Abstract
BACKGROUND
Selpercatinib, a highly selective potent and brain-penetrant RET inhibitor, was shown to have efficacy in patients with advanced RET fusion–positive non–small-cell lung cancer (NSCLC) in a nonrandomized phase 1–2 study.
METHODS
In a randomized phase 3 trial, we evaluated the efficacy and safety of first-line selpercatinib as compared with control treatment that consisted of platinum-based chemotherapy with or without pembrolizumab at the investigator’s discretion. The primary end point was progression-free survival assessed by blinded independent central review in both the intention-to-treat–pembrolizumab population (i.e., patients whose physicians had planned to treat them with pembrolizumab in the event that they were assigned to the control group) and the overall intention-to-treat population. Crossover from the control group to the selpercatinib group was allowed if disease progression as assessed by blinded independent central review occurred during receipt of control treatment.
RESULTS
In total, 212 patients underwent randomization in the intention-to-treat–pembrolizumab population. At the time of the preplanned interim efficacy analysis, median progression-free survival was 24.8 months (95% confidence interval [CI], 16.9 to not estimable) with selpercatinib and 11.2 months (95% CI, 8.8 to 16.8) with control treatment (hazard ratio for progression or death, 0.46; 95% CI, 0.31 to 0.70; P<0.001). The percentage of patients with an objective response was 84% (95% CI, 76 to 90) with selpercatinib and 65% (95% CI, 54 to 75) with control treatment. The cause-specific hazard ratio for the time to progression affecting the central nervous system was 0.28 (95% CI, 0.12 to 0.68). Efficacy results in the overall intention-to-treat population (261 patients) were similar to those in the intention-to-treat–pembrolizumab population. The adverse events that occurred with selpercatinib and control treatment were consistent with those previously reported.
CONCLUSIONS
Treatment with selpercatinib led to significantly longer progression-free survival than platinum-based chemotherapy with or without pembrolizumab among patients with advanced RET fusion–positive NSCLC. (Funded by Eli Lilly and others; ClinicalTrials.gov number, NCT04194944.)
Ret gene fusions, which lead to increased oncogenic signaling, are a targetable alteration in patients with RET fusion–positive non–small-cell lung cancer (NSCLC). Selpercatinib, a highly selective and potent RET kinase inhibitor with central nervous system (CNS) penetration, has previously been shown to have marked efficacy in nonrandomized studies involving patients with RET-driven lung, thyroid, and other solid tumors.1–3 Given the magnitude of efficacy together with the safety profile, selpercatinib received health authority approvals in several countries for the treatment of adult patients with RET-driven cancers, including advanced RET fusion–positive NSCLC.
On the basis of the results of the KEYNOTE-189 trial, pembrolizumab with a platinum-based drug and pemetrexed has become one of the standard treatments for patients with untreated advanced or metastatic NSCLC without EGFR or ALK alterations.4–7 Patients with NSCLC harboring EGFR or ALK alterations were excluded from the KEYNOTE-189 trial on the basis of preclinical and clinical data, which suggested that patients with tumors bearing these alterations were unlikely to benefit from treatment with the immune-checkpoint inhibitors.8–10 Retrospective analyses suggest that checkpoint inhibitors may also have limited effectiveness for patients with RET fusion–positive NSCLC.10,11 To define the treatment regimen to be used for newly diagnosed advanced RET fusion–positive NSCLC, a randomized trial was designed to evaluate selpercatinib as compared with platinum-based chemotherapy with or without pembrolizumab. Because of the uncertainty over the contribution of programmed cell death 1 (PD-1) inhibition in RET fusion–positive NSCLC, the decision for patients to receive pembrolizumab was left to the investigator’s discretion.
METHODS
PATIENTS
We enrolled patients who were 18 years of age or older with pathologically confirmed unresectable stage IIIB, IIIC, or IV nonsquamous NSCLC who had not previously received systemic treatment for metastatic disease. Additional eligibility criteria included measurable disease in accordance with Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 to 2 (scores range from 0 to 5, with higher numbers reflecting greater disability); and adequate organ function. The presence of a RET gene fusion was required to be identified by next-generation sequencing or polymerase chain reaction (PCR) with a local certified laboratory or sponsor-enabled testing. RET fusion testing results were validated by the sponsor before enrollment. Patients were excluded from the trial if they had additional validated oncogenic drivers in NSCLC, if they had received previous systemic therapy for advanced disease, and if they had active cardiovascular disease, active uncontrolled infections requiring treatment, or uncontrolled disease-related pericardial effusion or pleural effusion. Patients with known brain metastases were eligible if they were asymptomatic or had been neurologically stable for at least 2 weeks before randomization.
The trial was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and general principles for planning and design of multi-regional clinical trials, as well as with the principles of the Declaration of Helsinki and all applicable country and local regulations.12 The protocol was approved by the institutional review board or independent ethics committee at each site and is available with the full text of this article at NEJM.org. All patients provided written informed consent.
TRIAL DESIGN AND TREATMENT
This trial was designed jointly by the sponsor (Loxo Oncology, a wholly owned subsidiary of Eli Lilly) and the investigators. The sponsor collected, analyzed, and interpreted the trial data in collaboration with the authors. The authors provided input to revise the manuscript, and writing assistance with the submitted manuscript was funded by the sponsor. The authors vouch for the completeness and accuracy of the data and for the adherence of the trial to the protocol.
Patients were randomly assigned to receive either selpercatinib (160 mg twice daily) in continuous 21-day cycles or pemetrexed (500 mg per square meter of body-surface area) with vitamin supplementation along with the investigator’s choice of platinum therapy (carboplatin [area under the concentration–time curve, 5; maximum dose, 750 mg] or cisplatin [75 mg per square meter]) with or without pembrolizumab (200 mg) every 21 days. Patients were stratified according to geographic region (East Asia vs. elsewhere), status with respect to brain metastases at baseline (absent or unknown vs. present), and whether the investigator had intended (before randomization) to treat the patient with pembrolizumab or without pembrolizumab. Initially, eligible patients were randomly assigned in a 1:1 ratio to the selpercatinib group or the control group; however, on amendment of the protocol, patients were randomly assigned in a 2:1 ratio to the selpercatinib group or the control group, and therefore the final ratio of randomization was 1.6:1. After the completion of four cycles of control treatment without progressive disease, patients in the control group could continue to receive pemetrexed with or without pembrolizumab. Pembrolizumab was administered for a maximum of 35 cycles. Because of differences in treatment administration between the groups, the trial was open label to patients and investigators; however, the sponsor did not review or analyze aggregate data and response assessments or the assessments of disease progression conducted by blinded independent central review and by the investigator in accordance with RECIST, version 1.1.13 Patients were allowed to continue selpercatinib treatment after the occurrence of disease progression at the discretion of the investigator and with sponsor approval if there was clinical benefit. Patients who were randomly assigned to the control group and who had disease progression confirmed by blinded independent central review were eligible for optional crossover to selpercatinib. The efficacy and safety results among the patients who crossed over are not reported here.
