Abstract
Background
Spontaneous coronary artery dissection (SCAD) is a relatively rare condition affecting predominantly young adults, with a prevalence of female sex. The best management of SCAD is still unclear and not adequately evidence-based both in the acute phase but especially over the long-term. We therefore aimed to evaluate the impact of medical therapy usually adopted for coronary artery disease on long-term outcome in SCAD patients.
Methods
We performed a meta-regression analysis including all the studies evaluating the long-term outcome of patients affected by SCAD. We used long-term mortality, recurrent SCAD, admission for angina and major adverse cardio-vascular events (MACE) as dependent variables and the rates of discharge drug rates (beta-blockers, statins, renin-angiotensin-aldosterone system inhibitors, aspirin, dual antiplatelet therapy (DAPT)) as independent variables.
Results
Fourteen observational studies were included with a long-term follow-up of 3.5 ± 1.7 years. No statistically significant correlations between drug therapy (beta-blockers, statins, calcium channel blockers, nitrates, renin-angiotensin-aldosterone inhibitors) and mortality, MACE, admission for angina, and SCAD recurrence were found. Higher aspirin use rates were significantly correlated with lower admission rates for angina (p < 0.05); DAPT, however, showed a borderline correlation with higher rates of SCAD recurrence (p = 0.068).
Conclusions
In a meta-regression analysis including observational studies aspirin use rates correlated with lower long-term rates of admission for angina, while a borderline correlation between DAPT and rates of SCAD recurrence was found. Other drugs usually used for the treatment of coronary artery disease do not seem to impact long-term outcome of SCAD patients.
Keywords: Medical therapy, Spontaneous coronary artery dissection, Long-term prognosis, Meta-regression
1. Background
Spontaneous Coronary Artery Dissection (SCAD) is a rare but increasingly recognized nonatherosclerotic, nontraumatic cause of acute coronary syndrome (ACS) and sudden cardiac death [1], [2]. Women between 44 and 53 years with no or few traditional cardiovascular risk factors are more commonly affected. Etiopathogenesis of SCAD is multifactorial and not completely understood, involving both extrinsic and intrinsic factors; these include hormonal changes, genetic predisposition, underlying arteriopathies and environmental, physical and emotional triggers [1], [2].
Due to its relative rarity and the absence of specific electrocardiographic or clinical features, the diagnosis of SCAD is challenging. Clinical presentation may include symptoms similar to ACS, as angina and dyspnea [2], [3]. Atypical symptoms such as nausea, vomiting, and fatigue might as well be present [4].
Coronary angiography is the diagnostic gold standard, but noninvasive imaging techniques have emerged as effective diagnostic tools for SCAD. These include magnetic resonance imaging (MRI) and computed tomography angiography (CTA) [5]. Moreover, detailed information about the morphology and location of the dissection are usually obtained through intravascular imaging techniques: optical coherence tomography (OCT) and intravascular ultrasound (IVUS) [6].
The optimal management of SCAD is unclear and challenging. According to expert consensus, since there are limited trials to guide decision making, in the acute phase a conservative approach should be preferred over a percutaneous coronary intervention (PCI), unless electrical/hemodynamic instability or ongoing ischemia are present [7]. Long-term strategies to prevent re-SCAD and mortality are not defined and not evidence based. Beta-blockers are associated with lower rates of recurrent SCAD [8]. The optimal type and duration of antiplatelet treatment is unclear.
In the early phase, mortality of SCAD is generally low, but urgent revascularization is often required, up to 14 % [3]. Even if long-term mortality is generally low [8], at 2–3 years follow-up the reported major adverse cardiac events (MACE) are between 10 and 30 %, mostly caused by recurrent myocardial infarction from recurrent SCAD [3]. At longer-term follow-up, MACE have been reported in 15–37 %, and up to 50 % at 10 years follow-up, driven by recurrent SCAD or de novo SCAD [3]. The presence of genetic factors, peripartum status and extracoronary muscular fibrodysplasia are independent predictors of 3-year MACE. [1].
Even if a relatively rare condition, SCAD affects relatively young patients so long-term management represents an important issue both in terms of mortality and in terms of hospitalizations. We therefore aim to assess the impact of the medical therapy commonly used for long-term treatment of SCA patients, in the setting of SCAD.
