Abstract
Background:
Obesity and adiposity are associated with an increased risk of heart failure with preserved ejection fraction (HFpEF), yet specific underlying mechanisms remain unclear.
We sought to examine the association of obesity-related biomarkers including adipokines (leptin, resistin, adiponectin), inflammatory markers (CRP, IL-6), and insulin resistance (HOMA-IR) with HFpEF status, exercise capacity and cardiovascular outcomes.
Methods:
We studied 509 consecutive patients with LVEF ≥ 50% and chronic dyspnea, who underwent clinically indicated cardiopulmonary exercise test (CPET) with invasive hemodynamic monitoring between 2006–2017. We defined HFpEF based on the presence of elevated left ventricular filling pressures at rest or during exercise. Fasting blood samples collected at the time of CPET were used to assay obesity-related biomarkers. We examined the association of log-transformed biomarkers with HFpEF status and exercise traits using multivariable-adjusted logistic regression models.
Results:
We observed associations of obesity-related biomarkers with measures of impaired exercise capacity including peak VO2 (p ≤0.002 for all biomarkers). The largest effect size was seen with leptin, where a 1-SD higher leptin was associated with a 2.35 ml/kg/min lower peak VO2 (β −2.35 ± 0.19, p<0.001). In addition, specific biomarkers were associated with distinct measures of exercise reserve including blood pressure (HOMA-IR, leptin, adiponectin, p ≤0.002 for all), and chronotropic response (CRP, IL-6, HOMA-IR, leptin and resistin, p<0.05 for all). Our findings suggest that among the obesity-related biomarkers studied, higher levels of leptin and CRP are independently associated with increased odds of HFpEF, with odds ratios of 1.36 (95% CI 1.09–1.70) and 1.25 (95% CI 1.03–1.52), respectively.
Conclusions:
Specific obesity-related pathways including inflammation, adipokine signaling, and insulin resistance may underlie the association of obesity with HFpEF and exercise intolerance.
Keywords: heart failure, HFpEF, obesity, inflammation, CPET
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) accounts for nearly half of all heart failure cases and its prevalence continues to rise.1 This complex and heterogeneous clinical disorder is characterized by global impairments in both cardiac and extracardiac reserve.2 Obesity and associated metabolic risk factors have been identified as key contributors to the development of HFpEF.3,4 Over 80% of patients with HFpEF are either overweight or obese, highlighting the crucial role that obesity plays in the pathogenesis of HFpEF.5
The symptoms of HFpEF and functional limitations in obese individuals may be attributed, in part, to deconditioning or to the mechanical stress associated with higher BMI.6 However, growing evidence suggests that adipose tissue may actively contribute to important metabolic and hormonal derangements through the production of adipokines and promote a pro-inflammatory state that may further contribute to the development of HFpEF.6–8 For example, long standing obesity stimulates leptin signaling,9 which increases circulating plasma volume and promotes systemic inflammation, leading to adverse ventricular remodeling and cardiac fibrosis.10 Similarly, obesity reduces levels of the cardioprotective adipokine, adiponectin, potentially increased cardiovascular stress among obese individuals with HFpEF.10
Although obesity is recognized as a central risk factor for HFpEF, recent studies have suggested the existence of an obesity-associated phenotype in HFpEF distinct from other clinical presentations.7 However, the exact mechanisms of obesity and cardiovascular dysfunction in HFpEF remain unclear. In order to gain further insights into this relationship, we assessed obesity-related biomarkers representing pathways of inflammation, adiposity, and metabolic dysfunction. Analyses were performed on individuals who underwent cardiopulmonary exercise testing (CPET) with invasive hemodynamic monitoring, allowing us to examine the associations between obesity-related pathways and impairments in cardiorespiratory fitness that characterize HFpEF (Figure 1).
Figure 1. Study design and approach to explore the associations of obesity-related biomarkers with exercise intolerance and HFpEF.
Diagram shows assessment of adiposity-related biomarkers representing adipokines, inflammation and insulin resistance to explore possible mechanistic pathways in which obesity may associate with HFpEF and exercise intolerance.
METHODS
Statistical code and analytic methods will be made available upon request. The data and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study sample
We studied patients with preserved ejection fraction (LVEF ≥ 50%) and with chronic dyspnea who underwent clinically indicated CPET with invasive hemodynamic monitoring between 2006–2017 at Massachusetts General Hospital. From this sample (n = 869), we excluded participants with missing biomarker measurements (n=48), submaximal exercise performance (defined as respiratory exchange ratio [RER] <1.0, n = 68), duplicate studies (n = 18), LVEF <50% or missing (n = 74), history of pulmonary arterial hypertension (n = 14); previous heart or lung transplant (n = 15), current evaluation for heart or lung transplantation (n = 9), complex adult congenital heart disease (n = 14), mitochondrial disease (n = 11), significant aortic or mitral valve disease (n = 65), oxygen-dependent lung disease (n = 24), yielding a final sample of n = 509. The study protocols received proper ethical approval by the appropriate institutional review boards and all participants provided informed consent.
Clinical assessment and biomarker collection
All participants underwent history and physical examination, including anthropometric assessment and overnight fasting phlebotomy at the time of CPET. Obesity was defined as a body-mass index (BMI) > 30 kg/m2. Blood samples were processed and stored at −80°C until assay. An immunoturbidimetric assay (Roche) was used to ascertain high sensitivity C-reactive protein (CRP; intra-assay coefficient of variation [CV], 0.4%−8.4%). For the following biomarkers Simple plex (ProteinSimple) assays were utilized: adiponectin (intra-assay CV, 6.5%−8.9%), interleukin 6 (IL-6; intra-assay CV, 2.4%- 3.9%), leptin (intra-assay CV, 3.3%−9.3%), and resistin (intra-assay CV, 6.4%−9.9%). In exploratory analyses, we examined exercise trait associations with N-terminal B-type natriuretic peptide (NT-proBNP) to compare effect sizes. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated by multiplying insulin (uIU/mL) × fasting glucose (mg/dL)/405].11 Echocardiographic data including the presence of left ventricular (LV) hypertrophy, left atrial enlargement and diastolic dysfunction obtained within 1 year of CPET was abstracted from clinical studies (available for n=403). We conducted review of medical records to ascertain cardiovascular event-free survival. This included ascertainment of all-cause mortality assessed by electronic hospital records and the social security death index, and incident cardiovascular disease (CVD) outcomes through 2018, including unplanned hospitalizations for acute coronary syndrome, coronary revascularization, and heart failure.
