 mg (−)-epicatechin; Mars Edge] on the prevention of CVD and a multivitamin supplement (Centrum Silver; Pfizer Consumer Healthcare, now Haleon) on the prevention of cancer. The sample size and statistical power were calculated for each objective separately. A total of 21,442 U.S. adults were randomized into the trial, including 12,666 women aged ≥65 years and 8,776 men aged ≥60 years, who were free of myocardial infarction, stroke, and recently diagnosed cancer (except for nonmelanoma skin cancer) within the past 2 years (24–26).
A detailed description of participant recruitment and enrollment has been published elsewhere (24). In brief, the recruitment was conducted from June 2015 to March 2018 with mailings to active participants in the Women’s Health Initiative (WHI) Extension Study, men and women contacted for but not randomly assigned to the Vitamin D and Omega-3 Trial (VITAL), and volunteers who heard about the trial through various sources. Eligible participants agreed to forgo taking personal cocoa extract and multivitamin supplements during the trial, restrict vitamin D to ≤1,000 IU/day and calcium to ≤1,200 mg/day from all supplements, and complete a placebo run-in phase of at least 2 months with good compliance indicated by taking ≥75% of the study pills.
A total of 21,442 participants met all eligibility requirements and were randomly assigned to the following four groups with equal probability from April 2016 to March 2018: 1) active cocoa extract and active multivitamin, 2) active cocoa extract and multivitamin placebo, 3) cocoa extract placebo and active multivitamin, or 4) both placebos. The study pills were mailed to participants as monthly calendar packs. Treatment assignments were blinded to both investigators and participants and stratified by sex, age (blocks of 5 years), and recruitment sources (WHI and non-WHI). The randomized intervention phase ended as scheduled on 31 December 2020.
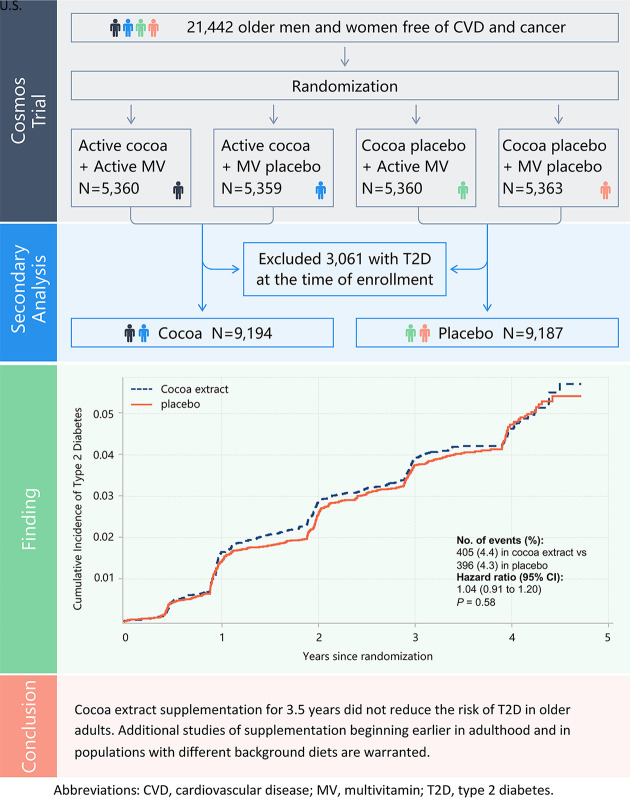
In the current study, we performed a secondary analysis to investigate the effects of cocoa extract supplementation on T2D risk. After excluding 3,061 participants with self-reported T2D at the time of enrollment, a total of 18,381 participants were included in the analysis. The participants who were randomly assigned to groups 1 and 2 were merged into the cocoa extract group (n = 9,194), while those assigned to groups 3 and 4 were categorized as the placebo group (n = 9,187) (Graphical Abstract).
Participant compliance was evaluated from self-reports on semiannual questionnaires that indicated the frequency of pill-taking, with good compliance defined as taking ≥75% of the study pills. A subset of 5,627 COSMOS participants (30.6%) was asked to provide optional biospecimens, including spot urines, through either a mailed biospecimen kit; home-based visit by Examination Management Services, Inc.; or at a Quest Diagnostics clinic visit. Furthermore, 1,750 participants also provided spot urine samples at 1, 2, and 3-year follow-up assessments. Urinary 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (gVL)-3′/4′-sulfate (gVL3S), and gVL-3′/4′-O-glucuronide (gVLG) metabolites (summed as gVLM) in humans has been considered to be specific for consumption of flavan-3-ol (27), which is one of the main bioactive components of cocoa extract. In the COSMOS trial, we measured baseline and follow-up urinary gVLM as a biomarker of compliance with the cocoa extract intervention. We previously demonstrated that participants randomized to the COSMOS cocoa extract intervention had a threefold increase in urinary gVLM compared with placebo (25).