2. Methods
Following the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) document, we searched Pubmed for studies involving patients with SCAD and investigating the long-term outcome, at least 1 year follow-up. As search term we used (spontaneous coronary artery dissection[Title/Abstract]) AND (long-term[Title/Abstract]), (spontaneous coronary artery dissection[Title/Abstract]) AND (medical therapy[Title/Abstract]), (spontaneous coronary artery dissection[Title/Abstract]) AND (outcome[Title/Abstract]), taking into account English language articles. Furthermore, we found meta-analyses and reviews using (spontaneous coronary artery dissection[Title]) as search terms, in order to add studies investigating long-term outcomes. We selected all the studies specifying all-cause mortality, recurrent SCAD (re-SCAD), admission for angina and major adverse cardio-vascular events (MACE) including myocardial infarction, ischemic stroke, death, hospitalization. We excluded the studies investigating the short-term prognosis, <1 year, Moreover, we excluded the studies not providing data on the use of beta-blockers, statins, renin-angiotensin-aldosterone system inhibitors, aspirin, dual anti-platelet therapy (DAPT), nitrates and calcium channel blockers. All titles and abstracts were searched and independently evaluated by 5 authors (MM, ET, RC, AG, SP) and 2 authors graded all studies for bias (Cochrane Handbook for Systematic Review of Interventions). Number of participants, sex distribution, average age, main cardiovascular risk factors, triggering factors for SCAD, treatment with PCI in acute phase, rates of cardiovascular drugs use (beta-blockers, statins, renin-angiotensin-aldosterone system inhibitors, aspirin, dual anti-platelet therapy, nitrates and calcium channel blockers), mortality, MACE, rates of admission for angina and SCAD recurrence were collected (Table 1).
Table 1.
Baseline characteristics of the selected studies.
| Study | Year | Design | N. of patients (n) | Mean Follow-up (y) | Age (y) | Female (%) | Ipertension (%) | Diabetic (%) | Smokers(%) | Dislipidemia (%) | Stress related SCAD | Pregnancy, hormone related (%) |
Aspirin (%) | DAPT (%) | ACE/AT II (%) | Statins (%) | Beta-blockers (%) (%) |
PCI (%) | Mortality (%) | MACE (%) | RE-SCAD (%) | Admission for angina (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortensen et al. [10] | 2009 | retrospective | 22 | 2,9 | 48,7 | 82 | 43 | 0 | 73 | 19 | – | 12 | 68 | 59 | 43 | 43 | 68 | 59 | 4,5 | 27,2 | 1 | 22 |
| Alfonso et al [11] | 2012 | prospective | 45 | 2 | 53 | 58 | 33 | 11 | 62 | 38 | – | 4 | – | 60 | 53,3 | – | 80 | 26,7 | 4,4 | 8,9 | 0 | 4,44 |
