Abstract
Introduction:
Interstitial lung disease (ILD) is the leading cause of mortality in patients with systemic sclerosis (SSc). Risk of developing progressive ILD is highest among patients with diffuse cutaneous disease, positive anti-topoisomerase I antibody, and elevated acute phase reactants. With the FDA approval of two medications and a pipeline of novel therapeutics in trials, early recognition and intervention is critical. High resolution computed tomography of the chest is the current gold standard test for diagnosis of ILD. Yet, it is not offered as a screening tool to all patients due to which ILD can be missed in up to a third of patients. There is need to develop and validate more innovative screening modalities.
Areas covered:
In this review, we provide an overview of screening and diagnosis of SSc-ILD, highlighting the recent innovations particularly the role of soluble serologic, radiomic (quantitative lung imaging, lung ultrasound), and breathomic (exhaled breath analysis) biomarkers in the early detection of SSc-ILD.
Expert opinion:
There is remarkable progress in the development of new radiomics and serum biomarkers in diagnosing SSc-ILD. There is an urgent need for conceptualizing and testing composite ILD screening strategies that incorporate these biomarkers.
Keywords: systemic sclerosis, interstitial lung disease, SSc-ILD, screening, diagnosis, imaging, HRCT, biomarkers, lung ultrasound, oscillometry
1. Introduction
Systemic sclerosis (SSc) is a heterogenous chronic inflammatory autoimmune disease with the highest case fatality among all systemic rheumatic diseases. Interstitial lung disease (ILD) can affect 50–70% of patients with SSc irrespective of the subtype [1]. Progressive ILD remains the leading cause of mortality in SSc, responsible for 17–35% of SSc-related deaths [2,3]. SSc-ILD related clinical symptoms occur late and are nonspecific. Sensitive diagnostic tests are essential as early detection and intervention is critical. Hence, early screening for ILD is essential in all SSc patients at initial evaluation. The clinical presentation can range from subclinical disease (with no clinical symptoms and normal lung function) on high resolution CT-scan (HRCT) to major pulmonary disease, respiratory failure requiring lung transplant and even death.
2. Screening for interstitial lung disease in systemic sclerosis is vital
The high prevalence of ILD in SSc patients supports screening all SSc patients. The risk of developing ILD is highest in the first 5 years after onset of SSc symptoms. There is high inter-individual variability in onset, severity, clinical trajectory, and risk of progression of ILD over time [4,5], necessitating ongoing monitoring during the disease course. Among those with progression in their ILD in the first year following diagnosis, nearly half continue to progress over the next 5 years [6]. Baseline screening has allowed the early identification of mild or ‘subclinical’ disease and when combined with risk factors that predisposed sees progression prompt initiation of targeted intervention to prevent progression. In fact, new treatment paradigms aim towards considering intervention prior to FVC decline occurs, with a goal to preserving lung function prior to irreversible fibrosis or scar setting in which may be a stage too late to intervene for potential gain. The clinical trajectory of SSc-ILD can be extremely heterogenous. In a report from the EUSTAR cohort assessing greater than 800 SSc patients longitudinally, majority (58%) with progressive ILD had a pattern of slow lung function decline, with more periods of stability or improvement than decline, whereas only 8% showed rapid, continuously declining FVC; 178 (33%) experienced no episode of FVC decline. The strongest predictive factors for FVC decline over 5 years include male sex, positive anti-topoisomerase I antibody (ATA) , higher modified Rodnan skin score, and reflux/dysphagia symptoms [6,7]. In a US cohort of 312 well characterized SSc-ILD patients, ATA was the only predictor in the multivariable model that predicted statistically significant decline in FVC over time [7].
3. Current screening modalities
3.1. High resolution CT-scan
The gold standard test to diagnose ILD is an HRCT. But fewer than 60% of physicians utilize HRCT in their practice to screen for ILD in newly diagnosed SSc patients [8]. Despite widespread educational efforts to increase awareness of HRCT as a key diagnostic tool, a recent international survey showed that only 65% physician responders are utilizing it as a routine screening tool at SSc diagnosis, while 30.7% pursue it based on clinical suspicion (presence of crackles on physical examination in 95% respondents or change in pulmonary physiology)[9].
Specific to SSc-ILD patients, detailed practical recommendations on performing HRCT scans have been published[10]. Volumetric acquisition with thin slices using the supine inspiratory scan acquisition is recommended to allow reconstruction in the coronal and sagittal planes; reconstructions should preferably be performed with less than 1.25 mm thick slices. Supine expiratory scans allow better detection of air trapping. It is important to provide specific breathing instructions to patients to allow repeatability of the scans. Prone images are recommended to assess for early ILD vs. gravitational changes. On prone imaging, atelectasis resolves whereas true opacities related to underlying ILD persist. Understanding the underlying diagnosis in patients with ILD often necessitates a multidisciplinary discussion, with thorough evaluation of clinical, imaging, and serological data, and in many cases a broncho-alveolar lavage and lung biopsy which can be time consuming and invasive with associated risks. Up to 25% of ILDs are known to occur in patients with undiagnosed CTD [11]. Patients with CTD-ILD are very highly likely to benefit from immunosuppressive medications, which may not impact disease course or could even be deleterious in some other ILDs, like IPF. HRCT also identifies other significant pulmonary conditions contributing to morbidity and mortality, including enlarged pulmonary arteries (signifying possible pulmonary hypertension), infection, esophageal dilation, emphysema, or lymphadenopathy. There is a clear need to identify accurate, safe, non-invasive, and quick high yield screening and diagnostic modalities for early identification, treatment planning and optimizing long term outcomes in these patients.
Among SSc patients without ILD on baseline HRCT, there is very limited guidance on repeating future HRCTs to detect ILD. In the above-mentioned survey, 170 of 819 (21%) SSc patients with negative baseline HRCT developed ILD during a 3.8 ±3 year follow up [9]. Only 14% survey respondents utilized follow-up HRCT in baseline negative cases routinely, and 65% repeated an HRCT based on development of dyspnea, crackles on physical exam and/or decline in lung function [9], an approach that can be a missed opportunity for early detection and intervention. Approximately 80% of SSc patients and ILD have a histologic pattern of non-specific interstitial pneumonia (NSIP) [Figure 1]. Other histologic patterns of ILD in SSc patients include usual interstitial pneumonia (UIP) [Figure 2: 2A, 2B, 2C, 2D], hypersensitivity pneumonitis (HP), organizing pneumonia (OP) [Figure 3], and acute interstitial pneumonia (AIP). HRCT is regarded as the gold standard for detection and characterization of ILD in SSc patients. Approximately 50% of patients diagnosed with SSc will have ILD on the initial screening HRCT. Findings of NSIP may range from early interstitial abnormalities (ground glass opacity and interlobular septal thickening) to end stage fibrosis (traction bronchiectasis and bronchiolectasis and honeycomb cyst formation).
Figure 1.
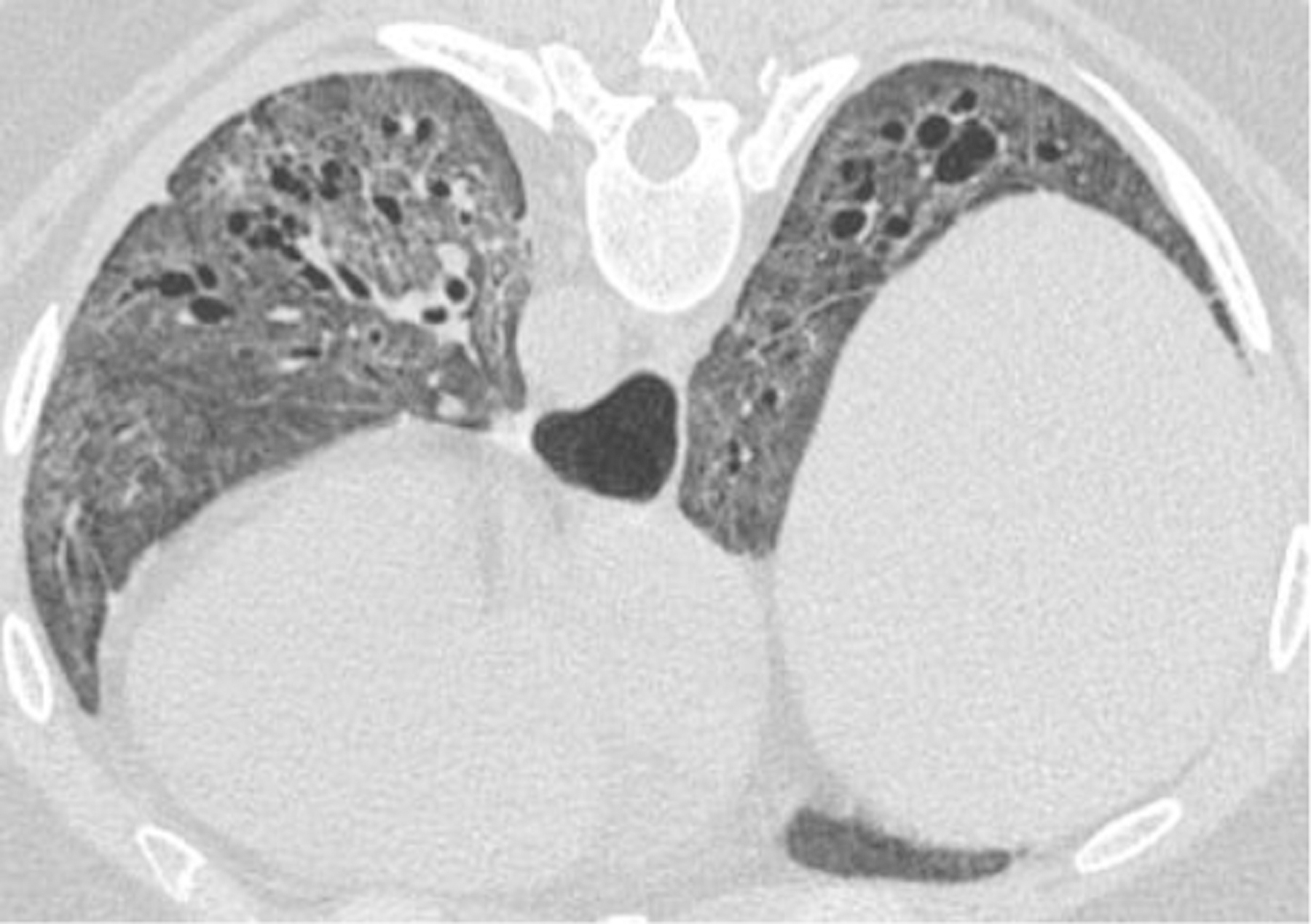
37-year-old female with dysphagia, sclerodactyly, Raynaud’s phenomenon, and calcinosis. Prone images with diffuse ground glass opacity, architectural distortion, interlobular septal thickening, and traction bronchiectasis, favoring fibrotic non-specific interstitial pneumonia pattern. Patulous changes noted in the distal esophagus.
