Highlights
-
•
Ceramide metabolism-related signature predict prognosis in osteosarcoma.
-
•
ST3GAL1 associated with TAMs-induced inhibition of CD8+ T cytotoxic function.
-
•
ST3GAL1 regulated TAMs-CD8+ T interaction through α2,3-linked sialic acid receptors.
-
•
ST3GAL1 in tumor cells regulated TAMs differentiation.
Keywords: Osteosarcoma, Ceramide metabolism, ST3GAL1, TAMs, Siglec receptor
Abstract
Osteosarcoma is the most common primary malignant bone tumor with elevated disability and mortality rates in children and adolescents and the therapeutic effect for osteosarcoma has remained stagnant in the past 30 years. Emerging evidence has shown ceramide metabolism plays a vital role in tumor progression, but its mechanisms in osteosarcoma progression remain unknown. Through consensus clustering and LASSO regression analysis based on the osteosarcoma cohorts from TARGET database, we constructed a ceramide metabolism-related prognostic signature including ten genes for osteosarcoma, with ST3GAL1 exhibiting the highest hazard ratio. Biological signatures analysis demonstrated that ceramide metabolism was associated with immune-related pathways, immune cell infiltration and the expression of immune checkpoint genes. Single-cell profiling revealed that ceramide metabolism was enriched in myeloid, osteoblast and mesenchymal cells. The interaction between TAMs and CD8+ T cells played an essential role in osteosarcoma. ST3GAL1 regulated the SPP1-CD44 interaction between TAMs and CD8+ T cells and IL-10 secretion in TAMs through α2,3 sialic acid receptors, which inhibited CD8+ T cell function. IHC analysis showed that ST3GAL1 expression correlated with the prognosis of osteosarcoma patients. Co-culture assay revealed that upregulation of ST3GAL1 in tumor cells regulated the differentiation of TAMs and cytokine secretion. Collectively, our findings demonstrated that ceramide metabolism was associated with clinical outcome in osteosarcoma. ST3GAL1 facilitated tumor progression through regulating tumor immune microenvironment, providing a feasible therapeutic approach for patients with osteosarcoma.
Introduction
Osteosarcoma is the most common primary malignant bone tumor in children and adolescents, characterized by elevated disability and mortality rates [1]. The application of neoadjuvant chemotherapy combined with surgical treatment significantly improves the 5-year overall survival rate of patients with osteosarcoma to 60–70 %. However, the therapeutic effect for osteosarcoma has remained stagnant in the past 30 years. Approximately 35 % of patients experience recurrence and metastasis during treatment, resulting in an unfavorable prognosis [2], [3], [4], [5], [6], [7]. Consequently, it becomes imperative to investigate the molecular mechanisms driving osteosarcoma progression and identify potential therapeutic targets.
Tumor cells exhibit unchecked proliferation and invasive behavior. These malignant phenotypes are associated with metabolic reprogramming, with metabolic dysregulation influencing tumorigenesis and progression [8]. Previous research has demonstrated multiple dysregulated metabolic pathways in cancer, implying that abnormal metabolites or intermediates could also exert a significant influence on the regulation of immune cell proliferation, differentiation, activation, and function [9,10]. Interestingly, cancer cells have the capacity to suppress anti-tumor immune responses through mechanisms such as resource competition, depletion of essential nutrients, or interference with the metabolic adaptability of immune cells infiltrating the tumor microenvironment [10,11]. These findings suggest the potential of metabolic interventions to improve the effectiveness of immunotherapy.
Ceramide is a sphingolipid metabolite engaged in complex metabolic processes. It is produced through three main pathways: the de novo pathway, the salvage pathway, and the sphingomyelin pathway [12]. Ceramide plays a crucial role as potential tumor suppressor, influencing various cellular processes such as cell proliferation, differentiation, and apoptosis [13], [14], [15], [16], [17], [18]. Moreover, emerging research has indicated that ceramide may also be implicated in immunomodulation. Studies have demonstrated its importance as a component of the T-cell receptor (TCR) signaling mechanism, impacting interleukin 2 production [19]. Furthermore, ceramide has the potential to modulate the immune-tolerant tumor microenvironment (TME) [5]. These intriguing findings have shown the potential of targeting ceramide metabolic pathways for cancer treatments.
In this study, we found a novel ceramide metabolism-related prognostic signature for osteosarcoma and identified the vital genes in a prognostic risk score model. Through Cox regression analysis, CERS1, ST3GAL1 and B4GALNT1 were found correlated with a poor prognosis in osteosarcoma patients. Based on the risk score model, patients were clustered into high-risk and low-risk groups. We further explored the biological signatures and characteristics of the tumor microenvironment in different risk groups. Through single-cell RNA sequencing (scRNA-seq) analysis of primary osteosarcoma samples, we found that tumor associated macrophages (TAMs) played an essential role in ceramide metabolism-related changes of immune microenvironment. We further analyzed the characteristics and functions of TAMs, including the cell communication. Among the genes in the risk score model, we identified the relationship between ST3 Beta-Galactoside Alpha-2,3-Sialyltransferase 1 (ST3GAL1) with TAMs-CD8+ T cells interaction and explored the clinical significance of ST3GAL1. Co-culture assay revealed the upregulation of ST3GAL1 in osteosarcoma cells contributed to the differentiation of TAMs with an immune-suppressive phenotype. Our study demonstrated the association between ceramide metabolism and the immune microenvironment in osteosarcoma and suggested that ST3GAL1 was associated with TAMs-induced inhibition of CD8+ T cytotoxic function through α2,3 sialic acids, providing new insight into the importance of metabolism in tumor immune microenvironment.
Material and methods
Dataset source and preprocessing
The osteosarcoma dataset (n = 85) was obtained from the TARGET database, which includes RNA expression data and clinical information and was utilized for survival analysis and clustering. Single-cell sequencing data of osteosarcoma from the GSE152048 and GSE162454 datasets were obtained from the Gene Expression Omnibus (GEO) databases. We used seven primary osteosarcoma samples from GSE152048 and all samples from GSE162454 to investigate cellular composition and the tumor immune microenvironment. Ceramide metabolism-related genes (GOBP_CERAMIDE_METABOLIC_PROCESS) were obtained from the MSigDB database.
Unsupervised clustering and functional enrichment analysis
We employed expression data from TARGET osteosarcoma cohort and clinical data to classify patients into distinct molecular subtypes using unsupervised clustering methods. The determination of the number of clusters and their stability was achieved using the “ConsensusClusterPlus” R package [20]. We used the consensus matrix, cumulative distribution function (CDF), and relative change in the area under the CDF curve to identify the optimal number of clusters. Kaplan‒Meier survival analysis was generated to assess the survival rates of each subtype. Furthermore, we employed GSEA [21] and GSVA [22] using the Gene Ontology or KEGG database for functional enrichment analysis in different groups based on gene expression profiles. The differences in the immune-related pathways were calculated and compared using the “IOBR” package [23].
