Abstract
BACKGROUND
Deep vein thrombosis (DVT) of the lower extremity is one of the most common postoperative complications, especially after craniocerebral surgery. DVT may lead to pulmonary embolism, which has a devastating impact on patient prognosis. This study aimed to investigate the incidence and risk factors of DVT in the lower limbs following craniocerebral surgery.
AIM
To identify independent risk factors for the development of postoperative DVT and to develop an effective risk prediction model.
METHODS
The demographic and clinical data of 283 patients who underwent craniocerebral surgery between December 2021 and December 2022 were retrospectively analyzed. The independent risk factors for lower extremity DVT were identified by univariate and multivariate analyses. A nomogram was created to predict the likelihood of lower extremity DVT in patients who had undergone craniocerebral surgery. The efficacy of the prediction model was determined by receiver operating characteristic curve using the probability of lower extremity DVT for each sample.
RESULTS
Among all patients included in the analysis, 47.7% developed lower extremity DVT following craniocerebral surgery. The risk of postoperative DVT was higher in those with a longer operative time, and patients with intraoperative intermittent pneumatic compression were less likely to develop postoperative DVT.
CONCLUSION
The incidence of lower extremity DVT following craniocerebral surgery is significant, highlighting the importance of identifying independent risk factors. Interventions such as the use of intermittent pneumatic compression during surgery may prevent the formation of postoperative DVT.
Keywords: Deep vein thrombosis, Craniotomy surgery, Risk factors, Nomogram
Core Tip: Deep vein thrombosis (DVT) of the lower extremity is one of the most common postoperative complications, especially after craniocerebral surgery. DVT may lead to pulmonary embolism, which has a devastating impact on patient prognosis. Therefore, preventing the formation of lower limb Deep vein thrombosis is crucial after surgery. This study aimed to investigate the incidence and risk factors of DVT in the lower limbs following craniocerebral surgery and develop effective risk prediction models.
INTRODUCTION
Deep vein thrombosis (DVT) is one of the most frequent complications of surgery that can lead to serious consequences, such as pulmonary embolism, post-thrombotic syndrome, and venous gangrene, all of which can affect postoperative recovery and the quality of life of patients[1,2]. Lower extremity deep veins are frequently affected by DVT, with the muscular calf vein being the most affected, followed by the popliteal, superficial, and common femoral veins[3].
Age, body mass index (BMI), smoking history, platelet count, D-dimer, and surgical-related factors have been identified as risk factors for postoperative DVT[4-16]. Craniocerebral surgery is associated with a higher risk of DVT than other surgical procedures due to its lengthy operation time and the release of many inflammatory factors from brain tissues during operation[17,18]. The incidence of postoperative thrombosis in craniocerebral surgery is 5%-60%[3,19]. The use of anticoagulants after surgery for the prevention of DVT formation remains inconclusive. Previous studies[20,21] have demonstrated that immediate anticoagulation intervention after surgery can effectively prevent low extremity DVT. However, this approach significantly increases the risk of fatal cerebral hemorrhage and the cost of treatment. Conversely, some studies[22] have suggested that postoperative application of anticoagulants does not result in a reduction in the incidence of DVT. It has also been suggested that mechanical techniques alone may be superior to anticoagulation intervention in preventing DVT after surgery[10,17,23].
Many previous studies have identified risk factors for the occurrence of DVT after orthopedic, gastrointestinal, or urological surgeries[2-8]. Several studies[12,24-26] have also specifically investigated the risk factors for DVT following brain surgery. However, they were either date back to earlier periods or were conducted in a single center in different countries. Intermittent pneumatic compression (IPC) device is a limb compression treatment system that applies periodic pressure changes to the peripheral tissues and vasculature of the body, preventing blood stasis, improving blood circulation, and preventing postoperative lower extremity DVT. In the present work, we aimed to identify the risk factors for lower extremity DVT and investigate the effect of intraoperative IPC on the formation of postoperative DVT in Chinese patients. A risk prediction model was also developed for predicting lower extremity DVT following craniocerebral surgery.
MATERIALS AND METHODS
Patients
Patients who underwent craniocerebral surgery between December 2021 and December 2022 in the First Affiliated Hospital of Jilin University were screened for enrollment. The inclusion criteria were as follows: (1) Aged ≥ 18 years; (2) had undergone their first craniocerebral surgery; and (3) had received a venous color ultrasound to check the DVT before and after the surgery. Patients were excluded from analysis if they had: (1) Existing DVT during the preoperative evaluation; (2) taken anticoagulant drugs or received other thrombus prevention measures; and (4) a history of interventional surgery.
