Abstract
Rationale
The development and progression of alcohol use disorder (AUD) are widely viewed as maladaptive neuroplasticity. The transmembrane alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) regulatory protein γ8 (TARP γ-8) is a molecular mechanism of neuroplasticity that has not been evaluated in AUD or other addictions.
Objective
To address this gap in knowledge, we evaluated the mechanistic role of TARP γ-8 bound AMPAR activity in the basolateral amygdala (BLA) and ventral hippocampus (vHPC) in the positive reinforcing effects of alcohol, which drive repetitive alcohol use throughout the course of AUD, in male C57BL/6 J mice. These brain regions were selected because they exhibit high levels of TARP γ-8 expression and send glutamate projections to the nucleus accumbens (NAc), which is a key nucleus in the brain reward pathway.
Methods and results
Site-specific pharmacological inhibition of AMPARs bound to TARP γ-8 in the BLA via bilateral infusion of the selective negative modulator JNJ-55511118 (0–2 μg/μl/side) significantly decreased operant alcohol self-administration with no effect on sucrose self-administration in behavior-matched controls. Temporal analysis showed that reductions in alcohol-reinforced response rate occurred > 25 min after the onset of responding, consistent with a blunting of the positive reinforcing effects of alcohol in the absence of nonspecific behavioral effects. In contrast, inhibition of TARP γ-8 bound AMPARs in the vHPC selectively decreased sucrose self-administration with no effect on alcohol.
Conclusions
This study reveals a novel brain region-specific role of TARP γ-8 bound AMPARs as a molecular mechanism of the positive reinforcing effects of alcohol and non-drug rewards.
Keywords: Alcohol use, AMPA receptor, TARP γ-8, Basolateral amygdala, JNJ-55511118
Introduction
Alcohol use disorder (AUD) is a multiphasic neuropsychiatric condition that impacts the health and well-being of over 14 million adults each year in the USA (SAMHSA 2019). During the binge-intoxication stage of AUD, the positive reinforcing properties of the drug promote a pattern of chronic repetitive use, followed by escalated intake over time and the development of physical dependence (Koob and Volkow 2010; Wise and Koob 2013). For these reasons, research that identifies neural mechanisms that regulate the positive reinforcing properties of alcohol is crucial to understanding the etiology and progression of AUD.
The behavioral process of positive reinforcement increases the rate of adaptive behavior; however, substances of abuse can usurp neural mechanisms of reward and reinforcement and promote drug-seeking behavior. This form of maladaptive plasticity is mediated, in part, through glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) activity in reward-related brain regions, including the amygdala (Kalivas 2009; Kauer and Malenka 2007; Koob 2003; 2009; Koob and Volkow 2010; Loweth et al. 2014; Marty and Spigelman 2012; McCool 2011; Roberts et al. 1996; Weiss and Koob 2001; Winder et al. 2002). Our prior research shows that CaMKII-dependent AMPAR signaling in the amygdala is required for the positive reinforcing effects of alcohol in both rats and mice (Cannady et al. 2016; Salling et al. 2016). We have also shown that AMPAR activation drives escalated alcohol self-administration and cue-induced reinstatement (Cannady et al. 2013), the latter of which is associated with increased CaMKII activity in the BLA (Salling et al. 2017). These findings indicate that CaMKII-AMPAR signaling is a neural target of alcohol that is required for the positive reinforcing effects of the drug. However, despite these and numerous other advances in understanding AMPAR regulation of alcohol drinking (reviewed by Woodward Hopf and Mangieri (2018)), the molecular mechanism(s) by which AMPAR activity regulates the reinforcing effects of alcohol remains to be fully elucidated.
Toward that goal, emerging evidence indicates that transmembrane AMPAR regulatory protein gamma-8 (TARP γ-8) is critical for glutamate-mediated plasticity. TARP γ-8 is a member of the calcium channel gamma subunit (Cacng1-8; γ1-8) family, which consists of the first discovered auxiliary subunits of the AMPAR or any other ligand-gated ion channel (Bissen et al. 2019). The broad family of TARPs can differentially control AMPAR pharmacology and physiology depending on cytological and neuroanatomical expression patterns (Kato et al. 2016, 2010). TARP γ-8 has a unique and selective anatomical expression which is highly restricted to forebrain regions such as the frontal cortex, hippocampus (HPC), and BLA (Fukaya et al. 2005; Maher et al. 2016). Each of these brain regions is sensitive to alcohol exposure and influences AUD-related behaviors and physiology (Talani et al. 2014; White and Swartzwelder 2004; Woodward Hopf and Mangieri 2018). At the cellular level, TARP γ-8 is enriched in the postsynaptic density (PSD) and plays a vital role in the surface expression, trafficking, and activity of AMPARs in these brain regions (Bissen et al. 2019; Jackson and Nicoll 2011) by binding the C-terminus of GluA1 and anchoring it in the PSD (Patriarchi et al. 2018). In the forebrain, a majority of TARP γ-8 interactions with AMPARs occur at GluA1/2 heteromers (Gill et al. 2011; Herguedas et al. 2022; Schwenk et al. 2014) where the TARP γ-8 transmembrane helix 4 (TM4) associates with GluA1 and TM3 attaches between GluA1 and GluA2 to modulate both the structural and functional properties of the receptor (Herguedas et al. 2022).
This mechanistic link between TARP γ-8 and AMPAR GluA1 activity is highly relevant to understanding how alcohol may induce maladaptive plasticity during the development of AUD and other forms of addiction. GluA1-containing AMPARs are phosphorylated (e.g., activated) at serine-831 (pGluA1-S831) in the amygdala by plasticity-inducing events (Lee et al. 2013), including exposure to drugs of abuse (Conrad et al. 2008; Mameli et al. 2011; Wolf and Tseng 2012). We have shown that alcohol self-administration increases pGluA1-S831 expression and synaptic insertion of calcium-permeable GluA2-lacking AMPARs (CP-AMPARs) in the BLA, and that membrane insertion of GluA1-containing AMPARs is required for the positive reinforcing effects of alcohol (Faccidomo et al. 2021). Moreover, we recently found that systemic pharmacological inhibition of TARP γ-8 bound AMPARs significantly reduced operant alcohol self-administration by male, but not female, C57BL/6 J mice (Hoffman et al. 2021), which was the first evidence that TARP γ-8 mediates any form of substance abuse. Together, these findings suggest the novel hypothesis that TARP γ-8 regulates the positive reinforcing properties of alcohol via modulation of AMPAR activity in specific reward-related brain regions.
To address this question, the present experiments were designed to determine the effects of reversible pharmacological inhibition of TARP γ-8 bound AMPARs in the BLA and vHPC on operant alcohol self-administration by male C57BL/6 J mice. We focused on the BLA and vHPC as potential sites of action based on high levels of TARP γ-8 expression (Maher et al. 2017, 2016) and our prior work demonstrating that glutamate activity in these regions regulates alcohol-related behavioral pathologies (Cannady et al. 2016; Faccidomo et al. 2021, 2020; Salling et al. 2016, 2017; Spanos et al. 2012). C57BL/6 J mice were trained to self-administer alcohol in operant conditioning chambers using our well-characterized method that compares responding reinforced by sweetened alcohol to parallel behavior-matched sucrose-only controls (Faccidomo et al. 2009, 2020, 2016b, 2015, 2018; Salling et al. 2016). To assess mechanistic regulation of self-administration behavior, the selective inhibitor of TARP γ-8 bound AMPARs, JNJ-55511118 (JNJ-5), was microinjected in the BLA or vHPC prior to operant self-administration sessions. JNJ-5 is a high affinity negative modulator of GluA1 containing AMPARs bound to TARP γ-8 that disrupts the interaction between TARP γ-8 and AMPAR GluA subunits, effectively inhibiting postsynaptic AMPAR activity (Maher et al. 2016); this selective disruption results in strong dose-dependent inhibition of AMPAR-mediated transmission and anticonvulsant properties in rodent models (Maher et al. 2016). Following self-administration studies, an effective dose of JNJ-5 was also evaluated for potential nonspecific effects on locomotor and anxiety-like (thigmotaxis) behavior.