Construction and validation of the prognostic signature
We conducted LASSO analysis using the “glmnet” package [24]. Candidate genes were identified based on the optimal penalty parameter λ, determined by the 1-SE (standard error) criterion. Risk scores were calculated by combining the expression levels and corresponding coefficients of each gene. Patients were then categorized as either low-risk or high-risk based on their median risk scores. We evaluated the prediction performance of the prognostic signature using Kaplan‒Meier analysis and time-dependent ROC analysis. The generalization of the prognostic signature was confirmed using data from an independent validation cohort.
Quantitative RT-PCR
Total RNA was extracted using TRIzol (Invitrogen) following the manufacturer's instructions. RNA was then reverse-transcribed into cDNA using the cDNA Synthesis Kit (Vazyme, R111). Real-time reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed with the Real-time Fluorescent Quantitative PCR Kit (Vazyme, Q321), with GAPDH as the endogenous control. Primer sequences were listed in Supplementary Table 1. The comparative Ct(2−△△CT) method was applied to calculate the relative expression.
Human samples and cell lines
The osteosarcoma and adjacent normal tissues used in this study were collected from the Department of Musculoskeletal Oncology, The First Affiliated Hospital of Sun Yat-sen University, with informed consent. The study was approved by the Research Medical Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (Approval number: [2023]182). The cell lines used in this study, including 143B, THP-1 and HEK293T, were obtained from the First Affiliated Hospital of Sun Yat-sen University. THP-1 cells were cultured in RPMI 1640 (Gibco) culture medium containing 10 % fetal bovine serum (FBS, Gibco), 0.2 mM l-glutamine (Gibco) and 10 mM HEPES (Gibco). Other cells were cultured in high-glucose DMEM (Gibco) with 10 % FBS and 1 % penicillin/streptomycin (Gibco) at 37 °C with 5 % CO2. All cell lines were certified mycoplasma-free.
Western blot analysis
Proteins were extracted from cells and tissues using RIPA buffer supplemented with 1 mM phenylmethanesulfonyl fluoride (PMSF). Protein concentration was determined by the bicinchoninic acid (BCA) assay. Total proteins were separated by SDS-PAGE using 10 % polyacrylamide gel and then transferred to a PVDF membrane. The membrane was blocked with 5 % non-fat milk and incubated overnight at 4 °C with primary antibodies (CERS1: ab85696, Abcam; B4GALNT1: LS-C200171, LSBio; ST3GAL1: LS-C185763, LSBio). Following, the membrane was incubated with species-matched secondary antibodies at room temperature for 1 h. The visualization of target protein was achieved using High-sig ECL substrate (Tanon, China).
Plasmid construction and lentiviral transduction
To overexpress ST3GAL1, the coding sequence of ST3GAL1 were obtained from the NCBI and cloned into the pCDH-CMV-MCS-EF1-Puro vector plasmid. ST3GAL1 overexpression plasmid was co-transfected with the 3rd generation packaging plasmids (#12251, #12253 and #12259, Addgene) into HEK293T cells using Lipofectamine 3000 (Invitrogen). After 48 h, supernatants were collected and were used to infect 143B cells. Following puromycin selection (2 μg/mL) for 7 days, the ST3GAL1 protein level was assessed via western blot analysis.
Immunohistochemistry (IHC)
For IHC, osteosarcoma specimen tissue sections were initially deparaffinized, followed by antigen retrieval with an EDTA Antigen Retrieval Solution. Subsequently, the sections were treated with 3 % H2O2 and blocked with 5 % goat serum. The sections were then incubated overnight at 4 °C with primary antibodies, (anti-ST3GAL1, 1:200, HPA040466, Sigma-Aldrich). IHC staining was performed using 3,3′-diaminobenzidine (DAB) (DAKO). The levels of IHC staining were quantified according to immunoreactive score (IRS) systems and the cut-off value for defining high or low ST3GAL1 expression in IHC was the median IHC score.
Single-cell analysis and cell communication
Single-cell analysis was performed in the R statistical environment (v4.2.3). The raw data for all samples were processed and the “Seurat” package was used for quality control and further analysis. For quality control, genes expressed in a minimum of 3 cells were included and cells with more than 10 % mitochondrial reads and less than 5 % ribosomal reads were excluded. In addition, we deleted cells with fewer than 200 genes or more than 5000 genes and doublets that were detected with DoubletFinder [25]. Harmony [26] was used to control batch effects. We applied the same parameters for clustering and generating the UMAP. The annotation of all cells was performed through manual labeling based on gene expression. CellChat [27] was used to identify cell–cell communication based on a human database. The Slingshot [28] algorithm was used to analyze the trajectory of TAMs.
Macrophage stimulation and co-culture assay
THP-1 cells were differentiated into macrophages by incubation with 150 nM phorbol 12-myristate 13-acetate (PMA, Sigma, P8139) for 24 h. For co-culture assay, THP-1 monocytes were differentiated in 6-well plate and 143B cells were cultured in 6-well Transwell inserts (0.4 μm, Falcon, 353090). Macrophages were co-cultured with indicated 143B cells for 48 h and indicated markers (CD206: 551135, BD Biosciences; CD86: 557343, BD Biosciences) was analyzed by flow cytometry, FlowJo was used for data analysis.
ELISA-based assay
For α2,3-sialylated glycans level detection, Maackia Amurensis Lectin II (MAL-II, Vector Laboratories) was used as a probe, and ELISA assays were conducted following established methods [29]. For intracellular α2,3-sialylated glycans detection, cells were washed five times with PBS and cell lysates were obtained using RIPA buffer supplemented with protease inhibitors (Roche). For secreted α2,3-sialylated glycans detection, cells were incubated for 48 h with RPMI 1640 without FBS, and supernatants were harvested and lyophilized. Protein concentration was determined using BCA assay. Nunc MaxiSorp plates (Thermo) were coated with cell lysates or supernatant overnight at room temperature. Plates were then washed with TPBS and blocked with Carbo Free Blocking Buffer (Vector Laboratories). Biotinylated MAL-II was added to each well and incubating for 30 min at room temperature. Quantification was performed using VECTASTAIN Elite ABC-HRP (Vector Laboratories) and TMB Substrate Kit (Vector Laboratories) following the manufacturer's instructions. For cytokine assay, supernatants were collected 12 h after co-culture assays, and IL-6 or IL-10 were conducted using ELISA kit (IL-6: ab178013, Abcam; IL-10: D1000B, R&D Systems) according to the manufacturer's instructions.
Statistical analysis
All statistical analysis and graph visualizations were performed using R v4.2.1 (http://www.r-project.org). Representative data from one experiment are presented. We conducted statistical analysis using Student's t-test. Overall survival was analyzed using the Kaplan‒Meier method and compared using the log-rank test. Differences with P values < 0.05 were considered statistically significant.