| Buja et al. [12] | 2013 | retrospective | 38 | 1,4 | 51,4 | 84,2 | 52,6 | 5,3 | 36,8 | – | – | 15,8 | 73,7 | 57,9 | 47,4 | – | 36,8 | 44,7 | 5,3 | 7,9 | – | – |
| Rogowski et al. [13] | 2015 | prospective | 64 | 4,5 | 53 | 94 | 45 | 0 | 28 | 52 | 2 | 5 | 97 | 92 | 36 | 89 | 86 | 10,9 | 0 | 6,2 | 6,2 | 0 |
| Mc Grath-Cadel et al [14] | 2016 | retrospective | 40 | 3 | 45 | 95 | 18 | 5 | 8 | 10 | 22,5 | 7,5 | 60 | 50 | 40 | 27,5 | 52,5 | 30 | 0 | 10 | 10 | – |
| Saw et al. [8] | 2017 | prospective | 327 | 4,8 | 52,5 | 90,8 | 36,4 | 4,6 | 9,8 | 25,7 | 76 | 23,5 | 88,1 | – | 49,2 | 36,7 | 80,4 | 16,5 | 0,3 | 5,3 | 2,8 | 2 |
| Chen et al. [15] | 2019 | retrospective | 111 | 3 | 48,1 | 92,8 | 28,8 | 7,2 | 12,6 | 37,8 | 12,6 | 20,3 | 94 | – | 63 | 81 | 88 | 48,6 | 0 | 8,1 | 3,3 | 8,88 |
| Clare et al. [16] | 2019 | retrospective | 208 | 4,7 | 49 | 88,9 | 30,8 | 8,2 | – | 27,9 | 13,9 | – | – | – | 57,2 | 80,8 | 83,2 | 11,1 | 3,3 | 13,9 | 10,6 | – |
| Seidl et al [17] | 2021 | prospective | 105 | 7,5 | 53,4 | 93 | 44 | 0 | 30 | 60 | 4,7 | 4,7 | 97 | 90 | 42 | 91 | 80 | 7 | 1,9 | 11,4 | 7,6 | 0 |
| Cerrato et al. [18] | 2021 | retrospective | 199 | 1 | 52,3 | 88,9 | 39,7 | 3,5 | 26,1 | 37,2 | 49,5 | 13,6 | 31,1 | 66,3 | – | 71,3 | 78,9 | 0 | 0,5 | 14,6 | 6 | 11 |
| Yongcheol Kim et al. [19] | 2021 | retrospective | 13 | 2,6 | 52,1 | 100 | 23,1 | 7,7 | 23,1 | 7,7 | – | – | 53,8 | 46,2 | 76,9 | 69,2 | 69,2 | 61,5 | 0 | 7,7 | 7,7 | – |
| Saw et al. [20] | 2022 | prospective | 750 | 3 | 51,7 | 88,5 | 32,1 | 6,9 | 11,6 | 20,3 | 60,1 | 25,2 | 93,7 | – | – | – | 84,3 | 14,1 | 0,7 | 8,5 | 3,3 | 1,06 |
| Proenca et al. [21] | 2023 | retrospective | 36 | 3,4 | 51 | 94 | 53 | 6 | 31 | 39 | 8,3 | 25,2 | 92 | 83 | 61 | 72 | 78 | – | – | 19 | 14 | 6 |
| Salamanca et al. [23] | 2023 | prospective | 348 | 5 | 54 | 88 | 34 | 6 | 44 | 34 | 36,2 | 0,9 | 92 | 56 | 51 | 75 | 80 | 21 | 2,5 | 12 | 1,6 | 6 |
2.1. Statistical analysis
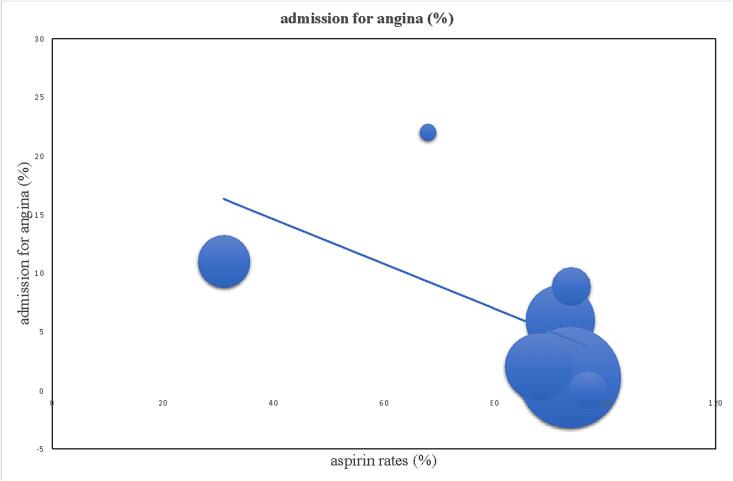
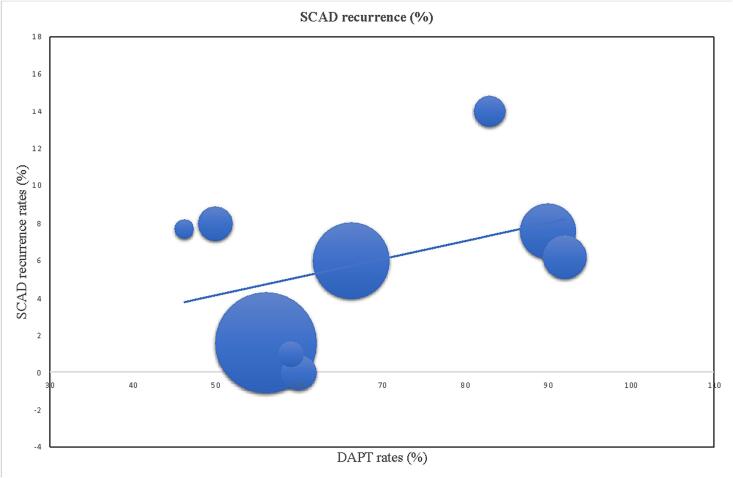
Meta-regression linear graphs were created by plotting medical treatment rates on the x-axis and all-cause mortality, re-SCAD, admission for angina and major cardiovascular adverse events (MACE) on the y-axis (Fig. 2, Fig. 3). The circles on the graphic represent an included study while the diameter of every circle is proportioned to the weight of study in the regression analysis. The line in the center represents the regression line. Linear regression was calculated and weighted for study size. A p < 0.05 was considered as statistically significant. Quality of studies was assessed with the Newcastle - Ottawa scale for bias assessment (Table 2) [9].