This study is the first to examine TARP γ-8 as a driving force of the reinforcing effects of any drug of abuse via activity within a brain reward pathway and provides further support for the premise that targeting this AMPAR subclass may be a viable strategy for developing medications to treat behavioral pathologies associated with AUD (Hoffman et al. 2021) or other neurological conditions (Maher et al. 2017).
Materials and methods
Animals
Male C57BL/6 J mice (The Jackson Laboratory, Bar Harbor, ME, USA) arrived in our colony room at 8–10 weeks old and habituated to the environment for 1 week. Mice were group-housed in Techniplast cages (28 × 17 × 14 cm) containing a plastic hut and nestlet for environmental enrichment. Food (Purina chow) and water were available ad libitum unless otherwise indicated. The colony and behavioral testing rooms were temperature (21 ± 1 °C) and humidity (40 ± 2%) controlled and maintained on a 12 h:12 h reverse light/dark cycle (dark at 0700). All experimental procedures involving mice were approved by the Institutional Care and Use Committee at the University of North Carolina at Chapel Hill and conducted as recommended by the Guide for the Care and Use of Laboratory Animals (National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. et al. 2011).
Drugs
Reinforcing solutions
Sweetened alcohol (alcohol 9% v/v + sucrose 2% w/v) was diluted from a 95% ethanol stock (Pharmco Products Inc., Brookfield, CT, USA) to 9% v/v with distilled water and sweetened with sucrose (2% w/v). The sucrose-only solution (2% w/v) was also prepared with sucrose and distilled water.
JNJ-55511118
5-[2-Chloro-6-(trifluoromethoxy)phenyl]-1,3-dihydro-2H-benzimidazol-2-one (JNJ-5; Tocris, Minneapolis, MN) is a high affinity, negative modulator of TARP γ-8 bound AMPA receptors (Maher et al. 2016). JNJ-5 (0.0, 0.3, 1.0, and 2.0 μg/μl) was suspended in aCSF and bilaterally microinfused into target brain regions.
Apparatus
Operant chambers (Med-Associates, St. Albans, VT) were housed in sound-attenuating cubicles to reduce extraneous noise. Chambers were computer-interfaced for automated control of inputs (e.g., recording of mouse behavior) and outputs (e.g., delivery of solutions) using commercially available software (MED-PC for Windows v5.0). Each chamber was equipped with two ultra-sensitive retractable response levers, positioned on opposite walls below a cue light. Levers were programmed to be either “active” (reinforced) or “inactive” and responses were recorded for both. A syringe pump was connected to a drinking trough positioned in the center of the chamber and adjacent to the active lever. Reinforced, active lever presses were accompanied by secondary cues including a cue light (800 ms) and the pump sound. Responses during pump run time were recorded but produced no programmed consequences.
Homecage exposure to reinforcing solutions
After habituation to the colony room (1 week), two drinking bottles were attached to each mouse homecage; one bottle contained sweetened alcohol (9% v/v + sucrose 2% w/v) or sucrose only (2% w/v) and the other bottle contained water. Mice were able to freely consume the reinforcing solution vs. water for 2 weeks prior to operant training reducing neophobic responses to the solutions during operant training (Faccidomo et al. 2015).
Procedural sequence
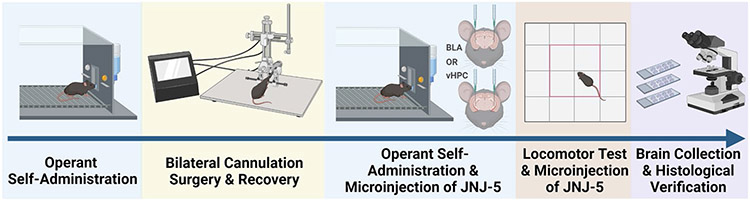
The following sections describe the experimental procedures shown in Fig. 1.
Fig. 1.
Sequence of experimental procedures. Mice were first trained to self-administer sweetened alcohol or sucrose only in operant conditioning chambers. Training was followed by bilateral cannulation surgery and recovery and then a return to operant responding. Mice were then microinjected with JNJ-5 (0, .3, 1, 2 μg/μl) into the BLA or vHPC prior to operant self-administration to determine the effects of inhibiting TARP γ-8 bound AMPAR on alcohol or sucrose self-administration. The JNJ-5 microinjections were repeated prior to an open-field test to account for any nonspecific locomotor or anxiety-like (thigmotaxis) effects. Finally, brains were removed for histological verification of cannula placement
Operant self-administration
Training and acquisition of mouse operant self-administration of sweetened alcohol (9% v/v + sucrose 2% w/v) or sucrose (2% w/v) in separate groups of mice were conducted as described previously (Faccidomo et al. 2009; Hoffman et al. 2021; Salling et al. 2008). Briefly, initial training occurred during 3–4 consecutive overnight (16 h) sessions; the number of sessions was determined by learning contingent lever pressing. Mice were water restricted 24 h prior to the initial training session. Initial lever press responses were reinforced on a fixed ratio 1 (FR1) schedule that increased to FR2, FR3, and to a final value of FR4 over the training session(s) with each increment of 25 reinforcers earned at each FR value. Active lever presses associated with a reinforcer activated the syringe pump to deliver 0.014 ml of the reinforcing solution into the drinking trough. After completing the session, an experimenter confirmed fluid intake via visual inspection of the drinking trough. All subsequent operant self-administration sessions took place during the dark phase of the light/dark cycle (between 1200 and 1600) and lasted for 60 min and were on an FR4 schedule. These 1-h daily sessions were conducted M-F unless otherwise noted. Our previous work using this protocol has resulted in pharmacologically relevant blood alcohol content (BAC) (Faccidomo et al. 2009) levels, though blood samples were not taken during this study to minimize behavioral disruption and possible stress-induced alterations of AMPARs (Bats et al. 2013; Kuniishi et al. 2020). Behavioral measures recorded included active lever responses, headpokes into the drinking receptacle (beam break), and inactive lever responses. Headpokes per reinforcer are calculated as a simple ratio as a measure of reinforcer contingent consumption of the reinforcing solution.
Surgery
After a baseline of 40–42 operant self-administration sessions, mice underwent surgery for bilateral cannula implantation. Mice were anesthetized with vaporized isoflurane (1.5–2.5%) and placed into a stereotaxic frame (Kopf Instruments, Tujunga, CA). Hair was removed from the scalp, and the surface was scrubbed with ethanol and beta iodine to sterilize the skin prior to the incision. Microclips were used to pull back skin to expose the skull to identify bregma. Using a digital arm, holes were drilled bilaterally at the following coordinates derived from Paxinos and Franklin’s Mouse Brain Atlas (Franklin and Paxinos 2019): BLA: AP = − 1.2 and ML = + / − 3.3 from bregma, DV = − 2.6 mm from dura; vHPC: AP = − 3.0 and ML = + / − 3.3 from bregma, DV = − 2.0 mm from dura. Next, 26-gauge guide cannula (Plastics One, Roanoke, VA) were implanted, positioned 2 mm above either the BLA or vHPC, and cemented to the skull with dental cement (Durelon, Butler Schein, Dublin, OH). A 33-gauge obturator extending 0.5 mm beyond the cannula tip was inserted into the guide cannula after surgery and moved daily to prevent blockage and scarring. Site-specific microinjections were performed through a 33-guage injector needle that extended 2 mm beyond the cannula tip. Mice were monitored and kept warm until awakening from anesthesia, returned to the colony room, and administered ibuprofen for acute pain. A bitter tasting deterrent solution was brushed onto the head-mount and obturator to discourage damage from cage-mates. After a 7-day recovery period, mice returned to operant self-administration.
