Highlights
-
•
Stereotactic ablative radiotherapy (SAbR) is an emerging non-invasive definitive treatment option for primary renal cell carcinoma (RCC), particularly when surgery is not ideal.
-
•
Primary RCC SAbR has been investigated in retrospective and prospective settings showing encouraging safety and efficacy.
-
•
Emerging evidence suggests possible immunomodulatory effects, leading to ongoing clinical investigations of cytoreductive primary RCC SAbR in combination with systemic therapy in patients with metastatic disease.
Keywords: Renal cell carcinoma, Stereotactic ablative radiotherapy, Stereotactic body radiotherapy, RCC, SABR, SBRT
Abstract
Stereotactic ablative radiotherapy (SAbR) is an emerging non-invasive definitive treatment option for primary renal cell carcinoma (RCC), particularly when surgery is not ideal. Employing ablative doses, SAbR delivered in one to five fractions to the primary tumor has been shown to achieve high local control rates with favorable toxicity profile in multiple retrospective and prospective series, and has dispelled previous notions of RCC radio-resistance. Moreover, emerging evidence suggests possible immunomodulatory effects, leading to clinical investigations of SAbR in combination with systemic and surgical management in patients with metastatic disease. In this review, we summarize key evidence supporting SAbR delivered to the primary tumor including preclinical rationale, dose escalation studies, recent prospective trials, and outcomes from ongoing multi-institutional registries. We also discuss areas of active clinical investigation including the use of primary SAbR in combination with systemic therapies in patients with metastatic disease. The accumulated body of evidence supports SAbR as promising indication being increasingly incorporated into the multi-disciplinary management of primary RCC.
Introduction
There is increasing incidence of renal cell carcinoma (RCC), with an estimated over 81,800 cases of kidney cancers diagnosed in 2023 in the United States.[1] RCC represents a heterogenous group of disease, ranging from indolent clinical course to widespread metastasis and rapid progression, with radiation being increasingly incorporated into its multi-disciplinary management. While RCC was historically considered a radioresistant tumor when treated with conventionally fractionated radiation, more recent data has demonstrated the effectiveness of ablative doses of radiotherapy in its management. The advent of stereotactic ablative radiotherapy (SAbR), also known as stereotactic body radiotherapy (SBRT), has been shown to achieve high local control rates with an attractive safety profile.[2]
The role for SAbR in the management of primary RCC includes definitive treatment, particularly if surgery is not an ideal option, and its use is now supported in national and international practice guidelines.[3], [4] Furthermore, there is emerging evidence to suggest that radiotherapy has immunomodulatory effects, leading to clinical investigations of SAbR in combination with surgery or systemic therapy for patients with metastatic disease. In these contexts, SAbR has emerged as a promising non-invasive therapeutic option for multiple stages of RCC (see Fig. 1).
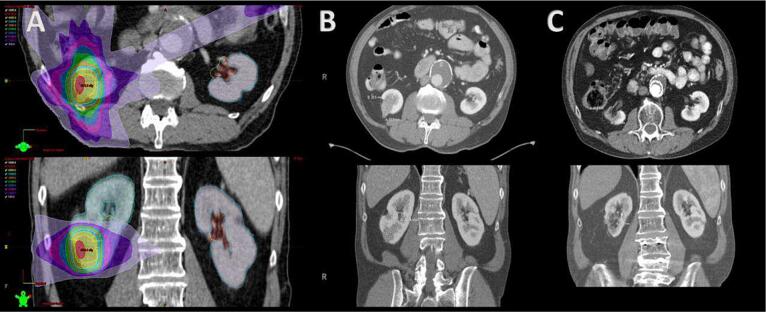
Fig. 1.
