Abstract
Background
Smith-Lemli-Opitz syndrome (SLOS) is an inherited disorder of cholesterol biosynthesis associated with congenital malformations, growth delay, intellectual disability and behavior problems. SLOS is caused by bi-allelic mutations in DHCR7, which lead to reduced activity of 7-dehydrocholesterol reductase that catalyzes the last step in cholesterol biosynthesis. Symptoms of SLOS are thought to be due to cholesterol deficiency and accumulation of its precursor 7-dehydrocholesterol (7-DHC) and 8-dehydrocholesterol (8-DHC), and toxic oxysterols. Therapy for SLOS often includes dietary cholesterol supplementation, but lipids are poorly absorbed from the diet, possibly due to impaired bile acid synthesis. We hypothesized that bile acid supplementation with cholic acid would improve dietary cholesterol absorption and raise plasma cholesterol levels.
Methods
Twelve SLOS subjects (10 M, 2F, ages 2–27 years) who had plasma cholesterol ≤125 mg/dL were treated with cholic acid (10 mg/kg/day) divided twice daily for 2 months. Plasma cholesterol, 7-DHC and 8-DHC were measured by GC–MS. Oxysterols were measured by ultra-high-performance LC-MS/MS. Data were analyzed using paired t-tests.
Results
At baseline, plasma cholesterol was 75 ± 24 mg/dL (mean ± SD; range 43–125, n = 12). After 2 months on cholic acid, mean plasma cholesterol increased to 97 ± 29 mg/dL (p = 0.011). Eleven of 12 subjects showed an increase in plasma cholesterol that varied from 3.8% to 85.7% (mean 38.7 ± 23.3%). 7-Hydroxycholesterol decreased by 20.6% on average (p = 0.013) but no significant changes were seen in 7-DHC or 8-DHC. Mean body weight tended to increase (3.6% p = 0.069). Subjects tolerated cholic acid well and experienced no drug-related adverse events.
Conclusions
In this pilot study, cholic acid supplementation was well tolerated and safe and resulted in an increase in plasma cholesterol in most SLOS subjects. Further controlled longitudinal studies are needed to look for the sustainability of the biochemical effect and possible clinical benefits.
Keywords: Bile acid, Oxysterol, Intellectual disability, Malformations, DHCR7
1. Introduction
Smith-Lemli-Opitz Syndrome (SLOS) is an autosomal recessive disorder associated with growth failure, intellectual disability, developmental delay, cataracts, autistic behavior, and congenital malformations with 2,3-toe syndactyly, dysmorphic facial features and hypospadias [1]. SLOS is caused by mutations in the DHCR7 gene for 7-dehydrocholesterol reductase (DHCR7), the enzyme that catalyzes the final step of cholesterol biosynthesis. Patients with SLOS demonstrate both cholesterol deficiency and elevations in the sterol precursors 7-dehydrocholesterol (7-DHC) and 8-dehydrocholesterol (8-DHC) (See Fig. 1) [[2], [3], [4]]. The many medical issues seen in patients with SLOS are thought to arise from the pre- and postnatal cholesterol deficiency, the downstream depletion of cholesterol metabolic products, and the oxidation of the sterol precursors into neurotoxic oxysterols [[5], [6], [7], [8]]. Therapeutic approaches to SLOS, therefore, include increasing cholesterol levels to allow its normal utilization for biosynthetic reactions, and reducing the levels of sterol precursors and toxic oxysterols [4,9,10].
Fig. 1.
Abbreviated biochemical pathway for cholesterol metabolism. Dashed arrows indicate regulatory relationships for feedback inhibition of cholesterol synthesis or bile acid synthesis. Bile acids stimulate dietary cholesterol absorption.