All participants provided written informed consent before enrollment in the trial. COSMOS was approved and overseen by the institutional review board of Brigham and Women’s Hospital/Mass General Brigham. The COSMOS website is https://www.cosmostrial.org.
Ascertainment of Incident T2D
Incident T2D cases were self-reported from questionnaires completed every year during the trial, as well as the study closeout follow-up questionnaire in January 2021. Participants were asked, “In the past year, have you been newly diagnosed with diabetes? If yes, please provide the month/year of the diagnosis.” In addition, they were asked, “Are you currently taking any diabetes medications?” If participants provided the date of diagnosis, the occurrence of incident T2D was marked on that date. In cases where participants did not provide this information but indicated current use of diabetes medication, the date of incident T2D was assigned as the date of questionnaire return. As a sensitivity analysis, we also ascertained incident T2D cases as self-reported incident T2D plus use of hypoglycemic medications. Compared with confirmation through use of medical records and biomarkers, self-reported diabetes has been found to have high accuracy and reliability in validation studies (28–30).
Assessment of Covariates
Demographic characteristics and lifestyle factors for each participant collected at baseline using questionnaires were included in the analysis as covariates, such as sex, age, race/ethnicity, education, smoking status, alcohol intake, physical activity, alternate healthy eating index (AHEI), BMI, and family history of diabetes. Because of the 2 × 2 factorial design, we also included randomized multivitamin assignment in our analyses. Participants who had smoked >100 cigarettes in their lifetime and were currently smoking were categorized as current smokers, those who had smoked <100 cigarettes in their lifetime and were not currently smoking were categorized as never smokers, and those who had smoked >100 cigarettes in their lifetime but were not currently smoking were categorized as previous smokers. Participants indicated their average consumption of beer, red wine, white wine, and liquor over the past year through a dietary questionnaire, which was then converted into grams of alcohol per day. Finally, participants reported the average time per week spent engaging in 10 recreational activities, as well as the daily average of steps climbed, which were subsequently converted into total physical activity in MET-h/week.
The proportions of missing data for covariates were 0.95% for education, 1.18% for physical activity, 1.39% for smoking, 1.90% for BMI, 4.94% for family history of diabetes, and 11.2% for AHEI. We applied median and mode imputation to replace the missing data for continuous and categorical covariates, respectively.
Statistical Analysis
Our primary analyses were based on the intention-to-treat principle. Follow-up was censored at the date of the occurrence of incident T2D, last contact, or end of the trial on 31 December 2020, whichever came first. We used Cox proportional hazards models to allow for variable follow-up length and estimated the hazard ratio (HR) for the randomized cocoa extract intervention, controlling for age, sex, race/ethnicity (White, African American, Hispanic, Asian/Pacific Islander, or other), randomized multivitamin assignment (yes or no), education (high school or less, college, or postcollege), smoking (never, previous, or current), alcohol intake (in g/day, continuous), physical activity in MET-h/week (continuous), dietary quality evaluated by AHEI (continuous), family history of diabetes (yes or no), and BMI (in kg/m2, continuous).
To assess the potential bias introduced by missing data imputation, we conducted an additional analysis by excluding participants with missing covariate data. To evaluate whether findings were biased by poor compliance, we censored follow-up at the time of noncompliance by taking <75% of the study pills in per-protocol analyses.
A series of subgroup analyses examined whether the effects of cocoa extract on T2D were modified by sex (male or female), race/ethnicity (White or non-White), BMI (≥30 or <30 kg/m2), smoking (never, previous, or current), physical activity (greater than or equal to or less than the median), AHEI (greater than or equal to or less than the median), baseline urinary gVLM (greater than or equal to or less than the median), and randomized multivitamin treatment assignment (active or placebo). Analyses were performed using SAS 9.4 statistical software (SAS Institute).
Results
Baseline Characteristics
Among the 18,381 participants included in the analyses, 7,459 were men with a mean ± SD age of 68.9 ± 6.2 years, 10,922 were women with a mean age of 74.2 ± 6.0 years, 90% were White, 90% had college degree or above, and 4% were current smokers. The mean BMI was 27.1 ± 5.0 kg/m2. The distribution of sociodemographic, lifestyle factors, family history of diabetes, statin use, and urinary gVLM at baseline was balanced between the randomized cocoa extract and placebo groups (Table 1).
Table 1.