Data collection
The following demographic and clinical data were collected to identify the risk factors for the onset and development of DVT: (1) Basic demographic characteristics, including age, gender, BMI, smoking history, blood glucose index, platelet count, and D-dimer[4-10]; (2) surgery-related factors, including operation time, blood transfusion during operation, use of IPC during operation, and pathological nature[10,12,18]; and (3) postoperative conditions, including postoperative Caprini score, infection, days of hemostatic application, and cortisol application[12,19].
Diagnosis of DVT
All patients underwent an ultrasound examination of their lower extremity veins on the day of admission and seven days after surgery. Venous ultrasound was performed on both lower extremities to observe the presence of DVT. Each patient was evaluated by two experienced ultrasound physicians.
Ethical statement
This study was approved by the local ethics committee and performed per the Declaration of Helsinki. All participants provided written informed consent.
Statistical analysis
AKIBM SPSS Statistics 23.0 (IBM Corp., Armonk, NY, United States) was used for data analysis. Continuous data are shown as means ± standard deviations (SD) and compared by Student’s t-test. Categorical data are expressed as numbers and percentages and were compared by χ2 test. Univariate and multivariate analyses were performed to identify the risk factors for lower extremity DVT. Factors with a P value of less than 0.05 were included in the final binary logical regression equation. A binary logistic regression model was created to identify the independent risk factors for DVT. Meanwhile, the partial maximum likelihood estimation method, namely Forward: LogisticRegression, was used to screen variables, making this model more reliable. The degree of correlation was quantified using odds ratio (OR) and 95% confidence interval (CI). The statistical significance level was set at P < 0.05. The goodness of fit of the final model was assessed using the Hosmer-Lemeshow (H-L) test, where a P value of greater than 0.05 indicated an acceptable fit. The RMS package in the R program (version 3.6.1) was used to build the nomogram model for risk evaluation. The reliability of the model was verified internally. The discriminative power of the nomogram was evaluated by the receiver operating characteristic curve (ROC) and the area under the curve (AUC). The calibration curve used to verify model consistency represents the line of fit between predicted and actual incidence. The clinical validity of the nomogram model was assessed using decision curve analysis and clinical impact curves, which quantify the net benefit of different risk threshold probabilities.
RESULTS
Postoperative deep vein thrombosis of lower limbs in patients
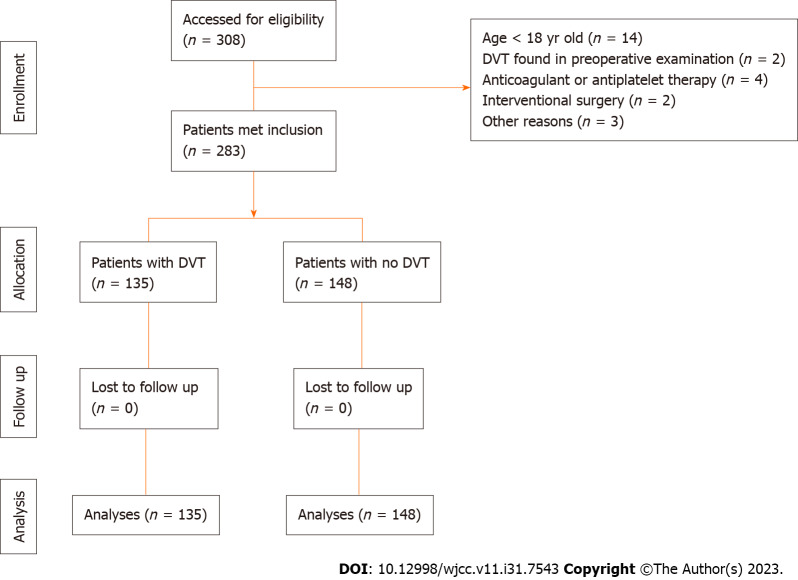
Out of 308 patients screened for enrollment, 25 were excluded due to matching the exclusion criteria. As a result, 283 patients were finally included in the analysis (Figure 1). Among them, 135 (47.7%) developed DVT, and 1 (0.4%) developed pulmonary embolism. There were no deaths within the first seven days after surgery.
Figure 1.
Screening process of sample. DVT: Deep vein thrombosis.
Factors influencing the formation of lower limb deep vein thrombosis in patients after surgery
The basic demographic characteristics, surgical-related factors, and postoperative conditions of patients were included in the univariate analysis. The results revealed that age, fasting blood glucose levels, D-dimer, postoperative infection, malignant tumor, postoperative Caprini score, postoperative cortisol use, operation time, intraoperative blood transfusion, and intraoperative IPC use (all P < 0.05) were significantly correlated with the occurrence of postoperative DVT in the lower extremities, while gender, BMI, smoking history, platelet count, and postoperative use of hemostatic drugs were not (P > 0.05, Table 1).