Dietary cholesterol supplementation via egg yolk leads to an increase in plasma cholesterol and a reduction in harmful precursors [11]. Raising cholesterol levels allows cholesterol to be available for physiologically important processes including bile acid production, cortisol synthesis, membrane stability, and growth. Although SLOS is listed as a treatable metabolic disorder causing intellectual disability [12], the complex clinical benefits of cholesterol supplementation in SLOS remain to be demonstrated in rigorously controlled intervention trials.
7-DHC-derived oxysterols cause premature neurogenesis [7,8], increased neuronal processes and retinal degeneration in animal and cell models of DHCR7 deficiency [13]. Treatment with antioxidants has been shown to reduce the formation of toxic oxysterols and improve some of these phenotypes, including neurogenesis in vitro and retinal degeneration in rats [7,8,13,14]. Simvastatin has also been used to inhibit the cholesterol biosynthetic pathway and lower cholesterol precursors (7-DHC) in SLOS but it did not lead to objective clinical improvement [15,16]. Thus, to date, there is no definitive treatment for SLOS. Nevertheless, patients are routinely put on cholesterol supplementation as a standard of care and may take multivitamin-mineral supplements with antioxidants.
Cholesterol is a major lipid component of cellular membranes and the precursor to steroid hormones, neurosteroids and bile acids. Through its covalent modification of Sonic Hedgehog, cholesterol is also critical for certain cell signaling pathways that function in fetal and postnatal development [17,18]. Cholesterol synthesis is highly regulated by feedback inhibition of the early rate-limiting step in cholesterol biosynthesis catalyzed by HMG-CoA reductase (Fig. 1). Under physiologic circumstances, changes in cholesterol levels result in compensatory variations in HMG-CoA reductase activity and bile acid biosynthesis, with bile acids playing a major positive role in intestinal cholesterol absorption and HMG-CoA reductase controlling the synthesis of mevalonate and intracellular cholesterol precursors [19]. Low cholesterol levels normally trigger a compensatory increase in HMG-CoA reductase and endogenous cholesterol synthesis to restore cholesterol homeostasis. In SLOS, however, the expected compensatory increase in HMG-CoA reductase is not observed, probably because cholesterol precursors such as 7-DHC, 8-DHC, or other precursors accumulating as a result of DHCR7 deficiency substitute for cholesterol in down-regulating HMG-CoA reductase [20].
In individuals with intact functional cholesterol homeostasis (biosynthesis and dietary intake), only about 50% of dietary cholesterol is absorbed [21]. In SLOS, absorption of dietary cholesterol may be further compromised due to impaired bile acid synthesis, thus contributing to overall cholesterol deficiency. Individuals with SLOS can exhibit reduced production of bile acids [22,23], or have normal bile acid synthesis in mildly affected individuals [24]. It is thus likely that clinically significant bile acid deficiency that would result in impaired cholesterol absorption may be limited to more severely affected SLOS patients with very low cholesterol levels. In spite of near total deficiency of normal bile acids in some patients, clinical evidence of fat malabsorption and fat-soluble vitamin deficiencies is rare [25].
We hypothesized that cholic acid treatment would improve intestinal cholesterol absorption in SLOS patients with impaired bile acid synthesis, simply by increasing the bioavailability of supplemental dietary cholesterol. In this study, we report on the efficacy of cholic acid to increase plasma cholesterol levels.
2. Methods
This research was approved by the institutional review boards at the University of Nebraska Medical Center and Children's Hospital Colorado. All subjects or their legal representatives gave written consent. Subjects were biochemically confirmed to have SLOS and most were also confirmed to carry pathogenic mutations in DHCR7. SLOS subjects who had plasma cholesterol ≤125 mg/dL were studied. They were treated with cholic acid (Cholbam®, Travere Therapeutics) given at a dose of 10 mg/kg/day divided twice daily for 2 months. Target doses were obtained using a combination of 50 mg and 250 mg cholic acid capsules. In the event that target doses could not be achieved using available capsule strengths, caregivers were provided education on appropriate partial-capsule dose preparation using a solid-in-liquid aliquot method. When occurring, caregivers' technique was observed by a study pharmacist to ensure safety and accuracy. Caregivers were instructed to administer cholic acid with feedings. Caregivers kept daily drug administration logs and were contacted weekly by telephone to elicit any adverse effects. Leftover cholic acid was collected and quantitated at the end of the study to monitor compliance.
