Summary
Background
The use of artificial intelligence (AI) in detecting colorectal neoplasia during colonoscopy holds the potential to enhance adenoma detection rates (ADRs) and reduce adenoma miss rates (AMRs). However, varied outcomes have been observed across studies. Thus, this study aimed to evaluate the potential advantages and disadvantages of employing AI-aided systems during colonoscopy.
Methods
Using Medical Subject Headings (MeSH) terms and keywords, a comprehensive electronic literature search was performed of the Embase, Medline, and the Cochrane Library databases from the inception of each database until October 04, 2023, in order to identify randomized controlled trials (RCTs) comparing AI-assisted with standard colonoscopy for detecting colorectal neoplasia. Primary outcomes included AMR, ADR, and adenomas detected per colonoscopy (APC). Secondary outcomes comprised the poly missed detection rate (PMR), poly detection rate (PDR), and poly detected per colonoscopy (PPC). We utilized random-effects meta-analyses with Hartung-Knapp adjustment to consolidate results. The prediction interval (PI) and I2 statistics were utilized to quantify between-study heterogeneity. Moreover, meta-regression and subgroup analyses were performed to investigate the potential sources of heterogeneity. This systematic review and meta-analysis is registered with PROSPERO (CRD42023428658).
Findings
This study encompassed 33 trials involving 27,404 patients. Those undergoing AI-aided colonoscopy experienced a significant decrease in PMR (RR, 0.475; 95% CI, 0.294–0.768; I2 = 87.49%) and AMR (RR, 0.495; 95% CI, 0.390–0.627; I2 = 48.76%). Additionally, a significant increase in PDR (RR, 1.238; 95% CI, 1.158–1.323; I2 = 81.67%) and ADR (RR, 1.242; 95% CI, 1.159–1.332; I2 = 78.87%), along with a significant increase in the rates of PPC (IRR, 1.388; 95% CI, 1.270–1.517; I2 = 91.99%) and APC (IRR, 1.390; 95% CI, 1.277–1.513; I2 = 86.24%), was observed. This resulted in 0.271 more PPCs (95% CI, 0.144–0.259; I2 = 65.61%) and 0.202 more APCs (95% CI, 0.144–0.259; I2 = 68.15%).
Interpretation
AI-aided colonoscopy significantly enhanced the detection of colorectal neoplasia detection, likely by reducing the miss rate. However, future studies should focus on evaluating the cost-effectiveness and long-term benefits of AI-aided colonoscopy in reducing cancer incidence.
Funding
This work was supported by the Heilongjiang Provincial Natural Science Foundation of China (LH2023H096), the Postdoctoral research project in Heilongjiang Province (LBH-Z22210), the National Natural Science Foundation of China’s General Program (82072640) and the Outstanding Youth Project of Heilongjiang Natural Science Foundation (YQ2021H023).
Keywords: Artificial intelligence, Computer-aided detection, Colonoscopy, Adenoma detection rate, Adenoma miss rate, Meta-analysis
Research in context.
Evidence before this study
A comprehensive electronic literature search was performed in Embase, Medline, and the Cochrane Library using MeSH terms and keywords until October 04, 2023. Additionally, reference lists of relevant studies, guidelines, and reviews were manually searched, and forward citation tracking via Web of Science was utilized to identify additional relevant studies.
Added value of this study
The analysis of 33 studies involving 27,404 randomized patients revealed significant findings. Individuals undergoing AI-aided colonoscopy experienced a 50.5% and 52.5% relative decrease in AMR and PMR, respectively, Moreover, there was a relative increase of 24.2% and 23.8% in ADR and PDR and a 39% and 38.8% relative increase in the rate of APC and PPC. Additionally, this resulted in an increment of 0.202 more APCs and 0.271 more PPCs, with a mean inspection time increase of 20 s.
