Key Points
Question
Among children who snore without frequent obstructive events, does early adenotonsillectomy compared with watchful waiting with supportive care improve neurodevelopment, behavior, or other symptoms at 12-month follow-up?
Findings
In this randomized clinical trial of 458 children with mild sleep-disordered breathing (SDB), adenotonsillectomy compared with watchful waiting resulted in no significant differences in executive function or attention at 12 months. The adenotonsillectomy group had improved quality of life, symptoms, behavior, and blood pressure, which were among the secondary outcomes measured.
Meaning
In children with mild SDB, adenotonsillectomy resulted in no statistically significant differences in changes in executive function or attention but led to improved secondary outcomes including symptoms, behavior, and blood pressure.
Abstract
Importance
The utility of adenotonsillectomy in children who have habitual snoring without frequent obstructive breathing events (mild sleep-disordered breathing [SDB]) is unknown.
Objectives
To evaluate early adenotonsillectomy compared with watchful waiting and supportive care (watchful waiting) on neurodevelopmental, behavioral, health, and polysomnographic outcomes in children with mild SDB.
Design, Setting, and Participants
Randomized clinical trial enrolling 459 children aged 3 to 12.9 years with snoring and an obstructive apnea-hypopnea index (AHI) less than 3 enrolled at 7 US academic sleep centers from June 29, 2016, to February 1, 2021, and followed up for 12 months.
Intervention
Participants were randomized 1:1 to either early adenotonsillectomy (n = 231) or watchful waiting (n = 228).
Main Outcomes and Measures
The 2 primary outcomes were changes from baseline to 12 months for caregiver-reported Behavior Rating Inventory of Executive Function (BRIEF) Global Executive Composite (GEC) T score, a measure of executive function; and a computerized test of attention, the Go/No-go (GNG) test d-prime signal detection score, reflecting the probability of response to target vs nontarget stimuli. Twenty-two secondary outcomes included 12-month changes in neurodevelopmental, behavioral, quality of life, sleep, and health outcomes.
Results
Of the 458 participants in the analyzed sample (231 adenotonsillectomy and 237 watchful waiting; mean age, 6.1 years; 230 female [50%]; 123 Black/African American [26.9%]; 75 Hispanic [16.3%]; median AHI, 0.5 [IQR, 0.2-1.1]), 394 children (86%) completed 12-month follow-up visits. There were no statistically significant differences in change from baseline between the 2 groups in executive function (BRIEF GEC T-scores: −3.1 for adenotonsillectomy vs −1.9 for watchful waiting; difference, −0.96 [95% CI, −2.66 to 0.74]) or attention (GNG d-prime scores: 0.2 for adenotonsillectomy vs 0.1 for watchful waiting; difference, 0.05 [95% CI, −0.18 to 0.27]) at 12 months. Behavioral problems, sleepiness, symptoms, and quality of life each improved more with adenotonsillectomy than with watchful waiting. Adenotonsillectomy was associated with a greater 12-month decline in systolic and diastolic blood pressure percentile levels (difference in changes, −9.02 [97% CI, −15.49 to −2.54] and −6.52 [97% CI, −11.59 to −1.45], respectively) and less progression of the AHI to greater than 3 events/h (1.3% of children in the adenotonsillectomy group compared with 13.2% in the watchful waiting group; difference, −11.2% [97% CI, −17.5% to −4.9%]). Six children (2.7%) experienced a serious adverse event associated with adenotonsillectomy.
Conclusions
In children with mild SDB, adenotonsillectomy, compared with watchful waiting, did not significantly improve executive function or attention at 12 months. However, children with adenotonsillectomy had improved secondary outcomes, including behavior, symptoms, and quality of life and decreased blood pressure, at 12-month follow-up.
Trial Registration
ClinicalTrials.gov Identifier: NCT02562040
This randomized clinical trial evaluates whether early adenotonsillectomy, compared with watchful waiting and supportive care, improves neurodevelopmental, behavioral, health, and polysomnographic outcomes in US children with mild sleep-disordered breathing.
Introduction
Sleep-disordered breathing (SDB) includes a spectrum of disorders ranging from habitual snoring to frequent episodes of obstructive breathing during sleep. The disorder affects 6% to 17% of children, with a higher prevalence among children from racially minoritized or low-income backgrounds.1,2,3 Consequences of untreated SDB potentially include behavior problems; daytime sleepiness; impairment of growth, neurodevelopment, and quality of life; and increased prevalence of cardiovascular and metabolic diseases.4
Adenotonsillar hypertrophy is the most recognized risk factor for pediatric SDB. Consequently, adenotonsillectomy is the first-line treatment for SDB in otherwise healthy children. Of more than 560 000 tonsillectomies performed in the US in 2019, the majority were performed for obstructive breathing using diagnostic categories of adenotonsillar hypertrophy (79%) and obstructive sleep apnea (OSA) (74%).5 However, there are limited data addressing the benefits of surgery. The first large randomized clinical study of adenotonsillectomy for children with SDB, the Childhood Adenotonsillectomy Trial (CHAT), did not show improvement in objective measures of attention and executive function but reported improved behavior and symptoms. CHAT enrolled children with OSA (median obstructive apnea-hypopnea index [AHI], 4.6), excluding approximately one-half of all children who were adenotonsillectomy candidates but who had infrequent obstructive events.6 Although children who have habitual snoring without frequent obstructive events (mild SDB) may be at risk for neurodevelopmental impairment,7 the effect of adenotonsillectomy on behavioral and health outcomes in these children is not known. CHAT also did not address the role of surgery in children younger than 5 years, who may be more sensitive than older children to sleep disturbances.8
The current study, the Pediatric Adenotonsillectomy Trial for Snoring (PATS), was designed to assess the effectiveness of early adenotonsillectomy compared with watchful waiting and supportive care (watchful waiting) in children aged 3 to 12.9 years with mild SDB.
Methods
Study Design and Patients
This multicenter, single-blind, randomized trial enrolled children from June 29, 2016, to February 1, 2021, at 7 US academic sleep centers and completed follow-up examinations on February 25, 2022. Methodologic details have been published9; see the Protocol in Supplement 1 and the Supplemental Methods in Supplement 2 for details. Eligible children were aged 3 to 12.9 years, had tonsillar hypertrophy and mild SDB (defined by habitual snoring [occurring most of the night on at least 3 nights per week, for at least 3 months], an obstructive apnea index [number of complete obstructive breathing pauses per hour of sleep] <1, and an obstructive AHI [number of complete and partial obstructive episodes per hour of sleep] <3), and were considered appropriate candidates for adenotonsillectomy by an otolaryngologist. Exclusion criteria included recurrent tonsillitis, a z score based on the body mass index (BMI, calculated as weight in kilograms divided by the square of the height in meters) of 3 or more, and severe comorbidities.
All participants received supportive care that included standardized education on healthy sleep and lifestyle and referral for untreated allergies or asthma. Participants were randomly assigned to early adenotonsillectomy (within 4 weeks after randomization) or watchful waiting with supportive care.
Study Oversight
The study was approved by the institutional review board at each site. Written informed consent was obtained from caregivers and assent from children older than 7 or 8 years (according to each site’s local requirements). An independent data and safety monitoring board reviewed interim data on safety and study quality. An independent medical monitor adjudicated potential treatment failures (ie, a new or worsening condition adjudicated by the medical monitor as a potential need to change treatment).
Assessments
Assessments were made by staff masked to the child’s treatment assignment. At baseline and 12 months after randomization, children underwent standardized polysomnographic evaluation scored at a centralized sleep reading center, and on a day following their typical sleep at home, participants underwent anthropometry, neurodevelopmental testing, and other clinical evaluations. Race and ethnicity were based on caregiver responses to categories provided in a questionnaire and were collected as proxies for structural and social disadvantage. At each examination, caregivers were asked to complete standardized questionnaires. Selective assessments were repeated at an interim 6-month evaluation.