Results
Identification of ceramide metabolism molecular subtypes and related biological signatures in osteosarcoma
We conducted a consensus clustering analysis based on 105 ceramide metabolism-related genes within the osteosarcoma cases from the TARGET database, aiming to explore the connection between ceramide metabolism and osteosarcoma. The analysis revealed three distinct clusters (k = 3), based on the consensus matrix (Figs. 1A-B), changes in the area under cumulative distribution function (CDF) curve (Fig. 1C), and the tendency in CDF curve (Fig. 1D). Accordingly, the entire cohort was stratified into three clusters. Survival analysis further demonstrated a significant difference in prognosis within 3 clusters, patients in cluster B exhibiting poorer outcomes than those in cluster C (P = 0.036) (Fig. 1E). The clustered heatmap demonstrated the relationship between ceramide metabolism-related gene expression and various clinical characteristics, such as sex, age, metastatic status, and primary tumor site. However, no significant differences were observed among the three clusters in terms of clinical characteristics, indicating a similarity in these aspects among the three clusters (Fig. 1F). Then, we conducted the Gene Set Variation Analysis (GSVA) and corresponding differential analysis among the three clusters (Fig. 1G). In Cluster A, there were significant changes in various metabolic pathways, including fatty acid metabolism and GABA metabolism, as well as alterations in ligand-receptor-mediated signaling pathways (Fig. 1H). In Cluster B, characterized by a poorer prognosis, two B-cell receptor (BCR)-related genes were significantly downregulated. However, in Cluster C, which has a better prognosis, the CD22-mediated BCR regulation pathway showed a significant upregulation (Fig. 1I-J). These findings suggest that ceramide metabolism plays an important role in osteosarcoma and has a potential impact on immune cell infiltration.
Fig. 1.
Clustering based on ceramide metabolism-related genes in osteosarcoma and corresponding biological signatures.
(A) Consensus clustering matrix of the TARGET-OS cohort for K = 3. (B) Sample distribution tracking plot with K values from 2 to 10. (C) The relative change in area under the CDF curve. (D) Consensus clustering CDF curve for K values ranging from 2 to 10. (E) Kaplan‒Meier survival analysis of the three clusters. (F) Heatmap showing the relationship between ceramide metabolism-related gene expression and clinical characteristics. (G) Heatmap presenting GSVA analysis of three clusters. (H-J) Top 10 upregulated and downregulated pathways in three clusters.
Construction of a prognostic risk score model for osteosarcoma based on ceramide metabolism-related genes and survival analysis
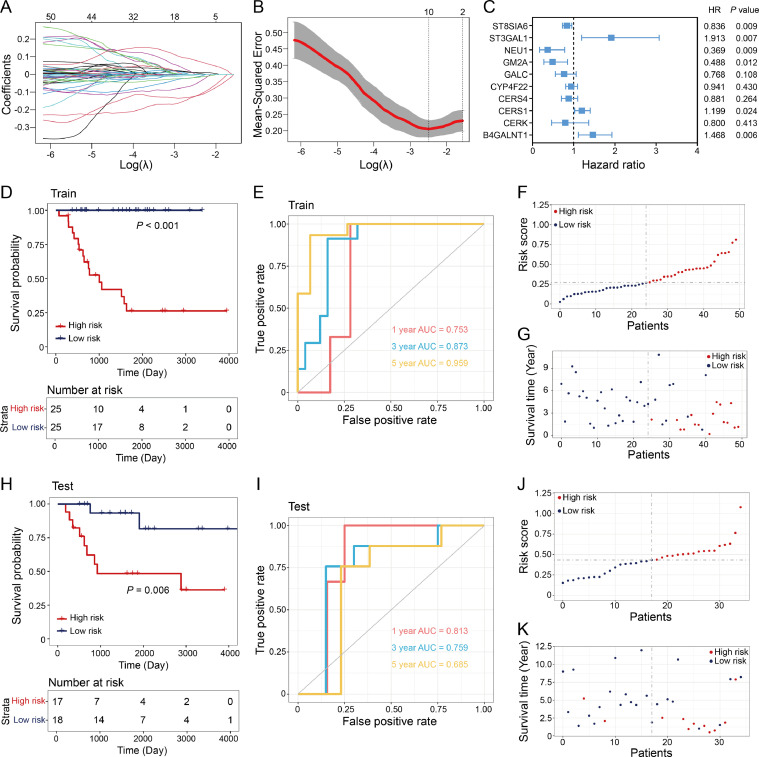
To further understand the role of ceramide metabolism in osteosarcoma progression and clinical outcomes. We randomly divided the TARGET osteosarcoma cohort into a training group (n = 50) and a test group (n = 35). We applied least absolute shrinkage and selection operator (LASSO) penalized Cox regression analysis to identify potential prognosis-related ceramide metabolism-related genes (Fig. 2A, B). The selection of the penalty parameter was based on the minimum criterion. Subsequently, we identified ten noteworthy prognosis-related genes (ST8SIA6, ST3GAL1, NEU1, GM2A, GALC, CYP4F22, CERS4, CERS1, CERK, and B4GALNT1). These genes were employed to construct a prognostic risk scoring model, with ST3GAL1 exhibiting the highest hazard ratio of 1.913 (P = 0.007) (Fig. 2C). In the training group, patients were stratified into high-risk and low-risk groups based on the median risk score. Kaplan‒Meier survival analysis revealed a notably shorter overall survival time in the high-risk group compared to low-risk group (Fig. 2D). The prognosis model exhibited high sensitivity and specificity, with an area under the receiver operating characteristic (ROC) curve of 0.753 for 1-year survival prediction, 0.873 for 3-year survival prediction, and 0.959 for 5-year survival prediction (Fig. 2E). The risk score and survival status clearly indicated that the high-risk group exhibited lower survival rates and shorter survival times compared to low-risk group (Fig. 2F-G). Furthermore, an independent test group was used for validation, and similar results were observed using the same prediction model (Fig. 2H–K).
Fig. 2.
Construction of a prognostic risk score model for osteosarcoma.
(A) Coefficient profiling of ceramide metabolism-related genes. (B) Identification of the optimal parameter (lambda) in LASSO. (C) Cox analysis of identified 10 genes in LASSO regression to construct the risk model. (D) Kaplan–Meier analysis of overall survival in training group. (E) ROC analysis of the risk model for predicting the 1, 3, and 5-year overall survival in training cohort. (F-G) Distribution plots of the risk score (F) and survival time (G) in the training group. (H) Kaplan–Meier analysis of overall survival in testing group. (I) ROC analysis of the risk model for predicting the 1, 3, and 5-year overall survival in testing cohort. (J-K) Distribution plots of the risk score (J) and survival time (K) in the testing group.
Then we performed survival analysis for the genes (P < 0.05) in Cox regression analysis. Kaplan–Meier analysis showed that the expression of ST8SIA6, NEU1 and GM2A had a positive impact on patient prognosis, as the high-expression group exhibited a more favorable prognosis (Fig. 3A–C). The high-expression group in CERS1, ST3GAL1 and B4GALNT1 exhibited a poor prognosis in osteosarcoma cohort while the hazard ratio of those genes was greater than 1 (Fig. 3D–F). We further investigated the expression of CERS1, ST3GAL1, and B4GALNT1 in osteosarcoma samples by quantitative reverse transcription PCR (qRT‒PCR) and immunoblotting. Results revealed that the mRNA and protein levels of CERS1, ST3GAL1, and B4GALNT1 were significantly increased in tumor samples (Fig. 3G-H). These results demonstrated that the risk scoring model based on ceramide metabolism-related genes can be used for predicting the prognosis of osteosarcoma patients. Additionally, we found CERS1, ST3GAL1, and B4GALNT1 were upregulated in osteosarcoma.
