Abstract
Background
Conventional cytotoxic drugs are not effective in alveolar soft-part sarcoma (ASPS). Immune checkpoint (programmed cell death protein 1/programmed death-ligand 1) inhibitors (ICIs) are promising drugs in ASPS. A worldwide registry explored the efficacy of ICI in ASPS.
Materials and methods
Data from adult patients diagnosed with ASPS and treated with ICI for advanced disease in expert sarcoma centers from Europe, Australia and North America were retrospectively collected, including demographics and data related to treatments and outcome.
Results
Seventy-six ASPS patients, with a median age at diagnosis of 25 years (range 3-61 years), were registered. All patients received ICI for metastatic disease. Immunotherapy regimens consisted of monotherapy in 38 patients (50%) and combination in 38 (50%) (23 with a tyrosine kinase inhibitor). Among the 68 assessable patients, there were 3 complete responses and 34 partial responses, translating into an overall response rate of 54.4%. After a median follow-up of 36 months [95% confidence interval (CI) 32-40 months] since the start of immunotherapy, 45 (59%) patients have progressed on ICI, with a median progression-free survival (PFS) of 16.3 months (95% CI 8-25 months). Receiving ICI in first line (P = 0.042) and achieving an objective response (P = 0.043) correlated with a better PFS. Median estimated overall survival (OS) from ICI initiation has not been reached. The 12-month and 24-month OS rates were 94% and 81%, respectively.
Conclusions
This registry constitutes the largest available series of ASPS treated with ICI. Our results suggest that the ICI treatment provides long-lasting disease control and prolonged OS in patients with advanced ASPS, an ultra-rare entity with limited active therapeutic options.
Key words: immunotherapy, alveolar soft-part sarcoma, progression-free survival, immune checkpoint
Highlights
-
•
ICIs are active in the treatment of advanced ASPS.
-
•
Achieving an objective radiological response after therapy with ICIs in ASPS significantly correlates with a better outcome.
-
•
This registry is the largest available series on systemic therapies in ASPS.
Introduction
Alveolar soft-part sarcoma (ASPS) is an ultra-rare sarcoma,1 molecularly characterized by the genetic rearrangement t(X;17)(p11;q25), resulting in the ASPSCR1-TFE3 fusion protein.2 This fusion protein acts as an aberrant transcription factor, up-regulating the expression of angiogenesis and cell proliferation-related genes.3 ASPS affects predominantly young patients, and, although it shows an indolent growth pattern, it has a high metastatic potential, with more than half of the patients developing metastasis, mainly in lungs, bones and characteristically in the central nervous system, even many years after initial diagnosis.4,5 ASPS is generally not responsive to classic cytotoxic drugs,5,6 while some activity has been described with vascular endothelial growth factor-based multitargeted tyrosine kinase inhibitors (TKIs), such as pazopanib,6 anlotinib,7 sunitinib8 or cediranib,9,10 the latter two assessed in a recently published randomized phase II study.11
In the past years, several immunomodulatory drugs have been widely developed in oncology, showing improvements in patient survival, especially in melanoma, lung and genitourinary carcinomas.12,13
Immune checkpoint inhibitors (ICIs) have been tested in sarcoma in several small clinical trials. One of the most attractive sarcoma subtypes for immunomodulation with ICIs is ASPS. Hints of activity in this histologic subtype were previously described in small prospective studies including sarcoma patients with different histologic subtypes.14, 15, 16, 17, 18 More recently, a phase II study assessed the efficacy of atezolizumab in a cohort of 49 patients with advanced ASPS, showing objective responses in 24% of the patients. These results have recently granted the Food and Drug Administration (FDA) approval of atezolizumab in this context. Geptanolimab, another programmed cell death protein 1 (PD-1) antibody, was prospectively tested in a cohort of 37 Chinese patients with advanced ASPS, with more than half of the patients free of progression at 6 months.19 The aim of this worldwide registry is to merge the available evidence for the activity of ICI in ASPS in expert sarcoma centers in order to explore its activity in a big cohort of this ultra-rare sarcoma.
Materials and methods
Patients
Patients diagnosed with ASPS, starting therapy with PD-1/programmed death-ligand 1 (PD-L1) inhibitors between October 2014 and October 2021, managed in any of the 18 participating sarcoma expert centers from Europe, Australia and North America and treated with ICI for advanced disease (aged >18 years at the time of start of therapy) were retrospectively reviewed. Pathological diagnosis was confirmed by expert sarcoma pathologist at local site as per institutional guidelines (based on immunohistochemistry and/or molecular demonstration of the expression/rearrangement of TFE3). ICI therapy had a palliative intent. Patients consented for systemic therapy as per institutional guidelines. In all participating centers, the approval from ethics committee was obtained for this international registry as per institutional guidelines.