A. Representative patient treatment plan axial (top) and coronal (bottom) prescirbed to 36 Gy in three fractions. Dose color wash values are 50 Gy (white), 45 Gy (red), 40 Gy (yellow), 35 Gy (cyan), 30 Gy (orange), 25 Gy (green), 20 Gy (blue), 15 Gy (pink), 10 Gy (purple), and 5 Gy (lavender); B. Pre-treatment Computed Tomography (CT) obtained two weeks before stereotactic ablative body radiation (SAbR) describing axial (top) and coronal (bottom) views; C. Post-treatment CT obtained five years after SAbR describing axial (top) and coronal (bottom) views demonstrating size reduction.
In this review, we will summarize key contemporary evidence, and in doing so also describe emerging clinical strategies and areas of active investigation for incorporating SAbR in primary RCC management.
Pre-clinical rationale
RCC was previously considered to be a radioresistant tumor at conventional dose-fractionation. The basis of such perception is unclear, and may have originated from earlier studies using human cell lines demonstrating one renal cell line to be the least radiosensitive among those tested.[3] The myth of RCC radioresistance was further consolidated by a number of adjuvant RCC studies of conventional radiotherapy given post-operatively to the nephrectomy bed that failed to demonstrate any improvement, with some even showing worse outcome due to bowel toxicity.[4] However, these studies utilized conventional radiotherapy at low doses per fraction and older techniques, and subsequent studies have demonstrated that when delivered at higher doses per fraction, there was increased cell kill for RCC. In an early study, Ning et. al. demonstrated that in vitro cell survival curves had an initial “shoulder” at low-dose regions, followed by exponential decreases in survival at higher doses, suggesting dose rate-dependent effects.[5] Walsh et. al. at UT Southwestern subsequently investigated the effectiveness of ablative high-dose per fraction radiation for implanted RCC in vivo using a mouse model. Tumor-bearing nude mice were irradiated with 48 Gy total in 3 weekly 16 Gy fractions, and after an initial moderate size increase, treated tumors subsequently demonstrated progressive decreased to 30 % of pre-treatment volumes, compared to progressive tumor growth in control animals. Remarkably, all tumors from mice sacrificed at more than 4 weeks post-treatment demonstrated no mitotic activity.[6].
In addition, in the context of modern doublet immunotherapy (IO) and targeted therapies, there is evidence to suggest that radiotherapy may potentiate the systemic immune response. While the mechanistic underpinnings of SAbR’s immunomodulatory effects remain to be fully elucidated, in an analysis of RCC samples from patients treated with SAbR, Chow et. al. demonstrated intra-tumoral immune remodeling including enrichment of pathways indicative of T cell activity, and at 2 weeks post-radiation treatment, there was a transient expansion of tumor-resistant clones observed in the peripheral blood.[7] There have also been subsequent pre-clinical and early phase clinical studies investigating the sequencing of SAbR and immunotherapy, and as later discussed in this review, the combination of SAbR and IO in the context of primary RCC is the subject of ongoing multi-institutional cooperative group trials.[8], [9] Taken together, these findings provided mechanistic support for SAbR as an effective local treatment modality with possible additive or synergistic effects with emerging standard of care systemic therapies.
Primary RCC SABR
Multiple studies to date have demonstrated effective local control rates of SAbR utilized in the primary treatment setting. Initial prospective clinical studies have demonstrated the safety and efficacy of dose escalation. In one study from Sweden published as early as 2006, SAbR prescribed to 8 Gy x 4, 10 Gy x 4, 15 Gy x 2, or 15 Gy x 3 demonstrated local control rates of 98 %, although the study included primary and metastatic lesions, and follow up was limited with 19 % of lesions in patients with less than 6 months of follow up. In a prospective dose-escalation study from McBride and Kaplan et. al., doses from 7 Gy x 3 up to 16 Gy x 3 were used in patients with stage IA/B RCC. There were no dose-limiting toxicities, and with a minimum follow up of 2 years, there were only two local failures which were both observed in the low dose arms of 7 Gy x 3 and 9 Gy x 3.[10], [11] Ponski et. al. published a phase I dose-escalation study using 24, 32, 40, and 48 Gy in 4 fractions in 15 patients, with none of the evaluable patients developing recurrence or progression at a median follow up of 13.7 months. The authors concluded there were no dose-limiting toxicities, although one patient did develop grade 4 duodenal ulcer in which the contoured bowel had received a maximum of 54 Gy in 4 fractions.[12], [13] In a recent update, the authors further dose escalated from 48 Gy up to 60 Gy in 3 fractions. From the dose escalation cohort, five patients underwent post-treatment biopsy, with all biopsies reported as positive based on standard hematoxylin and eosin staining. However, there were no local failures in the dose escalation arms on follow up, and this is discordant with the over 90 % local control rates seen on serial imaging and reported in the literature, suggesting that biopsies should not be routinely performed post-treatment without evidence of radiographic progression.[14].