Figure 2.




69-year-old female with CREST syndrome (Calcinosis, Raynaud’s phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasia) and known interstitial lung disease. Usual Interstitial Pneumonia pattern noted.
2A. Anterior predominant fibrosis at the lung apices (anterior upper lobe sign).
2B. Exuberant honeycombing in the lower lobes (exuberant honeycombing sign).
2C. Sharply demarcated fibrosis on coronal images (straight-edge sign).
2D. Patulous esophagus and basilar reticular interstitial lung markings on Barium Esophagram. Note patulous esophagus in 2A and 2B.
Figure 3.

61-year-old female with systemic sclerosis related interstitial lung disease. Architectural distortion, patchy ground glass opacities, and traction bronchiectasis. Pattern Indeterminate for usual interstitial pneumonia. Organizing pneumonia pattern also noted.
CT scans use ionizing radiation, which does carry some risks. These risks depend on the levels of radiation exposure associated with CT scans and are primarily related to radiation induced carcinogens basis. Although, these risks are small, they need to be acknowledged. Particularly, risks are higher in women, and they decrease with age [12]. Keeping these risks in consideration, there have been important advances to reduce HRCT associated radiation exposure including the use of more efficient detector systems, automatic exposure control, advanced image reconstruction methods which produced high-quality images, and other change in the techniques such as acquiring thin slices with large intervals (such as 1 mm with acquisition spaced every 10–20 mm)[13]. It is important to be aware of comparative radiation exposures from different imaging modalities and in specific population with occupational exposures. The estimated effective dose of radiation exposure to a standard HRCT scan is 2–4 mSv. This is lower than the estimated effective dose of routine diagnostic thoracic CT exam (estimated to be 5–7 mSv), but slightly higher to the low-dose lung cancer screening CT exam (1–2 mSv) or routine chest radiograph (0.02–0.1 mSv)[13]. In the United States, the estimated naturally occurring background annual radiation exposure is 3.1 mSv and for airline grew operating long-haul flights, the annual exposure is 2–3 mSv. These exposures or comparable to what is estimated from an HRCT scan. The maximum allowable annual exposure to radiation for radiation workup in the health care sector (including radiologists, radiation technologists, medical physicists) is 50 mSv per year[13].
In summary, HRCT is the current gold standard to diagnose ILD in patients with SSc. With advances in the technique and the procedural protocol (images with supination, pronation, inspiration, expiration), the benefits in diagnosing ILD at baseline evaluation outweigh the risks of radiation exposure. Repetition of HRCT in patients negative for ILD at their baseline evaluation should certainly be based on consideration of the overall risk of progression including the clinical evaluation and PFT data.
3.2. Pulmonary function tests (PFT)
PFTs are widely available, noninvasive, and safe. PFTs are integral in the assessment of restrictive lung disease in SSc-ILD and are obtained at baseline evaluation. Given the low sensitivity for detecting SSc-ILD and notably high false negative rate, PFTs are not to be used as sole screening tests to diagnose SSc-ILD but in combination with HRCT [14]. Once diagnosed with SSc-ILD, serial monitoring of the forced vital capacity (FVC) on PFTs is a robust marker of progression and survival [7].
3.3. Chest radiography
For the evaluation of dyspnea and cough especially in the primary care setting, chest radiographs are commonly used. The advantage is that chest radiography is commonly available and inexpensive. However, compared to HRCT it has good specificity but modest sensitivity for detecting ILD [15]. Therefore, up to 30% of the cases of fibrotic ILD could be missed if chest radiography is relied on as the sole diagnostic tool. Further, failure to mention ILD features on chest radiography is associated with a delay in pulmonology evaluation. Hence, chest radiography is not recommended as a screening tool for SSc-ILD.
Recently, consensus recommendations on different aspects of SSc-ILD management were independently developed in the United States and Europe [16,17]. In both these exercises, a modified Delphi process was completed by pulmonologists, rheumatologists, and internists with SSc-ILD expertise. Table 1 describes the recommendations pertaining to SSc-ILD screening and diagnosis based on these expert consensuses.
Table 1.
Consensus screening and diagnosis recommendations for interstitial lung disease in patients with systemic sclerosis
| Screen all SSc patients for ILD (Current screening modalities) |
|---|
|
| Expert consensus agreement statements on SSc-ILD screening and diagnosis * |
|
|
|
SSc = systemic sclerosis, ILD = interstitial lung disease, PFT = pulmonary function test, DLco = diffusion capacity for carbon monoxide, TLC = total lung capacity, HRCT = high-resolution CT scan, 6MWD = 6-minute walk distance, dcSSc = diffuse cutaneous systemic sclerosis, FVC = Forced Vital Capacity
Expert consensus statements from United States and Europe based pulmonologists, rheumatologists, and internists with expertise in SSc-ILD (from a primary Delphi exercise)
4. Innovative modalities for screening of SSc-ILD
4.1. Newer imaging modalities in SSc-ILD
4.1.1. Low dose CT
Volumetric protocols acquired during full inspiration are the preferred method of scanning for ILD, due to enhanced sensitivity to spatial heterogeneity. This is often augmented with volumetric images at expiration, and interval images in the prone position. HRCT imaging is limited by radiation dose, especially in young females most affected in SSc-ILD. Low dose techniques including low dose CT and interval imaging are limited by noise and spatial heterogeneity of disease, respectively [18]. There are no standardized approaches to low dose CT. Dose modulation is actively applied on modern CT scans, and iterative reconstruction decreases noise (increasing sensitivity and specificity). These variables may depend on different vendor algorithms for CT scanners. Additional approaches to decreasing dose and reducing noise applied for low dose CT include decreasing tube currents t below 50 mA (decrease dose) and increasing slice thickness to 3–5 mm (decrease noise). Volumetric LDCT sensitivity for ILD approaches HRCT with significant decrease in radiation dose. Alternatively, the number of slices can be reduced by performing a sequential CT protocol. In a cohort of 170 patients with SSc, Frauenfelder et.al, performed whole-chest HRCT (standard) and low-dose HRCT with nine slices allocated according to basal-apical gradient. The low-dose HRCT had a high sensitivity (88.3%), accuracy (91.8%, reader 1; 94.7%, reader 2), diagnostic confidence (98.8%, reader 1; 95.3%, reader 2) and image quality. Both minimal and extensive ILD were correctly quantified by visual read. Importantly, the reduced HRCT had a significantly lower radiation dose. Sequential LDCT is suboptimal for screening due to suboptimal characterization of spatial heterogeneity. While not for screening, low-dose CT when combined with Computer-Aided Lung Informatics for Pathology Evaluation and Ratings (CALIPER) technology can serve as an important adjunct to PFTs in disease monitoring in patients with idiopathic pulmonary fibrosis [19].
4.1.2. Quantitative and texture Analysis of ILD
Computer-aided diagnostic algorithms to better characterize HRCT patterns and quantify ILD was first reported in 2010 in patients with SSc[20]. These algorithm times were developed using a machine learned texture feature classification to detect and quantify the amount of ground-glass changes (QGG), lung fibrosis (QLF), ILD (QLD), and honeycomb cysts in patients with SSc-ILD and have been utilized in Scleroderma Lung II and tocilizumab phase 3 trial. Quantitative CT (QCT) analysis techniques using measurements related to the density of discrete regions of the lung (voxels) allow for more precise quantification of different CT findings of interest (reticulations, traction bronchiectasis/bronchiolectasis, honeycombing, lung density, pulmonary vessel volume, etc). From whole-lung density histograms, parameters such as mean lung attenuation (MLA), skewness, kurtosis, and proportion of high attenuation areas (HAA) can be calculated which have demonstrated association with lung function and visual fibrosis scores in SSc-ILD [21]. Independent of the staging systems for quantifying interstitial lung disease (visual or QLF), the extent of QLD is a predictor of decline in the FVC over 1-year period [22,23]. In a retrospective study of 146 SSc-ILD patients, Ariani et al demonstrated the ability of QCT parameters in differentiating high vs low mortality subgroups as assessed by clinical mortality-risk prediction models (ILD-Gender, Age, Physiology score and DuBois index) supporting a prognostic role for QCT in SSc-ILD [24]. In addition to determining types of parenchymal abnormalities and lung density maps, texture analysis software have been designed to recognize HRCT patterns. Robust and validated analysis may remove subjectivity inherent in qualitative reporting and allow greater objective measurements of change over time. This may more optimally characterize and quantify response to therapy and disease progression, from which morbidity and mortality may be inferred.
