Abstract
The Wolf Creek Conferences on Cardiac Arrest Resuscitation began in 1975, and have served as an important forum for thought leaders and scientists from industry and academia to come together with the common goal of advancing the field of cardiac arrest resuscitation. The Wolf Creek XVII Conference was hosted by the Max Harry Weil Institute of Critical Care Research and Innovation in Ann Arbor, Michigan on June 14–17, 2023. A new component of the conference was the Wolf Creek Innovator in Cardiac Arrest and Resuscitation Science Award competition. The competition was designed to recognize early career investigators from around the world who’s science is challenging the current paradigms in the field. Finalists were selected by a panel of international experts and invited to present in-person at the conference. The winner was chosen by electronic vote of conference participants and awarded a $10,0000 cash prize. Finalists included Carolina Barbosa Maciel from the University of Florida, Adam Gottula from the University of Michigan, Rajat Kalra from the University of Minnesota, Ryan Morgan from the Children’s Hospital of Philadelphia, Mitsuaki Nishikimi form Hiroshima University, and Jacob Sunshine from the University of Washington. Ryan Morgan from the Children’s Hospital of Philadelphia was selected as the 2023 Wolf Creek Innovator Awardee. This manuscript provides a summary of the work presented by each of the finalists and provides a preview of the future of resuscitation science.
Keywords: Cardiac Arrest, Resuscitation Science, ECPR, Neuroprognosication, Physiology guided CPR
Introduction
The Wolf Creek Conference has been a cornerstone of cardiac arrest resuscitation science since its founding in 1975. This event has consistently brought together the brightest minds and innovators in the field, serving as a catalyst for the exchange of groundbreaking ideas and the exploration of cutting-edge research, all united by the common goal of advancing the field of cardiac arrest resuscitation. Wolf Creek XVII marked another significant milestone with the inaugural Wolf Creek Early Career Innovator Award. The Wolf Creek Early Innovator Award celebrates the contributions of early-career investigators who are challenging conventional paradigms and shaping the future of resuscitation science.
The Wolf Creek Innovator in Cardiac Arrest and Resuscitation Science Award process began with an international call for applicants to be received by January 13, 2023. Applicants were asked to submit a one-page personal statement describing the individual's innovations in cardiac arrest resuscitation science, PDFs of relevant publication(s)/written works, and 3 letters of support (2 must be from outside the applicant's organization) describing the impact the applicant's work has or will potentially have on the field of cardiac arrest resuscitation. Applications were scored by an international panel of experts and 6 finalists were invited to present their work at the Wolf Creek XVII Conference. Presentations were followed by a question-and-answer session moderated by the review panel. Conference participants selected the winner by electronic vote. The winner was awarded a $10,000 cash prize. Fig. 1 lists the six Wolf Creek Innovator In Cardiac Arrest Resuscitation Science Award Finalists.
Fig. 1.
The six Wolf Creek Innovator in Cardiac Arrest Resuscitation Science Award Finalists with competition judge, Dianne Atkins. (Left to right: Mitsuaki Nishikimi, Ryan W. Morgan, Adam L. Gottula, Dianne Atkins, Carolina B. Maciel, Jacob Sunshine, and Rajat Kalra).
The purpose of this manuscript is to provide an opportunity for the broader scientific community to learn more about the work of these early career innovators.
Using smart phones and speakers to detect cardiac arrest: Jacob Sunshine, University of Washington
Background
Out-of-hospital cardiac arrest (OHCA) claims hundreds of thousands of lives each year, with nearly half of victims experiencing an unwitnessed event. OHCA is often characterized by specific patterns of breathing, chest movements, and other perturbations in vital status that aid recognition. The most vexing challenge of unwitnessed OHCA is the absence of a human bystander, however the last decade’s proliferation of low cost, intelligent computing platforms (e.g., smartphones and smart speakers) provide an unrealized opportunity to identify signs of arrest and connect victims to resuscitation faster.