Baseline characteristics of 18,381 COSMOS participants with no history of T2D according to randomized treatment assignment
| Characteristic | Total | Cocoa extract | Placebo | P |
|---|---|---|---|---|
| Participants, n | 18,381 | 9,194 | 9,187 | |
| Men, n (%) | 7,459 (40.58) | 3,746 (40.74) | 3,713 (40.42) | 0.65 |
| Age, years | 72.0 ± 6.6 | 72.0 ± 6.6 | 72.0 ± 6.6 | 0.96 |
| BMI, kg/m2 | 27.1 ± 5.0 | 27.1 ± 5.0 | 27.1 ± 5.0 | 0.33 |
| Self-identified race/ethnicity, n (%) | 0.11 | |||
| White | 16,481 (89.66) | 8,240 (89.62) | 8,241 (89.70) | |
| African American | 793 (4.31) | 394 (4.29) | 399 (4.34) | |
| Hispanic | 415 (2.26) | 188 (2.04) | 227 (2.47) | |
| Asian/Pacific Islander | 369 (2.01) | 199 (2.16) | 170 (1.85) | |
| Other | 323 (1.76) | 173 (1.88) | 150 (1.63) | |
| Education, n (%) | 0.84 | |||
| High school or less | 1,840 (10.01) | 926 (10.07) | 914 (9.95) | |
| College | 7,302 (39.73) | 3,633 (39.51) | 3,669 (39.94) | |
| Postcollege | 9,239 (50.26) | 4,635 (50.41) | 4,604 (50.11) | |
| Smoking status, n (%) | 0.26 | |||
| Never | 10,247 (55.75) | 5,093 (55.39) | 5,154 (56.10) | |
| Previous | 7,434 (40.44) | 3,765 (40.95) | 3,669 (39.94) | |
| Current | 700 (3.81) | 336 (3.65) | 364 (3.96) | |
| Total caloric intake, kcal/day | 1,641 ± 825 | 1,640 ± 831 | 1,643 ± 819 | 0.82 |
| Alcohol intake, g/day | 9.45 ± 14.30 | 9.45 ± 14.20 | 9.45 ± 14.40 | 0.99 |
| Total carbohydrates, g/day | 181.3 ± 100.4 | 180.8 ± 101.6 | 181.8 ± 99.2 | 0.50 |
| Saturated fat, g/day | 21.2 ± 12.2 | 21.2 ± 12.1 | 21.3 ± 12.3 | 0.40 |
| Monounsaturated fat, g/day | 22.1 ± 13.1 | 22.1 ± 13.3 | 22.0 ± 13.0 | 0.66 |
| Polyunsaturated fat, g/day | 13.3 ± 9.6 | 13.3 ± 9.5 | 13.3 ± 9.6 | 0.86 |
| Animal protein, g/day | 56.6 ± 37.3 | 56.7 ± 38.6 | 56.4 ± 36.0 | 0.52 |
| Vegetable protein, g/day | 22.4 ± 14.2 | 22.5 ± 14.8 | 22.3 ± 13.6 | 0.31 |
| AHEI | 42.8 ± 10.3 | 42.9 ± 10.2 | 42.8 ± 10.4 | 0.58 |
| Recreational physical activity, MET-h/week | 25.04 ± 25.22 | 25.28 ± 25.64 | 24.81 ± 24.80 | 0.21 |
| Family history of diabetes, n (%) | 5,500 (29.92) | 2,718 (29.56) | 2,782 (30.28) | 0.46 |
| History of high blood pressure, n (%) | 9,924 (54.15) | 4,955 (54.06) | 4,969 (54.24) | 0.80 |
| Statin use, n (%) | 7,325 (40.13) | 3,698 (40.44) | 3,627 (39.81) | 0.38 |
| Regular multivitamin use before run-in, n (%) | 7,436 (40.61) | 3,757 (41.00) | 3,679 (40.21) | 0.28 |
| Randomized to multivitamin, n (%) | 9,205 (50.08) | 4,601 (50.04) | 4,604 (50.11) | 0.92 |
| Urinary gVLM at baseline, μmol/La | 10.0 (15.4) | 9.8 (15.2) | 10.2 (15.5) | 0.43 |
Data are mean ± SD unless otherwise indicated.
Urine gVLM levels at baseline were measured in 2,836 participants in the cocoa extract group and 2,791 in the placebo group.