Fig. 2.
Significant reverse correlation between aspirin rates and admission rates for angina at follow-up (p < 0.05).
Fig. 3.
Borderline direct correlation between DAPT rates and SCAD recurrence at follow-up (p = 0.068).
Table 2.
Newcastle-Ottawa study evaluation: the quality of the studies included in the analysis in resumed by the final score.
| Study | Case definition adequate | Representativeness of the cases | Selection of controls | Definition of controls | Comparability based on design analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | Total Score |
|---|---|---|---|---|---|---|---|---|---|
| Mortensen et al. [10] | * | ○ | ○ | * | * | * | * | ○ | 5 |
| Alfonso et al [11] | * | * | * | * | ** | * | ○ | ○ | 7 |
| Buja et al. [12] | * | * | ○ | ○ | * | * | * | ○ | 5 |
| Rogowski et al. [13] | * | ○ | * | * | * | * | * | ○ | 7 |
| Mc Grath-Cadel et al [14] | * | ○ | * | * | * | * | * | ○ | 6 |
| Saw et al. [8] | * | * | * | * | ** | * | * | ○ | 8 |
| Chen et al. [15] | * | ○ | ○ | * | * | * | * | ○ | 5 |
| Clare et al. [16] | * | * | * | * | * | * | ○ | ○ | 6 |
| Seidl et al [17] | * | ○ | ○ | ○ | * | * | * | ○ | 4 |
| Cerrato et al. [18] | * | * | * | * | * | * | * | ○ | 7 |
| Yongcheol Kim et al. [19] | * | ○ | * | * | * | * | * | ○ | 6 |
| Saw et al. [20] | * | * | * | * | ** | * | * | ○ | 8 |
| Proenca et al. [21] | * | ○ | ○ | * | * | * | * | ○ | 5 |
| Salamanca et al. [22] | * | * | * | * | * | * | ○ | ○ | 6 |
3. Results
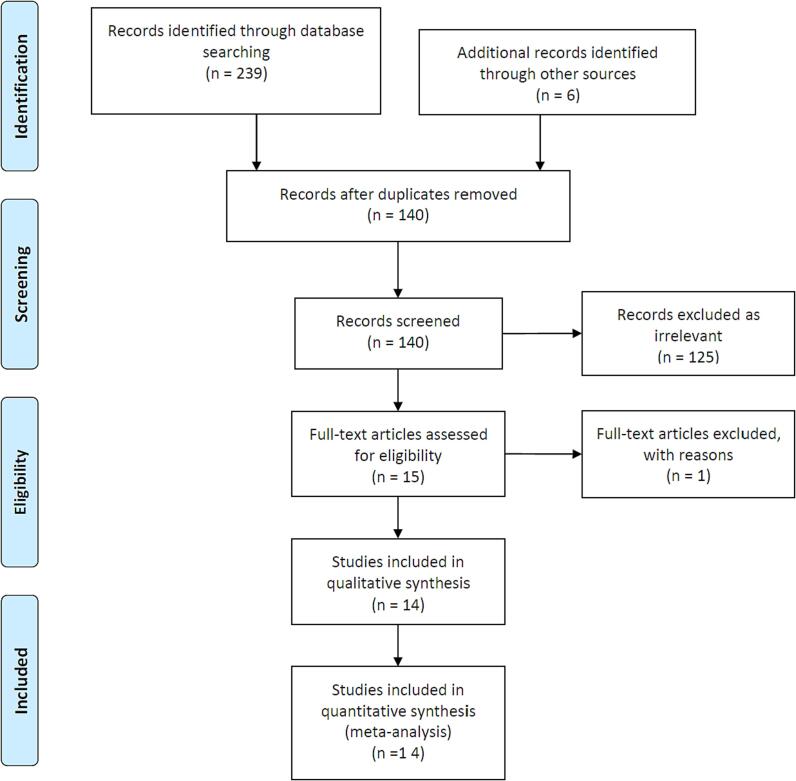
We reviewed 249 studies, adding 6 further studies from evaluation of the reviews and meta-analyses. We excluded one study as substantial duplicate results from the same population even though with different follow-up periods. After study selection, 14 studies were selected for long-term follow-up, including 2306 SCAD patients (Table 1, Fig. 1). Publication dates ranged from 2009 to 2023. All the studies were observational.
