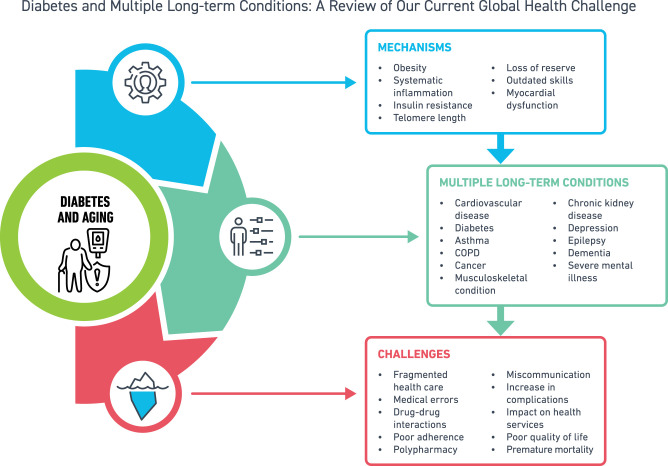
Abstract
Use of effective treatments and management programs is leading to longer survival of people with diabetes. This, in combination with obesity, is thus contributing to a rise in people living with more than one condition, known as multiple long-term conditions (MLTC or multimorbidity). MLTC is defined as the presence of two or more long-term conditions, with possible combinations of physical, infectious, or mental health conditions, where no one condition is considered as the index. These include a range of conditions such as cardiovascular diseases, cancer, chronic kidney disease, arthritis, depression, dementia, and severe mental health illnesses. MLTC has major implications for the individual such as poor quality of life, worse health outcomes, fragmented care, polypharmacy, poor treatment adherence, mortality, and a significant impact on health care services. MLTC is a challenge, where interventions for prevention and management are lacking a robust evidence base. The key research directions for diabetes and MLTC from a global perspective include system delivery and care coordination, lifestyle interventions and therapeutic interventions.
Graphical Abstract
Introduction
Diabetes is one of the leading global health challenges of our times. The number of adults living with diabetes globally is 537 million and is predicted to rise to 783 million by 2045 (1). With an increase in the number of effective treatments and management programs, the survival of people with diabetes is therefore contributing to a rise in people living with more than one condition, known as multiple long-term conditions (MLTC) or multimorbidity. This includes a range of conditions such as cardiovascular diseases, chronic kidney disease (CKD), arthritis, depression, and severe mental health illnesses (2). MLTC have major implications for the individual such as fragmented care, polypharmacy, poor quality of life, mortality, and a significant impact on health care services. Clinicians are aware that MLTC are impacting their patients with diabetes but are not able to fully support them, as the current health care system is still designed around the single-disease model (3,4). Thus, clinical decisions are based on multiple guidelines, and clinicians are often faced with many difficulties in balancing the benefits and risks of recommended treatments and consequently give varying advice to patients. For example, younger patients may have complex physical and mental health complications, and older patients may experience a high number of comorbidities with the need for treatment intensification or deintensification and social and quality of life support (5,6).
Previous health reports and reviews to date have highlighted the need to prioritize MLTC (2,7), where the presence of diabetes is associated with a higher likelihood of other long-term conditions being present (8,9). In this review, therefore, we aim to provide a summary of the epidemiology of MLTC in diabetes, challenges for the patient and service delivery, and the current interventions for prevention and management. Furthermore, key research directions for diabetes and MLTC are provided.
Epidemiology of MLTC in Diabetes
Definition of MLTC
MLTC is defined as the presence of two or more long-term conditions, with possible combinations of physical, infectious, or mental health conditions, where no one condition is considered as the index (10). The term multimorbidity has previously been used; however, following a patient and stakeholder consultation, the preferential description is now MLTC (11). MLTC measures are highly heterogeneous, either based on simple counts of chronic conditions or based on indices to assess the burden by using weights on the range of conditions, duration, severity, and resource utilization such as the Charlson Comorbidity Index or the Elixhauser Comorbidity Index (12,13). However, data on such indices are often limited (14). In a recent systematic review of 566 studies, investigators reported that the number of conditions measured in MLTC studies ranged from 2 to 285 conditions, median 17, in most cases including a cardiovascular condition (97.6%), metabolic and endocrine condition (97.3%), respiratory condition (93.4%), musculoskeletal condition (87.6%), and mental health condition (78.5%) (15).
Due to the limitations of inconsistent definitions and approaches to measuring MLTC (15), a recent Delphi consensus study included a panel of international experts who identified a definition of MLTC following a criteria relating to the impact of the condition (16). The consensus was reached for defining MLTC as two or more long-term conditions with a duration of ≥6 months, with a selected 24 conditions including diabetes to be always included in the MLTC measure and 35 to usually be included (Table 1). In future studies in MLTC investigators should use this definition by the Delphi study to help facilitate consistency and comparison of studies and to build on the evidence of MLTC research.
Table 1.
Conditions with consensus to always or usually be included in the MLTC measure, based on Delphi survey (16)
| Body system (based on ICD-10 chapters) | Always include, N = 24 | Usually include (unless there is a good reason not to in a particular context), N = 35* |
|---|---|---|
| Cardiovascular disease | Stroke, coronary artery disease, heart failure, peripheral artery disease | Heart valve disorders, arrhythmia, venous thromboembolic disease, aneurysm, hypertension (treated and untreated) |
| Metabolic and endocrine disease | Diabetes, Addison disease, cystic fibrosis | Thyroid disorders |
| Respiratory disease | Chronic obstructive pulmonary disease, asthma | Bronchiectasis |
| Neurological disease | Parkinson disease, epilepsy, multiple sclerosis, paralysis | Transient ischemic attack, peripheral neuropathy, chronic primary pain |
| Cancer | Solid organ cancers, hematological cancers, metastatic cancers | Melanoma, benign cerebral tumors that can cause disability |
| Mental and behavioral disorder | Dementia, schizophrenia | Depression, anxiety, bipolar disorder, drug or alcohol misuse, eating disorder, autism, Posttraumatic stress disorder |
| Musculoskeletal disease | Connective tissue disease | Osteoarthritis, long-term musculoskeletal problems due to injury, osteoporosis, gout |
| Digestive disease | Chronic liver disease, inflammatory bowel disease | Chronic pancreatic disease, peptic ulcer |
| Urogenital disorder | CKD, end-stage kidney disease | Endometriosis, chronic urinary tract infection |
| Hematological disorder | Anemia (including pernicious anemia, sickle cell anemia) | |
| Eye disease | Vision impairment that cannot be corrected | |
| Ear disease | Hearing impairment that cannot be corrected, Meniere disease | |
| Infectious disease | HIV/AIDS | Chronic Lyme disease, TB, post–acute COVID-19 |
| Congenital disease | Congenital disease and chromosomal abnormalities |
Untreated and treated hypertension were combined. Both sets of panelists agreed to include conditions that require surveillance (including cancers), and thus “cancers that require surveillance” was not stated separately in the list.