HFpEF and CPET definitions
All participants underwent right heart catheterization via the internal jugular vein, and insertion of a radial arterial line, followed by exercise testing using an upright cycle ergometer. The exercise protocol consisted of 3-minutes of unloaded exercise followed by a maximal effort-limited test using a continuous ramp protocol in increment of 5–20 watt/min.12 Gas-exchange (MedGraphics) and hemodynamic measurements were obtained at rest and during exercise including oxygen consumption (VO2). Hemodynamic assessment included serial measurements of pulmonary capillary wedge pressure (PCWP), blood pressure, cardiac output (calculated using the direct Fick formula) and peripheral oxygen utilization (measured by assessing the arteriovenous O2 content difference (ΔC[a-v]O2) at rest and exercise). We defined HFpEF using the following physiologic criteria: elevated LV filling pressures at rest (supine PCWP ≥15mm Hg) or during upright exercise with an abnormal steep increment in PCWP compared with cardiac output (ΔPCWP/ΔCO slope of >2.0 mm Hg/L/min, calculated using repeated measures of CO and PCWP during exercise) as previously described.13 We assessed exercise responses including cardiac output (ΔCO), heart rate (ΔHR), blood pressure (ΔBP) with secondary components [stroke volume (ΔSV) and systemic vascular resistance (ΔSVR)] as change from rest to peak exercise.
Statistical analysis
Baseline characteristics and CPET measures were reported using frequencies (%) or means and standard deviation (SD) as appropriate. Biomarker levels and HOMA-IR were natural log-transformed and summarized using medians and interquartile ranges due to right-skewed distributions. Baseline differences between participants with and without HFpEF were determined using χ2, t- tests, or the Wilcoxon rank sum test as appropriate. In primary analyses, we evaluated the association of obesity-related biomarkers (HOMA-IR, adipokines, and inflammatory biomarkers) with exercise parameters using multivariable linear regression models. Analyses were adjusted for clinical covariates including age, sex, hypertension, diabetes, smoking status, and previous myocardial infarction. Primary results were deemed statistically significant at a Bonferroni corrected p-value threshold of 0.05/9 exercise traits tested <0.006 and were considered suggestive at a p-value of <0.05. Analyses of HOMA-IR excluded individuals with prevalent diabetes. In secondary analyses, we separately examined the association of each cardiometabolic trait (diabetes, obesity, and BMI) and each obesity-related biomarkers (CRP, IL-6, HOMA-IR, adiponectin, leptin, resistin) with HFpEF status using multivariable logistic regression models adjusting for the aforementioned covariates used in primary analyses and further adjusting for BMI as an exploratory factor.
As further exploratory analyses, we used Cox proportional hazards models to evaluate the association of obesity-related biomarkers with clinical outcomes. Survival analyses were adjusted for the same covariates used in primary analysis, as well as HFpEF status. The primary clinical outcome was CV-event free survival. Upon inspection of Schoenfeld residuals and Kaplan-Meier curves across biomarker tertiles, we did not detect major violations of proportional hazards in the Cox regression analyses. Results from secondary and exploratory analyses were considered suggestive at a p-value of <0.005. Standardization was employed in all analyses meaning that the predictors were expressed per 1-SD deviation change, in order to facilitate the comparison of associations and enhance the interpretability of regression coefficients, given that the predictor variables had different scales and units. Statistical analyses were performed with STATA version 17 and SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
We studied 509 participants with LVEF ≥50% and chronic dyspnea, with a mean age of 55±16 years and 61% women. Of this sample, 228 (45%) participants met hemodynamic criteria for HFpEF. The baseline clinical characteristics of participants are shown in Table 1. Participants with HFpEF had a higher BMI (30.6 ± 6.5 vs. 27.5 ± 5.9 kg/m2) and were more likely to have obesity (48% vs. 29%), hypertension (75% vs. 50%) and diabetes (18% vs. 7%), when compared with participants without HFpEF (p for difference p<0.001 for all).
Table 1.
Clinical characteristics of individuals with and without HFpEF
| No HFpEF (n=281) | HFpEF* (n=228) | p-value | |
|---|---|---|---|
| Clinical Characteristic | |||
| Age, years | 50 ± 16 | 61 ± 13 | <0.001 |
| Female, sex | 178 (63) | 133 (58) | 0.25 |
| Current smoking | 10 (4) | 4 (2) | 0.22 |
| Prevalent MI | 9 (3) | 11 (5) | 0.35 |
| Beta-blocker use | 56 (20) | 100 (44) | <0.001 |
| Hypertension medication use | 112 (40) | 148 (65) | <0.001 |
| Chronic obstructive pulmonary disease | 15 (5) | 22 (10) | 0.062 |
| Obstructive sleep apnea | 45 (16) | 59 (26) | 0.006 |
| Connective tissue disease | 27 (10) | 20 (9) | 0.75 |
| Pulmonary Embolism | 24 (9) | 14 (7) | 0.31 |
| NT-pro BNP, pg/L | 45 (24 – 106) | 93 (46 – 263) | <0.001 |
| Cardiometabolic Traits | |||
| BMI, kg/m2 | 27.5 ± 5.9 | 30.6 ± 6.5 | <0.001 |
| Obesity | 82 (29) | 110 (48) | <0.001 |
| Diabetes mellitus | 21 (7) | 41 (18) | <0.001 |
| Hypertension | 139 (49) | 170 (75) | <0.001 |
| Adiposity-related biomarkers | |||
| CRP, mg/L | 1.35 (0.6 – 3.4) | 2.3 (0.9 – 5.1) | <0.001 |
| IL-6, pg/mL | 3.7 (2.4 – 6.4) | 5.0 (3.1 – 8.1) | <0.001 |
| HOMA-IR, mg·IU/dL·mL | 1.73 (0.96–3.13) | 2.06 (1.18 –3.86) | 0.02 |
| Leptin, pg/mL | 14313 (5834 – 27082) | 20027 (9603 – 37409) | <0.001 |
| Resistin, pg/mL | 10971 (8998 – 14571) | 11857 (8910 – 14922) | 0.19 |
| Adiponectin, ng/mL | 6280 (4213 – 8676) | 6274 (4209 – 9681) | 0.48 |
| Echocardiographic measures | |||
| LV ejection fraction, % | 65 ± 6 | 66 ± 7 | 0.14 |
| LV hypertrophy | 21 (10) | 50 (27) | <0.001 |
| Left atrial enlargement | 45 (21) | 67 (36) | 0.001 |
| Diastolic dysfunction | 57 (24) | 64 (37) | 0.006 |
Values are mean ± SD, n (%), or median (interquartile range).
p-value indicates comparison between HFpEF and non-HFpEF groups. Hypertension medication defined as use of any of the following: calcium channel blockers, beta-blockers, angiotensin-converting enzyme-inhibitors, angiotensin II receptor blockers.
