Abstract
Surgical resection of brain tumors is challenging because of the delicate balance between maximizing tumor removal and preserving vital brain functions. Functional MRI (fMRI) offers noninvasive preoperative mapping of widely distributed brain areas and is increasingly used in presurgical functional mapping. However, its impact on survival and functional outcomes is still not well-supported by evidence. Task-based fMRI (tb-fMRI) maps blood oxygen level–dependent (BOLD) signal changes during specific tasks, while resting-state fMRI (rs-fMRI) examines spontaneous brain activity. rs-fMRI may be useful for patients who cannot perform tasks, but its reliability is affected by tumor-induced changes, challenges in data processing, and noise. Validation studies comparing fMRI with direct cortical stimulation (DCS) show variable concordance, particularly for cognitive functions such as language; however, concordance for tb-fMRI is generally greater than that for rs-fMRI. Preoperative fMRI, in combination with MRI tractography and intraoperative DCS, may result in improved survival and extent of resection and reduced functional deficits. fMRI has the potential to guide surgical planning and help identify targets for intraoperative mapping, but there is currently limited prospective evidence of its impact on patient outcomes. This review describes the current state of fMRI for preoperative assessment in patients undergoing brain tumor resection.
Keywords: MR–Functional Imaging, CNS, Brain/Brain Stem, Anatomy, Oncology, Functional MRI, Functional Anatomy, Task-based, Resting State, Surgical Planning, Brain Tumor
© RSNA, 2023
Keywords: MR–Functional Imaging, CNS, Brain/Brain Stem, Anatomy, Oncology, Functional MRI, Functional Anatomy, Task-based, Resting State, Surgical Planning, Brain Tumor
Summary
This review highlights the current state of functional MRI for preoperative assessment in patients undergoing surgical intervention for brain tumors, with a brief pictorial review of functional anatomy.
Essentials
■ Mapping of blood oxygen level–dependent, or BOLD, signal changes during the performance of a particular task is referred to as task-based functional MRI (fMRI), and mapping of these signal changes at rest is referred to as resting-state fMRI.
■ Studies demonstrate a wide range of concordance between preoperative fMRI and direct cortical stimulation (DCS), particularly for higher cognitive functions such as language, in patients undergoing tumor resection.
■ Advances in fMRI algorithms may be useful adjuncts to intraoperative DCS, but it remains to be determined whether these modalities meaningfully impact glioma surgery independently.
Introduction
The central nervous system is the most common tumor site for persons aged 0–14 years, second most common in ages 15–39 years, and seventh most common in persons older than 40 years. Across all ages, astrocytomas account for more than half of malignant primary brain tumors, and their infiltrative nature presents a major challenge to safe and complete surgical resection. Malignant primary brain tumors commonly occur in the frontal (24.6%), temporal (17.6%), and parietal (10.4%) lobes, which frequently places them in or near eloquent brain regions (1). While surgical resection is a mainstay of treatment for many brain tumors, operating within the brain presents multiple challenges because of a delicate trade-off between maximizing tumor resection and minimizing the loss of vital cerebral functions. The risk of recurrence is greatly influenced by the extent of cytoreduction, with one study demonstrating improved patient survival with supramarginal resection of gliomas beyond conventional imaging-positive tumor boundaries (2). Meanwhile, quality of life is highly dependent on the sparing of functionally relevant brain regions, such as those involved in sensorimotor, vision, language, and other cognitive functions. The paradox of maximal safe resection that limits the impact of functional deficits, known as oncofunctional balance, has led to an increase in awake surgery with intraoperative brain mapping. Recently, these intraoperative mapping approaches have been greatly aided by noninvasive functional neuroimaging.
To ensure maximal safe resection, neurosurgeons have used a variety of techniques, including intraoperative imaging, direct cortical stimulation (DCS), transcranial magnetic stimulation, magnetoencephalography, functional MRI (fMRI), and MR tractography. Furthermore, there is growing interest in using three-dimensional printing, cinematic rendering models, and real-time augmented reality applications derived from preoperative MRI data for surgical planning (3). Intraoperative DCS is currently considered the reference standard technique for functional mapping of the brain (4). Nevertheless, there are several limitations of DCS. First, it can only be used intraoperatively, preventing detailed preplanning of the intervention. Second, electrical current spread to more distant areas may falsely attribute function to the area stimulated and can potentially trigger seizures. Once a seizure has occurred, further intraoperative stimulation is limited, which highlights the value of tools that minimize the amount of stimulation testing needed. Last, the cessation of function at DCS does not entirely predict a lasting clinical deficit after resection.
Over the past 20 years, fMRI has become a valuable tool for noninvasive mapping of brain function, with the goal of guiding surgical resections to reduce complications (5,6). In contrast to the established standard DCS brain mapping techniques, fMRI can be performed preoperatively to inform surgical decision-making, is noninvasive, and allows mapping of widely distributed brain areas beyond those limited areas exposed by the craniotomy (7). These functional data, in theory, could improve surgical planning and extent of surgical resection, broaden surgical indications for lesions in classically eloquent areas, and reduce intraoperative duration, which may ultimately improve functional outcome and tumor control (6,8). However, despite its widespread use, there is currently a limited level of evidence to support the impact of fMRI on survival and functional outcomes.
In this review, we focus on the current state of fMRI for preoperative assessment in patients undergoing surgical intervention for brain tumors.
Overview of fMRI Technique
fMRI uses blood oxygen level–dependent (BOLD) signal, which relies on the deoxyhemoglobin-to-oxyhemoglobin ratio. On activation in a region of brain, there is consequent increased local perfusion as a result of capillary vasodilation. Perfusion increase exceeds the metabolic demands of the active brain region. As a result, the increasing level of oxyhemoglobin relative to deoxyhemoglobin increases the T2* signal, which can be measured using an MRI sequence sensitive to the T2* change. Importantly, the BOLD signal change serves as a proxy for neuronal activity and is predicated on a predictable vascular response to neuronal activity (neurovascular coupling) (7,9).
Mapping of BOLD signal changes during the performance of a particular task is referred to as task-based fMRI (tb-fMRI). In contrast, resting state fMRI (rs-fMRI) is a more recently described imaging technique that examines spontaneous brain function at rest, in which BOLD images are acquired without the patient performing a task (10). Discrete resting-state brain networks are identified by correlating spontaneously occurring fluctuations of BOLD signal across spatially separated brain regions. The advantage over tb-fMRI is that rs-fMRI does not require compliance in performing tasks and may be more feasible than tb-fMRI in young pediatric patients who cannot cooperate or perform intended tasks, or in patients with language barriers or physical, visual, hearing, or cognitive impairment that would limit task performance. As multiple individual tasks do not need to be performed, rs-fMRI may hypothetically require less time than tb-fMRI to obtain similar network information (11). Despite these advantages, the adoption of single-subject rs-fMRI has been limited in clinical medicine because of several barriers. First, because rs-fMRI is reliant on spontaneous fluctuations in BOLD signal, the effect of tumors on this signal source is not well understood. Additionally, the frequency of these spontaneous fluctuations overlaps with many sources of physiologic noise, making it challenging to distinguish true neuronal-related changes from other signals of no interest; this is not the case with tb-fMRI, where physiologic noise can be easily filtered in most cases. Ultimately, this leads to greater functional signal-to-noise ratio in tb-fMRI. Next, rs-fMRI lacks the specificity of tb-fMRI, as activation patterns are primarily driven by the task and control. This could lead to unexpected results in which synchrony between resting networks is misinterpreted as functionally relevant to a task, such as overlap between language and attentional networks that may lead to miscalculation of language laterality index (see below). Last, while the absence of patient participation during the acquisition of rs-fMRI data may be advantageous to tb-fMRI, the processing and analysis of rs-fMRI is much more complex, and there is currently no reference standard.