Rising Prevalence of MLTC
The prevalence of MLTC to date is highly dependent on the population setting, social determinants such as age-groups and deprivation, health care access and diagnosis, and the number and types of conditions that have been included in the definitions of MLTC. Changes in prevalence may be further influenced by the coding of conditions or the source of data such as primary care, hospitalization, pharmacy, or laboratory. For this reason, it has been difficult to understand the full scale and burden of MLTC (7). Studies usually in the general population report a prevalence between 20 and 30%, and between 55 and 98% in older adults (17,18). Regardless of the variability in MLTC prevalence, with the aging population there has been an overall steady rise in MLTC over the years (19) and an increase in targeting MLTC epidemiological studies at younger age-groups and socioeconomically deprived populations and in low- to-middle-income countries (LMIC) (7). Patterns of MLTC have indicated a major increase particularly in cardiometabolic conditions; a cluster analysis study with use of UK Biobank data showed that diabetes was at the center of the disease cluster for MLTC and directly or indirectly related to several other chronic conditions (8,20).
Diabetes-Related MLTC
The classical complications resulting from diabetes are acute metabolic, microvascular, and cardiovascular. Therefore, the development of hyperglycemic states such as diabetic ketoacidosis, and the development of eye disease, kidney disease, foot disease, coronary heart disease, cerebrovascular disease, and peripheral vascular disease in people with diabetes, has long been characterized clinically as “the complications of diabetes.” However, more recently it has been appreciated that many other long-term conditions co-occur in people with diabetes, including conditions that arise by chance and conditions that are associated with diabetes but not traditionally identified as cardinal complications. The presence or absence of the classical complications of diabetes in those with MLTC can allow further categorization of MLTC into concordant and discordant MLTC, respectively, in people with diabetes, where concordant MLTC refers only to the classical complications of diabetes outlined above and discordant MLTC refers to all other long-term conditions.
Examples of conditions associated with diabetes but not traditionally identified as cardinal complications include mental health conditions such as schizophrenia (21), infective diseases such as tuberculosis (TB) and coronavirus disease 2019 (COVID-19) (22), and other physical long-term conditions such as many common cancers, fatty liver disease, obstructive sleep apnoea, dementia, and osteoarthritis of knee and hip. A study of 4,545 primary care patients with type 2 diabetes in Finland showed that 93% of people with type 2 diabetes had MLTC, 21% had a concordant disease, 8% had a discordant disease, and 64% had both concordant and discordant diseases (23). The concordant diseases included cardiometabolic diseases such as obesity and CKD and discordant diseases included cancer, musculoskeletal disease, respiratory disease, prostatic disease, hypothyroid, dementia or Alzheimer disease, depression, and sleep disorder (23).
Those with long duration of diabetes have a higher MLTC burden mainly characterized by complications such as cardiovascular disease and end-stage renal disease (24). From one U.K. study investigators reported a 33.3% prevalence of one or more comorbid conditions among subjects with type 2 diabetes for the least deprived population and 32.7% for the most deprived (25). In this study hypertension was the most common condition with type 2 diabetes, with the second most prevalent condition in women being depression and for men being coronary heart disease (25). Findings from a U.S. study showed that African American individuals had a higher cardiovascular risk among people with type 2 diabetes compared with White populations, particularly in early-onset type 2 diabetes, with the prevalence of cardiometabolic disease, depression, and diabetes increasing over the past two decades in both African American and White populations (26).
In one recent large study investigators reported an MLTC prevalence of 77% among subjects with type 2 diabetes (27). In this study there was a twofold higher likelihood of schizophrenia, chronic heart failure, hypertension, peripheral vascular disease, and myocardial infarction among those with type 2 diabetes in comparison with those without type 2 diabetes (27). In a U.S. cohort study of 892,233 veterans with diabetes, 52% of subjects had two or more comorbidities, where the odds of having three or more comorbidities were fourfold higher for those age ≥75 years and older in comparison with those age <50 years (28).
Role of Obesity in Diabetes and MLTC
Obesity is increasing in prevalence and is one of the key drivers for the development of diabetes and MLTC. In a recent study where investigators used data from Finland and the U.K. with a 12-year follow-up, obesity was associated with 21 nonoverlapping cardiometabolic, digestive, respiratory, neurological, musculoskeletal, and infectious disease conditions, with obesity being associated with a near 3-fold higher risk for developing at least one obesity-related disease, 5-fold higher risk for developing two diseases, and 12-fold higher risk for developing complex MLTC. The highest risk associated with obesity is the development of cardiometabolic multimorbidities (type 2 diabetes, coronary heart disease, and stroke) (29). During a mean follow-up of 10.7 years, investigators for one study found that in comparisons with individuals of healthy weight, risk of developing cardiometabolic MLTC in overweight individuals was twice as high (odds ratio [OR] 2.0, 95% CI 1.7–2.4), almost five times higher for individuals with class one obesity (4.5, 3.5–5.8), and almost 15 times higher for individuals with class 2 or class 3 obesity (14.5, 10.1–21.0) (29). The study showed that diabetes-related MLTC was more closely associated with BMI followed by vascular disease (29).
The exact pathways linking MLTC with obesity is unclear but may be related to dyslipidemia and systemic and vascular inflammation, insulin resistance, loss of reserves or frailty, increased thrombogenic factors, and general old age (30,31). Further understanding of causal mechanisms is required, for example, Mendelian randomization studies regarding the central role of obesity in pathogenesis of MLTC (32).
Communicable Diseases and Their Relationship With Diabetes
With the current epidemiological transition and rapid urbanization occurring in LMIC, LMIC are experiencing simultaneous epidemics of both communicable and noncommunicable diseases. We shed light on the complex interplay between key communicable diseases and MLTC.