Implications of all the available evidence
Our study demonstrates that AI-aided colonoscopy significantly enhances the detection of patients with advanced adenomas, especially those with non-neoplastic lesions. Moreover, we observed a significant increase in the detection of diminutive and small adenomas, potentially influencing surveillance strategies and reducing the risk of interval Colorectal cancer. Our findings suggest that endoscopists with lower ADR (or PDR) and shorter inspection times, as well as patients with lower BMI and younger age, tend to benefit more from AI-aided colonoscopy. Furthermore, factors such as bowel preparation, anesthesia, and the time of day also exhibited some influence on the efficacy of AI-aid colonoscopy.
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer globally, representing a significant cause of cancer-related deaths.1,2 The pivotal approach to reducing CRC incidence and mortality involves the detection and removal of adenomatous polyps during colonoscopy.3, 4, 5, 6 Every 1% increase in the adenoma detection rate (ADR), the primary quality indicator of colonoscopy, correlated with a 3% decrease in the risk of interval CRC.7,8 Nevertheless, wide variability exists in ADR Among different endoscopy services, with up to 27% of adenomatous polyps potentially being overlooked due to technical and cognitive limitations associated with the standard colonoscope.9, 10, 11
Several artificial intelligence (AI)-aided systems have been developed to standardize polyp detection among endoscopists and mitigate human error.12, 13, 14 However, the impact of these AI tools on colonoscopy outcomes has been shown inconsistent across studies. Concerns include the potential negative effects on endoscopic training, endoscopist distraction, and the risk of polyp overdiagnosis,15,16 which could lead to increased cost and patient burden without significant additional benefits.16,17 Thus, more reliable evidence is essential for supporting the clinical application of AI-aided colonoscopy.
Recent meta-analyses have highlighted significantly improved detection rates using AI-aided systems.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 However, certain limitations undercut the strength of their conclusions. Many meta-analyses encompassed only a few small RCTs,18, 19, 20, 21,24,25,27,29,30 and some studies even incorporated non-RCTs,22,23,26,28,30 leading to underpowered and unreliable pooled estimates. Moreover, the pooled outcomes reported in previous studies exhibit substantial and unexplained heterogeneity.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 Notably, while meta-analysis aims to provide pooled estimates, understanding variance amidst considerable heterogeneity is equally critical, with the summary effect becoming less significant.32 Furthermore, most published studies disregarded other important colonoscopy quality indicators, such as the adenoma miss rate (AMR),18, 19, 20, 21,23, 24, 25,27, 28, 29, 30 which better reflects the clinical utility of this emerging technology.9,33
To address the limitations of previous studies and extend our knowledge of AI-aided colonoscopy on colorectal neoplasia, we conducted a comprehensive systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the potential advantages and disadvantages of AI-aided systems during colonoscopy compared to standard colonoscopy. This comprehensive meta-analysis aims to increase statistical power, reduce bias, explore potential heterogeneity sources, and facilitate exploratory analyses to formulate hypotheses for future research.34
Method
Registration
This systematic review and meta-analysis is registered with PROSPERO (CRD42023428658) and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Assessing the Methodological Quality of Systematic Reviews (AMSTAR) guidelines.35,36 This study was independently conducted by two authors (SHL and FQD), with any disagreements resolved through consensus. In cases of unresolved disagreements, a third reviewer (WJS) was consulted.
Search strategy
Using Medical Subject Headings (MeSH) terms and keywords, a comprehensive electronic literature search was performed in Embase, Medline, and the Cochrane Library until October 04, 2023. The detailed search strategy is provided in File S1. Additionally, reference lists of relevant studies, guidelines, and reviews were manually searched, and forward citation tracking using the Web of Science database was conducted to identify additional pertinent studies.
Eligibility criteria
To be included in this study, trials had to meet the following inclusion criteria: (1) Enrollment of patients undergoing colonoscopy for primary screening, surveillance, or symptoms; (2) Comparison of real-time AI-aided colonoscopy with conventional colonoscopy; (3) Reporting of outcomes of interest; (4) Conducted as RCTs.
Excluded studies were those that: (1) Presented no original data; (2) Had no full text available; and (3) Were conducted in patients with inflammatory bowel disease or hereditary polyposis syndromes. No restrictions were imposed on language, publication date, study location, or sample size.