Data regarding patient demographics, tumor characteristics and details on previous treatments (surgery, systemic therapies, including chemotherapy and TKIs) were retrospectively recorded. Details regarding the context of the ICI treatment (clinical trial or not, line of systemic therapy, type of ICI, regimen—monotherapy or combination), as well as details on the course of treatment {date of start and end, best RECIST 1.1 response,20 date of best response, relevant adverse events [based on Common Terminology Criteria for Adverse Events (CTCAE) version 5.0], reason for treatment discontinuation, date of progression to ICI}, were collected. Information regarding further lines of immunotherapy and survival was also recorded.
Statistical analysis
Progression-free survival (PFS) of each therapy was calculated from the date of start of treatment and estimated by the Kaplan–Meier method. For PFS, the event was considered at the time of radiological progression or death due to any cause, whichever occurs first. In the event of starting any other therapy without evidence of progression, patients were censored for PFS. For overall survival (OS), the event was recorded at the date of the last contact, and it was calculated from the date of start of each treatment and estimated by the Kaplan–Meier method. Comparisons between the variables of interest were carried out by the log-rank test. When including response in the survival analysis, a landmark analysis from the date of response assessment was carried out. Multivariate analysis, with the variables significant in the univariate analysis, was carried out according to the Cox proportional hazards regression model. The validity of proportional hazards assumption was verified by adding a time-dependent variable to each model to confirm that hazard ratio (HR) for each covariate did not increase or decrease over time. Statistical analysis was carried out with SPSS version 26.0 (IBM, Chicago, IL).
Results
Data from 76 patients, with a median age at diagnosis of 25 years (range 3-61 years), were registered. There was a slight predominance of males (1 : 1.22) and 53 patients (70%) were metastatic at diagnosis. The complete demographic characteristics are included in Table 1.
Table 1.
Patient characteristics (n = 76)
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 42 (55) |
| Female | 34 (45) |
| Age at diagnosis (years), median (range) | 25 (3-61) |
| Site of primary tumor | |
| Lower limb | 51 (67) |
| Upper limb | 8 (12) |
| Head and neck | 6 (8) |
| Trunk | 5 (6.5) |
| Pelvis | 2 (2.5) |
| Other | 3 (4) |
| Stage at diagnosis | |
| Localized/locally advanced | 23 (30) |
| Metastatic | 53 (70) |
| Metastasis-free interval, months, median (range) | 13.4 (1-312) |
| Site of metastasis | |
| Lungs | 71 (93) |
| Brain | 12 (16) |
| Number of metastatic sites, median (range) | 2 (1-5) |
| Any previous surgery | |
| No | 29 (38) |
| Yes | 47 (62) |
| Any previous systemic therapy | |
| No | 17 (22) |
| Yes | 59 (78) |
| Number of previous systemic therapies, median (range) | 1 (0-6) |
| Any previous chemotherapy | |
| No | 55 (72) |
| Yes | 21 (28) |
| Any previous TKI | |
| No | 20 (26) |
| Yes | 56 (74) |
| Previous systemic therapy | |
| Sunitinib | 39 |
| Pazopanib | 26 |
| Anthracycline-based chemotherapy | 13 |
| Crizotinib | 2 |
| Gemcitabine-based | 1 |
| Trabectedin | 1 |
| Oral vinorelbine | 1 |
| Ifosfamide-etoposide | 1 |
| Belinostat-bortezomib | 1 |
TKI, tyrosine kinase inhibitor.
Data from previous systemic therapies
Fifty-nine patients (78%) had received previous systemic therapy, including chemotherapy in 21 (28%) and TKIs in 56 (74%), with a median of one previous line (range 0-6). Thirty-nine patients (51%) had previously received sunitinib. Overall response rate (ORR) was 43% (12/29 assessable patients) and the median PFS was 14.8 months [95% confidence interval (CI) 1-31.5 months] (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.102045). Twenty-six patients (34%) had received pazopanib with an ORR of 19% and a median PFS of 9.4 months (95% CI 4.8-14 months).
Data from immune checkpoint inhibitor
All patients received ICI in the context of advanced disease. Therapy started between October 2014 and October 2021. Immunotherapy regimens consisted of anti-PD-1/PD-L1 antibody monotherapy in 38 patients (50%) and combination in other 38 (50%) (23 with a TKI). More details on the regimen are provided in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.102045. Regarding toxicity, in 17 patients (22.3%), there was any adverse event grade 3-4, according to CTCAE, which led to therapy permanent discontinuation in 6 (7.8%) patients [liver enzyme elevation in 2 patients (in 1 case together with pneumonitis) and immune-mediated arthritis, pancreatitis, colitis and skin toxicity in 1 patient each]. More details on grade 3-4 toxicity are depicted in Table 2. In this series, two patients underwent surgery during ICI: in one patient the metastatic disease was resected (and follow-up was censored at the moment of surgery), and in another patient the primary tumor was resected (with complete pathological response in the specimen), but therapy was maintained, as the patient also presented pulmonary unresectable nodules. Another patient received palliative (analgesic) radiotherapy in a non-target lesion.