Abbreviations: MI = myocardial infarction; BMI = body mass index; eGFR = estimated glomerular filtration rate; NT-pro BNP = N-terminal pro-b-type natriuretic peptide; CRP = high-sensitivity C-reactive protein; IL-6 = interleukin-6; HOMA-IR= homeostatic model assessment of insulin resistance; LV = left ventricular. Echocardiographic measures available in n=403, and diastolic function reported in n=247.
We examined 6 adiposity-related biomarkers, including 3 adipokines (adiponectin, leptin and resistin), 2 inflammatory biomarkers (CRP and IL-6) and a measure of insulin resistance (HOMA-IR) as shown in Figure 1. Participants with HFpEF had higher baseline levels of CRP, IL-6, HOMA-IR, and leptin (Table 1, Figure 2), as well as lower peak oxygen uptake (VO2, 15.4 ± 4.2 vs. 19.4 ± 5.8 ml/kg/min) at similar respiratory exchange ratio (RER, 1.18 ± 0.11 vs. 1.19 ± 0.10) when compared to individuals without HFpEF, with additional exercise parameters summarized in Table S1.
Figure 2. Differences in obesity-related biomarkers in participants with HFpEF vs. without HFpEF.
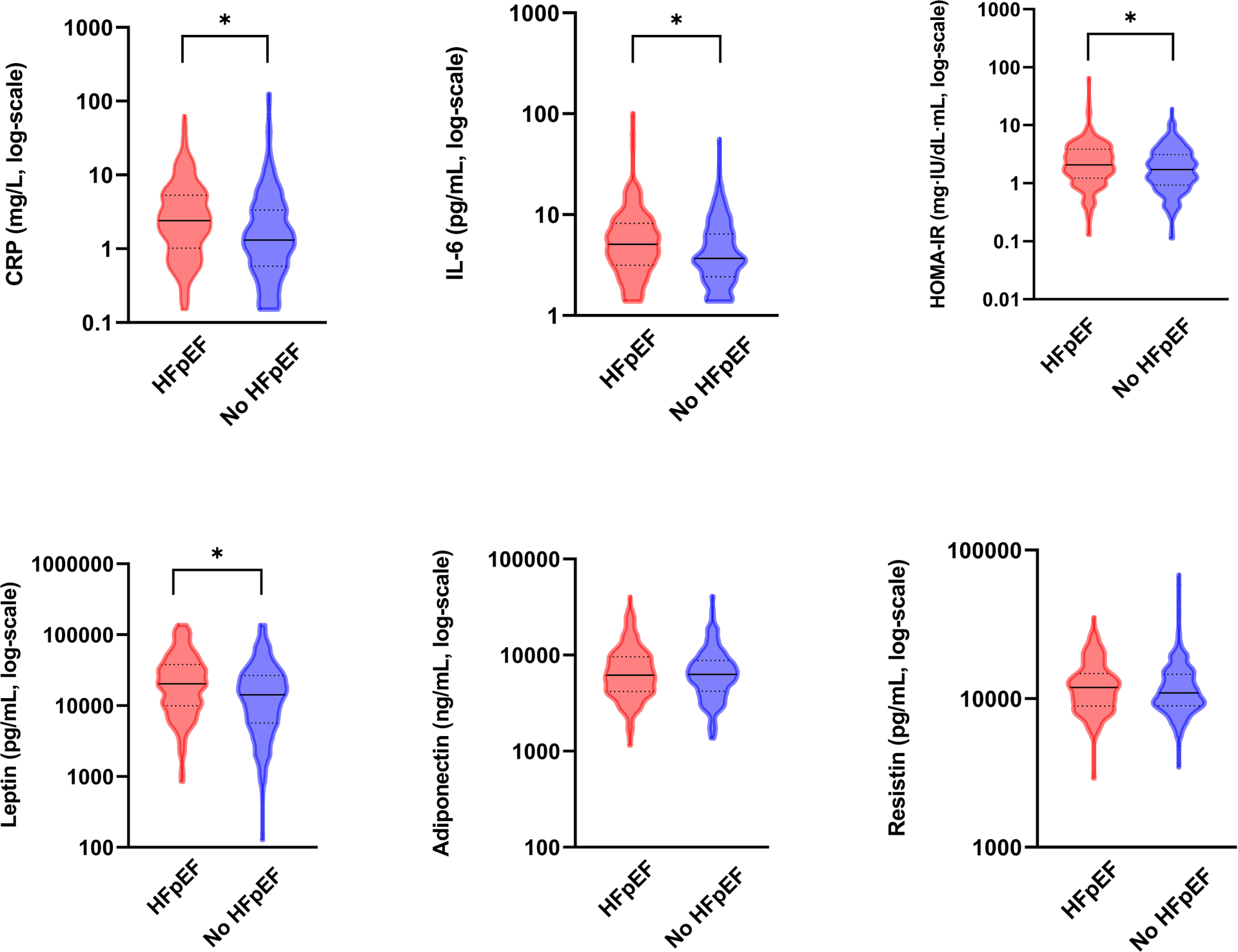
Violin plots displays between group differences in baseline values for biomarkers in participants with (red) and without HFpEF (blue). * Indicates p ≤ 0.0.5 via Mann-Whitney test. HOMA-IR levels calculated only in patients without diabetes. Lower detection limit for CRP is <0.15 mg/L and for IL-6 is <1.38 pg/mL.