Fig. 1.
PRISMA flow diagram showing search strategy.
Meta-regression analysis did not show any statistically significant relationship between medical therapy (beta-blockers, statins, calcium channel blockers, nitrates, renin-angiotensin-aldosterone inhibitors) and mortality, MACE, admission for angina, re-SCAD. Regarding aspirin, no statistically significant correlation was detected with mortality, MACE and SCAD recurrence. However, a statistically significant reverse correlation with admission for angina was found (p < 0.05, Fig. 2). A borderline statistically significant between rates of DAPT and recurrence of SCAD was also found (p = 0.068, Fig. 3). No other correlations were found with other variables included in the study.
4. Discussion
To the best of our knowledge, this is the first meta-regression study investigating the impact of the medical therapy usually used for coronary heart disease on long-term outcome in patients with SCAD. We did not find a statistically significant impact of medical therapy on long-term mortality and MACE. Furthermore, we found a statistically significant positive impact of aspirin on the long-term rate of admission for angina. Interestingly, we detected a trend suggesting a negative impact of DAPT on long-term incidence of re-SCAD.
According to the ‘inside-out” hypothesis, SCAD is determined by the entry of blood into the subintimal space of the vessel from the true lumen. So, endothelial-intimal disruption and failure to repair are at the basis of the formation of a flap. On the other hand, according to “outside-in” hypothesis, the disruption of adventitial micro-vessels is the key event for the creation of an intramural hematoma within the media [2]. Recent observations based on Optical Coherence Tomography analysis [22] and the observed absence of communication between true and false lumen support the ‘outside-in’ over the inside-out hypothesis [23], [11], [22]. The higher incidence of SCAD observed in patients affected by fibro-muscular dysplasia (FMD) may favor the ‘inside-out’ theory. In fact, FMD is characterized by a failure of endothelial healing [24]. The relationship between SCAD and sexual estrogens is also well-known [3], [25]. Some observations suggest that fluctuations/reduction in estrogen levels may determine a reduction of circulating progenitor cells with a subsequent inability to repair the endothelium [26], [27]. Based on this, the estrogen-related higher incidence of SCAD may support the ‘inside-out’ hypothesis.
Unlike ACS, the physiopathology of SCAD is controversial so there is a lack of definite pharmacological targets. Some observational data suggest a beneficial effect of beta-blocker on long-term outcome [8]. The rationale of beta-blockers treatment is the reduction of vessel shear stress [8] and the observation that arterial hypertension is an independent predictor of recurrent SCAD [28], [29]. Moreover, beta-blockers have shown a significant reduction in terms of mortality and risk of recurrent aortic dissection [30]. However, in our analysis there is the lack of any beneficial effect of beta blockers. It is conceivable that a beneficial effect of beta-blockers may be restricted to a subset of SCAD, such as those triggered by emotional or physical stress. The ongoing controlled randomized BA-SCAD trial aims to investigate the long-term effect of beta-blockers in patients with SCAD [31].
We did not find any evidence supporting the use of statins and angiotensin-converting enzymes in SCAD [2]. The SAFER-SCAD trial, aiming to investigate the role of statins and angiotensin converting enzymes on recurrence of SCAD, was interrupted prematurely for funding problems [31].
The optimal long-term antiplatelet treatment is controversial [28], [8]. Some authors suggest a DAPT for 12 months also in case of conservative strategy, others suggest a shorter DAPT (1–3 months) followed by aspirin alone [3]. The ongoing BA-SCAD trial investigates on the effect of a a short-term (1 month) vs a long-term antiplatelet treatment on a 1-year composite endpoint (death, myocardial infarction, stroke, coronary revascularization, recurrent SCAD, and unplanned hospitalization for acute coronary syndrome or heart failure) [30].
