Key Points
-
•
The bleeding phenotype of patients with mild hemophilia A is heterogeneous according to the mean lifelong FVIII level.
-
•
We identified the trough FVIII levels needed to prevent lifelong joint bleeds and at each quartile of age.
Visual Abstract
Abstract
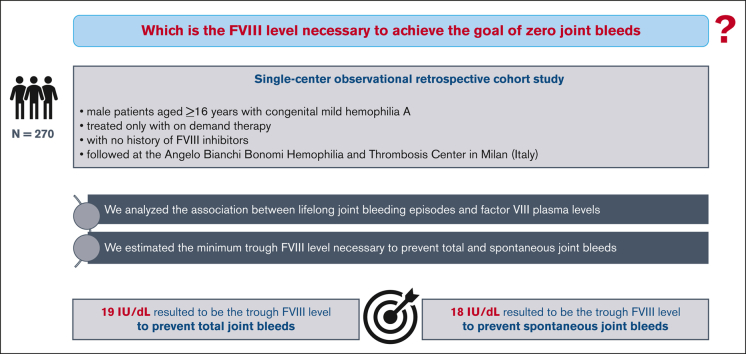
The severity of the bleeding phenotype in patients with hemophilia A (HA) broadly correlates with the degree of coagulation factor VIII (FVIII) deficiency in plasma. However, the FVIII level necessary to achieve the goal of zero joint bleeds remains unclear. This study aimed to identify the minimum FVIII level necessary to prevent joint bleeds in patients with HA. In this retrospective study, patients with congenital mild HA treated on demand, aged ≥16 years, with no history of FVIII inhibitors, followed at the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center in Milan, were enrolled. We investigated 270 male patients with a median age of 45 years (16-88) and median lifelong FVIII of 21 IU/dL. One hundred patients (37%) had a lifelong history of at least 1 joint bleed. The mean annualized joint bleeding rate (AJBR) and spontaneous AJBR were 0.016 (standard deviation [SD], 0.032) and 0.001 (SD, 0.010), respectively. After adjusting for age, for each IU/dL increase in FVIII, there was a 6% reduction in AJBR and an 11% reduction in spontaneous AJBR. The minimum FVIII levels needed to prevent lifelong any joint bleeds and spontaneous joint bleeds resulted to be 19.2 IU/dL and 17.7 IU/dL, respectively. In this large cohort of persons with mild HA, we identified the minimum FVIII levels needed to prevent total and spontaneous joint bleeds (19.2 IU/dL and 17.7 IU/dL, respectively). These findings could suggest important implications for the accurate design of prophylactic therapies for persons with moderate and severe HA, including gene therapy.
Introduction
Hemophilia A (HA) is an inherited bleeding disorder caused by the deficiency or dysfunction of coagulation factor VIII (FVIII). Low FVIII in plasma causes decreased thrombin generation, leading to defective clot formation and bleeding episodes, which mainly affect joints and muscles.1 The bleeding phenotype in HA (ie, onset, frequency, and severity of bleeds) broadly correlates with the degree of FVIII deficiency. Patients with severe HA (plasma FVIII levels <1 IU/dL) have spontaneous hemorrhages and bleed after minor trauma starting early in life, whereas patients with moderate and mild HA (FVIII between 1 and 5 and between 6 and 40 IU/dL) usually bleed later in life and mostly after trauma or surgery. Moreover, the annualized joint bleeding rate (AJBR), a widely used index of the severity of bleeding phenotype, decreases with increasing baseline FVIII.2, 3, 4, 5
In recent years, the management of HA has strikingly changed, improving the patient’s quality of life and life expectancy.6 Extended half-life (EHL) FVIII products, as well as nonreplacement strategies, such as emicizumab, have become therapeutic options.7, 8, 9 In this changing landscape, gene therapy may allow to achieve a continuous expression of the FVIII transgene for several years in patients with severe disease. Gene therapy is designed to provide constitutively expressed endogenous factor VIII at consistent levels, mimic a mild HA phenotype, provide better protection from bleeding than exogenous factor VIII prophylaxis, and eliminate the need for frequent infusions.10 However, it is not clear which FVIII level is necessary to achieve the goal of zero joint bleeds, and thus, the target to be achieved in the frame of prophylaxis regimens and gene therapy.11, 12, 13 In a Dutch cohort of 411 persons with HA (122 with mild HA), the number of joint bleeds decreased to ∼0 in patients with FVIII levels >12 IU/dL.2 Another nationwide Dutch study enrolling 119 persons with moderate HA and 314 with mild HA treated on demand suggested that those with residual FVIII levels ≥15 IU/dL experienced no joint bleeds.14 A very large multicenter US study that collected data on 3315 persons with mild or moderate HA without inhibitors receiving demand factor replacement therapy showed that a target FVIII of 15 IU/dL was insufficient to prevent joint bleeds.15 However, the wide heterogeneity in the study design and samples, including those with moderate or severe HA, limits the comparison and consistency of the findings. Indeed, severe, moderate, and mild HA are phenotypically different diseases characterized by different lifelong bleeding tendencies. Persons with severe or moderate HA on prophylaxis show FVIII troughs and peaks, whereas steady-state FVIII activity levels characterize persons with mild disease. Moreover, arthropathic joints exhibiting vascular remodeling are more prevalent in persons with severe or moderate HA and contribute to an increased joint bleeding frequency.16