While phase III data is currently lacking, there have been additional prospective trials and retrospective series further supporting these findings. A feasibility clinical trial conducted by Siva et. al. enrolled 37 patients with inoperable primary RCC who were treated with SAbR using 26 Gy in one fraction or 14 Gy x 3 fractions. With a median follow up of 24 months, local control was 100 %, with one grade 3 (3 %) event and no grade 4 or 5 toxicity, and eGFR decline was modest at 11 mL/min.[15] Hannan et. al. reported one of the first phase II trial of SAbR delivered in 12 Gy x 3 or 8 Gy x 5 fractions, and found 94 % local control rates at 3 years. The investigators enrolled enlarging biopsy-confirmed RCC and used a rigorous local control definition that incorporated both radiographic and pathologic evidence of tumor control. There was no observed grade 2 or greater acute or late toxicities. Uniquely, the study included spatial proteomic and transcriptomic analysis on pre- and post-treatment tumor samples demonstrating molecular findings consistent with radiation-induced cellular senescence.[16] These analyses shed light on the seemingly contradictory findings of “positive” biopsies post-SAbR and low local recurrence rates, suggesting that the presence of tumor cells post-treatment may reflect a terminally differentiated, non-viable tumor state, further confirming the limited utility of routine biopsy after RCC SAbR.[17].
Meta-analyses performed by Correa et. al. of retrospective and prospective series, which included 383 tumors in 372 patients, also showed that modern kidney SAbR had effective local control rates and was associated with low rates of toxicity.[18] Despite a population of older patients (mean age of 70.4) with larger tumor sizes (mean 4.6 cm) who were most considered inoperable, SAbR achieved an estimated local control rate of 97.2 %, ranging from 70 % to 100 % in the studies included for analyses. While a variety of dose-fractionations were used in the included studies, 26 Gy in one fraction and 30 to 40 Gy in 3 to 5 fractions were most common. Treatment was generally reported as well tolerated across the studies examined, with an overall estimated rate of 1.5 % for grade 3–4 toxicity, ranging from 0 % to 25 % in the studies included for analyses. Among 287 patients, only eight grade 3 events and two grade 4 events were reported, and there was no treatment-related mortality.
The International Radiosurgery Consortium of the Kidney (IROCK) is another landmark international effort formed to harmonize RCC treatment approaches and further patient research, which led to an initial publication of IROCK consensus statement covering aspects of patient selection, treatment techniques, and dose constraints for varying fractionation schedules.[19] The consortium subsequently demonstrated important insights into the efficacy and safety of SAbR for primary RCC. Correa et. al. analyzed 81 patients from IROCK with solitary kidneys who underwent SAbR and found a 2-year local control rate of 98 %, with an associated mean eGFR decrease of 5.8 mL/min (-9%). There were no statistically significant differences between solitary and bilateral kidney tumor cohorts.[20] Siva et. al. also demonstrated the efficacy and tolerability of SAbR for larger (>4 cm) RCC tumors among a mostly medically inoperable cohort. After treatment with SAbR, 95 patients from a pooled IROCK cohort demonstrated a local failure rate at 4 years of 2.9 %. There were no reported grade 3 to 5 toxicities.[21].
