Highlights
-
•
Current guidelines recommend either supraglottic airway or endotracheal intubation for advanced airway management during in-hospital cardiac arrest.
-
•
The evidence base guiding cardiac arrest advanced airway management draws primarily from clinical trials conducted for out-of-hospital cardiac arrest.
-
•
The Hospital Airway Resuscitation Trial (HART) is a pragmatic clinical trial being conducted in an in-hospital cardiac arrest population comparing a strategy of first choice endotracheal intubation to a strategy of first choice supraglottic airway.
Keywords: In-hospital cardiac arrest, Supraglottic airway, Advanced airway management, Clinical trial
Abstract
Guidelines for the management of in-hospital cardiac arrest resuscitation are often drawn from evidence generated in out-of-hospital cardiac arrest populations and applied to the in-hospital setting. Approach to airway management during resuscitation is one example of this phenomenon, with the recommendation to place either a supraglottic airway or endotracheal tube when performing advanced airway management during in-hospital cardiac arrest based mainly in clinical trials conducted in the out-of-hospital setting. The Hospital Airway Resuscitation Trial (HART) is a pragmatic cluster-randomized superiority trial comparing a strategy of first choice supraglottic airway to a strategy of first choice endotracheal intubation during resuscitation from in-hospital cardiac arrest. The design includes a number of innovative elements such as a highly pragmatic design drawing from electronic health records and a novel primary outcome measure for cardiac arrest trials—alive-and-ventilator free days. Many of the topics explored in the design of HART have wide relevance to other trials in in-hospital cardiac arrest populations.
Introduction and rationale
The modality of advanced airway management during in-hospital cardiac arrest is an integral aspect of resuscitation, with substantial implications for both the patient and the resuscitation team. While large, randomized trials of different airway management strategies have been performed in the out-of-hospital cardiac arrest (OHCA) setting, there are no randomized data to guide inpatient practice.1, 2, 3
The American Heart Association (AHA) has published distinct chains of survival for in-hospital cardiac arrest (IHCA) and OHCA.4, 5 These separate chains of survival reflect significant differences between OHCA and IHCA. As compared to OHCA, IHCA outcomes are more dependent upon surveillance and prevention of clinical decompensation. IHCA response is led by responders who are not bystanders, but professionals who can rapidly initiate advanced life support interventions and continue care for the patient after succesful resuscitation. IHCAs are also more likely to result from respiratory failure and occur in patients with existing acute and chronic medical conditions.6, 7 Despite the patient and provider differences between OHCA and IHCA, guidelines for airway management during cardiac arrest are drawn primarily from OHCA studies and extrapolated to the in-hospital population. Present guidelines from the AHA recommend either advanced airway placement or no advanced airway placement with bag-valve mask ventilation only during cardiac arrest resuscitation. When an advanced airway is placed in the inpatient setting by expert providers, either endotracheal intubation or supraglottic airway (SGA) is recommended.8 Although placement of a SGA is less likely to result in disruptions to cardiopulmonary resuscitation and may be placed earlier in an arrest, the fact that IHCA is more frequently of primary respiratory etiology and airway management is performed by trained physicians in the hospital environment may favor endotracheal intubation—leading to equipoise for this critical resuscitation practice.
The Hospital Airway Resuscitation Trial (HART) is designed to test whether a strategy of first-choice endotracheal intubation or first-choice SGA is superior for IHCA. HART is a highly pragmatic trial, designed to leverage existing clinical procedures and clinical data collection as part of a learning healthcare system.9 HART will add to the in-hospital resuscitation evidence base and allow for guidelines built on a foundation more relevant to IHCA. We describe the protocol and proposed statistical analysis plan (SAP) for the HART trial, which was designed by the trial chief investigators and statisticians.