Effect of Cocoa Extract Supplementation on Incident T2D
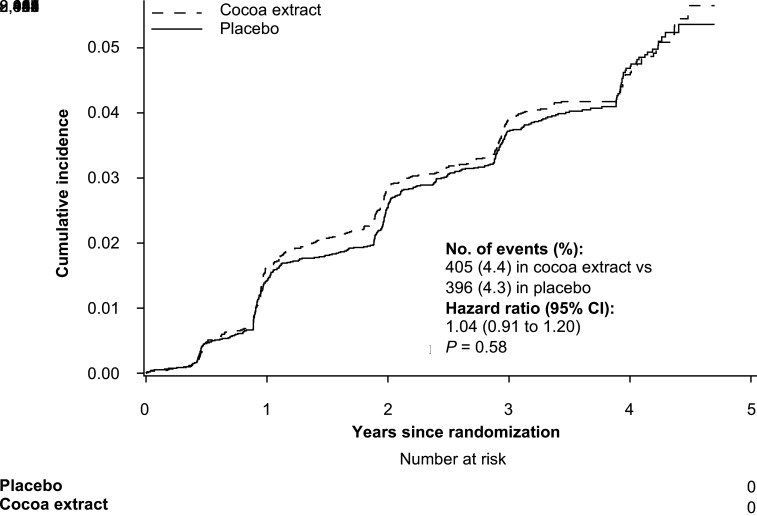
During a median follow-up of 3.5 (interquartile range 3.3–4.2) years, a total of 801 incident T2D cases were reported by participants. Compared with the participants taking placebo, those taking cocoa extract supplement did not have a reduced risk of T2D (HR 1.03, 95% CI 0.89–1.18, P = 0.73). Further adjustment or multiple covariables did not materially change the results (HR 1.04, 95% CI 0.91–1.20, P = 0.58) (Table 2 and Fig. 1). For 557 participants with incident T2D treated with hypoglycemic medications (insulin or other injectable or oral hypoglycemic agent), the HR was 1.04 (95% CI 0.88–1.22, P = 0.68) (Table 2). Excluding participants with missing data in analyses did not materially change the results. In the per-protocol analysis with follow-up censored at the time of noncompliance based on initial report of taking <75% of the study pills, the results were consistent with our intention-to-treat findings (Table 2).
Table 2.
Effect of randomized cocoa extract supplementation on incident T2D in intention-to-treat and per-protocol analyses
| Number of events of total | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Cocoa extract | Placebo | Model | HR1 (95% CI) | P | HR2 (95% CI) | P |
| Intention-to-treat analysis | |||||||
| Total incident T2D | 405 of 9,194 | 396 of 9,187 | 1 | 1.03 (0.89–1.18) | 0.73 | 1.03 (0.89–1.18) | 0.73 |
| 2 | 1.04 (0.91–1.20) | 0.58 | 1.06 (0.91–1.24) | 0.46 | |||
| Medication-treated T2D | 282 of 9,194 | 275 of 9,187 | 1 | 1.02 (0.87–1.21) | 0.79 | 1.02 (0.87–1.21) | 0.79 |
| 2 | 1.04 (0.88–1.22) | 0.68 | 1.06 (0.88–1.27) | 0.55 | |||
| Per-protocol analysis | |||||||
| Total incident T2D | 374 of 9,194 | 372 of 9,187 | 1 | 1.02 (0.88–1.17) | 0.84 | 1.02 (0.88–1.17) | 0.84 |
| 2 | 1.03 (0.90–1.19) | 0.66 | 1.06 (0.91–1.24) | 0.47 | |||
| Medication-treated T2D | 262 of 9,194 | 258 of 9,187 | 1 | 1.02 (0.86–1.21) | 0.81 | 1.02 (0.86–1.21) | 0.82 |
| 2 | 1.04 (0.87–1.23) | 0.67 | 1.07 (0.88–1.29) | 0.51 | |||
HRs and their 95% CIs were calculated by using Cox proportional hazards regression models. We imputed missing values for covariables before modeling. For the HR1 analysis, all participants were included, while for the HR2 analysis, participants with missing data were excluded. Model 1 is adjusted for sex, age, race/ethnicity, and randomized multivitamin assignment. Model 2 is model 1 plus education, smoking, alcohol intake, physical activity, AHEI, family history of diabetes, and BMI. In the per-protocol analysis, follow-up was censored on time of noncompliance evaluated by taking <75% of the study pills.
Figure 1.
Cumulative incidence of T2D in cocoa extract supplementation and placebo groups during the trial period. The HR of T2D was calculated by using Cox proportional hazards regression models with adjustment for sex, age, race/ethnicity, randomized multivitamin assignment, education, smoking, alcohol intake, physical activity, dietary quality, family history of diabetes, and BMI (intention-to-treat analysis).
Further stratification analyses showed that cocoa extract supplementation was associated with a nonsignificant 45% lower risk of T2D among current smokers (HR 0.55, 95% CI 0.29–1.05, P = 0.07), while never smokers (HR 1.08, 95% CI 0.90–1.30, P = 0.42) and former smokers (HR 1.06, 95% CI 0.85–1.32, P = 0.63, P for interaction = 0.17) had no suggestive effects. Otherwise, the effects of randomized cocoa extract supplementation on incident T2D did not significantly differ by sex (P for interaction = 0.99), race/ethnicity (P for interaction = 0.46), BMI (P for interaction = 0.40), physical activity (P for interaction = 1.00), dietary quality based on AHEI (P for interaction = 0.37), randomized multivitamin intervention (P for interaction = 0.86), or urinary gVLM at baseline (P for interaction = 0.65) (Table 3).