In a recent 2022 publication, the 5-year outcomes after SAbR for primary RCC from IROCK are now available.[2], [22] Individual data from 190 patients were prospectively and retrospectively collected from 12 institutions across five countries. Patients were treated from between 2007 and 2018 with a median tumor diameter of 4.0 cm, and of the patients with available operability details, 75 % were deemed inoperable. No patients received adjuvant or concurrent systemic therapy. Among this cohort, the cumulative incidence of local failure at 5 years was 5.5 %, there were no grade 3 toxicities, and only one patient developed grade 4 toxicity (acute duodenal ulcer and late gastritis). While a number of dose-fractionation schedules were included in the IROCK cohort, intriguingly, single-fraction SAbR using 25 Gy was associated with less local failure than multi-fraction SAbR, although the authors caution that further evidence from randomized trials are needed to elucidate optimal dose-fractionation regimens.[2] Data from multi-institutional clinical trials are also currently pending, including the TROG 15.03 FASTRACK II study (NCT02613819) and multi-institutional prospective IROCK registry trial.[23], [24], [25].
SAbR is increasingly being utilized in the primary setting and is supported by current consensus practice guidelines. Guidelines from the National Comprehensive Cancer Network (NCCN Version 1.2024) state that “Stereotactic body radiation therapy (SBRT) may be considered for medically inoperable patients with stage I kidney cancer (category 2B) or with stage II/III kidney cancer (both category 3).”[26] The European Society for Medical Oncology (ESMO) guidelines also support the use of radiotherapy in unresectable local or recurrent disease, stating that “For patients in whom surgery cannot be carried out due to poor PS or unsuitable clinical condition, RT can be an alternative if other local therapies such as RFA are not appropriate.”[27].
SABR in combination with surgery
The use of fractionated radiotherapy had previously been investigated in the 1960s to 1990s in the neoadjuvant and adjuvant settings in relation to nephrectomy. In a historical clinical trial published by Juusela et. al. in 1977, pre-operative radiotherapy delivered to 33 Gy in 15 fractions did not improve patient survival.[28] In a meta-analysis by Tunio et. al. in 2010, post-operative radiotherapy decreased locoregional failure but did not impact disease free survival or overall survival, although the included studies were limited by small sample sizes and utilized older radiotherapy techniques no longer commonly used in modern practice.[4] In the setting of SAbR demonstrating effective local control and excellent safety profile, there is emerging interest in investigating the role of radiotherapy in combination with nephrectomy.
Singh et. al. published a single-arm feasibility study of primary site SAbR followed by nephrectomy in metastatic RCC.[29] Patients were treated with 15 Gy in a single fraction to the primary lesion, followed by cytoreductive nephrectomy 4 weeks later. Among 14 patients treated, treatment was well tolerated and did not increase surgical complications. Compared to untreated archival controls, the treated tumors demonstrated increased expression of tumor-associated antigens and markers of T-cell infiltration, providing evidence for immunomodulation following neoadjuvant SAbR. This concept is being further investigated in the ongoing prospective, multi-center phase II clinical trial NAPSTER (NCT05024318). Patient with aggressive primary clear cell RCC will be randomized to SAbR 42 Gy in 3 fractions followed by nephrectomy, versus SAbR and pembrolizumab followed by nephrectomy. The study is expected to enroll 26 patients and in case of encouraging results, the regimen of combined neoadjuvant SAbR and immunotherapy will likely be further tested in larger phase III randomized studies.[30], [31].
