Abstract
Objective
Controlling of low-density lipoprotein cholesterol (LDL-C) in patients with acute coronary syndrome (ACS) remains a challenge. Health information technology (HIT) is increasingly being applied to close quality gaps in chronic illness care. The aim of this study was to perform a qualitative review of the association of implementing HIT on lipid management processes of care and LDL-C goal attainment in patients with ACS.
Method
Eligible patients with a discharge diagnosis of ACS from January 2018 to December 2021 at a tertiary medical center were retrospectively reviewed. An HIT system with a multidisciplinary approach including initiating high-intensity statin therapy, periodic laboratory follow-up, titration of lipid-lowering agents, patient education, patient-level and system-level interventions involving database monitoring and outreach by centralized care teams was introduced in October 2018. Electronical medical records including data on medications and laboratory findings at discharge and within 1 year were compared before and after implementing the HIT system.
Results
A total of 2001 ACS patients (average age 63 ± 12.7 years, 79.66 % men) were analyzed. The LDL-C < 70 mg/dL goal attainment rates (36.52 %, 53.57 %, 59.22 %, 62.18 % in 2018–2021) and medium serum LDL-C levels (80.5 mg/dL, 68 mg/dL, 65 mg/dL, 64 mg/dL in 2018–2021) significantly improved within 6 months (2018 as the reference, all p<0.001). The LDL-C attainment rate at 12 months also steadily increased (53.80 %, 61.82 %, 64.21 % in 2019–2021, p = 0.019). Most of the patients switched to a high-intensity statins regimen at discharge (0.57 %, 63.67 %, 72.41 %, 84.44 %, in 2018–2021, p<0.001 with 2018 as the reference), with low adverse event rates. The maintenance rates of high-intensity statin regimens at 12 months continued to improve (41.36 %, 49.04 %, 61.39 % in 2019–2021, p<0.001).
Conclusions
Efforts to control LDL-C should be increased in ACS patients by initiating and intensifying statin treatment earlier. Our results confirmed that a team-based strategy with HIT improved LDL-C target achievement for most patients with ACS.
Keywords: Acute coronary syndrome, Health information technology, High intensity statin, Lowdensity lipoprotein cholesterol
Graphical abstract
1. Introduction
Acute coronary syndrome (ACS) is one of the most common cardiac emergencies with substantial morbidity and mortality. More strict control of low-density lipoprotein cholesterol (LDL-C) by high-intensity statin therapy has been shown to reduce major vascular events in ACS patients, and consequently clinical practice guidelines updated the LDL-C goal to 70 mg/dL in 2018 [1,2]. However, despite the prompt in-hospital initiation of high-intensity statin therapy in patients with ACS, LDL-C target levels are often not attained, including in Taiwan where almost half of patients with ACS do not achieve this goal [3], [4], [5]. Physician inertia, statin intolerance and concerns over adverse events with high-intensity statin regimens have been associated with suboptimal adherence rates [6,7]. In addition, comorbidities and polypharmacy may also lead to suboptimal LDL-C control. Health information technology (HIT) is increasingly being applied to close quality gaps in chronic illness care by improving self-care, cross-team integration, and clinical decision support. However, there are limited data on the effect of HIT in a multidisciplinary approach to improve control of chronic illness by improving treatment target achievement and patient compliance. Therefore, we performed this qualitative review to evaluate the association of implementing HIT on lipid management processes of care and LDL-C goal attainment in patients with ACS.
2. Methods
2.1. Study patients and design
We retrospectively enrolled consecutive patients who were discharged with a diagnosis of ACS, including ST-elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI), at a tertiary medical center from January 1, 2018, to December 31, 2021. These patients underwent revisualization and defined as having type 1 myocardial infarction based on fourth universal definition of myocardial infarction [8]. Patients who died in the hospital, and those who were transferred to other hospitals or discharged against medical advice were excluded. All procedures and clinical care were performed in accordance with the updated guidelines and regulations [9]. The HIT system was introduced at our hospital in October 2018. Eligible patients were enrolled into the HIT system at admission and received multidisciplinary care for one year, including high-intensity statin therapy, periodic laboratory follow-up, titration of lipid-lowering therapy, patient education, comorbidity control at the patient level, and system-level interventions that involved database monitoring and outreach by centralized care teams (Fig. 1).
Fig. 1.
The working algorithm of health information technology (HIT).
The HIT system screens patients with discharge diagnosis of ACS who do not achieve their LDL-C goal and enrolls them into the system, after which high-intensity statins and periodic laboratory data follow-up are initiated at both the provider and system level. In addition, the system offers multidisciplinary care, including pharmaceutical consultations to address potential statin side effects, low-fat diet suggestions, exercise recommendations, and smoking cessation support. At the patient level, the system provides outreach services through various platforms such as messaging apps, video call, and combined care with family members.