Table 3.
Subgroup analyses of the effect of randomized cocoa extract supplementation on incident T2D
| Number of events of total | |||||
|---|---|---|---|---|---|
| Cocoa extract | Placebo | HR (95% CI) | P | P for interaction | |
| Sex | |||||
| Male | 136 of 3,746 | 131 of 3,713 | 1.05 (0.82–1.33) | 0.71 | 0.99 |
| Female | 269 of 5,448 | 265 of 5,474 | 1.04 (0.88–1.23) | 0.68 | |
| Race | |||||
| White | 357 of 8,240 | 342 of 8,241 | 1.06 (0.92–1.23) | 0.42 | 0.46 |
| Non-White | 48 of 954 | 54 of 946 | 0.90 (0.61–1.33) | 0.59 | |
| BMI, kg/m2 | |||||
| <30 | 229 of 7,097 | 234 of 7,098 | 0.99 (0.82–1.19) | 0.89 | 0.40 |
| ≥30 | 176 of 2,097 | 162 of 2,089 | 1.11 (0.90–1.37) | 0.34 | |
| Smoking status | |||||
| Never | 226 of 5,093 | 217 of 5,154 | 1.08 (0.90–1.30) | 0.42 | 0.17 |
| Previous | 164 of 3,765 | 151 of 3,669 | 1.06 (0.85–1.32) | 0.63 | |
| Current | 15 of 336 | 28 of 364 | 0.55 (0.29–1.05) | 0.07 | |
| Physical activity | |||||
| Less than median (19.1 MET-h/week) | 240 of 4,636 | 238 of 4,646 | 1.03 (0.86–1.24) | 0.73 | 1.00 |
| Greater than or equal to median (19.1 MET-h/week) | 165 of 4,558 | 158 of 4,541 | 1.03 (0.83–1.29) | 0.77 | |
| Dietary quality | |||||
| Less than median AHEI score (42.5) | 218 of 4,903 | 227 of 4,889 | 0.98 (0.82–1.18) | 0.85 | 0.37 |
| Greater than or equal to median AHEI score (42.5) | 187 of 4,291 | 169 of 4,298 | 1.13 (0.91–1.39) | 0.26 | |
| Multivitamin intervention | |||||
| Yes | 202 of 4,601 | 196 of 4,604 | 1.06 (0.87–1.29) | 0.56 | 0.86 |
| No | 203 of 4,593 | 200 of 4,583 | 1.03 (0.85–1.25) | 0.79 | |
| Urinary gVLM at baselinea | |||||
| Less than median (3.5 μmol/L) | 53 of 1,441 | 50 of 1,372 | 1.03 (0.70–1.52) | 0.89 | 0.65 |
| Greater than or equal to median (3.5 μmol/L) | 52 of 1,395 | 60 of 1,419 | 0.90 (0.62–1.31) | 0.58 | |
HRs and their 95% CIs were calculated by using Cox proportional hazards regression models, with adjustment for sex, age, race/ethnicity, randomized multivitamin assignment, education, smoking, alcohol intake, physical activity, AHEI, family history of diabetes, and BMI.
Urine gVLM levels at baseline were measured in 2,836 participants in the cocoa extract group and 2,791 in the placebo group.
Conclusions
In this large, randomized, double-blind, placebo-controlled trial, we found no beneficial or adverse effect of cocoa extract supplementation on self-reported incidence of T2D among older U.S. men and women free of diabetes at baseline during a median follow-up of 3.5 years. Previous prospective cohort studies that examined the associations between dietary flavonoid intake and risk of T2D have shown inconsistent findings. In the Nurses’ Health Study (NHS) of 70,359 women, NHS II of 89,201 women, and the Health Professionals Follow-Up Study of 41,334 men, the highest versus lowest quintile of dietary anthocyanins, a major flavonoid subclass, was associated with a lower risk of T2D. However, no significant associations were found for total flavonoid intake or other flavonoid subclasses (31). The European Prospective Investigation into Cancer and Nutrition (EPIC)–InterAct case-cohort study found that individual flavonoids were differentially associated with risk of T2D, with the highest versus lowest quintiles of flavan-3-ol monomers, proanthocyanidins with a low polymerization degree, and the flavonol myricetin inversely associated with a lower risk of T2D (32). However, in the Women’s Health Study of 38,018 women with 8.8 years of follow-up, there was no significant association between intake of flavonols and flavones and T2D risk (33). Finally, a meta-analysis of six prospective cohort studies that included 284,806 participants and 18,146 T2D cases showed that a 500 mg/day increase in total flavonoids intake was associated with a 5% lower risk of T2D (34). The interpretation of results from these and other observational studies is likely limited because of residual confounding by other dietary and lifestyle factors.