TRIAL ASSESSMENTS
Baseline radiologic scans were obtained up to 28 days before the initiation of treatment, and subsequent scans were performed at 6 and 12 weeks and then every 9 weeks for the first year. Thereafter, scans were required every 12 weeks until disease progression occurred. Initially, only patients with CNS lesions identified at baseline were required to undergo longitudinal magnetic resonance imaging (MRI) or computed tomography (CT) for intracranial disease evaluation; however, the protocol was later amended to include serial intracranial imaging for all patients. Adverse events were graded in accordance with Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, and were summarized with the use of Medical Dictionary for Regulatory Activities, version 25.0, preferred terms. Safety analyses were performed in the population of all patients who underwent randomization and received at least one dose of treatment (safety population).
The primary end point — progression-free survival assessed by blinded independent central review — was sequentially tested, first in patients in the intention-to-treat–pembrolizumab population, which consisted of patients who underwent randomization and whose physicians had planned to treat them with pembrolizumab in the event that they were assigned to the control group; if the results of that analysis were positive, then the primary end point was tested in the overall intention-to-treat population (which included all patients who underwent randomization). Progression-free survival assessed by blinded independent central review was defined as the time from randomization to the occurrence of disease progression or death. Overall survival in the intention-to-treat population was a key, alpha-controlled, secondary end point. Progression-free survival according to investigator assessment in both the intention-to-treat–pembrolizumab population and the overall intention-to-treat population, as well as the percentage of patients with a response and the duration of response assessed by blinded independent central review and by investigator assessment, were secondary end points. Intracranial response and the time to progression assessed by blinded independent central review in accordance with RECIST, version 1.1, were assessed in all patients in the intention-to-treat–pembrolizumab population who had a baseline CNS assessment (CNS–pembrolizumab population) and at least one evaluable postbaseline assessment.
PATIENT-REPORTED OUTCOMES
Time to confirmed worsening (deterioration) of pulmonary symptoms was evaluated as a secondary end point in all treated patients who had completed a baseline assessment and at least one postbaseline assessment.14 Pulmonary symptoms were assessed with the use of the NSCLC–Symptom Assessment Questionnaire (SAQ) total score (range, 0 [no symptoms] to 20 [worst symptoms]). The time to confirmed worsening of symptoms was defined as the time from the date of randomization to the date of the first increase of 2 or more points (the threshold for clinically meaningful change) in the NSCLC-SAQ total score with a confirmed increased score at the next subsequent assessment.15
STATISTICAL ANALYSIS
All analyses were conducted in accordance with the statistical analysis plan (available with the protocol). We calculated that a total of 140 events of disease progression or death in the intention-to-treat–pembrolizumab population would be required to achieve 89% overall statistical power with a two-sided type 1 error of 0.05. The preplanned interim efficacy analysis occurred after 98 events of disease progression assessed by blinded independent central review or death in the intention-to-treat–pembrolizumab population, with data cutoff on May 1, 2023. The nominal two-sided alpha level was fixed at 0.012 at the interim analysis. With the gated testing strategy, progression-free survival in the intention-to-treat population was tested conditionally on achievement of significance for progression-free survival in the intention-to-treat–pembrolizumab population, which acted as a gatekeeper for testing overall survival in the intention-to-treat population. Descriptive results of all other analyses, including subgroup analyses, are reported as point estimates and 95% confidence intervals; the confidence intervals in these analyses were not adjusted for multiplicity and should not be used to infer definitive treatment effects. The Kaplan–Meier method was used to estimate medians and percentages of patients at various time points for each group for progression-free survival, overall survival, and the time to confirmed worsening of symptoms. Hazard ratios for time-to-event end points were estimated with a stratified Cox regression model. The assumption of proportionality was assessed graphically by evaluating whether the estimated log-minus-log survival curves were parallel.
RESULTS
PATIENTS AND TREATMENT
From March 2020 through August 2022, a total of 261 patients with RET fusion–positive advanced NSCLC were enrolled at 103 sites across 23 countries (Fig. S1 in the Supplementary Appendix, available at NEJM.org). Of the 261 patients who underwent randomization, 5 discontinued participation before the start of treatment (1 in the selpercatinib group and 4 in the control group). As a result, 256 patients received at least one dose of treatment (158 in the selpercatinib group and 98 in the control group). With a median follow-up time of approximately 19 months, the median (±SD) time spent receiving treatment was 16.7±8.3 months in the selpercatinib group and 9.8±7.2 months in the control group (Table S2).
The intention-to-treat–pembrolizumab population included 212 patients who had been randomly assigned to receive selpercatinib (129 patients) or chemotherapy plus pembrolizumab (83 patients). The majority of the patients were women, younger than 65 years of age, and never smokers. The baseline clinical and demographic characteristics of the patients in the intention-to-treat–pembrolizumab population were generally well balanced between the groups, although more patients from East Asia were enrolled in the selpercatinib group than in the control group (58% vs. 49%) (Table 1). In the majority of patients (58%), RET fusions were identified by next-generation sequencing: from primary tumor in 56%, from metastatic tumor in 33%, and from blood-based testing in 10%. The most common RET fusion partners were KIF5B (in 45% of the patients) and CCDC6 (10%). In addition, the RET fusions in 42% of the patients were identified by PCR, which does not specify the RET fusion partner. Baseline clinical and demographic characteristics were similarly balanced in the overall intention-to-treat population (Table 1).
Table 1.