Diabetes, MLTC, and HIV
People living with HIV (PLHIV) are at increased risk of developing several metabolic diseases, including diabetes (33). Diabetes prevalence ranges from 1.3 to 18% in PLHIV in LMIC (34). These results are consistent with PLHIV in North America and Europe, where differences have been noted in cardiometabolic traits between HIV-infected and uninfected individuals, which may be further modified by use of antiretroviral therapy (ART) (35). Some documented risk factors for type 2 diabetes in PLHIV include traditional risk factors such as age and overweight/obesity and some that are HIV treatment specific including older-generation protease inhibitors (PIs) and nucleoside reverse transcriptase inhibitors, lipodystrophy, and hepatitis C co-infection (36).
Findings from observational studies have suggested that HIV infection itself may be a risk factor for type 2 diabetes (37,38). However, the direction of effect is unclear, especially with the use of PI-based antiretroviral regimens, which have been linked to insulin resistance and impaired glucose metabolism (39). Data from population-based surveys in Uganda and Tanzania have shown a large burden of hypertension and risk factors for diabetes in high–HIV burden settings (40). Similarly, in a South African cohort of patients attending primary care services, 75% of those with hypertension had comorbid diabetes (41), while 12% had HIV and type 2 diabetes comorbidity (41). Moreover, ART may adversely affect glucose metabolism through mitochondrial toxicity resulting in dysglycemia (42). Insulin resistance is caused by the direct or indirect effects of both PIs and nucleoside reverse transcriptase inhibitors on mitochondrial function and by chronic inflammatory changes induced by HIV (43), also known as antiretroviral-associated diabetes (44). However, introducing newer ARTs with less metabolic impact could lower diabetes-ART interaction in the future (45).
Some studies suggest that individuals with HIV-associated diabetes may be at a higher risk of developing metabolic syndrome. Evidence suggests that people living with HIV and diabetes are at increasingly higher risk of metabolic syndrome and dyslipidemia compared with people with diabetes but without HIV (46–48). Managing HIV infection and diabetes, as well as addressing other traditional risk factors, can help reduce the risk of developing metabolic syndrome. Regular monitoring, adherence to ART, lifestyle modifications, and appropriate medical management of both HIV and diabetes are crucial for minimizing the risk and complications.
Diabetes, MLTC, and TB.
Active TB increases the risk of developing diabetes, as diabetes has also been associated with a threefold increased risk of TB (49). TB/diabetes comorbidity leads to worse health outcomes and prognosis for both conditions (50). Diabetes is associated with a twofold risk of death during TB treatment, a fourfold risk of TB relapse after treatment completion, and a twofold risk of multidrug-resistant TB (51,52). In 2019, it was estimated that >15% of people with TB had comorbid diabetes globally, compared with 9.3% among the general adult population (53). In high–TB burden LMIC, it is important to have coordinated care and follow-up for both conditions.
Dual and Interacting Epidemics in Rapidly Urbanizing Spaces
Some LMIC such as Africa are currently undergoing an epidemiological transition with changes in disease patterns accompanied by changes in population composition and age distribution (54). The syndemic rise in obesity levels, hypertension, and young-onset diabetes in countries undergoing an epidemiological transition, with co-occurring infectious diseases and noncommunicable diseases, raises concerns around a perpetuated cycle of diabetes in high–HIV/TB burden LMIC (55,56). Settings where TB and HIV are endemic require routine monitoring and screening because there may be an undiagnosed burden of type 2 diabetes in the youth with added complexity, as HbA1c has been shown to be less reliable for diagnosis (57).
Diabetes, MTLC, and COVID-19
The World Health Organization declared COVID-19 as a pandemic on 11 March 2020 (58). During the early stages, findings from a modeling study based on the Global Burden of Diseases, Injuries, and Risk Factors Study data suggested that the population at the greatest risk for severe COVID-19 was people with diabetes, cardiovascular disease, CKD, or MLTC (59). Diabetes was reported as one of the most common comorbidities in patients diagnosed with COVID-19, with the prevalence of diabetes ranging from 5.3% to 20.7% (60). Diabetes was associated with a higher risk of death and/or invasive mechanical ventilation at 7 days (hazard ratio 1.42, 95% CI 1.17–1.72) and 28 days (1.30, 1.09–1.55) post-hospitalization in comparisons with those without diabetes irrespective of age and other comorbid conditions (61). Preliminary evidence suggests that people with COVID-19 may be at increased risk of developing type 2 diabetes (incidence rate ratio 1.28, 95% CI 1.05–1.57) in comparison with those with acute respiratory tract infection (62). The crude mortality due to COVID-19 among patients with MLTC was 50% more compared with those without MLTC (OR 1.48, 95% CI 1.26–1.75) (63). Although the most common combination of conditions was stroke and hypertension among patients with severe COVID-19 infection (hospitalization or death) (79%), those with combination of diabetes and CKD has the highest risk (OR 4.93, 3.36–7.22) of severe COVID-19 infection (64).
MLTC in Special Diabetes Groups
A major challenge of MLTC exists for individuals with diabetes at an earlier age, as they are living with a wide array of conditions for a longer duration.
Early-Onset Type 2 Diabetes
Early-onset type 2 diabetes (diagnosis before 40 years of age) is associated with significant adverse outcomes when compared with a diagnosis later in life (65). The early-onset presentation is concerning from the perspective of MLTC because 1) there is a greater lifetime exposure to hyperglycemia, increasing the risk of diabetes-related morbidity; 2) the phenotype is strongly associated with obesity (66), though not always (67), and appears to have aggressive cardiovascular risk at presentation (26,68), meaning the progression to MLTC may be accelerated in comparison with diagnosis later in life; and 3) the presence of MLTC in people of working age is likely to have a significant economic and societal burden in comparison with the traditional demography.
Taken together, those with early-onset type 2 diabetes should be regarded as the multimorbid population of the future, yet there is very little evidence and, consequently, guidance on best management approaches for prevention and treatment of MLTC and development of services to support affected individuals. These knowledge gaps are important to address, as early-onset type 2 diabetes cases are rising (69).