Contribution to the field
Smart speakers and smartphones are increasingly in proximity to people and have embedded sensors and computational capabilities that enable them to identify time-sensitive physiologic changes and, because of their connectivity, summon help. Previous pilot work has revealed that agonal breathing (in the setting of OHCA-induced severe hypoxia) can be accurately detected with these devices using machine-based classification.1 Separately, breathing cessation (sustained apnea) can be detected using active sonar on a smartphone with meaningful clinical accuracy using embedded sensors within existing commodity smart devices.2
Future directions
Given the near ubiquity of smart devices, there is now the potential to leverage these systems to identify unwitnessed OHCA such that nearly all events could be functionally witnessed and in turn more effectively resuscitated.
Physiology as a guide to building tomorrow’s resuscitation strategies*: Ryan Morgan, Children’s hospital of Philadelphia
Background
Current resuscitation paradigms are largely algorithmic, providing a teachable framework to provide reliable and reproducible CPR to all patients with cardiac arrest. However, cardiac arrest patients are diverse in terms of their characteristics, comorbidities, and arrest etiologies, and thus respond differentially to resuscitation therapies. As such, there is merit in developing resuscitation strategies that account for such heterogeneity and facilitate real-time titration of intra-arrest therapies to the individual patient’s response.
Contributions to the field
Our laboratory group developed a method of hemodynamic-directed CPR (“HD-CPR”) that leads to superior intra-arrest physiology and higher rates of survival in swine models of adult and pediatric IHCA.3, 4, 5 Additionally, we have conducted multicenter observational studies to determine and validate invasively measured diastolic blood pressure (DBP) targets during pediatric IHCA.6 Together, this work begins to form the foundation for blood pressure-directed CPR in clinical practice.
Recent work has focused on better understanding how epinephrine administration can be tailored to individual patients. Large animal laboratory studies by our team and others identified considerable inter-individual variability in the hemodynamic response to epinephrine during CPR with some animals demonstrating robust increases in DBP or coronary perfusion pressure and others failing to respond altogether.7, 8 A subsequent multicenter observational study identified similar physiologic variability in children with IHCA and an association between change in DBP after the first dose of epinephrine and return of spontaneous circulation.9
As any personalized method of CPR should also account for the pre-existing condition of the patient, we have studied how the physiologic derangements of specific disease states, such as sepsis and pulmonary hypertension, persist during CPR and how they can potentially be targeted.10, 11 Additionally, given the preponderance of pre-existing respiratory failure in children with IHCA, we have sought to better define pre-arrest gas exchange and respiratory mechanics in order to understand how intra-arrest ventilatory support may be accordingly individualized.12
Future directions
Ongoing and future work aims to: (1) continue to identify meaningful physiologic targets during CPR; (2) further develop physiology-directed approaches to CPR and epinephrine administration in particular; (3) elucidate pre-arrest phenotypic and physiologic characteristics that allow for individualization of resuscitation approaches; and (4) broaden the scope of physiology-directed CPR through the investigation of non-invasive modalities. To achieve these goals, we will continue to employ a translational approach, designing and conducting complementary clinical and large animal translational studies. Moreover, the ongoing development and refinement of high-fidelity, innovative physiologic data acquisition and analysis systems will greatly enhance the reliability and generalizability of such studies and facilitate translation to practice.
Scoop and go in extracorporeal cardiopulmonary resuscitation: A cluster wedge randomized trial: Rajat Kalra, University of Minnesota
Background
Restoration of normal flow is the priority in out-of-hospital cardiac arrest (OHCA). Traditionally, low- and no-flow time has been limited by the use of early defibrillation and high-quality cardiopulmonary resuscitation (CPR). Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) during OHCA is increasingly used as a means of limiting low-flow and no-flow time in OHCA.13, 14 However, there are still significant systems-based limitations that limit the rapid initiation of VA-ECMO as part of a extracorporeal cardiopulmonary resuscitation (ECPR) strategy. These barriers potentially leave patients with long low-flow and no-flow times prior to the initiation of VA-ECMO, thus negating the survival benefit of VA-ECMO.
Contribution to the field
The University of Minnesota’s Center for Resuscitation Medicine has previously demonstrated that the initiation of VA-ECMO, as part of a structured ECPR strategy, can improve cardiac recovery15 and neurologically favorable survival in refractory OHCA.16, 17, 18, 19 Importantly, we have also shown that the odds of neurologically favorable survival decline as the time to initiate VA-ECMO increases.20
Even in centers with established infrastructure and protocols, the time from emergency medical services (EMS) notification to the initiation of VA-ECMO can approach 60 minutes. Therefore, there is a critical need to reduce this no-flow/low-flow time and initiate VA-ECMO more rapidly. This may be an important means to further improve survival from refractory OHCA.