Study selection
All citations identified during the literature search were imported into reference management software, with automatic removal of duplicates. Titles and abstracts were initially screened for relevance, and full-text articles were reviewed for final inclusion. In cases where multiple reports existed for a single study, the most recent publication was used, supplemented with the complete version if necessary.
Outcomes of interest
Primary outcomes included AMR, ADR, and adenomas detected per colonoscopy (APC). A separate analysis for the primary outcomes stratified by pathology, morphology, size, and location was performed when data were available. Secondary outcomes consisted of the poly missed rate (PMR), poly detection rate (PDR), poly detected per colonoscopy (PPC), procedure time, adverse events, and false alarms. A detailed definition of the terms can be found in Table S1.
Data extraction
Data were extracted using standardized forms, encompassing study characteristics, patient characteristics, intervention characteristics, characteristics of detected adenomas and polyps, and outcomes of interest. Data about only the first colonoscopy for tandem trials were extracted to avoid a carryover effect. Data were obtained through direct extraction or indirect calculation.37 In cases of missing data, corresponding authors were contacted via e-mail.
Statistics
The Cochrane tool was used to assess the risk of bias,38 while publication bias was evaluated using Egger's weighted regression test.39 If publication bias was detected, the trim-and-fill method was used to estimate its potential impact on the overall effect sizes.40 The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.41
For dichotomous outcomes, we calculated risk ratios (RRs) or incidence rate ratios (IRRs), and for continuous outcomes, we computed mean differences, along with 95% confidence intervals (CIs). Given the anticipated considerable between-study heterogeneity, we utilized a random-effects model.42 The Sidik–Jonkman estimator with Hartaung-Knapp (HK) adjustments was employed to pool effect sizes.43,44
Between-study heterogeneity was assessed using Cochran's Q test, I2 statistics, and prediction intervals.45, 46, 47, 48 Meta-regression and subgroup analyses were performed to investigate potential sources of heterogeneity. The meta-regression analysis employed a random-effects model using the random effect maximum likelihood (REML) method with HK adjustment.49 Furthermore, subgroup interactions were tested with the chi-square test.
Sensitivity analyses were performed initially using the leave-one-out approach to assess the influence of individual trials on the overall effect estimate. Additionally, sensitivity analyses were performed to examine the robustness of findings by restricting analyses to (1) trials with data obtained by direct extraction or (2) trials with a low risk of bias.
Statistical analyses were conducted using Stata (version 17) and Comprehensive Meta-Analysis software (version 3.3.070). A two-tailed P-value of 0.05 was considered as the threshold for statistical significance.
Role of the funding source
This work was supported by the Heilongjiang Provincial Natural Science Foundation of China (LH2023H096), the Postdoctoral research project in Heilongjiang Province (LBH-Z22210), the National Natural Science Foundation of China's General Program (82072640), and the Outstanding Youth Project of Heilongjiang Natural Science Foundation (YQ2021H023).
The funders of this study played no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Result
Study selection
An initial search identified 1959 potential studies from the electronic databases, along with three additional studies via hand-searching reference lists. After eliminating duplicates, 1612 studies underwent title and abstract screening. Subsequently, 66 articles were selected for full-text review, from which 33 full-text articles were further excluded due to being study protocols, conference abstracts, or not RCTs. Finally, 33 unique RCTs were included in this meta-analysis.50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82 The study screening and selection process is depicted in Fig. 1.
Fig. 1.
PRISMA flowchart of study.
Study characteristics
Of the 27,404 individuals across the 33 included trials (sample size range: 96–3213), 13,580 patients underwent AI-aided colonoscopy, while 13,824 underwent standard colonoscopy. All studies were published between 2019 and 2023. Among these, 8 studies were designed as tandem studies, 18 were single-center experiences, and 14 were conducted in Western settings. Most studies utilized AI algorithms designed for colorectal lesion identification. Notably, two studies focused on assessing the colonoscopy quality by monitoring the withdrawal time and scope speed, while two others used the algorithm for both lesion detection and quality assurance. Study characteristics are summarized in Table 1 and Table S2, and the geographical distribution of the 33 trials is depicted in Fig. 2.