Other studies have investigated the association between FVIII levels and bleeding frequency in persons with moderate or severe HA on prophylaxis, reporting controversial findings. During prophylaxis, the risk of bleeding significantly increases with time when factor VIII activity is <1% and a higher level may prevent bleeds in most patients.17 However, it is not possible to identify a trough FVIII level that can prevent bleeds in all patients receiving prophylaxis, and a personalized approach is required. Indeed, the patient bleeding tendency changes over time and is affected lifelong by several factors, such as age, variability in individual pharmacokinetics, and physical activity, and is mainly due to joint disease (presence or absence of synovitis and vascular remodeling).16,18, 19, 20
There are suggestions by some authors, associations of patients and pharmaceutical companies that higher FVIII thresholds are necessary for gene therapy, potentially requiring a higher dose of adeno-associated virus (AAV) vectors. However, this may increase the risk of liver toxicity.21,22 Based on these controversial findings and related open questions, the FVIII level required to prevent joint bleeds remains undefined. Persons with mild HA are suitable natural models to answer this important question. This study was conducted to analyze the joint bleeding frequency observed lifelong according to FVIII levels in a large cohort of persons with mild HA, with the goal to identify the minimum level necessary to prevent joint bleeding.
Methods
Study design, patients, and definitions
In this single-center retrospective cohort study, male patients aged ≥16 years in August 2021 (data collection starting date) with a diagnosis of congenital mild HA, treated only with on-demand therapy and followed at the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center in Milan until the end of July 2021 (study end date) were enrolled. Patients with other coagulation disorders, history of FVIII inhibitors, missing data on FVIII levels, or joint bleeding phenotypes were excluded. The diagnosis of congenital mild HA was based on FVIII plasma levels between 6 and 40 IU/dL, as measured by a 1-stage or chromogenic clotting assay, in the presence of normal von Willebrand factor levels (Ag and RCo) and normal FVIII:VWF binding assay.
Data on age, blood group (0 or non-0), FVIII levels, and bleeds were collected from clinical records. All FVIII levels measured lifelong by 1-stage clotting assays were recorded under baseline conditions (ie, in the absence of an acute bleed and a state of inflammation, and after more than 1 week from any previous FVIII treatment). All joint bleeds that occurred lifelong were recorded, as well as all the other major and clinically relevant non-major bleeds (CRNMB) that occurred between January 2005 and the last follow-up visit (within the study end date). For each bleed, the date, type, situation (spontaneous or trauma-, surgery-, or dental procedure-related), and related treatments were recorded.
Major bleeding episodes and CRNMB were defined according to the International Society on Thrombosis and Hemostasis (ISTH) criteria.23 Major bleeding episodes were bleeds with a symptomatic presentation: i) fatal, and/or ii) occurring in a critical area or organ, and/or iii) causing a drop in hemoglobin level of at least 2 g/dL, or leading to the transfusion of at least 2 units of whole blood or red cells. CRNMB was defined as any bleeding episode that failed to meet the criteria for major bleeds but presented with at least 1 of the following criteria: requiring medical intervention by a health care professional, leading to hospitalization, increasing the level of care, or prompting a face-to-face evaluation.
Two different AJBRs were estimated, including total and spontaneous joint bleeds. For both rates, patient’s life duration (age at the last follow-up) was considered as the observation time. Two additional annualized bleeding rates (ABRs) were estimated, including only other than joint bleeds (major or CRNMB) and including all (joint and other bleeds). For both types, ABR was also estimated only for spontaneous bleeds, with a total of 4 ABRs. For all ABRs and each patient, the period from January 2005 to the last follow-up was the observation time.