Baseline Clinical and Demographic Characteristics of Patients.*
| Characteristic | Intention-to-Treat–Pembrolizumab Population | Overall Intention-to-Treat Population | ||
|---|---|---|---|---|
| Selpercatinib (N = 129) | Control (N = 83) | Selpercatinib (N = 159) | Control (N=102) | |
| Age — yr | ||||
| Median | 60.0 | 62.0 | 61.0 | 62.5 |
| Range | 31–84 | 31–83 | 31–87 | 31–83 |
| Age distribution — no. (%) | ||||
| <65 yr | 82 (64) | 49 (59) | 100 (63) | 57 (56) |
| ≥65 yr | 47 (36) | 34 (41) | 59 (37) | 45 (44) |
| Sex — no. (%) | ||||
| Female | 65 (50) | 48 (58) | 86 (54) | 57 (56) |
| Male | 64 (50) | 35 (42) | 73 (46) | 45 (44) |
| Race — no. (%)† | ||||
| Asian | 76 (59) | 41 (49) | 92 (58) | 52 (51) |
| White | 49 (38) | 37 (45) | 58 (36) | 43 (42) |
| Black | 2 (2) | 0 | 2 (1) | 0 |
| Other‡ | 2 (2) | 1 (1) | 3 (2) | 1 (1) |
| Missing data | 0 | 4 (5) | 4 (3) | 6 (6) |
| Region of enrollment — no. (%) | ||||
| East Asia | 75 (58) | 41 (49) | 91 (57) | 51 (50) |
| Europe | 31 (24) | 36 (43) | 42 (26) | 42 (41) |
| North America | 4 (3) | 1 (1) | 6 (4) | 2 (2) |
| Other§ | 19 (15) | 5 (6) | 20 (13) | 7 (7) |
| Smoking status — no. (%) | ||||
| Never smoked | 85 (66) | 59 (71) | 108 (68) | 68 (67) |
| Former smoker | 40 (31) | 22 (27) | 45 (28) | 32 (31) |
| Current smoker | 4 (3) | 2 (2) | 6 (4) | 2 (2) |
| ECOG performance-status score — no. (%)¶ | ||||
| 0 | 45 (35) | 27 (33) | 58 (36) | 40 (39) |
| 1 | 81 (63) | 52 (63) | 97 (61) | 58 (57) |
| 2 | 3 (2) | 4 (5) | 4 (3) | 4 (4) |
| NSCLC histologic type — no. (%) | ||||
| Adenocarcinoma | 128 (99) | 80 (96) | 158 (99) | 99 (97) |
| NSCLC not otherwise specified | 1 (1) | 3 (4) | 1 (1) | 3 (3) |
| Stage of disease — no. (%) | ||||
| IIIB or IIIC | 7 (5) | 7 (8) | 9 (6) | 8 (8) |
| IVA | 51 (40) | 35 (42) | 67 (42) | 45 (44) |
| IVB | 71 (55) | 41 (49) | 83 (52) | 49 (48) |
| Metastases identified at baseline — no. (%) | ||||
| Brain metastases | ||||
| No or unknown | 104 (81) | 65 (78) | 129 (81) | 81 (79) |
| Yes | 25 (19) | 18 (22) | 30 (19) | 21 (21) |
| Liver metastases | ||||
| No | 109 (84) | 65 (78) | 137 (86) | 80 (78) |
| Yes | 19 (15) | 17 (20) | 21 (13) | 20 (20) |
| PD-L1 status — no. (%) | ||||
| Negative | 31 (24) | 12 (14) | 38 (24) | 20 (20) |
| Positive | 55 (43) | 39 (47) | 66 (42) | 47 (46) |
| <1% | 8 (6) | 8 (10) | 8 (5) | 9 (9) |
| 1–49% | 25 (19) | 17 (20) | 29 (18) | 21 (21) |
| ≥50% | 22 (17) | 14 (17) | 29 (18) | 17 (17) |
| Missing data | 43 (33) | 32 (39) | 55 (35) | 35 (34) |
| RET fusion result — no. (%) | ||||
| Positive∥ | 58 (45) | 31 (37) | 69 (43) | 39 (38) |
| KIF5B–RET | 54 (42) | 41 (49) | 70 (44) | 50 (49) |
| CCDC6–RET | 13 (10) | 8 (10) | 16 (10) | 9 (9) |
| NCOA4–RET | 0 | 1 (1) | 0 | 2 (2) |
| KIF13A–RET | 0 | 1 (1) | 0 | 1 (1) |
| KIAA1549L–RET | 1 (1) | 0 | 1 (1) | 0 |
| KIAA1468–RET | 1 (1) | 0 | 1 (1) | 0 |
| PRKAR1A–RET | 0 | 1 (1) | 0 | 1 (1) |
| Other** | 2 (2) | 0 | 2 (1) | 0 |
Percentages may not total 100 because of rounding. The intention-to-treat–pembrolizumab population included patients whose physicians had planned to treat them with pembrolizumab in the event that they were assigned to the control group. Treatment in the control group was the investigator’s choice of platinum-based chemotherapy plus pembrolizumab (intention-to-treat–pembrolizumab population) or the investigator’s choice of platinum-based chemotherapy with or without pembrolizumab (intention-to-treat population). NSCLC denotes non–small-cell lung cancer, and PD-L1 programmed death ligand 1.
Race was reported by the patients. Data are missing for patients who did not disclose their race.
“Other” included American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, and multiple races.
“Other” included the following countries of enrollment: Argentina, Australia, Brazil, Israel, and Turkey.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher numbers reflecting greater disability.
In this category, RET fusion was indicated by molecular analysis but the RET fusion partner was not identified.
Two patients had multiple RET fusion partners identified (KIF5B–RET and CDKAL1–RET in one patient and NCOA4–RET and ZNF32-AS3–RET in the other).
EFFICACY
The preplanned interim efficacy analysis was performed after 98 events of death or disease progression assessed by blinded independent central review had occurred in the intention-to-treat–pembrolizumab population. In this population, median progression-free survival assessed by blinded independent central review was 24.8 months (95% confidence interval [CI], 16.9 to not estimable) with selpercatinib and 11.2 months (95% CI, 8.8 to 16.8) with control treatment, corresponding to a hazard ratio for progression or death of 0.46 (95% CI, 0.31 to 0.70; P<0.001) (Fig. 1). The percentage of patients with an objective response as assessed by blinded independent central review was higher in the selpercatinib group than in the control group (84% [95% CI, 76 to 90] vs. 65% [95% CI, 54 to 75]) (Table 2). The median time to response was 1.45 months with selpercatinib and 1.53 months with control treatment. Responses were durable, as indicated by a median response duration of 24.2 months (95% CI, 17.9 to not estimable) in the selpercatinib group, as compared with 11.5 months (95% CI, 9.7 to 23.3) in the control group (Table 2). In the preplanned subgroup analyses, progression-free survival assessed by blinded independent central review was longer with selpercatinib than with control treatment across all subgroups, including those based on race, geographic region, ECOG performance-status score, fusion partner (KIF5B, CCDC6, or other), programmed death ligand 1 (PD-L1) status, and status with respect to intracranial disease at baseline (Fig. S3).