Table 1.
Univariate analysis of the risk factors for deep vein thrombosis
| Risk factors | No (%) of DVT (n = 135) | No (%) of No-DVT (n = 148) |
Univariate
|
|
|
HR (95%CI)
|
P value
|
|||
| Gender (male) | 52 (38.5) | 74 (50.0) | 0.627 (0.390-1.006) | 0.053 |
| Age (yr) | NA | NA | 1.072 (1.048-1.097) | < 0.001 |
| BMI (kg/m2) | NA | NA | 0.988 (0.927-1.054) | 0.718 |
| Smoking history | 31 (23.0) | 31 (20.9) | 1.125 (0.640-1.977) | 0.682 |
| Glucose | NA | NA | 1.276 (1.109-1.468) | 0.001 |
| PLT | NA | NA | 0.999 (0.995-1.002) | 0.474 |
| D-dimer (≥ 1.08 mg/L) | 88 (65.2) | 48 (32.4) | 3.901 (2.381-6.391) | < 0.001 |
| Infections | 44 (32.6) | 17 (11.5) | 3.726 (2.004-6.929) | < 0.001 |
| Malignant tumor | 61 (45.2) | 38 (25.7) | 2.386 (1.446-3.938) | 0.001 |
| Carprini | NA | NA | 1.810 (1.511-2.168) | < 0.001 |
| Hemostatic drugs (days) | NA | NA | 0.926 (0.782-1.096) | 0.371 |
| Steroid | 61 (45.2) | 33 (22.3) | 2.873 (1.717-4.805) | < 0.001 |
| Operation time | NA | NA | 1.786 (1.486-2.148) | < 0.001 |
| Transfusion | 16 (11.9) | 5 (3.4) | 8.525 (3.207-22.664) | < 0.001 |
| IPC | 9 (6.7) | 62 (41.9) | 0.200 (0.109-0.366) | < 0.001 |
This table shows the proportion of each included factor in the outcome event, Continuous data are shown as means ± standard deviations (SD) and compared by Student’s t-test. Categorical data are expressed as numbers and percentages and were compared by χ2 test. BMI: Body mass index; DVT: Deep vein thrombosis; IPC: Intermittent pneumatic compression; PLT: Platelet; HR: Hazard ratio
Multivariate analysis showed that age (OR = 1.064, 95%CI: 1.029–1.100, P < 0.001), D-dimer (OR = 5.368, 95%CI: 2.575–11.190, P < 0.001), operation time (OR = 1.446, 95%CI: 1.093–1.914, P = 0.010), intraoperative blood transfusion (OR = 3.828, 95%CI: 1.056–13.873, P = 0.041), intraoperative use of IPC (OR = 0.094, 95%CI: 0.036–0.242, P < 0.001), postoperative infection (OR = 3.553, 95%CI: 1.397–9.039, P = 0.008), postoperative Caprini score (OR = 1.731, 95%CI: 1.308–2.290 P < 0.001), and postoperative steroid use (OR = 2.619, 95%CI: 1.203–5.706, P = 0.015) were independent risk factors for low extremity DVT (Table 2).
Table 2.
Multivariate analysis of the risk factors for deep vein thrombosis
|
Risk factors
|
β
|
Sx
|
P value
|
OR
|
95%CI
|
|
|
Lower limit
|
Upper limit
|
|||||
| Age | 0.062 | 0.017 | < 0.001 | 1.064 | 1.029 | 1.100 |
| D-dimer | 1.681 | 0.375 | < 0.001 | 5.368 | 2.575 | 11.190 |
| Infections | 1.268 | 0.476 | 0.008 | 3.553 | 1.397 | 9.039 |
| Carprini | 0.548 | 0.143 | < 0.001 | 1.731 | 1.308 | 2.290 |
| Steroid | 0.963 | 0.397 | 0.015 | 2.619 | 1.203 | 5.706 |
| Operation time | 0.369 | 0.143 | 0.010 | 1.446 | 1.093 | 1.914 |
| Transfusion | 1.342 | 0.657 | 0.041 | 3.828 | 1.056 | 13.873 |
| IPC | -2.370 | 0.486 | < 0.001 | 0.094 | 0.036 | 0.242 |
The P value and 95% confidence interval of the final included factors were calculated using the binary metalogic regression equation. IPC: Intermittent pneumatic compression.