Quantitative CT has been used in IPF and SSc-ILD to access response to immunosuppressive therapy [25,26] and antifibrotics [27], and to predict disease progression and subsequent death [28–31]. In the scleroderma lung study II, treatment of SSc-ILD with either cyclophosphamide for 1 year, followed by placebo for a second year, or mycophenolate for 2 years was associated with a significant reduction (improvement) in the extent of HRCT SSc-ILD (QILD) assessed by computer-aided diagnosis scores, which correlated well with one or more other measures of treatment response [25].
Another computer-aided CT image post-processing lung texture analalysis with the use of CALIPER (Computer-Aided Lung Informatics for Pathology Evaluation and Ratings) technology has been used to identify CT features predictive of mortality and FVC decline, and to predict response to Pirfenidone therapy in IPF patients [27,30]. In SSc-ILD patients, CALIPER can quantify ILD features and asses for worsening of PFT parameters [32]. In this subgroup, ground glass opacity was identified as the main radiological finding to predict worsening of pulmonary function as assessed by PFTs. CALIPER has also been used to demonstrate radiographic response to mycophenolate therapy in SSc-ILD patients [33]. Pulmonary arterial hypertension (PAH) when co-exists with ILD in SSc patients worsens the overall prognosis [34]. The use of CALIPER has found it application in simultaneously quantification of pulmonary vascular parameters alongside ILD. Vascular parameters for total and separated pulmonary vascular volume (PVV) significantly correlate with functional parameters and increase in parallel with ILD extent and functional impairment [35]. Further, CALIPER technology allowed to identify heterogenous patterns of pulmonary vascular changes with PVV significantly increased in SSc patients with DLCO% reduction. Concomitant quantification of ILD patterns and PVV changes may serve as radiomic biomarkers for assessing disease severity, therapeutic response, and outcome measure in trials [36].
While availability of QCT is still limited clinically and believed to be expensive, there are free software, such as OsiriX and its 64-bit version, Horos, which are more accessible and can help provide limited vs extensive SSc-ILD quantification [37]. Integration of QCT into ongoing clinical trials will also help better understand its utility in assessing progression over time in patients treated with placebo and understanding change in various QCT parameters in response to therapeutics in SSc-ILD to guide more focussed practice recommendations and its utility in day-to-day clinical care in the future.
4.1.3. Lung ultrasound
Lung ultrasound (LUS) for assessment of ILD was described as early as 2009 [38] and its usage has dramatically increased in the last decade [39–47]. Advantages of LUS include its smaller footprint improving patient accessibility as well as the lack of ionizing radiation. Aerated lung has a large acoustic impedance making it a strong acoustic reflector. Therefore, LUS assesses the parenchyma by means of imaging artifacts. A-lines, commonly seen in healthy lungs, are reverberation artifacts and are reproductions of the pleural line (Figures 4A and 4B). These A lines manifest as equidistant echogenic lines parallel to the pleural line. In contradistinction, B-lines have been described with various diseased conditions such as pulmonary edema, consolidation seen with pneumonia, or fibrosis. B-lines are vertical artifacts which arise from the pleural line that extend to the image edge (Figures 5A and 5B). These echogenic lines move with the underlying parenchyma during respiration. There is no definite anatomic correlate for B-lines but several studies have suggested that it is caused by structural alterations in the subpleural parenchyma causing discontinuity of the pleural surface[48]. This can be caused by several pathologies such as fluid within the interlobular septa or thickening of the interstitium seen with fibrosis. B- lines can have varied appearances and can be sharp and thin or may be coalescent and thick [48].
Figure 4.


4A. Lung ultrasound using a curvilinear transducer showing A-line artifacts (arrows)
4B. Corresponding lung CT image
Figure 5.


5A. Lung ultrasound using a curvilinear transducer demonstrating B-line artifact (arrow).
5B. Corresponding lung CT image
Early studies assessed the role of LUS in detecting ILD associated with CTD-ILD [40]. Evaluation of LUS specifically for SSc has been promising on several fronts. Some researchers have shown the potential for detecting early-stage ILD and have proposed its use as a screening tool [39–43]. Others have focused on assessing the degree of pulmonary fibrosis based on the number of B line artifacts [44,45]. Gigante et al compared LUS with HRCT and PFTs and found that the number of B-lines correlated with the results of HRCT [46]. Gargani et al compared the accuracy of LUS in sixty-nine patients with SSc who also underwent HRCT. Results showed that the number of B-lines significantly correlated with the HRCT score and concluded that a threshold of > 10 B- lines could be used to screen for ILD. A reticular pattern at HRCT correlated with B line artifacts and irregular pleural lines. Honeycombing at HRCT also correlated with B-line artifacts as well [47]. Although this research is promising, it still must be determined what role LUS is best suited for in the assessment of this disease. Further work is needed to validate these earlier studies as well as in imaging protocol and interpretation standardization.
4.1.4. Magnetic resonance imaging
High resolution CT-scans assess the character and spatial heterogeneity of ILD in SSc patients and is the current standard to assess and quantify morphological abnormalities. Recently, an interest in the role of MRI in evaluation of SSc-ILD has emerged. Lung MRI STIR and T1 values are significantly different between patients with and without SSc-ILD and may predict worsening lung involvement over time independent to HRCT assessment [49]. CT is also limited in its ability to provide functional information. MRI can provide functional information without the use of ionizing radiation. MRI sequences can identify ILD and subtle changes in disease distribution and provide functional information, although it remains suboptimal in the definition of the underlying pattern of ILD. Ground-glass opacities (GGOs) defined as “hazy increased opacity, with preservation of bronchial and vascular margins” are a frequent CT scan finding in patients with SSc-ILD [50]. It is hard to differentiate whether GGOs are due to underlying inflammation or fibrosis. Other modalities like LUS cannot identify GGOs. Many sequences have been applied in MRI evaluation of ILD. The most promising sequences to date include Ultrashort-time-to-Echo (UTE) MR sequence which allows imaging of the pulmonary parenchyma and contrast enhanced MR sequences. UTE Spiral VIBE-MRI sequence may differentiate between areas of active inflammation (reversible) and fibrosis (non-reversible) and have shown to be reliable in assessing both the extent of ILD and GGO in SSc-ILD [51]. Areas of inflammation (alveolitis) demonstrate high intensity on T2 weighted sequences and early enhancement on post contrast sequences. Fibrosis demonstrates low signal intensity on T2 and late enhancement on post contrast sequences [52].
Gaseous non proton inhaled contrast agents (Xenon and Helium) have also been applied to MRI imaging of pulmonary function and structure. These modalities represent an emerging area of future advancement. Xenon MRI has demonstrated “functional” resolution with increased sensitivity for diffusion across the blood gas barrier, which is limited in ILD and may precede spatial changes seen on HRCT.
4.1.5. Positron emission tomography – CT studies
PET-CT studies have shown increased pulmonary FDG uptake in patients with SSc-ILD than in dose without SSc-ILD and positively correlated with early severity [53–55]. 18F-FDG PET/CT may not differentiate the intensity of metabolic activity across HRCT patterns in chronic SSc patients especially in areas of ground-glass opacities and honeycombing [56]. A novel PET-CT approach with the use of 68Gallium-labeled selective inhibitor of fibroblast activation protein (68Ga-FAPI-04) showed that fibroblast activation assessed by FAPI correlated well with fibrotic activity and disease progression of SSc-ILD[57]. Increased 68Ga-FAPI-04 uptake at baseline was associated with progression of ILD within the next 6 months. Further, 68Ga-FAPI-04 uptake was higher in patients with extensive lung disease with previous ILD progression. In a related but different disease population (idiopathic inflammatory myositis associated ILD), PET-CT based scoring system showed superior performance and discriminating between rapidly progressive ILD and non-rapidly progressive ID compared with HRCT visual analysis, and could stratify the risk of a subset of rapidly progressive ILD with positive anti MDA 5 antibody [58]. These findings guide us to explore combining imaging modalities in certain subsets of patients with SSc-ILD, like in the early phase or in patients with overlap of myositis.
4.1.6. Dual energy CT scan (DECT)
parameters have recently shown to achieve excellent performance in terms of differentiating extensive from limited extent of ILD associated with connective tissue disease (CTD ILD) and correlated with severity as assessed by symptoms, CT scan, and PFT [59].
4.2. Impulse oscillometry
The forced oscillation technique (FOT) is commonly used in routine PFT due to the minimal need for patient co-operation[60]. The technique is meant to be useful in acquiring data of the respiratory system’s mechanical properties. The main components of an oscillometer include the loudspeaker, which produces a pulsatile stimulus through the adapter, through which pressures waves are transmitted to the airways with the airflow, and the pneumotachograph, usually attached to a mouthpiece, a face mask, or an endotracheal tube. Oscillometry may be a useful tool in assessment of small airways disease in ILD [61]. Oscillometry can be a sensitive complementary tool for diagnosing early stages of lung fibrosis.
4.3. The role of novel biomarkers in screening
Biomarkers are defined as indicators of normal and pathogenic biological processes and hold promise in the detection of ILD in patients with SSc. [62] The ideal SSc-ILD screening biomarker should have high accuracy for detecting early lung disease, prior to the development of respiratory symptoms or lung function decline. Blood-based biomarkers represent a promising non-invasive screening modality, carrying only the minimal risks of phlebotomy without the procedural risks of bronchoscopy or surgical lung biopsy or radiation risks of HRCT. Blood-based SSc-ILD biomarkers can be categorized into two categories: autoantibodies currently used in practice and novel biomarkers under study.