Two widely used approaches to analyze rs-fMRI data include seed-based analysis and independent component analysis. Seed-based analysis includes placing regions of interest in selected areas of the brain, extracting a signal time course for the region, and correlating this with the remainder of the brain to determine regions sharing similar signal fluctuations—presumably indicating they belong to the same network. Seed-based analysis is also dependent on a detailed and accurate knowledge of functional neuroanatomy to guide placement of regions of interest to target specific networks, which can be problematic because of the pervasiveness of outdated and incorrect models in clinical medicine (eg, Wernicke–Lichtheim model of language). Anatomic distortion from surgery also makes this approach challenging. While seed placement may be more reliable in assessing motor areas, language mapping can be challenging because language areas are more widely distributed anatomically across individuals. Furthermore, prior surgery or tumor infiltration may lead to reorganization of language function, which is a poorly understood process and can substantially impair the appropriate placement of seed regions (12). Independent component analysis is another common approach, which is a more data-driven method that is not reliant on manual seed placement. In this method, the time series of rs-fMRI data are separated into maximally independent components. These component maps each represent some contributor to the overall BOLD fluctuations and may also represent various sources of noise, signals of no interest (eg, cardiac activity, breathing, etc), or brain networks. Determining which maps represent brain networks can be challenging. One approach is the manual identification of network components by visual inspection, but automated techniques (eg, template matching) have also been proposed. Unfortunately, the accuracy of template-based approaches is substantially affected by neural plasticity, loss of normal function, and anatomic distortion (11).
Technical Challenges in Preoperative Brain Tumor Surgical Planning
fMRI is reliant on the detection of very small BOLD signal changes, often in the range of 1%–5%, which necessitates a sufficient signal-to-noise ratio to detect these changes (9). However, measured fMRI activation can be compromised by many factors, such as patient motion, task performance, pathologically altered neurovascular coupling, or various effects of data processing. Due to the resultant possibility of false-positive and false-negative findings, MRI sequence design and optimization, task design, image quality, and data processing techniques must be carefully addressed (7).
The interpretation of fMRI is also challenged by pathologic changes, such as tumor infiltration, anatomic distortion, edema, and, sometimes, functional reorganization. Tumor infiltration, angiogenesis, and perilesional edema can decrease the BOLD signal in functionally intact tissue, resulting in false-negative results (13). Susceptibility artifacts related to blood products from tumoral hemorrhage can also lead to signal loss and image distortion in the surrounding BOLD signal (14). Additionally, assumptions are made regarding the modeling of the neurovascular response, which can be nonlinearly increased because of tumor-induced neovascularization and produce a ceiling effect on the BOLD signal (15). The chronic mismatch between demand and perfusion can result in loss of local cerebrovascular autoregulation, vasomotor paralysis, and, ultimately, a loss of variability in BOLD signal. Further, in some studies, more than half of patients with brain tumors had a paradoxical change in deoxyhemoglobin level in the lesional hemisphere measured, leading to impaired activation on fMRI maps (16). In patients undergoing resection, false-negative results can be problematic because the assumption of an eloquent region no longer having function could lead to substantial harm (7). For these reasons, fMRI is best suited to provide guidance and help identify targets for intraoperative mapping rather than being relied on solely for preoperative decision-making because of the high incidence of false-negative activation and the possibility of false-positive activation.
In addition to these limitations, one of the other major challenges of using fMRI data intraoperatively stems from the fact that parenchymal shift occurs following craniotomy and durotomy, resulting in errors in registration with preoperative MRI. Last, fMRI is useful for mapping the cortical surface but does not aid with the mapping of subcortical white matter tracts and, thus, is complementary to tractography and subcortical stimulation techniques.
Validation Studies on tb-fMRI
Validating clinical fMRI against the reference standard DCS is crucial for independent application in presurgical planning. There is a wide range of concordance between fMRI and DCS reported in the literature, depending on patient characteristics and task selection. There is a high degree of concordance between the techniques when it comes to mapping sensorimotor functions. For example, Fang et al (17) reported a sensitivity of 84.6% and specificity of 77.8% in depicting motor areas by using tb-fMRI. However, the concordance is more variable when mapping higher cognitive functions. For example, sensitivity and specificity between the methods ranged from 22% to 100% and from 40% to 85%, respectively, in localizing language functions (18–21). Moreover, direct comparison of DCS and preoperative fMRI for language localization in patients with brain tumors showed a wide range of performance across institutions (pooled sensitivity, 67% [95% CI: 51%, 80%] and pooled specificity, 55% [95% CI: 25%, 82%]) and across patients (pooled sensitivity, 44% [95% CI: 14%, 78%] and pooled specificity, 80% [95% CI: 54%, 93%]) (22). This wide variation is likely attributable to the heterogeneity of the studies, including surgical indications, magnet strength, fMRI paradigm design, and intraoperative testing techniques (22).
One possible driving factor that may explain the variable concordance in the literature is that tb-fMRI is an activation technique, while DCS is an inhibition or disruptive technique. Also, tb-fMRI elicits activation of all brain regions that contribute to the performance of a task; however, not all nodes within these networks are necessarily critical to function, or they may be easily compensated for after damage. As such, fMRI may not differentiate between crucial and accessory areas. During DCS, these supportive areas may not produce an observable neurologic deficit, as opposed to the more critical nodes. Importantly, positive clinical deficits at DCS also do not necessarily imply a permanent deficit after resection; however, it is typical to preserve these potentially crucial areas to reduce the likelihood of permanent neurologic deficits (7).
As opposed to localization of function, language lateralization is a commonly used metric in epilepsy surgery. Language lateralization can be quantified using a laterality index (LI), which can be calculated by various methods. Most commonly, volume of activation is compared between hemispheres using either whole-hemisphere or region of interest measures of frontal or temporal language areas. There is abundant literature on fMRI in predicting naming and verbal memory outcomes in temporal lobe surgery for epilepsy, including class I evidence for the ability of LI to predict naming outcomes (23). However, these data cannot be easily translated to tumor surgery, for a variety of reasons. First, the data are specific to patients undergoing anterior temporal lobectomy and are not necessarily applicable to patients undergoing surgery in other brain regions. Second, LIs have been shown to be much more variable in patients with tumors because of a variety of factors, such as neurovascular uncoupling, brain distortion, and susceptibility artifact from blood products or prior surgery (24). Despite the high level of evidence for LI in predicting outcomes after temporal lobe surgery in epilepsy, there is no such high-level evidence for LIs in predicting outcomes after tumor surgery. LIs may give some preoperative confirmation of the dominant hemisphere, but tumor surgery is somewhat unique in that the surgeon is generally interested in language localization rather than in lateralization alone. Additionally, while low-level evidence suggests that proximity of tumor and resection to areas of fMRI activation may predict risk of postoperative functional deficit (25), studies with a high level of evidence for fMRI in accurately predicting surgical outcomes from voxel-level localization are currently lacking.