Statistical analysis
Categorical variables were expressed as counts and percentages, and continuous variables as means and standard deviations (SDs). For each patient, the mean of all FVIII levels measured lifelong was calculated and considered the study exposure. The 2 primary endpoints (all and spontaneous joint bleeds) were categorized as “at least 1 total/spontaneous joint bleed that occurred lifelong” and their association with exposure was assessed using a logistic regression model. Considering that the patients had different ages at the last follow-up and thus different observation periods, the joint bleeding endpoint was also categorized according to the population age distribution. When the 2 primary endpoints were considered continuous variables (AJBRs), 4 count data models with offset adjustment were tested: Poisson regression model, negative binomial regression model (NB), zero-inflated Poisson regression model, and zero-inflated negative binomial regression model. Among these models, a selection was carried out to assess the model goodness-of-fit using the Akaike’s Information Criterion value. The most suitable model to explain the variability in joint bleeding frequency as a function of the mean FVIII level resulted to be NB. The association between the mean FVIII level and joint bleeding was assessed in terms of incidence rate ratios, such as the AJBRR (annualized joint bleeding rate ratio). The spontaneous AJBR was explored using the same count data model. The relationships between AJBRR (all and spontaneous) and FVIII coagulation activity were also adjusted for age and blood group. The aforementioned analyses using the NB model were also performed for all bleeds (joint and other) in terms of the ABRR (annualized bleeding rate ratio). For the 2 categorized first endpoints (at least 1 total/spontaneous joint bleed occurred lifelong), receiver operating characteristic (ROC) curve analysis was performed to assess which FVIII level distinguished better patients with and without the aforementioned endpoints. The sensitivity and specificity of the different FVIII levels were also calculated. The area under the ROC curve (AUC) with 95% confidence interval (CI) was used to estimate the predictive capacity of the logistic model. The Youden Index was used to estimate which FVIII level was the optimal cutoff for optimizing both sensitivity and specificity in the model. Using ROC analyses, the minimum FVIII level needed to prevent total and spontaneous joint bleeds was estimated for each quartile of age. The analyses were performed using JMP Pro16 (SAS Institute Inc, Cary, NC) and R 4.1.0. Graphs were generated using the ggplot2 package. Negative Binomial analyses were performed using the MASS package and zero-inflated models were performed using the pscl package. Further details are reported in the supplemental material.
The study was approved by the institutional ethics committee and written informed consent was obtained from all subjects, in accordance with the Declaration of Helsinki.
Results
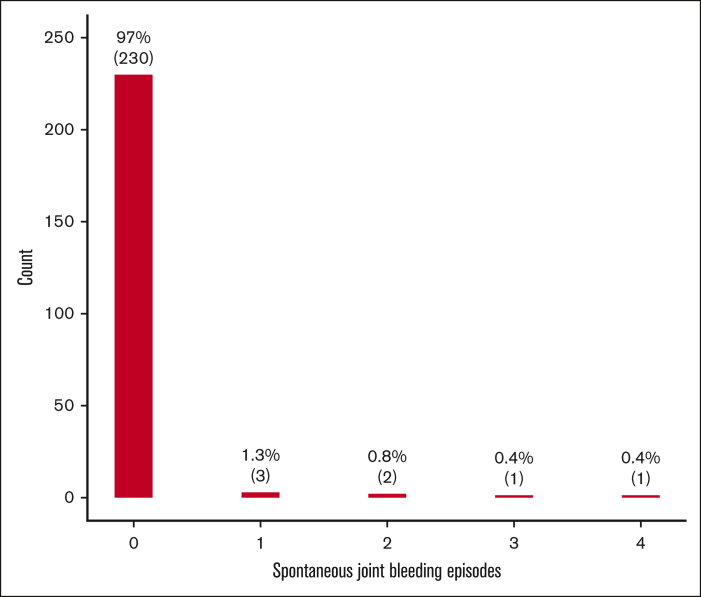
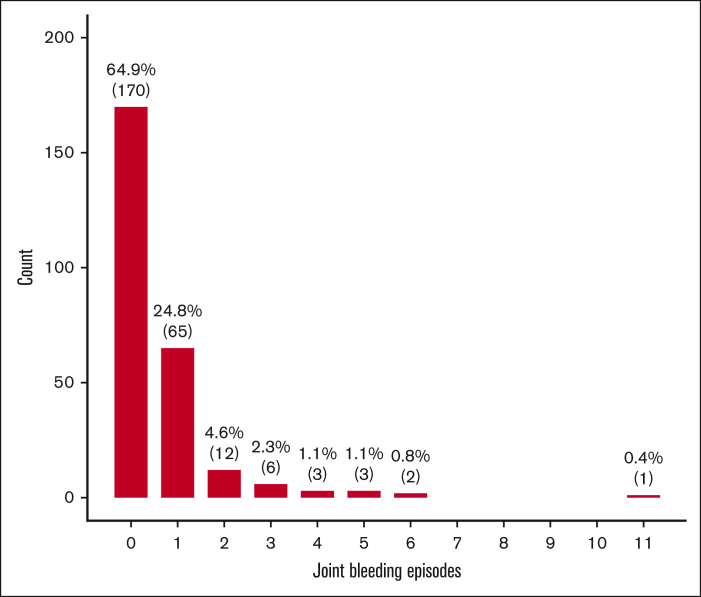