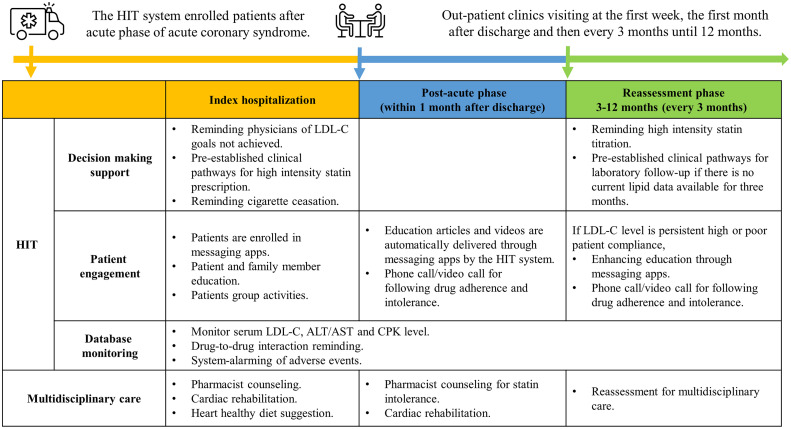
The HIT system was configured with LDL-C goals based on Taiwanese guidelines and enrolled patients after the acute phase of ACS [10,11]. Once enrolled, the HIT system provided pre-established clinical pathways for prescribing high-intensity statins and reminders of consultations for multidisciplinary care during hospitalization. Patient engagement was promoted by setting up messaging apps before discharge and phone contacts by case managers, facilitating education and communication through various media (message apps, video call, phone calls, etc.). During the first month after discharge, the HIT system focused on drug adherence and statin intolerance, through scheduled outpatient clinics visits and pharmacist counseling, education from message apps, and phone calls by case managers. From the 3rd to the 12th month, the HIT system changed its emphasis to reassessment, dose titration of treatment and the importance of goal achievement. If there were no lipid profile data available in the EMR system within three months, automatic reminders for periodic laboratory follow-up were displayed on the EMR system of the outpatient clinic when patients returned. If the lipid goal remained persistently high (LDL > 135 mg/dL) or unsatisfactory compliance or adherence are suspected, the HIT system not only suggested the physicians to up-titrate the lipid lowering agents, but also reinforced the patient-engagement and education program through messaging apps, and alerted case managers to conduct follow-up phone calls (Fig. 2).
Fig. 2.
Patient journey of health information technology (HIT) over 12 months period.
The intervention from the HIT is categorized into three main aspects: decision-making support, patient engagement, and database monitoring. The period over 12 months is divided into three phases: index hospitalization, post-acute phase (1 month after discharge), and reassessment phase (3–12 months). During index hospitalization, the HIT system focuses on high-intensity statins prescription, patient engagement and education, and comprehensive management of comorbidities. In the post-acute phase, the emphasis changes to monitor drug adherence and statin intolerance. Finally, during the reassessment phase, the HIT system efforts on tracking lipid profiles, titration of optimal medical therapy, reassessing patient compliance, and activating multidisciplinary care at appropriate timing and situations.
ACS, Acute coronary syndrome; ALT, Alanine transaminase; AST, Aspartate transaminase; CPK, Creatine phosphokinase; HIT, Health information technology; LDL-C, Low-density lipoprotein cholesterol.
2.2. Data collection and outcomes
The following characteristics were recorded from electronic medical records at baseline and over a one-year period after the index hospitalization: age, sex, body mass index, and cardiovascular risk factors and comorbidities including hypertension, type 2 diabetes mellitus, hyperlipidemia, chronic kidney disease; history of myocardial infarction, coronary artery disease or cerebral vascular accident; intensity of lipid-lowering therapy, and laboratory data including serum LDL-C level, liver enzymes (alanine transaminase [ALT], aspartate transaminase [AST]), and creatine kinases (CPK). The intensity of lipid-lowering therapy including statins, ezetimibe, and Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors was classified according to the 2019 ESC guideline-suggested dosage [2] and the target level of LDL-C was defined as < 70 mg/dL. The very high-risk group was defined by 2018 AHA/ACC guideline and the target level of LDL-C was defined as <55 mg/dL [1]. Adverse events associated with high-intensity statins, including hepatitis (ALT or AST > 3-fold upper normal limits) and myopathy (CPK > 10-fold upper normal limits), were also recorded.
This study aimed to evaluate the association of implementing an HIT system on lipid control in ACS patients. The primary outcomes were median LDL-C serum level and goal attainment rate at six and twelve months after discharge. The conversion of lipid-lowering therapy after implementing the HIT system according to the prescription rate of high-intensity statins at discharge and maintenance rate at twelve months was defined as the secondary outcome. The outcomes were compared before and after the introduction of the HIT system and team-based care for ACS patients at our institute. The factors affecting the LDL-C target and attainment rate were also analyzed.
2.3. Statistical analysis
Continuous variables were summarized as mean values with standard deviation (SD) or median values with interquartile range (IQR) by distribution. Categorical variables were summarized as numbers and percentages. The LDL-C goal attainment rate, prescription rate, and maintenance rate of high-intensity statins were compared using the one-way ANOVA test. Serum LDL-C levels were summarized as median values with IQR and compared using Kruskal-Wallis test. In addition, we also assessed LDL-C goal attainment rates among different subgroups based on an age of 60 years, sex, and major comorbidities. Statistical significance was defined as a p-value < 0.05. The statistical analysis was conducted using SPSS version 25.0 (IBM Inc., Armonk, NY, USA).