Future directions
We hypothesize that reducing the time to VA-ECMO initiation in an established ECPR strategy will further improve neurological favorable survival in OHCA due to shockable rhythms.
We propose to test a ‘scoop and go’ strategy in the setting of a stepped cluster wedge randomized controlled trial to reduce the time at the scene of the OHCA. We aim for the ‘scoop and go’ intervention to limit the time on the scene to 10 minutes. This will allow for the EMS teams to apply critical maneuvers but prioritize transfer to a hub center for VA-ECMO cannulation. We hypothesize that this will promote rapid transfer to a hospital hub for VA-ECMO initiation, thus reducing the time to VA-ECMO initiation and improving neurologically favorable survival in OHCA.
In this trial, each EMS agency will serve as a cluster to be randomized to the ‘scoop and go strategy’. Once randomized, each EMS agency will be trained in the ‘scoop and go’ strategy. EMS agencies (clusters) will be sequentially trained and then will serve as a control for themselves and other EMS agencies. The primary population will be adults aged 18–75 with OHCA due to shockable rhythms. The primary outcome will be survival to hospital discharge. The secondary outcomes will be survival at 30 and 180 days and functional status.
Helicopter-based ECPR for refractory OHCA: A system-based intervention to improve OHCA outcomes: Adam Gottula, University of Michigan
Background
OHCA treated by EMS in the United States (U.S.) has a neurologically intact survival to hospital discharge rate of 7.2%.21 ECPR has demonstrated the ability to improve outcomes in patients suffering refractory OHCA.17, 18 However, ECPR is only available at specialized medical centers for OHCA patients meeting specific criteria, limiting the availability of ECPR.22
Contributions to the field
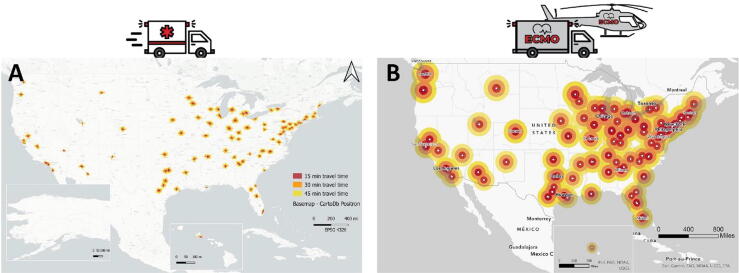
We constructed a Geographic Information System (GIS) model to estimate the number of ECPR candidates in the U.S. (Fig. 2A). We utilized the Resuscitation Outcome Consortium (ROC) database to model time-dependent rates of ECPR eligibility and the Cardiac Arrest Registry to Enhance Survival (CARES) registry to determine the total number of OHCA patients who meet pre-specified ECPR criteria. The combined model was used to estimate the total ECPR candidates and the effects of patient and hospital factors on eligibility. In our model, only 1.68% of OHCA patients in the U.S. were eligible for ECPR based on a 45-minute transportation time to an ECMO-ready center model.22
Fig. 2.
Displays the 15-minute, 30-minute, and 45-minute response time buffers around ECMO-ready centers for OHCA transport by (A) ground transport and (B) helicopter transport.
We then utilized our GIS model to develop a model to estimate the number of potential ECPR candidates in the U.S. in the current hospital-based system, in a prehospital ECPR ground-based system, and in a prehospital ECPR HEMS-based system (Fig. 2B) based on a time to cannulation of 60 minutes. Our study demonstrated a two-fold increase in ECPR eligibility could be achieved with a prehospital ECPR ground-based system and a four-fold increase in ECPR eligibility with a prehospital ECPR HEMS-based system when compared to the current hospital-based ECPR system.
Future directions
While prehospital ECPR systems demonstrate increased eligibility, there are many challenges to implementing such a system. Before large scale implementation of prehospital ECPR systems of care can be implemented we must first demonstrate that ECPR is cost effective, that we can effectively train ECPR teams, and how to best transport patients following ECPR.