Adiposity-related biomarkers and exercise reserve
We found that all obesity-related biomarkers were associated with overall exercise capacity as measured by peak VO2 (p ≤0.002 for all), with higher biomarker levels associated with worse fitness except for adiponectin, where higher levels were associated with better peak VO2 (Table 2). The largest effect size was seen with leptin, where a 1-SD higher leptin was associated with a 2.35 ml/kg/min lower peak VO2 (β −2.35 ± 0.19, p<0.001). This was higher than effect sizes observed with NT-proBNP (β −0.66 ± 0.22, p=0.004, refer to Table S2 for details). In exploratory analysis after adjusting the multivariable model for BMI, associations of biomarkers with peak VO2 persisted except for adiponectin and IL-6 (Table S3). We also examined the association of obesity-related biomarkers with peak VO2 stratified by HFpEF status (Table S4). This indicated consistent results among those with and without HFpEF, though multiplicative interaction terms suggested more pronounced effect of HOMA-IR and leptin among those without vs with HFpEF (p for interaction ≤0.002 for both).
Table 2.
Association of obesity-related biomarkers with exercise parameters
| Exercise Parameter | CRP | IL-6 | HOMA-IR | |||
|---|---|---|---|---|---|---|
| β (SE) | p value | β (SE) | p value | β (SE) | p value | |
| Peak VO2 | −1.32 (0.19) | <0.001 | −0.60 (0.20) | 0.002 | −1.85 (0.23) | <0.001 |
| ΔCO | 0.09 (0.11) | 0.40 | −0.07 (0.11) | 0.49 | 0.20 (0.13) | 0.12 |
| ΔPCWP/ΔCO | 0.06 (0.08) | 0.40 | 0.002 (0.08) | 0.98 | −0.12 (0.09) | 0.16 |
| exPCWP | 1.10 (0.33) | 0.001 | 0.039 (0.34) | 0.91 | 1.00 (0.41) | 0.014 |
| ΔHR | −3.48 (0.85) | <0.001 | −3.31 (0.86) | <0.001 | −2.63 (1.06) | 0.01 |
| ΔBP | 0.56 (1.07) | 0.60 | −1.72 (1.07) | 0.11 | 5.39 (1.27) | <0.001 |
| ΔSV | 2.19 (0.93) | 0.02 | 2.16 (0.93) | 0.02 | 4.41 (1.10) | <0.001 |
| ΔSVR | 43.59 (19.77) | 0.03 | −1.47 (20.68) | 0.94 | 29.49 (23.9) | 0.22 |
| ΔC(a-v) O2 | −0.23 (0.08) | 0.002 | −0.13 (0.08) | 0.08 | −0.20 (0.09) | 0.03 |
| Exercise Parameter | Adiponectin | Leptin | Resistin | |||
| β (SE) | p value | β (SE) | p value | β (SE) | p value | |
| Peak VO2 | 0.74 (0.21) | <0.001 | −2.35 (0.19) | <0.001 | −0.86 (0.19) | <0.001 |
| ΔCO | −0.11 (0.11) | 0.32 | 0.34 (0.11) | 0.004 | −0.06 (0.11) | 0.56 |
| ΔPCWP/ΔCO | 0.01 (0.08) | 0.94 | 0.04 (0.08) | 0.60 | −0.01 (0.08) | 0.87 |
| exPCWP | −0.37 (0.36) | 0.31 | 1.30 (0.36) | <0.001 | −0.01 (0.34) | 0.99 |
| ΔHR | 1.25 (0.92) | 0.17 | −1.93 (0.94) | 0.04 | −2.73 (0.85) | 0.001 |
| ΔBP | −3.84 (1.13) | <0.001 | 3.54 (1.16) | 0.002 | −1.41 (1.06) | 0.19 |
| ΔSV | −2.30 (0.99) | 0.02 | 4.30 (1.00) | <0.001 | 1.75 (0.92) | 0.06 |
| ΔSVR | 19.34 (21.04) | 0.36 | 19.72 (22.49) | 0.38 | 12.89 (19.88) | 0.52 |
| ΔC(a-v) O2 | 0.11 (0.08) | 0.17 | −0.21 (0.08) | 0.01 | −0.20 (0.08) | 0.01 |
ß-Coefficient represents SD unit difference in exercise parameter per 1-SD unit change in log-transformed biomarker in multivariable regression model adjusted for age, sex, hypertension, diabetes, current smoking, and previous myocardial infarction. Abbreviations: Δ = delta; VO2= oxygen consumption; CO = Cardiac Output; PCWP = Pulmonary Capillary Wedge Pressure; exPCWP = PCWP at peak exercise; HR = Heart Rate; BP = blood pressure; SV = stroke volume, SVR = systemic vascular resistance; C[a-v]O2= peripheral oxygen extraction; CRP = high-sensitivity C-reactive protein; IL-6 = interleukin-6; HOMA-IR= homeostatic model assessment of insulin resistance.
We next examined the associations of obesity-related biomarkers with cardiac and extracardiac responses to exercise using multivariable-adjusted models. We found that higher leptin was associated with greater systolic reserve as measured by change in CO (ΔCO: 0.034 ± 0.11, p=0.004). In regard to measures of diastolic reserve, we did not find an association between obesity-related biomarkers and ΔPCWP/ΔCO slope (p>0.05 for all), but we did find a significant association of CRP and leptin with exercise PCWP (1.10± 0.33, p = 0.001 and 1.30±0.36, p <0.001; respectively). Many obesity-related biomarkers were associated with chronotropic response, including CRP, IL-6, and resistin (ΔHR: p≤0.001 for all), with suggestive associations for HOMA-IR, leptin (ΔHR: p<0.05). With respect to blood pressure response, we found that worse HOMA-IR, lower adiponectin, and higher leptin concentrations were associated with greater blood pressure response (ΔBP: p≤0.002 for all). When examining determinants of BP response including SV and SVR response, we found that all obesity-associated biomarkers but resistin displayed suggestive associations with ΔSV but not ΔSVR (except CRP). Furthermore, higher CRP was found to be significantly associated with worse peripheral oxygen utilization (ΔC[a-v]O2: p=0.002), with suggestive associations for HOMA-IR, leptin, and resistin (ΔC[a-v]O2: p<0.05).