Outcomes
The 2 primary study outcomes were the Behavior Rating Inventory of Executive Function (BRIEF) and an objective test of attention, the Go/No-go (GNG) test. Caregivers completed age-appropriate versions of the BRIEF (BRIEF-Preschool Edition or the BRIEF 2nd Edition).10,11 The primary outcome from the BRIEF was the Global Executive Composite (GEC) T score, comprising summary measures of behavioral regulation and metacognition based on population mean scores of 50 and a standard deviation of 10 (higher, worse). The GNG test is a computer-based attention test developed for longitudinal studies of children aged 3 to 12 years. The primary outcome is d-prime, a signal detectability parameter that assesses the child’s ability to correctly identify targets corrected for their response bias (higher, better).12
Secondary outcomes included an objective test of fine motor control, the National Institutes of Health Toolbox 9-Hole Dexterity Test, with times for the child to complete the task averaged across hands; caregiver ratings of behavior assessed by the Childhood Behavior Checklist (CBCL) total problem score, a composite of scores of all specific behavior problem scales obtained from the preschool or school-age versions according to the child’s age; SDB symptom burden assessed with the Pediatric Sleep Questionnaire–Sleep-Related Breathing Disorder (PSQ-SRBD) scale, scores 0 to 1; sleepiness assessed with the Epworth Sleepiness Scale modified for children (mESS), scores 0 to 24; disease-specific quality of life assessed by the Obstructive Sleep Apnea 18 (OSA-18) assessment tool, scores 18 to 126; global quality of life assessed from the Pediatric Quality of Life Inventory (PedsQL), scores 0 to 100; resting systolic and diastolic blood pressure (age-, sex-, and height-adjusted percentiles13); and BMI. Higher scores indicate worse outcomes for all measures except the PedsQL.
Statistical Analysis
A sample size of 460 children, randomized 1:1 between adenotonsillectomy and watchful waiting, assuming attrition rates of 15% at 6 months and an additional 5% at 12 months, provided 80% power to detect a minimal effect size of 2.6 points for the BRIEF end point and a minimal effect size of 0.22 points for the GNG end point, with a family-wise type I error rate of 5%.14 The study provided 98% power to detect a difference of 3.7 points in the BRIEF end point (the change score observed in the CHAT study6) and a difference of 0.33 points in the GNG end point, with a family-wise type I error rate of 5%14 The Holm procedure was used to adjust for multiplicity for the 2 primary end points.14 Outcomes were evaluated using a mixed-effects model, with adjustment for study site and randomization stratification factors of age (≤5 vs >5 years), race (Black/African American vs other race or ethnicity), and BMI (≤85 vs >85th percentile). The mixed-effects models account for missing data using a maximum-likelihood-based approach, incorporate all data from participants who had at least 1 measurement from the 3 study visits, and are recommended as a preferred method for analyzing clinical trials with incomplete longitudinal data.15,16 (Details on sensitivity analyses based on imputation are provided in Supplement 2.)
Additional analyses included prevalence at 12 months of elevated AHI (≥3, ≥5) and of elevated symptom scores, using Poisson regression models (for relative risk) and linear regression (for risk difference) with robust variance estimators; assessing potential treatment modifications (family and child characteristics); and evaluating the effect of the COVID-19 pandemic on analyses of primary end points. The P values for the 22 key secondary analyses were adjusted controlling for a 5% false discovery rate17; the nominal coverage of the confidence intervals for the effect estimates of these outcomes was adjusted based on the corresponding false coverage-statement rate control.18 The P value and confidence intervals reported for exploratory analyses were not adjusted for multiple comparisons. Analyses were conducted using R version 4.1.0 (R Project for Statistical Computing).
Results
Study Overview
The flow of participants is shown in the Figure. Between June 29, 2016, and February 1, 2021, 458 children were randomized. Twelve-month follow-up visits were conducted for 395 children (86%), 355 of whom had both 6- and 12-month visits. There were no differences in the proportion of children who had complete BRIEF GEC or GNG end point data by study group (eTable 1 in Supplement 2).
Figure. Flow of Patients Through the Pediatric Adenotonsillectomy Trial for Snoring.
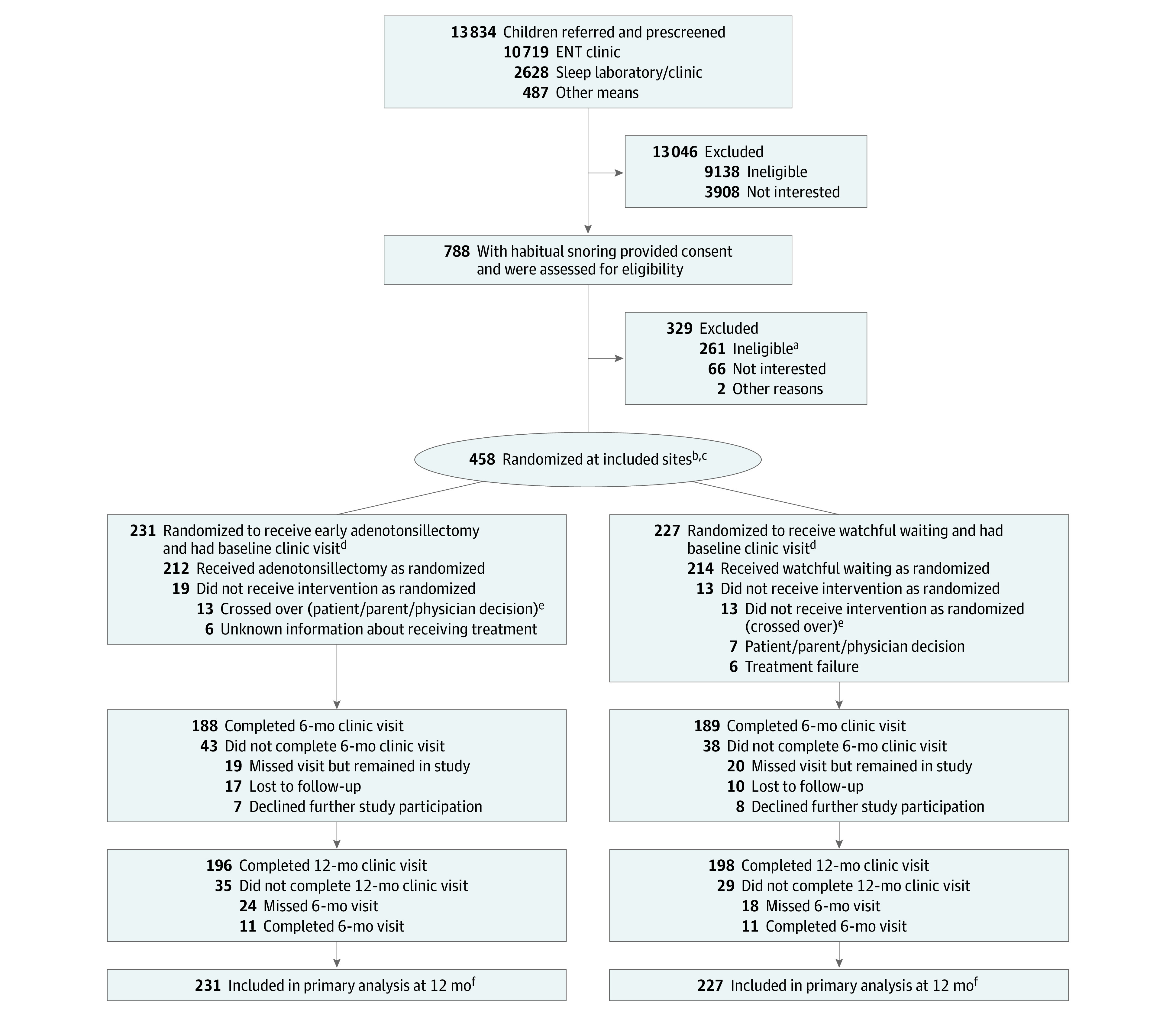
ENT indicates ear, nose, and throat.
aReasons for exclusion included apnea-hypopnea index level out of range; severe, chronic health problems; use of study-restricted medications; no report of habitual snoring; tonsillar size less than 2 on Brodsky scale; and lack of clinical equipoise.
bRandomization was stratified by age (≤5 vs >5 years), race (Black/African American vs other race or ethnicity), and body mass index (≤85 vs >85th percentile) within each study site.
cOne child randomized to watchful waiting was excluded due to site withdrawal but remained in the study and completed 12-month follow-up.
dVisit defined as completing either of the primary outcomes (BRIEF [Behavior Rating Inventory of Executive Function] or GNG [Go/No-go]).
eAnalyses reported are based on intention-to-treat; participants who crossed over were analyzed based on their randomized treatment assignments.
fThe primary analysis used a mixed-effects modeling approach that included observations from study participants at all included sites who had at least 1 measurement.