Cost effectiveness
Previous studies in Australia and Japan have demonstrated ECPR to be a cost-effective resuscitative strategy.23, 24 It remains unclear if ECPR is cost effective within the U.S. healthcare system. Ongoing work to determine the cost effectiveness of ECPR in the U.S. healthcare system will guide future resuscitation strategies within the U.S.
ECPR team training
ECPR is a high-stakes procedure, requiring fast cannulation for optimal patient outcomes. Therefore, it is important to develop effective training modalities outside the clinical setting. Extended reality training has demonstrated success in multiple invasive procedures. Future studies to develop an extended reality platform for ECPR could provide feasible training for prehospital ECPR teams.
Transport of patients supported by ECMO
Prehospital ECPR systems of care require patients be transported following ECMO initiation. While existing data is limited, a previous retrospective analysis demonstrated a 27.3% incidence of adverse events.25 Future work is needed to describe the current state of ECMO transport and determine the best practices of ECMO transport.
Addressing these knowledge gaps will allow for further investigation into the broader implementation of prehospital ECPR systems of care.
Saving the survivors of cardiac arrest—A multimodal transdisciplinary scientific approach to hypoxic-ischemic brain injury: Carolina Barbosa Maciel, University of Florida
Background
Hypoxic-ischemic brain injury remains a pervasive threat to OHCA patients who survive to hospital admission; the degree of neurologic injury is the main determinant of outcomes and challenges any potential impact that advances in resuscitation science may have on cardiac arrest survival and functional outcomes. A comprehensive approach to hypoxic-ischemic brain injury is needed, encompassing mechanisms of primary and secondary brain injury and refining neuroprognostication methods — this is paramount even for the selection of patients for therapeutic trials unrelated to neurologic injury as the aftermath of hypoxic-ischemic insults may jeopardize chances for a positive clinical trial even when the desired clinical effect is noted.
Contribution to the field
We propose a multipronged approach to hypoxic-ischemic brain injury that addresses the main mechanism implicated in primary ischemic insult (i.e., anoxic depolarization), the most common neurologic complication in the post-cardiac arrest period linked to secondary brain injury (i.e., post-anoxic status epilepticus), and the key bias plaguing neuroprognostic studies (i.e., self-fulfilling prophecy).
Primary and secondary injury mechanisms
Provoked spreading depolarizations by hypoxia or hypotension, both extremely common insults during cardiac arrest and in the post-cardiac arrest period, may lead to failure of microcirculation and ongoing ischemia to neurons; hence, they can be targeted in novel neuroprotective therapies, most of which are already widely available medications.
Post-anoxic status epilepticus
Early augmentation of GABAergic pathway with blockade of GABA breakdown by irreversible inhibition of GABA transaminase with vigabatrin is a promising novel strategy for post-anoxic status epilepticus,26 an essentially orphan entity as anoxic etiology of seizures is the most common exclusion criteria in clinical trials of status epilepticus. Vigabatrin has the potential for synergism with the anesthetics commonly used in the post-cardiac arrest period; hence, possibly leading to reduced cumulative doses of sedating medications. VIGAB-STAT, a phase IIa pilot trial completing recruitment in 2023, aims to demonstrate the feasibility of a single load of vigabatrin as an adjunctive treatment for electroclinical or electrographic status epilepticus.
Future directions
Anoxic depolarizations
We will expand experiments to furnish translational data supporting the role of modulation of spreading depolarizations in the overall burden of hypoxic-ischemic brain injury across different cardiac arrest models and include sex as a biologic variable.
GABA augmentation in hyperexcitability post-cardiac arrest
Upon demonstration of adequate absorption of vigabatrin in the post-cardiac arrest period, we will conduct ViPER, a phase IIb study aimed at identifying the best dose strategy for a large scale clinical trial of vigabatrin and standardized electroencephalogram-based tiered therapy for post-anoxic status epilepticus.