Microinjections
Site-specific microinjections were conducted as previously reported (Besheer et al. 2012; Faccidomo et al. 2016a; Hodge et al. 1996; Samson and Hodge 1993; Schroeder et al. 2003). After re-establishing baseline self-administration, mice were habituated to the microinjection procedure with sham injections, which involved the insertion of the injector and running the pump for 4 min (not connected to the injector). After the sham injection, mice were immediately placed into the operant chamber for the 60-min session. Once habituated, separate groups of mice were administered JNJ-5 (0.0, 0.3, 1.0, and 2.0 μg/0.5 μl/side) via bilateral microinjection in the BLA or vHPC according to a randomized Latin-square dosing design with a minimum of 3 days between injections. Mice were unrestrained during the JNJ-5 infusions; the infusions lasted for 4 min at a rate of 0.125 μl/min/side using a 1-μl Hamilton syringe connected to a syringe pump (Harvard Apparatus, Holliston, MA). After the infusion, the injector remained in place for 1 min to allow drug diffusion and minimize vertical capillary action. Mice were then immediately placed into the operant chamber for the 60-min self-administration session.
Locomotor activity
Locomotor testing was conducted to evaluate potential nonspecific motor or anxiety-like effects of JNJ-5 infusion as previously reported (Agoglia et al. 2016, 2015b; Besheer et al. 2006; Faccidomo et al. 2015; Hodge et al. 2002; Kelley et al. 2003). Mice were habituated to open-field chambers (Med-Associates) for 2 h, 1 week prior to locomotor testing. Microinjections of JNJ-5 (0.0, 0.3, 1.0, and 2.0 μg/side; counterbalanced design) into the BLA or vHPC were conducted immediately prior to a 60-min locomotor activity assessment. Distance traveled (cm) was computer-recorded every 100 ms in the open-field chamber via two sets (x and y axes) of 16 pulse-modulated infrared photobeams. Thigmotaxis (distance in periphery vs. center) was derived as a measure of anxiety-like behavior. Locomotor activity assessments occurred at least 4 days apart.
Histological verification
After completion of all microinjections and behavioral procedures, mice were deeply anesthetized with sodium pentobarbital and intracardially perfused with 0.9% phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA). Brains were extracted, coronally sliced (50 μm) on a vibratome (Lecia VT100S, Leica Biosystems, Wetzlar, Germany), and stained with cresyl violet and slide mounted. Histological verification of injection site was assessed visually by an experimenter both during tissue sectioning and by examination under light microscopy. Data were used only from mice verified to have received bilateral infusion in the BLA.
Statistical analysis
All analyses were conducted with Prism 9 (GraphPad Software, Boston, MA). Repeated-measures (RM) ANOVA or mixed-effects model (REML) analyses were used to compare groups with corrected multiple comparisons (Holm-Šídák) when appropriate.
Results
EtOH reinforcement is regulated by TARP γ-8 bound AMPARs in the BLA
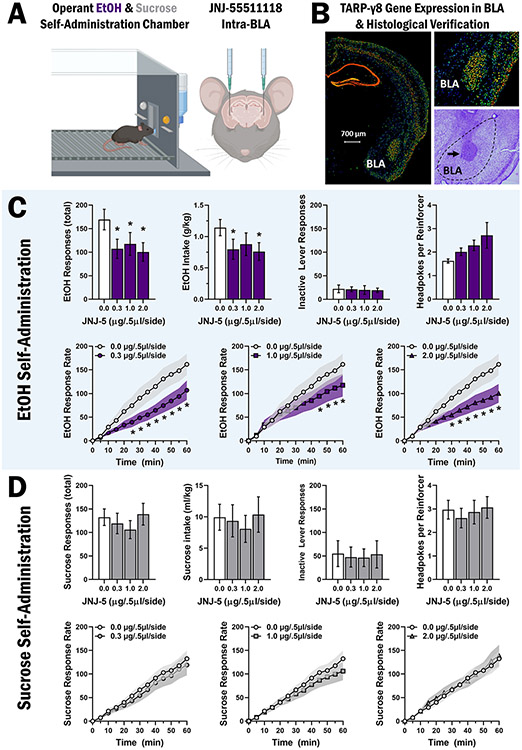
To determine if the activity of TARP γ-8 bound AMPARs in the BLA is required for EtOH reinforcement, the selective inhibitor JNJ-5 (0–2.0 μg/0.5 μl/side) was microinjected in the BLA prior to operant self-administration sessions in male C57BL/6 J mice (Fig. 2A). Data are from mice (n = 8) verified by histological examination of coronal brain sections to have injector placements in the BLA (AP stereotaxic coordinate range of − 1.07 to − 1.43 from bregma; Fig. 2B).
Fig. 2.
Inhibition of TARP γ-8 bound AMPARs in the BLA selectively decreases EtOH-reinforced responding. A Schematic showing mouse operant conditioning chamber and cannula placement for intra-BLA microinjections of JNJ-55511118 prior to self-administration of sweetened alcohol (n = 8) and sucrose only (n = 9). B Coronal sections of adult (P56) male C57BL/6 J mouse brain showing the expression pattern of the TARP γ-8 gene (Cacng8) by in situ hybridization (Allen Mouse Brain Atlas) and cresyl violet stained section showing representative injector placement in the BLA. C-D Bar graphs (top rows) showing MEAN ± SEM parameters of operant ethanol (C) and sucrose (D) self-administration plotted as a function of dosage of JNJ-5 infused in the BLA. Line graphs (bottom rows) show MEAN ± SEM rate of ethanol (C) and sucrose (D) reinforced responding (cumulative responses/5-min interval) as compared to vehicle control for each dose of JNJ-5 tested. Asterisk denotes statistically significant difference as compared to vehicle, p < .05
RM-ANOVA showed that intra-BLA infusion of JNJ-5 selectively decreased total EtOH-reinforced responding, F(3,21) = 5.481, p = 0.01. Follow-up analysis with Holm-Šídák’s multiple comparison tests shows that all doses of JNJ-5 decreased total EtOH-reinforced responses (Fig. 2C). Reduced total EtOH-reinforced responses were associated with a significant decrease in EtOH intake (g/kg), F(3,21) = 3.564, p = 0.03, which was a function of decreases following JNJ-5 (0.3 and 2.0 μg/0.5 μl/side) doses (Fig. 2C). RM-ANOVA showed no differences in inactive lever responding, F(3,21) = 0.111, p = 0.95, or total headpokes per reinforcer, F(3,21) = 2.519, p = 0.086 for mice consuming EtOH (Fig. 2C), suggesting that the reduction in EtOH-reinforced responding was not associated with nonspecific motor or consummatory effects, respectively.