Table 2.
Grade 3-4 adverse events (based on CTCAE 5.0)
| Adverse event | n (%) |
|---|---|
| Alanine/aspartate aminotransferase increased | 4 (5) |
| Pneumonitis | 3 (4) |
| Arthritis | 2 (3) |
| Pancreatitis | 2 (3) |
| Colitis | 2 (3) |
| Fatigue | 2 (3) |
| Othera | 3 (4) |
Seventeen out of 76 (22.3%) patients presented grade 3-4 toxicity.
CTCAE, Common Terminology Criteria for Adverse Events.
Other: one case of hematological toxicity, one case of immune-mediated skin toxicity, one case of seizures.
Among the 68 RECIST-assessable patients, there were 3 (4.4%) complete responses (CRs) and 34 (50%) partial responses (PRs), translating into an ORR of 54.4%. Fifty-three (78%) patients had discontinued therapy at the time of the current analysis [due to progressive disease in 35 (51%), adverse events in 6 (9%), surgery of all macroscopic disease in 1 (1.5%) and other reasons in 11 (16%) patients]. Among the six patients who withheld therapy due to toxicity, four achieved an objective response (ORR 67%, three PRs, one CR), one patient remained with stable disease (SD) and the response was not evaluable in another. Only one of these patients progressed (duration of response 11.5 months and PFS 13.7 months), 5.8 months after stopping therapy due to immune-mediated pancreatitis. The patient received a rechallenge of therapy (pembrolizumab), with maintained disease control (PFS 34.1 months+) at the time of the last follow-up. The other patients who stopped therapy were free of progression (treatment-free interval) at 7, 10, 21, 22 and 57 months of follow-up.
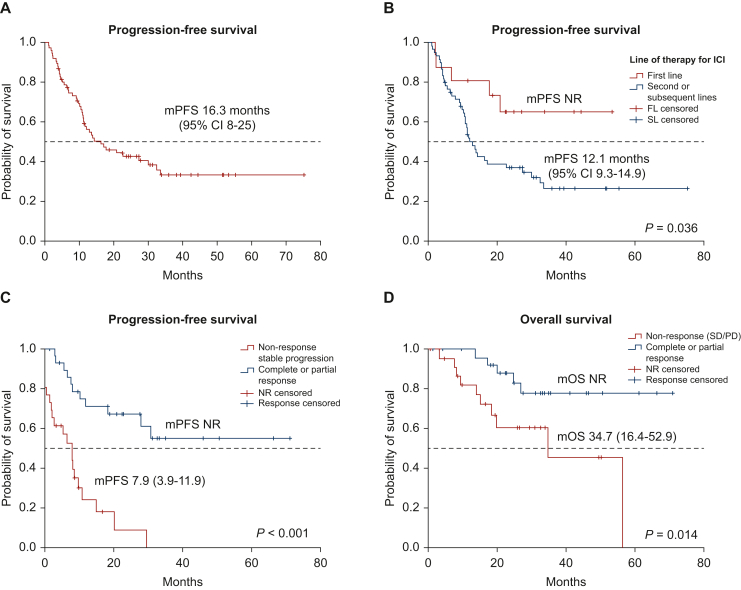
After a median follow-up of 36 months (95% CI 32-40 months) since the start of immunotherapy, 45 (66%) patients have progressed on ICI, with a median PFS of 16.3 months (95% CI 8-25 months) (Figure 1A). PFS was not statistically different between those patients receiving PD-1 inhibitor versus PD-L1 inhibitor (P = 0.18), neither due to the fact of receiving ICI in monotherapy or in combination (P = 0.38) nor according to other clinical factors such as age, stage at diagnosis or presence of brain metastasis (Table 3). However, those patients receiving ICI in first line had a longer median PFS when compared with patients previously treated with other systemic therapy before ICI [median PFS not reached (NR) versus 12.1 months (95% CI 9.3-14.9 months, P = 0.036)] (Figure 1B). We also found a trend toward a better PFS with ICI for those patients not previously treated with TKIs than for those who had already received any TKI drug [median PFS NR versus 12.9 months (95% CI 9.5-16.3 months), P = 0.071].
Figure 1.
Survival analyses. (A) Progression-free survival for all the series (n = 76); (B) progression-free survival according to line of therapy (ICI in first line versus subsequent lines); (C) progression-free survival according to response achieved by RECIST (complete/partial versus SD/PD); and (D) overall survival according to response achieved by RECIST (complete/partial versus SD/PD). CI, confidence interval; FL, first line; ICI, immune checkpoint inhibitor; mOS, median overall survival; mPFS, median progression-free survival; NR, not reached; PD, progressive disease; SD, stable disease; SL, subsequent line.