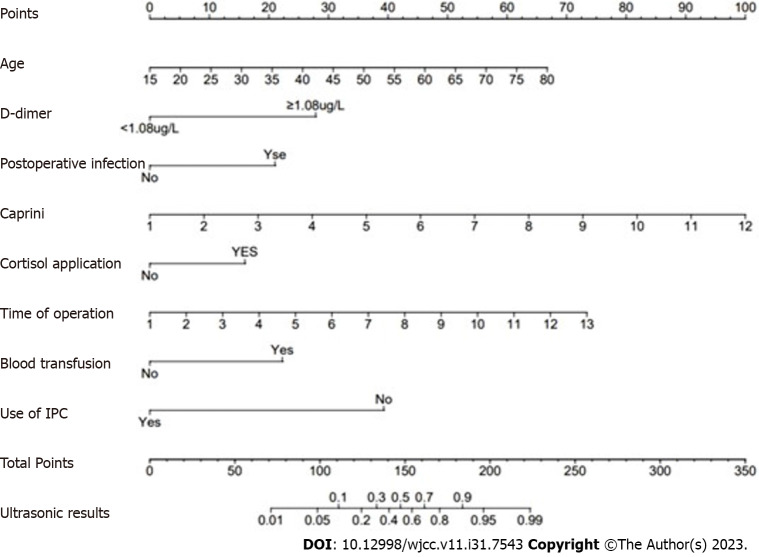
Based on the results of multifactorial logistic regression analyses of the study population, a nomogram model (Figure 2) was constructed using eight independent risk factors, including age, D-dimer, postoperative infection, caprini score, postoperative steroidal cortisol application, operative time, intraoperative blood transfusion, and intraoperative IPC use. The predicted probability corresponding to the total score was the risk of postoperative DVT in patients undergoing craniotomy. For instance, there is a 50-year-old patient who underwent a 4-hour surgery without the use of IPC and received a blood transfusion during the operation. On postoperative day 3, the patient had a D-dimer level of 0.68 (mg/L) and a Caprini score of 4. There were no postoperative infections, and no cortisol-based medications were administered. The sum of the scores for each predictor was 131, resulting in a calculated risk of postoperative DVT at 28%.
Figure 2.
Predictive nomogram for postoperative deep vein thrombosis in patients with craniotomy. IPC: Intermittent pneumatic compression.
Verification of the nomogram model calibration
The nomogram model was internally validated, as shown by the calibration curve for postoperative DVT nomogram prediction in patients. The X-axis represents the nomogram-predicted probability of DVT in patients, and the Y-axis represents the actual probability of DVT (Figure 3). The predicted probability of the nomogram highly aligned with the actual probability.
Figure 3.
Calibration curve for nomogram of postoperative deep vein thrombosis in patients with craniotomy.
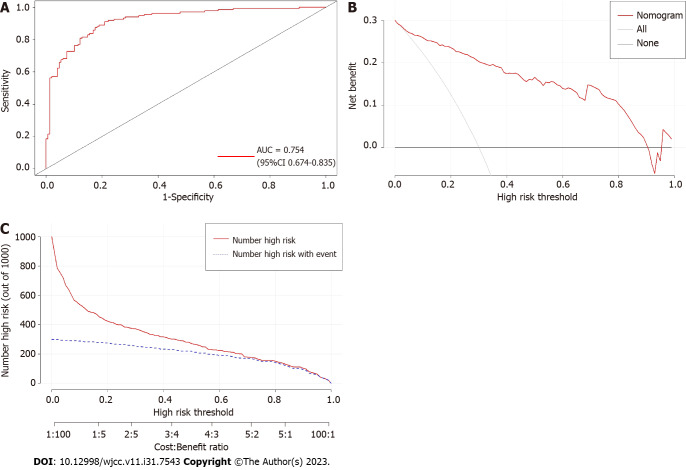
Validation of nomogram efficiency
The nomogram predictive model demonstrated strong discrimination, as indicated by the ROC curve with an AUC value of 0.754 (95%CI: 0.674–0.835) (Figure 4A). To evaluate the availability and benefits of the prediction model, decision curve analysis and clinical impact curve were used (Figures 4B and C). As for the risk of postoperative DVT for patients undergoing craniotomy, the clinical efficacy of the nomogram model is promising.
Figure 4.
Validation of predictive model performance. A: The receiver operating characteristic curve of the nomogram model. The area under the curve was used to evaluate the discrimination of the model; B: The decision curve analysis for nomogram to predict the risk of postoperative deep vein thrombosis in patients with craniotomy; C: The clinical impact curves for nomogram to predict the risk of postoperative deep vein thrombosis in patients with craniotomy. AUC: Area under the curve.