Several studies have now shown the presence of complications at diagnosis and faster progression to complications in those with early-onset type 2 diabetes. In the landmark Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study of youth with type 2 diabetes (aged <20 years at diagnosis) investigators published 10-year follow-up data on 572 participants; at baseline 19.2% had evidence of hypertension, 20.8% dyslipidemia, and 9.0% presence of a microvascular complication (70). By the end of follow-up, 46.8% of participants had hypertension, 44.4% had dyslipidemia, and 80.1% had one more microvascular complication. With adjustment for sex, race, age, and diabetes duration, the OR for the development of multiple microvascular complications was highest for the presence of hypertension, 3.18 (95% CI 2.35–4.3), or dyslipidemia, 2.77 (2.05–3.72), and both were higher than the OR for every 1% increase in HbA1c, 1.80 (1.65–1.95). In another study of electronic medical records in the U.S. investigators examined the prevalence of cardiovascular MLTC and depression among individuals with type 2 diabetes diagnosed at ages 18–39 years. At the time of diagnosis, 11% of Black American and non-Hispanic White individuals already had evidence of MLTC, including two or more of the following: myocardial infarction, stroke, heart failure, peripheral vascular disease, CKD, cancer, depression, hypertension, and dyslipidemia (26).
MLTC in early-onset type 2 diabetes may not only be restricted to traditional cardiometabolic comorbidities; results of a recent prospective study of 18,290 incident type 2 diabetes cases developing during follow-up over 38 years demonstrated an association between type 2 diabetes (diagnosed before age 40 years) and risk of cancer, with higher hazard ratio for risk of early cancer diagnosis (1.47, 95% CI 1.06–2.04), diabetes-related cancer (2.11, 1.38–3.23), and obesity-related cancer (1.75, 1.08–2.82), but this was restricted to people with high BMI at age 18 years (71).
Type 1 Diabetes
Type 1 diabetes is an autoimmune condition that does not share traditional cardiometabolic risk factors with many of the comorbidities that predominate in those with MLTC who have type 2 diabetes. Type 1 diabetes does, however, have several facets that predispose to the development of MLTC. First, like early-onset type 2 diabetes, lifetime exposure to chronic hyperglycemia and risks from hypoglycemia increases risk of cardiovascular morbidity from microvascular complications including kidney failure. Second, people with type 1 diabetes carry a higher risk of other autoimmune conditions reflecting underlying genetic predisposition to autoimmunity, and third, it is recognized that there is a significant psychological burden in people with type 1 diabetes that can cause diabetes distress, burnout, depression, and anxiety.
Type 1 diabetes carries a significant risk of cardiovascular disease and mortality that is in excess of the predicted burden from traditional risk factors (72). This risk may be conferred by the long duration of diabetes; for example, in a Swedish registry study, diagnosis of type 1 diabetes before the age of 10 years was associated with a 30-fold increased risk of cardiovascular disease; women in particular had a 90-fold increased risk of myocardial infarction (72). However, there is evidence of undertreatment of cardiovascular risk in people with type 1 diabetes (73), and the role of cardiac-directed autoimmunity as a proinflammatory state may play a role (74).
The earlier onset of type 1 diabetes results in long-duration type 1 diabetes by early to mid-adulthood. This means people with early-onset type 1 diabetes may be affected by MLTC by their third or fourth decade of life. In a German study with examination of the cross-sectional prevalence of MLTC in 6,967 adult outpatients with type 1 diabetes, investigators demonstrated MLTC in 63.7% of patients. The most common conditions were depression (10.4%), stress reactions (11.7%), hypertension (10.8%), back pain (14.5%), and dyslipidemia (12.1%) (75).
Despite the high risk, systematic studies assessing the breadth of MLTC in people with type 1 diabetes are lacking. However, the clustering of other autoimmune diseases in people with type 1 diabetes has been studied more comprehensively. Of 25,759 individuals studied in the type 1 diabetes network study, 28% had one or more additional autoimmune conditions (76). Factors associated with two or more autoimmune conditions (including type 1 diabetes) included female sex, older current age (and longer duration), earlier age at diagnosis, and White ethnicity. Results of another study of individuals with type 1 diabetes in a U.S. insurance database of 179,248 showed that 27% (95% CI 26.8–27.2%) had at least one concurrent autoimmune disease, hypothyroidism, rheumatoid arthritis, and celiac disease being the most common. In the same study the presence of additional autoimmune diseases was independently associated with increased risk of end-stage renal disease, stroke, and myocardial infarction and these observations were amplified in women.
The current evidence on the epidemiology of MLTC in diabetes has shown MLTC to be a major global health challenge; therefore, understanding the implications for the patient and service delivery is key.
Challenges for the Patient and Service Delivery
MLTC is associated with poor quality of life, worst health outcomes, and mortality as well as an impact on health care services (7). Fragmented care is one of the key challenges, where patients are seen by several different health care professionals, for each of their conditions. This can be burdensome for the individual in terms of attending multiple appointments at various locations with limited time for the consultation and may create miscommunication and result in inconsistent health care messages (20). Moreover, polypharmacy, defined as the simultaneous use of five or more chronic medications, can have adverse implications due to the drug-drug or drug-disease interactions; for example, metformin and impaired kidney function are well-known for drug-disease interaction (77). Additionally, multiple drugs can lead to potential side effects, medical errors, and poor adherence.
There is a great impact on health care service delivery, diabetes is associated with a higher number of comorbidities and risk of cardiovascular events, requiring frequent general practitioner and specialist consultations, and hospital appointments or admissions. Results from a recent study from England of 120,499 adults with newly diagnosed type 2 diabetes between 2000 and 2018 showed that the mean annual inflation–adjusted cost for face-to-face consultations was £412.70 per patient without comorbidities, £516.80 for one comorbidity, £620.75 for two comorbidities, and £778.83 for three or more comorbidities (78). In another Spanish study of nearly half a million people with type 2 diabetes, investigators reported that 70% had MLTC and also reported an increase in cost related to MLTC, where the highest was for insulin (average cost €153) or oral antidiabetes agents (€306) and combinations, for people with diabetes who also had cardiovascular disease and neoplasm (79).
Current Interventions for Prevention and Management
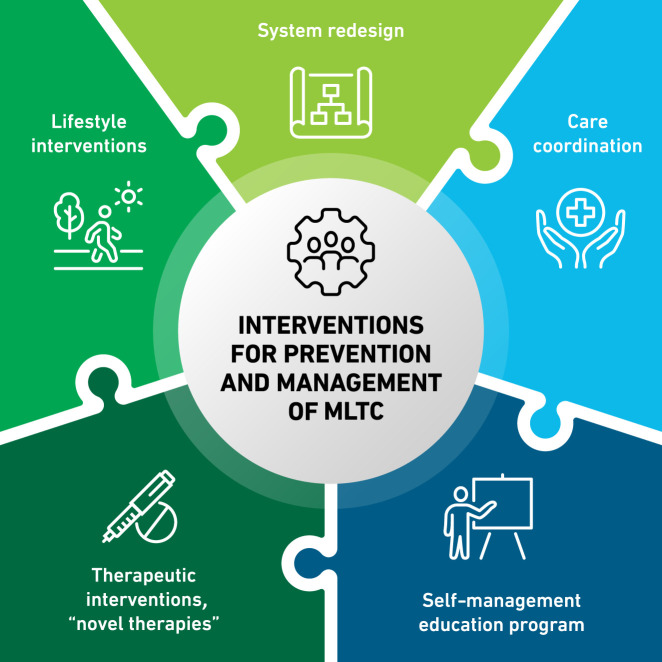
Recognition of the range of MLTC associated with diabetes has motivated researchers and clinicians to develop and implement several management approaches, which can be framed as 1) system delivery/care coordination, 2) lifestyle interventions, and 3) therapeutic interventions (Fig. 1).