Fig. 3.
Survival analysis of identified genes in Cox analysis.
(A-F) Kaplan–Meier survival analysis (left) and hazard ratio (right) plot of ST8SIA6 (A), NEU1 (B), GM2A (C), CERS1 (D), ST3GAL1 (E) and B4GALNT1 (F). (G) CERS1, B4GALNT1 and ST3GAL1 mRNA expression measured in 10 matched pairs of osteosarcoma and adjacent normal tissues by qRT‒PCR. (H) CERS1, B4GALNT1 and ST3GAL1 protein levels measured in 5 matched pairs of osteosarcoma and adjacent normal tissues. *P < 0.05 and **P < 0.01, by Student's t-test (G).
The biological signatures and immune landscape of ceramide metabolism-related risk groups
We performed GSVA and conducted corresponding differential expression analysis on each risk group to further investigate the biological characteristics of osteosarcoma patients within these distinct risk groups. In the low-risk group, we observed upregulation in B cell chemotaxis and myeloid dendritic cell chemotaxis pathway (Fig. 4A). Additionally, various glycosaminoglycan metabolism and nucleotide synthesis-related metabolic pathways showed significant alterations (Fig. 4B). We performed single-sample Gene Set Enrichment Analysis (ssGSEA) to calculate the signature scores of ceramide metabolic pathways and 15 immune-related pathways [30] for each sample. Additionally, we conducted correlation analysis among these pathways. Results demonstrated a significant association between ceramide metabolism and multiple immune-related pathways (Fig. 4C). Then, we compared the immune pathway scores between the high-risk and low-risk groups, and results showed significant differences in immune-related pathways, including cytokine receptors, TNF family members, and TNF family member receptor pathways (P < 0.05) (Fig. 4D). CIBERSORT [31] was performed to assess the composition of immune cells in each sample. This approach enabled us to delineate the tumor immune microenvironment (TIME) of the TARGET osteosarcoma cohort (Fig. 4E). The results indicated that, the low-risk score group exhibited higher levels of CD4 T cells and monocytes, whereas in the high-risk group, there was an elevation in the levels of gamma delta T cells (Fig. 4F). Additionally, differential expression analysis of the immune checkpoint genes showed that in the low-risk group, the expression of CD160, CTLA4, TIGIT, TNFSF14, and VTCN1 was significantly higher than that in the high-risk group (Fig. 4G). These results demonstrated an association between ceramide metabolism and immune regulation in osteosarcoma and suggested its potential impact on the tumor immune microenvironment.
Fig. 4.
Signatures and immune landscape of ceramide metabolism-related risk groups
(A-B) Heatmaps showing the functional ontology (A) and KEGG (B) signatures between different risk groups. (C) The heatmap presenting the correlation (left) and P value (right) between signature score of ceramide metabolic pathway and immune-related pathways. (D) Signature scores of immune-related pathways between different risk groups. (E) Immune cell fraction analysis by CIBERSORT. (F) Signature score of different immune cells between different risk groups. (G) Expression of immune checkpoint genes between the different risk groups.
Immune microenvironment profiling and ceramide metabolism analysis of osteosarcoma tissues
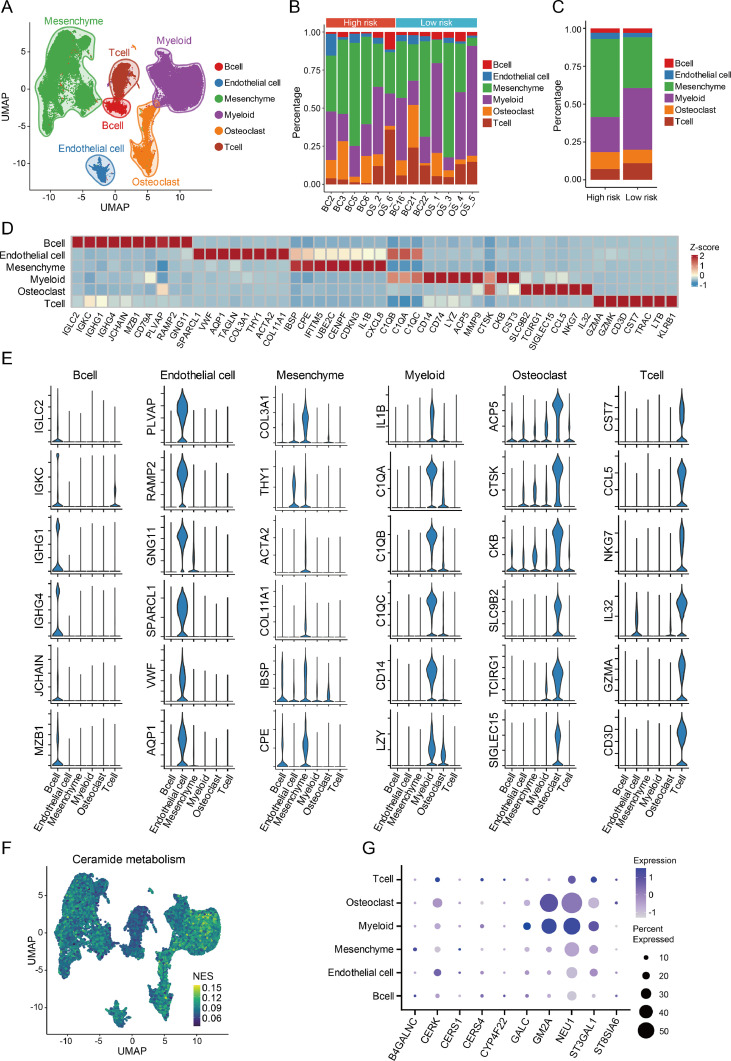
To further investigate the influence of ceramide metabolism on the immune microenvironment in osteosarcoma, single-cell RNA sequencing (scRNA-seq) datasets of primary osteosarcoma tissues in GSE152048 and GSE162454 were included in tumor immune microenvironment analysis. After quality control, a total of 85,057 cells were retained for further analysis. These cells were classified into six cell lineages, including mesenchymal cells, T cells, B cells, myeloid cells, osteoblasts, and endothelial cells (Fig. 5A). The 16 samples were also stratified into high-risk and low-risk groups using the previously mentioned risk-scoring model, and each sample exhibited diverse proportions of the different cell types (Fig. 5B). Compared to the high-risk group, low-risk group samples demonstrated a higher composition of myeloid and T cells and a decreased proportion of mesenchymal cells. (Fig. 5C). Each cell cluster displayed distinct gene expression patterns (Fig. 5D-E). Then, we conducted GSVA analysis focused on ceramide metabolism. Results revealed a significant upregulation of ceramide metabolism in myeloid, osteoblast and mesenchymal cells (Fig. 5F). The genes included in the risk score model exhibited higher expression in myeloid and osteoblastic cells (Fig. 5G).
Fig. 5.
Single-cell landscape and ceramide metabolic process in osteosarcoma.