DISCUSSION
DVT is a common postoperative complication after craniocerebral surgery, which can lead to pulmonary embolism[19], post-thrombotic syndrome, and venous gangrene[1,2], posing a serious threat to patients’ lives. Brain tissue damage during craniocerebral surgery may lead to the release of various tissue factors and cause brain retraction injuries, both of which can promote blood coagulation and increase the risk of DVT occurrence[11,12,19]. In the present study, the incidence of postoperative DVT was 47.7%, which was in line with previous studies investigating the incidence of postoperative DVT after neurosurgery[3,12,14,20]. Furthermore, eight independent risk factors for DVT after craniocerebral surgery were identified, including age, D-dimer, postoperative infection, postoperative Caprini score, postoperative cortisol application, operation time, intraoperative blood transfusion, and intraoperative IPC application.
Serum D-dimer measurement is a more convenient and cost-effective method for detecting DVT than lower extremity venous ultrasound[1,16]. However, the clinical application of D-dimer is limited by its high false positive rate, which can be elevated in the elderly and patients with tumors or infections[2]. The specificity is significantly increased by slightly reducing the sensitivity. When a patient presents with an exceptionally high D-dimer, it is recommended that they undergo a lower extremity venous ultrasound to confirm the presence of lower extremity DVT[27-30].
Infection is also a risk factor for the development of DVT. When infections invade the body, the production of cytokines by neutrophils increases, triggering the release of tissue factors by monocytes and platelets, which induces blood coagulation and fibrin formation. Previous evidence has revealed that the risk of infection-associated DVT can persist for six months[12]. Therefore, it is essential to closely monitor patients with any signs of postoperative infection.
The Caprini score is a reliable tool for predicting the risk of DVT. Previous studies have demonstrated that individuals with a Caprini score higher than[4] are at increased risk for developing DVT[2]. Venous dilations during surgery are considered a pivotal contributor to venous thrombosis. The prolonged surgical procedure results in significant blood stasis in the venous system, causing veinous distension. Intraoperative fluid infusion and muscle relaxants can also lead to various degrees of venous dilation. These factors collectively induce the occurrence of DVT[2].
DVT prevention strategies are often implemented after surgery rather than during the perioperative period[10,17,20,23,31,32]. However, taking preventive measures during the surgery is crucial to prevent postoperative DVT in the lower extremities. While some scholars suggest that the combination of physical and chemical prevention may be more effective in preventing DVT[20], the use of anticoagulants may increase the risk of postoperative hemorrhage, especially fatal cerebral hemorrhage. Therefore, physical measures are commonly used alone to prevent DVT[10,17,23]. In this study, we found that perioperative use of IPC effectively prevented the occurrence of DVT after craniocerebral surgery.
IPC was first applied in clinical practice by Calnan et al[33] in 1970. They attached the IPC to the patient’s lower legs and used regular inflation and deflation to simulate the contraction of leg muscles, promoting blood flow back to the heart[33]. IPC is commonly used to prevent thrombosis after surgery. The plasma concentration of the thrombin-antithrombin III complex peaks three hours after operation[13]. As craniocerebral surgery often lasts for a long period, it may increase the risk of thrombosis during the operation rather than after it[19]. Therefore, we suggest using IPC during the surgery to prevent DVT. IPC causes contractions of the muscle, which reduce venous pressure, increase the arteriovenous gradient, promote arterial blood flow, and reduce stasis. The pressure within the subcutaneous tissues also rises, facilitating the entry of tissue fluid into the blood circulation and reducing subcutaneous edema[10]. In addition, IPC can also promote the production of nitric oxide and prostacyclin by vascular endothelial cells, leading to a decrease in the plasminogen activator inhibitor and inhibition of the tissue factor pathway. However, this effect can only last 30 min after the device stops[10,19,32]. During craniocerebral surgeries involving functional regions, it is important to maintain critical craniocerebral functions, during which patients are often awakened during the procedure and asked to assist in performing limb exercises to prevent DVT. A previous study has also shown that IPC applied to only one lower limb is equally effective in preventing DVT[32].
The nomogram model has been widely used in clinical prediction, offering an intuitive and visually accessible means of presenting results. Few models are currently available to predict the risk of perioperative DVT in patients with lung cancer brain metastases. In this study, we established a nomogram model for predicting the risk of postoperative DVT in this population, which provided valuable insights for DVT risk assessment among them.
The limitations of this study should also be acknowledged. Firstly, the retrospective nature may introduce selection bias to the results. In addition, only internal validation was performed. Further investigations with a multicenter design and a large sample size were needed at a later stage for external validation.
CONCLUSION
Age, D-dimer, postoperative infection, postoperative Caprini score, postoperative cortisol application, operation time, intraoperative blood transfusion, and intraoperative IPC application are risk factors for lower limb DVT after craniocerebral surgery. It is crucial to recognize the independent risk factors and take proactive measures to prevent DVT, such as reducing operation time, maintaining strict aseptic protocols during the procedure, and preventing postoperative infection. Mechanical precautions, such as IPC, should be implemented during the operation to minimize the incidence of DVT.