Table 3.
Univariate analysis of factors related to progression-free and overall survival
| Median PFS from ICI (95% CI) | P | Median OS from ICI (95% CI) | P | |
|---|---|---|---|---|
| Age (years) | 0.18 | 0.37 | ||
| <26 | 12.1 (9.6-14.6) | NR | ||
| ≥26 | 22.6 (6.7-38.5) | 57.9 (NA) | ||
| Sex | 0.29 | 0.36 | ||
| Male | 17.7 (3.7-31.7) | NR | ||
| Female | 12.1 (9.7-14.4) | NR | ||
| Stage at diagnosis | 0.64 | 0.23 | ||
| Localized/LA | 16.3 (6.3-26.4) | 38.3 (16.4-60.3) | ||
| Metastatic | 14.3 (0-28.8) | 57 (NA) | ||
| Brain metastasis | 0.23 | 0.27 | ||
| Yes | 10.9 (8.4-13.3) | 57.9 (NA) | ||
| No | 20.8 (6.5-35.1) | NR | ||
| Previous antiangiogenic | 0.071 | 0.85 | ||
| Yes | 12.9 (9.5-16.3) | NR | ||
| No | NR | NR | ||
| Previous lines | NR 12.1 (9.3-14.9) |
0.036 | NR NR |
0.67 |
| 0 | ||||
| 1 or more | ||||
| Regimen | 0.38 | 0.60 | ||
| Monotherapy | 12.7 (9-16) | NR | ||
| Combination | 16.8 (8-26) | 57 (25-89) | ||
| Type of ICI | 0.18 | 0.17 | ||
| Anti-PD-1 | 14.3 (7.7-20.8) | NR | ||
| Anti-PD-L1 | NR | NR | ||
| RECIST best response (n = 56 landmark analysis) | <0.001 | 0.014 | ||
| CR/PR | NR | NR | ||
| SD/PD | 7.9 (3.9-11.9) | 34.7 (16.4-52.9) |
CR, complete response; ICI, immune checkpoint inhibitor; LA, locally advanced; NA, not available; NR, not reached; OS, overall survival; PD, progressive disease; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; PR, partial response; SD, stable disease.
Among those patients assessable by RECIST (n = 68, n = 56 for landmark analysis), the fact of achieving an objective response (CR/PR) correlated with a significantly better PFS when compared with those patients not responding [median PFS NR versus 7.9 months (95% CI 3.9-11.9 months), P < 0.001] (Figure 1C). Receiving ICI beyond the first line of advanced disease (HR 3.48, P = 0.042) and achieving an objective RECIST response on ICI (HR 0.44, P = 0.043) were found to be statistically significantly related to PFS in the multivariate analysis. In the univariate analysis, toxicity grade 3-4 did not significantly correlate with response: among patients experiencing grade 3-4 toxicity ORR was 65% (11/17) and in the other patients it was 44% (25/57), P = 0.31.
After a median follow-up of 36 months (95% CI 32-40 months) since the start of immunotherapy, 20 (26%) patients have died. The median estimated OS from ICI was NR. The 12-month and 24-month OS rates were 94% (95% CI 89% to 100%) and 81% (95% CI 72% to 90%), respectively. OS was not significantly impacted by the type of ICI (PD-1 inhibitor versus PD-L1 inhibitor, P = 0.17), or the fact of receiving ICI in first versus subsequent line of therapy (P = 0.67), but the achievement of an objective response was related to an statistically significant increase in OS [NR versus 34.7 months (95% CI 16.4-52.9 months, P = 0.014)] (Table 3, Figure 1D).
Sixteen patients received a subsequent line of immunotherapy after progressing to the first-line ICI. In 13 patients (81%), the regimen was a combination (8 with another ICI, 5 with a TKI), while 3 patients received ICI in monotherapy (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.102045).
With a median follow-up of 23.7 months (95% CI 3.1-59.3 months) since the start of second line of immunotherapy, nine (56%) patients have progressed, with a median PFS of 9.9 months (95% CI 4-15.7 months). Information about RECIST response was available in nine (56%) patients: three (33%) obtained a PR, four (44%) SD and two (22%) patients progressed as their best response. At the time of the current analysis, five (31%) of these patients have died, with a median OS from the start of second line of immunotherapy of 43 months (95% CI 26.3-59.7 months).
Discussion
Here we present the results of the first international retrospective registry on the efficacy of ICI in ASPS. To our knowledge, this is the largest series ever communicated in this histology, exploring the activity of systemic therapy in general and immunotherapy specifically. As expected for this disease, patients were young at diagnosis, the primary tumor arose in the lower limb in the majority of cases and almost three-quarters of the population harbored metastasis at diagnosis, confirming its aggressiveness.