Raising standards for neuroprognostic studies
Finally, the SPIN-CA — a systematic review of the methodology of neuroprognostic studies which grades the degree to which they have accounted for factors related to self-fulfilling prophecy bias influencing their results — will lay the foundation for more clarity in reporting for future studies evaluating the prediction performance of neuroprognostic tools.27
A new therapeutic strategy for PCAS: Basic and clinical research in New York and Japan: Mitsuaki Nishikimi, Hiroshima University
Background
Brain ischemic reperfusion injury after cardiac arrest (CA) is a major public health problem. The mechanisms responsible for brain damage have not been completely understood,28 which has hindered the development of successful therapeutic options. We have conducted basic and clinical research to develop a new therapeutic approach for improving the outcome of CA patients.
Contribution to the field
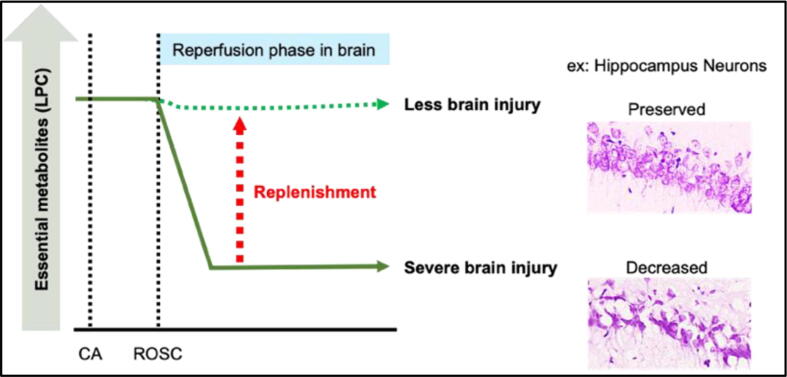
The main objective was to investigate the beneficial effect of the replenishment of essential metabolites after CA. The pathology of ischemic reperfusion injury after CA is triggered by loss of blood circulation which limits the supply of essential metabolites to vital organs. However, little attention has been given to essential metabolites that are diminished in the circulating plasma (Fig. 3). Lysophosphatidylcholine (LPC) is known to be one of the most abundant plasma phospholipids in mammalian species. We have found that LPC was decreased in post-reperfusion plasma in both humans and animal models using a phospholipid screening approach.29 Moreover, our recent study found that supplementing LPC alleviated neuronal cell death, activation of astrocytes, and expression of various inflammatory and mitochondrial dysfunction genes.30 This data highlights the importance of restoring plasma metabolites to achieve physiological normalcy after CA, which may be a new horizon in the endeavor to improve resuscitation medicine.
Fig. 3.
Demonstrates the observed morphological change and the effects of the replenishment of essential metabolites in the hippocampus in a post-cardiac arrest state.
Additionally, we developed a novel clinical risk classification tool for post-CA; the revised version of the “post-Cardiac Arrest Syndrome for Therapeutic hypothermia scoring system” (rCAST). The rCAST is a score that is calculated by using 5 clinical factors measured prior to the initiation of therapeutic hypothermia. Our recent study showed that the effect size of therapeutic hypothermia might vary according to the severity of post-CA on the rCAST. Therapeutic hypothermia was associated with a significantly higher rate of good neurologic outcomes in the moderate-severity group, but not in the low- or high- severity group. This may imply an importance for individualized management of the therapeutic hypothermia.31
Future directions
Currently a randomized clinical trial is ongoing in Japan to evaluate the beneficial effect of therapeutic hypothermia for post-CA patients classified into moderate severity group based on the rCAST.
Conclusion
The Wolf Creek Innovator in Cardiac Arrest and Resuscitation Science Award was created to celebrate and support early career investigators whose work is challenging the current paradigms in cardiac arrest resuscitation. The inaugural 2023 award was presented to Dr. Ryan Morgan MD for his work focused on using real-time physiology as a guide to building tomorrow’s resuscitation strategies. The work of all six finalists presented here highlights what innovators in the field envision as the future of resuscitation science.
Disclosures
CBM received funding from the American Heart Association (AHA 20IPA35380013), Claude D. Pepper Older Americans Independence Center, NINDS (1R13NS111956-01, and 1U01NS124613-01A1), McKnight Brain Institute at the University of Florida, and speaker honoraria from Natus medical and American Academy of Neurology. ALG has no disclosures. RK received funding from the American Heart Association (23TPA1140962). RWM received funding from the NIH NHLBI (K23HL148541).