To directly evaluate the regulation of EtOH-reinforcer function, we also examined the impact of intra-BLA infusion of JNJ-5 on EtOH-reinforced response rate. A mixed-effects model analysis of EtOH-reinforced response rate revealed a time × dose interaction, F(33,231) = 5.156, p < 0.0001, and main effects of time, F(11,77) = 30.68, p < 0.0001, and dose, F(3,21) = 3.722, p = 0.03. Post hoc Holm-Šídák’s multiple comparisons reveal statistically significant differences between JNJ-5 0.0 μg/0.5 μl/side and 0.3 μg/0.5 μl/side doses from 25 to 60 min, additional differences between 0.0 μg/0.5 μl/side and 2. μg/0.5 μl/side from 30 to 60 min, and additional differences between 0. μg/0.5 μl/side and 1.0 μg/0.5 μl/side from 45 to 60 min (Fig. 2C, bottom row).
TARP γ-8 bound AMPAR inhibition in the BLA has no effect on sucrose reinforcement
To assess EtOH-reinforcer specificity, we inhibited TARP γ-8 bound AMPARs in the BLA in behavior-matched controls trained to self-administer sucrose only (2% w/v). Data are from mice (n = 9) verified histologically to have injector placement in the BLA in AP stereotaxic coordinate range of − 1.07 ≤ × ≥ − 1.43 from bregma (Fig. 2B).
Intra-BLA infusion of JNJ-5 (0–2 μg/0.5 μl/side) has no effect on sucrose-reinforced responses F(3,23) = 1.143, p = 0.35 or on sucrose intake (ml/kg), F(3,23) = 0.599, p = 0.62 (Fig. 2D). Mixed-effects model analysis shows no differences in inactive lever responding, F(3,23) = 0.364, p = 0.78, or headpokes per reinforcer, F(3,23) = 1.915, p = 0.15 (Fig. 2D) indicating that JNJ-5 administered in the BLA had no effect on non-drug reinforcement, motor performance, or consummatory behavior. Mixed-effects model analysis of sucrose-reinforced response shows a main effect of time, F(11,88) = 40.88, p < 0.0001 (Fig. 2D, bottom row), indicating no effect of JNJ-5 in the dose range tested.
TARP γ-8 bound AMPAR inhibition in vHPC has no effect on EtOH reinforcement
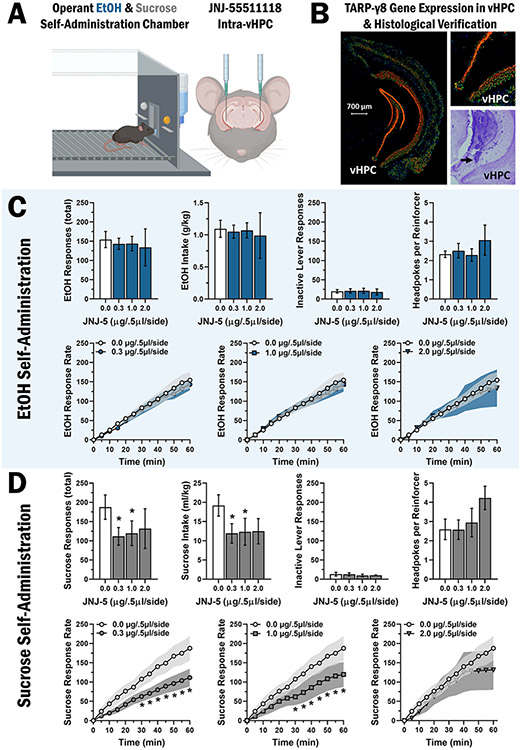
To evaluate brain regional specificity of TARP γ-8 regulation of EtOH reinforcement, JNJ-5 was microinjected in vHPC prior to operant self-administration sessions in C57BL/6 J mice (Fig. 3A). Data are from mice (n = 8) verified by histological examination of coronal brain sections to have injector placements in the vHPC (AP range − 2.79 to − 3.07 from bregma; Fig. 3B).
Fig. 3.
TARP γ-8 bound AMPAR inhibition in vHPC selectively decreases sucrose-reinforced responding. A Schematic showing mouse operant conditioning chamber and cannular placement for intra-vHPC microinjections of JNJ-55511118 prior to operant self-administration of sweetened alcohol (n = 8) and sucrose only (n = 9). B Coronal sections of adult (P56) male C57BL/6 J mouse brain showing the expression pattern of the TARP γ-8 gene (Cacng8) by in situ hybridization (Allen Mouse Brain Atlas) and cresyl violet stained section showing representative injector placement in the vHPC. C-D Bar graphs (top rows) showing MEAN ± SEM parameters of operant ethanol (C) and sucrose (D) self-administration plotted as a function of dosage of JNJ-5 infused in the vHPC. Line graphs (bottom rows) show MEAN ± SEM rate of ethanol (C) and sucrose (D) reinforced responding (cumulative responses/5-min interval) as compared to vehicle control for each dose of JNJ-5 tested. Asterisk denotes statistically significant difference as compared to vehicle, p < .05
In contrast to the BLA, mixed-effects model analysis shows that intra-vHPC JNJ-5 infusion has no effect on total EtOH-reinforced responding, F(3,20) = 0.1614, p = 0.92 (Fig. 3C). Similarly, RM-ANOVA showed no effect on EtOH intake (g/kg), F(3,20) = 0.055, p = 0.982 (Fig. 3C). Mixed-effects model shows no differences in inactive lever responding, F(3,20) = 0.3407, p = 0.80, or headpokes per reinforcer, F(3,20) = 1.028, p = 0.40, for mice consuming EtOH (Fig. 3C). An additional mixed-effects model analysis of the rate of EtOH-reinforced response rate shows a main effect of time, F(11,99) = 55.70, p < 0.0001 (Fig. 3C), indicating no effect of JNJ-5.
TARP γ-8 bound AMPAR inhibition in vHPC decreases sucrose-reinforced responding
We also sought to determine the potential regulation of sucrose reinforcement by TARP γ-8 bound AMPARs in the vHPC. Data are from mice (n = 9) verified by histological examination of coronal brain sections to have injector placements in the vHPC (AP range − 2.79 to − 3.07 from bregma; Fig. 3B).
Results showed that intra-vHPC infusion of JNJ-5 (0–2 μg/0.5 μl/side) decreased sucrose-reinforced responding, F(3,14) = 4.288, p = 0.02 and intake (ml/kg), F(3,14) = 5.856, p = 0.01 (Fig. 3D). Mixed-effects model analysis shows no differences in inactive lever responding, F(3,14) = 0.1173, p = 0.95, and no difference in headpokes per reinforcer, F(3,14) = 0.5789, p = 0.64 (Fig. 3D). Evaluation of sucrose-reinforced response rate by mixed-model analysis shows a time × dose interaction F(33,147) = 2.413, p < 0.0001, and main effects of time, F(11,77) = 34.84, p < 0.0001, and dose, F(3,21) = 5.407, p < 0.01. Post hoc Holm-Šídák’s multiple comparisons revealed statistically significant differences between JNJ-5 0.0 μg/0.5 μl/side and 0.3 μg/0.5 μl/side doses from 25 to 60 min, additional differences between 0.0 μg/0.5 μl/side and 2.0 μg/0.5 μl/side from 30 to 60 min, and additional differences between 0.0 μg/0.5 μl/side and 1.0 μg/0.5 μl/side from 45 to 60 min (Fig. 3D, bottom row).