Figure 1.
Interventions for prevention and management of diabetes and MLTC.
System Delivery and Care Coordination
There has been a limited number of interventions that have targeted the coordination of care and self-management support for MLTC (not diabetes specific) since 2011, when the first randomized controlled trial was published (80). One of the largest studies to date involved a three-dimensional review of the patient’s health, including information on depression and medications, during an appointment with a nurse and a records-based medication review by a pharmacist (81). A responsible physician then received this information and discussed a collaborative treatment plan with the patient and provided a printed plan of agreed goals. The three-dimensional intervention versus usual care was tested in a cluster randomized trial in England and Scotland with a 15-month follow-up with health-related quality of life as the primary outcome. Although no differences were found in health-related quality of life, patients perceived improvements in the quality of their care (81). In an updated Cochrane review of trials testing the effects of system intervention versus usual care investigators similarly found that patient perceptions of health care may improve, but the evidence was considered limited because of the variation in study participants and interventions (82).
Two trials in primary care networks including people with type 2 diabetes and MLTC involved in-home visits, care coordination by nurses or specially trained medical assistants, and periodic team case conferencing to enhance self-care (83,84). Comparisons with usual-care control subjects showed no significant differences in patient-reported physical and mental functioning or health-related quality of life. The variation in comorbidities and participants may be responsible, but thus far system delivery/care coordination has had only modest success.
Lifestyle Interventions
There is good evidence from randomized controlled trials that behavioral interventions to support people at high risk of developing type 2 diabetes to lose weight, adopt a healthy diet, and increase physical activity can significantly decrease the incidence of type 2 diabetes (85,86). Translation of this evidence to diabetes prevention programs delivered at scale in the real world and across national footprints has now demonstrated similar benefit (87,88). Evidence that lifestyle interventions directly affect the development of MLTC per se, rather than specific disease progression, is also now emerging. There is evidence for intensive lifestyle interventions preventing MLTC in both those with type 2 diabetes and those with prediabetes. The Look AHEAD (Action for Health in Diabetes) trial included middle-aged overweight or obese individuals with type 2 diabetes and achieved its primary goal to produce a 7% weight loss and cardiovascular fitness. Although no differences were found for cardiovascular outcomes, the intensive lifestyle intervention slowed the rate of increase in MLTC with time (89). In the 30-year follow-up of the Da Qing Diabetes Prevention Outcome Study, those with impaired glucose tolerance who underwent a lifestyle intervention still had lower type 2 diabetes incidence as well as reduced incidence of cardiovascular events, microvascular complications, and cardiovascular and all-cause mortality (90).
Lifestyle interventions tend to offer more positive evidence than system delivery. The choice of primary end point for interventional studies aiming to improve outcomes in those with type 2 diabetes and MLTC is important, but international agreement has been lacking. Although a recent Delphi consensus study was focused on which disease states to include in the MLTC definition (16), agreement on which end points to apply for interventional studies for MLTC is lacking, and choice of an end point is to some extent likely to be disease combination specific. Health-related quality of life has received somewhat more study, which is consistent with the promotion of patient-centered care, but health-related quality of life may be insufficiently sensitive to differentiate what is or is not a contributory intervention.
Therapeutic Interventions
Therapeutic interventions for risk factor management in people with diabetes can involve multiple different drugs and classes of drugs to be taken daily—usually including several glucose- and blood pressure–lowering agents as well as a statin. With concurrent diabetes complications and/or more diverse long-term conditions, the number of drugs to be taken will be even greater. The major determinant of progressive MLTC is aging, and many older people are on weight loss trajectories, associated, for example, with cognitive impairment, frailty, or cancer diagnoses. Without de-intensification in medications, aging and polypharmacy in those with diabetes and MLTC can therefore be associated with harms due to overtreatment, specifically related to hypoglycemia, postural hypotension, and falls. As a result, clinical guidelines that relate to MLTC often incorporate medicines management protocols with the aim of deintensification of therapy where deemed appropriate (2,91). This has been highlighted as important in those with diabetes where hypoglycemic risk is significant, particularly in people with CKD and dementia treated with sulfonylureas or insulin (92,93), and diabetes overtreatment has been associated with increased mortality (94). However, investigators of a recent systematic review of deprescribing antihyperglycemic medications in older adults with type 2 diabetes found that while deprescribing is likely feasible and safe and benefits may outweigh the harms, the evidence indicated very low certainty (95).
Newer classes of glucose-lowering medications that carry lower risks of hypoglycemia and have multiple beneficial targets of action have therefore been suggested as more attractive in the context of MLTC (30). For example, sodium–glucose cotransporter 2 inhibitors, in addition to lowering glucose, have been shown to reduce cardiovascular mortality, heart failure hospitalizations, and kidney complications (96) and feature prominently now in national and international guidelines (97,98). Similarly, glucagon-like peptide 1 receptor antagonists, in addition to lowering glucose, reduce weight and reduce risk of cardiovascular disease (99), although whether the associated weight loss is advantageous in more elderly cohorts with MLTC, who may already be on weight loss trajectories, is unclear.
The role of metformin in the prevention of MLTC is also currently being indirectly explored: the aim of the Targeting Aging with Metformin (TAME) is to test the hypothesis that human aging can be targeted and a number of age-related outcomes prevented, potentially delaying the aging process to improve human health span by modulating pathways involved in aging (100).
Key Research Directions
The existing literature to date has demonstrated significant progress in identifying the issues relating to diabetes and MLTC. However, further research is crucial to be able to develop a robust evidence base.
The key research directions for diabetes and MLTC from a global perspective are as follows:
Identifying the commonest clusters of MLTC in people with diabetes.