Design and setting
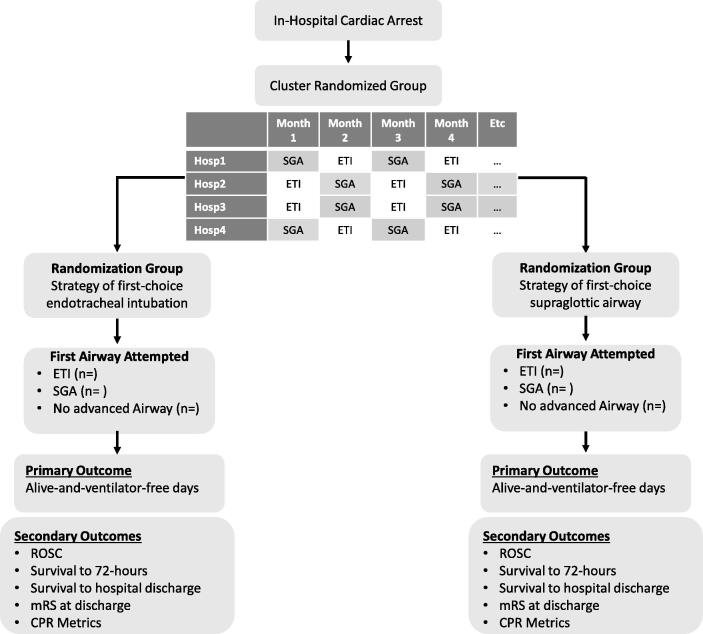
HART is a pragmatic, cluster-randomized with crossover superiority trial. The SPIRIT guidelines were followed in the creation of the trial protocol.10 Clinical trial registration information is summarized in Table 1. Flow through the trial is described in Fig. 1.
Table 1.
HART registration information.
| Data Category | Information |
|---|---|
| Trial Name | Hospital Airway Resuscitation Trial |
| Short Trial Name | HART |
| Registration and Approval Information | |
| Primary Registry | Clinicaltrials.gov |
| Clinical Trial Registration Number | NCT05520762 |
| Institutional Review Board and IRB Number | Office of Human Research Affairs at Albert Einstein College of Medicine |
| Principal Investigator/Sponsor | Ari Moskowitz, MD MPH |
| Funding Source | National Heart Lung and Blood Institute (NHLBI) |
| Funding Source Number | R61 HL162980; R33HL162980 |
| Enrollment Settings | |
| Country | United States of America |
| Locations | Montefiore Health System The Bronx, New York City • Moses Campus • Jack D. Weiler Campus • Wakefield Campus White Plains, New York • White Plains Hospital |
Fig. 1.
Trial flow diagram.
Inclusion and exclusion criteria
The inclusion and exclusion criteria are listed in Table 2.
Table 2.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adult aged ≥ 18 years | Cardiac arrest in the Operating Room or other area not responded to by critical care or emergency department teams. Arrests responded to by non-critical care physicians (e.g. emergency physicians) at White Plains Hospital are not included. Cardiac arrests in which the critical care or emergency department airway manager have opted out of trial procedures will not be included. |
| Admitted to the hospital for any condition | Cardiac arrest in which an invasive airway (i.e. endotracheal tube, tracheostomy tube) is already in place. This will include arrests that occur in the immediate peri-intubation period. |
| Suffered in-hospital cardiac arrest (loss of pulse and ≥ 2 minutes of chest compressions) | Patients with Do Not Resuscitate or Do Not Intubate orders |
| Need for assisted ventilation | Non-index cardiac arrest. |
Consent
HART is being conducted under a Waiver of Informed Consent as it meets all requirements under the Human Subjects Research Protections (OHRP) 45 CFR 46.116 and follows previously described criteria for the application of a waiver of informed consent to critical care trials.11 HART compares two advanced airway management strategies recommended equally by the American Heart Association. Thus, the trial will inform care variation that is otherwise arbitrary. The trial is not blinded and clinicians are encouraged to deviate from assigned ‘first choice’ airway at any time they feel that one airway approach is not appropriate in a given clinical scenario. As the trial is conducted in a population of patients suffering cardiac arrest, and the intervention is cluster randomized by hospital, consenting patients in advance of study procedures is not feasible. Although cardiac arrest is a high-risk scenario and many patients do not achieve return of spontaneous circulation, the added risk of study procedures above usual care is minimal. Prior to the start of study procedures, the investigators conducted clinician and community stakeholder consultation and public disclosure.
The trial was approved by the Einstein Institutional Review Board (IRB# 2021-13691).
Randomization, enrollment, and data collection
HART is a cluster-randomized with crossover trial. Each hospital is considered a cluster and crossover occurs on a monthly basis. A randomization list was created by the trial statistician prior to the start of enrollment.