Validation Studies on rs-fMRI
In theory, rs-fMRI reflects intrinsic functional networks and can be used to guide surgical resection. However, there is limited evidence to support this hypothesis. The major limitation of rs-fMRI is its reproducibility. Gujar et al (11) investigated the accuracy of visual inspection of subject-level rs-fMRI language networks by using tb-fMRI language activation maps as the reference standard. The accuracy of human identification of rs-fMRI language networks varied among different raters and years of fMRI experience. The most experienced rater achieved a peak accuracy of 72% in identifying rs-fMRI language maps, while raters with fewer years of experience had overall accuracy no better than chance (50%). Therefore, caution is advised when language map identification is crucial for decision-making, such as determining the surgical approach or the extent of tumor resection. It is critical to confirm the selected language component by using additional methods (eg, tb-fMRI or intraoperative mapping) when possible (11).
The agreement between rs-fMRI and other techniques, including tb-fMRI and intraoperative mapping, is not yet optimal. For example, when assessing language laterality, rs-fMRI commonly reveals greater activation in the nondominant hemisphere, compared with tb-fMRI (26). In children, Phillips et al (27) found that language lateralization concordance between rs-fMRI and tb-fMRI was 64%–73%, with none of the patients having atypical language lateralization (right lateralized or bilateral) being classified correctly by rs-fMRI. Future studies are needed to improve and standardize rs-fMRI processing techniques before this method can be further integrated into clinical assessment.
Impact of fMRI on Postoperative Outcomes
Despite its widespread use in presurgical planning for tumor resection, the data on fMRI as it relates to surgical outcomes are limited. In a meta-analysis of fMRI studies, patients who underwent preoperative fMRI were less likely to have a postoperative functional deficit and had slightly higher postoperative Karnofsky performance status scores (28). Importantly, most fMRI studies also used other mapping techniques, and the authors were unable to delineate outcome improvements from fMRI versus other factors known to independently improve outcomes, such as diffusion-tensor imaging (DTI), awake mapping with DCS, and/or intraoperative MRI (28). Any studies using these other techniques without patients solely randomized to fMRI or no fMRI must be viewed with caution as the outcomes may have been primarily driven by other mapping techniques. For example, a prospective study of rs-fMRI, tb-fMRI, and DTI showed that fMRI techniques explained the lowest amount of variance in the outcomes, while DTI was superior in explaining 32% of observed variance (29). As such, these studies present limited data to support the independent role of fMRI in predicting outcome from tumor surgery.
Of the many studies on fMRI in tumor surgery, few directly compare patients who have and have not undergone fMRI, and most have shown mixed results. Most of these studies are retrospective, lack randomization, and are confounded by the use of other mapping techniques. For example, Panigrahi et al (30) conducted a retrospective study of surgical resection of insular gliomas in patients who underwent both fMRI and DTI and compared results with a historical cohort. They did not find a difference in extent of resection or functional outcome in patients who did and those who did not undergo fMRI plus DTI. Zhang et al (31) investigated gliomas in language areas and categorized the patients into two groups: One group included patients who underwent fMRI and DTI along with intraoperative MRI, while the other group comprised patients who did not undergo fMRI, DTI, or intraoperative MRI. While they found that the study group had a slightly increased extent of resection and lower incidence of language deficits, the data specific to fMRI were severely compromised because surgical plans were commonly modified by the added information from the intraoperative structural MRI scan (31). Sun et al (32) explored the use of fMRI and DTI in patients undergoing surgery for lateral ventricular meningiomas. Compared with controls, the study group showed no difference in extent of resection but a lower rate of visual field deficits and slightly fewer cases of transient aphasia. There was no difference in long-term Karnofsky performance status score. There are a few important considerations in this study. First, the authors used a transparieto-occipital cortical approach for all patients, which primarily places white matter tracts at risk, specifically the optic radiations, superior longitudinal fasciculus (SLF) II, and arcuate fasciculus (33). As such, it is likely that DTI played a more substantial role in the outcomes compared with fMRI, which is confirmed by the authors’ statements that the optic radiations were the primary structure of avoidance. The authors also highlight that the Wernicke area and arcuate fasciculus “were relatively distant from the lesion as compared with the optic radiation and were therefore less affected by edema resulting from surgical manipulation” (32). Kosteniuk et al (34) used a retrospective propensity-matched cohort of patients with low-grade gliomas who did and did not undergo preoperative fMRI. They found no evidence of a difference in outcomes, extent of resection, or mean survival in the fMRI cohort. Vysotski et al (35) retrospectively reviewed a cohort of patients with brain tumors who did and did not undergo preoperative fMRI and found that survival was greater in the fMRI group, with a lower rate of motor and language decline postoperatively. However, the data must be interpreted with caution. There was no explicitly stated rationale for why some patients underwent fMRI and others did not. The fMRI cohort was younger, had a greater number of low-grade tumors, and had tumors with greater proximity to certain eloquent areas. Overall survival benefits are therefore limited because of the higher number of low-grade gliomas in the fMRI group, and this was by far the greatest independent predictor of survival. In subgroup analysis, benefits of fMRI seemed to be present only in patients with high-grade gliomas. Follow-up assessments were limited to review of clinical notes with no formal neuropsychological testing, which may underestimate the true extent of postoperative deficits. Additionally, the authors did not assess extent of resection; therefore, it is possible that the fMRI cohort had better functional outcomes because of more conservative resection, particularly as there were more dominant hemisphere lesions and closer proximity to eloquent cortex in the fMRI group. Last, DCS was also variably used in both cohorts, and patients with DCS had a much greater 3-year survival rate regardless of undergoing fMRI or not (35).
To summarize, there are limited studies directly comparing patients undergoing tumor resection with and without fMRI and no class I evidence. Most fMRI studies do not include a comparison group who did not undergo fMRI. Of those studies with comparison groups, many failed to identify a significant outcomes advantage to the use of fMRI. While some did show a difference, nearly all were heavily confounded by study designs that did not randomize for other factors that are well-established in providing benefits to survival, extent of resection, and functional outcomes (eg, awake craniotomy with DCS, intraoperative MRI, DTI, etc). Evidence supports that a combination of fMRI, DTI, and DCS leads to improved extent of resection, increased survival, and lower rates of postoperative functional deficits; however, the evidence for fMRI as an independent predictor is currently limited.
Modern Theories of Functional Neuroanatomy for Presurgical Mapping
Eloquent brain regions have historically been defined as those areas in which damage is expected to result in observable impairment in neurologic function. The most commonly assessed functions in surgical planning include motor, language, and vision. Here, we discuss relevant neuroanatomy for these functions.
Hand Motor Area
Primary hand motor function has been traditionally depicted as an omega-shaped region of the precentral gyrus, referred to as the hand knob (Fig 1). However, the specific location of task-based functional activation within the hand motor regions may not always coincide with the hand knob. This discrepancy can occur because of individual differences in combination with the mass effect caused by tumors, which can result in the relocation of eloquent cortices (36–38).