Not surprisingly, three-quarters of the patients included in this series had previously received any TKI drug, highlighting the relevance of this therapeutic family in the management of ASPS. Up to now, TKIs were the drugs with the best data of activity in ASPS, with reported median PFS ranging from 10.1 to 23.6 months in the existing small series and ORR ranging from 19.3% to 55%.6, 7, 8,21, 22, 23 Data of efficacy from TKIs in the current series are in line with those previously reported, supporting the consistency of our data. In our series, ICI showed a median PFS longer than 16 months and an ORR of 54.4%, which seem in line with the results obtained with TKIs in previous series. It is important to highlight that almost 75% of our patients had been previously treated with a TKI, and, in those patients receiving ICI in the context of first line, the median PFS with ICI was NR at the time of the current analysis. Patients with previous exposure to TKIs could potentially experience less benefit from ICIs. This is something already shown in melanoma with ICI following therapy with BRAF/MEK inhibitors,24,25 and could be related to the modulation of tumor microenvironment. One of the potential mechanisms related to secondary resistance to antiangiogenic drugs is related to macrophages, which could activate mechanisms of immune evasion.26 In any case, direct prospective comparisons between ICI and TKIs would be needed to elucidate the superiority of any of these families of drugs in this disease, as well as the best sequence. The fact that in our series combination regimens did not show superiority when compared with ICI monotherapy (with the limitations of our small size), as well as the activity data on subsequent lines of immunotherapy, could suggest that sequential therapy with immunotherapy and TKIs could be the best approach for the treatment of advanced ASPS. Another interesting finding of our series was that an objective response obtained during the therapy with ICI correlated with a better PFS and OS when compared with those patients not achieving responses. Dimensional tumor reductions induced by therapy are a surrogate of efficacy of cancer therapies,27 of special interest in entities with indolent biological behavior, such as ASPS, in which disease control results could be influenced by the nature of the disease. Similar findings (correlation between PFS and ORR) were obtained, for example, in a prospective study on pazopanib in extraskeletal myxoid chondrosarcoma, another ultra-rare sarcoma with indolent natural history,28 and in a phase II study assessing the combination of sunitinib and nivolumab in selected sarcoma subtypes.14
The results of this series confirm the previous findings reported in literature and establish the activity of ICI in ASPS, collected in retrospective series or as subgroup analysis of small trials including several sarcoma subtypes (Table 4). These studies reported ORRs ranging from 7.1% to 75% and median PFS ranging from 6.9 to 23.06 months.14, 15, 16,19,29, 30, 31, 32, 33 Two published clinical trials have assessed the activity of ICI specifically in ASPS. The first study explored the activity of PD-1 inhibitor geptanolimab, in a cohort of 37 Chinese patients with advanced ASPS. In that series, median PFS was 6.9 months (95% CI 5 months-NR) and one-third of patients (37.8%) achieved an objective response.19 More recently, the results from an academic phase II study developed in USA institutions tested the activity of atezolizumab in 52 patients. The results from this study (ORR 37%, median PFS 20.8 months) have granted the FDA approval of atezolizumab for patients with advanced ASPS.34
Table 4.
Reported results on immune checkpoint inhibitors in alveolar soft-part sarcoma
| Regimen | Type of study | n | mPFS (months) | 3-month PFS | 6-month PFS | ORR (RECIST) | mOS (months) | Ref |
|---|---|---|---|---|---|---|---|---|
| Nivolumab-ipilimumab | Phase II trial including several sarcoma subtypes | 2 (1 monotherapy arm, 1 combo) | NA | NA | NA | 1/2 (50%) in nivolumab arm | NA | D’ Angelo et al.16 |
| Axitinib-pembrolizumab | Phase II trial including several sarcoma subtypes | 12 | 12.4 | 72.7% | 38.1% | 6/11 (54.5%) | NR | Wilky et al.15 |
| Nivolumab-sunitinib | Phase I/II trial including several sarcoma subtypes | 7 | 11.3 | NA | NA | 57% | NR | Martin-Broto et al.14 |
| Toripalimab | Phase I trial including several tumors | 12 | 11.1 | ∼80% | ∼75% | 25% | 34.7 | Yang et al.29 |
| Geptanolimab | Phase II trial ASPS only | 37 | 6.9 | 70.3% | 56.1% | 37.8% | NA | Shi et al.19 |
| Nivolumab | Phase II study, with a cohort of ASPS | 14 | 6.0 | NA | NA | 7.1% | NA | Kawai, CTOS 2020, unpublished data |
| Atezolizumab | Phase II trial, ASPS only | 52 | 20.8 | NA | NA | 37% | NA | Chen et al.34 |
| TQB2450-anlotinib | Phase II trial including several sarcoma subtypes | 12 | 23.06 | NA | NA | 75% | NR | Liu et al.30 |
| Durvalumab-tremelimumab | Phase II trial, including several sarcoma subtypes, one cohort of ASPS | 10 | 80% | 40% | Somaiah et al.31 | |||
| Immunotherapy | Retrospective series, single center | 4 | NA | NA | NA | 50% | NA | Groisberg et al.32 |
| PD-1 inhibitor + antiangiogenic | Retrospective series, single center | 7 | NA | NA | NA | 28.6% | NA | You et al.33 |
| Current series | Retrospective series, ASPS only | 76 | 16.3 | 54.4 | NR |
ASPS, alveolar soft-part sarcoma; mPFS, median progression-free survival; NA, not available; NR, not reached; ORR, overall response rate; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
Currently, there are several studies, open to recruitment, assessing the efficacy of ICI in ASPS (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.102045). The Axiom study (NCT05333458), ongoing in the USA, explores atezolizumab as monotherapy or in combination with selinexor in ASPS, and IMMUNOSARC (NCT03277924), an European study, sponsored by the Spanish Group for Research in Sarcoma (GEIS), is assessing the activity of the combination of sunitinib and nivolumab in several sarcoma cohorts, one of them being ASPS. The prospective information of these trials will be very useful for a better definition of the role of ICI in this ultra-rare disease.