Systematic studies of MLTC in diverse populations with early-onset type 2 diabetes are lacking. In particular, a better understanding of the trajectory for the development of MLTC and the clusters that form would be useful to help design studies that can prevent MLTC but also tailor services to these groups.
The economic impact of people of working age developing MLTC in type 2 diabetes is also unknown.
The epidemiology of the evolution of MLTC in type 1 diabetes requires further study, but the question of how best to prevent and support MLTC in type 1 diabetes is very important.
Answers to simple questions are unknown, e.g., optimal age for starting statin therapy, especially in those with early-onset type 1 diabetes who already have long-duration well before the age of 40 years, or whether the presence of other autoimmune diseases should prompt more aggressive risk reduction.
Do the risk factors for MLTC differ qualitatively from the risk factors of the constituent conditions of MLTC?
Do the interventions that affect MLTC differ from the interventions that are effective in reducing the progression of the constituent conditions of MLTC?
In LMIC how can primary health care systems be strengthened to address the growing burden of MLTC?
How can existing single disease–focused national programs (e.g., HIV, TB) be integrated with noncommunicable disease programs to provide MLTC care in LMIC?
What are the most cost-effective models of care for people with diabetes and MLTC?
Evaluation of therapeutic target(s) for prevention and management of MLTC.
Evaluation of health delivery models; e.g., remote to virtual (mHealth) technologies might be used to send text messages to inform and prompt patients concerning topics such as medical adherence and increasing physical activity.
Conclusion
The main clinical and research directions for diabetes and MLTC from a global perspective will include system delivery and care coordination, lifestyle interventions, and therapeutic interventions. This will require a multidisciplinary approach, including clinical experts, specialists, researchers, and people with MLTC, to develop interventions to improve the lives of people with MLTC and to build future cost-effective health care systems to deliver.
Article Information
Funding. N.S.V. was supported by the Fogarty International Center of the National Institutes of Health under award no. D43 TW011404. S.M. is a Wellcome Trust Career Development Fellow (223024/Z/21/Z). K.K. is supported by the National Institute for Health Research (NIHR), Applied Research Collaboration East Midlands (ARC EM), NIHR Global Centre for MLTC, and the NIHR Leicester Biomedical Research Centre (BRC).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. K.K. is the National Lead for multiple long-term conditions for National Institute for Health Research Applied Research Collaboration (NIHR ARC) and leads the NIHR Global Research Centre for Multiple Long Term Conditions. K.K. has acted as a consultant or speaker or received grants for investigator-initiated studies from AstraZeneca, Abbott, Amgen, Bayer, Novartis, Novo Nordisk, Roche, Servier, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Oramed Pharmaceuticals, and Applied Therapeutics. S.M. has received investigator-initiated research funds from DexCom and is a Trustee of the Diabetes Research & Wellness Foundation, U.K. J.V. is National Clinical Director for Diabetes and Obesity at NHS England. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.K. contributed to the review concept and design. All authors contributed to the first draft of the manuscript. K.K. and Y.V.C. contributed to editing and management of the manuscript. All authors contributed to critical revision for the study and the manuscript draft. All authors provided final approval of the version to publish.
Funding Statement
N.S.V. was supported by the Fogarty International Center of the National Institutes of Health under award no. D43 TW011404. S.M. is a Wellcome Trust Career Development Fellow (223024/Z/21/Z). K.K. is supported by the National Institute for Health Research (NIHR), Applied Research Collaboration East Midlands (ARC EM), NIHR Global Centre for MLTC, and the NIHR Leicester Biomedical Research Centre (BRC).
Footnotes
This article is featured in podcasts available at diabetesjournals.org/care/pages/diabetes_care_on_air.
References
- 1. Key global findings 2021, 2021. Accessed 20 April 2023. Available from https://diabetesatlas.org/
- 2. Multimorbidity: clinical assessment and management: NICE guideline [NG56], 2016. Accessed 20 April 2023. Available from https://www.nice.org.uk/guidance/NG56
- 3. Guthrie B, Payne K, Alderson P, McMurdo MET, Mercer SW. Adapting clinical guidelines to take account of multimorbidity. BMJ 2012;345:e6341. [DOI] [PubMed] [Google Scholar]
- 4. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 2006;29:725–731 [DOI] [PubMed] [Google Scholar]
- 5. Older people with social care needs and multiple long-term conditions: NICE guideline [NG22], 2015. Accessed 20 April 2023. Available from https://www.nice.org.uk/guidance/ng22/chapter/Recommendations-for-research
- 6. Hong CS, Atlas SJ, Ashburner JM, et al. Evaluating a model to predict primary care physician-defined complexity in a large academic primary care practice-based research network. J Gen Intern Med 2015;30:1741–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Multimorbidity: a priority for global health research, 2018. Accessed 19 January 2023. Available from https://acmedsci.ac.uk/file-download/82222577
- 8. Zemedikun DT, Gray LJ, Khunti K, Davies MJ, Dhalwani NN. Patterns of multimorbidity in middle-aged and older adults: an analysis of the UK Biobank data. Mayo Clin Proc 2018;93:857–866 [DOI] [PubMed] [Google Scholar]
- 9. Cicek M, Buckley J, Pearson-Stuttard J, Gregg EW. Characterizing multimorbidity from type 2 diabetes: insights from clustering approaches. Endocrinol Metab Clin North Am 2021;50:531–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akker VD. Comorbidity or multimorbidity: what’s in a name? A review of literature. Eur J Gen Pract 1996;2:65–70 [Google Scholar]
- 11. Whitty CJM, MacEwen C, Goddard A, et al. Rising to the challenge of multimorbidity. BMJ 2020;368:l6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Reste JY, Nabbe P, Manceau B, et al. The European General Practice Research Network presents a comprehensive definition of multimorbidity in family medicine and long term care, following a systematic review of relevant literature. J Am Med Dir Assoc 2013;14:319–325 [DOI] [PubMed] [Google Scholar]
- 13. Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci 2011;66:301–311 [DOI] [PubMed] [Google Scholar]
- 14. Suls J, Bayliss EA, Berry J, et al. Measuring multimorbidity: selecting the right instrument for the purpose and the data source. Med Care 2021;59:743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho IS, Azcoaga-Lorenzo A, Akbari A, et al. Examining variation in the measurement of multimorbidity in research: a systematic review of 566 studies. Lancet Public Health 2021;6:e587–e597 [DOI] [PubMed] [Google Scholar]
- 16. Ho ISS, Azcoaga-Lorenzo A, Akbari A, et al. Measuring multimorbidity in research: Delphi consensus study. BMJ Medicine 2022;1:e000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011;10:430–439 [DOI] [PubMed] [Google Scholar]