Figure 1:
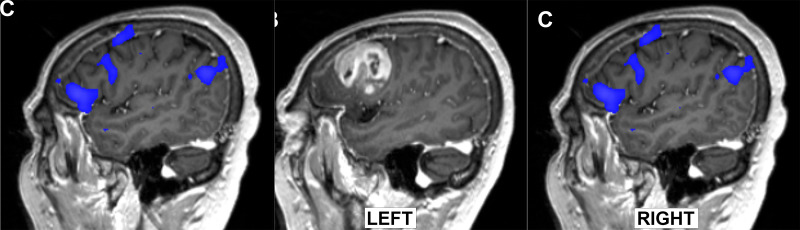
Images from presurgical functional MRI in a 30-year-old man with grade 3 IDH-mutant astrocytoma. (A) Axial image of right foot motor function (magenta) shows localization along the medial aspect of the lesion, with supplementary motor area activation (arrow) in the right hemisphere, suggesting reorganization. (B) Right hand motor function (green) is shown in the left hemisphere hand knob area along the posterolateral border of the lesion. (C) Tongue motor function (blue) is present in the lateral precentral gyrus along the lateral border of the lesion.
Foot Motor Area
The primary foot motor area is situated along the medial precentral gyrus along the interhemispheric fissure. This region is less anatomically defined than the hand motor region, as it lacks distinct anatomic landmarks on structural MRI studies. Furthermore, slight variations in the patient’s head position, plus mass effect from a tumor or edema, leads to difficulty in accurately identifying the foot motor region. Additionally, the foot motor area’s proximity to and positioning beneath the superior sagittal sinus makes it challenging to access during DCS. Consequently, fMRI plays a crucial role in localization for presurgical mapping (Fig 1) (39).
Lip and Tongue Motor Area
The primary motor area responsible for tongue and lip movements is situated along the lateral precentral gyrus within the corticobulbar region. However, there are no discrete macroscopic features that delineate the specific location at structural imaging, making it challenging to anatomically locate. The functional activation maps for lip and tongue movements are found in the area where the pre- and postcentral gyri merge at the base of the sensorimotor cortex. Dorsally, this region is bound by the posterior subcentral sulcus on the sagittal plane. Tongue movement evokes activation in the lateral aspect of the precentral gyrus, which is slightly lower and overlaps with the activation produced by lip puckering (Fig 1) (40).
Supplementary Motor Area
The supplementary motor area (SMA) is located in the posterior portion of the superior frontal gyrus just anterior to the paracentral lobule. The SMA consists of a posterior SMA proper (herein, referred to as SMA) and the anterior pre-SMA. The SMA and pre-SMA do not have a distinct macroscopic border, but the division can be approximated by a vertical line through the anterior commissure that is perpendicular to the line connecting the anterior and posterior commissures (41). The SMA and pre-SMA have overlapping functions and presumably form a continuum of function versus discrete segregation. The caudal SMA has complex motor and sensory connections and is involved in the planning, initiation, and timing of complex motor functions and action sequences. The SMA is believed to be involved in bilateral or bimanual motor coordination by maintaining a tonic interhemispheric balance between initiation and suppression. There is somatotopic organization in the SMA proper, with the face, upper limbs, and lower limbs being represented in an anteroposterior direction. Speech and language are located more anteriorly at the border of the SMA and in the pre-SMA. The pre-SMA has been implicated in cognitive control, including inhibition and task switching, and is commonly activated during language tasks. Complex motor and sensory tasks, such as finger tapping, during fMRI result in activation within the contralateral SMA proper (Fig 1).
Surgical resection of a unilateral SMA can result in SMA syndrome, which is characterized by temporary contralateral global akinesia with normal tone. If the lesion is in the dominant hemisphere, SMA aphasia can also result in impaired verbal fluency and initiation and maintenance of speech. Confrontation naming, repetition, and spoken speech comprehension are spared. Improvement or recovery is common in the ensuing weeks to months after surgery, and the extent of preservation of anatomic connections to the contralateral SMA can increase the likelihood of recovery (42–44). Nearly all patients make a full functional recovery, except for deficits in alternating bimanual movements.
Speech and Language Areas
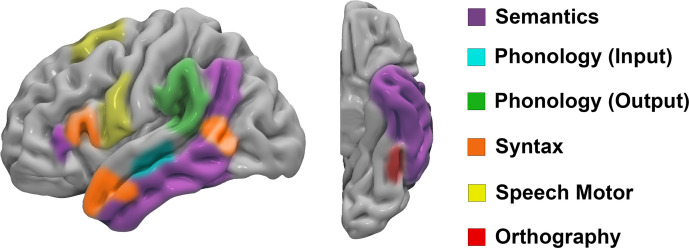
The advent of fMRI has considerably advanced our understanding of the functional neuroanatomy of speech and language functions. The classic 19th-century localization model developed by Wernicke focused on an area in the superior temporal gyrus where sound images were held, known as the Wernicke area, and an area in the frontal lobe that contained representations of movements governing speech production, known as the Broca area. While this model played an important role in understanding and localizing clinical aphasia presentations, more recent research has shown that it is overly simplistic, incomplete, and, in some cases, inaccurate in terms of functional neuroanatomy. For example, a large body of evidence indicates that language is far more widely distributed than originally thought, including large networks (Fig 2) in the frontal, temporal, and parietal lobes that are specialized in processing different linguistic information (eg, orthography, phonology, semantics, syntax, pragmatics). These cortical centers are highly interconnected via white matter tracts, allowing dynamic feedforward and feedback communication needed for the production and comprehension of language. The classic model was modular in approach and focused on limited cortical structures, with little consideration for subcortical white matter connections. Further, decades of research have challenged the originally conceived functional roles of the Broca and Wernicke areas. Data suggest that the Broca area (pars operculum and triangularis) is not involved in actual speech production but rather is involved in prearticulatory processing, perhaps gating the flow of phonetic motor codes to the ventral premotor and primary motor cortex for speech motor execution (45). Data also suggest that the Wernicke area, as defined by the posterior superior temporal gyrus, is not involved in auditory comprehension but rather in the selection and retrieval of phonetic codes for word production (46). These concepts have important clinical implications, as it has been shown that tumors can often be safely resected within the Broca area with no long-term speech deficits, while injury to the more posteriorly located ventral premotor cortex has a high association with permanent speech deficits (Fig 3) (47,48).
Figure 2:
Cortical representation of the dual stream model of language illustrates functional areas for semantic, phonologic, syntactic, orthographic, and speech motor functions.
Figure 3:
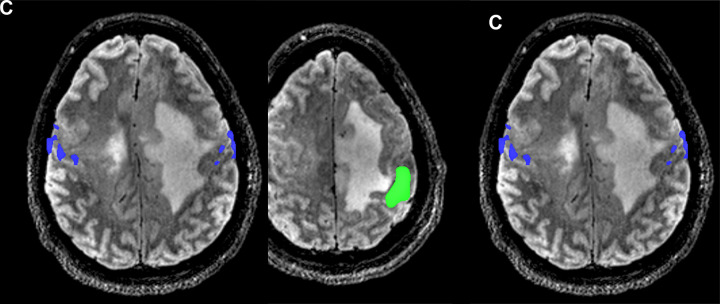
Images from presurgical functional MRI (fMRI) for language mapping. (A) Sagittal fMRI scan in a 45-year-old woman with a left frontal low-grade glioma (arrow) shows close proximity of the lesion with the ventral premotor cortex (red) from a sentence completion task. Activation from a reading comprehension task is shown in magenta, with activation in the inferior frontal gyrus corresponding to the traditional Broca area. (B) In a second patient, a 73-year-old woman with a left frontal high-grade glioma, there is no activation present in the left hemisphere during a sentence completion task. (C) In the same patient, there is right hemisphere–dominant language function (blue) on the sentence completion task showing the ability of task-based fMRI to correctly help lateralize this atypical language organization, which was confirmed intraoperatively.