Autoantibodies represent the serologic hallmark of SSc, well-studied as diagnostic and prognostic markers of disease-related complications. They have also been shown to be associated with ILD in patients with known SSc. Among these, the autoantibody most often associated with the presence of ILD is ATA. [63–67] Additionally, anti Th/To ribonucleoprotein antibodies and anti PM/Scl have also been shown to be associated with ILD, though these antibodies are not as highly prevalent in SSc [68,69]. In two large multi-center cohorts, the Canadian Scleroderma Research Group registry and the German Network for Systemic Scleroderma Registry, the presence of Anti-SSA/Ro was found to be associated with at least a two-fold increased odds of ILD.[70,71] On the contrary, anti-centromere antibodies appear to be ‘relatively protective’ and associated with decreased likelihood of progressive ILD.[64,72] While these antibodies carry an association with the presence of ILD, they are not unique to lung-specific disease activity and also correlate with extrapulmonary SSc complications. Therefore, the use of blood-based biomarkers alone cannot replace gold-standard HRCT to evaluate for ILD. While we recommend that all patients with SSc be screened for ILD, there still exists some variation in practice pattern, with some clinicians and patients choosing to defer HRCT imaging. In these situations, elevated levels of these ILD-associated autoantibodies can be used as a risk stratification tool and when present should signal a higher risk of ILD in patients with SSc prompting HRCT acquisition.
There are ongoing investigations for new molecular biomarkers that may better detect ILD (Table 2). Among those with the best described test performance is Krebs von den Lungen 6 (KL-6), which is thought to be a marker of epithelial injury [73]. Set at various cutoff thresholds, the sensitivity for detecting ILD in patients in SSc ranges 73%−86%, while the specificity ranges 70–90%.[74–76] Another well-studied biomarker of lung epithelial damage and turnover is surfactant protein D (SP-D), whose sensitivity for detecting SSc-ILD ranges from 68% to 90% and specificity 70% to 80% depending on the cutoff threshold used [77]. There are several other emerging candidate diagnostic biomarkers, that include biomarkers of lung epithelial cell dysfunction, [76,78–82] aberrant immunity, [83–94] and abnormal lung remodeling. [95–103] Taken together, many novel blood-based biomarkers hold promise in SSc-ILD screening, but none have yielded adequate test performance or been validated for clinical implementation.
Table 2.
Novel blood-based biomarkers in patients with systemic sclerosis associated interstitial lung disease
| Biomarker | Abbreviation | Increased expression / association / correlation | Reference(s) |
|---|---|---|---|
| Clara Cell 16-kDa | CC16 | Active ILD | (1) |
| Chemokine (C-C motif) ligand 2 | CCL2 | Skin and lung disease activity | (2) |
| Chemokine (C-C motif) ligand 18 | CCL18 | Fibrotic activity in the lungs | (3, 4) |
| Connective Tissue Growth Factor | CTGF | Extent of skin and lung fibrosis | (5) |
| Chemokine (C-X3-C motif) ligand 1 | CX3CL1 | Progressive ILD | (6) |
| Chemokine (C-X-C motif) ligand 10 | CXCL10 | Active / progressive ILD | (7, 8) |
| Chemokine (C-X-C motif) ligand 16 | CXCL16 | Active ILD | (9) |
| Chemokine (C-X-C motif) ligand 4 | CXCL4 | Progressive ILD | (10) |
| Chemokine (C-X-C motif) ligand 9 | CXCL9 | ILD | (11) |
| E-Selectin | E-Selectin | Progressive ILD | (12–14) |
| Endothelin-1 | ET-1 | ILD | (14) |
| Growth Differentiation Factor-15 | GDF-15 | Extent of skin and lung fibrosis | (15–18) |
| Gremlin-1 | Gremlin-1 | ILD in early diffuse systemic sclerosis | (19) |
| Human epididymis protein 4 | HE-4 | ILD | (20) |
| Intercellular Adhesion Molecule 1 | ICAM-1 | ILD | (21) |
| Interleukin-4 | IL-4 | ILD | (22) |
| Interleukin −6 | IL-6 | ILD | (23) |
| Interleukin-22 | IL-22 | ILD | (23) |
| Interleukin-23 | IL-23 | ILD | (24) |
| Interleukin-33 | IL-33 | Extent of skin and lung fibrosis | (18) |
| Interleukin-35 | IL-35 | Severity of lung fibrosis | (25) |
| Krebs von den Lungen-6 | KL-6 | Active ILD and severity of fibrosis | (1, 3, 26–28) |
| Matrix Metalloproteinase-12 | MMP-12 | Severity of skin and lung fibrosis | (29) |
| Matrix Metalloproteinase-7 | MMP-7 | Poor baseline lung function and transplant-free survival | (30) |
| Pulmonary and activation-regulated chemokine | PARC | Fibrotic activity in the lungs | (28) |
| Surfactant Protein-A | SP-A | ILD | (31) |
| Surfactant Protein-D | SP-D | Active ILD | (1, 26, 27, 32–34) |
| Tissue inhibitors of metalloproteinase 1 | TIMP-1 | ILD | (35) |
| Vascular Endothelial Growth Factor | VEGF | ILD | (14) |
| YKL-40 | YKL-40 | ILD | (36) |
With the rise of large -omics platforms, we anticipate that larger numbers of blood-based biomarkers will be studied. We have seen that modeling large numbers of biomarkers in aggregate can achieve better test performance compared to single biomarkers in ILD associated with other connective tissue diseases, [89,122] and we anticipate that a similar approach will be essential to screening for SSc-ILD. Lastly, prior to clinical implementation in SSc-ILD screening, validation of biomarker test performance will need to be performed. Collaborations between scleroderma centers will be essential for external validation prior to biomarker implementation.
4.4. Nailfold capillaroscopy in ILD
Nailfold capillaroscopy (NFC) is a simple, non-invasive, low-cost tool that is now integrated into the classification criteria for SSc [123]. Besides its role in the early diagnosis of SSc, it has been recognized as an important tool to identify SSc patients at high risk for visceral complications and death[124]. Originally described by Maricq et al, the ‘Scleroderma’ pattern comprising a set of typical NFC features including dilated capillary loops (ectasias and/or giant capillary loops), loss of capillary density, avascular areas, microhemorrhages and neoangiogenesis can be seen in 83–98% patients with SSc [125]. Cutolo et al classified these changes into 3 stages – early, active and late reflecting theoretically the progression of SSc-related microangiopathy with disease evolution and suggesting a strong association with disease duration, but it is controversial if these stages correlate with disease duration or severity in SSc [126]. A recently published meta-analysis of 21 studies found that the prevalence of nailfold capillary abnormalities by nailfold videocapillaroscopy (NVC) was highest in CTD-ILD at 80.4% (95 CI 74.3%, 85.3%), followed by IPAF 27.4% (95% CI 10.9%, 53.7%), and lowest in IPF 13.8% (95% CI 5.7%, 29.9%) [127]. Late SSc pattern was the most prevalent pattern in the CTD-ILD cohort 40.4% (28.1%, 54.1%). Among the CTDs, NVC abnormalities were highest among SSc-ILD (N=569 patients, from 7 studies) at 92.7% (88.8%, 95.3%). Despite these interesting findings, the role and utility of nailfold capillaroscopy in ILD evaluation remains unclear.
4.5. E-Nose technology
Exhaled air contains a complex mixture of volatile organic compounds (VOCs) that have recently been identified as a novel biomarker to discriminate healthy controls from patients with a variety of chronic lung conditions including asthma, COPD, and ventilator-associated pneumonia [128,129]. A recently published pilot study [130] using a thermal desorption gas chromatography-time of flight-mass spectrometry analysis [131] on concentrated samples of exhaled air demonstrated that the VOC profiles in exhaled breath from 53 patients with CTD-ILD and 53 patients with IPF could differentiate these groups from each other and from 51 age-matched healthy controls. In patients with CTD-ILD, a set of 16 VOCs discriminated them from IPF patients, and their VOC profile strongly correlated with total lung capacity and the 6 min walk distance. In another study, VOC signatures obtained by electronic nose technology (Aeonose®) in 174 ILD patients, 23 patients with COPD and 33 healthy controls showed potential in separating those with ILD from heathy controls but showed low specificity in distinguishing ILD subgroups (IPF, CTD-ILD, Cryptogenic organizing pneumonia) from one another [132]. Currently, there is no data to show if VOC profiles are different in SSc patients with or without ILD. It is also difficult to say if the difference in VOC profiles among CTD-ILD and IPF patients may be associated with medication usage (immunosuppressive and/or antifibrotic) which has previously been reported as a confounding factor affecting VOCs in other studies [133]. These breathomics biomarkers could potentially prove to be an important non-invasive step in precision medicine for patients with CTD-ILD but warrant further detailed study in larger cohorts of individual CTDs to understand their role and significance in screening, diagnosis, and monitoring of ILD.
4.6. Composite risk scores
Bruni et al recently developed and validated a composite risk score that can guide physicians in ordering both baseline and follow up HRCT to screen for SSc-ILD (ILD-RISC)[134]. This model using FVC%, DLCO%, digital ulcer (ever), age, and SSc autoantibody status in a derivation cohort of 533 SSc patients (43% ILD) showed an Odds ratio (OR) of 133.9 (95% CI 53.4–335.9) with an AUC of 79.1% (95% CI 75.3–83%) for ILD on HRCT. A score of ≥ 0.3 had a sensitivity of 85.6%, specificity of 53.6%, NPV 83.2% and PPV of 58.2%; that was replicated in a validation cohort of 247 SSc patients (48% ILD) and had similar performance when used for longitudinal follow-up. Using a composite risk-score strategy such as this, can be advantageous in resource limited practices and can help avoid unnecessary imaging and health care utilization.