Among the entire cohort of persons with mild HA (n=442), 270 (61%) were eligible for this study, with a median age at the last follow-up of 45 years (interquartile range [IQR], 28-59) and a median lifelong FVIII level of 21 IU/dL (IQR, 14-32). One hundred of them (37%) had a history of at least 1 joint bleed in life and only 10 (4%) had a history of at least 1 spontaneous joint bleed. Moreover, the first and fourth age quartiles (<28 and ≥59 years) showed the highest proportions of joint bleeds (Table 1). The mean annualized total and spontaneous joint bleeding rates (AJBR) were 0.016 (SD, 0.032) and 0.001 (SD, 0.010), respectively. The distributions of both total and spontaneous joint bleeds were highly skewed to the right, with a large proportion of zero values (Figures 1-2). Lower mean FVIII levels were observed in patients with a history of at least 1 lifelong joint bleed than in those with no previous joint bleeds [mean difference: –9 IU/dL (95% CI, –11.7 to –6.2)] (Table 2). The logistic regression model showed a 7% and 12% reduction in total and spontaneous joint bleeding odds, respectively, for each IU/dL increase in FVIII after adjusting for age. When analyzing the endpoint as a continuous variable by mean of the NB regression model, for each unitary increase of FVIII there was a 6% reduction of AJBR and an 11% reduction of spontaneous AJBR after adjusting for age. In the multivariate models adjusted for blood group, the effect of FVIII levels on spontaneous joint bleeds increased, but with a more limited sample size (168 patients had data on blood group) (Table 3).
Figure 2.
Barplot of the number of spontaneous joint bleeds (n=237) in persons with mild HA.
Table 1.
Main patient characteristics and joint bleeding phenotype
| N=270 | |
|---|---|
| Age at last follow-up, mean (SD), y | 45.6 (19.9) |
| FVIII:C, mean (SD), IU/dL∗ | 23.5 (12.1) |
| Time of observation for other bleeds, mean (SD), y† | 13.4 (3.8) |
| No zero blood group, n (%)‡ | 115 (59.9) |
| All joint bleeding episodes | |
| At least 1 joint bleeding episode in life, n (%) | 100 (37) |
| At least 1 joint bleeding episode in the 1st quartile of age (<28), n (%)§ | 60 (22.6) |
| At least 1 joint bleeding episode in the 2nd quartile of age (28 ≤ x < 45), n (%)‖ | 25 (12.3) |
| At least 1 joint bleeding episode in the 3rd quartile of age (45 ≤ x < 59), n (%)¶ | 10 (7.7) |
| At least 1 joint bleeding episode in the 4th quartile of age (≥59), n (%)# | 14 (21.2) |
| Spontaneous joint bleeding episodes | |
| At least 1 spontaneous joint bleeding episode in life, n (%)∗∗ | 10 (4.2) |
| At least 1 spontaneous joint bleeding episode in the 1st quartile of age (<28), n (%)†† | 3 (1.3) |
| At least 1 spontaneous joint bleeding episode in the 2nd quartile of age (28 ≤ x < 45), n (%)‡‡ | 3 (1.7) |
| At least 1 spontaneous joint bleeding episode in the 3rd quartile of age (45 ≤ x < 59), n (%)§§ | 0 |
| At least 1 spontaneous joint bleeding episode in the 4th quartile of age (≥59), n (%)‖‖ | 3 (5.4) |
FVIII:C, factor VIII coagulant activity.
For each patient, the mean level of all the FVIII:C dosages performed in life by 1 stage clotting assay.
For each patient, the period between January 2005 and the date of last follow-up. This time of observation was considered for other than joint bleeds. For joint bleeds we considered as time of observation the whole life (age at last follow-up).
Available in 192 patients.
Available in 266 patients.
Available/applicable in 203 patients.
Available/applicable in 130 patients.
Available/applicable in 66 patients.
Available in 240 patients.
Available in 238 patients.
Available/applicable in 180 patients.
Available/applicable in 115 patients.