Figure 1. Progression-Free Survival Assessed by Blinded Independent Central Review.
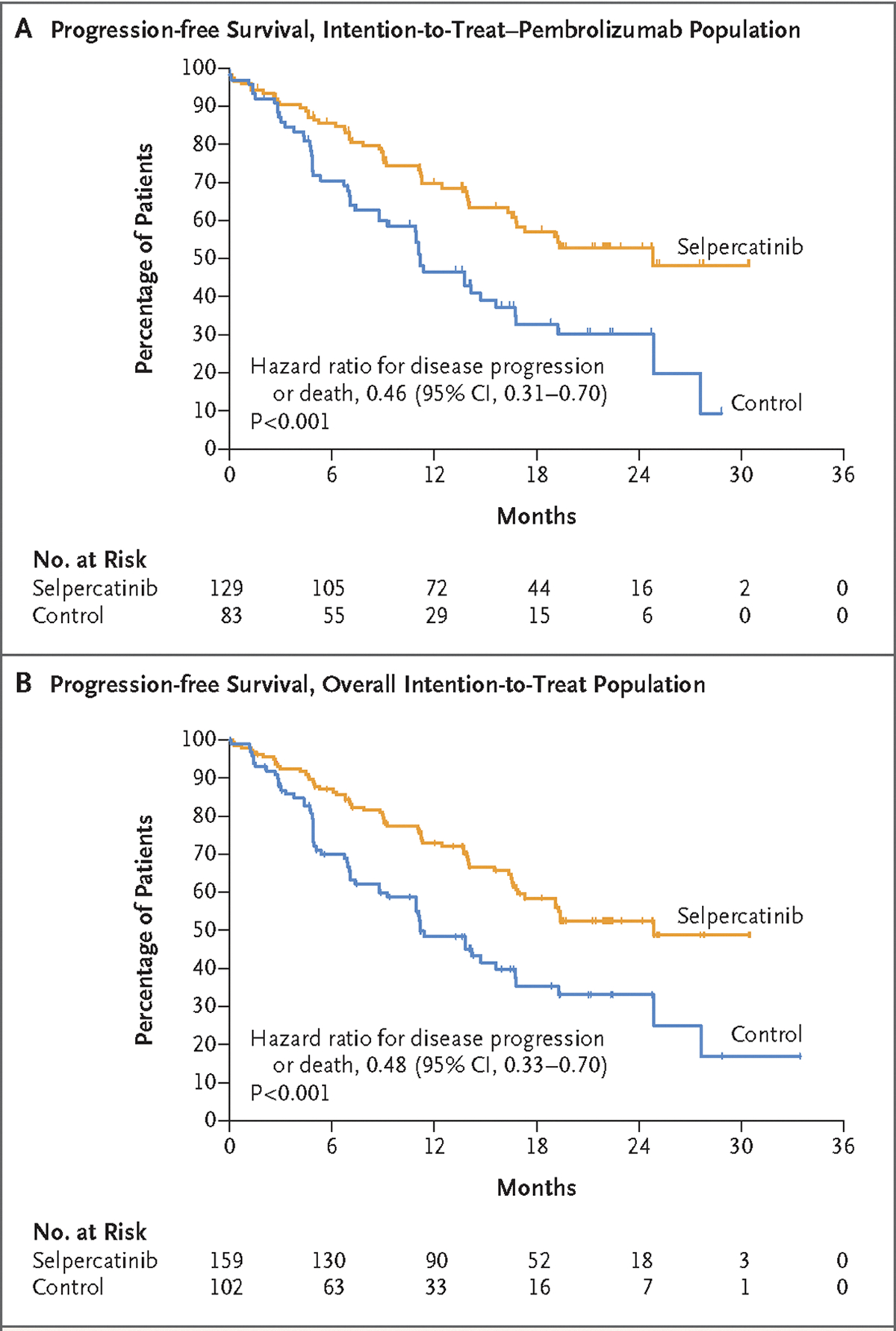
Panel A shows Kaplan–Meier estimates of progression-free survival assessed by blinded independent central review in the intention-to-treat–pembrolizumab population (i.e., patients whose physicians had planned to treat them with pembrolizumab in the event that they were assigned to the control group). Panel B shows Kaplan–Meier estimates of progression-free survival assessed by blinded independent central review in overall intention-to-treat population. Tick marks on the survival curves indicate censoring of data.
Table 2.
Summary of End Points Assessed by Blinded Independent Central Review.*
| End Point | Intention-to-Treat–Pembrolizumab Population | Overall Intention-to-Treat Population | ||
|---|---|---|---|---|
| Selpercatinib (N = 129) | Control (N = 83) | Selpercatinib (N = 159) | Control (N=102) | |
| Progression-free survival — mo | ||||
| Median progression-free survival (95% CI) | 24.8 (16.9–NE) | 11.2 (8.8–16.8) | 24.8 (17.3–NE) | 11.2 (8.8–16.8) |
| Median duration of follow-up (95% CI) | 19.4 (16.7–19.7) | 18.9 (14.2–22.3) | 19.4 (16.7–19.6) | 16.5 (13.6–21.0) |
| Objective response (95% CI) — % of patients | 84 (76–90) | 65 (54–75) | 84 (77–89) | 63 (53–72) |
| Best overall response — no. (%) | ||||
| Complete response | 9 (7) | 5 (6) | 12 (8) | 5 (5) |
| Partial response | 99 (77) | 49 (59) | 121 (76) | 59 (58) |
| Stable disease | 14 (11) | 20 (24) | 17 (11) | 26 (25) |
| Progressive disease | 2 (2) | 5 (6) | 2 (1) | 7 (7) |
| Not evaluable | 5 (4) | 4 (5) | 7 (4) | 5 (5) |
| Duration of response | ||||
| Patients with a response — no. | 108 | 54 | 133 | 64 |
| Patients with a response and censored data — no. (%) | 74 (69) | 25 (46) | 43 (32) | 31 (48) |
| Median duration of response (95% CI) — mo | 24.2 (17.9–NE) | 11.5 (9.7–23.3) | 24.2 (17.9–NE) | 12.0 (9.7–23.3) |
| Median duration of follow-up (95% CI) — mo | 18.0 (16.5–19.5) | 14.6 (11.2–19.8) | 17.9 (15.7–18.7) | 12.7 (11.1–16.6) |
Percentages may not total 100 because of rounding. Confidence intervals have not been adjusted for multiplicity and cannot be used to infer treatment effects. Efficacy outcomes were assessed with the use of Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, and were confirmed by blinded independent radiologic review. NE denotes not estimable.