Baseline Characteristics
Table 1 reports the baseline characteristics by study group. In total, 139 children (30.3%) were younger than 5 years and 127 (30.0%) were from households with an annual income less than $30 000. The sample included 123 Black/African American children (26.9%) and 75 (16.4%) of Hispanic ethnicity. Among the sample, 169 children (36.9%) were overweight or obese, 108 (23.6%) had a history of asthma, 282 (61.6%) had tonsils that occupied more than 50% of the oropharyngeal width,19 and 331 (72.3%) had an AHI less than 1 (median, 0.5; IQR, 0.2-1.1). Baseline demographic and clinical characteristics were balanced between groups.
Table 1. Baseline Characteristics of Trial Participantsa.
| No. (%) | ||
|---|---|---|
| Early adenotonsillectomy (n = 231) | Watchful waiting (n = 227) | |
| Age, median (IQR), y | 6 (4-8) | 6 (4-8) |
| Sex, No. (%) | ||
| Female | 119 (51.5) | 111 (48.9) |
| Male | 112 (48.5) | 116 (51.1) |
| Race, No./total (%)b | ||
| American Indian/Alaska Native | 2/230 (0.9) | 1/227 (0.4) |
| Asian | 3/230 (1.3) | 5/227 (2.2) |
| Black/African American | 60/230 (26.1) | 63/227 (27.6) |
| Multiracial | 10/230 (4.3) | 10/227 (4.4) |
| White | 155/230 (67.4) | 148/227 (65.2) |
| Ethnicity, No. (%)b | ||
| Hispanic/Latinx | 42 (18.2) | 33 (14.5) |
| Non-Hispanic/Latinx | 189 (81.8) | 194 (85.5) |
| Maternal education, No./total (%) | ||
| High school diploma or less | 46/229 (20.1) | 40/227 (17.6) |
| Some college | 91/229 (39.7) | 93/227 (41.0) |
| 4-Year college or greater | 92/229 (40.2) | 94/227 (41.4) |
| Annual household income <$30 000 | 71/213 (33.3) | 56/210 (26.7) |
| Childhood Opportunity Index, median (IQR)c | 51 (22-75) [n = 229] | 58 (29-78) [n = 226] |
| Study site location, No. (%) | ||
| Ann Arbor, Michigan | 42 (18.2) | 42 (18.5) |
| Cincinnati, Ohio | 43 (18.6) | 41 (18.1) |
| Cleveland, Ohio | 33 (14.3) | 33 (14.5) |
| Dallas, Texas | 46 (19.9) | 45 (19.8) |
| Norfolk, Virginia | 33 (14.3) | 33 (14.5) |
| Philadelphia, Pennsylvania | 34 (14.7) | 33 (14.5) |
| Diagnosed asthma, No./total (%) | 53/230 (23.0) | 55/227 (24.2) |
| Current ADHD medication, No./total (%) | 11/230 (4.8) | 7/227 (3.1) |
| BMI (z score), median (IQR)d | 0.7 (−0.3 to 1.4) | 0.6 (−0.3 to 1.5) |
| BMI weight classification, No. (%)e | ||
| Healthy weight | 138 (59.7) | 132 (58.1) |
| Underweight | 7 (3.0) | 12 (5.3) |
| Overweight | 47 (20.3) | 34 (15.0) |
| Obese | 39 (16.9) | 49 (21.6) |
| Tonsil grade, No. (%)f | ||
| II | 84 (36.4) | 92 (40.5) |
| III | 127 (55.0) | 119 (52.4) |
| IV | 20 (8.7) | 16 (7.0) |
| Measures, No./total (%) | ||
| BRIEF GEC T score ≥65g | 52/230 (22.6) | 57/227 (25.1) |
| CBCL total problems T score ≥60h | 65/227 (28.6) | 62/224 (27.7) |
| PSQ-SRBD score ≥0.33i | 176/229 (76.9) | 167/227 (73.6) |
| mESS total score ≥10j | 50/227 (22.0) | 57/227 (25.1) |
| OSA-18 ≥60k | 66/229 (28.8) | 68/227 (30.0) |
| Frequent loud snoring, No./total (%)l | 125/229 (54.6) | 125/227 (55.1) |
| AHI (events/h), median (IQR)m | 0.5 (0.1-1.1) | 0.6 (0.3-1.2) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; BMI, body mass index; BRIEF, Behavior Rating Inventory of Executive Function; CBCL, Child Behavior Checklist; GEC, global executive composite; mESS, modified Epworth Sleepiness Scale; OSA, obstructive sleep apnea; PSQ-SRBD, Sleep-Related Breathing Disorder scale of the Pediatric Sleep Questionnaire.
Primary analysis included all participants in the Pediatric Adenotonsillectomy Trial for Snoring other than 1 child who was randomized from Boston Children’s Hospital (site started and closed recruitment early); this participant was excluded from this table.
Race and ethnicity were based on caregiver responses to categories provided in a questionnaire and were collected as proxies for structural and social disadvantage.
A census tract–level composite measure comprised of an education index, health and environment index, and social and economic index. Scores range from 1 to 100; higher scores indicate greater overall levels of opportunity.
BMI calculated as weight in kilograms divided by the square of height in meters. BMI z scores are age- and sex-standardized transformations of BMI that range from positive to negative infinity, with z scores 3 or greater indicating obesity.
Categories based on BMI percentile, which compares the child’s weight to that of other children of the same age and sex. Underweight: BMI in less than the fifth percentile; healthy weight: BMI in the 5th to 85th percentile; overweight: BMI in the 85th to 95th percentile; obese: BMI in the 95th percentile or greater.
Maximum Brodsky tonsil grade across both the left and right tonsils, determined by physical examination as the percentage of the oropharyngeal airway that the tonsil occupies: size I, 0% to 25%; size II, 26% to 50%; size III, 51% to 75%; size IV, more than 75%.
The BRIEF GEC comprises summary measures of behavioral regulation, emotion regulation, and cognitive regulation (BRIEF-2, for children ages 5 to 18 years) or inhibitory self-control, flexibility, and emergent metacognition (BRIEF-P, for preschool-aged children). Caregiver scores ranged from 33 to 102, with higher scores indicating worse functioning. A T score of 65 or greater is considered potentially clinically elevated.
The CBCL total problems summary scale comprises internalizing, externalizing, social, thought, and attention problems. Scores ranged from 24 to 84, with higher scores indicating greater emotional, social, and behavioral problems. A T score of 60 or greater indicates that the child is at risk for clinical problem behaviors.
Scores range from 0 to 1, with higher scores indicating greater severity. A score of 0.33 or greater suggests a high risk for a pediatric sleep-related breathing disorder.
Scores on the mESS range from 0 to 24, with higher scores indicating greater sleepiness. A score of 10 or greater represents excessive daytime sleepiness.
Scores on the OSA-18 quality of life survey range from 18 to 126, with higher scores indicating a greater negative effect of sleep-disordered breathing on quality of life. A score of 60 or greater represents a moderate to severe negative effect.
Snoring was assessed using item 1a of the OSA-18 quality of life survey, which uses a Likert scale to ask about the frequency of loud snoring over the last 4 weeks. Possible responses ranged from “none of the time” to “all of the time”; loud snoring was considered frequent if it occurred “a good bit of the time,” “most of the time,” or “all of the time.”
AHI defined as the average number of apneas or hypopneas (hypopneas defined ≥3% oxygen desaturation or arousal) per hour of sleep by polysomnography, with higher scores indicating more severe obstructive sleep apnea. In pediatric populations, AHI levels greater than 1 to 5 are considered indicative of obstructive sleep apnea. AHI levels were rounded to the nearest tenth.