- 18. Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012;10:142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhalwani NN, O’Donovan G, Zaccardi F, et al. Long terms trends of multimorbidity and association with physical activity in older English population. Int J Behav Nutr Phys Act 2016;13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chudasama YV, Khunti K, Davies MJ. Clustering of comorbidities. Future Healthc J 2021;8:e224–e229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holt RI, Bushe C, Citrome L. Diabetes and schizophrenia 2005: are we any closer to understanding the link? J Psychopharmacol 2005;19(Suppl.):56–65 [DOI] [PubMed] [Google Scholar]
- 22. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 2020;8:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heikkala E, Mikkola I, Jokelainen J, Timonen M, Hagnäs M. Multimorbidity and achievement of treatment goals among patients with type 2 diabetes: a primary care, real-world study. BMC Health Serv Res 2021;21:964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seng JJB, Kwan YH, Lee VSY, et al. Differential health care use, diabetes-related complications, and mortality among five unique classes of patients with type 2 diabetes in Singapore: a latent class analysis of 71,125 patients. Diabetes Care 2020;43:1048–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nowakowska M, Zghebi SS, Ashcroft DM, et al. The comorbidity burden of type 2 diabetes mellitus: patterns, clusters and predictions from a large English primary care cohort. BMC Med 2019;17:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dibato JE, Montvida O, Zaccardi F, et al. Association of cardiometabolic multimorbidity and depression with cardiovascular events in early-onset adult type 2 diabetes: a multiethnic study in the U.S. Diabetes Care 2021;44:231–239 [DOI] [PubMed] [Google Scholar]
- 27. Zghebi SS, Steinke DT, Rutter MK, Ashcroft DM. Eleven-year multimorbidity burden among 637 255 people with and without type 2 diabetes: a population-based study using primary care and linked hospitalisation data. BMJ Open 2020;10:e033866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lynch CP, Gebregziabher M, Axon RN, Hunt KE, Payne E, Egede LE. Geographic and racial/ethnic variations in patterns of multimorbidity burden in patients with type 2 diabetes. J Gen Intern Med 2015;30:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kivimäki M, Kuosma E, Ferrie JE, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017;2:e277–e285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet 2017;389:2239–2251 [DOI] [PubMed] [Google Scholar]
- 31. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–880 [DOI] [PubMed] [Google Scholar]
- 32. He C, Zhang M, Li J, et al. Novel insights into the consequences of obesity: a phenotype-wide Mendelian randomization study. Eur J Hum Genet 2022;30:540–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sarkar S, Brown TT. Diabetes in people living with HIV. Curr Diab Rep 2021;21:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel P, Rose CE, Collins PY, et al.; NIH HIV/NCD Project Disease Condition Technical Operating Group . Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis. AIDS 2018;32(Suppl. 1):S5–S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis 2007;44:1625–1631 [DOI] [PubMed] [Google Scholar]
- 36. Sarkar S, Brown TTJE. Diabetes in people living with HIV. 2019; [Google Scholar]
- 37. Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999-2011: epidemiology and public health implications. A systematic review. BMC Public Health 2011;11:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008;31:1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geffner ME, Patel K, Miller TL, et al.; Pediatric HIV/AIDS Cohort Study . Factors associated with insulin resistance among children and adolescents perinatally infected with HIV-1 in the pediatric HIV/AIDS cohort study. Horm Res Paediatr 2011;76:386–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kavishe B, Biraro S, Baisley K, et al. High prevalence of hypertension and of risk factors for non-communicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC Med 2015;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oni T, Youngblood E, Boulle A, McGrath N, Wilkinson RJ, Levitt NS. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis 2015;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Njuguna B, Kiplagat J, Bloomfield GS, Pastakia SD, Vedanthan R, Koethe JR. Prevalence, risk factors, and pathophysiology of dysglycemia among people living with HIV in sub-Saharan Africa. J Diabetes Res 2018;2018:6916497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010;33:2244–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dagogo-Jack S. HIV therapy and diabetes risk. Diabetes Care 2008;31:1267–1268 [DOI] [PubMed] [Google Scholar]
- 45. Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology 2018;29:431–441 [DOI] [PubMed] [Google Scholar]
- 46. Husain NE, Noor SK, Elmadhoun WM, et al. Diabetes, metabolic syndrome and dyslipidemia in people living with HIV in Africa: re-emerging challenges not to be forgotten. 2017;9:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Girma D, Dejene H, Geleta LA, et al. Metabolic syndrome among people living with HIV in Ethiopia: a systematic review and meta-analysis. Diabetol Metab Syndr 2023;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kiama CN, Wamicwe JN, Oyugi EO, et al. Prevalence and factors associated with metabolic syndrome in an urban population of adults living with HIV in Nairobi, Kenya. Pan Afr Med J 2018;29:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008;5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young F, Critchley JA, Johnstone LK, Unwin NC. A review of co-morbidity between infectious and chronic disease in Sub Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome, and the impact of globalization. Globah Health 2009;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Q, Li W, Xue M, et al. Diabetes mellitus and the risk of multidrug resistant tuberculosis: a meta-analysis. Sci Rep 2017;7:1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Noubiap JJ, Nansseu JR, Nyaga UF, et al. Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2·3 million patients with tuberculosis. Lancet Glob Health 2019;7:e448–e460 [DOI] [PubMed] [Google Scholar]
- 53. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 54. Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q 1971;49:509–538 [PubMed] [Google Scholar]
- 55. Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 56. Hanson MA, Gluckman PD, Ma RC, Matzen P, Biesma RG. Early life opportunities for prevention of diabetes in low and middle income countries. BMC Public Health 2012;12:1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coelho AR, Moreira FA, Santos AC, et al. Diabetes mellitus in HIV-infected patients: fasting glucose, A1c, or oral glucose tolerance test - which method to choose for the diagnosis? BMC Infect Dis 2018;18:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020 [article online], 2020. Accessed 9 March 2023. Available from https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 59. Clark A, Jit M, Warren-Gash C, et al.; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group . Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health 2020;8:e1003–e1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Singh AK, Khunti K. COVID-19 and diabetes. Annu Rev Med 2022;73:129–147 [DOI] [PubMed] [Google Scholar]