3. Results
A total of 2248 consecutive patients discharged with a diagnosis of ACS were reviewed between January 1, 2018, and December 31, 2021. After excluding patients who died during the index hospitalization (n = 218), referred to another hospital (n = 6) and discharged against medical advice (n = 53), the remaining 2001 patients were included for analysis (n = 526, 545, 493, and 437 from 2018 to 2021, respectively) (Fig. 3). There were 1761 (88.01 %) patients classified to very high-risk group. The clinical characteristics of the patients are presented in Table 1, and similar demographics were observed in each year. The average age of the patients was 63 ± 12.7 years, more were male (n = 1594, 79.66 %), and there were high prevalence rates of hypertension (n = 1190, 59.47 %), hyperlipidemia (n = 1173, 58.62 %) and type 2 diabetes mellitus (n = 766, 38.28 %). The baseline lipid profile was quite similar except minimal but significant difference of high-density lipoprotein cholesterol (HDL-C) between years (40.0 mg/dL, 40.0 mg/dL, 41.0 mg/dL, 38.0 mg/dL from 2018 to 2021, p<0.001 between groups). The prevalence of hyperlipidemia decreased over time from 61.60 % in 2018 to 54.00 % in 2021, with a notable decrease observed after 2020. This decrease in prevalence may have been due to the approval of high-strength statins for ACS patients by the National Health Insurance program of Taiwan in February 2019. However, the prevalence of hyperlipidemia remained similar in each year when a strict LDL-C target of 70 mg/dL was applied (81.75 %, 82.02 %, 80.73 %, and 77.80 % in 2018, 2019, 2020, and 2021, respectively, p = 0.323). (Table 1)
Fig. 3.
Patient disposition.
ACS, Acute coronary syndrome.
Table 1.
Patient demographics and baseline characteristics.
| All (n = 2001) | 2018 (n = 526) | 2019 (n = 545) | 2020 (n = 493) | 2021 (n = 437) | P-value | |
|---|---|---|---|---|---|---|
| Age, years old (mean ± SD) | 63±12.70 | 61.83±12.63 | 63.51±12.91 | 63.81±12.45 | 62.83±12.72 | 0.058 * |
| ≤ 40 years old, n (%) | 66(3.30) | 22(4.18) | 14(2.57) | 12(2.43) | 18(4.12) | 0.232 * |
| 41–60 years old, n (%) | 771(38.53) | 219(41.63) | 205(37.61) | 180(36.51) | 167(38.22) | 0.385 * |
| 61–80 years old, n (%) | 976(48.78) | 241(45.82) | 270(49.54) | 253(51.32) | 212(48.51) | 0.37 * |
| ≥ 81 years old, n (%) | 188(9.40) | 44(8.37) | 56(10.28) | 48(9.74) | 40(9.15) | 0.747 * |
| Sex, male, n (%) | 1594(79.66) | 413 (78.52) | 426 (78.17) | 397 (80.53) | 358 (81.92) | 0.396 * |
| BMI (mean ± SD) | 25.64±4.29 | 25.58±4.07 | 25.80±4.11 | 25.36±4.73 | 25.84±4.28 | 0.334 * |
| Current smoking, n (%) | 871(43.53) | 241(45.82) | 232 (42.57) | 217(44.02) | 181(41.42) | 0.205 * |
| Hypertension, n (%)a | 1190 (59.47) | 304(57.79) | 314(57.61) | 303(61.46) | 269 (61.56) | 0.269 * |
| Diabetes mellitus, n (%)a | 766 (38.28) | 220 (41.83) | 205(37.61) | 173(35.09) | 168(38.44) | 0.17 * |
| Coronary artery disease, n (%) a | 424(21.19) | 105 (19.96) | 120(22.02) | 96(19.47) | 103(23.57) | 0.384 * |
| Cerebrovascular disease, n (%) a | 124(6.20) | 33(6.27) | 36(6.61) | 29(5.88) | 26(5.95) | 0.131 * |
| Chronic kidney disease, n (%) a | 443(22.14) | 109(20.72) | 110(20.18) | 109(22.11) | 115(26.32) | 0.1 * |
| Hyperlipidemia, LDL-C ≥ 100 mg/dL, n (%) | 1173(58.62) | 324(61.60) | 336(61.65) | 277(56.19) | 236(54.00) | 0.029 * |
| Hyperlipidemia, LDL-C ≥ 70 mg/dL, n (%) | 1615(80.71) | 430(81.75) | 447(82.02) | 398(80.73) | 340(77.80) | 0.323 * |
| Baseline lipid profile | ||||||
| LDL-C, Median (IQR), mg/dL | 112.0 (83.0, 141.0) | 115.0 (85.0, 144.0) | 114.0 (86.0, 140.0) | 110.0 (80.0, 141.0) | 106.0 (78.25, 138.5) | 0.056 ⁎⁎ |
| HDL-C, Median (IQR), mg/dL | 39.0 (33.0, 48.0) | 40.0 (33.0, 48.0) | 40.0 (34.0, 49.0) | 41.0 (34.0, 49.0) | 38.0 (32.0, 45.0) | <0.001 ⁎⁎ |
| Total Cholesterol, Median (IQR), mg/dL | 162.0 (133.0, 193.0) | 162.0 (134.0, 194.0) | 161.0 (136.0, 191.0) | 164.0 (131.0, 195.0) | 161.5 (131.25, 193.0) | 0.759 ⁎⁎ |
| Triglycerides, Median (IQR), mg/dL | 105.0 (70.0, 150.0) | 108.0 (72.0, 156.0) | 106.0 (67.0, 152.0) | 103.0 (71.0, 150.0) | 103.0 (73.0, 146.75) | 0.669 ⁎⁎ |
| Types of acute coronary syndrome | ||||||
| STEMI, n (%) | 1001(50.02) | 270(51.33) | 276(50.64) | 251(50.91) | 200(45.77) | 0.288 * |
| NSTEMI, n (%) | 1000(49.98) | 256(48.67) | 269(49.36) | 242(49.09) | 237(54.23) | 0.288 * |
Comorbidities are defined by International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) recording by electronical medical records.