Our findings support a protective role of aspirin in terms of admission for angina. This may be explained by the anti-thrombotic and anti-inflammatory effect on endothelium and is consistent with the inside-out hypothesis. Moreover, the positive role of aspirin in the setting of secondary prevention in patients with coronary artery disease is well-established [32]. Aspirin may provide a protective, antithrombotic, not SCAD-specific action on the coronary artery tree, determining a positive impact on episodes of angina over a long-term follow-up.
An interesting observation is the border-line significant negative impact of DAPT on recurrence of SCAD. This finding is consistent with the outside-in hypothesis, as the probability of IMH creation and extension is considered higher in patients with DAPT. For this reason, an aggressive antithrombotic with parenteral antiplatelet drugs is discouraged in the acute phase [3]. However, in the absence of an adequate multivariable analysis, a possible role for DAPT as an identifier of high risk patients characterized by higher recurrence rates may not be excluded.
The long-term management of SCAD is complex and probably completely different from the classical CAD. SCAD may probably be considered a common final pathological finding that derives from different etiologies, risk factors and physiopathology. The understanding of what leads to a SCAD is poor and incomplete so far, consequently the optimal secondary prevention is unknown. Based on our findings, the medical treatment usually utilized for secondary prevention of acute coronary syndromes seems not to fit for SCAD. Furthermore, a long-term SCAD strategy seems to be harmful while long-term aspirin shows a protective effect. Further observations and controlled trial are needed to explore this gap in knowledge and the impact of specific drugs in this specific subset of patients.
5. Conclusions
In a meta-regression analysis, aspirin showed a positive impact on long-term rate of admission for angina, while DAPT showed a borderline association with higher rates of re-SCAD. Except for anti-platelet medications, other drugs usually adopted for the treatment of coronary artery disease do not seem to impact on long-term outcome of SCAD patients.
6. Limitations
The collected data refer to the rates of medical therapy at discharge of the index hospitalization for SCAD. Factors such as the duration of DAPT and therapeutic adherence may affect the impact of cardio-vascular drugs on long-term outcome in SCAD patients. The lack of a uniform understanding of SCAD's underlying mechanisms can make it challenging to generalize treatment recommendations. The study does not account for these etiological variations and their potential impact on treatment outcomes. The analysis is primarily based on observational studies and lacks data from well-designed randomized controlled trials. The absence of RCTs can introduce biases, and the effects of specific medications may not be accurately assessed due to potential confounding factors. There is considerable heterogeneity in the medical treatments prescribed to SCAD patients. This diversity in treatment regimens, including the choice of antiplatelet agents and other medications, can influence the study results. A lack of standardized treatment protocols for SCAD makes it difficult to draw definitive conclusions. SCAD is a rare condition, and long-term follow-up studies are limited. As a result, the study may not capture the full spectrum of long-term outcomes, and it may not account for evolving treatment strategies and their impact on patient outcomes over time. The borderline significance of some findings, such as the negative impact of DAPT on SCAD recurrence, should be interpreted cautiously. The borderline nature of these associations suggests the need for further research to confirm or refuse these trends. The results of this meta-regression analysis are primarily based on the available literature, which may not fully represent the diversity of SCAD patients. Generalizing the findings to all SCAD patients should be done with caution, as specific patient subgroups may respond differently to medical therapy. The study primarily focuses on efficacy outcomes, such as long-term admission for angina and SCAD recurrence. However, it does not provide comprehensive data on potential adverse events associated with different medications, which is essential for a thorough assessment of their safety profiles.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Marco Mele, Email: drmelemarco@yahoo.it.
Erika Tabella, Email: erika.tabella@unifg.it.
Raffaele Capasso, Email: raffaele.capasso@unifg.it.
Adriano Grillo, Email: adriano.grillo@unifg.it.
Simone Puglisi, Email: simone.puglisi@unifg.it.
Antonietta Mele, Email: antonietta.mele@uniba.it.
Andrea Cuculo, Email: andr.cuculo@tiscali.it.
Antonella Liantonio, Email: antonella.liantonio@uniba.it.
Paola Imbrici, Email: paola.imbrici@uniba.it.
Francesco Santoro, Email: dr.francesco.santoro.it@gmail.com.
Natale Daniele Brunetti, Email: natale.brunetti@unifg.it.