Table 1.
Characteristics of included studies.
| Study, year | Study design | No. of center | Study region | AI system |
Indication (%) |
Sample size |
Sex (Male, %) |
Age (mean, years) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Function | Screening | Surveillance | Symptomatic | FIT positive | AI | Control | Total | Male | % | AI | Control | Total | ||||
| Ahmad 2023 | Parallel | 1 | Western | GI-Genius | CADe | 100.00% | 308 | 306 | 614 | 208 | 33.88% | 66.2 | 66.4 | 66.30 | |||
| Aniwan 2023 | Parallel | 1 | Asia | CAD-EYE | CADe | 88.75% | 11.25% | 312 | 310 | 622 | 266 | 42.77% | 62.8 | 62 | 62.40 | ||
| Engelke 2023 | Parallel | 1 | Western | GI-Genius | CADe | 3.88% | 7.76% | 88.36% | 122 | 110 | 232 | 113 | 48.71% | 63.54 | 65.24 | 64.35 | |
| Gimeno-García 2023 | Parallel | 1 | Western | ENDO-AID | CADe | 6.09% | 32.37% | 27.88% | 33.65% | 155 | 157 | 312 | 165 | 52.88% | 62.99 | 64.71 | 63.86 |
| Glissen 2022 | Tandem | 4 | Western | EndoScreener | CADe | 59.64% | 40.36% | 113 | 110 | 223 | 122 | 54.71% | 61.18 | 60.51 | 60.85 | ||
| Gong 2020 | Parallel | 1 | Asia | ENDOANGEL | CAQ | 17.47% | 5.11% | 77.42% | 355 | 349 | 704 | 345 | 49.01% | 48.25 | 47.25 | 47.75 | |
| Hüneburg 2023 | Parallel | 1 | Western | CAD-EYE | CADe | 50 | 46 | 96 | 41 | 42.71% | 50.3 | 46.3 | 48.38 | ||||
| Kamba 2021 | Tandem | 4 | Asia | Locally system | CADe | 46.76% | 35.77% | 17.47% | 178 | 177 | 355 | 272 | 76.62% | 61.63 | 61.44 | 61.54 | |
| Karsenti 2023 | Parallel | 1 | Western | GI-Genius | CADe | 14.94% | 43.47% | 26.10% | 7.10% | 1003 | 1012 | 2015 | 979 | 48.59% | 58.4 | 58.4 | 58.40 |
| Liu PX 2020 | Parallel | 1 | Asia | EndoScreener | CADe | 23.04% | 76.96% | 393 | 397 | 790 | 374 | 47.34% | 49.84 | 48.79 | 49.31 | ||
| Liu WN 2020 | Parallel | 1 | Asia | Locally system | CADe | 6.43% | 93.57% | 508 | 518 | 1026 | 551 | 53.70% | 51.02 | 50.13 | 50.57 | ||
| Lui 2023 | Tandem | 3 | Asia | Locally system | CADe | 54.17% | 45.83% | 108 | 108 | 216 | 104 | 48.15% | 62 | 61.4 | 61.70 | ||
| Mangas-Sanjuan 2023 | Parallel | 6 | Western | GI-Genius | CADe | 100.00% | 1610 | 1603 | 3213 | 1717 | 53.44% | 60.7 | 60.6 | 60.65 | |||
| Nakashima 2023 | Tandem | 1 | Asia | CAD-EYE | CADe | 7.71% | 38.07% | 54.22% | 207 | 208 | 415 | 298 | 71.81% | 54.9 | 55.9 | 55.40 | |
| Repici 2020 | Parallel | 3 | Western | GI-Genius | CADe | 22.34% | 23.94% | 23.50% | 30.22% | 341 | 344 | 685 | 337 | 49.20% | 61.5 | 61.1 | 61.30 |
| Repici 2022 | Parallel | 5 | Western | GI-Genius | CADe | 29.09% | 37.12% | 26.52% | 7.27% | 330 | 330 | 660 | 330 | 50.00% | 61.9 | 62.6 | 62.25 |