Primary Outcomes
The baseline executive function (BRIEF GEC) T score was close to the population normative value in both study groups (normative values are not available for the GNG). The mean BRIEF GEC score improved from baseline to 12 months by 1.9 (SD, 8.6) points in the watchful waiting group and by 3.1 (SD, 9.4) points in the adenotonsillectomy group. The difference between groups (−0.96 points [95% CI, −2.66 to 0.74]) was not statistically significant (P = .27). The GNG sustained-attention d-prime value improved slightly from baseline to 12 months in both groups without evidence of group differences (0.05 [95% CI, −0.18 to 0.27]; P = .68) (Table 2; eFigure in Supplement 2). Sensitivity analysis based on multiple imputation yielded similar results (eTables 3 and 4 in Supplement 2).
Table 2. Outcome Measures.
| Mean (SD) | Effect size: difference in 12-mo changes (95% CI)a (n = 458) | P valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Normative values | Early adenotonsillectomy (n = 231) | Watchful waiting (n = 227) | |||||||
| Baseline | 12 mo | Change from baseline to 12 mo | Baseline | 12 mo | Change from baseline to 12 mo | ||||
| Coprimary outcomes | |||||||||
| Caregiver BRIEF GEC T scoreb | 50 (10) | 55.3 (12.2) [n = 230] |
52.1 (11.3) [n = 196] |
−3.1 (9.4) [n = 195] |
56.0 (12.5) | 53.7 (11.2) [n = 196] |
−1.9 (8.6) [n = 196] |
−0.96 (−2.66 to 0.74) | .27 |
| Go/No-go sustained attentionc,d | NA | 2.0 (1.1) [n = 229] |
2.2 (1.1) [n = 184] |
0.2 (1.2) [n = 182] |
2.1 (1.0) [n = 222] |
2.3 (1.0) [n = 184] |
0.1 (1.2) [n = 182] |
0.05 (−0.18 to 0.27) [n = 455] |
.68 |
| Secondary outcomes | Effect size: difference in 12-mo changes (FDR-adjusted CI)d | FDR-adjusted P valued | |||||||
| Pegboard dexterity (average)e | NA | 32.5 (14.6) [n = 227] |
27.4 (9.1) [n = 187] |
−5.3 (7.4) [n = 183] |
32.8 (11.9) | 26.7 (6.4) [n = 187] |
−5.9 (8.0) [n = 187] |
0.76 (−0.92 to 2.43) | .37 |
| Caregiver-reported CBCLf | 50 (10) | ||||||||
| Total problems T score | 53.0 (11.0) [n = 227] |
48.4 (10.8) [n = 186] |
−4.5 (9.0) [n = 183] |
53.3 (11.3) [n = 224] |
51.6 (10.9) [n = 182] |
−1.4 (7.5) [n = 182] |
−3.09 (−4.90 to −1.28) [n = 454] |
<.001 | |
| Externalizing problems T score | 51.1 (10.8) [n = 227] |
48.1 (10.3) [n = 186] |
−3.2 (8.5) [n = 183] |
51.2 (11.7) [n = 224] |
49.6 (11.2) [n = 182] |
−1.6 (8.2) [n = 182] |
−1.54 (−3.34 to 0.26) [n = 454] |
.09 | |
| Internalizing problems T score | 51.8 (11.2) [n = 227] |
47.8 (10.9) [n = 186] |
−3.8 (10.0) [n = 183] |
52.1 (11.3) [n = 224] |
51.1 (11.0) [n = 182] |
−0.7 (8.3) [n = 182] |
−3.05 (−5.07 to −1.04) [n = 454] |
.003 | |
| Attentional problems T score | 57.5 (8.2) [n = 227] |
55.2 (6.6) [n = 186] |
−2.3 (7.1) [n = 183] |
57.3 (7.8) [n = 224] |
56.0 (6.7) [n = 182] |
−1.1 (5.8) [n = 182] |
−1.19 (−2.55 to 0.17) [n = 454] |
.09 | |
| PSQ-SRBDg | 0.2 (0.1) | 0.5 (0.2) [n = 229] |
0.2 (0.2) [n = 189] |
−0.2 (0.2) [n = 187] |
0.5 (0.2) | 0.4 (0.2) [n = 193] |
−0.1 (0.2) [n = 193] |
−0.16 (−0.20 to −0.12) | <.001 |
| mESSh | 5.4 (3.7) | 6.9 (4.7) [n = 227] |
5.0 (5.3) [n = 188] |
−1.8 (4.9) [n = 184] |
6.9 (4.6) | 6.2 (5.1) [n = 193] |
−0.7 (4.4) [n = 193] |
−1.18 (−2.15 to −0.21) | .01 |
| OSA-18i | NA | 51.2 (15.7) [n = 229] |
35.6 (13.9) [n = 188] |
−15.8 (14.4) [n = 186] |
52.7 (17.4) | 46.5 (17.3) [n = 193] |
−6.0 (14.6) [n = 193] |
−9.75 (−12.84 to −6.65) | <.001 |
| Caregiver-reported PedsQLj | |||||||||
| Total score | 75.9 (13.2) [n = 229] |
78.4 (16.0) [n = 189] |
2.1 (14.9) [n = 187] |
77.7 (12.8) [n = 226] |
75.0 (15.9) [n = 193] |
−2.6 (15.0) [n = 193] |
4.76 (1.44 to 8.09) [n = 457] |
.005 | |
| Physical score | 79.5 (19.1) [n = 229] |
81.1 (21.9) [n = 189] |
0.7 (23.5) [n = 187] |
82.1 (16.3) [n = 226] |
76.4 (23.2) [n = 193] |
−5.3 (24.3) [n = 193] |
6.53 (1.29 to 11.78) [n = 457] |
.01 | |
| Psychosocial score | 73.9 (13.6) [n = 229] |
77.0 (14.8) [n = 189] |
2.8 (14.2) [n = 187] |
75.3 (14.1) [n = 226] |
74.3 (14.5) [n = 193] |
−1.1 (13.3) [n = 193] |
3.88 (0.89 to 6.88) [n = 457] |
.01 | |
| BMI (percentile) | 50 (25-75)k | 65.0 (30.0) | 70.4 (27.4) [n = 187] |
5.1 (14.3) [n = 187] |
62.0 (32.1) | 66.2 (31.4) [n = 188] |
3.2 (13.3) [n = 188] |
1.86 (−0.88 to 4.60) | .18 |
| Systolic blood pressure (percentile) | 50 (25-75)k | 63.6 (23.0) [n = 218] |
58.6 (27.9) [n = 180] |
−4.5 (28.9) [n = 173] |
57.6 (27.0) [n = 216] |
61.6 (26.0) [n = 184] |
4.8 (31.4) [n = 177] |
−9.02 (−15.49 to −2.54) [n = 451] |
.006 |
| Diastolic blood pressure (percentile) | 50 (25-75)k | 55.5 (20.5) [n = 218] |
51.5 (22.2) [n = 179] |
−4.9 (22.5) [n = 172] |
52.5 (21.5) [n = 216] |
54.6 (22.1) [n = 184] |
2.2 (20.8) [n = 177] |
−6.52 (−11.59 to −1.45) [n = 451] |
.01 |
| Heart rate, /min | NA | 80.8 (9.1) | 78.8 (8.9) [n = 154] |
−2.0 (7.7) [n = 154] |
81.2 (9.5) | 79.7 (9.5) [n = 152] |
−1.8 (7.9) [n = 152] |
−0.23 (−2.13 to 1.67) | .82 |
| AHIl | Baseline, No. (%) | 12 mo, No./total (%) | Baseline, No. (%) | 12 mo, No./total (%) | Effect size: risk difference at 12 mo, % (FDR-adjusted CI) d , m (n = 458) | ||||
| ≥3 | NA | 2 (0.9) | 2/154 (1.3) | 1 (0.4) | 20/152 (13.2) | −11.2 (−17.5 to −4.9) [n = 306] |
<.001 | ||
| ≥5 | NA | 0 | 0/154 | 0 | 11/152 (7.2) | −7.1 (−11.8 to −2.5) [n = 306] |
.002 | ||
Abbreviations: AHI, apnea-hypopnea index; BMI, body mass index; BRIEF, Behavior Rating Inventory of Executive Function; CBCL, Child Behavior Checklist; FDR, false discovery rate; GEC, global executive composite; mESS, modified Epworth Sleepiness Scale; NA, not available or not applicable; OSA, obstructive sleep apnea; PedsQL, Pediatric Quality of Life Inventory; PSQ-SRBD, Pediatric Sleep Questionnaire–Sleep-Related Breathing Disorder.