(A) Clustering of all cell types in osteosarcoma datasets. (B) Cell proportions in different samples. (C) Cell proportions in different risk groups. (D) Heatmap showing expression of different markers in various cell types. (E) Violin plot of representative markers in various cell types. (F) GSVA of ceramide metabolism in different cells. (G) Expression of genes in the risk score model among different cells.
We conducted a more detailed clustering analysis for the myeloid cells and lymphocytes. Myeloid cells were divided into three clusters: tumor associated macrophages (TAMs), monocytes, and proliferative cells. Lymphocytes were further categorized into various subtypes, including regulatory T cells (T-regs), T cells, CD8+ T cells, natural killer T cells (NKTs), B cells, plasma cells, proliferative cells, dendritic cells (DCs), and plasmacytoid dendritic cells (pDCs) (Fig. 6A). There was no significant difference in composition of myeloid cells between the high- and low-risk groups (Fig. 6B). The abundance of plasma cells in the low-risk group was significantly lower than that in the high-risk group (Fig. 6C). Each cluster of cells showed distinct gene expression patterns (Fig. 6D-E). GSVA demonstrated ceramide metabolism was enriched in TAMs cell (Fig. 6F), and the ceramide metabolic score of the low-risk group was significantly higher than that of the high-risk group (Fig. 6G). The findings demonstrated that ceramide metabolism was predominantly centered in macrophages, and ceramide metabolism was significantly upregulated in the low-risk group compared to the high-risk group.
Fig. 6.
Characterization of myeloid cells and lymphocytes
(A) Clustering of myeloid cells, B cells and T cells in osteosarcoma. (B) Cell proportion of myeloid cells. (C) Cell proportions of B and T cells. (D) Heatmap showing the expression of markers in B cells and T cells. (E) Heatmap showing the expression of markers in myeloid cells. (F) GSVA of ceramide metabolism in different risk groups. (G) GSVA score of ceramide metabolism in different risk groups.
Communication of TAMs with lymphocytes
The expression patterns of ceramide metabolic process and changes in immune microenvironment showed that intercellular cross-talk played an essential role in osteosarcoma immune regulation. Consequently, we explored the ligand‒receptor interactions in immune cells using CellChat. Results revealed that the interaction frequency and strength were higher in TAMs and CD8+ T cells. CD8+ T cells received more signals than other cells, whereas TAMs sent a higher number of signals than other cell types, influencing the function of CD8+ T cells (Fig. 7A-B). To find the key gene in the risk model and explore its role in regulating immune microenvironment, we focused on the gene with the highest hazard ratio, ST3GAL1 (ST3 Beta-Galactoside Alpha-2,3-Sialyltransferase 1). ST3GAL1 belongs to the sialyltransferase family that responsible for adding sialic acids to an elongating glycan structure, catalyzing the synthesis of α2,3-sialylated glycans [32]. Sialic acids added to glycans can be recognized by a family of receptors called Siglecs (sialic acid-binding immunoglobulin-type lectins). Sialylated glycans, when engaged with Siglec receptors, can trigger tolerance programs in various immune cells, including macrophages, T cells and NK cells [33]. We further profiled the expression of Siglec receptors in different cell types and observed that Siglecs receptors were predominantly expressed in TAMs, except for Siglec-2, which was primarily expressed in B cells (Fig. 7C). Therefore, the overexpression of ST3GAL1 in osteosarcoma led to an increase in the levels of α2,3-linked sialic acids in tumor microenvironment. The binding of α2,3 sialic acids to the Siglec receptors on TAMs regulated immune microenvironment, promoting the progression of osteosarcoma. We further conducted a more detailed analysis of the interactions between TAMs and various cells (Fig. 7D). TAMs exhibited interactions with various lymphocytes, with an emphasis on interactions with CD8+ T cells. Apart from the interaction between HLA and CD8, other prominent interactions included SPP1-CD44 and LGALS9-CD44 (Figs. 7E-F). These results showed that the interaction between TAMs and CD8+ T cells played an essential role in osteosarcoma.
Fig. 7.
Cell–cell interactions of TAMs and their expression
(A) Network of cell communication; Number of interactions (top); Strength of interactions (bottom). (B) Heatmap showing cell–cell interactions; Number of interactions (left); Strength of interactions (right). (C) Expression of Siglec receptors genes in different cells. (D) Network of cell communication between TAMs and other cells. (E) Bubble heatmap showing different cell-cell interactions between TAMs and other cells. (F) Chord diagram showing multiple cell interactions between TAMs and other cells.
ST3GAL1 associated with TAMs-induced inhibition of CD8+ T cytotoxic function and the clinical significance of ST3GAL1
To further investigate the relationship between ST3GAL1 and TAMs-induced regulation of CD8+ T cells, we conducted a detailed clustering analysis of macrophages (Fig. 8A). We stratified the samples into ST3GAL1 high-expression and ST3GAL1 low-expression groups. Then, we compared the composition of macrophages in the different ST3GAL1 expression groups. Results indicated that the proportion of M2 macrophages was significantly higher in the ST3GAL1 high-expression group (Fig. 8B-C), and there was also an increase in the expression of markers associated with M2 macrophages (Fig. 8D-E). Next, diffusion maps were used to analyze the differentiation trajectories of macrophages. Our analysis revealed that M1 and M2 macrophages in osteosarcoma exhibited clear and distinct differentiation pathways (Fig. 8F). We further conducted a comparison of intercellular communication between the high and low ST3GAL1 expression groups. The results indicated that macrophages in the ST3GAL1 high-expression group emitted a higher number of signals and more robust signals to CD8+ T cells (Fig. 8G). Among these cell interactions, there were significant alterations in the SPP1-CD44 and NECTIN2-TIGIT interactions, demonstrating the potential impact of ST3GAL1 expression on the communication between macrophages and CD8+ T cells (Fig. 8H). Furthermore, we conducted an analysis of the expression of regulatory cytokines IL-10 and IL-6. The results revealed that M2 macrophages expressed higher levels of IL-10, and the expression of IL-10 was significantly upregulated in the ST3GAL1 high-expression group (Fig. 8I-K). We also examined the pathways upregulated in the high ST3GAL1 expression group, and found that the SPP1-CD44 pathway was significantly upregulated and played an essential role in the interactions between macrophages and CD8+ T cells (Fig. 8L).
Fig. 8.
Cell communication in the ST3GAL1-associated groups and clinical significance of ST3GAL1
(A) Clustering of TAMs in osteosarcoma. (B) Cell proportion of TAMs in different samples. (C) Cell proportion of TAMs in different ST3GAL1 expression groups. (D) Feature plots of marker genes in M1 and M2 macrophages (CD80, CD86, CD206 and CD163). (E) Dot plot of the expression of marker genes in M1 and M2 macrophages. (F) Analysis of the differentiation of macrophages towards M1 and M2 using diffusion map. (G) Heatmap of cell–cell interactions between TAMs and CD8+ T cells. (H) Differential expression of interactions between TAMs and CD8+ T cells. (I) Feature plots showing expression of regulatory cytokines genes (IL10 and IL6). (J) Expression of IL10 and IL6 in TAMs. (K) Expression of IL10 and IL6 in different ST3GAL1 expression groups. (L) Chord diagram showing upregulated interaction between TAMs and CD8+ T cells in high-ST3GAL1 expression group. (M) Correlation between the expression of ST3GAL1, SIGLEC1, CD33, SIGLEC7 and SPP1, IL10, IL6. (N) Representative images showing high or low expression of ST3GAL1 IHC in osteosarcoma cohort; Scale bars: 50 μm (left), 25 μm (right). (O) Overall survival (left) and lung metastasis-free survival (right) of patients according to the expression levels of ST3GAL1.