The underlying mechanism for the efficacy of ICI in ASPS is still unclear. ASPS is a translocation-related sarcoma, and consistent with this, the majority of ASPS harbor a stable molecular background, with low tumoral mutation burden (TMB),35 although isolated cases with high TMB have also been reported.36 PD-L1 expression has been reported in 29.7%-100% of ASPS samples,15,19,31 as well as T-lymphocyte infiltration. However, the lack of a clear correlation among these factors and PFS or OS after therapy with ICI do not confirm its predictive role.15,31
Microsatellite instability (MSI) is also a molecular predictive factor for response to ICI. MSI has been described in a proportion of patients with ASPS. Loss of expression of MSH2 or MLH1 genes was observed in 18.2% and 27.3% of cases in a series of 11 ASPS patients.37 Mutational signatures in the mismatch repair (MMR) deficiency pathway were detected in five out of seven cases of ASPS analyzed in another small series.38 Interestingly, two of these patients had received and responded to ICI. However, protein expression of MLH1, MSH2, MSH6 and PMS2 was preserved in all cases, suggesting that this MMR deficiency could be related to the non-canonical components of this pathway.
Finally, the product of the ASPS-TFE3 rearrangement could also be directly involved in the immune response of ASPS patients, explaining the predisposition of this histologic subtype for immune-modulating strategies. TFE3 acts as a transcription factor, which is directly involved in the activation of CD40 expression, a key factor in the T-cell-dependent antibody response.39 Additionally, it is also related to transforming growth factor-β, a wide-spectrum immune regulator.40 Despite all these hypotheses, much is still to be unveiled in the mechanisms of action of ICI in ASPS, and this could only be done through well-designed correlative studies within specific clinical trials, probably in the context of international collaborations.
This registry constitutes the largest available series of ASPS treated with ICI. Our results suggest that treatment with ICI provides long-lasting disease control and prolonged OS in patients with advanced ASPS, an ultra-rare entity with limited active therapeutic options.
Acknowledgements
DSM is a recipient of a Sara Borrell postdoctoral fellowship funded by the National Institute of Health Carlos III (ISCIII) (CD20/00155). The authors thank SELNET project. SELNET has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 825806.
Funding
None declared.