Plasma cholesterol, 7-DHC, and 8-DHC were measured by GC–MS at Kennedy-Krieger Institute, Baltimore, MD. Oxysterols were measured by LC-MS/MS as described [7]. Oxysterols measured included 7-Hydroxycholesterol (7-OH-cholesterol), 7-Keto-cholesterol, 4α-OH-7-DHC, 4ß-OH-7-DHC, 24-Keto-cholesterol, 24-OH-7-DHC, 24/25-OH-cholesterol, 7-Keto-8-DHC, 7,27-diOH-cholesterol, DHCEO (3ß,5α-dihydroxycholest-7-en-6-one), and 7-Keto-OH-cholesterol.
Plasma 25-hydroxy-vitamin D was measured at Mayo Clinic Laboratories, Rochester, MN. Safety lab tests including comprehensive chemistry panel and complete blood count were performed using standard automated analysis methods. Data were analyzed by paired t-tests and Pearson correlation coefficients using Prism 9.5 software (GraphPad Software, LLC). Statistical significance was established at p < 0.05.
3. Results
The clinical characteristics of the subjects are summarized in Table 1. Thirteen subjects were screened for enrollment in the study; one subject was excluded because of a plasma cholesterol level > 125 mg/dL. The remaining subjects, 10 males and 2 females, had plasma cholesterol levels of ≤125 mg/dL (range 43–125 mg/dL) and were studied further. Their ages ranged from 2 to 27 years. Five subjects were fed orally, 4 subjects were fed exclusively by G-tube, and 3 subjects were fed by a combination of oral and G-tube. Four subjects received cholesterol supplementation only given by egg yolk consumption, 5 subjects were taking cholesterol suspension exclusively and 2 subjects took a combination of egg yolks and cholesterol suspension. One subject received no supplemental cholesterol. Supplemental cholesterol intake ranged widely from 0 to 230 mg/kg body weight/day.
Table 1.
Characteristics of the study population.
| Subject |
Sex |
Age (yrs) |
Weight (kg) |
Fed by G-tube (GT) or Oral (PO) |
Daily supplemental cholesterol intake |
|||
|---|---|---|---|---|---|---|---|---|
| Given as Suspension (S) or Eggs (E) | From Eggs (mg) | From Susp (mg) | Cholesterol Intake (mg/kg body wt) | |||||
| 1 | F | 4 | 11.4 | GT | E + S | 372 | 500 | 76.5 |
| 2 | M | 27 | 84.1 | PO | – | – | – | 0 |
| 3 | M | 5 | 14.9 | GT | S | – | 1600 | 107 |
| 4a | M | 7 | 22.1 | PO | S | – | 1200 | 54.3 |
| 5a | M | 7 | 24.3 | PO | S | – | 1200 | 49.4 |
| 6 | F | 15 | 37.5 | PO and GT | S | – | 1023 | 27.3 |
| 7 | M | 26 | 56.4 | PO | E + S | 465 | 12,000 | 221 |
| 8 | M | 5 | 15.6 | PO | S | – | 3600 | 230 |
| 9 | M | 5 | 23.5 | PO and GT | E | 558 | – | 23.7 |
| 10 | M | 9 | 28.6 | GT | E | 1050 | – | 36.7 |
| 11 | M | 2 | 8.9 | GT | E | 93 | – | 10.4 |
| 12 | M | 2 | 9.7 | PO and GT | E | 251 | – | 25.9 |
Twin siblings.