The connectomic model is now widely accepted as the most comprehensive, up-to-date model of human language, having replaced the outdated localizationist models of cognitive function. The “dual stream” concept posits that speech and language involve intricate processes that are widely distributed throughout the brain. However, they are generally supported by two main pathways: a dorsal “phonologic” stream and a ventral “semantic” stream (Fig 2). According to the dual-stream neurocognitive model, different neuroanatomic networks are responsible for the processing of word meaning and language comprehension (ventral stream) and the production of speech sounds (dorsal stream) (49).
Initial input for language streams varies depending on the form of language. An area in the ventral temporo-occipital region, referred to as the visual word form area, is critical in decoding orthographic or written information, and stimulation or damage to this area can result in pure alexia or the inability to read but retained writing and other linguistic functions. The superior temporal gyri are involved in a bilateral network specialized in processing or decoding speech sounds, where stimulation or damage can result in pure word deafness or the inability to understand spoken speech, with intact written language comprehension and other linguistic functions. Phonologic retrieval and assembly of phonetic codes for speech output localize to the left posterior superior temporal gyrus, where stimulation or damage can result in phonologic paraphasic errors and conduction aphasia. Lexical semantic processing involves a left-lateralized distributed network of frontotemporoparietal areas, where stimulation or damage can result in anomia and degradation of semantic conceptual knowledge. Further, processing sentence-level structure or syntax involves areas in the inferior frontal gyrus, anterior temporal pole, and posterior middle temporal gyrus.
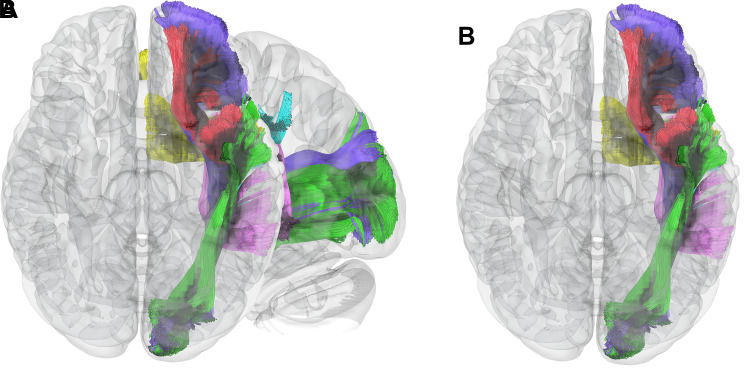
The ventral stream largely functions in semantic processing and involves widely distributed areas extending from the angular gyrus, through the lateral temporal lobe and temporal pole, and to the frontal lobe. Using more robust tasks, the semantic network has been shown to have a relatively symmetric representation across both hemispheres (50). Nevertheless, there are differences in hemispheric function and susceptibility to injury. Patients with damage to the semantic system of the nondominant hemisphere typically demonstrate deficits in nonverbal tasks, often of lower severity than those resulting from damage to their dominant hemisphere counterparts. Meanwhile, verbal semantic performance is generally intact after nondominant hemisphere injury (51). As it relates to surgical planning, we can expect to see greater semantic network activation in the dominant hemisphere with typical clinically utilized tasks, and patients would be expected to have a much greater risk of clinically relevant semantic impairment after damage to the dominant hemisphere. Key white matter pathways in the ventral stream include the inferior fronto-occipital fasciculus, uncinate fasciculus, and inferior longitudinal fasciculus (Fig 4). Stimulation or damage to the inferior fronto-occipital fasciculus and inferior longitudinal fasciculus can result in anomia or semantic paraphasias (52); injury to the latter may also result in impaired reading or alexia (53). The uncinate fasciculus appears to play a role in retrieval of unique names or proper nouns, and disruption or damage to this tract has been associated with proper noun anomia (54).
Figure 4:
Critical white matter connections of the language systems. (A) Sagittal and (B) inferior axial MR tractography images show the relationship of several of the primary connections of the language system. The ventral semantic stream primarily consists of the inferior fronto-occipital fasciculus (purple), inferior longitudinal fasciculus (green), and uncinate fasciculus (red). The dorsal phonologic stream primarily consists of the arcuate fasciculus (pink) and the lateral portion of the superior longitudinal fasciculus (cyan). The frontal aslant tract (yellow) connects the frontal opercular language areas with the presupplementary motor area and is a critical component of verbal fluency.
The dorsal stream primarily engages in processing phonologic information. It starts in the superior temporal gyrus and superior temporal sulcus, where the initial auditory input related to phonologic information occurs. From there, it extends dorsoposteriorly, passing through the supramarginal gyrus and reaching the inferior frontal gyrus and premotor cortex (49). The main fiber pathways of the dorsal stream consist of the arcuate fasciculus, the SLF III, and the frontal aslant tract. Damage to the arcuate fasciculus can lead to conduction aphasia, repetition disorders, or phonologic paraphasias (55). A phonologic working memory loop is subserved by the most lateral portion of the SLF (SLF III), connecting the frontal opercular areas with the supramarginal gyrus. SLF III stimulation or damage can result in anarthria or dysarthria, which can cause severe speech output disorders (56). The frontal aslant tract, a more recently described pathway, is a critical component of verbal fluency and can produce deficits in speech initiation and articulatory planning (57).
It is important to note that language-related fMRI tasks often activate primary and secondary language areas, with the latter potentially playing a less critical role in function. Therefore, an understanding of the functional anatomy of language is imperative so that the neuroradiologist has a better understanding of the importance of different regions of language activation. By appropriately communicating this information to the neurosurgeon, damage to these more critical areas can potentially be avoided and the incidence of postoperative functional deficits can be reduced.
Visual Areas
The primary visual cortex is located in the occipital lobe and situated in the vicinity of the calcarine fissure. The cortical magnification phenomenon allocates a larger area of the cortex for processing the central visual field, specifically the fovea, as compared with the peripheral visual field. This phenomenon provides enhanced perception and visual acuity. Visual inputs from the retinogeniculocortical pathways are segregated and recombined in the primary visual cortex, which then transmits to various extrastriate regions. Lesions in the occipital lobe can lead to visual field deficits, and there is a risk of such deficits when lesions disrupt the geniculocalcarine pathway, even as distant as the temporal lobe. Moreover, the visual cortex extends beyond the primary visual cortex to include portions of the parietal, temporal, and frontal lobes. Damage along any part of this pathway can produce a range of visual deficits that can often be localized further by the parts of the visual field that are affected. Preoperative planning in patients with lesions involving the visual pathway may be enhanced by retinotopic mapping with fMRI (Fig 5) (58).