Investigator-assessed efficacy measures were generally consistent with those assessed by blinded independent central review in the intention-to-treat–pembrolizumab population (Table S1). Median progression-free survival assessed by the investigators was 24.8 months (95% CI, 19.1 to not estimable) with selpercatinib and 14.0 months (95% CI, 10.9 to 22.3) with control treatment, corresponding to a hazard ratio for progression or death of 0.53 (95% CI, 0.34 to 0.80) (Fig. S2).
Similar results were observed in the overall intention-to-treat population for end points assessed by blinded independent central review and investigator-assessed end points and across all prespecified subgroups. Median progression-free survival assessed by blinded independent central review was more than 13 months longer in the selpercatinib group than in the control group (24.8 months [95% CI, 17.3 to not estimable] vs. 11.2 months [95% CI, 8.8 to 16.8]; hazard ratio for progression or death, 0.48; 95% CI, 0.33 to 0.70; P<0.001) (Fig. 1).
Data on overall survival are not yet mature; the information fraction at this interim analysis is 28.6% (50 deaths), based on the target number of 175 deaths in the intention-to-treat population. The hazard ratios for death in the intention-to-treat–pembrolizumab and overall intention-to-treat populations were 0.96 (95% CI, 0.50 to 1.83) and 1.04 (95% CI, 0.58 to 1.87), respectively (Fig. S8). With approximately 21 months of median follow-up time, more than 76% of the patients in each group were still alive at the cutoff date. Among the patients who had been randomly assigned to the control group and who stopped receiving control treatment or discontinued participation in the control group before receiving treatment, approximately 60% crossed over to receive selpercatinib within the trial, and an additional 15% went on to receive a selective RET inhibitor outside the trial.
INTRACRANIAL EFFICACY
Intracranial baseline assessments were available for evaluation by neuroradiologic blinded independent central review in accordance with RECIST, version 1.1, for 192 patients in the CNS–pembrolizumab population (120 patients in the selpercatinib group and 72 patients in the control group). The cause-specific hazard ratio for the time to CNS disease progression was 0.28 (95% CI, 0.12 to 0.68); 8 patients (7%) receiving selpercatinib had a first event of CNS progression, as compared with 13 (18%) receiving control treatment (Table S2). The 12-month cumulative incidence of CNS progression, with adjustment for the competing risks of non-CNS progression and death, was 6% (95% CI, 2 to 11) in the selpercatinib group and 20% (95% CI, 11 to 31) in the control group (Fig. S9).
Overall, 42 of the 192 patients (22%) were confirmed to have brain metastases at baseline, 29 of whom had measurable metastases (17 in the selpercatinib group and 12 in the control group). Among the patients with measurable brain metastases at baseline, intracranial response occurred in 82% (95% CI, 57 to 96) of those in the selpercatinib group and 58% (95% CI, 28 to 85) of those in the control group (Table S3). Complete responses occurred in 6 of the 17 patients (35%) in the selpercatinib group and 2 of the 12 patients (17%) in the control group (Fig. 2). Data on the median duration of intracranial response were immature, but at 12 months, 76% of patients continued to have a response with selpercatinib, as compared with 63% with control treatment.
Figure 2. Best Overall Responses Assessed by Blinded Independent Central Review.
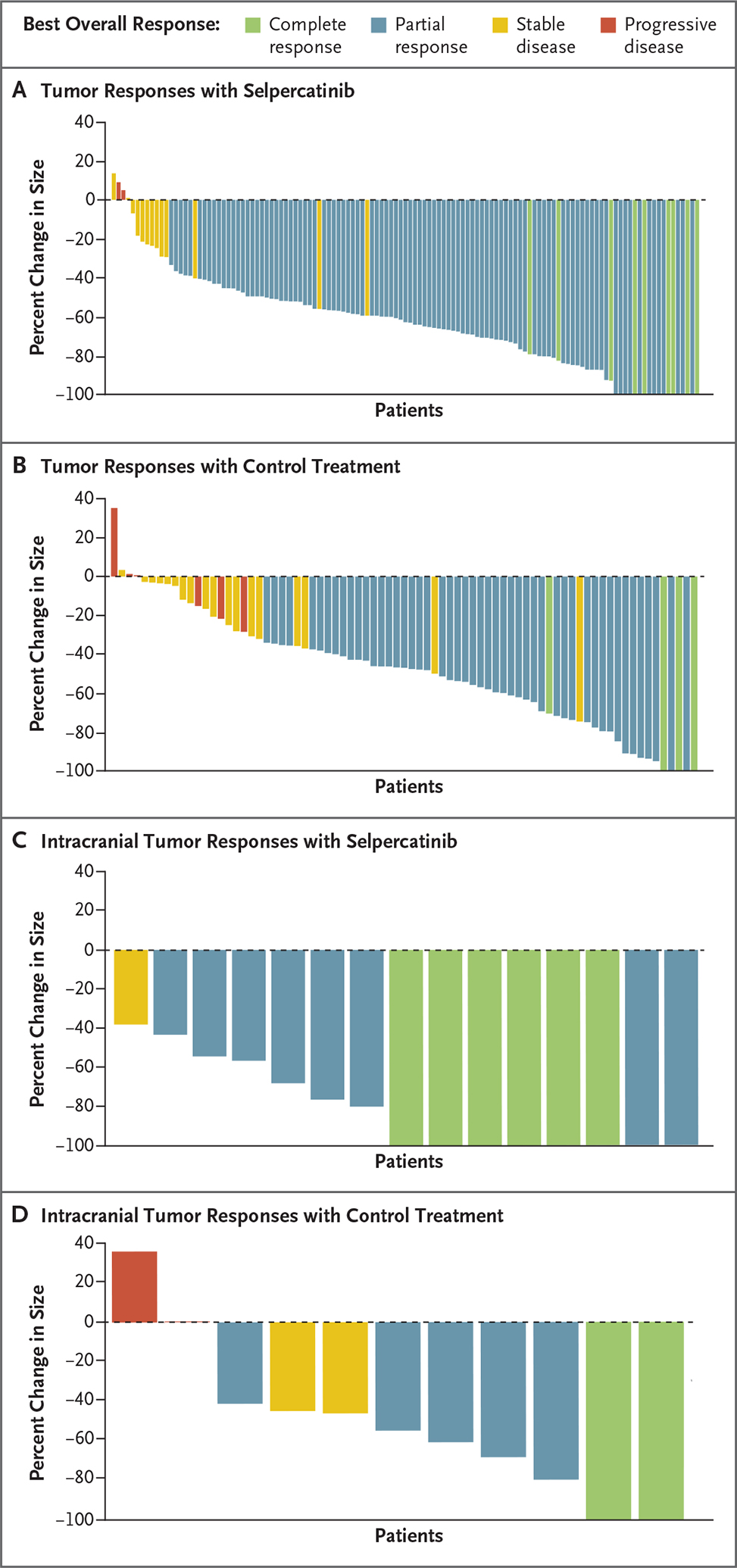
Panels A and B show waterfall plots of the maximum change from baseline in tumor size for patients with at least one evaluable postbaseline assessment according to blinded independent central review; data were available for 123 patients in the selpercatinib group and 77 patients in the control group in the intention-to-treat–pembrolizumab population. Panels C and D show waterfall plots of the maximum change from baseline in intracranial tumor size according to blinded independent central review for 15 patients in the selpercatinib group and 11 patients in the control group who had measurable brain metastases at baseline and at least one evaluable postbaseline assessment.