References
- 1.Saw J., Mancini G.B., Humphries K.H. Contemporary Review on Spontaneous Coronary Artery Dissection. J. Am. Coll. Cardiol. 2016;68:297–312. doi: 10.1016/j.jacc.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Hayes S.N., Tweet M.S., Adlam D., Kim E.S.H., Gulati R., Price J.E., et al. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020 Aug;25(76):961–984. doi: 10.1016/j.jacc.2020.05.084. [DOI] [PubMed] [Google Scholar]
- 3.S.N. Hayes, E.S.H. Kim, J. Saw, D. Adlam, C. Arslanian-Engoren, K.E. Economy et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2018 May 8;137:e523-e557. [DOI] [PMC free article] [PubMed]
- 4.Okoroafor C.D., Akram A., Ong A. Atypical Presentation of Spontaneous Coronary Artery Dissection. Cureus. 2020;12:e9543. doi: 10.7759/cureus.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan N.Y., Hayes S.N., Young P.M., Gulati R., Tweet M.S. Usefulness of Cardiac Magnetic Resonance Imaging in Patients With Acute Spontaneous Coronary Artery Dissection. Am. J. Cardiol. 2018;122:1624–1629. doi: 10.1016/j.amjcard.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 6.Tweet M.S., Gulati R., Hayes S.N. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 7.J.P. Collet, H. Thiele, E. Barbato, O. Barthélémy, J. Bauersachs, D.L. Bhatt, et al ; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. [DOI] [PubMed]
- 8.Saw J., Humphries K., Aymong E., Sedlak T., Prakash R., Starovoytov A., et al. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J. Am. Coll. Cardiol. 2017;70:1148–1158. doi: 10.1016/j.jacc.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010 Sep;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 10.Mortensen K.H., Thuesen L., Kristensen I.B., Christiansen E.H. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter. Cardiovasc. Interv. 2009;74:710–717. doi: 10.1002/ccd.22115. [DOI] [PubMed] [Google Scholar]
- 11.Alfonso F., Paulo M., Lennie V., Dutary J., Bernardo E., Jiménez-Quevedo P., et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. J. Am. Coll. Cardiol. Intv. 2012;5:1062–1070. doi: 10.1016/j.jcin.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Buja P., Coccato M., Fraccaro C., Tarantini G., Isabella G., Almamary A., et al. Management and outcome of spontaneous coronary artery dissection: conservative therapy versus revascularization. Int. J. Cardiol. 2013;168:2907–2908. doi: 10.1016/j.ijcard.2013.03.116. [DOI] [PubMed] [Google Scholar]
- 13.McGrath-Cadell L., McKenzie P., Emmanuel S., Muller D.W., Graham R.M., Holloway C.J. Outcomes of patients with spontaneous coronary artery dissection. Open Heart. 2016 Aug;24(3) doi: 10.1136/openhrt-2016-000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S., Merchant M., Mahrer K.N., Lundstrom R.J., Naderi S., Goh A.C. Spontaneous Coronary Artery Dissection: Clinical Characteristics, Management, and Outcomes in a Racially and Ethnically Diverse Community-Based Cohort. Perm. J. 2019;23:18 .278. doi: 10.7812/TPP/18.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clare R., Duan L., Phan D., Moore N., Jorgensen M., Ichiuji A., et al. Characteristics and Clinical Outcomes of Patients With Spontaneous Coronary Artery Dissection. J. Am. Heart Assoc. 2019;8:e012570. doi: 10.1161/JAHA.119.012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidl S., Rickli H., Rogowski S., Weilenmann D., Ammann P., Haager P.K., et al. Long-term follow-up of medically treated patients with spontaneous coronary artery dissection: a prospective, Swiss single-centre cohort study. Swiss Med. Wkly. 2021;151 doi: 10.4414/smw.2021.w30067. [DOI] [PubMed] [Google Scholar]