High-dimensional image analysis or ‘radiomics’ utilizes computationally retrieved, quantitative data derived from radiological images that describe lung tissue in terms of its texture, intensity and statistical properties adding the ability to capture tissue phenotypes on different spatial scales. This adds noninvasive, novel, and complementary information to capture extent and severity of the heterogeneity of lung disease in SSc-ILD identifying phenotypes that provide a prognostic value add to risk stratification beyond that can be done by clinical, serological parameters and/or standard CT imaging alone. In a recently published study, using two independent, prospectively followed SSc-ILD cohorts (Zurich, derivation cohort, n=90; Oslo, validation cohort, n=66), the authors identified 1355 radiomic features from standard-of-care CT images, performed unsupervised clustering [135]. They identified based on radiomic features, two clinically and prognostically distinct SSc-ILD patient clusters, and derived a clinically applicable prognostic quantitative radiomic risk score (qRISSc) composed of 26 features that accurately predicted progression-free survival (PFS) and significantly enhanced clinical risk stratification in multivariable Cox regression analysis [135]. Radiomics-based risk stratification approaches using readily available standard CT images could thus significantly impact clinical decision making in SSc-ILD, using a quantitative risk score that improves upon conventional risk factors.
4.7. Conclusion
There are myriads of radiological and biomarker innovations in the recent years to diagnose SSc-ILD. Although HRCT remains a gold standard, alternative modalities like lung ultrasound, MRI, PET-CT, and incorporation of image processing algorithms expand the investigative options, their specific applications in different clinical scenarios, and the potential to combine them with serum / lung biomarkers and breathomics. The future holds promise in testing and validating composite screening strategies.
5. Expert opinion
In a serious and complex disease such as SSc-ILD, an ideal screening test should reliably identify ILD even before symptoms develop. HRCT fits this requirement and is the current gold standard in diagnosing ILD. Screening for ILD in SSc is essential for early identification of ILD to initiate treatment and change the trajectory of the lung disease. PFTs, 6MWD, and patient reported outcome measurements complement in characterizing other dimensions of the ILD like lung function, functional capacity, and impact on the quality of life in SSc patients (Figure 6). The availability of HRCT should not prevent us from exploring innovative screening modalities. There is continued quest in identifying screening strategies that are inexpensive, safe (minimize radiation exposure), easy to administer, sensitive, reliable, valid, and cause minimal discomfort. A screening test with a high specificity should also prevent the need for repetitive ILD screening in a low-risk SSc patient. With the exciting new modalities described in this article (also refer Table 3), we can envision a composite multi-tiered approach to ILD screening that could incorporate the above-mentioned test characteristics and may be available even in resource limited settings in the future (Future state in Figure 6). The future state should encompass cost-effective screening strategies with comparable efficacy that can be used in different healthcare settings. Break-through developments in radiomics (lung ultrasound, computer-aided CT interpretations, PET-CT, MRI) and biomarker discovery should force us to think beyond the current paradigm and address the unmet needs. There is immense potential in applying these new technologies in a composite approach to sub-type SSc-ILD phenotypes, differentiate inflammatory-predominant and fibrotic-predominant subsets, and through this differentiation direct the choice or combination of drug therapies (immunomodulatory with or without anti-fibrotic medications). Many imaging modalities have been described in this article. More guidance is needed in the unique case-based application of these modalities. For example, the computer-aided CT-scans allow quantitative texture analysis of fibrosis, ground glass opacities, and lung disease. These interpretations when amplified by the artificial intelligence algorithms could pave the way to consistency in baseline CT-chest interpretations and longitudinal comparisons. MRI of the lungs seem to better define the ground glass opacities. PET-CT chest, based on the marker used, could differentiate fibrotic from inflammatory lung diseases. This could potentially guide the choice of pharmacotherapy. Lung ultrasound has the advantage of portability and limited office space requirement make it an attractive option to be used in office visit and complement clinical evaluation. Impulse oscillometry is a relatively inexpensive technique that can be combined with PFTs in identifying early disease and concomitant small airway disease. The evolution of new biomarkers that predict ILD (like KL-6) strengthen the composite approach in screening ILD. Continuing research activities and collaborations between different centers is critical in defining the optimal screening / diagnosis algorithms in SSc-ILD that will help in sub-typing high-risk vs low-risk patients and identifying progressors vs non-progressors and treatment responders vs non-responders, at baseline evaluation. These innovations thus have a vital role in prognosticating the course of ILD at the time of diagnosis enabling decision making and advise to the SSc patients.
Figure 6.

Screening for interstitial lung disease in systemic sclerosis: current vs. future state
ILD = interstitial lung disease, SSc = systemic sclerosis, HRCT= high resolution CT-scan, PFT = pulmonary function test, PRO= patient reported outcomes measures, PET = positron emission tomography
*HRCT is the current gold standard to diagnose ILD
^Defined as having positive anti-SCL70, male gender, high baseline skin score, early diffuse SSc with high CRP
ayet to be defined
Table 3.
Pros and cons of interstitial lung disease screening modalities in systemic sclerosis
| Modality | Pros | Cons | |
|---|---|---|---|
| Current approach to screening | HRCT at baseline |
|
|
| Pulmonary function test |
|
|
|
| Autoantibody testing |
|
|
|
| Newer innovations | Low-dose CT scan |
|
|
| Computer-aided CT scans |
|
|
|
| PET-CT scans |
|
|
|
| Lung ultrasound |
|
|
|
| Lung MRI |
|
|
|
| Novel biomarkers* |
|
|
|
| E-nose |
|
|
KL-6, SP-D, and biomarkers of lung epithelial cell dysfunction, aberrant immunity, and abnormal lung remodeling
Article highlights:
Interstitial lung disease (ILD) is highly prevalent in patients with systemic sclerosis (SSc) and early screening is essential.
Despite the availability of high-resolution CT-scans of the chest universal baseline screening is not performed routinely.
Recent interests and innovations in different radiologic evaluation modalities expand the diagnostic options for screening.
Computer-aided image processing algorithms allow consistency in quantifying aspects of ILD.
An expanding repertoire of serum or blood-based biomarkers link to different dimensions of the ILD.
Funding
D Khanna was supported by NIAMS K24 ARAR063120.
Footnotes
Declaration of interest
A Makol reports consultancy fees from Boehringer Ingelheim and Sanofi. V Nagaraja reports consultancy fees from Boehringer Ingelheim. D Khanna reports grant support from Bayer, BMS, Horizon, Immune Tolerance Network, NIH, and Pfizer; consultancy fees from AbbVie, Acceleron, Actelion, Amgen, Bayer, Chemomab, Boehringer Ingelheim, CSL Behring, Genentech/Roche, Horizon, Merck, Mitsubishi Tanabe Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Sankar S, Habib M, Jaafar S et al. Hospitalisations related to systemic sclerosis and the impact of interstitial lung disease. Analysis of patients hospitalised at the University of Michigan, USA. Clin Exp Rheumatol, 39 Suppl 131, 43–51 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elhai M, Meune C, Boubaya M et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis, 76, 1897–1905 (2017) [DOI] [PubMed] [Google Scholar]
- 3.Tyndall AJ, Bannert B, Vonk M et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis, 69, 1809–15 (2010) [DOI] [PubMed] [Google Scholar]
- 4.Man A, Davidyock T, Ferguson LT, Ieong M, Zhang Y, Simms RW. Changes in forced vital capacity over time in systemic sclerosis: application of group-based trajectory modelling. Rheumatology (Oxford), 54, 1464–71 (2015) [DOI] [PubMed] [Google Scholar]
- 5.Vonk MC, Walker UA, Volkmann ER, Kreuter M, Johnson SR, Allanore Y. Natural variability in the disease course of SSc-ILD: implications for treatment. Eur Respir Rev, 30 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann-Vold AM, Allanore Y, Alves M et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis, 80, 219–27 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramahi A, Lescoat A, Roofeh D et al. Risk factors for lung function decline in systemic sclerosis-associated interstitial lung disease in a large single-center cohort. Rheumatology (Oxford), (2022): keac639* * This study highlights the prognostic significance of anti-topoisomerase antibodies in patients with systemic sclerosis associated interstitial lung disease.
- 8.Bernstein EJ, Khanna D, Lederer DJ. Screening High-Resolution Computed Tomography of the Chest to Detect Interstitial Lung Disease in Systemic Sclerosis: A Global Survey of Rheumatologists. Arthritis Rheumatol, 70, 971–72 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruni C, Chung L, Hoffmann-Vold AM et al. High-resolution computed tomography of the chest for the screening, re-screening and follow-up of systemic sclerosis-associated interstitial lung disease: a EUSTAR-SCTC survey. Clin Exp Rheumatol, 40, 1951–55 (2022) [DOI] [PubMed] [Google Scholar]
- 10.Chung JH, Walker CM, Hobbs S. Imaging Features of Systemic Sclerosis-Associated Interstitial Lung Disease. J Vis Exp, (160) (2020) [DOI] [PubMed] [Google Scholar]
- 11.Decker P, Sobanski V, Moulinet T et al. Interstitial pneumonia with autoimmune features: Evaluation of connective tissue disease incidence during follow-up. Eur J Intern Med, 97, 62–68 (2022) [DOI] [PubMed] [Google Scholar]
- 12.Council NR. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2 (2006) [PubMed]
- 13. Khanna D, Distler O, Cottin V et al. Diagnosis and monitoring of systemic sclerosis-associated interstitial lung disease using high-resolution computed tomography. J Scleroderma Relat Disord, 7, 168–78 (2022)* * This paper comprehensively reviews the diagnosis and monitoring requirements for patients with systemic sclerosis-associated interstitial lung disease. Besides that, there is comparative analysis of risks with ionizing radiations in routine testing.