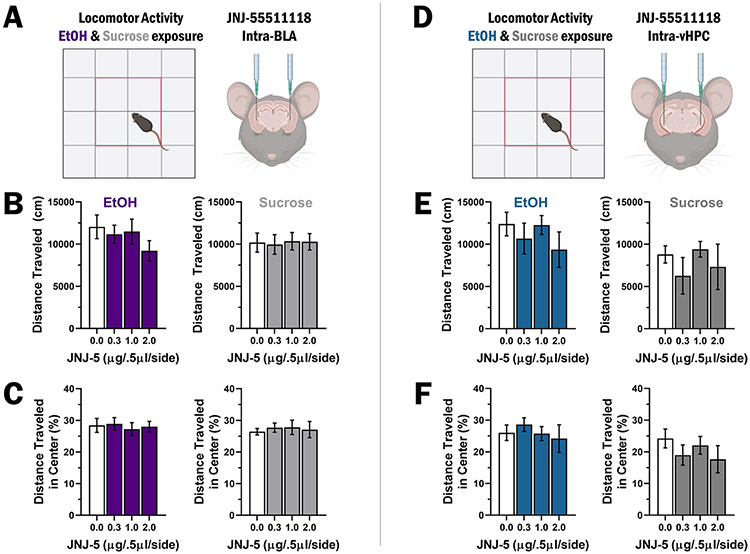
Site-specific TARP γ-8 bound AMPAR inhibition does not alter locomotor activity or thigmotaxis
To determine if decreases in operant self-administration induced by site-specific inhibition of TARP γ-8 bound AMPAR activity are due to changes in motor function, additional microinjections of JNJ-5 into the BLA (Fig. 4A-C) and vHPC (Fig. 4D-F) were performed prior to tests of open-field locomotor activity. RM-ANOVA shows no difference in distance traveled after BLA microinjection of JNJ-5 in mice exposed to EtOH, F(3,24) = 2.728, p = 0.07, or sucrose, F(3,27) = 0.04578, p = 0.99 (Fig. 4B). Similarly, mixed-effects model analysis of % distance traveled in the center of an open field following BLA JNJ-5 microinjection showed no differences in mice exposed to EtOH, F(3,31) = 0.1254, p = 0.94, or sucrose, F(3,35) = 0.1348, p = 0.94 (Fig. 4C). Likewise, mixed-effects model analysis of vHPC microinjection of JNJ-5 showed no differences in distance traveled in mice exposed to EtOH, F(3,12) = 1.15, p = 0.37, or sucrose, F(3,13) = 0.9423, p = 0.45 (Fig. 4E). Finally, mixed-effects model analysis also showed no effect of vHPC microinjection of JNJ-5 in % distance traveled in the center of an open field for mice exposed to EtOH, F(3,13) = 0.2354, p = 0.87, or sucrose, F(3,13) = 1.402, p = 0.30 (Fig. 4F).
Fig. 4.
Site-specific inhibition of TARP γ-8 bound AMPAR has no effect on locomotor activity or anxiety-like behavior. A Experimental schematic showing open field with center zone and intra-BLA microinjections prior to the open-field test. B Motor activity is shown as MEAN ± SEM distance traveled (cm) during each 60-min session for EtOH (left, blue bars, n = 9) and sucrose (right, gray bars, n = 10) exposed mice plotted as a function of JNJ-5 dosage in the BLA. C Thigmotaxis shown as MEAN ± SEM distance traveled (cm) in the center of the open field for EtOH (left, blue bars) and sucrose (right, gray bars) exposed mice plotted as a function of JNJ-5 dosage in the BLA. D Experimental schematic of the apparatus and intra-vHPC microinjections prior to the open-field test. E Motor activity plotted as MEAN ± SEM distance traveled (cm) during 60-min sessions for EtOH (left, blue bars, n = 9) and sucrose (right, gray bars, n = 9) exposed mice plotted as a function of JNJ-5 dosage in the VHPC. F Thigmotaxis shown as MEAN ± SEM distance traveled (cm) in the center of the open field for EtOH (left, blue bars) and sucrose (right, gray bars) exposed mice plotted as a function of JNJ-5 dosage in the vHPC
Discussion
Our prior research shows that inhibition of TARP γ-8 bound AMPARs via systemic administration of the selective inhibitor JNJ-5 significantly decreases operant alcohol self-administration in male, but not female, C57BL/6 J mice (Hoffman et al. 2021). The present study extends this finding and identifies TARP γ-8 in the BLA as a novel site-specific molecular mechanism of the positive reinforcing effects of alcohol. Here we show that microinjection of JNJ-5 (0–2 μg/0.5 μl/side), a high affinity negative modulator of TARP γ-8 bound AMPARs, in the BLA significantly reduced the total amount and rate of operant alcohol (sweetened) self-administration by male C57BL/6 J mice. By contrast, JNJ-5 infusion in the BLA had no effect on sucrose-only self-administration in parallel behavior-matched controls, which suggests alcohol reinforcer specificity. Critical control measures within the operant procedure and separate tests found no disruption of motor (open-field activity), consummatory (headpokes per reinforcer), or anxiety-like (thigmotaxis) behavior. This selective reduction in alcohol-reinforced response rate indicates that TARP γ-8-modulated AMPAR activity in the BLA is required for the full expression of the positive reinforcing properties of alcohol.
This conclusion is bolstered by the high level of TARP γ-8 expression in the BLA relative to the central amygdala and other adjacent nuclei (Fig. 2B (Maher et al. 2016)). However, evidence indicates that drug solutions diffuse down a concentration gradient as a function of time and distance from the site of intracranial infusion with a maximum lateral diffusion range of 1–1.5 mm at 15 and 60 min, respectively (Edeline et al. 2002). As can be seen in Fig. 2B, TARP γ-8 is also expressed within this range lateral to the BLA in layer 1 of the piriform cortex. We have shown that cue-induced reinstatement of alcohol-seeking behavior is associated with increased expression of phosphorylated CaMKII-T286 in the piriform cortex (Salling et al. 2017). Since CaMKII phosphorylation is required for the functional effects of TARP γ-8 (Park et al. 2016) and the reinforcing effects of alcohol (Agoglia et al. 2015a; Cannady et al. 2016; Faccidomo et al. 2016b), it is plausible that JNJ-5 diffusion to the piriform cortex disrupted CaMKII-TARP γ-8 signaling and played a partial role in the observed reduction in self-administration.
The BLA plays a prominent role in reinforcement processes through glutamatergic projections to the NAc (Wright et al. 1996), which are both necessary and sufficient to promote reward-seeking behavior (Stuber et al. 2011). We have shown previously that operant alcohol self-administration increases postsynaptic insertion of GluA1-containing AMPARs in BLA neurons that project to the NAc, and that this membrane trafficking of GluA1 in the BLA is required for the reinforcing effects of alcohol (Faccidomo et al. 2021). TARP γ-8 modulates postsynaptic glutamate signaling and behavioral plasticity by binding the AMPAR GluA1 subunit C-terminus and anchoring it in the PSD (Park et al. 2016; Patriarchi et al. 2018). Thus, TARP γ-8 may regulate alcohol-induced synaptic insertion of GluA1-containing AMPARs in BLA projection neurons and in alcohol reinforcement processes via modulation of excitatory projections from the BLA to reward-related neural structures including the NAc.
Although the present results show a significant role for TARP γ-8 in the BLA, they also suggest that TARP γ-8 modulation of AMPAR activity in the vHPC may not regulate the reinforcing properties of alcohol. Site-specific infusion of JNJ-5 in the vHPC had no effect on any measure of operant alcohol self-administration, or measures of motor activity and anxiety-like behavior. This finding was surprising as TARP γ-8 is highly abundant in the projection regions of the hippocampus (Maher et al. 2016) where CaMKII and TARP γ-8 have been shown to regulate synaptic and behavioral plasticity (Park et al. 2016) and alcohol enhances glutamatergic output from the vHPC to NAc neurons expressing dopamine D1 receptors (Kircher et al. 2019), which are known to regulate alcohol self-administration (Hodge et al. 1997). Moreover, the hippocampus and NAc interactively regulate the glutamatergic component of alcohol’s discriminative stimulus properties (Hodge and Cox 1998), which are fundamental to reinforcement processes (Stolerman 1992). Thus, these results are consistent with the conclusion that there is brain region-specific regulation of alcohol reinforcement by AMPARs associated with TARP γ-8 in the BLA. However, since TARP γ-8 and AMPARs are expressed throughout the hippocampus and various cortical areas, further research is warranted to assess this hypothesis.