The estimated between-group difference in mean change from baseline to 12 months and the corresponding P value are from a linear mixed-effects model with prespecified adjustment for stratification factors (age ≥6 years, overweight/obese, Black/African American) and site effect. The analysis incorporates information from all study participants with at least 1 measurement across the 3 study visits.
The BRIEF GEC section comprises summary measures of behavioral regulation, emotion regulation, and cognitive regulation (BRIEF-2, for children aged 5 to 18 years) or inhibitory self-control, flexibility, and emergent metacognition (BRIEF-P, for preschool-aged children). Caregiver scores ranged from 33 to 102, with higher scores indicating worse functioning. Thirty-five patients were lost to follow-up at 12 months in the early adenotonsillectomy group and 31 in the watchful waiting with supportive care group. All 458 patients contributed information to the difference-in-differences analysis.
The Go/No-go sustained attention d-prime is a signal detection measure that combines a child’s true positive rate on an attention task (correct response to the target stimuli) with their false-alarm rate (incorrect response to the nontarget stimuli). Scores ranged from −0.9 to 4.5, with higher scores indicating greater sustained attention. Forty-seven patients were lost to follow-up at 12 months in the adenotonsillectomy group and 43 in the watchful waiting group. Three patients were excluded from the difference-in-differences analysis.
P values for all secondary outcomes are adjusted so that the set of hypotheses with P values below .05 corresponds to the set of hypotheses that would be rejected under the Benjamini and Hochberg procedure for controlling the FDR at .05.17 This means that, among all effects considered statistically significant at the .05 level (using the adjusted P values), 5% would be expected to be truly null. Twenty-two P values were included in the adjustment set: all 17 secondary analyses presented in Table 2, as well as analyses of 5 caregiver BRIEF subscale scores (eTable 6 in Supplement 2). The 95% confidence intervals for all 22 of these secondary end points are adjusted for multiple comparisons following the procedure of Benjamini and Yekutieli,18 with a nominal coverage level of 97%.
The average of National Institutes of Health Toolbox 9-hole pegboard dexterity test times (in seconds) from both the dominant and nondominant hand. Average times ranged from 15.5 seconds to 118 seconds, with higher average times indicating lower manual dexterity. Forty-four patients were lost to follow-up at 12 months in the adenotonsillectomy group and 40 in the watchful waiting group. All 458 patients contributed information to the difference-in-differences analysis.
Scores ranged from 28 to 86 on the CBCL externalizing problems scale, from 29 to 88 on the CBCL internalizing problems scale, from 50 to 97 on the CBCL attentional problems scale, and from 24 to 84 on the CBCL total problems scale (comprising all 3 of the previous scales plus social and thought problems). On each scale, higher scores indicate greater problems. Forty-five patients were lost to follow-up at 12 months in both the adenotonsillectomy group and the watchful waiting group. Four patients were excluded from the difference-in-differences analysis.
Scores range from 0 to 1, with higher scores indicating greater severity. Forty-two patients were lost to follow-up at 12 months in the adenotonsillectomy group and 34 in the watchful waiting group. All 458 patients contributed information to the difference-in-differences analysis.
Scores range from 0 to 24, with higher scores indicating greater sleepiness. Forty-three patients were lost to follow-up at 12 months in the adenotonsillectomy group and 34 in the watchful waiting group. All 458 patients contributed information to the difference-in-differences analysis.
Scores on the OSA-18 quality of life survey range from 18 to 126, with higher scores indicating a greater negative effect of sleep-disordered breathing on quality of life. Forty-three patients were lost to follow-up at 12 months in the adenotonsillectomy group and 34 in the watchful waiting group. All 458 patients contributed information to the difference-in-differences analysis.
The PedsQL total score comprises performance on 4 subscales: emotional functioning, social functioning, and school functioning (summarized by the psychosocial functioning score) and physical functioning (summarized by the physical functioning score). Scores on all scales range from 0 to 100, with higher scores indicating better quality of life. Forty-two patients were lost to follow-up at 12 months in the adenotonsillectomy group and 34 in the watchful waiting group. One patient was excluded from the difference-in-differences analysis.
Normative values reported as median (IQR).
AHI defined as the average number of apnea or hypopnea (hypopneas with ≥3% oxygen desaturation or arousal) events per hour of sleep, with higher scores indicating more severe obstructive sleep apnea. AHIs were rounded to the nearest tenth. An AHI of 3 or greater at 12 months indicates a progression of disease over the course of the study; an AHI of 5 or greater at 12 months indicates progression to moderate obstructive sleep apnea. Seventy-seven patients were lost to follow-up at 12 months in the adenotonsillectomy group and 75 in the watchful waiting group. One hundred fifty-two patients were excluded from the risk difference analysis.
The between-group difference in prevalence at 12 months was estimated using a linear regression model with robust standard errors, fit to all patients with complete baseline and 12-month information. Point estimates and confidence intervals adjust for baseline apnea-hypopnea index, stratification factors (age ≥6 years, overweight/obese, Black/African American) and site effect. The confidence intervals are also adjusted for multiple comparisons.
Key Secondary Outcomes
Key secondary outcomes are reported in Table 2.
Neurodevelopment and Behavior
The CBCL total problems score (−3.09 [97% CI, −4.90 to −1.28]) and the CBCL internalizing subscale (−3.05 [97% CI, −5.07 to −1.04]) improved more in children randomized to adenotonsillectomy. However, no intervention differences were observed for the pegboard dexterity performance test (0.76 [97% CI, −0.92 to 2.43]).
SDB Symptoms and Quality of Life
Symptoms of SDB (PSQ-SRBD score, −0.16 [97% CI, −0.20 to −0.12]), sleepiness (mESS score, −1.18 [97% CI, −2.15 to −0.21]), disease-specific quality of life (OSA-18 score, −9.75 [97% CI, −12.84 to −6.65]), and generic quality of life (PedsQL total score, 4.76 [97% CI, 1.44-8.09]) improved more in the adenotonsillectomy group. The PedsQL improvements were observed for both the physical subscale (6.53 [97% CI, 1.29-11.78]) and the psychosocial subscale (3.88 [97% CI, 0.89-6.88]).
Blood Pressure
At 12 months, systolic and diastolic blood pressure percentile levels decreased more in the adenotonsillectomy compared with the watchful waiting group (−9.02 [97% CI, −15.49 to −2.54] and −6.52 [97% CI, −11.59 to −1.45], respectively).
Polysomnography
Twelve-month polysomnography was conducted for 306 children; no differences in baseline characteristics were observed for children who did and did not undergo follow-up polysomnography. At 12 months, the AHI exceeded the study’s upper entry criteria (AHI = 3) for 1.3% of children in the adenotonsillectomy group compared with 13.2% of children in the watchful waiting group (risk difference, −11.2% [97% CI, −17.5% to −4.9%]). AHI levels progressed to moderate OSA (AHI ≥5) in 7.2% of children in the watchful waiting group and in no child in the adenotonsillectomy group (−7.1% [97% CI, −11.8% to −2.5%]).
Similar results were observed when analyses were conducted without adjustment for stratification factors (eTable 6 in Supplement 2).
Exploratory Analyses
Other Polysomnography Changes
Median AHI increased from 0.6 to 0.7 in the watchful waiting group but declined from 0.5 to 0.3 in adenotonsillectomy group (difference in changes [log-transformed], −0.6 [95% CI, −0.93 to −0.27]). Changes in the percentage time in each sleep stage did not differ for children by study group although arousal index (a measure of sleep fragmentation) decreased more in the adenotonsillectomy group (eTable 5 in Supplement 2).