- 14.Suliman YA, Dobrota R, Huscher D et al. Brief Report: Pulmonary Function Tests: High Rate of False-Negative Results in the Early Detection and Screening of Scleroderma-Related Interstitial Lung Disease. Arthritis Rheumatol, 67, 3256–61 (2015) [DOI] [PubMed] [Google Scholar]
- 15.Ghodrati S, Pugashetti JV, Kadoch MA, Ghasemiesfe A, Oldham JM. Diagnostic Accuracy of Chest Radiography for Detecting Fibrotic Interstitial Lung Disease. Ann Am Thorac Soc, 19, 1934–37 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahaghi FF, Hsu VM, Kaner RJ et al. Expert consensus on the management of systemic sclerosis-associated interstitial lung disease. Respir Res, 24, 6 (2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann-Vold A-M, Maher TM, Philpot EE et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. The Lancet Rheumatology, 2, e71–83 (2020) [DOI] [PubMed] [Google Scholar]
- 18.Frauenfelder T, Winklehner A, Nguyen TD et al. Screening for interstitial lung disease in systemic sclerosis: performance of high-resolution CT with limited number of slices: a prospective study. Ann Rheum Dis, 73, 2069–73 (2014) [DOI] [PubMed] [Google Scholar]
- 19.Koo CW, Larson NB, Parris-Skeete CT et al. Prospective machine learning CT quantitative evaluation of idiopathic pulmonary fibrosis in patients undergoing anti-fibrotic treatment using low- and ultra-low-dose CT. Clin Radiol, 77, e208–14 (2022) [DOI] [PubMed] [Google Scholar]
- 20.Kim HG, Tashkin DP, Clements PJ et al. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol, 28(Suppl 62), S26–35 (2010) [PMC free article] [PubMed] [Google Scholar]
- 21.Saldana DC, Hague CJ, Murphy D et al. Association of Computed Tomography Densitometry with Disease Severity, Functional Decline, and Survival in Systemic Sclerosis-associated Interstitial Lung Disease. Ann Am Thorac Soc, 17, 813–20 (2020) [DOI] [PubMed] [Google Scholar]
- 22.Watadani T, Sakai F, Johkoh T et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology, 266, 936–44 (2013) [DOI] [PubMed] [Google Scholar]
- 23.Khanna D, Nagaraja V, Tseng CH et al. Predictors of lung function decline in scleroderma-related interstitial lung disease based on high-resolution computed tomography: implications for cohort enrichment in systemic sclerosis-associated interstitial lung disease trials. Arthritis Res Ther, 17, 372 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ariani A, Silva M, Seletti V et al. Quantitative chest computed tomography is associated with two prediction models of mortality in interstitial lung disease related to systemic sclerosis. Rheumatology (Oxford), 56, 922–27 (2017) [DOI] [PubMed] [Google Scholar]
- 25. Goldin JG, Kim GHJ, Tseng CH et al. Longitudinal Changes in Quantitative Interstitial Lung Disease on Computed Tomography after Immunosuppression in the Scleroderma Lung Study II. Ann Am Thorac Soc, 15, 1286–95 (2018)** **This paper demonstrates the application of a computer aided diagnostic model in longitdunially quantifying interstitial lung disease in a clinical trial / therapuetics setting.
- 26.Roofeh D, Lin CJF, Goldin J et al. Tocilizumab Prevents Progression of Early Systemic Sclerosis-Associated Interstitial Lung Disease. Arthritis Rheumatol, 73, 1301–10 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob J, Bartholmai BJ, Rajagopalan S et al. Predicting Outcomes in Idiopathic Pulmonary Fibrosis Using Automated Computed Tomographic Analysis. Am J Respir Crit Care Med, 198, 767–76 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clukers J, Lanclus M, Belmans D et al. Interstitial lung disease in systemic sclerosis quantification of disease classification and progression with high-resolution computed tomography: An observational study. Journal of Scleroderma and Related Disorders, 6, 154–64 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob J, Bartholmai BJ, Rajagopalan S et al. Evaluation of computer-based computer tomography stratification against outcome models in connective tissue disease-related interstitial lung disease: a patient outcome study. BMC Med, 14, 190 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacob J, Bartholmai BJ, Rajagopalan S et al. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J, 49 (2017) [DOI] [PubMed] [Google Scholar]
- 31.Jankharia BG, Angirish BA. Computer-Aided quantitative analysis in interstitial lung diseases - A pictorial review using CALIPER. Lung India, 38, 161–67 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrazza AM, Gigante A, Gasperini ML et al. Assessment of interstitial lung disease in systemic sclerosis using the quantitative CT algorithm CALIPER. Clin Rheumatol, 39, 1537–42 (2020)** ** This paper describes another post-study image processing algorithm and its application in interstitial lung disease.
- 33.Baqir M, Makol A, Osborn TG, Bartholmai BJ, Ryu JH. Mycophenolate mofetil for scleroderma-related interstitial lung disease: A real world experience. PLoS One, 12, (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nihtyanova SI, Schreiber BE, Ong VH et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol, 66, 1625–35 (2014) [DOI] [PubMed] [Google Scholar]
- 35.Bruni C, Occhipinti M, Pienn M et al. Lung vascular changes as biomarkers of severity in systemic sclerosis-associated interstitial lung disease. Rheumatology (Oxford), 62, 696–706 (2023) [DOI] [PubMed] [Google Scholar]
- 36.Occhipinti M, Bruni C, Camiciottoli G et al. Quantitative analysis of pulmonary vasculature in systemic sclerosis at spirometry-gated chest CT. Ann Rheum Dis, 79, 1210–17 (2020) [DOI] [PubMed] [Google Scholar]
- 37.Ariani A, Lumetti F, Silva M et al. Systemic sclerosis interstitial lung disease evaluation: comparison between semiquantitative and quantitative computed tomography assessments. J Biol Regul Homeost Agents, 28, 507–13 (2014) [PubMed] [Google Scholar]
- 38.Gargani L, Doveri M, D’Errico L et al. Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology (Oxford), 48, 1382–87 (2009) [DOI] [PubMed] [Google Scholar]
- 39. Bruni C, Mattolini L, Tofani L et al. Lung Ultrasound B-Lines in the Evaluation of the Extent of Interstitial Lung Disease in Systemic Sclerosis. Diagnostics (Basel), 12 (2022)* * This paper describes the application of lung ultrasound in systemic sclerosis associated interstitial lung disease and the common ultrasonographic findings.
- 40.Gutierrez M, Salaffi F, Carotti M et al. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders--preliminary results. Arthritis Res Ther, 13 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferro F, Delle Sedie A. The use of ultrasound for assessing interstitial lung involvement in connective tissue diseases. Clin Exp Rheumatol, 36 Suppl 114, 165–70 (2018) [PubMed] [Google Scholar]
- 42.Reyes-Long S, Gutierrez M, Clavijo-Cornejo D et al. Subclinical Interstitial Lung Disease in Patients with Systemic Sclerosis. A Pilot Study on the Role of Ultrasound. Reumatol Clin (Engl Ed), 17, 144–49 (2021) [DOI] [PubMed] [Google Scholar]
- 43.Delle Sedie A, Carli L, Cioffi E, Bombardieri S, Riente L. The promising role of lung ultrasound in systemic sclerosis. Clin Rheumatol, 31, 1537–41 (2012) [DOI] [PubMed] [Google Scholar]
- 44.Wells AU, Hansell DM, Rubens MB, Cailes JB, Black CM, du Bois RM. Functional impairment in lone cryptogenic fibrosing alveolitis and fibrosing alveolitis associated with systemic sclerosis: a comparison. Am J Respir Crit Care Med, 155, 1657–64 (1997) [DOI] [PubMed] [Google Scholar]
- 45.Salaffi F, Carotti M, Baldelli S et al. [Subclinical interstitial lung involvement in rheumatic diseases. Correlation of high resolution computerized tomography and functional and cytologic findings]. Radiol Med, 97, 33–41 (1999) [PubMed] [Google Scholar]
- 46.Gigante A, Rossi Fanelli F, Lucci S et al. Lung ultrasound in systemic sclerosis: correlation with high-resolution computed tomography, pulmonary function tests and clinical variables of disease. Intern Emerg Med, 11, 213–17 (2016) [DOI] [PubMed] [Google Scholar]
- 47.Gargani L, Romei C, Bruni C et al. Lung ultrasound B-lines in systemic sclerosis: cut-off values and methodological indications for interstitial lung disease screening. Rheumatology (Oxford), 61, SI56–64 (2022) [DOI] [PubMed] [Google Scholar]
- 48.Soldati G, Demi M, Inchingolo R, Smargiassi A, Demi L. On the Physical Basis of Pulmonary Sonographic Interstitial Syndrome. J Ultrasound Med, 35, 2075–86 (2016) [DOI] [PubMed] [Google Scholar]
- 49.Gargani L, Bruni C, De Marchi D et al. Lung magnetic resonance imaging in systemic sclerosis: a new promising approach to evaluate pulmonary involvement and progression. Clin Rheumatol, 40, 1903–12 (2021) [DOI] [PubMed] [Google Scholar]
- 50.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology, 246, 697–722 (2008) [DOI] [PubMed] [Google Scholar]
- 51.Landini N, Orlandi M, Occhipinti M et al. Ultrashort Echo-Time Magnetic Resonance Imaging Sequence in the Assessment of Systemic Sclerosis-Interstitial Lung Disease. J Thorac Imaging, (2022) [DOI] [PubMed]
- 52.Lonzetti L, Zanon M, Pacini GS et al. Magnetic resonance imaging of interstitial lung diseases: A state-of-the-art review. Respir Med, 155, 79–85 (2019) [DOI] [PubMed] [Google Scholar]