The p-value between each group was analyzed by the one-way ANOVA test. Statistical significance was defined as a p-value < 0.05.
Data are medians (Q1, Q3). The p-value between each group, was analyzed by the Kruskal-Wallis test. Statistical significance was defined as a p-value < 0.05.
BMI: Body Mass Index; HDL-C: High-density lipoprotein cholesterol; IQR, Interquartile range; LDL-C: Low-density lipoprotein cholesterol; NSTEMI: Non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction; SD: Standard deviation.
The LDL-C goal attainment rate at discharge was low, with a slight increase but not statistically significant from 2018 to 2021 (14.64 %, 15.96 %, 16.02 %, and 19.91 % in 2018, 2019, 2020, and 2021, p = 0.168 between groups). After initiating the HIT system, LDL-C goal attainment rates (36.52 %, 53.57 %, 59.22 %, and 62.18 % in 2018, 2019, 2020, and 2021) and the medium serum levels of LDL-C (80.5 mg/dL, 68 mg/dL, 65 mg/dL, and 64 mg/dL in 2018, 2019, 2020, and 2021) significantly improved within six months (2018 as the reference, all p<0.001). The improvement in LDL-C goal attainment rate within six to twelve months, although moderate, steadily increased after implementing the HIT system (53.80 %, 61.82 %, and 64.21 % in 2019, 2020, and 2021, p = 0.019) (Table 2 and Fig. 4). In the very high-risk group with an LDL-C target of <55 mg/dL, we observed a similar trend of improvement in LDL-C goal attainment rate at 6 months (17.05 %, 24.72 %, 29.93 %, and 30.59 %, in 2018, 2019, 2020, and 2021, p = 0.001 between groups) and 12 months (16.91 %, 21.45 %, 31.11 %, 36.36 % in 2018, 2019, 2020, and 2021, p<0.001 between groups) after the implementing the HIT system.(Table 2)
Table 2.
Primary and secondary outcomes after health information technology initiation.
| 2018 (n = 526) | 2019 (n = 545) | 2020 (n = 493) | 2021 (n = 437) | P-value | |
|---|---|---|---|---|---|
| Total patients returning OPD, n (%) | 498(94.68) | 529(97.06) | 456(92.49) | 423(96.8) | 0.88* |
| LDL-C goal attainment at discharge, n (%) | 77(14.64) | 87(15.96) | 79(16.02) | 87(19.91) | 0.168 * |
| LDL-C goal attainment at 6 months, n (%) | 103(36.52) | 210(53.57) | 183(59.22) | 171(62.18) | <0.001 * |
| LDL-C goal attainment at 12 months, n (%) | 118(38.19) | 184(53.80) | 183(61.82) | 183(64.21) | <0.001 * |
| Very high-risk groupb | |||||
| LDL-C goal attainment at discharge (n,%)b | 26(5.78) | 34(6.88) | 37(8.77) | 37(9.37) | 0.168 * |
| LDL-C goal attainment at 6 months (n,%)b | 44(17.05) | 89(24.72) | 85(29.93) | 78(30.59) | 0.001 * |
| LDL-C goal attainment at 12 months (n,%)b | 47(16.91) | 68(21.45) | 84(31.11) | 96(36.36) | <0.001 * |
| LDL-C level | |||||
| LDL-C level at discharge, mg/dL | 115.0 (85.0, 144.0) | 114.0 (86.0, 140.0) | 110.0 (80.0, 141.0) | 106.0 (78.25, 138.5) | 0.056⁎⁎ |
| LDL-C level at 6 months, mg/dL | 80.5 (63.0, 98.0) | 68.0 (55.0, 85.75) | 65.0 (51.5, 83.0) | 64.0 (52.0, 82.0) | <0.001⁎⁎ |
| LDL-C level at 12 months, mg/dL | 76.0 (62.0, 92.5) | 68.0 (56.0, 83.0) | 64.5 (51.0, 80.0) | 61.0 (50.0, 80.0) | <0.001⁎⁎ |
| LLT at discharge | |||||
| Without LLT treatment, (n,%) | 44(8.37) | 46(8.44) | 35(7.10) | 31(7.09) | 0.639 |
| Moderate intensity statin, n (%) | 401(76.24) | 114(20.92) | 54(10.95) | 28(6.41) | <0.001 * |
| Moderate intensity statin with ezetimibe, n (%) | 61(11.60) | 28(5.14) | 15(3.04) | 8(1.83) | <0.001 * |
| High intensity statin, n (%) | 3(0.57) | 347(63.67) | 357(72.41) | 369(84.44) | <0.001 * |
| High intensity statin with ezetimibe, n (%) | 0(0) | 5(0.92) | 5(1.01) | 4(0.92) | 0.166* |
| PCSK9 inhibitor, n (%) | 0(0) | 1(0.18) | 3(0.61) | 0(0) | 0.1* |
| High intensity LLT after 12 months | |||||
| Maintenance, n (%) | – | 146(41.36) | 179(49.04) | 229(61.39) | <0.001* |
| De-escalation, n (%) | – | 132(37.39) | 120(32.88) | 78(20.91) | <0.001* |
| Lost follow up, n (%) | – | 75(21.25) | 66(18.08) | 66(17.69) | 0.413* |
aThe target level of LDL-C <70 mg/dL.