It is important to note that the lack of involvement of TARP γ-8 bound AMPARs in the vHPC in the present study does not negate a role of the vHPC in alcohol self-administration. First, it is possible that a higher dosage of JNJ-5 in the vHPC may have altered alcohol self-administration. Second, the vHPC is a large structure consisting of multiple subregions and although the site-specific microinjection method provides a level of anatomical specificity, it is limited in targeting specific cell types or restricted loci; thus, alternative methods that allow manipulation of specific vHPC projections may show involvement. For example, recent evidence shows that inhibition of the vHPC with the inhibitory DRE-ADD hM4Di increases homecage alcohol drinking in non-dependent male mice and that DREADD-induced activation of nucleus accumbens terminals receiving glutamatergic input from the vHPC reduced alcohol drinking in dependent mice (Griffin et al. 2023). Thus, site-specific infusion of JNJ-5 in the vHPC may have targeted multiple subregions or circuits that differentially alter self-administration behavior through compensatory mechanisms. For this reason, it will be important to evaluate TARP γ-8 bound AMPAR activity in specific subregions of the vHPC and in the vHPC-to-NAc circuit as a potential mechanism of dependence-induced increases in alcohol self-administration.
A key finding from the present study is that the positive reinforcing properties of alcohol and those of non-drug rewards, such as sucrose, may be regulated by TARP γ-8 bound AMPARs in a brain region-dependent manner. We found that JNJ-5 infusion in the vHPC inhibited sucrose-reinforced responses with no effect on alcohol, whereas an opposite alcohol-specific effect was observed in the BLA. Although the mechanism for this dissociation is unclear, one plausible hypothesis is that alcohol exposure may differentially alter TARP γ-8 or AMPAR expression in a manner that alters the response to JNJ-5. As noted above, our prior work shows that alcohol self-administration upregulates both CP-AMPAR synaptic expression and GluA1 activity (GluA1-S831 phosphorylation) in the BLA to a greater extent than sucrose and, in turn, GluA1 membrane insertion is required for the positive reinforcing effects of alcohol (Faccidomo et al. 2021). Thus, if alcohol selectively upregulates TARP γ-8 expression or activity in the BLA, this may increase the population of TARP γ-8 bound AMPARs in the BLA and promote behavioral response to JNJ-5 via enhanced output to downstream projection regions. By contrast, chronic alcohol exposure has been shown to reduce GluA1 expression (Yao et al. 2021) in the hippocampus of mice, suggesting that a potential downregulation of TARP γ-8 by alcohol might decrease the population of TARP γ-8 bound AMPARs and blunt response to JNJ-5. However, others have reported increases in excitatory synaptic transmission in the ventral hippocampus, particularly in the CA1, and increases in GluA2 expression following chronic alcohol exposure in male rats (Ewin et al. 2019). As TARP γ-8 is abundantly expressed in both the CA1 and CA3 of the vHPC (Fig. 3B), examination of alcohol-induced and sucrose-induced changes in TARP γ-8 expression may be needed to fully determine the role of this novel neural target on alcohol and sucrose reinforcement.
Nonetheless, our finding that inhibition of TARP γ-8 bound AMPARs in the vHPC by JNJ-5 decreases the reinforcing effects of sucrose is consistent with evidence showing that glutamate neurotransmission in the vHPC regulates feeding (Kanoski and Grill 2017) and, specifically, that sucrose intake increases total AMPAR GluA1 subunit expression in the vHPC (Ross et al. 2019). Since TARP γ-8 modulates AMPAR activity via binding to the GluA1 subunit (Herguedas et al. 2022), upregulation of GluA1 in the vHPC by sucrose self-administration may increase the number of TARP γ-8 AMPARs, which would support both mechanistic regulation of behavior by this subgroup of AMPARs and sensitivity to inhibition by JNJ-5. Thus, we propose that TARP γ-8 bound AMPAR activity in the vHPC is a novel mechanism of the reinforcing effects of sucrose. However, this observation complicates the interpretation of the present results showing no effect of JNJ-5 infusion in the vHPC on sweetened alcohol self-administration, thus raising the possibility of differential brain region-dependent effects of alcohol and sucrose on this receptor population and requiring further research to clarify alcohol-specific effects in this brain region. Moreover, the present results showing inhibition of sucrose self-administration by JNJ-5 infusion in the vHPC are not consistent with our prior observation that systemic administration of JNJ-5 had no effect on sucrose self-administration by male, or female, mice (Hoffman et al. 2021). Although the reason(s) for this inconsistency is unclear, potential explanations include differential receptor occupancy in the vHPC following systemic or site-specific injection and inverse compensatory regulation by another brain region following systemic administration. To address these questions, future studies can evaluate the impact of alcohol and/or sucrose on brain regional TARP γ-8 gene and protein expression and assess a broader dose range of JNJ-5 in each brain region to account for potential differential sensitivity.
It is widely recognized that AMPAR antagonists produce significant side effects including locomotor and cognitive deficits. However, site-specific inhibition of TARP γ-8 bound AMPARs was without effect on motor activity or thigmotaxis (anxiety-like behavior). The highest dose of JNJ-5 into the BLA (2 μg/0.5 μl) for ethanol exposed mice exhibits a trend toward a decrease in total distance traveled; although this did not reach statistical significance, the possible interaction between higher site-specific doses of JNJ-5 and a history of ethanol exposure can be explored in future studies. Moreover, the lack of significant reductions in operant behavior during the initial onset of responding, or on reinforcer consumption (headpokes per reinforcer), suggests a lack of memory deficits or altered consummatory behavior. This is consistent with our prior observation that systemic administration of JNJ-5 reduced alcohol self-administration by male, but not female, mice in the absence of nonspecific effects (Hoffman et al. 2021). Thus, blocking only the subclass of AMPARs associated with TARP γ-8 may provide therapeutic efficacy in the absence of negative side effects; however, further work needs to explore the possibility of sexually dimorphic sensitivity to JNJ-5 (Hoffman et al. 2021) and the potential nonspecific impact of higher doses in females.
In conclusion, discovering mechanistically driven therapeutic interventions for behavioral pathologies associated with AUD remains a challenge. Despite a plethora of preclinical and clinical evidence identifying glutamate neurotransmission as a potential target for alcohol medications (Heilig and Egli 2006; Holmes et al. 2013), selective treatment approaches remain to be developed. Toward that goal, the use of AMPAR negative modulators is appealing for the pharmacological treatment of AUD and other CNS disorders involving increased neuronal excitability. However, nonspecific inhibition of AMPARs produces undesirable side effects including sedation and memory disruption (Rogawski 2011). Thus, there is a need for novel AMPAR-targeted therapeutic strategies that selectively modulate disease-specific brain regions or pathways. Given the restricted anatomical expression of TARP γ-8 (e.g., hippocampus, frontal cortex, basolateral amygdala), this AMPAR auxiliary protein has been proposed as a novel target for modulating excitability in specific brain regions via systemic treatment (Gill and Bredt 2011; Maher et al. 2017). In support of TARP γ-8 as a therapeutic target for AUD, we showed previously that systemic administration of JNJ-5 selectively reduces the reinforcing effects of alcohol in mice (Hoffman et al. 2021) and, in the present study, localized this therapeutic-like effect to the BLA. This suggests that systemic treatment with JNJ-5 reduces the reinforcing effects of alcohol by inhibition of TARP γ-8-associated AMPARs selectively within the amygdala, a key component of the brain’s reward pathway (Koob 1999; 2003). It will be important to determine if TARP γ-8 regulates other behavioral pathologies including relapse-like behavior and dependence-induced escalated alcohol use, both of which characterize advanced stages of AUD and lack treatment approaches.