Enrollment is performed through daily review of electronic code sheets by trained research coordinators. Patients meeting all inclusion and no exclusion criteria are entered into the electronic data capture tool (REDCap). Given the pragmatic nature of HART, all required data elements are abstracted directly from the Electronic Health Record. A standardized data dictionary was created and guides completion of the case report form. Double data entry of key variables and regular data audits are performed.
The clinician carrying out the airway interventions of each cardiac arrest enrolled is sent a brief survey after the arrest. The survey includes a number of questions regarding airway management during the cardiac arrest event.
Cardiopulmonary resuscitation (CPR) data is abstracted from Zoll CaseReview (Zoll R-Series Defibrillator, RescueNet® CaseReview), a comprehensive CPR quality assessment tool that is being used for resuscitation quality assessment at the study hospitals.
Trial interventions
Clusters in the trial are randomized to either 1) a strategy of first choice endotracheal intubation or 2) a strategy of first choice supraglottic airway. There is no requirement that a clinician place an advanced airway during the cardiac arrest nor is there recommended timing for advanced airway placement. If the clinician decides to place an advanced airway, they place the assigned first choice airway unless that airway type would not be appropriate for the clinician scenario. All care teams enrolling patients for HART primarily use the iGel supraglottic airway (https://www.intersurgical.com/info/igel). While this form of supraglottic airway is not required for the trial, it accounts for the vast majority of supraglottic airways placed at all hospitals.
Beyond the above trial intervention, all other elements of cardiac arrest care and post-arrest care are at the discretion of the clinical team. This includes any decision to change airway management device or approach.
Training and simulation
Airway management with both endotracheal intubation and SGA are standard elements of training for all emergency airway operators at the study hospitals. This practice pattern is supported by the American Society of Anesthesiologists Management of the Difficult Airway practice guideline.12 All airway operators in this trial are emergency or critical care trained physicians or their designees under direct supervision. As part of HART, additional simulation and training sessions were added to ensure all staff are fully comfortable with advanced airway management during IHCA. Training sessions additionally served as opportunities for education and reinforcement of trial procedures. These training sessions included 1) in situ task-trainer mannequin simulation for all clinicians participating in cardiac arrest advanced airway management 2) viewing of a training video detailing study procedures as well as advanced airway management techniques with both SGA and intubation during cardiac arrest 3) regular re-training of new staff as needed and 4) opportunities for simulation laboratory training as requested.
Outcomes
Primary outcome
The primary outcome is alive-and-ventilator free days (AVFD), defined as the number of days in the 28-day period after IHCA when the patient was alive and breathing independently of invasive mechanical ventilation. Measurement of AVFD follows a traditional first-on/last-off approach. Ventilation for any part of a day constitutes a ventilator day. The day of the arrest is considered day 0.
For the measurement of AVFD, death after return of spontaneous circulation (ROSC) but before 28-days is given a value of 0. Failure to achieve ROSC is given a value of −1. In this pragmatic trial, patients will not be followed once they have left the hospital. A patient who leaves the hospital alive and is not discharged to a hospice setting will be considered to have lived to 28-days. A patient discharged to a hospice facility will be assessed as having died on the day of hospital discharge. A patient who is discharged requiring invasive mechanical ventilation for any part of the day, will be assumed to have remained on invasive mechanical ventilation through 28-days.
AVFD is a composite outcome that measures the effect of an intervention on morbidity while also accounting for the competing risk of death.13, 14 In the case of cardiac arrest, ventilator dependence may reflect both pulmonary and neurologic dysfunction (i.e. a patient cannot be extubated due to poor neurologic status). Neurologic outcomes traditionally used in cardiac arrest interventions were considered, but may not apply given that a minority of IHCA victims succumb as a result of neurologic injury.15 This is an important contrast with out-of-hospital cardiac arrest where neurologic injury is the predominant reason for death.16 While the International Liaison Committee on Resuscitation (ILCOR) provides a core outcome set for cardiac arrest, these are geared primarily to the out-of-hospital arrest population.17 The decision to assign failure to achieve ROSC a score of −1 was made to reflect that attainment of ROSC is itself an important patient-centered outcome, and that failure to achieve ROSC is worse than the achievement of ROSC but subsequent death. This approach is similar to that taken by recent high-impact clinical trials in critical care.18, 19, 20
Secondary outcomes
Details and definitions of secondary outcomes can be found in Table 3.
Table 3.