Subjects were administered cholic acid at a daily dose of 10 mg/kg divided twice daily with formula or meals. Plasma cholesterol at baseline was 75 ± 24 mg/dL (mean ± SD; n = 12) and ranged from 43 to 125 mg/dL. After 2 months on cholic acid, mean plasma cholesterol increased to 97 ± 29 mg/dL (p = 0.011) (Fig. 2). One SLOS subject (Subject 1) had a febrile illness at the 2-month follow up visit and showed a reduction in plasma cholesterol from 125 to 89 mg/dL. The other 11 subjects had an increase in plasma cholesterol that varied from 3.8% to 85.7% (mean 38.7 ± 23.3%). No consistent or statistically significant changes were seen in 7-DHC, 8-DHC or the sum of these cholesterol precursors (Fig. 2).
Fig. 2.
Change in plasma sterols on cholic acid supplementation. Asterisks designates Subject 1.
There were no significant correlations between the baseline plasma cholesterol and the relative change in cholesterol on cholic acid, or the amount of supplemental cholesterol consumed (mg/kg/day) and the change in plasma cholesterol on cholic acid.
Oxysterols were measured at baseline and after 2 months on cholic acid. 7-OH-cholesterol, which is the precursor to bile acids, was decreased by 20.6% on average (p = 0.013) on cholic acid (Fig. 3). 7-Ketocholesterol trended lower but did not reach statistical significance (p = 0.06). Other oxysterols were not changed on cholic acid (Supplemental Table 1).
Fig. 3.
Change in plasma oxysterols on cholic acid supplementation. Asterisks designates Subject 1.
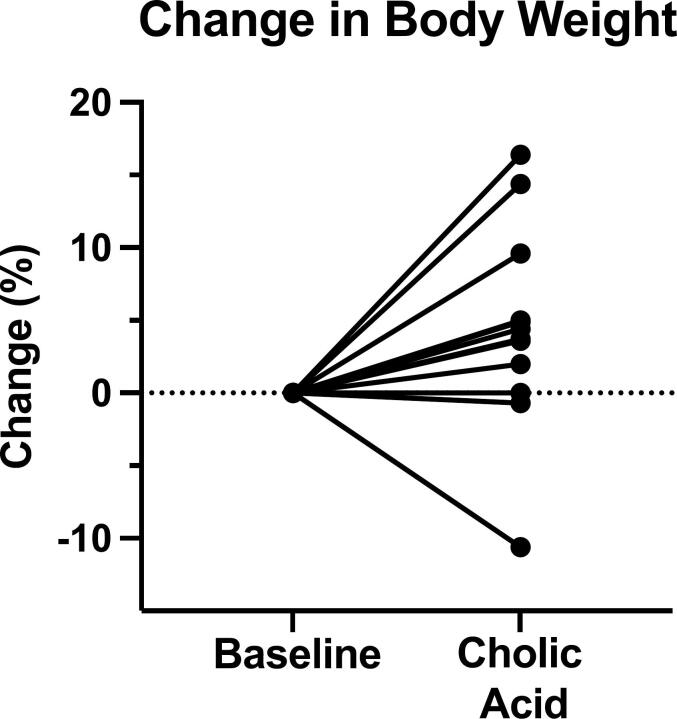
Mean body weight on cholic acid tended to increase by 3.6% but did not reach statistical significance (p = 0.069) (Fig. 4). Safety lab tests only showed a mild reduction in plasma chloride from 107 ± 2.0 mmol/L at baseline to 104 ± 2.9 on cholic acid (p = 0.016) and an increase in platelets from 314 ± 101 × 103/μL to 369 ± 164 (p = 0.034) (Table 2). Subjects tolerated cholic acid well and experienced no drug-related adverse events.
Fig. 4.
Body weight change on cholic acid. Baseline body weight varied from 8.9 kg to 84.1 kg.
Table 2.