Next, we explored the association between related genes and prognosis in the TARGET osteosarcoma cohort. Results exhibited that the expression of ST3GAL1 and Siglec receptors SIGLEC1, CD33 and SIGLEC7 was correlated with SPP1 expression. Furthermore, SIGLEC1, CD33, and SIGLEC7 expression was also correlated with the expression of IL-10 (Fig. 8M). To further assess the clinical significance of ST3GAL1, we conducted immunohistochemistry (IHC) staining on osteosarcoma samples of 74 patients from the First Affiliated Hospital of Sun Yat-sen University (Fig. 8N). Patients were categorized based on their ST3GAL1 expression, and survival analysis was performed. Results demonstrated that patients with high ST3GAL1 expression had a poorer overall survival (P = 0.001) and poorer lung metastasis-free survival (P = 0.005) (Fig. 8O). These findings suggested that ST3GAL1 promoted osteosarcoma progression by regulating the SPP1-CD44 interaction of macrophages with CD8+ T cells through α2,3-linked sialic acids and inhibiting CD8+ T cell function by IL-10. ST3GAL1 expression correlated with the prognosis of osteosarcoma patients.
ST3GAL1 in osteosarcoma cells influenced TAMs differentiation and cytokine secretion
To further investigate the role of ST3GAL1 in TAMs differentiation, we initially overexpressed ST3GAL1 (ST3GAL1-OE) in the 143B osteosarcoma cell line, resulting in a significant increase in α2,3-sialylated glycans (Fig. 9A–C). Next, we applied a co-culture system, where ST3GAL1-OE 143B cells were cultured with THP-1-derived macrophages for 48 h. TAMs were subsequently analyzed for CD206 and CD86 expression using flow cytometry (Fig. 9D). Results showed overexpression of ST3GAL1 facilitated the polarization of CD206+ M2-like macrophage and had no significant effect on differentiation to CD86+ macrophages (Fig. 9E-F). Moreover, overexpression of ST3GAL1 increased the mRNA level of arginase-1 (ARG1) while had no significant effect on the expression of inducible nitric oxide synthase (iNOS) in TAMs (Fig. 9G). We also measured cytokine secretion of IL-6 and IL-10 using ELISA, revealing that ST3GAL1 overexpression significantly increased the expression of IL-10 and IL-6 (Fig. 9H). These findings suggested that ST3GAL1 in osteosarcoma cells promoted M2-like macrophage polarization through α2,3-sialylated glycans secretion and these TAMs secreted higher level of IL-6 and IL-10.
Fig. 9.
ST3GAL1 influenced TAMs differentiation and cytokine secretion.
(A) Overexpression of ST3GAL1 in 143B cells. (B and C) Expression of intracellular (B) and extracellular (C) α2,3-sialylated glycans in 143B cell evaluated by ELISA. (D) Co-culture of ST3GAL1-OE 143B cells with macrophages derived from THP-1. (E) Surface expression of CD206 (top) and CD86 (bottom) in co-cultured TAMs analyzed by flow cytometry. (F) Percentages of CD206+ M2-like macrophages and CD86+ M1-like macrophages in co-cultured TAMs using flow cytometry. (G) Relative expression of ARG1 (top) and iNOS (bottom) mRNA analyzed using qRT-PCR. (H) Expression of IL-10 (top) and IL-6 (bottom) evaluated by ELISA. Data are presented as the mean ± SD, ns not significant *P < 0.05, **P < 0.01, and ***P <0.001, ****P < 0.0001, by Students’ t-test (B, C, F, G and H).
Discussion
Accumulating evidence indicates that ceramide metabolism is dysregulated in multiple cancers and plays an essential role in tumor progression [34], [35], [36]. Ceramide is a crucial signaling molecules in tumors, influencing pathways such as apoptosis, proliferation, cell migration, aging, and inflammation [37,38]. In lung cancer, ceramide synthase 1 (CERS1) is involved in mitochondrial ceramide generation and mitophagy-mediated cell death [39]. In glioblastoma, accumulation of ceramides and their hexosylmetabolites contributes to endoplasmic reticulum stress and secondary autophagy [40]. Although existing studies have reviewed the involvement of ceramide metabolism in osteosarcoma chemotherapy [41], [42], [43], a comprehensive analysis of key molecules within the ceramide metabolism pathway is still pending. In this study, we found a novel prognostic signature in ceramide metabolism pathway for osteosarcoma and identified the essential genes associated with clinical outcomes. CERS1, ST3GAL1 and B4GALNT1 were found upregulated in osteosarcoma and associated with a poor prognosis in osteosarcoma patients.
Emerging research on the tumor immune microenvironment has demonstrated the significance of immunotherapy in the treatment of multiple cancers and shown that ceramide metabolism is associated with tumor immune microenvironment [36,44,45]. In hepatocellular carcinoma, ceramide has the potential to impede the tolerance of CD8+ T cells while enhancing the immune response to tumor antigen stimulation [46]. In melanoma, the accumulation of ceramide induced by neutral sphingomyelinase 2 (SMPD3) was associated with tumor-infiltrating CD8+ lymphocytes and increased levels of IFNγ and CXCL9 [47]. In our study, we found that ceramide metabolism was associated with BCR signaling, myeloid DC chemotaxis and many other immune-related pathways. The composition of immune cells varies among different ceramide metabolism-related groups, furthermore, ceramide metabolism also influence the expression of immune checkpoint genes.
Macrophages serve as critical regulators of tumor immune homeostasis and are abundant within the tumor microenvironment. TAMs exhibit M1/M2 macrophage polarization, playing an essential role in immune microenvironment through various signaling pathways [48,49]. In breast cancer, a subset of FOLR2+macrophages efficiently activated CD8+ T cells, enhancing their cytotoxic function, and played a crucial antitumorigenic role in tumor immune microenvironment [50]. Shi et.al. reported that O-GlcNAc transferase in TAMs promoted cancer metastasis and chemoresistance through secretion of mature cathepsin B in melanoma [51]. In our study, we observed that ceramide metabolism was primarily enriched in myeloid, osteoblast, and mesenchymal cells. TAMs and CD8+ T cells play a pivotal role in cell communication within the osteosarcoma microenvironment. Our research connected ceramide metabolism with tumor immune microenvironment, offering a new perspective for understanding the role of ceramide metabolism in osteosarcoma progression.