Acknowledgements
The authors wish to thank our dedicated team of undergraduate research assistants and research technicians for their assistance. Specifically, we thank Eric Homberger, Julie Lee, and LC Wong for their help with behavioral tasks and laboratory upkeep.
Funding
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award numbers R01 AA028782 (CWH), P60 AA011605 (CWH), and F32 AA028993 (JLH), and by the Bowles Center for Alcohol Studies at The University of North Carolina at Chapel Hill.
Footnotes
Competing interests The authors declare no competing interests.
Data availability
Data from this study are available from the corresponding author upon reasonable request.
References
- Agoglia AE, Holstein SE, Eastman VR, Hodge CW (2016) Cannabinoid CB1 receptor inhibition blunts adolescent-typical increased binge alcohol and sucrose consumption in male C57BL/6J mice. Pharmacol Biochem Behav 143:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoglia AE, Holstein SE, Reid G, Hodge CW (2015a) CaMKIIalpha-GluA1 activity underlies vulnerability to adolescent binge alcohol drinking. Alcohol Clin Exp Res 39:1680–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoglia AE, Sharko AC, Psilos KE, Holstein SE, Reid GT, Hodge CW (2015b) Alcohol alters the activation of ERK1/2, a functional regulator of binge alcohol drinking in adult C57BL/6J mice. Alcohol Clin Exp Res 39:463–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bats C, Farrant M, Cull-Candy SG (2013) A role of TARPs in the expression and plasticity of calcium-permeable AMPARs: evidence from cerebellar neurons and glia. Neuropharmacology 74:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Cannady R, Grondin JJ, Hodge CW (2012) Intra-amygdala inhibition of ERK(1/2) potentiates the discriminative stimulus effects of alcohol. Behav Brain Res 228:398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Mole B, Hodge CW (2006) GABAA receptor regulation of voluntary ethanol drinking requires PKCepsilon. Synapse 60:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissen D, Foss F, Acker-Palmer A (2019) AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking. Cell Mol Life Sci 76:2133–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Durant B, Besheer J, Hodge CW (2013) Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addict Biol 18:54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Graham C, Crayle J, Besheer J, Hodge CW (2016) Potentiation of amygdala AMPA receptor activity selectively promotes escalated alcohol self-administration in a CaM-KII-dependent manner. Addict Biol 22:652–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Hars B, Hennevin E, Cotillon N (2002) Muscimol diffusion after intracerebral microinjections: a reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiol Learn Mem 78:100–124 [DOI] [PubMed] [Google Scholar]
- Ewin SE, Morgan JW, Niere F, McMullen NP, Barth SH, Almonte AG, Raab-Graham KF, Weiner JL (2019) Chronic intermittent ethanol exposure selectively increases synaptic excitability in the ventral domain of the rat hippocampus. Neuroscience 398:144–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Besheer J, Stanford PC, Hodge CW (2009) Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology 204:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Cogan ES, Hon OJ, Hoffman JL, Saunders BL, Eastman VR, Kim M, Taylor SM, McElligott ZA, Hodge CW (2021) Calcium-permeable AMPA receptor activity and GluA1 trafficking in the basolateral amygdala regulate operant alcohol self-administration. Addict Biol 26:e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Holstein SE, Santanam TS, Saunders BL, Swaim KS, Reid GT, O’Neill C, Eastman VR, Hodge CW (2020) Pharmacological inhibition of glycogen synthase kinase 3 increases operant alcohol self-administration in a manner associated with altered pGSK-3beta, protein interacting with C kinase and GluA2 protein expression in the reward pathway of male C57BL/6J mice. Behav Pharmacol 31:15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Reid GT, Agoglia AE, Ademola SA, Hodge CW (2016a) CaMKII inhibition in the prefrontal cortex specifically increases the positive reinforcing effects of sweetened alcohol in C57BL/6J mice. Behav Brain Res 298:286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Salling MC, Galunas C, Hodge CW (2015) Operant ethanol self-administration increases extracellular-signal regulated protein kinase (ERK) phosphorylation in reward-related brain regions: selective regulation of positive reinforcement in the prefrontal cortex of C57BL/6J mice. Psychopharmacology 232:3417–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Swaim KS, Saunders BL, Santanam TS, Taylor SM, Kim M, Reid GT, Eastman VR, Hodge CW (2018) Mining the nucleus accumbens proteome for novel targets of alcohol self-administration in male C57BL/6J mice. Psychopharmacology 235:1681–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Yamazaki M, Sakimura K, Watanabe M (2005) Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci Res 53:376–383 [DOI] [PubMed] [Google Scholar]
- Gill MB, Bredt DS (2011) An emerging role for TARPs in neuropsychiatric disorders. Neuropsychopharmacology 36:362–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MB, Kato AS, Roberts MF, Yu H, Wang H, Tomita S, Bredt DS (2011) Cornichon-2 modulates AMPA receptor-transmembrane AMPA receptor regulatory protein assembly to dictate gating and pharmacology. J Neurosci 31:6928–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Lopez MF, Woodward JJ, Becker HC (2023) Alcohol dependence and the ventral hippocampal influence on alcohol drinking in male mice. Alcohol 106:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M (2006) Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther 111:855–876 [DOI] [PubMed] [Google Scholar]
- Herguedas B, Kohegyi BK, Dohrke JN, Watson JF, Zhang D, Ho H, Shaikh SA, Lape R, Krieger JM, Greger IH (2022) Mechanisms underlying TARP modulation of the GluA1/2-gamma8 AMPA receptor. Nat Commun 13:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Cox AA (1998) The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology 139:95–107 [DOI] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Chappelle AM, Samson HH (1996) Effects of ventral tegmental microinjections of the GABAA agonist muscimol on self-administration of ethanol and sucrose. Pharmacol Biochem Behav 53:971–977 [DOI] [PubMed] [Google Scholar]
- Hodge CW, Raber J, McMahon T, Walter H, Sanchez-Perez AM, Olive MF, Mehmert K, Morrow AL, Messing RO (2002) Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. J Clin Investig 110:1003–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM (1997) Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res 21:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JL, Faccidomo S, Saunders BL, Taylor SM, Kim M, Hodge CW (2021) Inhibition of AMPA receptors (AMPARs) containing transmembrane AMPAR regulatory protein gamma-8 with JNJ-55511118 shows preclinical efficacy in reducing chronic repetitive alcohol self-administration. Alcohol Clin Exp Res 45:1424–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH (2013) Glutamatergic targets for new alcohol medications. Psychopharmacology 229:539–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA (2011) The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70:178–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572 [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Grill HJ (2017) Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol Psychiatry 81:748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AS, Burris KD, Gardinier KM, Gernert DL, Porter WJ, Reel J, Ding C, Tu Y, Schober DA, Lee MR, Heinz BA, Fitch TE, Gleason SD, Catlow JT, Yu H, Fitzjohn SM, Pasqui F, Wang H, Qian Y, Sher E, Zwart R, Wafford KA, Rasmussen K, Ornstein PL, Isaac JT, Nisenbaum ES, Bredt DS, Witkin JM (2016) Forebrain-selective AMPA-receptor antagonism guided by TARP gamma-8 as an antiepileptic mechanism. Nat Med 22:1496–1501 [DOI] [PubMed] [Google Scholar]
- Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS (2010) TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci 33:241–248 [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8:844–858 [DOI] [PubMed] [Google Scholar]
- Kelley SP, Bratt AM, Hodge CW (2003) Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol 461:19–25 [DOI] [PubMed] [Google Scholar]
- Kircher DM, Aziz HC, Mangieri RA, Morrisett RA (2019) Ethanol experience enhances glutamatergic ventral hippocampal inputs to D1 receptor-expressing medium spiny neurons in the nucleus accumbens shell. J Neurosci 39:2459–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (1999) The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci 877:445–460 [DOI] [PubMed] [Google Scholar]
- Koob GF (2003) Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol: J Eur College Neuropsychopharmacol 13:442–452 [DOI] [PubMed] [Google Scholar]
- Koob GF (2009) Brain stress systems in the amygdala and addiction. Brain Res 1293:61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology: Off Publ Am College Neuropsychopharmacol 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniishi H, Yamada D, Wada K, Yamada M, Sekiguchi M (2020) Stress induces insertion of calcium-permeable AMPA receptors in the OFC–BLA synapse and modulates emotional behaviours in mice. Transl Psychiatry 10:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Song B, Kim J, Park K, Hong I, An B, Song S, Lee J, Park S, Park D, Lee CJ, Kim K, Shin KS, Tsien RW, Choi S (2013) GluA1 phosphorylation at serine 831 in the lateral amygdala is required for fear renewal. Nat Neurosci 16:1436–1444 [DOI] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME (2014) Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology 76 Pt B:287–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher MP, Matta JA, Gu S, Seierstad M, Bredt DS (2017) Getting a Handle on neuropharmacology by targeting receptor-associated proteins. Neuron 96:989–1001 [DOI] [PubMed] [Google Scholar]
- Maher MP, Wu N, Ravula S, Ameriks MK, Savall BM, Liu C, Lord B, Wyatt RM, Matta JA, Dugovic C, Yun S, Ver Donck L, Steckler T, Wickenden AD, Carruthers NI, Lovenberg TW (2016) Discovery and characterization of AMPA receptor modulators selective for TARP-gamma8. J Pharmacol Exp Ther 357:394–414 [DOI] [PubMed] [Google Scholar]
- Mameli M, Bellone C, Brown MT, Luscher C (2011) Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci 14:414–416 [DOI] [PubMed] [Google Scholar]
- Marty VN, Spigelman I (2012) Long-lasting alterations in membrane properties, k(+) currents, and glutamatergic synaptic currents of nucleus accumbens medium spiny neurons in a rat model of alcohol dependence. Front Neurosci 6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA (2011) Ethanol modulation of synaptic plasticity. Neuropharmacology 61:1097–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington, D.C. [Google Scholar]
- Park J, Chavez AE, Mineur YS, Morimoto-Tomita M, Lutzu S, Kim KS, Picciotto MR, Castillo PE, Tomita S (2016) CaMKII phosphorylation of TARPgamma-8 is a mediator of LTP and learning and memory. Neuron 92:75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi T, Buonarati OR, Hell JW (2018) Postsynaptic localization and regulation of AMPA receptors and Cav1.2 by beta2 adrenergic receptor/PKA and Ca(2+)/CaMKII signaling. EMBO J 37:e99771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF (1996) Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res 20:1289–1298 [DOI] [PubMed] [Google Scholar]
- Rogawski MA (2011) Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr 11:56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A, Barnett N, Faulkner A, Hannapel R, Parent MB (2019) Sucrose ingestion induces glutamate AMPA receptor phosphorylation in dorsal hippocampal neurons: increased sucrose experience prevents this effect. Behav Brain Res 359:792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Faccidomo S, Hodge CW (2008) Nonselective suppression of operant ethanol and sucrose self-administration by the mGluR7 positive allosteric modulator AMN082. Pharmacol Biochem Behav 91:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, Agoglia AE, Kash TL, Hodge CW (2016) Moderate alcohol drinking and the amygdala proteome: identification and validation of calcium/calmodulin dependent kinase II and AMPA receptor activity as novel molecular mechanisms of the positive reinforcing effects of alcohol. Biol Psychiat 79:430–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Hodge CJ, Psilos KE, Eastman VR, Faccidomo SP, Hodge CW (2017) Cue-induced reinstatement of alcohol-seeking behavior is associated with increased CaMKII T286 phosphorylation in the reward pathway of mice. Pharmacol Biochem Behav 163:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (2019) Center for Behavioral Health Statistics and Quality. 2019 National Survey on Drug Use and Health. Table 5.4A – Alcohol Use Disorder in Past Year among Persons Aged 12 or Older, by Age Group and Demographic Characteristics: Numbers in Thousands, 2018 and 2019. https://www.samhsa.gov/data/sites/default/files/reports/rpt29394/NSDUHDetailedTabs2019/NSDUHDetTabsSect5pe2019.htm#tab5-4a. Accessed November 16, 2022 [Google Scholar]
- Samson HH, Hodge CW (1993) The role of the mesoaccumbens dopamine system in ethanol reinforcement: studies using the techniques of microinjection and voltammetry. Alcohol Alcohol Suppl 2:469–474 [PubMed] [Google Scholar]
- Schroeder JP, Olive F, Koenig H, Hodge CW (2003) Intra-amygdala infusion of the NPY Y1 receptor antagonist BIBP 3226 attenuates operant ethanol self-administration. Alcohol Clin Exp Res 27:1884–1891 [DOI] [PubMed] [Google Scholar]
- Schwenk J, Baehrens D, Haupt A, Bildl W, Boudkkazi S, Roeper J, Fakler B, Schulte U (2014) Regional diversity and developmental dynamics of the AMPA-receptor proteome in the mammalian brain. Neuron 84:41–54 [DOI] [PubMed] [Google Scholar]
- Spanos M, Besheer J, Hodge CW (2012) Increased sensitivity to alcohol induced changes in ERK Map kinase phosphorylation and memory disruption in adolescent as compared to adult C57BL/6J mice. Behav Brain Res 230:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman I (1992) Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci 13:170–176 [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talani G, Licheri V, Masala N, Follesa P, Mostallino MC, Biggio G, Sanna E (2014) Increased voluntary ethanol consumption and changes in hippocampal synaptic plasticity in isolated C57BL/6J mice. Neurochem Res 39:997–1004 [DOI] [PubMed] [Google Scholar]
- Weiss F, Koob GF (2001) Drug addiction: functional neurotoxicity of the brain reward systems. Neurotox Res 3:145–156 [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS (2004) Hippocampal function during adolescence: a unique target of ethanol effects. Ann N Y Acad Sci 1021:206–220 [DOI] [PubMed] [Google Scholar]
- Winder DG, Egli RE, Schramm NL, Matthews RT (2002) Synaptic plasticity in drug reward circuitry. Curr Mol Med 2:667–676 [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF (2013) The development and maintenance of drug addiction. Neuropsychopharmacology: Off Publ Am College Neuropsychopharmacol 39:254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY (2012) Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci 5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward Hopf F, Mangieri RA (2018) Do alcohol-related AMPA-type glutamate receptor adaptations promote intake? Handb Exp Pharmacol 248:157–186 [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer AV, Groenewegen HJ (1996) Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci: Off J Soc Neurosci 16:1877–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Shen H, Yu H, Wang C, Ding R, Lan X, Tash D, Wu X, Wang X, Zhang G (2021) Chronic ethanol exposure induced depressive-like behavior in male C57BL/6 N mice by downregulating GluA1. Physiol Behav 234:113387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study are available from the corresponding author upon reasonable request.