Available/applicable in 56 patients.
Figure 1.
Barplot of the number of all joint bleeds (n=263) in persons with mild HA.
Table 2.
Mean FVIII levels in patients with and without a history of joint bleeds
| At least 1 joint bleed (n=100) | No joint bleeds (n=170)∗ | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| FVIII:C† mean (SD) | 17.8 (10.8) | 26.8 (11.6) | –9.0 (–11.7 to –6.2) | <.0001 |
| At least 1 spontaneous joint bleed (n=10) | No spontaneous joint bleeds (n=230)‡ | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| FVIII:C† mean (SD) | 13.8 (9.9) | 25.0 (11.9) | –11.1 (–18.3 to –3.9) | .0063 |
Mean differences with CI and P values have been assessed using the t test.
FVIII:C, factor VIII coagulant activity.
Available in 270 patients.
For each patient, the mean level of all FVIII:C performed in life by 1 stage clotting assay.
Available in 240 patients.
Table 3.
Association between mean FVIII level and joint bleeding frequency
| Logistic regression models for “at least 1 joint bleed in life” |
||||||
|---|---|---|---|---|---|---|
| Univariate (n = 270) |
Multivariate∗ (n = 270) |
Multivariate† (n = 192) |
||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Mean FVIII:C | 0.93 (0.90-0.95) | <.0001 | 0.93 (0.90-0.95) | <.0001 | 0.92 (0.89-0.95) | <.0001 |
| Logistic regression models for “at least 1 spontaneous joint bleed in life” |
||||||
|---|---|---|---|---|---|---|
| Univariate (n = 240) |
Multivariate∗ (n = 240) |
Multivariate† (n = 170) |
||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Mean FVIII:C | 0.88 (0.80-0.97) | .001 | 0.88 (0.79 – 0.97) | .0008 | 0.76 (0.60-0.96) | .0005 |
| Negative binomial regression models for AJBR |
||||||
|---|---|---|---|---|---|---|
| Univariate (n = 262) |
Multivariate∗ (n = 262) |
Multivariate† (n = 187) |
||||
| AJBRR (95% CI) | P value | AJBRR (95% CI) | P value | AJBRR (95% CI) | P value | |
| Mean FVIII:C | 0.94 (0.92-0.96) | <.0001 | 0.94 (0.92-0.96) | <.0001 | 0.93 (0.91-0.96) | <.0001 |
| Negative binomial regression models for spontaneous AJBR |
||||||
|---|---|---|---|---|---|---|
| Univariate (n = 237) |
Multivariate∗ (n = 237) |
Multivariate† (n = 168) |
||||
| AJBRR (95% CI) | P value | AJBRR (95% CI) | P value | AJBRR (95% CI) | P value | |
| Mean FVIII:C | 0.892 (0.798-0.997) | .0437 | 0.89 (0.79-0.97) | .0453 | 0.66 (0.45-0.95) | .0264 |
AJBR was calculated considering the time of observation of the patient’s entire life (age at the last follow-up). For spontaneous AJBR, only spontaneous bleeds were considered.
AJBRR, annualized joint bleeding rate ratio; FVIII:C, factor VIII coagulant activity; OR, odds ratio.
Adjusted for age.
Adjusted for age and ABO blood group.
The minimum FVIII levels needed to prevent lifelong any and spontaneous joint bleeds were 19.2 IU/dL and 17.7 IU/dL, respectively, with up to 6 IU/dL lower FVIII levels needed to prevent joint bleeds in adults and older ages (ie, 2nd-4th quartile of age) (Table 4).
Table 4.
Minimum FVIII level to prevent total and spontaneous joint bleeds
| Outcomes for all joint bleeds | AUC | YI | FVIII level |
|---|---|---|---|
| At least 1 joint bleeding episode in life∗ | 0.73 | 0.39 | 19.2 |
| At least 1 joint bleeding episode in the 1st quartile of age (<28)† | 0.68 | 0.34 | 19.2 |
| At least 1 joint bleeding episode in the 2nd quartile of age (28 ≤ x < 45)‡ | 0.73 | 0.41 | 14.0 |
| At least 1 joint bleeding episode in the 3rd quartile of age (45 ≤ x < 59)§ | 0.71 | 0.41 | 12.8 |
| At least 1 joint bleeding episode in the 4th quartile of age (≥59)‖ | 0.80 | 0.47 | 14.3 |
| Outcomes for spontaneous joint bleeds | |||
| At least 1 spontaneous joint bleeding episode in life¶ | 0.80 | 0.58 | 17.7 |
| At least 1 spontaneous joint bleed in the 1st quartile of age (<28)# | 0.85 | 0.67 | 17.7 |
| At least 1 spontaneous joint bleed in the 2nd quartile of age (28 ≤ x < 45)∗∗ | 0.88 | 0.71 | 16.7 |
| At least 1 spontaneous joint bleed in the 3rd quartile of age (45 ≤ x < 59)†† | NA | NA | NA |
| At least 1 spontaneous joint bleed in the 4th quartile of age (≥59)‡‡ | 0.92 | 0.87 | 12.8 |
FVIII level indicates the minimum (trough) FVIII level to achieve zero joint bleeds (total or spontaneous).