Association of adiposity-related biomarkers with HFpEF and clinical trajectory
In secondary analyses, we confirmed that obesity (OR 1.96, 95% CI 1.30–2.96) and diabetes (OR 1.89, 95% CI 1.04–3.41) were significantly associated with increased odds of HFpEF (Table 3). Additionally, higher levels of leptin, and inflammation (CRP) were also associated with greater odds of HFpEF (p <= 0.03 for both, Figure 3). Specifically, a 1-SD higher leptin level was associated with 36% greater odds of HFpEF (OR 1.36, 95% CI 1.09–1.70), and a 1-SD higher CRP level was associated with 25% higher odds of HFpEF (OR 1.25, 95% CI 1.03–1.52). However, IL-6, HOMA-IR, adiponectin, and resistin were not associated with HFpEF status (p>0.05 for all).
Table 3.
Association of cardiometabolic traits and obesity-related biomarkers with HFpEF
| MV-adjusted model | ||
|---|---|---|
| OR (95% CI) | p-value | |
| Cardiometabolic Traits | ||
| Diabetes | 1.89 (1.04–3.41) | 0.04 |
| Obesity | 1.96 (1.30−2.96) | 0.001 |
| BMI | 1.06 (1.03−1.10) | <0.001 |
| Obesity-Related Biomarkers | ||
| CRP | 1.25 (1.03−1.52) | 0.03 |
| IL-6 | 1.15 (0.95−1.40) | 0.16 |
| HOMA-IR | 1.26 (0.98−1.62) | 0.07 |
| Adiponectin | 0.97 (0.79−1.19) | 0.77 |
| Leptin | 1.36 (1.09−1.70) | 0.007 |
| Resistin | 0.92 (0.75−1.11) | 0.37 |
| NT-pro BNP | 1.54 (1.21−1.94) | <0.001 |
Odds ratio represents point estimate and 95% confidence interval of HFpEF per 1 unit change in BMI and per 1-SD unit change in log-transformed biomarkers in multivariable regression models adjusted for age, sex, hypertension, diabetes, current smoking, and previous myocardial infarction. Analyses for HOMA-IR excludes individuals with diabetes. Abbreviations: MV = multivariable; OR = odds ratio; CI = confidence interval; CRP = high-sensitivity C-reactive protein; IL-6 = interleukin-6; HOMA-IR= homeostatic model assessment of insulin resistance; NT-pro BNP = N-terminal pro-B-type natriuretic peptide.
Figure 3. Association of obesity related biomarkers with HFpEF.
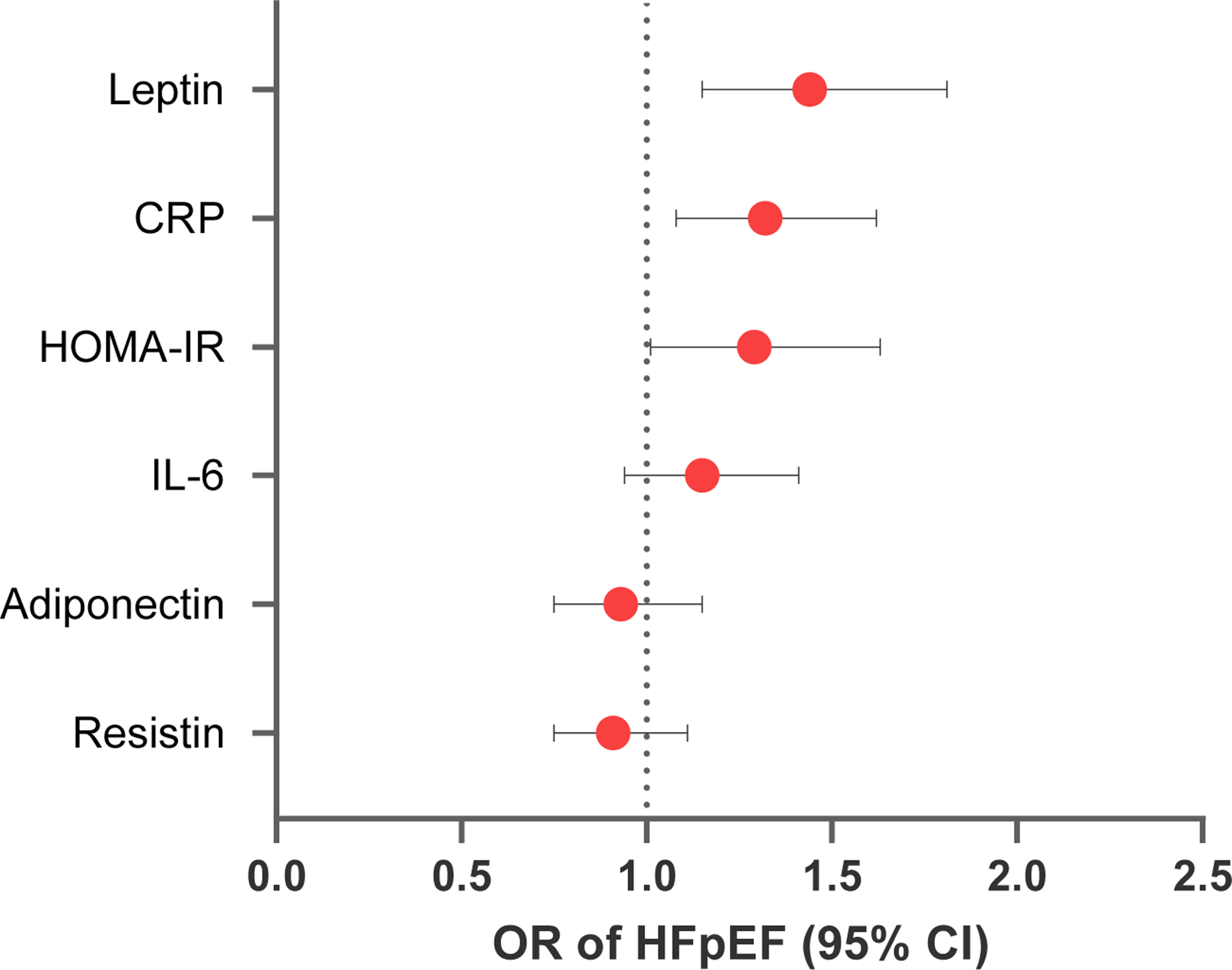
Forest plot shows odds ratio per 1-SD unit change in log-transformed biomarker with HFpEF using a multivariable model adjusted for age, sex, hypertension, diabetes, smoking, and previous MI. Abbreviations: OR = Odds ratio (point estimate and 95% CI) of HFpEF.