CRediT authorship contribution statement
Adam L. Gottula: Conceptualization, Writing – review & editing, Supervision. Carolina B. Maciel: Conceptualization. Mitsuaki Nishikimi: Conceptualization. Rajat Kalra: Conceptualization. Jacob Sunshine: Conceptualization. Ryan W. Morgan: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chan J., Rea T., Gollakota S., Sunshine J.E. Contactless cardiac arrest detection using smart devices. NPJ Digit Med. 2019;2:52. doi: 10.1038/s41746-019-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nandakumar R., Gollakota S., Sunshine J.E. Opioid overdose detection using smartphones. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau8914. [DOI] [PubMed] [Google Scholar]
- 3.Sutton R.M., Friess S.H., Naim M.Y., et al. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med. 2014;190:1255–1262. doi: 10.1164/rccm.201407-1343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan R.W., Kilbaugh T.J., Shoap W., et al. A hemodynamic-directed approach to pediatric cardiopulmonary resuscitation (HD-CPR) improves survival. Resuscitation. 2017;111:41–47. doi: 10.1016/j.resuscitation.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lautz A.J., Morgan R.W., Karlsson M., et al. Hemodynamic-directed cardiopulmonary resuscitation improves neurologic outcomes and mitochondrial function in the heart and brain. Crit Care Med. 2019;47:e241–e249. doi: 10.1097/CCM.0000000000003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg R.A., Morgan R.W., Reeder R.W., et al. Diastolic blood pressure threshold during pediatric cardiopulmonary resuscitation and survival outcomes: A multicenter validation study. Crit Care Med. 2023;51:91–102. doi: 10.1097/CCM.0000000000005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slovis J.C., Morgan R.W., Landis W.P., et al. The physiologic response to rescue therapy with vasopressin versus epinephrine during experimental pediatric cardiac arrest. Resusc Plus. 2020;4 doi: 10.1016/j.resplu.2020.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien C.E., Santos P.T., Reyes M., et al. Association of diastolic blood pressure with survival during paediatric cardiopulmonary resuscitation. Resuscitation. Oct 2019;143:50–56. doi: 10.1016/j.resuscitation.2019.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan R.W., Berg R.A., Reeder R.W., et al. The physiologic response to epinephrine and pediatric cardiopulmonary resuscitation outcomes. Crit Care. 2023;27:105. doi: 10.1186/s13054-023-04399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan R.W., Reeder R.W., Ahmed T., et al. Outcomes and characteristics of cardiac arrest in children with pulmonary hypertension: A secondary analysis of the ICU-RESUS clinical trial. Resuscitation. 2023;190 doi: 10.1016/j.resuscitation.2023.109897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan R.W., Sutton R.M., Karlsson M., et al. Pulmonary vasodilator therapy in shock-associated cardiac arrest. Am J Respir Crit Care Med. 2018;197:905–912. doi: 10.1164/rccm.201709-1818OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepard L.N., Reeder R.W., O'Halloran A., et al. Pediatric in-hospital cardiac arrest: Respiratory failure characteristics and association with outcomes. Resuscitation. 2023;188 doi: 10.1016/j.resuscitation.2023.109856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalra R., Kosmopoulos M., Goslar T., Raveendran G., Bartos J.A., Yannopoulos D. Extracorporeal cardiopulmonary resuscitation for cardiac arrest. Curr Opin Crit Care. 2020;26:228–235. doi: 10.1097/MCC.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsangaris A., Alexy T., Kalra R., et al. Overview of Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) support for the management of cardiogenic shock. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.686558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalra R., Bartos J.A., Kosmopoulos M., et al. Echocardiographic evaluation of cardiac recovery after refractory out-of-hospital cardiac arrest. Resuscitation. 2020;154:38–46. doi: 10.1016/j.resuscitation.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Yannopoulos D., Kalra R., Kosmopoulos M., et al. Rationale and methods of the Advanced R(2)Eperfusion STrategies for Refractory Cardiac Arrest (AR- REST) trial. Am Heart J. 2020;11:29–39. doi: 10.1016/j.ahj.