NA, non applicable; YI, Youden Index (sensitivity + specificity – 1).
Available in 270 patients.
Available in 266 patients.
Available/applicable in 203 patients.
Available/applicable in 130 patients.
Available/applicable in 66 patients
Available in 240 patients.
Available in 238 patients.
Available/applicable in 180 patients.
Available/applicable in 115 patients.
Available/applicable in 56 patients.
During a mean follow-up of 13 years, 201 of 252 patients (74%) presented with at least 1 other bleed (ie, major or CRNMB other than joint bleed), with a mean ABR (estimated including only other bleeds) of 0.160 (SD, 0.207). Forty patients (16%) had at least 1 spontaneous other bleed during follow-up, with a mean spontaneous ABR (including only other bleeds) of 0.008 (SD, 0.024). When ABR rates were estimated, including joint bleeds occurring during the same observation period, the mean and spontaneous ABR were 0.187 (0.232) and 0.009 (0.033), respectively. Higher mean FVIII levels were associated with lower odds of other bleeds in the univariate and multivariate NB models, showing a 2% reduction in ABR for each unitary increase in FVIII levels. This association was not confirmed when only spontaneous bleeds were considered in the ABR estimations (supplemental Tables 1 and 2).
Discussion
In this retrospective cohort study, the joint bleeding phenotype was evaluated in a large cohort of persons with mild HA treated only with on-demand therapy to identify the minimum FVIII level needed to prevent lifelong total and spontaneous joint bleeds.
Data regarding the natural history and bleeding phenotype of persons with mild HA are heterogeneous, with reported mean annual bleeding rates ranging from 0.4 to 4.5 episodes per patient year.3,5,24 This heterogeneity reflects differences in study designs, length of the observation period, geographical locations, and bleed definitions.5
In the present cohort, 37% of patients experienced lifelong at least 1 joint bleed, with a lower median AJBR (0.00; IQR, 0.00-0.02) than reported in a multicenter cohort study among 234 patients with mild hemophilia (193 with HA) (0.0; IQR, 0.0-0.1).3 The higher median FVIII level in this cohort (21 IU/dL vs 15 IU/dL) and the much longer observation time for AJBR estimation (the entire patient life vs a median follow-up of 11 years) may partially explain these differences. Over a mean follow-up of 13.4 years and when both joint and other bleeds were included in the ABR estimation, the median ABR observed by us [0.0; IQR, 0.1-0.2] was in line with that reported by Kloosterman et al [0.2; IQR, 0.0-0.4].3
As previously shown,14 the NB model is the most suitable model for analyzing low-frequency bleeding data. Using this model, the association between FVIII level and joint bleeding frequency was similar to that reported by Kloosterman et al who observed a 6% reduction in the joint bleeding rate and a 12% reduction in the spontaneous joint bleeding rate for each IU/dL increase in the FVIII level.3
We found that a mean trough FVIII level of 18-19 IU/dL is necessary to prevent joint bleeds, which is in partial agreement with the findings from a Dutch cohort of 433 persons with HA (73% mild), wherein the FVIII target level suggested was slightly lower (15%).14 Differences in the methodology of data collection and observation periods may partially explain the poor consistency between the 2 studies. In particular, in the study by den Uijl et al, a nationwide questionnaire was administered and data on bleeding frequency were only referred to the previous year. This limited observation period may have introduced more variations than those in this study. This variation may also be important considering the low frequency of the endpoint.14 In another Dutch single-center cohort study including 122 persons with mild HA, the FVIII trough level identified as needed to prevent joint bleeds was even lower (12 IU/dL).2 The limited sample size may have affected the findings. Moreover, a different exposure definition was used; among the FVIII measures obtained lifelong, the lowest value was considered instead of the mean value, as in this study. Our findings are partially in line with those reported in a large US cohort study, enrolling 3315 persons with nonsevere HA.15 In that study, data were collected over an 11-year period and the 6-month average bleeding rate was recorded during the study period. The authors evaluated the association between the number of joint bleeds and FVIII levels using multiple linear regression analysis, assuming a negative binomial distribution. Consistent with the results of this study, an FVIII level of 15% was insufficient to prevent joint bleeds, with 1.4 bleeds per year predicted at this level. Age was an important predictor of bleeding, with the highest joint bleeding rates observed in patients aged 25 to 44 years who required high trough FVIII levels.15 This is in contrast to the results of this study, wherein a trough level of 14 IU/dL was sufficient in this age class.