Changes in the Proportion of Children With Elevated Behavioral and Symptom Scores
The prevalence of elevated scores for the BRIEF GEC, CBCL-total problems, PSQ-SRBD, mESS, and OSA-18 at each examination are shown in eTable 7 in Supplement 2. At baseline, elevations (denoting potential clinical concern) of at least 1 SDB-related symptom or behavioral measure were found in almost 80% of children in each group. At 12 months, the proportion of study participants with at least 1 elevated score declined to 37.7% (95% CI, 30.7%-44.7%) in the adenotonsillectomy group and 64.6% (95% CI, 57.7%-71.6%) in the watchful waiting group. At 12 months, children in the adenotonsillectomy group compared with the watchful waiting group had a 7.6% (95% CI, −14.6% to−0.7%) lower prevalence of elevated scores for the CBCL total problems score, a 30.9% (95% CI, −39.1% to−22.8%) lower prevalence of elevated PSQ-SRBD scores, a 5.9% (95% CI, −12.7% to 1.0%) lower prevalence of elevated mESS score; and a 13.0% (95% CI, −19.1% to −7.0%) lower prevalence of elevated OSA-18 scores (Table 3). The adenotonsillectomy group also had a 27.3% (95% CI, −34.5% to −20.1%) lower prevalence of frequent loud snoring. Similar results were observed when analyses were unadjusted for stratification factors (eTable 8 in Supplement 2).
Table 3. Exploratory Analyses of the Prevalence of Dichotomous Behavioral and Sleep Symptom Outcomes at 12 Months, by Randomization Group.
| Symptom prevalence at 12 mo, No./total (%) | Risk difference, % (95% CI)a | Relative risk (95% CI)b | ||
|---|---|---|---|---|
| Early adenotonsillectomy (n = 231) | Watchful waiting (n = 227) | |||
| BRIEF GEC T score ≥65c | 25/196 (12.8) | 35/196 (17.9) | −4.0 (−10.2 to 2.3) [n = 391] | 0.83 (0.53-1.29) [n = 391] |
| CBCL total problems T score ≥60d | 30/186 (16.1) | 44/182 (24.2) | −7.6 (−14.6 to −0.7) [n = 365] | 0.66 (0.46-0.94) [n = 365] |
| PSQ-SRBD ≥0.33e | 48/189 (25.4) | 106/193 (54.9) | −30.9 (−39.1 to −22.8) [n = 380] | 0.43 (0.33-0.55) [n = 380] |
| mESS total score ≥10f | 30/188 (16.0) | 44/193 (22.8) | −5.9 (−12.7 to 1.0) [n = 377] | 0.65 (0.45-0.94) [n = 377] |
| OSA-18 ≥60g | 13/188 (6.9) | 41/193 (21.2) | −13.0 (−19.1 to −7.0) [n = 379] | 0.36 (0.21-0.62) [n = 379] |
| Frequent loud snoringh | 15/188 (8.0) | 69/193 (35.8) | −27.3 (−34.5 to −20.1) [n = 379] | 0.22 (0.13-0.38) [n = 379] |
Abbreviations: BRIEF, Behavior Rating Inventory of Executive Function; CBCL, Child Behavior Checklist; GEC, global executive composite; mESS, modified Epworth Sleepiness Scale; OSA, obstructive sleep apnea; PSQ-SRBD, Pediatric Sleep Questionnaire–Sleep-Related Breathing Disorder.
Difference in prevalence at 12 months was estimated using a linear regression model with robust standard errors, fit to all patients with complete baseline and 12-month information. Point estimates and confidence intervals adjust for the value of the symptom scale at baseline, stratification factors (age ≥6 years, overweight/obese, Black/African American), and site effect.
Relative risk was estimated using a Poisson regression model with log link and robust standard errors, fit to all patients with complete baseline and 12-month information. Point estimates and confidence intervals adjust for the value of the symptom scale at baseline, stratification factors (age ≥6, overweight/obese, Black/African American), and site effect.
The BRIEF GEC section comprises summary measures of behavioral regulation, emotion regulation, and cognitive regulation (BRIEF-2, for children aged 5 to 18 years) or inhibitory self-control, flexibility, and emergent metacognition (BRIEF-P, for preschool-aged children). Caregiver scores ranged from 33 to 102, with higher scores indicating worse functioning. A T score of 65 or greater is considered potentially clinically elevated.10
The CBCL total problems summary scale comprises internalizing, externalizing, social, thought, and attention problems. Scores ranged from 24 to 84, with higher scores indicating greater emotional, social, and behavioral problems. A T score of 60 or greater indicates that the child is at risk for clinical problem behaviors.20
Scores range from 0 to 1, with higher scores indicating greater severity. A score of 0.33 or greater suggests a high risk for a pediatric sleep-related breathing disorder.21
Scores range from 0 to 24, with higher scores indicating greater sleepiness. A score of 10 or greater represents excessive daytime sleepiness.22
Scores on the OSA-18 quality of life survey range from 18 to 126, with higher scores indicating a greater negative effect of sleep-disordered breathing on quality of life. A score of 60 or greater represents a moderate to severe negative effect.23
Snoring was assessed using item 1a of the OSA-18 quality of life survey, which uses a Likert scale to ask about the frequency of loud snoring over the last 4 weeks. Possible responses ranged from “none of the time” to “all of the time,” and loud snoring was considered frequent if it occurred “a good bit of the time,” “most of the time,” or “all of the time.”
Subgroup Differences
There was no evidence that intervention effects on the BRIEF GEC, GNG, or AHI differed across most baseline characteristics, including age and obesity (eTable 9 in Supplement 2). However, for the BRIEF end point, children with worse baseline CBCL scores had more improvement with adenotonsillectomy than those with fewer problems, while children with more baseline sleepiness had less improvement. Larger improvements in the GNG were observed in children with fewer SDB symptoms. AHI intervention differences were largest for children with a higher baseline AHI and more sleepiness.
Treatment effects on the primary end points were not affected by the COVID-19 pandemic (eTable 10 in Supplement 2).
Adverse Events
Six serious adverse events were related to perioperative complications (secondary hemorrhage requiring intervention); 1 occurred in a child randomized to watchful waiting who crossed over to surgery (Table 4). No long-term consequences of surgical intervention were seen. The risk of having at least 1 adverse event (related or unrelated to the study) did not differ between the 2 groups (adenotonsillectomy, 175/231 vs watchful waiting, 172/228) (eTable 11 in Supplement 2).
Table 4. Participants With Adverse Events Related to the Study, by Randomized Group and According to Seriousnessa.
| No. of participants | ||||
|---|---|---|---|---|
| Early adenotonsillectomy (n = 231) |
Watchful waiting (n = 227) |
|||
| Serious | Nonserious | Serious | Nonserious | |
| Adverse events related to adenotonsillectomyb | ||||
| Postoperative pain | 11 | |||
| Postoperative bleeding | 5 | 8 | 1 | |
| Dehydration | 3 | |||
| Aspiration pneumonia | 1 | |||
| Adverse events related to other study procedurec | ||||
| Hives (allergy to tape used in PSG) | 1 | |||
| Vomiting (immediately following blood draw) | 1 | |||
Abbreviation: PSG, polysomnography.
Adverse events were defined as any unfavorable or unintended sign, symptom, or disease occurring in a participant at any stage following consent (see Supplement 1 and Supplement 2).
The following events were prespecified as not requiring reporting: intraoperative blood loss 7 mL/kg or less; postoperative pain, hoarseness, or difficulty swallowing lasting less than 21 days and not requiring intravenous hydration or unscheduled evaluation or treatment; postoperative blood-tinged oral or nasal secretions lasting less than 72 hours; velopharyngeal insufficiency lasting less than 2 months and not requiring evaluation or treatment.
The following events were prespecified as not requiring reporting: skin irritation associated with adhesives used in PSGs lasting less than 2 days; temporary depigmentation under areas of PSG sensor attachment lasting less than 1 month; poor sleep during PSG; temporary pain at the site of the phlebotomy lasting less than 48 hours; bleeding or bruising at the site of the phlebotomy not requiring evaluation or treatment; anxiety surrounding behavioral testing not requiring psychiatric attention.
Discussion
To our knowledge, this is the first large, randomized trial to address the effect of adenotonsillectomy for children with mild SDB. Multiple outcomes of importance to children with SDB and their families were evaluated. Adenotonsillectomy compared with watchful waiting resulted in no statistically significant differences in improvement in the 2 primary outcomes of executive function and attention at 12-month follow-up. However, adenotonsillectomy resulted in greater improvements in several key secondary outcomes, including behavioral problems, symptom burden, daytime sleepiness, and quality of life, and greater decrease in blood pressure. AHI was less likely to progress with adenotonsillectomy compared with watchful waiting. Intervention-related AHI changes were observed for children regardless of age, race and ethnicity, or weight.