Another novel application of neoadjuvant SAbR is in the setting of locally advanced RCC with tumor thrombus. It is estimated that up to 10 % of patients with RCC may develop tumor thrombus that invades the inferior vena cava (IVC-TT). This can lead to complications such as Budd-Chiari syndrome or tumor emboli, with extirpative surgery being the only potential definitive treatment option.[32] In 2015, Hannan et. al. demonstrated feasibility in two cases of using SAbR for RCC IVC-TT, with both patient tolerating treatment well without acute or late treatment-related toxicity, and one patient had follow up imaging data that demonstrated radiographic response.[33] This was further investigated in a safety lead-in phase II clinical trial of neoadjuvant SAbR of 40 Gy in 5 fractions followed by radical nephrectomy and thrombectomy. Margulis et. al. reported initial results from six patients. All patients were alive after a median follow up of 24 months, and there were no grade 4 or 5 adverse events observed.[34] In a multi-center retrospective study reviewing experience from six institutions, Freifeld et. al. showed favorable safety profile of SAbR for IVC-TT among 15 patients with all patients experiencing palliative benefit and 58 % demonstrating radiographic response.[35].
Primary site SABR with systemic therapy
In the context of modern systemic therapy combinations with IO and targeted agents, the possible immunomodulatory effects of SAbR has led to increased interest in its use for cytoreduction of the primary tumor. While the CARMENA and SURTIME randomized trials have called into question the role of cytoreductive nephrectomy, “cytoreductive SAbR” delivered to the primary tumor is now being investigated in multi-institutional cooperative group trials.[36], [37] It has been postulated that SAbR combined with immunotherapy may enhance anti-tumor response through additive or synergistic systemic effects between these treatment modalities.
CYTOSHRINK (NCT04090710) is an ongoing phase II randomized clinical trial enrolling patients with previously untreated metastatic RCC with IMDC intermediate or poor risk disease. Patients are randomized 2:1 between ipilimumab and nivolumab with SAbR delivered to 30 to 40 Gy in 5 fractions, versus standard of care ipilimumab and nivolumab. The trial is enrolling in Canada and Australia with an accrual goal of 78 patients.[38], [39] The NRG GU-012 clinical trial SAMURAI (NCT05327686) is another phase II randomized trial enrolling patients with metastatic RCC with IMDC intermediate or poor risk disease. Patients are randomized 2:1 to SAbR 42 Gy in 3 fractions to the primary and standard immunotherapy, versus standard of care immunotherapy. A variety of combination immunotherapy and targeted therapies are allowed on the trial, and the study is enrolling through the NRG oncology cooperative group with a targeted accrual of 240 patients.[40], [41].
Conclusion
There is a growing body of evidence supporting the use SAbR for primary RCC management. In this setting, SAbR has been demonstrated to be safe and provide effective local control. Primary RCC SAbR in combination with surgical or systemic therapeutic options is a novel area of active investigation. While beyond the scope of this review, SAbR has also emerged as an attractive therapeutic option in the setting of metastatic disease, demonstrated in prospective clinical trials to achieve effective clinical outcomes for selected patients with oligometastases and oligoprogression, both instead of and in combination with systemic therapy.[42], [43], [44], [45].
Future investigations will continue to elucidate the optimal dose-fractionation regimen for primary RCC, particularly as it applies to personalized clinical factors and biomarkers. Clinical trials exploring SAbR in combination with IO and systemic therapies are also currently ongoing.[41], [46] Contrary to historical notions of radio-resistant disease, primary RCC SAbR is a promising indication being increasingly incorporated into the multi-disciplinary management of this disease.
Funding
Raquibul Hannan is funded by P50CA196516.