Disclosure
NH reports grants, personal fees and non-financial support from PharmaMar, personal fees from Lilly and Tecnopharma, grants from Eisai and Novartis, outside the submitted work and research funding for clinical studies (institutional) from PharmaMar, Eli Lilly and Company, AROG, Bayer, Eisai, Lixte, Karyopharm, Deciphera, GSK, Novartis, Blueprint, Nektar, Forma, Amgen and Daichii-Sankyo. RH reports travel grants from Lilly, Novartis and PharmaMar as well as personal fees from Lilly and PharmaMar outside of the submitted work. PR has received honoraria for lectures and advisory boards from BMS, MSD, Novartis, Pierre Fabre, Philogen, Astra Zeneca, Merck and Sanofi outside of the scope of this study. MB declares honoraria from Bayer, Amgen, Pharmamar and Deciphera outside of the scope of this study. AR reports honoraria and advisory/consultancy (MSD, AstraZeneca, GSK and PharmaMar); research grant/funding to his institution (Eisai, PharmaMar and Roche); travel/accommodation/expenses (AstraZeneca, PharmaMar and Roche); and speakers bureau (MSD, AstraZeneca, GSK, Clovis and PharmaMar), outside the submitted work. DSM reports institutional research grants from PharmaMar, Eisai, Immix BioPharma and Novartis outside the submitted work; travel support from PharmaMar, Eisai, Celgene, Bayer and Pfizer; and personal fees from Tecnopharma, outside the submitted work. JMB reports research grants from PharmaMar, Eisai, Immix BioPharma and Novartis outside the submitted work; honoraria for advisory board participation and expert testimony from PharmaMar, Eli Lilly and Company, Bayer, GSK, Novartis, Boehringer Ingelheim, Amgen, Roche, Tecnofarma and Asofarma; and research funding for clinical studies (institutional) from PharmaMar, Eli Lilly and Company, BMS, Pfizer, AROG, Bayer, Eisai, Lixte, Karyopharm, Deciphera, GSK, Celgene, Novartis, Blueprint, Adaptinmune, Nektar, Forma, Amgen, Daichii-Sankyo, Ran Therapeutics, INHIBRX, Ayala Pharmaceuticals, Philogen, Cebiotex, PTC Therapeutics, Inc. and SpringWorks Therapeutics. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Stacchiotti S., Frezza A.M., Blay J.Y., et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127(16):2934–2942. doi: 10.1002/cncr.33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladanyi M., Lui M.Y., Antonescu C.R., et al. The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene. 2001;20(1):48–57. doi: 10.1038/sj.onc.1204074. [DOI] [PubMed] [Google Scholar]
- 3.Lazar A.J., Das P., Tuvin D., et al. Angiogenesis-promoting gene patterns in alveolar soft part sarcoma. Clin Cancer Res. 2007;13(24):7314–7321. doi: 10.1158/1078-0432.CCR-07-0174. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman P.H., Brennan M.F., Kimmel M., et al. Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer. 1989;63(1):1–13. doi: 10.1002/1097-0142(19890101)63:1<1::aid-cncr2820630102>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Portera C.A., Jr., Ho V., Patel S.R., et al. Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer. 2001;91(3):585–591. doi: 10.1002/1097-0142(20010201)91:3<585::aid-cncr1038>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Stacchiotti S., Mir O., Le Cesne A., et al. Activity of pazopanib and trabectedin in advanced alveolar soft part sarcoma. Oncologist. 2018;23(1):62–70. doi: 10.1634/theoncologist.2017-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi Y., Fang Z., Hong X., et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. 2018;24(21):5233–5238. doi: 10.1158/1078-0432.CCR-17-3766. [DOI] [PubMed] [Google Scholar]
- 8.Stacchiotti S., Negri T., Zaffaroni N., et al. Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol. 2011;22(7):1682–1690. doi: 10.1093/annonc/mdq644. [DOI] [PubMed] [Google Scholar]
- 9.Kummar S., Allen D., Monks A., et al. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol. 2013;31(18):2296–2302. doi: 10.1200/JCO.2012.47.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judson I., Scurr M., Gardner K., et al. Phase II study of cediranib in patients with advanced gastrointestinal stromal tumors or soft-tissue sarcoma. Clin Cancer Res. 2014;20(13):3603–3612. doi: 10.1158/1078-0432.CCR-13-1881. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen J., Takebe N., Kummar S., et al. Randomized phase II trial of sunitinib or cediranib in alveolar soft part sarcoma. Clin Cancer Res. 2023;29(7):1200–1208. doi: 10.1158/1078-0432.CCR-22-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40(2):127–137. doi: 10.1200/JCO.21.02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Broto J., Hindi N., Grignani G., et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: a multicenter, single-arm, phase Ib/II trial. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilky B.A., Trucco M.M., Subhawong T.K., et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019;20(6):837–848. doi: 10.1016/S1470-2045(19)30153-6. [DOI] [PubMed] [Google Scholar]
- 16.D’Angelo S.P., Mahoney M.R., Van Tine B.A., et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19(3):416–426. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariuk-Jarema A., Koseła-Paterczyk H., Rogala P., et al. A durable complete response to immunotherapy in a patient with metastatic alveolar soft part sarcoma. Tumori. 2020;106(6):NP9–NP13. doi: 10.1177/0300891620928133. [DOI] [PubMed] [Google Scholar]