Neurodevelopmental Functioning
A recent meta-analysis described a wide range of neurodevelopmental deficits in children with SDB compared with controls.24 Deficits have been attributed to the effect of sleep loss, sleep fragmentation, and/or intermittent hypoxemia on the developing brain circuitry.25,26,27 The current study focused on attention and executive function because of the potential sensitivity of the frontoparietal attentional network to sleep disruption. However, neither a direct performance measure of attention (GNG) nor a caregiver report of executive function (BRIEF) improved more with adenotonsillectomy. The inability to detect improvement in a performance-based test is consistent with a meta-analysis and a recent clinical trial of adenotonsillectomy in preschool children that each reported few if any treatment-related improvements in structured measures of cognitive function in children with SDB.28,29
There are several potential explanations for the lack of observed effect on executive function and attention. Improvements may be difficult to discern, given the wide range of factors influencing cognitive function and test performance. Cognitive testing in controlled conditions may not accurately reflect performance in real-world situations when children need to attend to multiple tasks and may be influenced if sleep was suboptimal prior to assessment. Another possible explanation is that past studies that have identified an association between cognitive impairment and SDB may have been biased, as suggested by a large cross-sectional study that showed marked attenuation of an association between habitual snoring and cognition after accounting for multiple confounders.30
The lack of a treatment-related improvement in caregiver-reported executive function is consistent with a recent study of preschool children29 but differs from the small to medium improvement in BRIEF scores reported in the adenotonsillectomy group of children in CHAT.6 Although CHAT participants had higher AHI severity, they had better baseline BRIEF scores than children in the current study.31
Behavioral Problems
Behavioral problems are common parental concerns and can negatively affect children’s psychosocial, emotional, and academic functioning.32,33,34 At baseline, 28% of children in this study had clinically elevated behavioral problem scores (CBCL score ≥60), which suggests a high prevalence of behavioral comorbidity in children with mild SDB. At 12 months, the risk of an elevated behavioral problems score decreased by 7.6% in the adenotonsillectomy group compared with the watchful waiting group. A recent large study reported that smaller cortical gray matter volumes mediated the association between caregiver-reported SDB symptoms and behavioral problems,35 which suggests a biological basis for the treatment-related improvement in behavior observed in PATS.
Symptom Burden, Sleepiness, and Quality of Life
Standardized measurements of caregiver-reported outcomes of day and nighttime symptoms and disease-specific and generic quality of life improved more with adenotonsillectomy than with watchful waiting. Improvement in global quality of life included both physical and psychosocial domains. These broad improvements in symptom-based measures are consistent with studies of adenotonsillectomy performed in patient populations with a wide range of AHI.6,36,37,38 Notably, prior studies found that AHI was not associated or minimally associated with symptom burden or quality of life39,40 and that baseline AHI did not predict behavioral or symptom improvement (although baseline symptoms did).41 In total, the current findings and the prior literature support the importance of symptoms as both outcomes and predictors of treatment response and bring into question the role of the AHI for risk stratification in generally healthy children.
Blood Pressure
Resting systolic and diastolic blood pressure increased in the watchful waiting group but declined in the adenotonsillectomy group, suggesting that persistent mild SDB may negatively affect blood pressure. While adverse effects of recurrent obstructive events and intermittent hypoxemia on blood pressure in children have been reported,42 less is understood regarding the vascular impact of snoring without hypoxemia and whether treatment affects blood pressure in children with mild SDB.43 The current findings suggest that mechanisms apart from intermittent hypoxemia, such as sympathetic activation related to increased work of breathing, negatively influence vascular outcomes.
Polysomnography
Improvement of AHI levels, sleep-related hypoxemia, and sleep fragmentation are common treatment goals. In the current study, which included both preschool- and school-aged children, AHI levels worsened during 12-month follow-up in the watchful waiting group but not in the adenotonsillectomy group, a pattern consistent with previous studies.6,29,38 In exploratory analyses, the effect of adenotonsillectomy on AHI was unassociated with age, sex, socioeconomic status, or obesity but appeared larger in children with more sleepiness and higher AHI. Similar to a prior study,29 the current study found that arousal index declined with adenotonsillectomy, but sleep stage distributions did not change. The lack of improvement in depth of sleep may be due to the limitations of single-night polysomnography.
Safety
Among children randomized to adenotonsillectomy, 2.2% experienced a serious adverse event associated with surgery. In children randomized to watchful waiting, 2.6% were adjudicated to have a treatment failure. This study therefore supports the overall safety of both surgery and watchful waiting but suggests the need for ongoing monitoring of children treated conservatively.
Limitations
Study strengths include the randomized design; the large, geographically and racially diverse sample of preschool- and school-aged children; standardized measurements; masking of key research personnel; and high 12-month follow-up rates. However, caregivers could not be masked to the intervention, and this may have influenced several outcome assessments. Learning and memory, which may be sensitive to hippocampal damage secondary to OSA,44 were not assessed.
Clinical Implications
These findings do not provide support for adenotonsillectomy in children with mild SDB with the goal of improving cognition, although it is possible that differences may have been observed with additional follow-up. In contrast, surgery improved secondary outcomes, including behavior, symptom burden, quality of life, blood pressure, and AHI level over 12 months. The current findings suggest that a low AHI on polysomnography does not preclude the possible benefits of surgery in children with habitual snoring. The mechanisms by which mild SDB contribute to adverse outcomes are unclear but may relate to chronic effects of subtle periods of hypoventilation and increased work of breathing, which are not well characterized by AHI level but may improve with adenotonsillectomy.45 Although children in this study were identified by caregiver-reported snoring and infrequent obstructive events on polysomnography, the majority had at least 1 elevated score on several validated SDB symptom or behavioral questionnaires. Future research is needed to develop easy-to-use screening instruments for identifying which children are more likely to benefit from adenotonsillectomy compared with watchful waiting. There also is a need to identify objective measures that better characterize physiological stressors that mediate SDB-related health outcomes.
Conclusions
In children with mild SDB, adenotonsillectomy, compared with watchful waiting, did not significantly improve executive function or attention at 12 months. However, children with adenotonsillectomy had improved secondary outcomes including behavior, symptoms, and quality of life and decreased blood pressure and AHI, on 12-month follow-up.