Small-scale, short-term trials have also tested the effects of flavanols on cardiometabolic outcomes related to T2D. Interventions in these trials included monomers, proanthocyanidins, and foods and beverages rich in flavanol, such as fruit, dark chocolate, cocoa, tea, and red wine. Outcomes were typically limited to intermediate biomarkers of cardiometabolic health, including glucose metabolism via HbA1c, fasting glucose, and HOMA of insulin resistance (35,36). In a meta-analysis of RCTs evaluating the effect of flavan-3-ols on glucose metabolism, there were significant decreases in HbA1c and HOMA of insulin resistance but not in fasting glucose. Furthermore, meta-regression on these smaller and short-term RCTs did not reveal any dose-response associations between flavanol intake and net change in glucose metabolism (23).
In the COSMOS trial, with its large sample size and long-term follow-up, we found a null effect of cocoa extract supplementation on incident T2D in older men and women. COSMOS was designed with ≥80% power to detect an 11% relative hazard reduction in total CVD (25,26). As the incidence rate for T2D was higher than CVD for which COSMOS was designed, we believe that we had adequate power to evaluate the efficacy of cocoa flavanol supplementation on risk of T2D, a secondary outcome, in the current study. In addition, compliance with the cocoa extract intervention was high, with >80% of participants on average taking at least 75% of study pills and a more than threefold increase in urinary gVLM concentrations with cocoa intervention (25,26). Hence, we do not expect poor compliance to explain our lack of effect for cocoa extract on T2D.
Some prospective cohort studies to date suggested that the associations between chocolate consumption and risk of diabetes may be stronger in comparatively younger men and women. The Physicians’ Health Study included 18,235 men with a mean follow-up of 9 years and suggested an inverse association of chocolate intake with incident T2D in younger (<65 years) but not older (≥65 years) men (P for interaction for age = 0.03) (5). Furthermore, the WHI included 92,678 postmenopausal women with a mean follow-up of 13 years and found that moderate chocolate intake modestly decreased risk of T2D in the women aged <65 years but not in those aged ≥65 years (P for interaction for age = 0.048) (37). Although these observational analyses cannot exclude residual confounding and selection biases, it is possible that the older age of participants recruited in the COSMOS trial may have contributed to the null effect of cocoa extract supplementation observed in the current study. In addition, the 3.5-year follow-up period in the COSMOS trial is comparatively shorter than the 9–13 years of follow-up in the prospective cohorts. This discrepancy raises the possibility that the observed null results could be at least partially attributed to insufficient follow-up time.
In subgroup analyses, we found that most subgroups did not have a lower risk of T2D with cocoa extract supplementation, but current smokers had a 45% lower risk of T2D in the active versus placebo group. On the one hand, given multiple comparisons, this finding may have been due to chance and should be interpreted cautiously. On the other hand, it is possible that current smokers and others with unhealthy lifestyles and higher oxidative/inflammatory loads may be more likely to benefit from cocoa supplementation. Further research is necessary to examine the potential of cocoa supplementation in preventing T2D in populations characterized by unhealthy lifestyles.
Several strengths of the current study should be considered, including the large sample size, double-blind and placebo-controlled trial design, 2 × 2 factorial design, and good compliance with the cocoa extract intervention. These strengths made COSMOS the most rigorous trial to date to evaluate the efficacy of cocoa flavanol supplementation in reducing the incidence of T2D. Some limitations should also be considered. First, because the trial enrolled older U.S. men and women, our findings cannot be generalized to younger adults at risk for developing T2D or to populations with different background diets or risk factors for T2D. Second, the incident T2D cases were self-reported; however, self-reported T2D has previously been demonstrated to have high accuracy and reliability in validation studies (28–30). Third, cocoa has a high concentration of bioactive compounds, including antioxidants (38), although we were not able to determine the antioxidant activity of the cocoa extract supplement used in COSMOS. Finally, this secondary analysis that excluded participants with T2D at baseline may modestly alter the balance between intervention and placebo groups; however, our large sample size and randomization minimized potential bias for the main relative risk estimate comparing cocoa with placebo.
In conclusion, cocoa extract supplementation during a median of 3.5 years did not reduce the risk of T2D in older U.S. adults. Nonetheless, given previous research suggesting potential benefits for T2D in younger adults, additional studies of cocoa extract supplementation beginning earlier in adulthood and in countries or regions with different background diets are warranted.