- 18.Conry A., Peters M., Fried D.B., et al. Complete response to dual immunotherapy in a young adult with metastatic alveolar soft part sarcoma enabled by a drug recovery program in a community practice. J Adolesc Young Adult Oncol. 2020;9(3):449–452. doi: 10.1089/jayao.2019.0113. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y., Cai Q., Jiang Y., et al. Activity and safety of geptanolimab (GB226) for patients with unresectable, recurrent, or metastatic alveolar soft part sarcoma: a phase II, single-arm study. Clin Cancer Res. 2020;26(24):6445–6452. doi: 10.1158/1078-0432.CCR-20-2819. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Fan Z., Li S., et al. Target therapy for metastatic alveolar soft part sarcoma: a retrospective study with 47 cases. Ann Transl Med. 2020;8(22):1493. doi: 10.21037/atm-20-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Judson I., Morden J.P., Kilburn L., et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): a double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol. 2019;20(7):1023–1034. doi: 10.1016/S1470-2045(19)30215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagodzińska-Mucha P., Świtaj T., Kozak K., et al. Long-term results of therapy with sunitinib in metastatic alveolar soft part sarcoma. Tumori. 2017;103(3):231–235. doi: 10.5301/tj.5000617. [DOI] [PubMed] [Google Scholar]
- 24.Atkins M.B., Lee S.J., Chmielowski B., et al. Combination dabrafenib and trametinib versus combination nivolumab and ipilimumab for patients with advanced BRAF-mutant melanoma: the DREAMseq trial-ECOG-ACRIN EA6134. J Clin Oncol. 2023;41(2):186–197. doi: 10.1200/JCO.22.01763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas L., Elewaut A., Gerard C.L., et al. Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat Cancer. 2021;2(7):693–708. doi: 10.1038/s43018-021-00221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalton H.J., Pradeep S., McGuire M., et al. Macrophages facilitate resistance to anti-VEGF therapy by altered VEGFR expression. Clin Cancer Res. 2017;23(22):7034–7046. doi: 10.1158/1078-0432.CCR-17-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon B.J., Loong H.H., Summers Y., et al. Correlation between treatment effects on response rate and progression-free survival and overall survival in trials of targeted therapies in molecularly enriched populations. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stacchiotti S., Ferrari S., Redondo A., et al. Pazopanib for treatment of advanced extraskeletal myxoid chondrosarcoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20(9):1252–1262. doi: 10.1016/S1470-2045(19)30319-5. [DOI] [PubMed] [Google Scholar]
- 29.Yang J., Dong L., Yang S., et al. Safety and clinical efficacy of toripalimab, a PD-1 mAb, in patients with advanced or recurrent malignancies in a phase I study. Eur J Cancer. 2020;130:182–192. doi: 10.1016/j.ejca.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Gao T., Tan Z., et al. Phase II study of TQB2450, a novel PD-L1 antibody, in combination with anlotinib in patients with locally advanced or metastatic soft tissue sarcoma. Clin Cancer Res. 2022;28(16):3473–3479. doi: 10.1158/1078-0432.CCR-22-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somaiah N., Conley A.P., Parra E.R., et al. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: a single-centre phase 2 trial. Lancet Oncol. 2022;23(9):1156–1166. doi: 10.1016/S1470-2045(22)00392-8. [DOI] [PubMed] [Google Scholar]
- 32.Groisberg R., Hong D.S., Behrang A., et al. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J Immunother Cancer. 2017;5(1):100. doi: 10.1186/s40425-017-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You Y., Guo X., Zhuang R., et al. Activity of PD-1 inhibitor combined with anti-angiogenic therapy in advanced sarcoma: a single-center retrospective analysis. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.747650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen A.P., Sharon E., O’Sullivan-Coyne G., et al. Atezolizumab for advanced alveolar soft part sarcoma. N Engl J Med. 2023;389(10):911–921. doi: 10.1056/NEJMoa2303383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groisberg R., Roszik J., Conley A.P., et al. Genomics, morphoproteomics, and treatment patterns of patients with alveolar soft part sarcoma and response to multiple experimental therapies. Mol Cancer Ther. 2020;19(5):1165–1172. doi: 10.1158/1535-7163.MCT-19-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Liu Y., Qu Y., et al. Case report: Two cases of soft-tissue sarcomas: high TMB as a potential predictive biomarker for anlotinib combined with toripalimab therapy. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.832593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito T., Oda Y., Kawaguchi K., et al. Possible association between tumor-suppressor gene mutations and hMSH2/hMLH1 inactivation in alveolar soft part sarcoma. Hum Pathol. 2003;34(9):841–849. doi: 10.1016/s0046-8177(03)00343-5. [DOI] [PubMed] [Google Scholar]
- 38.Lewin J., Davidson S., Anderson N.D., et al. Response to immune checkpoint inhibition in two patients with alveolar soft-part sarcoma. Cancer Immunol Res. 2018;6(9):1001–1007. doi: 10.1158/2326-6066.CIR-18-0037. [DOI] [PubMed] [Google Scholar]
- 39.Huan C., Kelly M.L., Steele R., Shapira I., Gottesman S.R., Roman C.A. Transcription factors TFE3 and TFEB are critical for CD40 ligand expression and thymus-dependent humoral immunity. Nat Immunol. 2006;7(10):1082–1091. doi: 10.1038/ni1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hua X., Miller Z.A., Benchabane H., Wrana J.L., Lodish H.F. Synergism between transcription factors TFE3 and Smad3 in transforming growth factor-beta-induced transcription of the Smad7 gene. J Biol Chem. 2000;275(43):33205–33208. doi: 10.1074/jbc.C000568200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.