Safety blood tests monitored in the study.
| Lab test | Baseline | On cholic acid | P value⁎ |
|---|---|---|---|
| Sodium (mmol/L) | 139 ± 2.5 | 139 ± 2.1 | 0.295 |
| Potassium (mmol/L) | 4.3 ± 0.29 | 4.4 ± 0.39 | 0.396 |
| Chloride (mmol/L) | 107 ± 2.02 | 104 ± 2.87 | 0.0168 |
| Carbon dioxide (meq/L) | 24.3 ± 2.70 | 22.9 ± 3.87 | 0.112 |
| Aspartate aminotransferase (U/L) | 43 ± 11 | 47 ± 16 | 0.515 |
| Alanine aminotransferase (U/L) | 34 ± 20 | 36 ± 14 | 0.813 |
| Alkaline phosphatase (U/L) | 138 ± 50 | 133 ± 47 | 0.568 |
| Gamma-glutamyl transferase (U/L) | 22 ± 19 | 22 ± 18 | 0.884 |
| Bilirubin (mg/dL) | 0.34 ± 0.14 | 0.29 ± 0.14 | 0.191 |
| Albumin (g/dL) | 4.5 ± 0.29 | 4.5 ± 0.36 | 0.690 |
| Total protein (g/dL) | 7.0 ± 0.44 | 7.1 ± 0.56 | 0.582 |
| Creatinine (mgdL) | 0.43 ± 0.294 | 0.42 ± 0.28 | 0.411 |
| Blood Urea Nitrogen (mg/dL) | 14 ± 3.1 | 14 ± 3.8 | 0.999 |
| Calcium (mg/dL) | 9.7 ± 0.35 | 9.7 ± 0.16 | 0.781 |
| 25-OH-Vitamin D (ng/ml) | 69.9 ± 26.4 | 77.9 ± 28.9 | 0.129 |
| White Blood Count ((x103/μL) | 8.39 ± 2.19 | 9.43 ± 2.91 | 0.194 |
| Hemoglobin (g/dL) | 13.9 ± 1.61 | 14.1 ± 1.57 | 0.427 |
| Hematocrit (%) | 41.5 ± 4.57 | 42.4 ± 4.18 | 0.225 |
| Platelets (x103/ μL) | 314 ± 101 | 369 ± 164 | 0.034 |
| Body weight (kg) | 28.1 ± 22.2 | 29.1 ± 23.0 | 0.069 |
Paired t-test.
4. Discussion
Bile acid supplementation in SLOS was used in the mid-1990's along with cholesterol to promote improved cholesterol absorption [26,27]. The bile acids administered included chenodeoxycholic acid, ursodeoxycholic acid (ursodiol, Actigall®) [26] or cholic acid [27]. Chenodeoxycholic acid is associated with potential liver toxicity in children and it became unavailable. Cholic acid was started in some patients soon after the metabolic error in cholesterol biosynthesis causing SLOS was first identified, but there is little published evidence regarding effects of cholic acid alone on biochemical or clinical course. Due to the decision to cease manufacturing cholic acid, it became unavailable since the late 1990's. Recently, cholic acid (Cholbam®) has come back on the market but, to date, an organized study demonstrating its efficacy has not been reported and there is no data available to suggest that long term use of cholic acid has a sustained effect.
In our short-term pilot study, cholic acid supplementation led to an increase in plasma cholesterol. The single patient who did not show an increase in cholesterol had an intercurrent febrile illness at the time of her follow-up visit, which probably accounts for the discrepant response. Despite an increase in plasma cholesterol, the decrease in 7-OH-cholesterol, which is a metabolite of cholesterol via cytochrome P450 7A1 (CYP7A1), on cholic acid treatment is consistent with the downregulation of de novo bile acid biosynthesis due to the supplementation of cholic acid. Furthermore, 7 keto-cholesterol, which can be formed from cholesterol via autoxidation [28] or from 7-DHC via CYP7A1 [29,30], showed a decreasing trend with cholic acid treatment, particularly for patients with a high baseline level (p = 0.06). These findings could be potentially significant because the accumulation of toxic oxysterols is thought to contribute to progressive retinal and neurological pathologies in patients with SLOS and 7-keto-cholesterol is a well-established toxic oxysterol in various settings [5,7,31]. We used a conservative dose of cholic acid (10 mg/kg/day) because of a lack of safety data in the SLOS population. A higher dose (15 mg/kg) has been approved for other diseases and it is possible that this dose would result in a greater cholesterol response in SLOS.