Article Information
Acknowledgments. The authors express sincere gratitude to the 21,442 COSMOS participants for their steadfast and conscientious collaboration and to the members of the COSMOS Research Group for their commitment to the trial despite the challenges of the coronavirus 2019 pandemic. The authors specifically acknowledge the COSMOS Research Group for its scientific (Brigham and Women’s Hospital, Fred Hutchinson Cancer Research Center, WHI, data safety and monitoring board, and Mars Edge) and logistical (Brigham and Women’s Hospital, Fred Hutchinson Cancer Research Center, data monitoring and safety board, Mars Edge, Contract Pharmacal Corp., and Pfizer Consumer Healthcare, now Haleon) contributions.
Funding. The COSMOS trial is supported by an investigator-initiated grant from Mars Edge, a segment of Mars dedicated to nutrition research and products, which included infrastructure support and the donation of study pills and packaging. Pfizer Consumer Healthcare (now part of Haleon) provided support through the partial provision of study pills and packaging. COSMOS is also supported in part by National Institutes of Health grants AG050657, AG071611, EY025623, and HL157665. The WHI program is funded by National Heart, Lung, and Blood Institute contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, and 75N92021D00005.
Neither Mars Edge or Pfizer Consumer Healthcare, now Haleon, had a role in the trial design or conduct, data collection (other than blinded assays supported by Mars Edge and completed independently), data analysis, or manuscript preparation or review.
Duality of Interest. S.L. reported receiving consulting fees, payment, or honoraria for lectures and presentations from Barilla, TwinHealth, Johns Hopkins University, Fred Hutchinson Cancer Center, Harvard University, University at Buffalo, Guangdong Provincial People’s Hospital, Fuwai Hospital, Chinese Academy of Medical Sciences, National Institutes of Health, and American Society for Nutrition and participates on data safety monitoring boards for the Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT) trial sponsored by Novo Nordisk and a trial of pulmonary hypertension in patients with diabetes sponsored by Massachusetts General Hospital. H.D.S. and J.E.M. reported receiving investigator-initiated grants from Mars Edge and Pfizer Consumer Healthcare, now Haleon. H.D.S. additionally reported receiving investigator-initiated grants from Pure Encapsulations and Pfizer Inc. and honoraria and/or travel expenses for lectures from the Council for Responsible Nutrition, BASF, National Institutes of Health, and American Society of Nutrition during the conduct of the study. No other conflicts of interest relevant to this article were reported.
Author Contributions. J.L. and S.L. contributed to the data interpretation and writing of the first draft of the manuscript. H.D.S. and J.E.M. obtained study funding. H.D.S., J.E.M., G.F., A.C., T.C., A.H.S., J.W.-W., L.T., and S.L critically reviewed the manuscript. H.D.S., J.E.M., G.F., A.C., and T.C., and S.L. contributed to the data collection. H.D.S., J.E.M., and. S.L. contributed to the study design. E.K. performed the data analysis. All authors read and approved the final manuscript. S.L., J.E.M., and H.D.S. are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Funding Statement
The COSMOS trial is supported by an investigator-initiated grant from Mars Edge, a segment of Mars dedicated to nutrition research and products, which included infrastructure support and the donation of study pills and packaging. Pfizer Consumer Healthcare (now part of Haleon) provided support through the partial provision of study pills and packaging. COSMOS is also supported in part by National Institutes of Health grants AG050657, AG071611, EY025623, and HL157665. The WHI program is funded by National Heart, Lung, and Blood Institute contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, and 75N92021D00005.
Footnotes
Clinical trials reg. no. NCT02422745, clinicaltrials.gov
References
- 1. Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global Perspective. Endocrinol Metab Clin North Am 2021;50:337–355 [DOI] [PubMed] [Google Scholar]
- 2. Dillinger TL, Barriga P, Escárcega S, Jimenez M, Salazar Lowe D, Grivetti LE. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J Nutr 2000;130(Suppl.):2057S–2072S [DOI] [PubMed] [Google Scholar]
- 3. Greenberg JA. Chocolate intake and diabetes risk. Clin Nutr 2015;34:129–133 [DOI] [PubMed] [Google Scholar]
- 4. Oba S, Nagata C, Nakamura K, et al. Consumption of coffee, green tea, oolong tea, black tea, chocolate snacks and the caffeine content in relation to risk of diabetes in Japanese men and women. Br J Nutr 2010;103:453–459 [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto C, Petrone AB, Sesso HD, Gaziano JM, Djoussé L. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr 2015;101:362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal 2011;15:2779–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kerimi A, Williamson G. The cardiovascular benefits of dark chocolate. Vascul Pharmacol 2015;71:11–15 [DOI] [PubMed] [Google Scholar]
- 8. Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am J Clin Nutr 2008;88:58–63 [DOI] [PubMed] [Google Scholar]