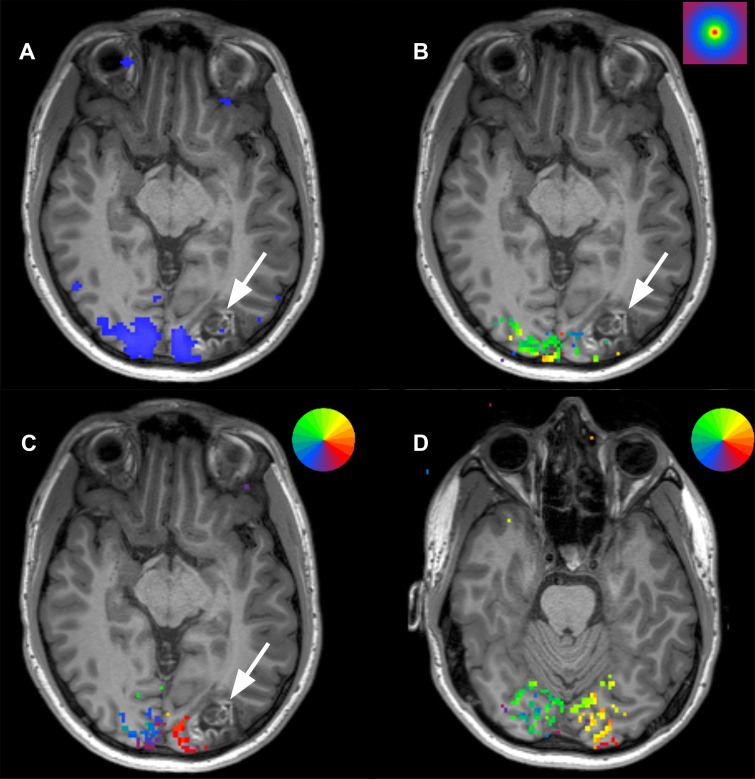
Figure 5:
Scans from presurgical retinotopic mapping functional MRI in a 24-year-old man with a left occipital lobe ganglioglioma (arrow). (A) Image obtained during axial checkerboard vision task shows the primary visual cortex (blue) to lie medial to the lesion. (B) Concentric ring paradigm shows the lesion to occupy cortical representation of areas outside of the fovea (stimulus key in right upper corner; fovea = red). (C) Rotating wedge paradigm maps at the level of the lesion show activation in the area of the patient’s inferior outer quadrant, suggesting surgical risk for right inferior quadrantanopia (stimulus key showing color representation of visual fields in right upper corner; the visual field representation in the brain is inverted and flipped right-to-left such that right inferior visual quadrant is represented in the superior left occipital lobe. (D) More inferior image shows that the superior quadrant visual field is not within proximity to the lesion.
Beyond the primary visual cortex are additional cortical regions that respond to visual stimuli, referred to as extrastriate and visual association cortices. These areas receive inputs from both the primary visual cortex and other extrastriate areas. These regions also lie primarily within the occipital lobe, with additional localization in the posterior temporal, parietal, and frontal lobes. The different cortical visual areas and the networks that connect them are poorly understood at the present time. Two major processing streams can be described as part of cortical visual processing, referred to as the what pathway and the where pathway. A slow ventral stream processes object recognition (the what pathway), and a faster dorsal stream processes spatial information and attention (the where pathway). The occipital cortex and inferior temporal cortex are connected via the ventral stream. A critical component of the ventral stream is the fusiform gyrus, which is located in the middle of the ventral temporal lobe and plays an important role in recognizing faces and objects. From the primary visual cortex, the dorsal stream projects to the parietal lobe and the middle temporal visual complex. There is also evidence that the dorsal stream is connected to the premotor and prefrontal cortex, as well as the mesial temporal lobe, which controls spatial navigation, spatial working memory, and visual guidance (58,59).
Conclusion
Advancements in fMRI techniques have resulted in widespread use for presurgical planning and intraoperative guidance in patients with brain tumors. However, it is uncertain whether these modalities truly have a substantial impact on glioma surgery, because of a low level of existing evidence. Unfortunately, the increasing reliance on fMRI has created a challenging environment to conduct reliable randomized studies where patients could be randomized to not undergo fMRI preoperatively. As such, there remains a void for evidence demonstrating that preoperative fMRI improves the extent of tumor removal, reduces complications, and expands the surgical possibilities in areas traditionally considered crucial for brain function (60). Additionally, further research is needed to assess the role of rs-fMRI in preoperative planning, as it has yet to prove as reliable as tb-fMRI (61–63). With further advancements in fMRI, such as high temporal and spatial resolution imaging, ultra-high-field-strength MRI (7 T), artificial intelligence, and new processing techniques, it remains plausible that fMRI could play a more critical role in guiding patient selection and surgical planning in the near future.
Authors declared no funding for this work.
Disclosures of conflicts of interest: D.A.L. Radiological Society of North America 2022 William W. Olmstead Fellow. D.S.S. Received honoraria (<$1000) for speaking about electrical brain mapping at the International Neuropsychological Society in San Diego in 2023. K.L.C. No relevant relationships. A.Q.H. No relevant relationships. E.H.M. Clinical trial funding from Varian Medical Systems and Vigil Neuroscience, paid to author’s institution; consulting fees from Varian Medical Systems, Boston Scientific, and Cortechs.ai; payment from Varian Medical Systems and Siemens Healthineers for lectures plus travel support.