Study Protocol and Statistical Analysis Plan
Supplemental Study Information
Supplemental Methods
Supplementary Results
eTables 1-11
eFigure
eReferences
Study Investigators
Data Sharing Statement
References
- 1.Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714-e755. doi: 10.1542/peds.2012-1672 [DOI] [PubMed] [Google Scholar]
- 2.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149(3):342-347. doi: 10.1016/j.jpeds.2006.04.061 [DOI] [PubMed] [Google Scholar]
- 3.Wang R, Dong Y, Weng J, et al. Associations among neighborhood, race, and sleep apnea severity in children: a six-city analysis. Ann Am Thorac Soc. 2017;14(1):76-84. doi: 10.1513/AnnalsATS.201609-662OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas S, Patel S, Gummalla P, Tablizo MA, Kier C. You cannot hit snooze on OSA: sequelae of pediatric obstructive sleep apnea. Children (Basel). 2022;9(2):261. doi: 10.3390/children9020261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RF, Zhang J, Chorney SR, et al. Estimations of inpatient and ambulatory pediatric tonsillectomy in the United States: a cross-sectional analysis. Otolaryngol Head Neck Surg. 2023;169(2):258-266. doi: 10.1002/ohn.268 [DOI] [PubMed] [Google Scholar]
- 6.Marcus CL, Moore RH, Rosen CL, et al. ; Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366-2376. doi: 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emancipator JL, Storfer-Isser A, Taylor HG, et al. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch Pediatr Adolesc Med. 2006;160(2):203-210. doi: 10.1001/archpedi.160.2.203 [DOI] [PubMed] [Google Scholar]
- 8.Weiss JT, Donlea JM. Roles for sleep in neural and behavioral plasticity: reviewing variation in the consequences of sleep loss. Front Behav Neurosci. 2022;15:777799. doi: 10.3389/fnbeh.2021.777799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R, Bakker JP, Chervin RD, et al. Pediatric Adenotonsillectomy Trial for Snoring (PATS): protocol for a randomised controlled trial to evaluate the effect of adenotonsillectomy in treating mild obstructive sleep-disordered breathing. BMJ Open. 2020;10(3):e033889. doi: 10.1136/bmjopen-2019-033889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function (BRIEF). PAR website. Published 1999. Accessed November 3, 2023. https://www.parinc.com/Products/Pkey/23
- 11.Gioia GA, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol. 2002;8(4):249-257. doi: 10.1076/chin.8.4.249.13513 [DOI] [PubMed] [Google Scholar]
- 12.Clark CAC, Cook K, Wang R, et al. Psychometric properties of a combined Go/No-go and continuous performance task across childhood. Psychol Assess. 2023;35(4):353-365. doi: 10.1037/pas0001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653-666. doi: 10.1093/aje/kwm348 [DOI] [PubMed] [Google Scholar]
- 14.Holm S. A simple sequentially rejective multiple test procedure. Scand J of Stat. 1979:65-70. [Google Scholar]
- 15.Ware JH, Harrington D, Hunter DJ, D’Agostino RB Sr. Missing data. N Engl J Med. 2012;367(14):1353-1354. doi: 10.1056/NEJMsm121004322475612 [DOI] [Google Scholar]
- 16.Molenberghs G, Thijs H, Jansen I, et al. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004;5(3):445-464. doi: 10.1093/biostatistics/kxh001 [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 18.Benjamini Y, Yekutieli D. False discovery rate–adjusted multiple confidence intervals for selected parameters. J Am Stat Assoc. 2005;100(469):71-81. doi: 10.1198/016214504000001907 [DOI] [Google Scholar]
- 19.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36(6):1551-1569. doi: 10.1016/S0031-3955(16)36806-7 [DOI] [PubMed] [Google Scholar]
- 20.Guerrera S, Menghini D, Napoli E, Di Vara S, Valeri G, Vicari S. Assessment of psychopathological comorbidities in children and adolescents with autism spectrum disorder using the child behavior checklist. Front Psychiatry. 2019;10:535. doi: 10.3389/fpsyt.2019.00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21-32. doi: 10.1016/S1389-9457(99)00009-X [DOI] [PubMed] [Google Scholar]
- 22.Janssen KC, Phillipson S, O’Connor J, Johns MW. Validation of the Epworth Sleepiness Scale for Children and Adolescents using Rasch analysis. Sleep Med. 2017;33:30-35. doi: 10.1016/j.sleep.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 23.Mitchell RB, Kelly J, Call E, Yao N. Long-term changes in quality of life after surgery for pediatric obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2004;130(4):409-412. doi: 10.1001/archotol.130.4.409 [DOI] [PubMed] [Google Scholar]
- 24.Menzies B, Teng A, Burns M, Lah S. Neurocognitive outcomes of children with sleep disordered breathing: a systematic review with meta-analysis. Sleep Med Rev. 2022;63:101629. doi: 10.1016/j.smrv.2022.101629 [DOI] [PubMed] [Google Scholar]
- 25.Krause AJ, Simon EB, Mander BA, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017;18(7):404-418. doi: 10.1038/nrn.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dang-Vu TT, Desseilles M, Peigneux P, Maquet P. A role for sleep in brain plasticity. Pediatr Rehabil. 2006;9(2):98-118. doi: 10.1080/13638490500138702 [DOI] [PubMed] [Google Scholar]
- 27.Brooks SJ, Katz ES, Stamoulis C. Shorter duration and lower quality sleep have widespread detrimental effects on developing functional brain networks in early adolescence. Cereb Cortex Commun. 2021;3(1):tgab062. doi: 10.1093/texcom/tgab062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinnadurai S, Jordan AK, Sathe NA, Fonnesbeck C, McPheeters ML, Francis DO. Tonsillectomy for obstructive sleep-disordered breathing: a meta-analysis. Pediatrics. 2017;139(2):e20163491. doi: 10.1542/peds.2016-3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waters KA, Chawla J, Harris M-A, et al. Cognition after early tonsillectomy for mild OSA. Pediatrics. 2020;145(2):e20191450. doi: 10.1542/peds.2019-1450 [DOI] [PubMed] [Google Scholar]
- 30.Isaiah A, Ernst T, Cloak CC, Clark DB, Chang L. Association between habitual snoring and cognitive performance among a large sample of preadolescent children. JAMA Otolaryngol Head Neck Surg. 2021;147(5):426-433. doi: 10.1001/jamaoto.2020.5712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu PK, Radcliffe J, Gerry Taylor H, et al. Neurobehavioral morbidity of pediatric mild sleep-disordered breathing and obstructive sleep apnea. Sleep. 2022;45(5):zsac035. doi: 10.1093/sleep/zsac035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams J, Klinepeter K, Palmes G, Pulley A, Foy JM. Diagnosis and treatment of behavioral health disorders in pediatric practice. Pediatrics. 2004;114(3):601-606. doi: 10.1542/peds.2004-0090 [DOI] [PubMed] [Google Scholar]
- 33.Ogundele MO. Behavioural and emotional disorders in childhood: a brief overview for paediatricians. World J Clin Pediatr. 2018;7(1):9-26. doi: 10.5409/wjcp.v7.i1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaiah A, Ernst T, Cloak CC, Clark DB, Chang L. Associations between frontal lobe structure, parent-reported obstructive sleep disordered breathing and childhood behavior in the ABCD dataset. Nat Commun. 2021;12(1):2205. doi: 10.1038/s41467-021-22534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldassari CM. Do young children with nonsevere obstructive sleep apnea benefit from adenotonsillectomy? the CHAT vs the KATE study. JAMA Otolaryngol Head Neck Surg. 2020;146(7):654-655. doi: 10.1001/jamaoto.2020.0878 [DOI] [PubMed] [Google Scholar]
- 37.Todd CA, Bareiss AK, McCoul ED, Rodriguez KH. Adenotonsillectomy for obstructive sleep apnea and quality of life: systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2017;157(5):767-773. doi: 10.1177/0194599817717480 [DOI] [PubMed] [Google Scholar]
- 38.Fehrm J, Nerfeldt P, Browaldh N, Friberg D. Effectiveness of adenotonsillectomy vs watchful waiting in young children with mild to moderate obstructive sleep apnea: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2020;146(7):647-654. doi: 10.1001/jamaoto.2020.0869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen CL, Wang R, Taylor HG, et al. Utility of symptoms to predict treatment outcomes in obstructive sleep apnea syndrome. Pediatrics. 2015;135(3):e662-e671. doi: 10.1542/peds.2014-3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell RB, Garetz S, Moore RH, et al. The use of clinical parameters to predict obstructive sleep apnea syndrome severity in children: the Childhood Adenotonsillectomy (CHAT) study randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(2):130-136. doi: 10.1001/jamaoto.2014.3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isaiah A, Spanier AJ, Grattan LM, Wang Y, Pereira KD. Predictors of behavioral changes after adenotonsillectomy in pediatric obstructive sleep apnea: a secondary analysis of a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2020;146(10):900-908. doi: 10.1001/jamaoto.2020.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Driscoll DM, Horne RSC, Davey MJ, et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. 2011;12(5):483-488. doi: 10.1016/j.sleep.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 43.Baker-Smith CM, Isaiah A, Melendres MC, et al. ; American Heart Association Athero, Hypertension and Obesity in the Young Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young . Sleep-disordered breathing and cardiovascular disease in children and adolescents: a scientific statement from the American Heart Association. J Am Heart Assoc. 2021;10(18):e022427. doi: 10.1161/JAHA.121.022427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cha J, Zea-Hernandez JA, Sin S, et al. The effects of obstructive sleep apnea syndrome on the dentate gyrus and learning and memory in children. J Neurosci. 2017;37(16):4280-4288. doi: 10.1523/JNEUROSCI.3583-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guilleminault C, Huang Y-S, Chin W-C, Okorie C. The nocturnal-polysomnogram and “non-hypoxic sleep-disordered-breathing” in children. Sleep Med. 2019;60:31-44. doi: 10.1016/j.sleep.2018.11.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol and Statistical Analysis Plan
Supplemental Study Information
Supplemental Methods
Supplementary Results
eTables 1-11
eFigure
eReferences
Study Investigators
Data Sharing Statement