Outcome definitions.
| Outcome | Definition |
|---|---|
| Return of spontaneous circulation (ROSC) | Rate of ROSC defined as at least 20 minutes of continuous spontaneous circulation without chest compressions. Patients cannulated for extracorporeal or placed on other mechanical support during the cardiac arrest will be categorized as having achieved ROSC. |
| 72-hour survival | Survival to 72-hours after time of ROSC. |
| Survival to hospital discharge | Survival to hospital discharge. Patients who are alive and remain in the hospital at 60 days after ROSC will be categorized as having survived to hospital discharge. |
| Prolonged pauses | Number of prolonged pauses (>5 seconds) in chest compressions during active Cardiopulmonary Resuscitation (CPR). |
| Longest pause | Longest pause in chest compressions. |
| Chest compression fraction | Percentage of total cardiac arrest time during which chest compressions are being performed. |
| Rate of ventilator-associated pneumonia (VAP) | Rate of VAP in the 7 days after cardiac arrest. VAP defined as new pneumonia while receiving mechanical ventilation after cardiac arrest. New pneumonia defined by 1) new pulmonary infiltrate on chest imaging 2) either new/worsening fever or leukocytosis 3) either change in sputum composition/frequency or worsening gas exchange or new/worsening cough or dyspnea. |
| Modified Rankin Scale | Modified Rankin Scale (mRS) at time of hospital discharge. |
| Time to epinephrine | Time from initiation of chest compressions to first epinephrine for cardiac arrest with initial non-shockable rhythm. |
| Time to advanced airway | Time from initiation of chest compressions to advanced airway placement. |
Statistical analysis plan
Sample size justification
We project that each of the four hospitals will enroll 6 subjects per month over 3.5 years for a total of 1008 patients. The assumptions underlying the assessment of power with this sample size were based on data from an observational cohort of IHCA patients at the study hospitals. The following was also assumed: hospitals (clusters) crossover to the alternate intervention on a month-to-month basis, a within-period intra-cluster correlation (WPICC) of 0.01 based on our preliminary data, and a between-period intra-cluster correlation of 0.008 (corresponding to cluster autocorrelation coefficient (CAC) of 0.008/0.01 = 0.8). We anticipate a ROSC rate of 52 % in the endotracheal intubation group and 62 % in the supraglottic airway group, a hospital survival rate of 8 % in the endotracheal intubation group and 13 % in the supraglottic airway group, and a ventilator free day average of 19 days (SD 10 days) in the endotracheal intubation group and 21 (SD 10 days) days in the supraglottic airway group among survivors.
Power to detect a difference between intervention arms with respect to the primary outcome of AVFD was estimated through simulation studies. To simulate the data, we applied a mixed effects multinomial logistic regression model to randomly generate death/ROSC/survival status for each individual, and a mixed effects beta-binomial regression model to randomly generate AVFD data for survivors. In both models, random effects for cluster and cluster-period were included to account for the cluster randomized cross-over design, and model parameters were specified to satisfy the assumed effects sizes and correlations. For each simulated data set, the AVFD outcome was analyzed using a mixed effects proportional odds logistic regression model with 30 ordinal categories (−1 to 28 days), as our earlier simulation studies indicated that this approach tended to have greater power than alternative statistical methods. Under these conditions, 1000 simulated data sets were generated and analyzed. Power, defined as the proportion of times the intervention effect was statistically significant (two-sided p < 0.05), was determined to be greater than 93 % with 1008 patients. Minimal drop-out is expected in this trial but to account for a 5 % missing data rate, 1060 patients will be enrolled.
General principles
The primary analysis will be conducted using an intention-to-treat approach, wherein each patient is evaluated as part of the group randomized—regardless of which (if any) advanced airway was placed. A secondary per-protocol analysis will also be performed.
Prespecified subgroup analyses will be conducted regardless of whether a statistically significant treatment effect on the primary outcome is observed in the overall sample. No formal adjustments for multiplicity of testing will be applied, but the outcome will be ordered by degree of importance and significant test results will be interpreted in light of the multiple comparisons.
All tests will be two-sided and the nominal level of statistical significance (α) will be 5 %. All CIs will have 95 % coverage.