ARTICLE HIGHLIGHTS
Research background
Lethal pulmonary embolism caused by deep vein thrombosis after surgery is a common cause of sudden death in postoperative patients.
Research motivation
Early identification and timely intervention in people at a high risk of postoperative deep vein thrombosis are essential for preventing the development of fatal pulmonary embolism.
Research objectives
The purpose of this study is to observe the factors affecting the development of deep vein thrombosis after cranio-cerebral surgery, to investigate the relationship between the general characteristics of patients, surgery-related factors and postoperative conditions and postoperative deep vein thrombosis, and to establish a reliable prediction model for postoperative deep vein thrombosis.
Research methods
In this study, data from 283 patients who underwent craniotomy were collected and analyzed retrospectively. Patients were classified into thrombotic and non-thrombotic groups based on the presence or absence of postoperative deep vein thrombosis, and the clinical data of the two groups were compared. Independent risk factors for deep vein thrombosis were screened by statistical analysis. A nomogram model was developed to predict the likelihood of deep vein thrombosis in patients undergoing cranial surgery based on the identified independent risk factors. The reliability of the model was verified.
Research results
Of the included patients, 47.7% developed deep vein thrombosis after craniotomy surgery. Statistical analysis yielded eight independent risk factors. A reliable nomogram model was developed to predict the risk of postoperative deep vein thrombosis after craniotomy.
Research conclusions
This study identified eight risk factors associated with postoperative lower extremity deep vein thrombosis after open heart surgery: Age, D-dimer, postoperative infection, postoperative Caprini score, postoperative cortisol application, operation time, intraoperative blood transfusion, and intraoperative intermittent pneumatic compression application. A reliable nomogram model was developed for the early identification of patients at a high risk of postoperative deep vein formation.
Research perspectives
Previous studies of postoperative deep vein thrombosis have primarily focused on analyzing risk factors without establishing an efficient and reliable predictive model. In this study, we enrolled patients who underwent craniotomy and established a risk prediction model based on the risk factors. The efficacy of the model was also verified. These findings provide an important reference for early detection of patients at a high risk of postoperative deep vein thrombosis.
Footnotes
Institutional review board statement: This retrospective study has been reviewed by the Ethics Committee of the First Hospital of Jilin University, and all procedures being performed were part of routine care.
Informed consent statement: Informed written consent was obtained from the patients for the release of clinical data involved in this study.
Conflict-of-interest statement: The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 14, 2023
First decision: September 26, 2023
Article in press: October 23, 2023
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shariati MBH, Iran S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
Contributor Information
Zhen-Jin Su, Department of Neurosurgery, The First Hospital of Jilin University, Changchun 130000, Jilin Province, China.
Hong-Rui Wang, Department of Operating Theater, The First Hospital of Jilin University, Changchun 130000, Jilin Province, China.
Li-Qin Liu, Department of Operating Theater, The First Hospital of Jilin University, Changchun 130000, Jilin Province, China.
Nan Li, Department of Operating Theater, The First Hospital of Jilin University, Changchun 130000, Jilin Province, China.
Xin-Yu Hong, Department of Neurosurgery, The First Hospital of Jilin University, Changchun 130000, Jilin Province, China. hongxy@jlu.edu.cn.
Data sharing statement
Data sharing statement: The authors state that raw data related to the study will be shared.