Abbreviations:
- BOLD
- blood oxygen level–dependent
- DCS
- direct cortical stimulation
- DTI
- diffusion-tensor imaging
- fMRI
- functional MRI
- LI
- laterality index
- rs-fMRI
- resting-state fMRI
- SLF
- superior longitudinal fasciculus
- SMA
- supplementary motor area
- tb-fMRI
- task-based fMRI
References
- 1. Ostrom QT , Price M , Neff C , et al . CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019 . Neuro-oncol 2022. ; 24 ( Suppl 5 ): v1 – v95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vivas-Buitrago T , Domingo RA , Tripathi S , et al . Influence of supramarginal resection on survival outcomes after gross-total resection of IDH-wild-type glioblastoma . J Neurosurg 2021. ; 136 ( 1 ): 1 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lakhani DA , Deib G . Photorealistic depiction of intracranial tumors using cinematic rendering of volumetric 3T MRI Data . Acad Radiol 2022. ; 29 ( 10 ): e211 – e218 . [DOI] [PubMed] [Google Scholar]
- 4. De Witt Hamer PC , Robles SG , Zwinderman AH , Duffau H , Berger MS . Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis . J Clin Oncol 2012. ; 30 ( 20 ): 2559 – 2565 . [DOI] [PubMed] [Google Scholar]
- 5. Chaudhry AA , Naim S , Gul M , et al . Utility of preoperative blood-oxygen-level-dependent functional MR imaging in patients with a central nervous system neoplasm . Neuroimaging Clin N Am 2021. ; 31 ( 1 ): 93 – 102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castellano A , Cirillo S , Bello L , Riva M , Falini A . Functional MRI for surgery of gliomas . Curr Treat Options Neurol 2017. ; 19 ( 10 ): 34 . [DOI] [PubMed] [Google Scholar]
- 7. Silva MA , See AP , Essayed WI , Golby AJ , Tie Y . Challenges and techniques for presurgical brain mapping with functional MRI . Neuroimage Clin 2017. ; 17 : 794 – 803 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dimou S , Battisti RA , Hermens DF , Lagopoulos J . A systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection . Neurosurg Rev 2013. ; 36 ( 2 ): 205 – 214 ; discussion 214. [DOI] [PubMed] [Google Scholar]
- 9. Parrish TB , Gitelman DR , LaBar KS , Mesulam MM . Impact of signal-to-noise on functional MRI . Magn Reson Med 2000. ; 44 ( 6 ): 925 – 932 . [DOI] [PubMed] [Google Scholar]
- 10. Barkhof F , Haller S , Rombouts SA . Resting-state functional MR imaging: a new window to the brain . Radiology 2014. ; 272 ( 1 ): 29 – 49 . [DOI] [PubMed] [Google Scholar]
- 11. Gujar SK , Manzoor K , Wongsripuemtet J , et al . Identification of the language network from resting-state fMRI in patients with brain tumors: how accurate are experts? AJNR Am J Neuroradiol 2023. ; 44 ( 3 ): 274 – 282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang N , Zeng W , Chen L . SACICA: a sparse approximation coefficient-based ICA model for functional magnetic resonance imaging data analysis . J Neurosci Methods 2013. ; 216 ( 1 ): 49 – 61 . [DOI] [PubMed] [Google Scholar]
- 13. Krings T , Reinges MH , Willmes K , et al . Factors related to the magnitude of T2* MR signal changes during functional imaging . Neuroradiology 2002. ; 44 ( 6 ): 459 – 466 . [DOI] [PubMed] [Google Scholar]
- 14. Thickbroom GW , Byrnes ML , Morris IT , Fallon MJ , Knuckey NW , Mastaglia FL . Functional MRI near vascular anomalies: comparison of cavernoma and arteriovenous malformation . J Clin Neurosci 2004. ; 11 ( 8 ): 845 – 848 . [DOI] [PubMed] [Google Scholar]
- 15. Hou BL , Bradbury M , Peck KK , Petrovich NM , Gutin PH , Holodny AI . Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex . Neuroimage 2006. ; 32 ( 2 ): 489 – 497 . [DOI] [PubMed] [Google Scholar]
- 16. Fujiwara N , Sakatani K , Katayama Y , et al . Evoked-cerebral blood oxygenation changes in false-negative activations in BOLD contrast functional MRI of patients with brain tumors . Neuroimage 2004. ; 21 ( 4 ): 1464 – 1471 . [DOI] [PubMed] [Google Scholar]
- 17. Fang S , Liang J , Qian T , et al . Anatomic location of tumor predicts the accuracy of motor function localization in diffuse lower-grade gliomas involving the hand knob area . AJNR Am J Neuroradiol 2017. ; 38 ( 10 ): 1990 – 1997 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roux FE , Boulanouar K , Lotterie JA , Mejdoubi M , LeSage JP , Berry I . Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation . Neurosurgery 2003. ; 52 ( 6 ): 1335 – 1345 ; discussion 1345–1347 . [DOI] [PubMed] [Google Scholar]
- 19. Meier MP , Ilmberger J , Fesl G , Ruge MI . Validation of functional motor and language MRI with direct cortical stimulation . Acta Neurochir (Wien) 2013. ; 155 ( 4 ): 675 – 683 . [DOI] [PubMed] [Google Scholar]
- 20. Bizzi A , Blasi V , Falini A , et al . Presurgical functional MR imaging of language and motor functions: validation with intraoperative electrocortical mapping . Radiology 2008. ; 248 ( 2 ): 579 – 589 . [DOI] [PubMed] [Google Scholar]
- 21. Austermuehle A , Cocjin J , Reynolds R , et al . Language functional MRI and direct cortical stimulation in epilepsy preoperative planning . Ann Neurol 2017. ; 81 ( 4 ): 526 – 537 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weng HH , Noll KR , Johnson JM , et al . Accuracy of presurgical functional MR imaging for language mapping of brain tumors: a systematic review and meta-analysis . Radiology 2018. ; 286 ( 2 ): 512 – 523 . [DOI] [PubMed] [Google Scholar]
- 23. Gross WL , Helfand AI , Swanson SJ , et al . Prediction of naming outcome with fMRI language lateralization in left temporal epilepsy surgery . Neurology 2022. ; 98 ( 23 ): e2337 – e2346 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Połczyńska MM , Beck L , Kuhn T , et al . Tumor location and reduction in functional MRI estimates of language laterality . J Neurosurg 2021. ; 135 ( 6 ): 1674 – 1684 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kundu B , Penwarden A , Wood JM , et al . Association of functional magnetic resonance imaging indices with postoperative language outcomes in patients with primary brain tumors . Neurosurg Focus 2013. ; 34 ( 4 ): E6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robinson LF , He X , Barnett P , et al . The temporal instability of resting state network connectivity in intractable epilepsy . Hum Brain Mapp 2017. ; 38 ( 1 ): 528 – 540 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Phillips NL , Shatil AS , Go C , Robertson A , Widjaja E . Resting-state functional MRI for determining language lateralization in children with drug-resistant epilepsy . AJNR Am J Neuroradiol 2021. ; 42 ( 7 ): 1299 – 1304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luna LP , Sherbaf FG , Sair HI , Mukherjee D , Oliveira IB , Köhler CA . Can preoperative mapping with functional MRI reduce morbidity in brain tumor resection? a systematic review and meta-analysis of 68 observational studies . Radiology 2021. ; 300 ( 2 ): 338 – 349 . [DOI] [PubMed] [Google Scholar]
- 29. Osipowicz K , Sperling MR , Sharan AD , Tracy JI . Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy . J Neurosurg 2016. ; 124 ( 4 ): 929 – 937 . [DOI] [PubMed] [Google Scholar]
- 30. Panigrahi M , Chandrasekhar YB , Vooturi S , Ram GA , Rammohan VS . Surgical resection of insular gliomas and roles of functional magnetic resonance imaging and diffusion tensor imaging tractography-single surgeon experience . World Neurosurg 2017. ; 98 : 587 – 593 . [DOI] [PubMed] [Google Scholar]