- 9. Magrone T, Russo MA, Jirillo E. Cocoa and dark chocolate polyphenols: from biology to clinical applications. Front Immunol 2017;8:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grassi D, Necozione S, Lippi C, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005;46:398–405 [DOI] [PubMed] [Google Scholar]
- 11. Mellor DD, Sathyapalan T, Kilpatrick ES, Beckett S, Atkin SL. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in Type 2 diabetes patients. Diabet Med 2010;27:1318–1321 [DOI] [PubMed] [Google Scholar]
- 12. Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012;35:226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desideri G, Kwik-Uribe C, Grassi D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 2012;60:794–801 [DOI] [PubMed] [Google Scholar]
- 14. Basu A, Betts NM, Leyva MJ, Fu D, Aston CE, Lyons TJ. Acute cocoa supplementation increases postprandial HDL cholesterol and insulin in obese adults with type 2 diabetes after consumption of a high-fat breakfast. J Nutr 2015;145:2325–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stote KS, Clevidence BA, Novotny JA, Henderson T, Radecki SV, Baer DJ. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur J Clin Nutr 2012;66:1153–1159 [DOI] [PubMed] [Google Scholar]
- 16. Muniyappa R, Hall G, Kolodziej TL, Karne RJ, Crandon SK, Quon MJ. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr 2008;88:1685–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes 2008;32:1289–1296 [DOI] [PubMed] [Google Scholar]
- 18. Rynarzewski J, Dicks L, Zimmermann BF, et al. Impact of a usual serving size of flavanol-rich cocoa powder ingested with a diabetic-suitable meal on postprandial cardiometabolic parameters in type 2 diabetics-a randomized, placebo-controlled, double-blind crossover study. Nutrients 2019;11:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dicks L, Kirch N, Gronwald D, et al. Regular intake of a usual serving size of flavanol-rich cocoa powder does not affect cardiometabolic parameters in stably treated patients with type 2 diabetes and hypertension-a double-blinded, randomized, placebo-controlled trial. Nutrients 2018;10:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr 2005;81:611–614 [DOI] [PubMed] [Google Scholar]
- 21. Grassi D, Desideri G, Necozione S, et al. Protective effects of flavanol-rich dark chocolate on endothelial function and wave reflection during acute hyperglycemia. Hypertension 2012;60:827–832 [DOI] [PubMed] [Google Scholar]
- 22. Sian TS, Din USU, Deane CS, et al. Cocoa flavanols adjuvant to an oral nutritional supplement acutely enhances nutritive flow in skeletal muscle without altering leg glucose uptake kinetics in older adults. Nutrients 2021;13:1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raman G, Avendano EE, Chen S, et al. Dietary intakes of flavan-3-ols and cardiometabolic health: systematic review and meta-analysis of randomized trials and prospective cohort studies. Am J Clin Nutr 2019;110:1067–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rist PM, Sesso HD, Johnson LG, et al.; COSMOS Research Group . Design and baseline characteristics of participants in the Cocoa Supplement and Multivitamin Outcomes Study (COSMOS). Contemp Clin Trials 2022;116:106728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sesso HD, Manson JE, Aragaki AK, et al.; COSMOS Research Group . Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: the Cocoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am J Clin Nutr 2022;115:1490–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sesso HD, Rist PM, Aragaki AK, et al.; COSMOS Research Group . Multivitamins in the prevention of cancer and cardiovascular disease: the Cocoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am J Clin Nutr 2022;115:1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ottaviani JI, Fong R, Kimball J, et al. Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies. Sci Rep 2018;8:9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med 2007;167:1676–1685 [DOI] [PubMed] [Google Scholar]
- 29. Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 30. Margolis KL, Lihong Qi, Brzyski R, et al.; Women Health Initiative Investigators . Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 2008;5:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wedick NM, Pan A, Cassidy A, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012;95:925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zamora-Ros R, Forouhi NG, Sharp SJ, et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr 2014;144:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr 2005;24:376–384 [DOI] [PubMed] [Google Scholar]
- 34. Liu YJ, Zhan J, Liu XL, Wang Y, Ji J, He QQ. Dietary flavonoids intake and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Clin Nutr 2014;33:59–63 [DOI] [PubMed] [Google Scholar]
- 35. Hooper L, Kay C, Abdelhamid A, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 2012;95:740–751 [DOI] [PubMed] [Google Scholar]
- 36. Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr 2011;141:1982–1988 [DOI] [PubMed] [Google Scholar]
- 37. Greenberg JA, Manson JE, Tinker L, et al. Chocolate intake and diabetes risk in postmenopausal American women. Eur J Clin Nutr 2017;71:1088–1093 [DOI] [PubMed] [Google Scholar]
- 38. Jenkins DJ, Kendall CW, Vuksan V, et al. Effect of cocoa bran on low-density lipoprotein oxidation and fecal bulking. Arch Intern Med 2000;160:2374–2379 [DOI] [PubMed] [Google Scholar]