- 53.Ledoult E, Morelle M, Soussan M et al. (18)F-FDG positron emission tomography scanning in systemic sclerosis-associated interstitial lung disease: a pilot study. Arthritis Res Ther, 23, 76 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peelen DM, Zwezerijnen B, Nossent EJ et al. The quantitative assessment of interstitial lung disease with positron emission tomography scanning in systemic sclerosis patients. Rheumatology (Oxford), 59, 1407–15 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henderson J, Duffy L, Stratton R, Ford D, O’Reilly S. Metabolic reprogramming of glycolysis and glutamine metabolism are key events in myofibroblast transition in systemic sclerosis pathogenesis. J Cell Mol Med, 24, 14026–38 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bastos AL, Ferreira GA, Mamede M et al. PET/CT and inflammatory mediators in systemic sclerosis-associated interstitial lung disease. J Bras Pneumol, 48 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergmann C, Distler JH, Treutlein C et al. 68Ga-FAPI-04 PET-CT for molecular assessment of fibroblast activation and risk evaluation in systemic sclerosis-associated interstitial lung disease: a single-centre, pilot study. The Lancet Rheumatology, 3, e185–94 (2021) [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Chen Z, Long Y et al. 18F-FDG PET/CT and HRCT: a combined tool for risk stratification in idiopathic inflammatory myopathy-associated interstitial lung disease. Clin Rheumatol, 41, 3095–3105 (2022) [DOI] [PubMed] [Google Scholar]
- 59.Chen L, Zhu M, Lu H et al. Quantitative evaluation of disease severity in connective tissue disease-associated interstitial lung disease by dual-energy computed tomography. Respir Res, 23, 47 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porojan-Suppini N, Fira-Mladinescu O, Marc M, Tudorache E, Oancea C. Lung Function Assessment by Impulse Oscillometry in Adults. Ther Clin Risk Manag, 16, 1139–50 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mikamo M, Fujisawa T, Oyama Y et al. Clinical Significance of Forced Oscillation Technique for Evaluation of Small Airway Disease in Interstitial Lung Diseases. Lung, 194, 975–83 (2016) [DOI] [PubMed] [Google Scholar]
- 62.Wu AC, Kiley JP, Noel PJ et al. Current Status and Future Opportunities in Lung Precision Medicine Research with a Focus on Biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute Research Statement. Am J Respir Crit Care Med, 198, e116–36 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reveille JD, Solomon DH, American College of Rheumatology Ad Hoc Committee of Immunologic Testing G. Evidence-based guidelines for the use of immunologic tests: anticentromere, Scl-70, and nucleolar antibodies. Arthritis Rheum, 49, 399–412 (2003) [DOI] [PubMed] [Google Scholar]
- 64.Nihtyanova SI, Schreiber BE, Ong VH et al. Prediction of Pulmonary Complications and Long-Term Survival in Systemic Sclerosis. Arthritis & rheumatology, 66(6), 1625–1635 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Jandali B, Salazar GA, Hudson M et al. The Effect of Scl‐70 Antibody Determination Method on Its Predictive Significance for Interstitial Lung Disease Progression in Systemic Sclerosis. ACR Open Rheumatology, 4, 345–51 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walker UA, Tyndall A, Czirjak L et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Annals of the Rheumatic Diseases, 66, 754–63 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liaskos C, Marou E, Simopoulou T et al. Disease-related autoantibody profile in patients with systemic sclerosis. Autoimmunity, 50, 414–21 (2017) [DOI] [PubMed] [Google Scholar]
- 68.Mitri GM, Lucas M, Fertig N, Steen VD, Medsger TA, Jr. A comparison between anti-Th/To- and anticentromere antibody-positive systemic sclerosis patients with limited cutaneous involvement. Arthritis Rheum, 48, 203–9 (2003) [DOI] [PubMed] [Google Scholar]
- 69.Lazzaroni M-G, Marasco E, Campochiaro C et al. The clinical phenotype of systemic sclerosis patients with anti-PM/Scl antibodies: results from the EUSTAR cohort. Rheumatology, 60, 5028–41 (2021) [DOI] [PubMed] [Google Scholar]
- 70.Hudson M, Pope J, Mahler M et al. Clinical significance of antibodies to Ro52/TRIM21 in systemic sclerosis. Arthritis research & therapy, 14 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mierau R, Moinzadeh P, Riemekasten G et al. Frequency of disease-associated and other nuclear autoantibodies in patients of the German network for systemic scleroderma: correlation with characteristic clinical features. Arthritis research & therapy, 13 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wangkaew S, Euathrongchit J, Wattanawittawas P, Kasitanon N, Louthrenoo W. Incidence and predictors of interstitial lung disease (ILD) in Thai patients with early systemic sclerosis: Inception cohort study. Modern Rheumatology, 26, 588–93 (2016) [DOI] [PubMed] [Google Scholar]
- 73.Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig, 50, 3–13 (2012) [DOI] [PubMed] [Google Scholar]
- 74.Asano Y, Ihn H, Yamane K et al. Clinical significance of surfactant protein D as a serum marker for evaluating pulmonary fibrosis in patients with systemic sclerosis. Arthritis & Rheumatism, 44, 1363–69 (2001) [DOI] [PubMed] [Google Scholar]
- 75.Hant FN, Ludwicka-Bradley A, Wang H-J et al. Surfactant Protein D and KL-6 as Serum Biomarkers of Interstitial Lung Disease in Patients with Scleroderma. The Journal of Rheumatology, 36, 773–80 (2009) [DOI] [PubMed] [Google Scholar]
- 76.Hasegawa M, Fujimoto M, Hamaguchi Y et al. Use of Serum Clara Cell 16-kDa (CC16) Levels as a Potential Indicator of Active Pulmonary Fibrosis in Systemic Sclerosis. The Journal of Rheumatology, 38, 877–84 (2011) [DOI] [PubMed] [Google Scholar]
- 77.Elhai M, Hoffmann-Vold AM, Avouac J et al. Performance of Candidate Serum Biomarkers for Systemic Sclerosis-Associated Interstitial Lung Disease. Arthritis Rheumatol, 71, 972–82 (2019) [DOI] [PubMed] [Google Scholar]
- 78.Prasse A, Pechkovsky DV, Toews GB et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis & Rheumatism, 56, 1685–93 (2007) [DOI] [PubMed] [Google Scholar]
- 79.Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: relationship to organ systemic involvement. Clinical Rheumatology, 24, 111–16 (2005) [DOI] [PubMed] [Google Scholar]
- 80.Ihn H, Sato S, Fujimoto M, Takehara K, Tamaki K. Increased serum levels of soluble vascular cell adhesion molecule-1 and E-selectin in patients with systemic sclerosis. Br J Rheumatol, 37, 1188–92 (1998) [DOI] [PubMed] [Google Scholar]
- 81.Kumanovics G, Minier T, Radics J, Palinkas L, Berki T, Czirjak L. Comprehensive investigation of novel serum markers of pulmonary fibrosis associated with systemic sclerosis and dermato/polymyositis. Clin Exp Rheumatol, 26, 414–20 (2008) [PubMed] [Google Scholar]
- 82.Hasegawa M, Asano Y, Endo H et al. Serum Adhesion Molecule Levels as Prognostic Markers in Patients with Early Systemic Sclerosis: A Multicentre, Prospective, Observational Study. PLoS ONE, 9(2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasegawa M, Fujimoto M, Matsushita T, Hamaguchi Y, Takehara K, Sato S. Serum chemokine and cytokine levels as indicators of disease activity in patients with systemic sclerosis. Clinical Rheumatology, 30, 231–37 (2011) [DOI] [PubMed] [Google Scholar]
- 84.Hoffmann-Vold A-M, Weigt SS, Palchevskiy V et al. Augmented concentrations of CX3CL1 are associated with interstitial lung disease in systemic sclerosis. PLOS ONE, 13 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tiev KP, Chatenoud L, Kettaneh A, Toledano C, Bach JF, Cabane J. [Increase of CXCL10 serum level in systemic sclerosis interstitial pneumonia]. Rev Med Interne, 30, 942–46 (2009) [DOI] [PubMed] [Google Scholar]
- 86.Antonelli A, Ferri C, Fallahi P et al. CXCL10 (alpha) and CCL2 (beta) chemokines in systemic sclerosis--a longitudinal study. Rheumatology (Oxford), 47, 45–9 (2008) [DOI] [PubMed] [Google Scholar]
- 87.Cossu M, Andracco R, Santaniello A et al. Serum levels of vascular dysfunction markers reflect disease severity and stage in systemic sclerosis patients. Rheumatology (Oxford), 55, 1112–16 (2016) [DOI] [PubMed] [Google Scholar]
- 88.Van Bon L, Affandi AJ, Broen J et al. Proteome-wide Analysis and CXCL4 as a Biomarker in Systemic Sclerosis. New England Journal of Medicine, 370, 433–43 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kass DJ, Nouraie M, Glassberg MK et al. Comparative Profiling of Serum Protein Biomarkers in Rheumatoid Arthritis-Associated Interstitial Lung Disease and Idiopathic Pulmonary Fibrosis. Arthritis Rheumatol, 72, 409–19 (2020) [DOI] [PubMed] [Google Scholar]
- 90.Khadilkar PV, Khopkar US, Nadkar MY et al. Fibrotic Cytokine Interplay in Evaluation of Disease Activity in Treatment Naive Systemic Sclerosis Patients from Western India. J Assoc Physicians India, 67, 26–30 (2019) [PubMed] [Google Scholar]
- 91.Olewicz-Gawlik A, Danczak-Pazdrowska A, Kuznar-Kaminska B et al. Interleukin-17 and interleukin-23: importance in the pathogenesis of lung impairment in patients with systemic sclerosis. International Journal of Rheumatic Diseases, 17, 664–70 (2014) [DOI] [PubMed] [Google Scholar]
- 92.Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum IL-33 levels are raised in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. Clinical Rheumatology, 30, 825–30 (2011) [DOI] [PubMed] [Google Scholar]
- 93.Tang J, Lei L, Pan J, Zhao C, Wen J. Higher levels of serum interleukin-35 are associated with the severity of pulmonary fibrosis and Th2 responses in patients with systemic sclerosis. Rheumatology International, 38, 1511–19 (2018) [DOI] [PubMed] [Google Scholar]