Author contribution
All authors contributed to the drafting the manuscript or revising it critically for intellectual content.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Siva S., Ali M., Correa R.J.M., et al. 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: an individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney) Lancet Oncol. 2022;23(12):1508–1516. doi: 10.1016/S1470-2045(22)00656-8. [DOI] [PubMed] [Google Scholar]
- 3.Deschavanne P.J., Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34(1):251–266. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 4.Tunio M.A., Hashmi A., Rafi M. Need for a new trial to evaluate postoperative radiotherapy in renal cell carcinoma: a meta-analysis of randomized controlled trials. Ann Oncol off J Eur Soc Med Oncol. 2010;21(9):1839–1845. doi: 10.1093/annonc/mdq028. [DOI] [PubMed] [Google Scholar]
- 5.Ning S., Trisler K., Wessels B.W., Knox S.J. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer. 1997;80(12 Suppl):2519–2528. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2519::aid-cncr26>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Walsh L., Stanfield J.L., Cho L.C., et al. Efficacy of ablative high-dose-per-fraction radiation for implanted human renal cell cancer in a nude mouse model. Eur Urol. 2006;50(4):795–800. doi: 10.1016/j.eururo.2006.03.021. discussion 800. [DOI] [PubMed] [Google Scholar]
- 7.Chow J., Hoffend N.C., Abrams S.I., Schwaab T., Singh A.K., Muhitch J.B. Radiation induces dynamic changes to the T cell repertoire in renal cell carcinoma patients. Proc Natl Acad Sci. 2020;117(38):23721–23729. doi: 10.1073/pnas.2001933117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannan R., Mohamad O., Diaz de Leon A., et al. Outcome and Immune Correlates of a Phase II Trial of High-Dose Interleukin-2 and Stereotactic Ablative Radiotherapy for Metastatic Renal Cell Carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27(24):6716–6725. doi: 10.1158/1078-0432.CCR-21-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z., Liu X., Chen D., Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. 2022;7(1):1–34. doi: 10.1038/s41392-022-01102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan ID, Redrosa I, Martin C, Collins C, Wagner A. Results of a Phase I Dose Escalation Study of Stereotactic Radiosurgery for Primary Renal Tumors. Int J Radiat Oncol. 2010;78(3, Supplement):S191. doi: 10.1016/j.ijrobp.2010.07.464.
- 11.McBride SM, Wagner AA, Kaplan ID. A Phase 1 Dose-Escalation Study of Robotic Radiosurgery in Inoperable Primary Renal Cell Carcinoma. Int J Radiat Oncol. 2013;87(2, Supplement):S84. doi: 10.1016/j.ijrobp.2013.06.218.
- 12.Svedman C., Sandström P., Pisa P., et al. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol Stockh Swed. 2006;45(7):870–875. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 13.Ponsky L., Lo S.S., Zhang Y., et al. Phase I dose-escalation study of stereotactic body radiotherapy (SBRT) for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2015;117(1):183–187. doi: 10.1016/j.radonc.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Grubb W.R., Ponsky L., Lo S.S., et al. Final results of a dose escalation protocol of stereotactic body radiotherapy for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2021;155:138–143. doi: 10.1016/j.radonc.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Siva S., Pham D., Kron T., et al. Stereotactic ablative body radiotherapy for inoperable primary kidney cancer: a prospective clinical trial. BJU Int. 2017;120(5):623–630. doi: 10.1111/bju.13811. [DOI] [PubMed] [Google Scholar]
- 16.Hannan R, McLaughlin MF, Pop LM, et al. Phase 2 Trial of Stereotactic Ablative Radiotherapy for Patients with Primary Renal Cancer. Eur Urol. Published online March 8, 2023:S0302-2838(23)02625-8. doi:10.1016/j.eururo.2023.02.016. [DOI] [PMC free article] [PubMed]