ADVERSE EVENTS
A summary of the safety profile among the 256 patients who received treatment is shown in Table S4. Adverse events that occurred at a higher incidence with selpercatinib than with control treatment (by ≥10 percentage points) included increases in aspartate aminotransferase (AST) levels (grade ≥3 in 13% of the patients in the selpercatinib group), increases in alanine aminotransferase (ALT) levels (grade ≥3 in 22%), hypertension (grade ≥3 in 20%), diarrhea (grade ≥3 in 1%), edema (grade ≥3 in 3%), dry mouth (no grade ≥3 events), increases in bilirubin levels (grade ≥3 in 1%), and a prolonged QTc interval on an electrocardiogram (grade ≥3 in 9%) (Table 3). Adverse events that occurred at a higher incidence with control treatment than with selpercatinib (by ≥10 percentage points) included anemia, fatigue, neutropenia, nausea, constipation, decreased appetite, pyrexia, vomiting, and pruritus (Table 3). Overall, the incidence of grade 3 or higher adverse events was higher with selpercatinib than with control treatment (70% vs. 57%) (Table S4). Adverse events leading to dose reductions occurred in 51% of the patients who received selpercatinib, as compared with 29% of those who received control treatment (Table S4).
Table 3.
Adverse Events That Occurred during Treatment (Safety Population).*
| Event | Selpercatinib (N = 158) | Control (N = 98) | ||
|---|---|---|---|---|
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| number of patients (percent) | ||||
| Any event | 158 (100) | 111 (70) | 97 (99) | 56 (57) |
| AST increase | 97 (61) | 20 (13) | 39 (40) | 1 (1) |
| ALT increase | 95 (60) | 35 (22) | 39 (40) | 3 (3) |
| Hypertension | 76 (48) | 32 (20) | 7 (7) | 3 (3) |
| Diarrhea | 70 (44) | 2 (1) | 24 (24) | 2 (2) |
| Edema | 65 (41) | 4 (3) | 27 (28) | 0 |
| Dry mouth | 62 (39) | 0 | 6 (6) | 0 |
| Blood bilirubin increase | 59 (37) | 2 (1) | 1 (1) | 0 |
| Rash | 52 (33) | 3 (2) | 29 (30) | 1 (1) |
| Fatigue | 51 (32) | 5 (3) | 49 (50) | 5 (5) |
| Thrombocytopenia | 42 (27) | 5 (3) | 28 (29) | 7 (7) |
| Abdominal pain | 40 (25) | 1 (1) | 19 (19) | 2 (2) |
| Leukopenia | 40 (25) | 2 (1) | 32 (33) | 7 (7) |
| Blood creatinine increase | 39 (25) | 2 (1) | 17 (17) | 1 (1) |
| Neutropenia | 36 (23) | 3 (2) | 44 (45) | 27 (28) |
| Constipation | 34 (22) | 0 | 39 (40) | 1 (1) |
| QT prolongation on ECG | 32 (20) | 14 (9) | 1 (1) | 0 |
| Decreased appetite | 27 (17) | 0 | 33 (34) | 2 (2) |
| Pyrexia | 21 (13) | 1 (1) | 23 (23) | 0 |
| Nausea | 20 (13) | 0 | 43 (44) | 1 (1) |
| Vomiting | 20 (13) | 0 | 23 (23) | 1 (1) |
| Anemia | 18 (11) | 2 (1) | 58 (59) | 10 (10) |
| Pruritus | 16 (10) | 0 | 22 (22) | 0 |
Shown are events that occurred during treatment in at least 20% of the patients in either group. The terms used to describe the adverse events are adapted from or composites of Medical Dictionary for Regulatory Activities, version 25.0, preferred terms. ALT denotes alanine aminotransferase, AST aspartate aminotransferase, and ECG electrocardiogram.
The median relative dose intensity was high for both selpercatinib and the control agents (88.8% and 92.2 to 97.7%, respectively). Patients in East Asia, who constituted 54% of the intention-to-treat population, had a higher incidence of grade 3 or higher adverse events, serious adverse events, and treatment discontinuations due to adverse events than patients not from East Asia (Table S5). Adverse events leading to permanent discontinuation of treatment were reported in 10% of the patients in the selpercatinib group and 2% of the patients in the control group. Fatal adverse events that occurred during participation in the trial either during treatment or within 30 days after treatment discontinuation occurred in 7 patients (4.4%) in the selpercatinib group and none of the patients in the control group; the deaths in 2 of 7 patients were judged by the investigators to be related to selpercatinib. Details of the deaths are provided in Table S6.