Our study has several limitations. Firstly, its retrospective design may have induced an underestimation of the reported bleeds (recall bias) and missing data, limiting the sample size in some models. To limit this bias, minor bleeds were excluded from the ABRs estimation; for other bleeds, only those that occurred since January 2005 were considered. To partially overcome intraindividual FVIII variability over time, the mean FVIII level, as estimated lifelong, was used as the exposure in all models. Among the effect modifiers, only the age and blood groups were considered. It is known that age may influence the bleeding rate in persons with HA. In this study, age at the last follow-up was also the time of observation for the joint bleeding end point and thus a key determinant of the odds of lifelong occurrence of joint bleeds. Therefore, the NB model was used to estimate bleeding frequency in terms of the AJBR, and an ROC model was defined for each quartile of age. The main strengths of this study were the lifelong evaluation of FVIII levels (measured by 1-stage clotting in a single laboratory) and the joint bleeding frequency in a regularly followed large cohort.
In conclusion, our study provides a FVIII target level needed to prevent joint bleeds and spontaneous joint bleeds for each age quartile. Results suggest that trough FVIII levels of 18 IU/dL and 19 IU/dL could be a good therapeutic target to prevent spontaneous and total joint bleeds at any age, even though these values may be lower in adults and older ages. Although these findings only apply to persons with mild HA, they may provide important implications to accurately design prophylactic therapies also for persons with moderate and severe HA, including gene therapy, designed to provide consistent FVIII levels without troughs and peaks. However, treatment decisions should be tailored and based not only on the residual clotting factor activity levels but also on all other potential factors influencing the risk of bleeding (eg, age, physical activity, and joint health status) in the framework of a comprehensive and personalized approach.
Conflict-of-interest disclosure: R.G. has received honoraria for participating as a speaker in education meetings organized by Pfizer, Novo Nordisk, Sobi, Takeda, and Roche and is a member of the scientific advisory boards of Bayer, Sobi, Roche, and Pfizer. F.P. has received honoraria for participating as a speaker in education meetings and symposia organized by Takeda and Spark. She is a consultant/member of the advisory boards for CSL Behring, Biomarin, Roche, Sanofi, and Sobi. The remaining authors declare no competing financial interests.
Acknowledgments
The authors gratefully acknowledge P.M. Mannucci for his critical revision.
This work was supported by the 2020 Fellowship Project Award-Bayer Hemophilia Awards Program. This study was partially supported by the Italian Ministry of Health - Bando Ricerca Corrente (RC2022). Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico is a member of the European Reference Network EuroBloodNet.
Authorship
Contribution: P.A. and F.P. designed the study; P.A., S.M.S., and A.T. collected data; P.A. and S.S. analyzed the data; P.A. wrote the manuscript; and P.A., S.M.S., S.S., A.T., R.G., and F.P. interpreted the data and carefully revised the results of the study and the content of the manuscript.
Footnotes
Presented in abstract form at the 31st Congress of the International Society on Thrombosis and Haemostasis, Montréal, Canada, 24-28 June 2023.