Cholic acid was well tolerated and safe in our SLOS patients. No dose reductions or interruptions in the drug were needed, and there were no reports of drug-related adverse effects. The mild reduction in plasma chloride and increase in blood platelets seen in subjects on cholic acid were not clinically significant. Reports of increases in transaminases in some patients with Zellweger spectrum disorder treated with cholic acid were not observed in the SLOS population.
It might be expected that the subjects with the lowest plasma cholesterol would respond to cholic acid better than those with higher cholesterol. However, this was not the case. There was no significant correlation between the plasma cholesterol response to cholic acid and the severity of cholesterol deficiency. Similarly, no significant correlation was noted between the amount of cholesterol supplementation (mg/kg/day) and the increase in plasma cholesterol. Both of these observations may reflect the complex nature of plasma cholesterol regulation, which depends on endogenous synthesis rates along with dietary intake and absorption. We hypothesized that plasma cholesterol levels in SLOS are reduced because of impaired biosynthesis along with decreased absorption from dietary sources owing in part to limited bile acid production. Bile acid synthesis in SLOS has been reported to be either normal or low [[22], [23], [24]]. The variation in dietary cholesterol supplementation in our SLOS subjects along with an unknown degree of bile acid deficiency in the gut may account for, and contribute to, the variation in plasma cholesterol response observed. In addition, it is possible that the addition of cholic acid to the diet raises the general efficiency of cholesterol absorption even in SLOS subjects who have normal bile acid production.
Although plasma cholesterol increased on cholic acid, there were no reductions in mean levels of sterol precursors, 7-DHC or 8-DHC, and other 7-DHC-derived oxysterols. Failure to significantly decrease sterol precursors may require higher cholesterol elevations than were achieved in this study or require a longer time to respond. Accumulations of sterol precursors along with their derived oxysterols are thought to be key contributors to the pathogenic mechanisms of SLOS [6]. Therefore, cholic acid may be one component, together with cholesterol supplementation and antioxidants, of a rational approach for treating SLOS [4,9,10].
For physicians diagnosing and managing SLOS patients, we emphasize the importance for measuring plasma cholesterol using methods that employ chromatography for separating cholesterol from sterol precursors. Cholesterol measurements by standard automated methods used by most hospitals provide a combined measure of total cholesterol with 7-DHC and 8-DHC precursors, which can be misleading for diagnosing SLOS and determining the response to cholic acid.
The results of our open-label pilot study were likely affected by several uncontrolled factors that limit our conclusions including the wide age range of SLOS subjects, small number of subjects studied with a male preponderance, variation in dietary cholesterol intake, and endogenous rates of cholesterol and bile acid synthesis. Further controlled longitudinal studies are needed to look for sustainability of cholic acid effects and possible clinical benefits.
Author statement
All authors have reviewed the manuscript and agree to its submission.
The revised manuscript incorporates changes suggested by the authors.
The order of authors is agreed upon by all authors.
CRediT authorship contribution statement
Ellen R. Elias: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. Lucas E. Orth: Investigation, Methodology. Amy Li: Data curation, Methodology, Writing – review & editing. Libin Xu: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Sara M. Jones: Project administration, Writing – review & editing. William B. Rizzo: Conceptualization, Formal analysis, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
None.
Acknowledgments
This research was funded by grant U54 HD061939 from the Eunice Kennedy Shriver National Institutes of Child Health & Human Development and National Center for Advancing Translational Sciences, NIH in support of the Sterol and Isoprenoid Research Consortium of the Rare Disease Clinical Research Network, and grant 1 U2C TR002818 01. AL and LX were supported by a grant from the NIH (R01HD092659). We thank the efforts of the Smith-Lemli-Opitz Foundation for invaluable assistance in recruiting SLOS subjects for this study. We also thank the many SLOS families for participating in this research.