- 94.Kodera M, Hasegawa M, Komura K, Yanaba K, Takehara K, Sato S. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis: A sensitive indicator of active pulmonary fibrosis. Arthritis & Rheumatism, 52, 2889–96 (2005) [DOI] [PubMed] [Google Scholar]
- 95.Moinzadeh P, Krieg T, Hellmich M et al. Elevated MMP-7 levels in patients with systemic sclerosis: correlation with pulmonary involvement. Experimental Dermatology, 20, 770–73 (2011) [DOI] [PubMed] [Google Scholar]
- 96.Matson SM, Lee SJ, Peterson RA et al. The prognostic role of matrix metalloproteinase-7 in scleroderma-associated interstitial lung disease. European Respiratory Journal, 58, (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manetti M, Guiducci S, Romano E et al. Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis: correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann Rheum Dis, 71, 1064–72 (2012) [DOI] [PubMed] [Google Scholar]
- 98.Kikuchi K, Kubo M, Sato S, Fujimoto M, Tamaki K. Serum tissue inhibitor of metalloproteinases in patients with systemic sclerosis. J Am Acad Dermatol, 33, 973–78 (1995) [DOI] [PubMed] [Google Scholar]
- 99.Sato S, Nagaoka T, Hasegawa M et al. Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol, 27, 149–54 (2000) [PubMed] [Google Scholar]
- 100.Lambrecht S, Smith V, De Wilde K et al. Growth differentiation factor 15, a marker of lung involvement in systemic sclerosis, is involved in fibrosis development but is not indispensable for fibrosis development. Arthritis Rheumatol, 66, 418–27 (2014) [DOI] [PubMed] [Google Scholar]
- 101.Gamal SM, Elgengehy FT, Kamal A et al. Growth Differentiation Factor-15 (GDF-15) Level and Relation to Clinical Manifestations in Egyptian Systemic Sclerosis patients: Preliminary Data. Immunol Invest, 46, 703–13 (2017) [DOI] [PubMed] [Google Scholar]
- 102.Yanaba K, Asano Y, Tada Y, Sugaya M, Kadono T, Sato S. Clinical significance of serum growth differentiation factor-15 levels in systemic sclerosis: association with disease severity. Mod Rheumatol, 22, 668–75 (2012) [DOI] [PubMed] [Google Scholar]
- 103.Nordenbæk C, Johansen JS, Halberg P et al. High serum levels of YKL‐40 in patients with systemic sclerosis are associated with pulmonary involvement. Scandinavian Journal of Rheumatology, 34, 293–97 (2005) [DOI] [PubMed] [Google Scholar]
- 104.Hasegawa M, Fujimoto M, Hamaguchi Y et al. Use of serum clara cell 16-kDa (CC16) levels as a potential indicator of active pulmonary fibrosis in systemic sclerosis. J Rheumatol, 38, 877–84 (2011) [DOI] [PubMed] [Google Scholar]
- 105.Hasegawa M, Fujimoto M, Matsushita T, Hamaguchi Y, Takehara K, Sato S. Serum chemokine and cytokine levels as indicators of disease activity in patients with systemic sclerosis. Clin Rheumatol, 30, 231–37 (2011) [DOI] [PubMed] [Google Scholar]
- 106.Prasse A, Pechkovsky DV, Toews GB et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum, 56, 1685–93 (2007) [DOI] [PubMed] [Google Scholar]
- 107.Hoffmann-Vold AM, Weigt SS, Palchevskiy V et al. Augmented concentrations of CX3CL1 are associated with interstitial lung disease in systemic sclerosis. PLoS One, 13, (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Bon L, Affandi AJ, Broen J et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. New England journal of medicine, 370, 433–443 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: relationship to organ systemic involvement. Clin Rheumatol, 24, 111–16 (2005) [DOI] [PubMed] [Google Scholar]
- 110.Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum IL-33 levels are raised in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. Clin Rheumatol, 30, 825–30 (2011) [DOI] [PubMed] [Google Scholar]
- 111.O’Reilly S Circulating Gremlin-1 is elevated in systemic sclerosis patients. Journal of Scleroderma and Related Disorders, 6, 286–89 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang M, Zhang L, E L et al. Increased levels of HE4 (WFDC2) in systemic sclerosis: a novel biomarker reflecting interstitial lung disease severity? Ther Adv Chronic Dis, 11, (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Truchetet ME, Brembilla NC, Montanari E, Allanore Y, Chizzolini C. Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res Ther, 13, R166 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olewicz-Gawlik A, Danczak-Pazdrowska A, Kuznar-Kaminska B et al. Interleukin-17 and interleukin-23: importance in the pathogenesis of lung impairment in patients with systemic sclerosis. Int J Rheum Dis, 17, 664–70 (2014) [DOI] [PubMed] [Google Scholar]
- 115.Tang J, Lei L, Pan J, Zhao C, Wen J. Higher levels of serum interleukin-35 are associated with the severity of pulmonary fibrosis and Th2 responses in patients with systemic sclerosis. Rheumatol Int, 38, 1511–19 (2018) [DOI] [PubMed] [Google Scholar]
- 116.Asano Y, Ihn H, Yamane K et al. Clinical significance of surfactant protein D as a serum marker for evaluating pulmonary fibrosis in patients with systemic sclerosis. Arthritis Rheum, 44, 1363–69 (2001) [DOI] [PubMed] [Google Scholar]
- 117.Hant FN, Ludwicka-Bradley A, Wang HJ et al. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol, 36, 773–80 (2009) [DOI] [PubMed] [Google Scholar]
- 118.Kodera M, Hasegawa M, Komura K, Yanaba K, Takehara K, Sato S. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis: a sensitive indicator of active pulmonary fibrosis. Arthritis Rheum, 52, 2889–96 (2005) [DOI] [PubMed] [Google Scholar]
- 119.Matson SM, Lee SJ, Peterson RA et al. The prognostic role of matrix metalloproteinase-7 in scleroderma-associated interstitial lung disease. Eur Respir J, 58 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Takahashi H, Kuroki Y, Tanaka H et al. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med, 162, 258–63 (2000) [DOI] [PubMed] [Google Scholar]
- 121.Nordenbaek C, Johansen JS, Halberg P et al. High serum levels of YKL-40 in patients with systemic sclerosis are associated with pulmonary involvement. Scand J Rheumatol, 34, 293–97 (2005) [DOI] [PubMed] [Google Scholar]
- 122.Doyle TJ, Patel AS, Hatabu H et al. Detection of Rheumatoid Arthritis–Interstitial Lung Disease Is Enhanced by Serum Biomarkers. American Journal of Respiratory and Critical Care Medicine, 191, 1403–12 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van den Hoogen F, Khanna D, Fransen J et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum, 65, 2737–47 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Minopoulou I, Theodorakopoulou M, Boutou A et al. Nailfold Capillaroscopy in Systemic Sclerosis Patients with and without Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. J Clin Med, 10 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maricq HR, LeRoy EC, D’Angelo WA et al. Diagnostic potential of in vivo capillary microscopy in scleroderma and related disorders. Arthritis Rheum, 23, 183–9 (1980) [DOI] [PubMed] [Google Scholar]
- 126.Cutolo M, Sulli A, Pizzorni C, Accardo S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol, 27, 155–60 (2000) [PubMed] [Google Scholar]
- 127.Umashankar E, Abdel-Shaheed C, Plit M, Girgis L. Assessing the role for nailfold videocapillaroscopy in interstitial lung disease classfication: a systematic review and meta-analysis. Rheumatology (Oxford), 61, 2221–34 (2022) [DOI] [PubMed] [Google Scholar]
- 128.Boots AW, Bos LD, van der Schee MP, van Schooten FJ, Sterk PJ. Exhaled Molecular Fingerprinting in Diagnosis and Monitoring: Validating Volatile Promises. Trends Mol Med, 21, 633–44 (2015) [DOI] [PubMed] [Google Scholar]
- 129.Boots AW, van Berkel JJ, Dallinga JW, Smolinska A, Wouters EF, van Schooten FJ. The versatile use of exhaled volatile organic compounds in human health and disease. J Breath Res, 6, (2012) [DOI] [PubMed] [Google Scholar]
- 130.Plantier L, Smolinska A, Fijten R et al. The use of exhaled air analysis in discriminating interstitial lung diseases: a pilot study. Respir Res, 23, 12 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Van Berkel JJ, Dallinga JW, Moller GM et al. Development of accurate classification method based on the analysis of volatile organic compounds from human exhaled air. J Chromatogr B Analyt Technol Biomed Life Sci, 861, 101–7 (2008) [DOI] [PubMed] [Google Scholar]
- 132.Krauss E, Haberer J, Maurer O et al. Exploring the Ability of Electronic Nose Technology to Recognize Interstitial Lung Diseases (ILD) by Non-Invasive Breath Screening of Exhaled Volatile Compounds (VOC): A Pilot Study from the European IPF Registry (eurIPFreg) and Biobank. J Clin Med, 8 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blanchet L, Smolinska A, Baranska A et al. Factors that influence the volatile organic compound content in human breath. J Breath Res, 11, (2017) [DOI] [PubMed] [Google Scholar]
- 134.Bruni C, Tofani L, Fretheim H et al. POS0388 Developing a screening tool for the detection of interstitial lung disease in systemic sclerosis: the ILD-RISC risk score. Annals of the Rheumatic Diseases, 81(Suppl 1), 449–50 (2022) [Google Scholar]
- 135.Schniering J, Maciukiewicz M, Gabrys HS et al. Computed tomography-based radiomics decodes prognostic and molecular differences in interstitial lung disease related to systemic sclerosis. Eur Respir J, 59 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]