- 17.Correa R.J.M., Appu S., Siva S. Stereotactic Radiotherapy for Renal Cell Carcinoma: The Fallacy of (False) Positive Post-treatment Biopsy? Eur Urol. 2023;84(3):287–288. doi: 10.1016/j.eururo.2023.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Correa R.J.M., Louie A.V., Zaorsky N.G., et al. The Emerging Role of Stereotactic Ablative Radiotherapy for Primary Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Eur Urol Focus. 2019;5(6):958–969. doi: 10.1016/j.euf.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Siva S., Ellis R.J., Ponsky L., et al. Consensus statement from the International Radiosurgery Oncology Consortium for Kidney for primary renal cell carcinoma. Future Oncol Lond Engl. 2016;12(5):637–645. doi: 10.2217/fon.16.2. [DOI] [PubMed] [Google Scholar]
- 20.Correa R.J.M., Louie A.V., Staehler M., et al. Stereotactic Radiotherapy as a Treatment Option for Renal Tumors in the Solitary Kidney: A Multicenter Analysis from the IROCK. J Urol. 2019;201(6):1097–1104. doi: 10.1097/JU.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 21.S S, Rjm C, A W, et al. Stereotactic Ablative Radiotherapy for ≥T1b Primary Renal Cell Carcinoma: A Report From the International Radiosurgery Oncology Consortium for Kidney (IROCK). Int J Radiat Oncol Biol Phys. 2020;108(4). doi:10.1016/j.ijrobp.2020.06.014. [DOI] [PubMed]
- 22.Siva S., Louie A.V., Warner A., et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK) Cancer. 2018;124(5):934–942. doi: 10.1002/cncr.31156. [DOI] [PubMed] [Google Scholar]
- 23.Siva S., Chesson B., Bressel M., et al. TROG 15.03 phase II clinical trial of Focal Ablative STereotactic Radiosurgery for Cancers of the Kidney - FASTRACK II. BMC Cancer. 2018;18(1):1030. doi: 10.1186/s12885-018-4916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trans Tasman Radiation Oncology Group. Focal Ablative STereotactic Radiosurgery for Cancers of the Kidney, a Phase II Clinical Trial (FASTRACK II). clinicaltrials.gov; 2022. Accessed August 4, 2023. https://clinicaltrials.gov/study/NCT02613819.
- 25.ACSQHC-ARCR-373 | Australian Commission on Safety and Quality in Health Care. Accessed August 21, 2023. https://www.safetyandquality.gov.au/acsqhc-arcr-373.
- 26.NCCN Clinical Practice Guidelines in Oncology. Kidney Cancer (Version 1.2024). https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
- 27.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up††Approved by the ESMO Guidelines Committee: September 2008, last update January 2019. This publication supersedes the previously published version—Ann Oncol 2016; 27 (Suppl 5): v58–v68. Ann Oncol. 2019;30(5):706-720. doi:10.1093/annonc/mdz056. [DOI] [PubMed]
- 28.Juusela H., Malmio K., Alfthan O., Oravisto K.J. Preoperative irradiation in the treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1977;11(3):277–281. doi: 10.3109/00365597709179965. [DOI] [PubMed] [Google Scholar]
- 29.Singh A.K., Winslow T.B., Kermany M.H., et al. A Pilot Study of Stereotactic Body Radiation Therapy Combined with Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma. Clin Cancer Res off J Am Assoc Cancer Res. 2017;23(17):5055–5065. doi: 10.1158/1078-0432.CCR-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali M., Wood S., Pryor D., et al. NeoAdjuvant pembrolizumab and STEreotactic radiotherapy prior to nephrectomy for renal cell carcinoma (NAPSTER): A phase II randomised clinical trial. Contemp Clin Trials Commun. 2023;33 doi: 10.1016/j.conctc.2023.101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peter MacCallum Cancer Centre, Australia. NeoAdjuvant Pembrolizumab and STEreotactic Radiotherapy Prior to Nephrectomy for Renal Cell Carcinoma: Investigating Induced Immune Context Changes. clinicaltrials.gov; 2023. Accessed August 4, 2023. https://clinicaltrials.gov/study/NCT05024318.