In exploratory analyses, we examined the association of adiposity-related biomarkers with cardiovascular event-free survival (Table S5 and Figure S1). During a median follow-up of 2.7 years (IQR 1.0–4.9), 96 subjects developed the primary endpoint. In multivariable Cox models, we found that CRP was associated with a 41% higher hazard of adverse events (HR 1.41, 95% CI 1.15–1.73) and IL-6 was associated with a 29% higher hazard (HR 1.29, 95% CI 1.06–1.57). We did not observe significant associations of HOMA-IR, adiponectin, leptin, or resistin with CV event-free survival.
DISCUSSION
In this study, we sought to better understand the association of obesity-related pathways with exercise intolerance and HFpEF by measuring circulating biomarkers related to adipokines, inflammation, and insulin resistance (Figure 1). We leveraged a unique sample of patients with dyspnea who had undergone CPET with invasive hemodynamic monitoring, allowing us to uniformly define HFpEF based on physiologic criteria, and to evaluate organ-specific contributions to exercise intolerance. Our main findings are as follows: first, we observed that all obesity-related biomarkers were associated with worse exercise capacity as measured by peak VO2. Further, we identified specific associations of CRP, IL-6, and resistin with chronotropic response, insulin resistance, adiponectin, and leptin with blood pressure response, and CRP with peripheral oxygen extraction. Finally, we observed that adipokine signaling pathways (leptin) and inflammation (CRP) pathways were associated with hemodynamic evidence of HFpEF. Taken together, our findings suggest that obesity-related pathways, including inflammation, adipokine signaling, and insulin resistance may play a role in exercise intolerance as a hallmark finding in HFpEF pathophysiology.
Obesity and abdominal adiposity are known risk factors for development of HFpEF,14 15 but the mechanisms underlying this association are not fully understood. Some of the effect may be due to the greater mechanical burden related of obesity, including greater metabolic oxygen demand, restricted chest wall expansion, and higher circulating blood volume.6 Perhaps more importantly, adipose tissue can mediate key metabolic and hormonal functions by secreting biologically active molecules such as inflammatory markers and adipokines that, when dysregulated, may promote HFpEF development.16 In this context, we studied the associations of adipokines, markers of systemic inflammation, and metabolic dysfunction with exercise intolerance as the hallmark symptom of HFpEF.17
We show that obesity-associated biomarkers across pathways of inflammation, insulin resistance, and adipokine signaling are associated with overall exercise capacity among individuals with dyspnea. Our findings build upon prior studies in largely asymptomatic samples that have demonstrated that inflammatory biomarkers (IL-6 and CRP) and insulin resistance are associated with worse cardiorespiratory fitness,18,19 and extend these observations to a hospital-based sample of individuals with and without HFpEF. In addition to inflammatory biomarkers, we also show that adipokines including higher leptin, higher resistin, and lower adiponectin concentrations are associated with worse exercise capacity. While the positive effects of exercise training interventions on adipokine profiles have been well documented,20 few studies have examined the association of adipokines with exercise capacity.21 Although our study helps to fill this knowledge gap and adds to our understanding of the relationship between obesity, adipokines, and exercise intolerance in HFpEF, we did not find an association between obesity-associated biomarkers and PCWP/CO. a defining feature of diastolic reserve. Nevertheless, higher levels of CRP and leptin did associate with a greater exercise PCWP and higher odds of HFpEF. While diastolic reserve is considered a hallmark of HFpEF, the pathophysiology of this syndrome is complex and likely involves multiple mechanisms beyond impaired diastolic function alone.22 Additionally, the definition of HFpEF is still evolving and there is ongoing debate regarding the diagnostic criteria and the importance of individual parameters in its characterization.23 Further studies are needed to elucidate the relationship between diastolic reserve and biomarkers in HFpEF.
Beyond overall cardiorespiratory fitness, we examined specific obesity-related biomarkers and their associations with both cardiac and extracardiac contributors to exercise intolerance. For example, inflammatory markers CRP and IL-6, as well as resistin were associated with worse chronotropic response to exercise, a common finding in HFpEF,24 though its association with obesity-related biomarkers has not been previously reported. Further, we observed that worse insulin resistance, higher leptin levels, and lower adiponectin concentrations were associated with higher blood pressure responses during exercise. These results are consistent with previous evidence linking insulin resistance to arterial stiffening and higher BP during exercise, which can lead to impaired ventricular-vascular coupling, exercise intolerance and ultimately HFpEF.25,26 While adiponectin is known to have cardioprotective effects,27 its association with BP response during exercise has not previously been described. Although an increase in BP during exercise may occur in the setting of increased CO, our data did only show a mild increase in response to changes in CO for leptin, suggesting that the associations between these biomarkers and a greater BP response during exercise may be due to intrinsic large artery stiffness rather than being dependent on flow. We also observed that CRP was significantly associated with worse peripheral oxygen extraction. In previous studies, central adiposity measures were found to associate with worse peripheral oxygen utilization in HFpEF,28 and our findings suggest that inflammation may play a role.
Furthermore, we observed that adipokine signaling pathways (leptin) and inflammatory (CRP) pathways were associated with hemodynamic evidence of HFpEF. Our findings are consistent with previous research demonstrating inflammation and adipokine signaling as contributors to the development of HFpEF. For example, one study found that baseline CRP and IL-6 levels were associated with incident HFpEF in a multi-ethnic study cohort,29 although both biomarkers were more strongly associated with HF with reduced ejection fraction (HFrEF).30
Another potential mechanism through which obesity may contribute to the development of HFpEF is through the adipokine leptin, a hormone produced by fat cells that plays a role in regulating energy balance and appetite.31 Previous studies have suggested that leptin levels increase as the heart fails, and may decline after ventricular unloading.32,33 The mechanisms by which leptin leads to HFpEF may be multifactorial, as it can stimulate aldosterone production directly and indirectly through the renin-angiotensin-aldosterone (RAAS) pathway,34–36 and may also exacerbate chronic inflammation.31 We now show that higher leptin levels are associated with worse exercise tolerance and hemodynamic evidence of HFpEF.