Deidentified data are available upon reasonable request from the corresponding author, Flora Peyvandi (flora.peyvandi@unimi.it).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Mannucci PM, Tuddenham EG. The hemophilias--from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 2.Den Uijl IE, Mauser Bunschoten EP, Roosendaal G, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. 2011;17(6):849–853. doi: 10.1111/j.1365-2516.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 3.Kloosterman FR, Zwagemaker AF, Bagot CN, et al. The bleeding phenotype in people with nonsevere hemophilia. Blood Adv. 2022;6(14):4256–4265. doi: 10.1182/bloodadvances.2022007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.den Uijl IE, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Clinical outcome of moderate haemophilia compared with severe and mild haemophilia. Haemophilia. 2009;15(1):83–90. doi: 10.1111/j.1365-2516.2008.01837.x. [DOI] [PubMed] [Google Scholar]
- 5.Peyvandi F, Tavakkoli F, Frame D, et al. Burden of mild haemophilia A: systematic literature review. Haemophilia. 2019;25(5):755–763. doi: 10.1111/hae.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan S, van Balen EC, Smit C, et al. Health and treatment outcomes of patients with hemophilia in the Netherlands, 1972-2019. J Thromb Haemost. 2021;19(10):2394–2406. doi: 10.1111/jth.15424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima Y, Mizumachi K, Shimonishi N, et al. Comparisons of global coagulation potential and bleeding episodes in emicizumab-treated hemophilia A patients and mild hemophilia A patients. Int J Hematol. 2022;115(4):489–498. doi: 10.1007/s12185-021-03276-7. [DOI] [PubMed] [Google Scholar]
- 8.Krumb E, Fijnvandraat K, Makris M, et al. Adoption of emicizumab (Hemlibra®) for hemophilia A in Europe: data from the 2020 European Association for Haemophilia and Allied Disorders survey. Haemophilia. 2021;27(5):736–743. doi: 10.1111/hae.14372. [DOI] [PubMed] [Google Scholar]
- 9.Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–818. doi: 10.1056/NEJMoa1703068. [DOI] [PubMed] [Google Scholar]
- 10.Thornburg CD, Simmons DH, von Drygalski A. Evaluating gene therapy as a potential paradigm shift in treating severe hemophilia. BioDrugs. 2023;37(5):595–606. doi: 10.1007/s40259-023-00615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer K, Berntorp E. Targeting factor replacement therapy in severe hemophilia: which level is important? Semin Thromb Hemost. 2015;41(8):860–863. doi: 10.1055/s-0035-1552562. [DOI] [PubMed] [Google Scholar]
- 12.Collins PW, Obaji SG, Roberts H, Gorsani D, Rayment R. Clinical phenotype of severe and moderate haemophilia: who should receive prophylaxis and what is the target trough level? Haemophilia. 2021;27(2):192–198. doi: 10.1111/hae.14201. [DOI] [PubMed] [Google Scholar]
- 13.Mahlangu J, Kaczmarek R, von Drygalski A, et al. Two-year outcomes of valoctocogene roxaparvovec therapy for hemophilia A. N Engl J Med. 2023;388(8):694–705. doi: 10.1056/NEJMoa2211075. [DOI] [PubMed] [Google Scholar]
- 14.den Uijl IE, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17(1):41–44. doi: 10.1111/j.1365-2516.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 15.Soucie JM, Monahan PE, Kulkarni R, Konkle BA, Mazepa MA, US Hemophilia Treatment Center Network The frequency of joint hemorrhages and procedures in nonsevere hemophilia A vs B. Blood Adv. 2018;2(16):2136–2144. doi: 10.1182/bloodadvances.2018020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou JY, Barnes RFW, Foster G, Iorio A, Cramer TJ, von Drygalski A. Joint bleeding tendencies in adult patients with hemophilia: it's not all pharmacokinetics. Clin Appl Thromb Hemost. 2019;25 doi: 10.1177/1076029619862052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdary P, Fischer K, Collins PW, et al. Modeling to predict factor VIII levels associated with zero bleeds in patients with severe hemophilia A initiated on tertiary prophylaxis. Thromb Haemost. 2020;120(5):728–736. doi: 10.1055/s-0040-1709519. [DOI] [PubMed] [Google Scholar]
- 18.Tiede A, Abdul Karim F, Jiménez-Yuste V, et al. Factor VIII activity and bleeding risk during prophylaxis for severe hemophilia A: a population pharmacokinetic model. Haematologica. 2021;106(7):1902–1909. doi: 10.3324/haematol.2019.241554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valentino LA, Pipe SW, Collins PW, et al. Association of peak factor VIII levels and area under the curve with bleeding in patients with haemophilia A on every third day pharmacokinetic-guided prophylaxis. Haemophilia. 2016;22(4):514–520. doi: 10.1111/hae.12905. [DOI] [PubMed] [Google Scholar]
- 20.Broderick CR, Herbert RD, Latimer J, et al. Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012;308(14):1452–1459. doi: 10.1001/jama.2012.12727. [DOI] [PubMed] [Google Scholar]
- 21.Ertl HCJ. Immunogenicity and toxicity of AAV gene therapy. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.975803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samelson-Jones BJ, George LA. Adeno-associated virus gene therapy for hemophilia. Annu Rev Med. 2023;74(1):231–247. doi: 10.1146/annurev-med-043021-033013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of Anticoagulation Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 24.Tagliaferri A, Di Perna C, Riccardi F, Pattacini C, Rivolta GF, Franchini M. The natural history of mild haemophilia: a 30-year single centre experience. Haemophilia. 2012;18(2):166–174. doi: 10.1111/j.1365-2516.2011.02617.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.