ST3GAL1 catalyzes the addition of sialic acids to a glycan structure, responsible for the synthesis of α2,3-sialylated glycans. Sialic acids can be recognized by Siglecs receptors, which can trigger tolerance programs in various immune cells, including macrophages, T cells and NK cells [33,36]. In immunotherapy, ST3GAL1 functions as a negative regulator of the cancer-specific migration of CAR-T cells, and CD18 serves as a primary effector of ST3GAL1 in activated CD8+ T cells [52]. In pancreatic ductal adenocarcinoma, ST3GAL1 and ST3GAL4 serve as primary contributors to the synthesis of α2,3 and α2,6 sialic acids, which trigger Siglec-7 and Siglec-9 receptors in macrophages. Activation of Siglec-9 in macrophages leads to a reduction in inflammatory programs, while increasing the expression of PD-L1 and IL-10, ultimately facilitating tumor progression [29]. In our study, we demonstrated that the expression of ST3GAL1 influenced the cell composition of TAMs and was associated with the upregulated inhibitory signaling of SPP1-CD44 and NECTIN2-TIGIT in the interaction between TAMs and CD8+ T cells. The IL-10 and IL-6 expression was also increased in ST3GAL1 high-expression group. IHC and survival analysis in our osteosarcoma cohort demonstrated that ST3GAL1 was associated with poor clinical outcomes. Furthermore, co-culture assays revealed that upregulation of ST3GAL1 in tumor cells promoted the polarization of M2-like macrophage, associated with α2,3-sialylated glycans secretion. These TAMs produced higher levels of IL-6 and IL-10, influencing the tumor immune microenvironment in osteosarcoma. Given that ST3GAL1 is the vital gene with highest hazard ratio and could be a potential target for osteosarcoma treatment, further exploration of inhibitors would be of great significance.
Conclusion
Collectively, we found that the ceramide metabolism was associated with prognosis in osteosarcoma. A signature containing ten essential genes was included in risk score model and CERS1, ST3GAL1 and B4GALNT1 were found correlated with a poor prognosis in osteosarcoma patients. Ceramide metabolism was found associated with tumor immune environment and the expression of immune checkpoint genes. Single-cell analysis demonstrated ST3GAL1 associated with TAMs-induced inhibition of CD8+ T cytotoxic function through α2,3 sialic acids. ST3GAL1 upregulation in tumor cells facilitated the M2-like macrophage polarization and promoted TAMs to produce higher levels of IL-6 and IL-10, thereby influencing the tumor immune microenvironment in osteosarcoma. These findings provided a novel insight into the importance of ST3GAL1 and α2,3 linked sialic acids in tumor immune microenvironment.
Funding
This work was funded by the National Natural Science Foundation of China (Grants 82273357, 81972510 and 81772864).
CRediT authorship contribution statement
Yutong Zou: Investigation, Validation, Writing – original draft. Siyao Guo: Methodology, Visualization, Writing – review & editing. Yan Liao: Data curation. Weidong Chen: Formal analysis. Ziyun Chen: Resources. Junkai Chen: Software. Lili Wen: Supervision, Writing – review & editing. Xianbiao Xie: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101840.
Contributor Information
Lili Wen, Email: wenll@sysucc.org.cn.
Xianbiao Xie, Email: xiexbiao@mail.sysu.edu.cn.
Appendix. Supplementary materials
Reference
- 1.Whelan J.S., Davis L.E. Osteosarcoma, chondrosarcoma, and chordoma. J. Clin. Oncol. 2018;36(2):188–193. doi: 10.1200/JCO.2017.75.1743. [DOI] [PubMed] [Google Scholar]
- 2.Daw N.C., Chou A.J., Jaffe N., Rao B.N., Billups C.A., Rodriguez-Galindo C., et al. Recurrent osteosarcoma with a single pulmonary metastasis: a multi-institutional review. Br. J. Cancer. 2015;112(2):278–282. doi: 10.1038/bjc.2014.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crompton J.G., Ogura K., Bernthal N.M., Kawai A., Eilber F.C. Local control of soft tissue and bone sarcomas. J. Clin. Oncol. 2018;36(2):111–117. doi: 10.1200/JCO.2017.75.2717. [DOI] [PubMed] [Google Scholar]
- 4.Gill J., Gorlick R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 2021;18(10):609–624. doi: 10.1038/s41571-021-00519-8. [DOI] [PubMed] [Google Scholar]
- 5.Harrison D.J., Geller D.S., Gill J.D., Lewis V.O., Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018;18(1):39–50. doi: 10.1080/14737140.2018.1413939. [DOI] [PubMed] [Google Scholar]
- 6.Ritter J., Bielack S.S. Osteosarcoma. Ann. Oncol. 2010;21(Suppl 7):VII320–VII325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer P.S., Helman L.J. New horizons in the treatment of osteosarcoma. N. Engl. J. Med. 2021;385(22):2066–2076. doi: 10.1056/NEJMra2103423. [DOI] [PubMed] [Google Scholar]
- 8.Smith R.L., Soeters M.R., Wüst R.C.I., Houtkooper R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018;39(4):489–517. doi: 10.1210/er.2017-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon J.Y., Zolnik C.P., Wang Z., Qiu Y., Usyk M., Wang T., et al. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. EBioMedicine. 2018;37:392–400. doi: 10.1016/j.ebiom.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronin S.J.F., Seehus C., Weidinger A., Talbot S., Reissig S., Seifert M., et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature. 2018;563(7732):564–568. doi: 10.1038/s41586-018-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra L., Bonetti L., Brenner D. Metabolic modulation of immunity: a new concept in cancer immunotherapy. Cell Rep. 2020;32(1) doi: 10.1016/j.celrep.2020.107848. [DOI] [PubMed] [Google Scholar]
- 12.Kitatani K., Idkowiak-Baldys J., Hannun Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 2008;20(6):1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morad S.A., Cabot M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer. 2013;13(1):51–65. doi: 10.1038/nrc3398. [DOI] [PubMed] [Google Scholar]
- 14.Moro K., Nagahashi M., Gabriel E., Takabe K., Wakai T. Clinical application of ceramide in cancer treatment. Breast Cancer. 2019;26(4):407–415. doi: 10.1007/s12282-019-00953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales A., Lee H., Goñi F.M., Kolesnick R., Fernandez-Checa J.C. Sphingolipids and cell death. Apoptosis. 2007;12(5):923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 16.Jeffries K.A., Krupenko N.I. Ceramide signaling and p53 pathways. Adv. Cancer Res. 2018;140:191–215. doi: 10.1016/bs.acr.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brachtendorf S., El-Hindi K., Grösch S. Ceramide synthases in cancer therapy and chemoresistance. Prog. Lipid Res. 2019;74:160–185. doi: 10.1016/j.plipres.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Kurz J., Parnham M.J., Geisslinger G., Schiffmann S. Ceramides as novel disease biomarkers. Trends Mol. Med. 2019;25(1):20–32. doi: 10.1016/j.molmed.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Stoffel B., Bauer P., Nix M., Deres K., Stoffel W. Ceramide-independent CD28 and TCR signaling but reduced IL-2 secretion in T cells of acid sphingomyelinase-deficient mice. Eur. J. Immunol. 1998;28(3):874–880. doi: 10.1002/(SICI)1521-4141(199803)28:03<874::AID-IMMU874>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Wilkerson M.D., Hayes D.N. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26(12):1572–1573. doi: 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hänzelmann S., Castelo R., Guinney J. GSVA: gene set variation analysis for microarray and RNA-Seq data. BMC Bioinf. 2013;14(1):7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng D., Ye Z., Shen R., Yu G., Wu J., Xiong Y., et al. IOBR: multi-omics immuno-oncology biological research to decode tumor microenvironment and signatures. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.687975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman J.H., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 25.McGinnis C.S., Murrow L.M., Gartner Z.J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 2019;8(4) doi: 10.1016/j.cels.2019.03.003. 329-337.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korsunsky I., Millard N., Fan J., Slowikowski K., Zhang F., Wei K., et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods. 2019;16(12):1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin S., Guerrero-Juarez C.F., Zhang L., Chang I., Ramos R., Kuan C.-H., et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021;12(1):1088. doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Street K., Risso D., Fletcher R.B., Das D., Ngai J., Yosef N., et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics. 2018;19(1):477. doi: 10.1186/s12864-018-4772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez E., Boelaars K., Brown K., Eveline Li RJ, Kruijssen L., Bruijns S.C.M., et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat. Commun. 2021;12(1):1270. doi: 10.1038/s41467-021-21550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Jiang T., Zhou W., Li J., Li X., Wang Q., et al. Pan-cancer characterization of immune-related lncRNAs identifies potential oncogenic biomarkers. Nat. Commun. 2020;11(1):1000. doi: 10.1038/s41467-020-14802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dall’Olio F., Malagolini N., Trinchera M., Chiricolo M. Sialosignaling: sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta. 2014;1840(9):2752–2764. doi: 10.1016/j.bbagen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Lübbers J., Rodríguez E., van Kooyk Y. Modulation of immune tolerance via Siglec-Sialic acid interactions. Front. Immunol. 2018;9:2807. doi: 10.3389/fimmu.2018.02807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laviad E.L., Kelly S., Merrill A.H., Jr., Futerman A.H. Modulation of ceramide synthase activity via dimerization. J. Biol. Chem. 2012;287(25):21025–21033. doi: 10.1074/jbc.M112.363580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reza S., Ugorski M., Suchański J. Glucosylceramide and galactosylceramide, small glycosphingolipids with significant impact on health and disease. Glycobiology. 2021;31(11):1416–1434. doi: 10.1093/glycob/cwab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tallima H., Azzazy H.M.E., El Ridi R. Cell surface sphingomyelin: key role in cancer initiation, progression, and immune evasion. Lipids Health Dis. 2021;20(1):150. doi: 10.1186/s12944-021-01581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullen T.D., Obeid L.M. Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med. Chem. 2012;12(4):340–363. doi: 10.2174/187152012800228661. [DOI] [PubMed] [Google Scholar]
- 38.Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer. 2018;18(1):33–50. doi: 10.1038/nrc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oleinik N., Kim J., Roth B.M., Selvam S.P., Gooz M., Johnson R.H., et al. Mitochondrial protein import is regulated by p17/PERMIT to mediate lipid metabolism and cellular stress. Sci. Adv. 2019;5(9):eaax1978. doi: 10.1126/sciadv.aax1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer N., Henkel L., Linder B., Zielke S., Tascher G., Trautmann S., et al. Autophagy activation, lipotoxicity and lysosomal membrane permeabilization synergize to promote pimozide- and loperamide-induced glioma cell death. Autophagy. 2021;17(11):3424–3443. doi: 10.1080/15548627.2021.1874208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu X., Du X., Deng X., Yi H., Cui S., Liu W., et al. C6 ceramide sensitizes pemetrexed-induced apoptosis and cytotoxicity in osteosarcoma cells. Biochem. Biophys. Res. Commun. 2014;452(1):72–78. doi: 10.1016/j.bbrc.2014.08.065. [DOI] [PubMed] [Google Scholar]
- 42.Yao C., Wu S., Li D., Ding H., Wang Z., Yang Y., et al. Co-administration phenoxodiol with doxorubicin synergistically inhibit the activity of sphingosine kinase-1 (SphK1), a potential oncogene of osteosarcoma, to suppress osteosarcoma cell growth both in vivo and in vitro. Mol. Oncol. 2012;6(4):392–404. doi: 10.1016/j.molonc.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhule S.S., Penfornis P., He J., Harris M.R., Terry T., John V., et al. The combined effect of encapsulating curcumin and C6 ceramide in liposomal nanoparticles against osteosarcoma. Mol. Pharm. 2014;11(2):417–427. doi: 10.1021/mp400366r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schenkel J.M., Pauken K.E. Localization, tissue biology and T cell state - implications for cancer immunotherapy. Nat. Rev. Immunol. 2023 doi: 10.1038/s41577-023-00884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Combes A.J., Samad B., Krummel M.F. Defining and using immune archetypes to classify and treat cancer. Nat. Rev. Cancer. 2023;23(7):491–505. doi: 10.1038/s41568-023-00578-2. [DOI] [PubMed] [Google Scholar]
- 46.Li G., Liu D., Kimchi E.T., Kaifi J.T., Qi X., Manjunath Y., et al. Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology. 2018;154(4) doi: 10.1053/j.gastro.2017.10.050. 1024-36.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montfort A., Bertrand F., Rochotte J., Gilhodes J., Filleron T., Milhès J., et al. Neutral sphingomyelinase 2 heightens anti-melanoma immune responses and anti-PD-1 therapy efficacy. Cancer Immunol. Res. 2021;9(5):568–582. doi: 10.1158/2326-6066.CIR-20-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bied M., Ho W.W., Ginhoux F., Blériot C. Roles of macrophages in tumor development: a spatiotemporal perspective. Cell. Mol. Immunol. 2023;20(9):983–992. doi: 10.1038/s41423-023-01061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen S., Saeed A., Liu Q., Jiang Q., Xu H., Xiao G.G., et al. Macrophages in immunoregulation and therapeutics. Signal Trans. Targeted Ther. 2023;8(1):207. doi: 10.1038/s41392-023-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nalio Ramos R., Missolo-Koussou Y., Gerber-Ferder Y., Bromley C.P., Bugatti M., Núñez N.G., et al. Tissue-resident FOLR2(+) macrophages associate with CD8(+) T cell infiltration in human breast cancer. Cell. 2022;185(7) doi: 10.1016/j.cell.2022.02.021. 1189-207.e25. [DOI] [PubMed] [Google Scholar]
- 51.Shi Q., Shen Q., Liu Y., Shi Y., Huang W., Wang X., et al. Increased glucose metabolism in TAMs fuels O-GlcNAcylation of lysosomal Cathepsin B to promote cancer metastasis and chemoresistance. Cancer Cell. 2022;40(10) doi: 10.1016/j.ccell.2022.08.012. 1207-22.e10. [DOI] [PubMed] [Google Scholar]
- 52.Hong Y., Walling B.L., Kim H.R., Serratelli W.S., Lozada J.R., Sailer C.J., et al. ST3GAL1 and βII-spectrin pathways control CAR T cell migration to target tumors. Nat. Immunol. 2023;24(6):1007–1019. doi: 10.1038/s41590-023-01498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.