Analysis of the primary outcome
For all patient outcomes, we will use the same overall analytic strategy of fitting generalized linear mixed models (GLMM) that include a fixed effect for the intervention, a random cluster (site) effect, and a random cluster-period effect to account for the within- and between-period intra-cluster correlations in the data. To analyze the primary outcome of AVFD, the GLMM will be specified as a proportional odds regression model. The covariance matrix of the cluster-period random effects in the model will follow an exchangeable correlation structure. The correlation among subjects from the same cluster but different periods will be analyzed as decaying as the time between periods increases according to a first order autoregressive model.
Robustness of the intervention effect observed in the primary analyses will be assessed by performing the following sensitivity analyses1: since randomization is at the cluster rather individual level, additional analyses will be performed to adjust for any imbalances across comparison groups in site and patient characteristics, such as arrest location, demographics, and receipt of vasopressors at the time of the arrest—including these as additional covariates in the model if there are differences between groups2; per protocol analysis including only the patients who actually received the assigned treatment. Patients whose resuscitation ended without an advanced airway (e.g. patients who received bag-valve-mask only), will be analyzed as having received care per protocol. Given the potential for bias in this approach, the results of the per-protocol analysis will be considered exploratory.
Analysis of secondary outcomes
Analysis of secondary outcomes will follow the same general GLMM strategy as for the primary outcome to account for the trial design. Logit or identity link functions will be applied as appropriate based on the distribution of the outcome.
Missing data will be addressed using both multiple imputation with chained equations and list-wise deletion. Sensitivity analyses using pattern mixture modeling will be applied to evaluate robustness of results to non-random missingness. CPR metrics will only be assessed for those patients in whom the data is available.
Analysis of pre-specified subgroups
Subgroup analyses will be performed to assess heterogeneity of treatment effect across subgroups defined by initial rhythm (non-shockable vs shockable), location of arrest (emergency department or inpatient), and arrest etiology (primary respiratory vs. non respiratory). Intervention effects will be estimated separately in each subgroup; in exploratory analyses, relevant interaction terms between the variables that define the subgroups and intervention status will also be included in the regression models.
Interim analysis
Two interim analyses of the data will be performed. Stopping boundaries for efficacy will be based on the O’Brien-Fleming criteria (Table 4) to maintain the overall Type I error rate at 5 %. There will be no early stopping for futility as obtaining point estimates in either direction will be informative in this trial. In addition, there are a number of exploratory outcomes which will be of interest even if we fail to reject the null hypothesis for the primary outcome.
Table 4.
O’Brien-Fleming stopping criteria rules for HART.
| Analysis | Proportion of statistical information | Cumulative sample size | α | Boundary |Z| |
|---|---|---|---|---|
| #1 | 0.33 | 346 | 0.0006 | 3.438 |
| #2 | 0.67 | 703 | 0.0151 | 2.431 |
| #3 | 1.00 | 1060 | 0.0471 | 1.985 |
Safety and adverse events
Data safety and monitoring
An independent, five member data safety and monitoring board was created to oversee the safety and conduct of the clinical trial. The data safety and monitoring board is comprised of investigators with expertise in the fields of critical care, cardiac arrest resuscitation, clinical trial design, bioethics, and statistics. A data safety and monitoring board charter was agreed upon prior to trial start.
Protocol specified exempt serious events and adverse events
Given that many patients suffering cardiac arrest experience severe morbidity and high rates of mortality, traditional approaches to adverse event monitoring do not apply.21 As such, a number of protocol-specified exempt serious events (PSESE) were identified as expected to occur as part of the natural history of cardiac arrest (Table 5). These are recorded in the electronic data capture and reported to the data safety and monitoring board at regular meetings. Suspected unexpected serious adverse events not included as PSESEs will be recorded and reported in a timely fashion.
Table 5.
Protocol specified exempt serious events.
| Protocol Specified Exempt Serious Events |
|---|
| • Failed first-pass airway attempt (for endotracheal intubation) |
| • Advanced airway placement in the esophagus or other extra-tracheal structure (both during the arrest and within 24-hours of the arrest) |
| • Advanced airway dislodgement after placement (both during the arrest and within 24-hours of the arrest) |
| • Aspiration of gastric contents (both during the arrest and within 24-hours of the arrest) |
| • Trauma to the oropharynx, trachea, or esophagus (both during the arrest and within 24-hours of the arrest) |
| • Pneumothorax (both during the arrest and within 24-hours of the arrest) |
| • Recurrent cardiac arrest (both during the arrest and within 24-hours of the arrest) |
Trial progress
The first patient was enrolled in HART on February 6th, 2023. As of October 5th, 2023 there have been 220 patients enrolled into the trial.