In exploratory analyses we found that higher CRP and IL-6 were associated with worse cardiovascular event-free survival. These results are consistent with findings from a large heterogenous cohort of HF patients, showing that higher IL-6 levels are not only associated with HFpEF vs. HFrEF, but also that higher IL-6 independently predicted incident HF hospitalizations, CV, and all-cause mortality.37 Similarly, a recent meta-analysis also showed that higher CRP levels predict the risk of adverse cardiovascular outcomes and all-cause mortality in HFpEF patients.38 While inflammation is believed to contribute to the development of incident HF,30,39 the role of anti-inflammatory therapies in HFpEF remains unclear. For example, biologic agents targeting TNF-alpha in HFrEF were in fact associated with adverse outcomes in early studies.40,41 By contrast, post-hoc analyses of the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) suggested that treatment with the IL-1 antibody canakinumab, may have a dose-dependent reduction in incident heart failure hospitalization and mortality rates in patients with prior MI and high CRP,42 though no distinction in HF subtypes was made. Further studies are needed to determine potential therapeutic implications of agents targeting inflammation in HFpEF.
There are several limitations to consider in our study. First, the sample of this study consisted of participants who were referred for clinically indicated CPET, limiting the generalizability of our findings due to potential referral bias. Second, the inflammatory biomarkers studied are not specific to obesity, and other comorbid conditions could have confounded our results, although we did adjust for most common comorbidities in multivariable analyses. Third, our study had limited power to examine the association of the explored biomarkers in a purely obese HFpEF phenotype, although we were able to examine associations across a range of BMIs. Fourth, suggestive findings that were not adjusted for multiple comparisons, and should be considered as preliminary and hypothesis-generating rather than conclusive. Fifth, while exercise traits such as lower peak VO2 and chronotropic incompetence are important features in individuals with HFpEF, we found overlap among those without HFpEF, showing that these metrics alone are not able to determine HFpEF status. Sixth, associations with clinical outcomes were limited due to sample size and associations were observational in nature, and causal inferences cannot be drawn. Finally, echocardiography was not systematically performed but rather abstracted from clinical studies at or around the time of right hearth catheterization. As such, limited information is available with respect to cardiac structure and function. In sum, in a rigorously phenotyped sample referred to CPET due to dyspnea, we found that obesity-related biomarkers were associated with exercise intolerance as a cardinal manifestation of HFpEF. These findings suggest that inflammation, adipocyte signaling, and insulin resistance may contribute to the association of obesity with HFpEF. Taken together, these findings highlight biological pathways that may explain the greater susceptibility to HFpEF among obese individuals. Further studies are needed to determine the potential therapeutic implications of these findings, considering the rising prevalence of obesity and the obese-HFpEF phenotype.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Obesity-related biomarkers representing inflammation, adipokine signaling, and insulin resistance are associated with exercise intolerance and HFpEF.
These findings highlight potential pathways that may contribute to the development of HFpEF in obesity.
What are the clinical implications?
This observational study provides insights into the complex relationship between obesity, inflammation, adipokine signaling, and insulin resistance pathways and how they may contribute to myocardial remodeling in obesity-related HFpEF.
These results may help improve and tailor future screening, diagnostic, and therapeutic approaches for obesity-related HFpEF.
Source of Funding:
Dr. Ho is supported by grants from the National Institutes of Health R01-HL134893, R01-HL140224, R01-HL160003, and K24-HL153669. Dr. Lau is supported by grants from the National Institutes of Health K23-HL159243 and the American Heart Association 853922. Dr. Malhotra is supported by grants from the National Institutes of Health R01HL142809 and R01HL159514, the American Heart Association 22TPA969625, and the Wild Family Foundation.
Disclosures:
Dr. Ho has received past research funding from Bayer, AG. Dr. Lau reports modest honoraria from Roche Diagnostics. Dr. Nayor has received modest honoraria from Cytokinetics. Dr. Malhotra receives research funding from Angea Biotherapeutics and Amgen and serves as a consultant for Myokardia/BMS, Renovacor, Epizon Pharma, and Third Pole. All other authors have declared that they have no relationships to disclose.
ABBREVIATIONS
- BMI
body mass index
- BP
blood pressure
- CO
cardiac output
- CPET
cardiopulmonary exercise testing
- CRP
high sensitivity C-reactive protein
- CV
cardiovascular
- CVD
cardiovascular disease
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HOMA_IR
homeostatic model assessment of insulin resistance
- HR
heart rate
- IL-6
interleukin-6
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- NT-pro BNP
N-terminal pro-b-type natriuretic peptide
- PCWP
pulmonary capillary wedge pressure
- VO2
oxygen consumption
Footnotes
REFERENCES
- 1.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines.; 2022: 1-e895–e1032. [DOI] [PubMed] [Google Scholar]
- 2.Ho JE, Zern EK, Wooster L, Bailey CS, Cunningham T, Eisman AS, Hardin KM, Zampierollo GA, Jarolim P, Pappagianopoulos PP, Malhotra R, Nayor M, Lewis GD. Differential Clinical Profiles, Exercise Responses, and Outcomes Associated with Existing HFpEF Definitions. Circulation. 2019;140:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Hear Fail. 2018;6:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, Lavie CJ. Physical Activity, Fitness, and Obesity in Heart Failure With Preserved Ejection Fraction. JACC Hear Fail. 2018;6:975–982. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Lam CSP, Kitzman W, Glenn K. Obese Heart Failure With Preserved Ejection Fraction Phenotype From Pariah to Central Player. Circulation. 2017;136:20–23. [DOI] [PubMed] [Google Scholar]
- 6.Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, Lavie CJ. Physical Activity, Fitness, and Obesity in Heart Failure With Preserved Ejection Fraction. JACC Hear Fail. 2018;6:975–982. [DOI] [PubMed] [Google Scholar]
- 7.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulus WJ, Zile MR. From Systemic Inflammation to Myocardial Fibrosis The Heart Failure With Preserved Ejection Fraction Paradigm Revisited. Circ Res. 2021;128:1451–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packer M. Derangements in adrenergic–adipokine signalling establish a neurohormonal basis for obesity-related heart failure with a preserved ejection fraction. Eur J Heart Fail. 2018;20:873–878. [DOI] [PubMed] [Google Scholar]