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Yannopoulos D., Bartos J., Raveendran G., et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396:1807–1816. doi: 10.1016/S0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belohlavek J., Yannopoulos D., Smalcova J., et al. Intraarrest transport, extracorporeal cardiopulmonary resuscitation, and early invasive management in refractory out-of-hospital cardiac arrest: an individual patient data pooled analysis of two randomised trials. EClinicalMedicine. 2023;59 doi: 10.1016/j.eclinm.2023.101988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexy T., Kalra R., Kosmopoulos M., et al. Initial hospital length of stay and long-term survival of patients successfully resuscitated using extracorporeal cardiopulmonary resuscitation for refractory out-of-hospital cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2023;12:175–183. doi: 10.1093/ehjacc/zuac141. [DOI] [PubMed] [Google Scholar]
- 20.Bartos J.A., Grunau B., Carlson C., et al. Improved survival with extracorporeal cardiopulmonary resuscitation despite progressive metabolic derangement associated with prolonged resuscitation. Circulation. 2020;141:877–886. doi: 10.1161/CIRCULATIONAHA.119.042173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CARES Summary Report – Demographic and Survival Characteristics of OHCA/Non-Traumatic Etiology (Date of Arrest: 01/01/20–12/31/20), https://mycares.net/sitepages/uploads/2021/2020Non-TraumaticNationalSummaryReport.pdf, accessed on June 10, 2021.
- 22.Gottula A.L., Shaw C.R., Gorder K.L., et al. Eligibility of out-of-hospital cardiac arrest patients for extracorporeal cardiopulmonary resuscitation in the United States: A geographic information system model. Resuscitation. 2022;180:111–120. doi: 10.1016/j.resuscitation.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Spangenberg T., Schewel J., Dreher A., et al. Health related quality of life after extracorporeal cardiopulmonary resuscitation in refractory cardiac arrest. Resuscitation. 2018;127:73–78. doi: 10.1016/j.resuscitation.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Matsuoka Y., Goto R., Atsumi T., et al. Cost-effectiveness of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: A multi-centre prospective cohort study. Resuscitation. 2020;157:32–38. doi: 10.1016/j.resuscitation.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Broman L.M., Holzgraefe B., Palmér K., Frenckner B. The Stockholm experience: interhospital transports on extracorporeal membrane oxygenation. Crit Care. 2015;19:278. doi: 10.1186/s13054-015-0994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maciel C.B., Teixeira F.J.P., Dickinson K.J., et al. Early vigabatrin augmenting GABA-ergic pathways in post-anoxic status epilepticus (VIGAB-STAT) phase IIa clinical trial study protocol. Neurol Res Pract. 2022;4:4. doi: 10.1186/s42466-022-00168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teixeira F.J.P., Ahmad B., Gibatova V., et al. Do neuroprognostic studies account for self-fulfilling prophecy bias in their methodology? The SPIN protocol for a systematic review. Crit Care Explor. 2023;5:e0943. doi: 10.1097/CCE.0000000000000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naito H., Nojima T., Fujisaki N., et al. Therapeutic strategies for ischemia reperfusion injury in emergency medicine. Acute Med Surg. 2020;7:e501. doi: 10.1002/ams2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikimi M., Yagi T., Shoaib M., et al. Phospholipid screening postcardiac arrest detects decreased plasma lysophosphatidylcholine: supplementation as a new therapeutic approach. Crit Care Med. 2022;50:e199–e208. doi: 10.1097/CCM.0000000000005180. [DOI] [PubMed] [Google Scholar]
- 30.Nishikimi M., Shoaib M., Choudhary R.C., et al. Preserving brain LPC-DHA by plasma supplementation attenuates brain injury after cardiac arrest. Ann Neurol. 2022;91:389–403. doi: 10.1002/ana.26296. [DOI] [PubMed] [Google Scholar]
- 31.Nishikimi M., Ogura T., Nishida K., et al. Outcome related to level of targeted temperature management in postcardiac arrest syndrome of low, moderate, and high severities: a nationwide multicenter prospective registry. Crit Care Med. 2021;49:e741–e750. doi: 10.1097/CCM.0000000000005025. [DOI] [PubMed] [Google Scholar]