Discussion
Advanced airway management is a complex but fundamental aspect of IHCA management, and a source of substantial practice variability.22, 23 Supraglottic airway placement has hypothesized advantages over endotracheal intubation including ease of placement, improved CPR metrics, fewer airway complications, and lower intrathoracic pressures which can improve venous return to the heart.1 Supraglottic airways, however, are less definitive airways than endotracheal tubes, and patients may have a higher risk of gastric contents aspiration and lower levels of oxygenation and ventilation. As endotracheal intubation during CPR is complex, there are limited clinicians in the hospital who are adequately trained in this procedure. Should supraglottic airways be an acceptable (or even superior) alternative, this would have important implications for the composition, training, and actions of hospital cardiac arrest response teams.
The Hospital Airway Resuscitation Trial seeks to expand the evidence base for IHCA by comparing a strategy of first choice endotracheal intubation to a strategy of first choice supraglottic airway. This trial is innovative not only with respect to the focus on IHCA, a heavily understudied disease entity relative to the burden of IHCA on public health24, but also in the highly pragmatic nature of the trial design. Use of the electronic health record for patient identification and data capture allows for a highly efficient trial, and is a step towards the more seamless evidence generation framework proposed by the concept of a Learning Health System. The primary outcome of alive-and-ventilator free days is also novel as an outcome measure for cardiac arrest trials, and the results of HART may provide further basis for this use of this outcome measure in future cardiac arrest trials.
HART joins the Airways-3 trial (ISRCTN17720457) in the effort to better understand optimal approaches to advanced airway management during IHCA.25 These clinical trials, in combination with an increasing focus on IHCA resuscitation practices, represent a step towards improving outcomes for IHCA patients worldwide.
Funding
The Hospital Airway Resuscitation Trial is funded by the National Heart, Lung, and Blood Institute (R33HL162980).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ari Moskowitz, Michelle Gong, Michael Donnino, David Esses, Mimi Kim, Ariel Shiloh, Daniel Fein, Samuel Rednor, and Henry Wang report financial support was provided by National Institutes of Health.
Acknowledgments
The authors acknowledge the contributions of the following key individuals:
-
•
HART senior administrator: Daniel Ceusters.
-
•
Research coordinators: Brenda Lopez, Martha Torres, Ofelia Garcia, Elizabeth Nelson, Michelle Dominguez.
-
•
Data Safety and Monitoring Board: Nicholas Johnson (chair), Johnathan Casey, Elizabeth Cheung, Jonah Rubin, Ariel Mueller
References
- 1.Wang H.E., Schmicker R.H., Daya M.R., et al. Effect of a strategy of initial laryngeal tube insertion vs endotracheal intubation on 72-hour survival in adults with out-of-hospital cardiac arrest: a randomized clinical trial. Jama. 2018;320:769–778. doi: 10.1001/jama.2018.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benger J.R., Kirby K., Black S., et al. Effect of a strategy of a supraglottic airway device vs tracheal intubation during out-of-hospital cardiac arrest on functional outcome: the AIRWAYS-2 randomized clinical trial. Jama. 2018;320:779–791. doi: 10.1001/jama.2018.11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabre P., Penaloza A., Pinero D., et al. Effect of bag-mask ventilation vs endotracheal intubation during cardiopulmonary resuscitation on neurological outcome after out-of-hospital cardiorespiratory arrest: a randomized clinical trial. Jama. 2018;319:779–787. doi: 10.1001/jama.2018.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg K.M., Cheng A., Panchal A.R., et al. Part 7: systems of care: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142:S580–S604. doi: 10.1161/CIR.0000000000000899. [DOI] [PubMed] [Google Scholar]
- 5.Cummins R.O., Ornato J.P., Thies W.H., Pepe P.E. Improving survival from sudden cardiac arrest: the “chain of survival” concept. A statement for health professionals from the advanced cardiac life support subcommittee and the emergency cardiac care committee, american heart association. Circulation. 1991;83:1832–1847. doi: 10.1161/01.cir.83.5.1832. [DOI] [PubMed] [Google Scholar]