- 10.Packer M. Leptin-Aldosterone-Neprilysin Axis: Identification of Its Distinctive Role in the Pathogenesis of the Three Phenotypes of Heart Failure in People with Obesity. Circulation. 2018;137:1614–1631. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary Exercise Testing in Heart Failure. JACC Hear Fail. 2016;4:607–616. [DOI] [PubMed] [Google Scholar]
- 13.Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, Malhotra R, Lewis GD. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ Heart Fail. 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation. 2021;:E984–E1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS, Michos ED. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Hear Fail. 2018;6:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Packer M, Kitzman DW. Obesity-Related Heart Failure With a Preserved Ejection Fraction: The Mechanistic Rationale for Combining Inhibitors of Aldosterone, Neprilysin, and Sodium-Glucose Cotransporter-2. JACC Hear. Fail. 2018;6. [DOI] [PubMed] [Google Scholar]
- 17.Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, Abbate A. Exercise Intolerance in Patients With Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2209–2225. [DOI] [PubMed] [Google Scholar]
- 18.Kullo IJ, Khaleghi M, Hensrud DD. Markers of inflammation are inversely associated with V̇O 2 max in asymptomatic men. J Appl Physiol. 2007;102:1374–1379. [DOI] [PubMed] [Google Scholar]
- 19.Leite SA, Monk AM, Upham PA, Chacra AR, Bergenstal RM. Low cardiorespiratory fitness in people at risk for type 2 diabetes: early marker for insulin resistance. Diabetol Metab Syndr. 2009;1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golbidi S, Laher I. Exercise Induced Adipokine Changes and the Metabolic Syndrome. J Diabetes Res. 2014;2014:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lendeckel F, Zylla S, Markus MRP, Ewert R, Gläser S, Völzke H, Albrecht D, Friedrich N, Nauck M, Felix SB, Dörr M, Bahls M. Association of Cardiopulmonary Exercise Capacity and Adipokines in the General Population. Int J Sports Med. 2022;43:616–624. [DOI] [PubMed] [Google Scholar]
- 22.Roh J, Hill JA, Singh A, Valero-Muñoz M, Sam F. Heart Failure With Preserved Ejection Fraction: Heterogeneous Syndrome, Diverse Preclinical Models. Circ Res. 2022;130:1906–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieske B, Tschöpe C, De Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 24.Nayor M, Houstis NE, Namasivayam M, Rouvina J, Hardin C, Shah RV, Ho JE, Malhotra R, Lewis GD. Impaired Exercise Tolerance in Heart Failure With Preserved Ejection Fraction. JACC Hear Fail. 2020;8:605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb DR, Khunti K, Silverman R, Gray LJ, Srinivasan B, Lacy PS, Williams B, Davies MJ. Impact of metabolic indices on central artery stiffness: Independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia. 2010;53:1190–1198. [DOI] [PubMed] [Google Scholar]
- 26.Huot M, Arsenault BJ, Gaudreault V, Poirier P, Pérusse L, Tremblay A, Bouchard C, Després JP, Rhéaume C. Insulin resistance, low cardiorespiratory fitness, and increased exercise blood: Pressure contribution of abdominal obesity. Hypertension. 2011;58:1036–1042. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamani P, Proto EA, Mazurek JA, Prenner SB, Margulies KB, Townsend RR, Kelly DP, Arany Z, Poole DC, Wagner PD, Chirinos JA. Peripheral Determinants of Oxygen Utilization in Heart Failure With Preserved Ejection Fraction: Central Role of Adiposity. JACC Basic to Transl Sci. 2020;5:211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albar Z, Albakri M, Hajjari J, Karnib M, Janus SE, Al-Kindi SG. Inflammatory Markers and Risk of Heart Failure With Reduced to Preserved Ejection Fraction. Am J Cardiol. 2022;167:68–75. [DOI] [PubMed] [Google Scholar]
- 30.De Boer RA, Nayor M, DeFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, Bahrami H, Van Der Harst P, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL, Shah SJ, Levy D, Herrington DM, Larson MG, Van Gilst WH, Gottdiener JS, Bertoni AG, Ho JE. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 2018;3:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poetsch MS, Strano A, Guan K. Role of Leptin in Cardiovascular Diseases. Front Endocrinol (Lausanne). 2020;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontes-Carvalho R, Pimenta J, Bettencourt P, Leite-Moreira A, Azevedo A. Association between plasma leptin and adiponectin levels and diastolic function in the general population. Expert Opin Ther Targets. 2015;19:1283–1291. [DOI] [PubMed] [Google Scholar]
- 33.McGaffin KR, Moravec CS, McTiernan CF. Leptin signaling in the failing and mechanically unloaded human heart. Circ Hear Fail. 2009;2:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: Does leptin have a role? J Am Coll Cardiol. 2011;58:1870–1877. [DOI] [PubMed] [Google Scholar]
- 35.Lieb W, Sullivan LM, Harris TB, Roubenoff R, Benjamin E, Levy D, Fox CS, Wang TJ, Wilson PW, Kannel WB, Vasan RS. Plasma leptin levels and incidence of heart failure, cardiovascular disease, and total mortality in elderly individuals. Diabetes Care. 2009;32:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin SS, Blaha MJ, Muse ED, Qasim AN, Reilly MP, Blumenthal RS, Nasir K, Criqui MH, McClelland RL, Hughes-Austin JM, Allison MA. Leptin and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, Dickstein K, Filippatos GS, van der Harst P, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, Zannad F, Zwinderman AH, Hillege HL, van Veldhuisen DJ, Kakkar R, Voors AA, van der Meer P. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21:965–973. [DOI] [PubMed] [Google Scholar]
- 38.Lakhani I, Wong MV, Hung JKF, Gong M, Waleed K Bin, Xia Y, Lee S, Roever L, Liu T, Tse G, Leung KSK, Li KHC. Diagnostic and prognostic value of serum C-reactive protein in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart Fail Rev. 2021;26:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chia YC, Kieneker LM, van Hassel G, Binnenmars SH, Nolte IM, van Zanden JJ, van der Meer P, Navis G, Voors AA, Bakker SJL, De Borst MH, Eisenga MF. Interleukin 6 and development of heart failure with preserved ejection fraction in the general population. J Am Heart Assoc. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH. Circulation. 2003;107:3133–3140. [DOI] [PubMed] [Google Scholar]
- 41.Mann DL, McMurray JJV, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, Van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted Anticytokine Therapy in Patients with Chronic Heart Failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–1602. [DOI] [PubMed] [Google Scholar]
- 42.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