- 61. Cariou B, Wargny M, Boureau AS, et al.; CORONADO investigators . Impact of diabetes on COVID-19 prognosis beyond comorbidity burden: the CORONADO initiative. Diabetologia 2022;65:1436–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rathmann W, Kuss O, Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia 2022;65:949–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Agrawal U, Azcoaga-Lorenzo A, Fagbamigbe AF, et al. Association between multimorbidity and mortality in a cohort of patients admitted to hospital with COVID-19 in Scotland. J R Soc Med 2022;115:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chudasama YV, Zaccardi F, Gillies CL, et al. Patterns of multimorbidity and risk of severe SARS-CoV-2 infection: an observational study in the U.K. BMC Infect Dis 2021;21:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol 2020;16:321–331 [DOI] [PubMed] [Google Scholar]
- 66. Wright AK, Kontopantelis E, Emsley R, et al. Life expectancy and cause-specific mortality in type 2 diabetes: a population-based cohort study quantifying relationships in ethnic subgroups. Diabetes Care 2017;40:338–345 [DOI] [PubMed] [Google Scholar]
- 67. Siddiqui MK, Anjana RM, Dawed AY, et al. Young-onset diabetes in Asian Indians is associated with lower measured and genetically determined beta cell function. Diabetologia 2022;65:973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sattar N, Rawshani A, Franzén S, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 2019;139:2228–2237 [DOI] [PubMed] [Google Scholar]
- 69. Perng W, Conway R, Mayer-Davis E, Dabelea D. Youth-onset type 2 diabetes: the epidemiology of an awakening epidemic. Diabetes Care 2023;46:490–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bjornstad P, Drews KL, Caprio S, et al.; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Y, Song M, Cao Y, et al. Incident early- and later-onset type 2 diabetes and risk of early- and later-onset cancer: prospective cohort study. Diabetes Care 2023;46:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018;392:477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shah VN, Grimsmann JM, Foster NC, et al. Undertreatment of cardiovascular risk factors in the type 1 diabetes exchange clinic network (United States) and the prospective diabetes follow-up (Germany/Austria) registries. Diabetes Obes Metab 2020;22:1577–1585 [DOI] [PubMed] [Google Scholar]
- 74. Sousa GR, Pober D, Galderisi A, et al. Glycemic control, cardiac autoimmunity, and long-term risk of cardiovascular disease in type 1 diabetes mellitus. Circulation 2019;139:730–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van den Boom L, Buchal G, Kaiser M, Kostev K. Multimorbidity among adult outpatients with type 1 diabetes in Germany. J Diabetes Sci Technol 2022;16:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hughes JW, Riddlesworth TD, DiMeglio LA, Miller KM, Rickels MR; T1D Exchange Clinic Network . Autoimmune diseases in children and adults with type 1 diabetes from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab 2016;101:4931–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dumbreck S, Flynn A, Nairn M, et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ 2015;350:h949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Coles B, Zaccardi F, Seidu S, et al. Rates and estimated cost of primary care consultations in people diagnosed with type 2 diabetes and comorbidities: a retrospective analysis of 8.9 million consultations. Diabetes Obes Metab 2021;23:1301–1310 [DOI] [PubMed] [Google Scholar]
- 79. Sancho-Mestre C, Vivas-Consuelo D, Alvis-Estrada L, Romero M, Usó-Talamantes R, Caballer-Tarazona V. Pharmaceutical cost and multimorbidity with type 2 diabetes mellitus using electronic health record data. BMC Health Serv Res 2016;16:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Boult C, Reider L, Leff B, et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Arch Intern Med 2011;171:460–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Salisbury C, Man M-S, Bower P, et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet 2018;392:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2021;1:CD006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miklavcic JJ, Fraser KD, Ploeg J, et al. Effectiveness of a community program for older adults with type 2 diabetes and multimorbidity: a pragmatic randomized controlled trial. BMC Geriatr 2020;20:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kamradt M, Ose D, Krisam J, et al. Meeting the needs of multimorbid patients with type 2 diabetes mellitus - a randomized controlled trial to assess the impact of a care management intervention aiming to improve self-care. Diabetes Res Clin Pract 2019;150:184–193 [DOI] [PubMed] [Google Scholar]
- 85. Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 86. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Valabhji J, Barron E, Bradley D, et al. Early outcomes from the English National Health Service Diabetes Prevention Programme. Diabetes Care 2020;43:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McManus E, Meacock R, Parkinson B, Sutton M. Population level impact of the NHS Diabetes Prevention Programme on incidence of type 2 diabetes in England: an observational study. Lancet Reg Health Eur 2022;19:100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Espeland MA, Gaussoin SA, Bahnson J, et al. Impact of an 8-year intensive lifestyle intervention on an index of multimorbidity. J Am Geriatr Soc 2020;68:2249–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gong Q, Zhang P, Wang J, et al.; Da Qing Diabetes Prevention Study Group . Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol 2019;7:452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Birtcher KK, Allen LA, Anderson JL, et al.; Writing Committee . 2022 ACC expert consensus decision pathway for integrating atherosclerotic cardiovascular disease and multimorbidity treatment: a framework for pragmatic, patient-centered care: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023;81:292–317 [DOI] [PubMed] [Google Scholar]
- 92. Hambling CE, Seidu SI, Davies MJ, Khunti K. Older people with type 2 diabetes, including those with chronic kidney disease or dementia, are commonly overtreated with sulfonylurea or insulin therapies. Diabet Med 2017;34:1219–1227 [DOI] [PubMed] [Google Scholar]
- 93. Seidu S, Kunutsor SK, Topsever P, Hambling CE, Cos FX, Khunti K. Deintensification in older patients with type 2 diabetes: a systematic review of approaches, rates and outcomes. Diabetes Obes Metab 2019;21:1668–1679 [DOI] [PubMed] [Google Scholar]
- 94. Christiaens A, Baretella O, Del Giovane C, et al. Association between diabetes overtreatment in older multimorbid patients and clinical outcomes: an ancillary European multicentre study. Age Ageing 2023;52:afac320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Deng Z, Thompson W, Korenvain C, et al. Benefits and harms of deprescribing antihyperglycemics for adults with type 2 diabetes: a systematic review. Can J Diabetes 2022;46:473–479 [DOI] [PubMed] [Google Scholar]
- 96. Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE-TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 97. Type 2 diabetes in adults: management: NICE guideline [NG28], 2022. Accessed 13 June 2023. Available from https://www.nice.org.uk/guidance/ng28
- 98. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022;45:2753–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab 2020;32:15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]