- 6.Andersen L.W., Holmberg M.J., Berg K.M., Donnino M.W., Granfeldt A. In-hospital cardiac arrest: a review. Jama. 2019;321:1200–1210. doi: 10.1001/jama.2019.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balakrishnan R., Andrea L., Moskowitz A. Closing the evidence gap for in-hospital cardiac arrest: a focus on advanced airway management. J Thorac Dis. 2023;15:4033–4039. doi: 10.21037/jtd-23-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panchal A.R., Bartos J.A., Cabañas J.G., et al. Part 3: adult basic and advanced life support: 2020 american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142:S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 9.Olsen L., Aisner D., McGinnis J.M. the learning healthcare system: workshop summary. National Academies Press; Washington (DC): 2007. Institute of medicine roundtable on evidence-based M. [PubMed] [Google Scholar]
- 10.Chan A.W., Tetzlaff J.M., Altman D.G., et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Rev Panam Salud Publica. 2015;38:506–514. [PMC free article] [PubMed] [Google Scholar]
- 11.Morris M.C., Nelson R.M. Randomized, controlled trials as minimal risk: an ethical analysis. Crit Care Med. 2007;35:940–944. doi: 10.1097/01.CCM.0000257333.95528.B8. [DOI] [PubMed] [Google Scholar]
- 12.Apfelbaum J.L., Hagberg C.A., Connis R.T., et al. 2022 american society of anesthesiologists practice guidelines for management of the ifficult airway. Anesthesiology. 2021 doi: 10.1097/ALN.0000000000004002. [DOI] [PubMed] [Google Scholar]
- 13.Yehya N., Harhay M.O., Curley M.A.Q., Schoenfeld D.A., Reeder R.W. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200:828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenfeld D.A., Bernard G.R. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Haywood K., Whitehead L., Nadkarni V.M., et al. COSCA (core outcome set for cardiac arrest) in adults: an advisory statement from the international liaison committee on resuscitation. Circulation. 2018;137:e783–e801. doi: 10.1161/CIR.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 16.Witten L., Gardner R., Holmberg M.J., et al. Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation. 2019;136:93–99. doi: 10.1016/j.resuscitation.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haywood K., Whitehead L., Nadkarni V.M., et al. COSCA (Core Outcome Set for Cardiac Arrest) in adults: an advisory statement from the international liaison committee on resuscitation. Resuscitation. 2018;127:147–163. doi: 10.1016/j.resuscitation.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Self W.H., Shotwell M.S., Gibbs K.W., et al. Renin-angiotensin system modulation with synthetic angiotensin (1–7) and angiotensin II Type 1 receptor-biased ligand in adults with COVID-19: two randomized clinical trials. Jama. 2023;329:1170–1182. doi: 10.1001/jama.2023.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskowitz A., Shotwell M.S., Gibbs K.W., et al. Oxygen-free days as an outcome measure in clinical trials of therapies for COVID-19 and other causes of new-onset hypoxemia. Chest. 2022;162:804–814. doi: 10.1016/j.chest.2022.04.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation with heparin in critically Ill patients with covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moskowitz A., Andersen L.W., Holmberg M.J., Grossestreuer A.V., Berg K.M., Granfeldt A. Identification, collection, and reporting of harms among non-industry-sponsored randomized clinical trials of pharmacologic interventions in the critically ill population: a systematic review. Crit Care. 2020;24:398. doi: 10.1186/s13054-020-03113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley S.M., Zhou Y., Ramachandran S.K., Engoren M., Donnino M., Girotra S. Retrospective cohort study of hospital variation in airway management during in-hospital cardiac arrest and the association with patient survival: insights from Get With The Guidelines-Resuscitation. Crit Care. 2019;23:158. doi: 10.1186/s13054-019-2426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen L.W., Granfeldt A., Callaway C.W., et al. Association between tracheal intubation during adult in-hospital cardiac arrest and survival. Jama. 2017;317:494–506. doi: 10.1001/jama.2016.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coute R.A., Panchal A.R., Mader T.J., Neumar R.W. National institutes of health-funded cardiac arrest research: a 10-year trend analysis. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watkins S., Chowdhury F.J., Norman C., et al. Randomised trial of the clinical and cost effectiveness of a supraglottic airway device compared with tracheal intubation for in-hospital cardiac arrest (AIRWAYS-3): Protocol, design and implementation. Resusc Plus. 2023;15 doi: 10.1016/j.resplu.2023.100430. [DOI] [PMC free article] [PubMed] [Google Scholar]