The very high-risk group was defined by 2018 AHA/ACC guideline and the target level of LDL-C<55 mg/dL. [1].
The p-value between each group was analyzed by the one-way ANOVA test.
Data are medians (Q1, Q3). The p-value between each group, was analyzed by the Kruskal-Wallis test.
Both statistical significances were defined as a p-value < 0.05.
IQR, Interquartile range; LDL-C, Low-density lipoprotein cholesterol; LLT, Lipid-lowering therapy; OPD, Outpatient department; PCSK9 inhibitor: Proprotein convertase subtilisin/kexin type 9 inhibitor.
Fig. 4.
Primary outcome after implementing health information technology (HIT).
ent rate is percentage and the p-value between each groups was analyzed using one-way ANOVA. B. Data for serum LDL-C level is median values with interquartile range and was analyzed using Kruskal-Wallis test. Statistical significance was defined as a p-value < 0.05.
Most of the patients initiated moderate-intensity statin therapy at discharge in 2018 (76.24 %, 20.92 %, 10.95 %, and 6.41 % in 2018, 2019, 2020, and 2021), and then switched to a high-intensity statin after initiation of the HIT system (0.57 %, 63.67 %, 72.41 %, and 84.44 % in 2018, 2019, 2020, and 2021) (2018 as the reference, all p<0.001). Although the maintenance rate of high-intensity statin therapy was suboptimal at twelve months initially, it slowly increased after implementing the HIT system (41.36 %, 49.04 %, and 61.39 % at 2019, 2020, and 2021, p<0.001) (Table 2).
In subgroup analysis (Table 3), we found that the patients who were younger than 60 years had a lower LDL-C goal attainment rate at discharge (10.50% vs. 22.06 %, p<0.001), as well as at six and twelve months compared to those who were older (44.57% vs. 50.26 %, p = 0.036 at six months and 45.59% vs. 51.92 %, p = 0.022 at 12 months). The patients with major comorbidities had better control of LDL-C at discharge. The LDL-C goal attainment rate was found to be independent of the number of comorbidities present. However, the intergroup differences in LDL-C control rate gradually diminished at six and twelve months; except that the patients with type 2 diabetes mellitus at 6 months (51.95% vs. 45.20 %, p = 0.017) and hypertension at twelve months (52.07% vs. 44.90 %, p = 0.01) had a better LDL-C goal attainment rate.
Table 3.
Subgroup analysis of LDL-C goal attainment rate.
| Subgroups | At discharge (n = 2001) | P-value * | At 6 months (n = 1406) | P-value * | At 12 months (n = 1370) | P-value * | |
|---|---|---|---|---|---|---|---|
| All patients, n (%) | 322(16.09) | 666(47.36) | 666(48.61) | ||||
| Age, years old, n (%) | ≥60 | 84(10.50) | <0.001 | 283(44.57) | 0.036 | 274(45.59) | 0.022 |
| >60 | 238(22.06) | 383(50.26) | 392(51.92) | ||||
| Sex, n (%) | Male | 228(15.21) | <0.001 | 543(47.59) | 0.945 | 542(48.87) | 0.725 |
| Female | 94(24.74) | 123(48.05) | 124(50.20) | ||||
| Hypertension, n (%) a | Yes | 218(19.62) | 0.001 | 402(49.33) | 0.158 | 415(52.07) | 0.01 |
| No | 104(13.54) | 264(45.36) | 251(44.90) | ||||
| Diabetes mellitus, n (%) a | Yes | 196(28.00) | <0.001 | 266(51.95) | 0.017 | 256(51.93) | 0.127 |
| No | 126(10.69) | 400(45.20) | 410(47.51) | ||||
| Coronary artery disease, n (%) a | Yes | 138(36.70) | <0.001 | 126(49.22) | 0.628 | 122(48.41) | 0.834 |
| No | 187(12.44) | 540(47.33) | 544(49.28) | ||||
| Cerebrovascular disease, n (%) a | Yes | 33(29.20) | 0.001 | 38(52.78) | 0.398 | 33(45.21) | 0.548 |
| No | 289(16.36) | 628(47.40) | 633(49.34) | ||||
| Chronic kidney disease, n (%) a | Yes | 129(33.42) | <0.001 | 118(46.27) | 0.628 | 111(47.44) | 0.615 |
| No | 193(12.93) | 548(47.99) | 555(49.47) | ||||
| The number of comorbidities, n (%) b | <3 | 131(19.07) | 0.098 | 246(49.70) | 0.263 | 238(50.85) | 0.361 |
| ≥3 | 191(16.02) | 420(46.56) | 428(48.20) |
Comorbidities are defined by International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) recording by electronical medical records.
The count of comorbidities is defined as the number of comorbidities the patient has enrolled in our study.
The p-value between each subgroup was analyzed by the chi-square test or Fisher's exact test. Statistical significance was defined as a p-value < 0.05.
The incidence of adverse events recorded from the EMRs was 2.5 %, and there was no statistically significant difference after implementing the HIT system. In addition, the incidence of hepatitis was slightly higher (n = 47, 2.35 %) than myopathy (n = 3, 0.15 %). Most of the patients with statin-induced hepatitis and myopathy were asymptomatic (Table 4).