- 31. Zhang J , Chen X , Zhao Y , Wang F , Li F , Xu B . Impact of intraoperative magnetic resonance imaging and functional neuronavigation on surgical outcome in patients with gliomas involving language areas . Neurosurg Rev 2015. ; 38 ( 2 ): 319 – 330 ; discussion 330 . [DOI] [PubMed] [Google Scholar]
- 32. Sun GC , Chen XL , Yu XG , et al . Functional neuronavigation-guided transparieto-occipital cortical resection of meningiomas in trigone of lateral ventricle . World Neurosurg 2015. ; 84 ( 3 ): 756 – 765 . [DOI] [PubMed] [Google Scholar]
- 33. Güngör A , Baydin S , Middlebrooks EH , Tanriover N , Isler C , Rhoton AL Jr . The white matter tracts of the cerebrum in ventricular surgery and hydrocephalus . J Neurosurg 2017. ; 126 ( 3 ): 945 – 971 . [DOI] [PubMed] [Google Scholar]
- 34. Kosteniuk SE , Gui C , Gariscsak PJ , Lau JC , Megyesi JF . Impact of functional magnetic resonance imaging on clinical outcomes in a propensity-matched low grade glioma cohort . World Neurosurg 2018. ; 120 : e1143 – e1148 . [DOI] [PubMed] [Google Scholar]
- 35. Vysotski S , Madura C , Swan B , et al . Preoperative FMRI associated with decreased mortality and morbidity in brain tumor patients . Interdiscip Neurosurg 2018. ; 13 : 40 – 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahdab R , Ayache SS , Brugières P , Farhat WH , Lefaucheur JP . The hand motor hotspot is not always located in the hand knob: a neuronavigated transcranial magnetic stimulation study . Brain Topogr 2016. ; 29 ( 4 ): 590 – 597 . [DOI] [PubMed] [Google Scholar]
- 37. Hlustík P , Solodkin A , Gullapalli RP , Noll DC , Small SL . Somatotopy in human primary motor and somatosensory hand representations revisited . Cereb Cortex 2001. ; 11 ( 4 ): 312 – 321 . [DOI] [PubMed] [Google Scholar]
- 38. Siero JC , Hermes D , Hoogduin H , Luijten PR , Ramsey NF , Petridou N . BOLD matches neuronal activity at the mm scale: a combined 7T fMRI and ECoG study in human sensorimotor cortex . Neuroimage 2014. ; 101 : 177 – 184 . [DOI] [PubMed] [Google Scholar]
- 39. Moreno RA , Holodny AI . Functional brain anatomy . Neuroimaging Clin N Am 2021. ; 31 ( 1 ): 33 – 51 . [DOI] [PubMed] [Google Scholar]
- 40. Xiao FL , Gao PY , Qian TY , et al . Cortical representation of facial and tongue movements: a task functional magnetic resonance imaging study . Clin Physiol Funct Imaging 2017. ; 37 ( 3 ): 341 – 345 . [DOI] [PubMed] [Google Scholar]
- 41. Bozkurt B , Yagmurlu K , Middlebrooks EH , et al . Microsurgical and tractographic anatomy of the supplementary motor area complex in humans . World Neurosurg 2016. ; 95 : 99 – 107 . [DOI] [PubMed] [Google Scholar]
- 42. Krainik A , Lehéricy S , Duffau H , et al . Postoperative speech disorder after medial frontal surgery: role of the supplementary motor area . Neurology 2003. ; 60 ( 4 ): 587 – 594 . [DOI] [PubMed] [Google Scholar]
- 43. Laplane D , Talairach J , Meininger V , Bancaud J , Orgogozo JM . Clinical consequences of corticectomies involving the supplementary motor area in man . J Neurol Sci 1977. ; 34 ( 3 ): 301 – 314 . [DOI] [PubMed] [Google Scholar]
- 44. Vergani F , Lacerda L , Martino J , et al . White matter connections of the supplementary motor area in humans . J Neurol Neurosurg Psychiatry 2014. ; 85 ( 12 ): 1377 – 1385 . [DOI] [PubMed] [Google Scholar]
- 45. Flinker A , Korzeniewska A , Shestyuk AY , et al . Redefining the role of Broca’s area in speech . Proc Natl Acad Sci USA 2015. ; 112 ( 9 ): 2871 – 2875 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Binder JR . The Wernicke area: Modern evidence and a reinterpretation . Neurology 2015. ; 85 ( 24 ): 2170 – 2175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andrews JP , Cahn N , Speidel BA , et al . Dissociation of Broca’s area from Broca’s aphasia in patients undergoing neurosurgical resections . J Neurosurg 2022. ; 138 ( 3 ): 847 – 857 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suarez-Meade P , Marenco-Hillembrand L , Sabsevitz D , et al . Surgical resection of gliomas in the dominant inferior frontal gyrus: Consecutive case series and anatomy review of Broca’s area . Clin Neurol Neurosurg 2022. ; 223 : 107512 . [DOI] [PubMed] [Google Scholar]
- 49. Hickok G , Poeppel D . Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language . Cognition 2004. ; 92 ( 1-2 ): 67 – 99 . [DOI] [PubMed] [Google Scholar]
- 50. Huth AG , de Heer WA , Griffiths TL , Theunissen FE , Gallant JL . Natural speech reveals the semantic maps that tile human cerebral cortex . Nature 2016. ; 532 ( 7600 ): 453 – 458 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thompson HE , Henshall L , Jefferies E . The role of the right hemisphere in semantic control: A case-series comparison of right and left hemisphere stroke . Neuropsychologia 2016. ; 85 : 44 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Middlebrooks EH , Yagmurlu K , Szaflarski JP , Rahman M , Bozkurt B . A contemporary framework of language processing in the human brain in the context of preoperative and intraoperative language mapping . Neuroradiology 2017. ; 59 ( 1 ): 69 – 87 . [DOI] [PubMed] [Google Scholar]
- 53. Herbet G , Zemmoura I , Duffau H . Functional anatomy of the inferior longitudinal fasciculus: from historical reports to current hypotheses . Front Neuroanat 2018. ; 12 : 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koay JM , Blackmon KE , Middlebrooks EH , et al . Examining the role of the uncinate fasciculus in proper noun naming: awake brain tumor resections and stereo EEG targeted electrical stimulation multiple case study . Neurocase 2022. ; 28 ( 5 ): 439 – 447 . [DOI] [PubMed] [Google Scholar]
- 55. Chang EF , Raygor KP , Berger MS . Contemporary model of language organization: an overview for neurosurgeons . J Neurosurg 2015. ; 122 ( 2 ): 250 – 261 . [DOI] [PubMed] [Google Scholar]
- 56. van Geemen K , Herbet G , Moritz-Gasser S , Duffau H . Limited plastic potential of the left ventral premotor cortex in speech articulation: evidence from intraoperative awake mapping in glioma patients . Hum Brain Mapp 2014. ; 35 ( 4 ): 1587 – 1596 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kinoshita M , de Champfleur NM , Deverdun J , Moritz-Gasser S , Herbet G , Duffau H . Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study . Brain Struct Funct 2015. ; 220 ( 6 ): 3399 – 3412 . [DOI] [PubMed] [Google Scholar]
- 58.Gill S, Ulmer J, DeYoe E. Vision and higher cortical function. In: Holodny A, ed. Functional Neuroimaging: A Clinical Approach. New York, NY: Taylor & Francis, 2008;67–80. [Google Scholar]
- 59. DeYoe EA , Raut RV . Visual mapping using blood oxygen level dependent functional magnetic resonance imaging . Neuroimaging Clin N Am 2014. ; 24 ( 4 ): 573 – 584 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Azad TD , Duffau H . Limitations of functional neuroimaging for patient selection and surgical planning in glioma surgery . Neurosurg Focus 2020. ; 48 ( 2 ): E12 . [DOI] [PubMed] [Google Scholar]
- 61. Elliott ML , Knodt AR , Cooke M , et al . General functional connectivity: Shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks . Neuroimage 2019. ; 189 : 516 – 532 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Finn ES , Bandettini PA . Movie-watching outperforms rest for functional connectivity-based prediction of behavior . Neuroimage 2021. ; 235 : 117963 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao W , Makowski C , Hagler DJ , et al . Task fMRI paradigms may capture more behaviorally relevant information than resting-state functional connectivity . Neuroimage 2023. ; 270 : 119946 . [DOI] [PMC free article] [PubMed] [Google Scholar]