References
- 1.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–3073. doi: 10.1016/S0140-6736(16)30514-1. [DOI] [PubMed] [Google Scholar]
- 2.Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3–10. doi: 10.1016/j.amjsurg.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Qiu T, Zhang T, Liu L, Li W, Li Q, Zhang X, Jiao Y, Ma H. The anatomic distribution and pulmonary embolism complications of hospital-acquired lower extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2021;9:1391–1398.e3. doi: 10.1016/j.jvsv.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Panpikoon T, Chuntaroj S, Treesit T, Chansanti O, Bua-Ngam C. Lower-Extremity Venous Ultrasound in DVT-Unlikely Patients with Positive D-Dimer Test. Acad Radiol. 2022;29:1058–1064. doi: 10.1016/j.acra.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Ellis HB Jr, Sabatino MJ, Clarke Z, Dennis G, Fletcher AL, Wyatt CW, Zia A, Wilson PL. The Importance of a Standardized Screening Tool to Identify Thromboembolic Risk Factors in Pediatric Lower Extremity Arthroscopy Patients. J Am Acad Orthop Surg. 2019;27:335–343. doi: 10.5435/JAAOS-D-18-00390. [DOI] [PubMed] [Google Scholar]
- 6.Tian Q, Li M. Risk factors of deep vein thrombosis of lower extremity in patients undergone gynecological laparoscopic surgery: what should we care. BMC Womens Health. 2021;21:130. doi: 10.1186/s12905-021-01276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan Z, Hu H, Deng X, Zhu J, Zhu Y, Ye D, Cheng X, Zhang Y. Incidence and risk factors for deep venous thrombosis of lower extremity after surgical treatment of isolated patella fractures. J Orthop Surg Res. 2021;16:90. doi: 10.1186/s13018-021-02240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Z, Mi B, Liu X, Panayi AC, Xiong Y, Xue H, Zhou W, Cao F, Liu J, Hu L, Hu Y, Chen L, Yan C, Xie X, Guo J, Hou Z, Sun Y, Zhang Y, Liu G. Nomogram for Predicting Deep Venous Thrombosis in Lower Extremity Fractures. Biomed Res Int. 2021;2021:9930524. doi: 10.1155/2021/9930524. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Chopard R, Albertsen IE, Piazza G. Diagnosis and Treatment of Lower Extremity Venous Thromboembolism: A Review. JAMA. 2020;324:1765–1776. doi: 10.1001/jama.2020.17272. [DOI] [PubMed] [Google Scholar]
- 10.Pranata R, Deka H, Yonas E, Vania R, Tondas AE, Lukito AA, July J. The use of intermittent pneumatic compression to prevent venous thromboembolism in neurosurgical patients-A systematic review and meta-analysis. Clin Neurol Neurosurg. 2020;191:105694. doi: 10.1016/j.clineuro.2020.105694. [DOI] [PubMed] [Google Scholar]
- 11.Shi S, Cheng J, Chen H, Zhang Y, Zhao Y, Wang B. Preoperative and intraoperative predictors of deep venous thrombosis in adult patients undergoing craniotomy for brain tumors: A Chinese single-center, retrospective study. Thromb Res. 2020;196:245–250. doi: 10.1016/j.thromres.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Nakano F, Matsubara T, Ishigaki T, Hatazaki S, Mouri G, Nakatsuka Y, Suzuki H. Incidence and risk factor of deep venous thrombosis in patients undergoing craniotomy for brain tumors: A Japanese single-center, retrospective study. Thromb Res. 2018;165:95–100. doi: 10.1016/j.thromres.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Chen H, Liu M, Lin Z, Ren X, Wang Y, Zou X, Gu Z. A risk prediction model for evaluating thrombosis extension of muscle calf venous thrombosis after craniotomy. Front Surg. 2022;9:992576. doi: 10.3389/fsurg.2022.992576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Zhao B, Xu G, Zhou J. A nomogram for individualized prediction of lower extremity deep venous thrombosis in stroke patients: A retrospective study. Medicine (Baltimore) 2022;101:e31585. doi: 10.1097/MD.0000000000031585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, He M, Jia W, Xie W, Song Y, Wang H, Peng J, Li Y, Wang Z, Lin Z. Analysis of high-risk factors for preoperative DVT in elderly patients with simple hip fractures and construction of a nomogram prediction model. BMC Musculoskelet Disord. 2022;23:441. doi: 10.1186/s12891-022-05377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Z, Zhao K, Jin L, Lian X, Zhang Z, Ma L, Hou Z. Combination of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio with plasma D-dimer level to improve the diagnosis of deep venous thrombosis (DVT) following ankle fracture. J Orthop Surg Res. 2023;18:362. doi: 10.1186/s13018-023-03840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinaldo L, Brown DA, Bhargav AG, Rusheen AE, Naylor RM, Gilder HE, Monie DD, Youssef SJ, Parney IF. Venous thromboembolic events in patients undergoing craniotomy for tumor resection: incidence, predictors, and review of literature. J Neurosurg. 2019;132:10–21. doi: 10.3171/2018.7.JNS181175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma J, Du P, Qin J, Zhou Y, Liang N, Hu J, Zhang Y, Zhu Y. Incidence and risk factors predicting deep venous thrombosis of lower extremity following spinal fractures. Sci Rep. 2021;11:2441. doi: 10.1038/s41598-021-82147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prell J, Schenk G, Taute BM, Scheller C, Marquart C, Strauss C, Rampp S. Reduced risk of venous thromboembolism with the use of intermittent pneumatic compression after craniotomy: a randomized controlled prospective study. J Neurosurg. 2018:1–7. doi: 10.3171/2017.9.JNS17533. [DOI] [PubMed] [Google Scholar]