Table 4.
Stain associated adverse events after health information technology initiation.
| All (n = 2001) | 2018(n = 526) | 2019(n = 545) | 2020(n = 493) | 2021(n = 437) | |
|---|---|---|---|---|---|
| ALT or AST>3 times of ULN, n (%) a | 47(2.35) | 16(3.04) | 15(2.75) | 12(2.43) |
4(0.92) |
| ALT or AST>3 times of ULN with symptoms, n (%)a | 14 (0.70) | 4 (0.76) | 7 (1.28) | 3 (0.61) | 0(0) |
| CPK>10 times of ULN, n (%)b | 3 (0.15) | 0 (0) | 2 (0.37) | 1 (0.20) | 0(0) |
| CPK>10 times of ULN with symptoms, n (%)b | 3 (0.15) | 0 (0) | 2 (0.37) | 1 (0.20) | 0(0) |
| Adverse event rates associated with high-intensity statin option | |||||
| Atorvastatin, n (%) | 31(3.99) | ||||
| Rosuvastatin, n (%) | 14(4.44) | ||||
The upper limit of normal (ULN) for ALT and AST is 40 U/L and exclude other demonstrable cause. Symptoms include fatigue, poor appetite, nausea, vomiting, abdominal pain, jaundice.
The upper limit of normal (ULN) for CPK is 220 U/L. Statin-associated muscle symptoms include myalgias, cramps and weakness.
ALT, Alanine transaminase; AST, Aspartate transaminase; CPK, Creatine phosphokinase; ULN, Upper limit normal.
4. Discussion
Statins are suggested as first-line therapy to manage dyslipidemia through their action in inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, and statin therapy has been shown to reduce LDL-C levels by 20 % to 65 % according to the intensity of treatment [7]. Previous studies demonstrated that a higher intensity of statin treatment resulted in a 23 % reduction in major coronary events and a 20 % reduction in cardiovascular death over a 5-year period for every 1 mmol/L decrease in LDL-C [12]. Thus, clinical practice guidelines were updated in 2018 to recommend the use of high-intensity statins to achieve an LDL-C target of 70 mg/dL in patients with ACS [1,2]. This change led to a comparable starting point for quality improvement programs in our hospital.
The rate of attaining a LDL-C level < 70 g/dL at discharge was still low from 2018 to 2021 in this study (14.64 %, 15.96 %, 16.02 %, and 19.91 % from 2018 to 2021, respectively), indicating that most patients with the presentation of ACS still had increased LDC-C levels, regardless of whether or not they had a prior history of coronary artery disease or myocardial infarction [13]. This finding highlights the need to improve cardiovascular risk control and therapeutic inertia in high-risk patients before ACS events occur in everyday clinical practice. In the subgroup analysis, we found that the LDL-C goal attainment rate at discharge was higher in the patients who were older, female, and had comorbidities such as hypertension, diabetes mellitus, coronary artery disease, cerebrovascular disease, and chronic kidney disease. Although we did not routinely record baseline medications before admission, these results suggest that they received lipid lowering therapy before ACS events to manage their comorbidities. The difference in LDL-C control between these subgroups then reduced at six and twelve months. This suggests that regardless of the baseline LDL-C level at discharge or underlying comorbidities, the quality improvement program worked well.
Despite guidelines suggest high intensity statins in reducing LDL-C levels, previous studies have reported that almost half of patients do not achieve their LDL-C goals after ACS, and approximately one-third of patients do not receive periodic follow-up in Taiwan [3], [4], [5]. Improving guideline adherence in patients with ACS has been associated with a reduction in adverse clinical outcomes following ACS [14]. The HIT system with decision-making support has been shown to help increase the initiation of high-intensity statin therapy in patients with ACS [15], [16], [17]. In this study, the prescription rate of high-intensity statins significantly increased after implementing the HIT system (0.57 % in 2018 and 84.44 % in 2021), and this led to significant improvement in LDL-C goal attainment rate within six months (36.52 % in 2018 and 62.18 % in 2021).
Combining patient education tools and system-based monitoring, which allow for connectivity to a health care provider, can be effective in improving lipid control [15]. Upon enrollment, our HIT system provides multidisciplinary care, including care from pharmacists, dietitians, and physical therapists. At the patient level, the system also provides outreach services through various platforms such as messaging apps, video call, and combined care with family members. The average number of outpatient clinic visits are 6.5 times per patient in one year after the HIT initiation. Before systematically implementing the HIT system, we did not utilize any social media or messaging apps, except for one phone call within 1 month after discharge. The HIT system actively engaged patients by sending an average of 8.28 messages via Line Bot per patient within a year. In specific situations, in patients with lower left ventricular ejection fraction (LVEF) less than 40 %, high LDL-C levels (> 135 mg/dL) even under high-potency statin and ezetimibe, irregular outpatient follow-up suggested unsatisfactory adherence, the HIT system would actively send message to the case managers, enabling them to call the patients, conduct phone-call consultations, or arrange outpatient clinic follow-ups. There was an average of ten calls per month prompted by HIT systems. By combining patient and system-level interventions in our HIT system, the maintenance rate of high-intensity statins gradually increased over time. The HIT system shares several similarities with the remote patient care program outlined in the study by Blood et al. [18], which improved hypertension and hyperlipidemia control through patient engagement and integrated multidisciplinary care using remote database monitoring and drug-treatment algorithms. Despite active reminders from our HIT system, the maintenance rate of high-intensity statins was still suboptimal at 12 months (41.36 % in 2019 and 61.39 % in 2021), although the reported adverse event rate was as low at 2.5 %. Among those who reported adverse events, 29 (58 %) patients discontinued the statin due to intolerance, and gastrointestinal upset (26.0 %) and myalgia (6.0 %) were the most common complaints. One patient was reported to have statin allergy. The reason for the suboptimal maintenance rate is believed associated with therapeutic inertia rather than statin intolerance, which may improve after initiation of team care and the implementation of the HIT system.