PATIENT-REPORTED OUTCOMES
In the intention-to-treat–pembrolizumab population, 71% of the patients in the selpercatinib group and 69% of the patients in the control group completed all items in the NSCLC-SAQ at baseline. The percentages of patients who completed all items in the NSCLC-SAQ at time points after baseline were greater than 80% across most assessed time points in both groups.
The percentage of patients with confirmed worsening of symptoms as defined with the NSCLC-SAQ total score was lower in the selpercatinib group than in the control group (30 patients [23%] vs. 36 patients [43%]) (Table S7). The median time to confirmed worsening of pulmonary symptoms was not yet reached in the selpercatinib group and was 1.9 months (95% CI, 0.7 to 6.6) in the control group (hazard ratio, 0.34, 95% CI, 0.20 to 0.55) (Fig. S10). Consistent results were reported in the overall intention-to-treat population.
DISCUSSION
In this randomized, controlled trial that directly evaluated the efficacy and safety of a targeted therapy as compared with platinum-based chemotherapy plus pembrolizumab as first-line therapy for patients with advanced NSCLC, selpercatinib resulted in significantly longer progression-free survival than the control treatment. The percentage of patients with a response to treatment and the duration of the response were greater with selpercatinib than with control treatment, and comparisons of progression-free survival in prespecified subgroups were directionally consistent with these results. The percentage of patients with an intracranial response, 82%, is consistent with findings in previous studies and shows that selpercatinib has the ability to treat existing CNS metastases.16 The data on the time to intracranial progression also indicate that selpercatinib may prevent or delay the formation of new intracranial metastases. Although treatment for advanced or metastatic NSCLC has improved in recent years, it has been reported that more than 40% of patients do not receive therapy after first-line treatment, which indicates the need for the most effective therapies to be used early in treatment.17–19 Given the benefit with respect to progression-free survival, our data support selpercatinib as first-line therapy for patients with RET fusion–positive advanced NSCLC, although the adverse-event profile should be kept in mind, and they confirm the findings from the LIBRETTO-001 study.1,20
Median progression-free survival in the selpercatinib group was more than 2 years, which was more than double the progression-free survival in the control group. This is particularly noteworthy given that outcomes in the control group were similar to or better than those previously reported in the KEYNOTE-189 trial.7,21 At the time of this preplanned interim efficacy analysis, overall survival data remain both immature and confounded by a high frequency of crossover both between the groups in the trial itself and to commercially available selective RET inhibitors. Follow-up is ongoing, although mature overall survival data are not expected for several years.
The adverse events reported with selpercatinib and with the KEYNOTE-189 regimen were generally consistent with those reported previously.1,7,20–22 The frequency of adverse events, including events with fatal outcomes, was higher in the selpercatinib group than in the control group. Elevated liver-function values were more commonly observed with selpercatinib than with control treatment, especially elevations in levels of ALT (grade ≥3 in 22% of the patients), AST (grade ≥3 in 13%), and bilirubin (grade ≥3 in 1%). The majority of these adverse events were managed with dose adjustments and did not result in treatment discontinuation (3 patients [2%] discontinued because of elevated liver-function values). No patients had hepatic failure. Elevations in liver-function values were previously reported in phase 2 studies of selpercatinib.20,22 Hematologic toxic effects were the most common adverse events with control treatment. The majority of adverse events reported in the selpercatinib group are monitorable with standard clinical assessments, and dose adjustments enabled most patients who had adverse events to continue receiving selpercatinib. A high incidence of certain adverse events (including increases in AST or ALT levels, hypertension, and QTc prolongation) were observed among patients from East Asia, a finding that was consistent with those of previous studies.1,20,22 Despite this finding, the benefit with respect to progression-free survival supports the positive benefit–risk balance in this population.
Disease-related symptoms of cough, dyspnea, and chest pain have been reported to have a negative effect on health-related quality of life of patients with lung cancer.23,24 The time to worsening of pulmonary symptoms was delayed in the selpercatinib group (because the median time was not estimable), whereas the median time to worsening of symptoms in the control group was 1.9 months. These results should be interpreted with caution because of the relatively low percentages of patients who completed the NSCLC-SAQ at baseline.
In this randomized trial of a targeted agent in comparison with a PD-1 inhibitor plus chemotherapy for patients with biomarker-defined, advanced NSCLC, the efficacy of selpercatinib was superior in patients with RET fusion–positive NSCLC. The outcomes in this trial highlight the importance of comprehensive genomic testing for RET fusions at the time of diagnosis to inform first-line therapy for this patient population.
Supplementary Material
Acknowledgments
Supported by Loxo Oncology, a wholly owned subsidiary of Eli Lilly, and by grants from the National Cancer Institute, the National Institutes of Health, and the LUNGevity Foundation.
We thank the investigators and site personnel for their participation in the trial; the trial participants and their families and caregivers, without whom this work would not be possible; and the following employees of Eli Lilly: David Hyman and Collin Churchill for their insights, guidance, and revisions of the manuscript files, Justin Williams for analysis and medical writing assistance with the submitted manuscript, Sara Broncano and Bente Frimodt-Moller for critical operational and data review support, and Adrienne Gilligan, who provided critical input related to data on patient-reported outcomes.
APPENDIX
The authors’ affiliations are as follows: Shanghai Pulmonary Hospital, Tongji University School of Medicine (C.Z.), and the Department of Respiratory and Critical Care Medicine, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University (B.H.), Shanghai, the Chinese University of Hong Kong, Hong Kong (H.H.L.), and the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou (J.Z.) — all in China; Peter MacCallum Cancer Institute, Melbourne, VIC, Australia (B.S.); Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea (K.P.); Centre Léon Bérard, Lyon, France (M.P.); Hospital del Mar, Barcelona (E.A.); the Department of Oncology, Azienda Ospedaliero–Universitaria San Luigi–Orbassano, University of Turin, Turin (S.N.), and IRCCS Azienda Ospedaliero–Universitaria di Bologna, Bologna (A.A.) — both in Italy; Instituto do Câncer do Estado de São Paulo, University of São Paulo Medical School and Instituto D’Or de Ensino e Pesquisa (M.P.M.), and the Oncology Center, Hospital Śırio Libanês (F.C.S.) — both in São Paulo; the University of Texas M.D. Anderson Cancer Center, Houston (Y.Y.E.); Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York (A.D.); the Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany (J.W.); Loxo@Lilly (H.H.) and Eli Lilly (N.P., M.K.U., D.R., T.P., V.S., A.B.L., B.K.L.) — both in Indianapolis; and National Cancer Center Hospital East, Kashiwa, Japan (K.G.).