- 32.Psutka S.P., Leibovich B.C. Management of inferior vena cava tumor thrombus in locally advanced renal cell carcinoma. Ther Adv Urol. 2015;7(4):216–229. doi: 10.1177/1756287215576443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hannan R., Margulis V., Chun S.G., et al. Stereotactic radiation therapy of renal cancer inferior vena cava tumor thrombus. Cancer Biol Ther. 2015;16(5):657–661. doi: 10.1080/15384047.2015.1026506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margulis V., Freifeld Y., Pop L.M., et al. Neoadjuvant SABR for Renal Cell Carcinoma Inferior Vena Cava Tumor Thrombus—Safety Lead-in Results of a Phase 2 Trial. Int J Radiat Oncol. 2021;110(4):1135–1142. doi: 10.1016/j.ijrobp.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freifeld Y., Pedrosa I., Mclaughlin M., et al. Stereotactic ablative radiation therapy for renal cell carcinoma with inferior vena cava tumor thrombus. Urol Oncol. 2022;40(4):166.e9–166.e13. doi: 10.1016/j.urolonc.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bex A., Mulders P., Jewett M., et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019;5(2):164–170. doi: 10.1001/jamaoncol.2018.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Méjean A., Ravaud A., Thezenas S., et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2018;379(5):417–427. doi: 10.1056/NEJMoa1803675. [DOI] [PubMed] [Google Scholar]
- 38.Ontario Clinical Oncology Group (OCOG). Cytoreductive Stereotactic Hypofractionated Radiotherapy With Combination Ipilimumab/Nivolumab for Metastatic Kidney Cancer. clinicaltrials.gov; 2023. Accessed August 4, 2023. https://clinicaltrials.gov/study/NCT04090710.
- 39.Lalani AKA, Swaminath A, Pond GR, et al. Phase II trial of cytoreductive stereotactic hypofractionated radiotherapy with combination ipilimumab/nivolumab for metastatic kidney cancer (CYTOSHRINK). J Clin Oncol. 2020;38(6_suppl):TPS761-TPS761. doi: 10.1200/JCO.2020.38.6_suppl.TPS761.
- 40.NRG Oncology. Randomized Phase II Stereotactic Ablative Radiation Therapy (SABR) for Metastatic Unresected Renal Cell Carcinoma (RCC) Receiving Immunotherapy (SAMURAI). clinicaltrials.gov; 2023. Accessed August 4, 2023. https://clinicaltrials.gov/study/NCT05327686.
- 41.Hall WA, Karrison T, McGregor BA, et al. NRG-GU012: Randomized phase II stereotactic ablative radiation therapy (SABR) for patients with metastatic unresected renal cell carcinoma (RCC) receiving immunotherapy (SAMURAI). J Clin Oncol. 2023;41(16_suppl):TPS4604-TPS4604. doi:10.1200/JCO.2023.41.16_suppl.TPS4604.
- 42.Tang C., Msaouel P., Hara K., et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-centre, feasibility, phase 2 trial. Lancet Oncol. 2021;22(12):1732–1739. doi: 10.1016/S1470-2045(21)00528-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaorsky N.G., Lehrer E.J., Kothari G., Louie A.V., Siva S. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): a meta-analysis of 28 studies. Eur Urol Oncol. 2019;2(5):515–523. doi: 10.1016/j.euo.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Hannan R., Christensen M., Christie A., et al. Stereotactic Ablative Radiation for Systemic Therapy-naïve Oligometastatic Kidney Cancer. Eur Urol Oncol. 2022;5(6):695–703. doi: 10.1016/j.euo.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hannan R., Christensen M., Hammers H., et al. Phase II Trial of Stereotactic Ablative Radiation for Oligoprogressive Metastatic Kidney Cancer. Eur Urol Oncol. 2022;5(2):216–224. doi: 10.1016/j.euo.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ECOG-ACRIN Cancer Research Group. Phase III Randomized Trial of Stereotactic Ablative Radiotherapy (SAbR) for Oligometastatic Advanced Renal Carcinoma (SOAR). clinicaltrials.gov; 2023. Accessed August 8, 2023. https://clinicaltrials.gov/study/NCT05863351.