The largest meta-analysis to date found inconsistent benefits of HIT on lipid control and clinical outcomes when applied solely by computerized decision support at the provider level [15]. The author suggested that therapeutic inertia due to negative physician attitudes and alert fatigue may be contributing factors. Therapeutic inertia, defined as failure to timely adjust medications according to evidence-based guidelines, has been identified as a key factor contributing to poor control of chronic illness [19,20]. A recent study analyzed the reasons for statin non-use and found that therapeutic inertia was a more common reason than statin-associated side effects or contraindications. The study also found that patient characteristics such as younger age and male sex were associated with a higher rate of therapeutic inertia, which is consistent with our findings [21]. Limited comprehension of the risk-benefit profile of statin usage may also contributed to therapeutic inertia, and extending the duration of clinical encounters was reported to improve the situation [22]. This suggests that providing decision-making algorithms alone is not sufficient for controlling dyslipidemia. Furthermore, the rates of addition of other drugs such as ezetimibe or PCSK9 inhibitors were also low, which may have been due to regulations of the National Health Insurance program 23]. Recently the treatment target of LDL-C for patients with atherosclerotic cardiovascular disease was modified from recently published clinical trials. The global guidelines recommend a lower LDL-C target < 55 mg/dL or minimum percentage LDL-C reduction (50 %) in very high-risk patients following ACS, which endorsed by the major medical societies in Taiwan, and emphasis is placed on optimizing the control of risk factors through preventive measures, including a lower LDL-C target <50 mg/dl for patients with extreme risk [1,2,11,24]. The regulation of Taiwan National Health Insurance had been revised in 2018, but there is still a gap between the guidelines and the payment regulation, which might result a relatively low rate in achieving the lower LDL-C target in Taiwan. Even though, the HIT system helped to increase the control rate of hyperlipidemia by implementation of clinical guidelines, and we believed it could improve the clinical cardiovascular outcomes of these high-risk patients in the future.
This study has several inherent limitations. First, it was a retrospective cohort design in a single tertiary medical center. There was no control group. Second, not all patients were completely followed at our institute. Third, some data were missing from the electronic medical records, which could potentially have affected our results. Fourth, although we observed a significant improvement in LDL-C levels and LDL-C goal attainment rate, we did not assess any hard outcomes such as cardiovascular events or mortality due to insufficient sample size and follow-up duration. Fifth, the brands, component, and dose of each pill of lipid lowering agents in our hospital changed during these years, which might affect the patient compliance. In addition, the function of our HIT continues upgrading, which may also improve the LDL-C attainment rate. Further research is needed to assess the long-term benefits of HIT system in a larger population.
5. Conclusions
The HIT system demonstrated promising results in enhancing lipid control through promoting the early initiation of high-intensity statin treatment and multidisciplinary team care in ACS patients. The integration of patient engagement tools and system-based monitoring with multidisciplinary care is cardinal of the HIT system to achieve better LDL-C control and may translate to improve the long-term clinical outcomes in the future.
Funding
This study was partly supported by grants FEMH-2013-D-030, 2013-HHC-002, 2014-HHC-002, 2018-C-007, 2019-C-024, 2020-C-006, 108DN26, 109DN25, 110DN1, and PI20210003 from Far Eastern Memorial Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statement
Yi-Sheng Chen: Methodology, Data curation, Formal analysis, Writing – review & editing. Heng Hsu Lin: Methodology, Data curation, Formal analysis, Writing – review & editing. Hao-Yuan Tsai: Data curation, Writing – review & editing. Chien-Lin Lee: Data curation, Writing – review & editing. Yen-Ting Yeh: Data curation, Writing – review & editing. Yen-Wen Wu: Methodology, Writing – review & editing.
Declaration of Competing Interest
All authors declare no conflicts of interest.