Footnotes
Contributor Information
Caicun Zhou, Shanghai Pulmonary Hospital, Tongji University School of Medicine, China
Benjamin Solomon, Peter MacCallum Cancer Institute, Melbourne, VIC in Australia
Herbert H. Loong, Shanghai, the Chinese University of Hong Kong, Hong Kong, China
Keunchil Park, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
Maurice Pérol, Centre Léon Bérard, Lyon, France
Edurne Arriola, Hospital del Mar, Barcelona
Silvia Novello, Department of Oncology, Azienda Ospedaliero–Universitaria San Luigi–Orbassano, University of Turin, Turin, Italy
Baohui Han, Department of Respiratory and Critical Care Medicine, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University, China
Jianying Zhou, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Andrea Ardizzoni, IRCCS Azienda Ospedaliero–Universitaria di Bologna, Bologna in Italy
M. Perez Mak, Instituto do Câncer do Estado de São Paulo, University of São Paulo Medical School and Instituto D’Or de Ensino e Pesquisa in São Paulo
Fernando C. Santini, Oncology Center, Hospital Śırio Libanês in São Paulo
Yasir Y. Elamin, University of Texas M.D. Anderson Cancer Center, Houston
Alexander Drilon, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York
Jürgen Wolf, Center for Integrated Oncology, University Hospital of Cologne, Cologne, Germany
Nalin Payakachat, Eli Lilly in Indianapolis
Minji K. Uh, Eli Lilly in Indianapolis
Deborah Rajakumar, Eli Lilly in Indianapolis
Hongmei Han, Loxo@Lilly in Indianapolis
Tarun Puri, Eli Lilly in Indianapolis
Victoria Soldatenkova, Eli Lilly in Indianapolis
A. Bence Lin, Eli Lilly in Indianapolis
Boris K. Lin, Eli Lilly in Indianapolis
Koichi Goto, National Cancer Center Hospital East, Kashiwa, Japan.
REFERENCES
- 1.Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion–positive non–small-cell lung cancer. N Engl J Med 2020; 383: 813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 2020; 383: 825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbiah V, Wolf J, Konda B, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol 2022; 23:1261–73. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Abreu D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol 2021; 32:881–95. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2022;20: 497–530. [DOI] [PubMed] [Google Scholar]
- 6.Planchard D, Popat S, Kerr K, et al. Correction to: “Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.” Ann Oncol 2019;30: 863–70. [DOI] [PubMed] [Google Scholar]
- 7.Garassino MC, Gadgeel S, Esteban E, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, nonsquamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2020; 21: 387–97. [DOI] [PubMed] [Google Scholar]
- 8.Berghoff AS, Bellosillo B, Caux C, et al. Immune checkpoint inhibitor treatment in patients with oncogene-addicted non-small cell lung cancer (NSCLC): summary of a multidisciplinary round-table discussion. ESMO Open 2019;4 (3): e000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016; 22: 4585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET Registry. Ann Oncol 2019; 30: 1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu C, Dong X-R, Zhao J, et al. Association of genetic and immuno-characteristics with clinical outcomes in patients with RET-rearranged non-small cell lung cancer: a retrospective multicenter study. J Hematol Oncol 2020; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Council for Harmonisation. E17: general principles for planning and design of multi-regional clinical trials (https://www.fda.gov/media/99974/download).
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–47. [DOI] [PubMed] [Google Scholar]
- 14.McCarrier KP, Atkinson TM, DeBusk KPA, Liepa AM, Scanlon M, Coons SJ. Qualitative development and content validity of the Non-small Cell Lung Cancer Symptom Assessment Questionnaire (NSCLC-SAQ), a patient-reported outcome instrument. Clin Ther 2016; 38: 794–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke N Meaningful change threshold estimation for the Non-Small Cell Lung Cancer Symptom Assessment Questionnaire (NSCLC-SAQ): psychometric analysis from a phase 3 trial. Presented at the International Society for Quality of Life Research (ISOQOL) 30th Annual Conference, Calgary, AB, Canada, October 18–21, 2023. poster. [Google Scholar]
- 16.Subbiah V, Gainor JF, Oxnard GR, et al. Intracranial efficacy of selpercatinib in RET fusion-positive non-small cell lung cancers on the LIBRETTO-001 Trial. Clin Cancer Res 2021; 27: 4160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazhenova L, Kish J, Cai B, Caro N, Feinberg B. Real-world observational study of current treatment patterns and outcomes in recurrent or locally advanced/metastatic non-small cell lung cancer. Cancer Treat Res Commun 2022; 33:100637. [DOI] [PubMed] [Google Scholar]
- 18.Davies J, Patel M, Gridelli C, de Mari-nis F, Waterkamp D, McCusker ME. Real-world treatment patterns for patients receiving second-line and third-line treatment for advanced non-small cell lung cancer: a systematic review of recently published studies. PLoS One 2017; 12(4): e0175679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess LM, Han Y, Zhu YE, Bhandari NR, Sireci A. Characteristics and outcomes of patients with RET-fusion positive non-small lung cancer in real-world practice in the United States. BMC Cancer 2021; 21: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drilon A, Subbiah V, Gautschi O, et al. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol 2023; 41: 385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med 2018; 378: 2078–92. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Y, Huang D, Zhou J, et al. Intracranial activity of selpercatinib in Chinese patients with advanced RET fusion-positive non-small-cell lung cancer in the phase II LIBRETTO-321 Trial. JCO Precis Oncol 2023; 7:e 2200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer S, Roughley A, Rider A, Taylor-Stokes G. The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer 2014; 22: 181–7. [DOI] [PubMed] [Google Scholar]
- 24.Iyer S, Taylor-Stokes G, Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer 2013; 81:288–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