- 20.Hoefnagel D, Kwee LE, van Putten EH, Kros JM, Dirven CM, Dammers R. The incidence of postoperative thromboembolic complications following surgical resection of intracranial meningioma. A retrospective study of a large single center patient cohort. Clin Neurol Neurosurg. 2014;123:150–154. doi: 10.1016/j.clineuro.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita Y, Morimoto T, Muraoka N, Oyakawa T, Umetsu M, Akamatsu D, Nishimoto Y, Sato Y, Takada T, Jujo K, Minami Y, Ogihara Y, Dohi K, Fujita M, Nishikawa T, Ikeda N, Hashimoto G, Otsui K, Mori K, Sueta D, Tsubata Y, Shoji M, Shikama A, Hosoi Y, Tanabe Y, Chatani R, Tsukahara K, Nakanishi N, Kim K, Ikeda S, Mo M, Yoshikawa Y, Kimura T ONCO DVT Study Investigators. Edoxaban for 12 Months Versus 3 Months in Cancer Patients With Isolated Distal Deep Vein Thrombosis (ONCO DVT study): An Open-label, Multicenter, Randomized Clinical Trial. Circulation. 2023 doi: 10.1161/CIRCULATIONAHA.123.066360. [DOI] [PubMed] [Google Scholar]
- 22.Lambrechts MJ, Fried T, D'Antonio ND, Karamian BA, Bodnar JG, Somers S, Canseco JA, Kaye ID, Woods BI, Hilibrand AS, Kepler CK, Vaccaro AR, Schroeder GD. Is Deep Vein Thrombosis Chemoprophylaxis Indicated After Spinal Irrigation and Débridement? World Neurosurg. 2022;168:e278–e285. doi: 10.1016/j.wneu.2022.09.111. [DOI] [PubMed] [Google Scholar]
- 23.Danish SF, Burnett MG, Ong JG, Sonnad SS, Maloney-Wilensky E, Stein SC. Prophylaxis for deep venous thrombosis in craniotomy patients: a decision analysis. Neurosurgery. 2005;56:1286–92; discussion 1292. doi: 10.1227/01.neu.0000159882.11635.ea. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Guo J, Long Y, Yin Y, Hou Z. Risk factors for preoperative deep venous thrombosis in hip fracture patients: a meta-analysis. J Orthop Traumatol. 2022;23:19. doi: 10.1186/s10195-022-00639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein NE. A review of the risks and benefits of differing prophylaxis regimens for the treatment of deep venous thrombosis and pulmonary embolism in neurosurgery. Surg Neurol. 2005;64:295–301; discussion 302. doi: 10.1016/j.surneu.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 26.Kshettry VR, Rosenbaum BP, Seicean A, Kelly ML, Schiltz NK, Weil RJ. Incidence and risk factors associated with in-hospital venous thromboembolism after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2014;21:282–286. doi: 10.1016/j.jocn.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Tang G, Qi L, Sun Z, Liu J, Lv Z, Chen L, Huang B, Zhu S, Liu Y, Li Y. Evaluation and analysis of incidence and risk factors of lower extremity venous thrombosis after urologic surgeries: A prospective two-center cohort study using LASSO-logistic regression. Int J Surg. 2021;89:105948. doi: 10.1016/j.ijsu.2021.105948. [DOI] [PubMed] [Google Scholar]
- 28.Barrosse-Antle ME, Patel KH, Kramer JA, Baston CM. Point-of-Care Ultrasound for Bedside Diagnosis of Lower Extremity DVT. Chest. 2021;160:1853–1863. doi: 10.1016/j.chest.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Goodacre S, Sampson F, Thomas S, van Beek E, Sutton A. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging. 2005;5:6. doi: 10.1186/1471-2342-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernardi E, Camporese G. Diagnosis of deep-vein thrombosis. Thromb Res. 2018;163:201–206. doi: 10.1016/j.thromres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Adeeb N, Hattab T, Savardekar A, Jumah F, Griessenauer CJ, Musmar B, Adeeb A, Trosclair K, Guthikonda B. Venous Thromboembolism Prophylaxis in Elective Neurosurgery: A Survey of Board-Certified Neurosurgeons in the United States and Updated Literature Review. World Neurosurg. 2021;150:e631–e638. doi: 10.1016/j.wneu.2021.03.072. [DOI] [PubMed] [Google Scholar]
- 32.Auguste KI, Quiñones-Hinojosa A, Berger MS. Efficacy of mechanical prophylaxis for venous thromboembolism in patients with brain tumors. Neurosurg Focus. 2004;17:E3. doi: 10.3171/foc.2004.17.4.3. [DOI] [PubMed] [Google Scholar]
- 33.Calnan JS, Pflug JJ, Mills CJ. Pneumatic intermittent-compression legging simulating calf-muscle pump. Lancet. 1970;2:502–503. doi: 10.1016/s0140-6736(70)90118-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing statement: The authors state that raw data related to the study will be shared.