Cholic acid (Cholbam) was kindly provided as a gift from Travere Therapeutics. Travere Therapeutics was not involved in the study design, analysis of data, or writing of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2023.101030.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- 1.Nowaczyk M.J.M., Irons M.B. Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology. Am. J. Med. Genet. Part C. 2012;160C:250–262. doi: 10.1002/ajmg.c.31343. [DOI] [PubMed] [Google Scholar]
- 2.Irons M., Elias E.R., Salen G., Tint G.S., Batta A.K. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 1993;341:1414. doi: 10.1016/0140-6736(93)90983-n. [DOI] [PubMed] [Google Scholar]
- 3.Tint G.S., Irons M., Elias E.R., Batta A.K., Friedan R., Chen T.S., Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. New Eng. J. Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 4.Svoboda M.D., Christie J.M., Eroglu Y., Freeman K.A., Steiner R.D. Treatment of Smith-Lemli-Opitz syndrome and other sterol disorders. Am. J. Med. Genet. 2012;160C:285–294. doi: 10.1002/ajmg.c.31347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L., Sheflin L.G., Porter N.A., Fliesler S.J. 7-Dehydrocholesterol derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim. Biophys. Acta. 2012;1821(6):877–883. doi: 10.1016/j.bbalip.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korade Z., Xu L., Shelton R., Porter N.A. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2010;51:3259–3269. doi: 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomita H., Hines K.M., Herron J.M., Li A., Baggett D.W., Xu L. 7-Dehydrocholesterol-derived oxysterols cause neurogenic defects in Smith-Lemli-Opitz syndrome. eLife. 2022;11 doi: 10.7554/eLife.67141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L., Mirnics K., Bowman A.B., Liu W., Da J., Poter N.A., Korade Z. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol. Dis. 2012;45:923–929. doi: 10.1016/j.nbd.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korade Z., Xu L., Harrison F.E., Ahsen R., Hart S.E., Oakleigh M.F., Mirnics K., Porter N.A. Antioxidant supplementation ameliorates molecular deficits in Smith-Lemli-Opitz syndrome. Biol. Psychiatry. 2014;75:215–222. doi: 10.1016/j.biopsych.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fliesler S.J. Antioxidants: the missing key to improved therapeutic intervention in the Smith-Lemli-Opitz syndrome? Hereditary Genet. 2013;2(2):119–124. doi: 10.4172/2161-1041.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linck L.M., Lin D.S., Flavell D., Connor W.E., Steiner R.D. Cholesterol supplementation with egg yolk increases plasma cholesterol and decreases plasma 7-dehydrocholesterol in Smith-Lemli-Opitz syndrome: thinking beyond cholesterol deficiency. Am. J. Med. Genet. 2000;93:360–365. doi: 10.1002/1096-8628(20000828)93:5<360::aid-ajmg4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.van Karnebeek C., Shevell M., Zschoke J., Moeschler J.B., Stockler A. The metabolic evaluation of the child with an intellectual developmental disorder: diagnostic algorithm for identification of treatable causes and new digital resource. Mol. Genet. Metab. 2014;111:428–438. doi: 10.1016/j.ymgme.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Fliesler S.J., Peachey N.S., Herron J., Hines K.M., Weinstock N.I., Ramachandra Rao S., Xu L. Prevention of retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Sci. Rep. 2018;8:1286. doi: 10.1038/s41598-018-19592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fliesler S.J. Retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome: thinking beyond cholesterol deficiency. Adv. Exp. Med. Biol. 2010;664:481–489. doi: 10.1007/978-1-4419-1399-9_55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan Y.M., Merkens L.S., Connor W.E., Roullet J.