Acknowledgments
We thank all cardiologists at the Division of Cardiology, Cardiovascular Medical Center, Far Eastern Memorial Hospital including Bing-Hsiean Tzeng, Chi-Cheng Huang, Chung-Ming Tu, Yu-Wei Chiu, Shin-Rong Ke, Wen-Po Chuang, Shan-Hui Huang, Yuan-Hung Liu, Chun-Hsien Chiang, Kei-Ip Cheong, Yi-Yao Chang, Kuo-Chin Chen, Jung-Cheng Hsu, Wan-Khey Chan, Pen-Chih Liao, and Ai-Hsien Li who joined the quality improvement program for acute coronary syndrome. We thank case managers Hsin-Chiao Chuang, Yun-En Chien, and research assistant Yung-Cheng Chen for conducting the quality improvement program and data collection. We also thank Dr. Shu-Hsun Chu, the Department of Medical Research of Far Eastern Memorial Hospital, for her assistance in statistical consultation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F., Baigent C., Catapano A.L., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 3.Yan B.P., Chiang F.T., Ambegaonkar B., et al. Low-density lipoprotein cholesterol target achievement in patients surviving an acute coronary syndrome in Hong Kong and Taiwan - findings from the dyslipidemia international study II. Int J Cardiol. 2018;265:1–5. doi: 10.1016/j.ijcard.2018.01.099. [DOI] [PubMed] [Google Scholar]
- 4.Navar A.M., Matskeplishvili S.T., Urina-Triana M., et al. Prospective evaluation of lipid management following acute coronary syndrome in non-Western countries. Clin Cardiol. 2021;44:955–962. doi: 10.1002/clc.23623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho L.T., Yin W.H., Chuang S.Y., et al. Determinants for achieving the LDL-C target of lipid control for secondary prevention of cardiovascular events in Taiwan. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien S.C., Chen P.S., Huang Y.H., et al. 2019 Taiwan society of lipids and atherosclerosis expert consensus statement on statin intolerance. J Formos Med Assoc. 2019;118:1385–1392. doi: 10.1016/j.jfma.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Krychtiuk K.A., Ahrens I., Drexel H., et al. Acute LDL-C reduction post ACS: strike early and strike strong: from evidence to clinical practice. A clinical consensus statement of the association for acute cardiovascular care (ACVC), in collaboration with the European association of preventive cardiology (EAPC) and the European society of cardiology working group on cardiovascular pharmacotherapy. Eur Heart J Acute Cardiovasc Care. 2022;11:939–949. doi: 10.1093/ehjacc/zuac123. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 9.Li Y.H., Lee C.H., Huang W.C., et al. 2020 Focused update of the 2012 guidelines of the Taiwan society of cardiology for the management of ST-segment elevation myocardial infarction. Acta Cardiol Sin. 2020;36:285–307. doi: 10.6515/ACS.202007_36(4).20200619A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y.H., Ueng K.C., Jeng J.S., et al. 2017 Taiwan lipid guidelines for high risk patients. J Formos Med Assoc. 2017;116:217–248. doi: 10.1016/j.jfma.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Chen P.S., Lee M., Tang S.C., et al. 2022 focused update of the 2017 Taiwan lipid guidelines for high risk patients: coronary artery disease, peripheral artery disease and ischemic stroke. J Formos Med Assoc. 2022;121:1363–1370. doi: 10.1016/j.jfma.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Silverman M.G., Ference B.A., Im K., et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 13.Li Y.H., Chen J.W., Lin T.H., et al. A performance guide for major risk factors control in patients with atherosclerotic cardiovascular disease in Taiwan. J Formos Med Assoc. 2020;119:674–684. doi: 10.1016/j.jfma.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Wada H., Ogita M., Suwa S., et al. Guideline adherence and long-term clinical outcomes in patients with acute myocardial infarction: a Japanese registry of acute myocardial infarction diagnosed by universal definition (J-MINUET) substudy. Eur Heart J Acute Cardiovasc Care. 2020;9:939–947. doi: 10.1177/2048872620902024. [DOI] [PubMed] [Google Scholar]
- 15.Aspry K.E., Furman R., Karalis D.G., et al. Effect of health information technology interventions on lipid management in clinical practice: a systematic review of randomized controlled trials. J Clin Lipidol. 2013;7:546–560. doi: 10.1016/j.jacl.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Samal L., Fu H.N., Camara D.S., et al. Health information technology to improve care for people with multiple chronic conditions. Health Serv Res. 2021;56(Suppl 1):1006–1036. doi: 10.1111/1475-6773.13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buonvino C., Chopard R., Guillon B., et al. An innovative lipid-lowering approach to enhance attainment of low-density lipoprotein cholesterol goals. Eur Heart J Acute Cardiovasc Care. 2020;9:879–887. doi: 10.1177/2048872620912639. [DOI] [PubMed] [Google Scholar]
- 18.Blood A.J., Cannon C.P., Gordon W.J., et al. Results of a remotely delivered hypertension and lipid program in more than 10,000 patients across a diverse health care network. JAMA Cardiol. 2023;8:12–21. doi: 10.1001/jamacardio.2022.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khunti K., Gomes M.B., Pocock S., et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427–437. doi: 10.1111/dom.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langer A., Mancini G.B.J., Tan M., et al. Treatment inertia in patients with familial hypercholesterolemia. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarraju A., Zammit A., Ngo S., et al. Identifying reasons for statin nonuse in patients with diabetes using deep learning of electronic health records. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.028120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheutlin A.R., Zhang M., Conroy M.B. Clinical encounter length and initiation of statin therapy for primary prevention among adults with elevated atherosclerotic cardiovascular disease risk. Am J Prev Cardiol. 2023;13 doi: 10.1016/j.ajpc.2022.100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin P.L., Wu Y.W., Lin C.F., et al. Real-world analyses of the treatment conditions in patients initiating proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor in Taiwan. J Atheroscler Thromb. 2023;30:1123–1131. doi: 10.5551/jat.63789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueng K.C., Chiang C.E., Chao T.H., et al. 2023 Guidelines of the Taiwan Society of Cardiology on the diagnosis and management of chronic coronary syndrome. 2023;39:4–96.AU Please provide complete details in Ref. [24]. [DOI] [PMC free article] [PubMed]