- 17.Cerrato E., Giacobbe F., Quadri G., Macaya F., Bianco M., Mori R., et al. DISCO Collaborators. Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur. Heart J. 2021;42:3161–3171. doi: 10.1093/eurheartj/ehab372. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y., Han X., Ahn Y., Kim M.C., Sim D.S., Hong Y.J., et al. Clinical characteristics of spontaneous coronary artery dissection in young female patients with acute myocardial infarction in Korea. Korean J. Intern. Med. 2021;36:106–113. doi: 10.3904/kjim.2019.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saw J., Starovoytov A., Aymong E., Inohara T., Alfadhel M., McAlister C., et al. Canadian Spontaneous Coronary Artery Dissection Cohort Study: 3-Year Outcomes. J. Am. Coll. Cardiol. 2022;80:1585–1597. doi: 10.1016/j.jacc.2022.08.759. [DOI] [PubMed] [Google Scholar]
- 20.M. de Sousa Almeida, Spontaneous coronary artery dissection: When so much is unknown, details matter for the right decision. Rev Port Cardiol. 2023 Mar;42(3):267-268. [DOI] [PubMed]
- 21.J. Salamanca, M. García-Guimarães, M. Sabaté, R. Sanz-Ruiz, F. Macaya, G. Roura, et al. Multivessel spontaneous coronary artery dissection: Clinical features, angiographic findings, management, and outcomes, Int J Cardiol 370 (2023) 65-71. [DOI] [PubMed]
- 22.Jackson R., Al-Hussaini A., Joseph S., van Soest G., Wood A., Macaya F., et al. Spontaneous Coronary Artery Dissection: Pathophysiological Insights From Optical Coherence Tomography. J. Am. Coll. Cardiol. Img. 2019;12:2475–2488. doi: 10.1016/j.jcmg.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Waterbury T.M., Tweet M.S., Hayes S.N., Eleid M.F., Bell M.R., Lerman A., et al. Early Natural History of Spontaneous Coronary Artery Dissection. Circ. Cardiovasc. Interv. 2018;11:e006772. doi: 10.1161/CIRCINTERVENTIONS.118.006772. [DOI] [PubMed] [Google Scholar]
- 24.Gornik H.L., Persu A., Adlam D., Aparicio L.S., Azizi M., Boulanger M., et al. Working Group ‘Hypertension and the Kidney’ of the European Society of Hypertension (ESH) and the Society for Vascular Medicine (SVM). First international consensus on the diagnosis and management of fibromuscular dysplasia. J. Hypertens. 2019;37:229–252. doi: 10.1097/HJH.0000000000002019. [DOI] [PubMed] [Google Scholar]
- 25.Tweet M.S., Miller V.M., Hayes S.N. The Evidence on Estrogen, Progesterone, and Spontaneous Coronary Artery Dissection. JAMA Cardiol. 2019;4:403–404. doi: 10.1001/jamacardio.2019.0774. [DOI] [PubMed] [Google Scholar]
- 26.Topel M.L., Hayek S.S., Ko Y.A., Sandesara P.B., Samman Tahhan A., Hesaroieh I., et al. Sex Differences in Circulating Progenitor Cells. J. Am. Heart Assoc. 2017;6:e006245. doi: 10.1161/JAHA.117.006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adlam D., Alfonso F., Maas A., Vrints C., Committee W. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur. Heart J. 2018;39:3353–3368. doi: 10.1093/eurheartj/ehy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoud A.N., Taduru S.S., Mentias A., Mahtta D., Barakat A.F., Saad M., et al. Trends of Incidence, Clinical Presentation, and In-Hospital Mortality Among Women With Acute Myocardial Infarction With or Without Spontaneous Coronary Artery Dissection: A Population-Based Analysis. J. Am. Coll. Cardiol. Intv. 2018;11:80–90. doi: 10.1016/j.jcin.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 29.R. Erbel, V. Aboyans, C. Boileau, E. Bossone, R. Di Bartolomeo, H. Eggebrecht, et al; Authors/Task Force members. Corrigendum to: 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J. 2015;36:2779. [DOI] [PubMed]
- 30.Alfonso F., de la Torre Hernández J.M., Ibáñez B., Sabaté M., Pan M., Gulati R., et al. Rationale and design of the BA-SCAD (Beta-blockers and Antiplatelet agents in patients with Spontaneous Coronary Artery Dissection) randomized clinical trial. Rev. Esp. Cardiol. (Engl Ed). 2022;75:515–522. doi: 10.1016/j.rec.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 31.T. Sedlak,. Statin and Angiotensin-converting Enzyme Inhibitor on Symptoms in Patients With SCAD (SAFER-SCAD). Available online: https://clinicaltrials.gov/ct2/show/NCT02008786?cond=scad&rank=6.
- 32.Patrono C., Morais J., Baigent C., Collet J.P., Fitzgerald D., Halvorsen S., et al. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J. Am. Coll. Cardiol. 2017;70:1760–1776. doi: 10.1016/j.jacc.2017.08.037. [DOI] [PubMed] [Google Scholar]