-B., Penfeld J.A., Jordan J.M., Steiner R.D., Jones P.J. Effects of dietary cholesterol and simvastatin on cholesterol synthesis in Smith-Lemli-Opitz syndrome. Pediatr. Res. 2009;65:681–685. doi: 10.1203/PDR.0b013e31819ea4eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassif C.A., Kratz L., Sparks S.E., Wheeler C., Bianconi S., Gropman A., Calis K.A., Kelley R.I., Tierney F.D. A placebo-controlled trial of simvastatin therapy in Smith-Lemli-Opitz syndrome. Genet. Med. 2016;19:1–9. doi: 10.1038/gim.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter J.A., Young K.E., Beachy P.A. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 18.Daggubati V., Raleigh D.D.R., Sever N. Sterol regulation of developmental and oncogenic Hedgehog signaling. Biochem. Pharmacol. 2022;196 doi: 10.1016/j.bcp.2021.114647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen D.E. Balancing cholesterol synthesis and absorption in the gastrointestinal tract. J. Clin. Lipidol. 2008;2(2):S1–S3. doi: 10.1016/j.jacl.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda M., Tint G.S., Honda A., Salen G., Shefer S., Batta A.K., Matsuzaki Y., Tanaka N. Regulation of cholesterol biosynthetic pathway in patients with Smith-Lemli-Opitz syndrome. J. Inherit. Metab. Dis. 2000;23:464–474. doi: 10.1023/a:1005660130109. [DOI] [PubMed] [Google Scholar]
- 21.Sehayek E. Genetic regulation of cholesterol absorption and plasma plant sterol levels: commonalities and differences. J. Lipid Res. 2003;44:2030–2038. doi: 10.1194/jlr.R300008-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Natowicz M.R., Evans J.E. Abnormal bile acids in the Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. 1994;50:364–367. doi: 10.1002/ajmg.1320500413. [DOI] [PubMed] [Google Scholar]
- 23.Honda A., Salen G., Shefer S., Batta A.K., Honda M., Xu G., Tint G.S., Matsuzaki Y., Shoda J., Tanaka N. Bile acid synthesis in the Smith-Lemli-Opitz syndrome: effects of dehydrocholesterols on cholesterol 7-alpha-hydroxylase and 27-hydroxylase activities in rat liver. J. Lipid Res. 1999;40(8):1520–1528. [PubMed] [Google Scholar]
- 24.Steiner R.D., et al. Sterol balance in the Smith-Lemli-Opitz syndrome: reduction in whole body cholesterol synthesis and normal bile acid production. J. Lipid Res. 2000;41:1437–1447. [PubMed] [Google Scholar]
- 25.Kelley R.I., Hennekam R.C. The Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irons M., Elias E.R., Abuelo D., Bull M.J., Greene C.L., Johnson V.P., Keppen L., Schanen C., Tint G.S., Salen G. Treatment of Smith-Lemli-Opitz syndrome: results of a multicenter trial. Am. J. Med. Genet. 1997;68:311–314. [PubMed] [Google Scholar]
- 27.Nwokoro N.A., Mulvihill J.J. Cholesterol and bile acid replacement therapy in children and adults with Smith-Lemli-Opitz (SLO/RSH) syndrome. Am. J. Med. Genet. 1997;68:315–321. doi: 10.1002/(sici)1096-8628(19970131)68:3<315::aid-ajmg13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Yin H., Xu L., Porter N.A. Free radical and lipid peroxidation: mechanisms and analysis. Chem. Rev. 2011;111:5942–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 29.Shinkyo R., Xu L., Tallman K.A., Cheng Q., Porter N.A., Guengerich F.P. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J. Biol. Chem. 2011;286:33021–33028. doi: 10.1074/jbc.M111.282434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu L., Liu W., Sheflin L.G., Fliesler S.J., Porter N.A. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: a model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011;52:1810–1820. doi: 10.1194/jlr.M018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown A.J., Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.