| Rondonotti 2022 | Parallel | 5 | Western | CAD-EYE | CADe | 100.00% | 405 | 395 | 800 | 409 | 51.13% | 62 | 61 | 61.51 | |||
| Shaukat 2022 | Parallel | 5 | Western | Locally system | CADe | 65.78% | 34.22% | 682 | 677 | 1359 | 723 | 53.20% | 60.6 | 59.9 | 60.25 | ||
| Shen 2021 | Parallel | 1 | Asia | Locally system | CADe | 64 | 64 | 128 | 62 | 48.44% | 58.6 | 59 | 58.80 | ||||
| Su 2020 | Parallel | 1 | Asia | Locally system | CADe and CAQ | 34.67% | 65.33% | 308 | 315 | 623 | 307 | 49.28% | 50.54 | 51.63 | 51.09 | ||
| Vilkoite 2023 | Parallel | 1 | Western | ENDO-AID | CADe | 194 | 206 | 400 | 193 | 48.25% | 50.1 | 51.2 | 50.67 | ||||
| Wallace 2022 | Tandem | 8 | Western | GI-Genius | CADe | 35.65% | 64.35% | 116 | 114 | 230 | 157 | 68.26% | 63 | 64.6 | 63.79 | ||
| Wang 2019 | Parallel | 1 | Asia | EndoScreener | CADe | 7.94% | 92.06% | 522 | 536 | 1058 | 512 | 48.39% | 51.07 | 49.94 | 50.50 | ||
| Wang 2020-a | Tandem | 1 | Asia | EndoScreener | CADe | 30.63% | 8.67% | 60.70% | 184 | 185 | 369 | 179 | 48.51% | 47.72 | 47.19 | 47.45 | |
| Wang 2020-b | Parallel | 1 | Asia | EndoScreener | CADe | 16.42% | 83.58% | 484 | 478 | 962 | 495 | 51.46% | 49.35 | 48.40 | 48.88 | ||
| Wang 2022 | Parallel | 1 | Asia | Locally system | CADe | 200 | 200 | 400 | 191 | 47.75% | 49.29 | 47.21 | 48.25 | ||||
| Wang 2023 | Parallel | 4 | Asia | EndoScreener | CADe | 16.97% | 5.55% | 77.48% | 636 | 625 | 1261 | 690 | 54.72% | 45.56 | 46.3 | 45.93 | |
| Wei 2023 | Parallel | 4 | Western | EndoVigilant | CADe | 71.78% | 28.22% | 387 | 382 | 769 | 392 | 50.98% | 58 | 57.6 | 57.80 | ||
| Xu 2021 | Parallel | 6 | Asia | Locally system | CADe | 8.55% | 91.45% | 1177 | 1175 | 2352 | 1198 | 50.94% | 50.9 | 51.7 | 51.30 | ||
| Xu 2023 | Parallel | 6 | Asia | Eagle-Eye | CADe | 95.85% | 4.15% | 1519 | 1540 | 3059 | 1435 | 46.91% | 57.49 | 57.03 | 57.26 | ||
| Yamaguchi 2023 | Tandem | 3 | Asia | CAD-EYE | CADe | 20.78% | 12.12% | 9.96% | 50.65% | 113 | 118 | 231 | 124 | 53.68% | 63.1 | 63.3 | 63.20 |
| Yao 2022 | Parallel | 1 | Asia | ENDOANGEL | CAQ | 88.89% | 10.00% | 1.10% | 269 | 271 | 540 | 241 | 44.63% | 49.91 | 50.85 | 50.38 | |
| Yao 2023 | Tandem | 3 | Asia | ENDOANGEL | CADe and CAQ | 63.80% | 8.18% | 28.02% | 227 | 458 | 685 | 357 | 52.12% | 50.63 | 49.86 | 50.12 | |
AI, artificial intelligence; FIT, fecal immunochemical test; CADe, computer assisted detection; CAQ, computer assisted quality.
Fig. 2.
Geographical distribution of the 33 trials. Note: the number or words in map means the PMID or DOI of every trial.
Risk of bias assessment
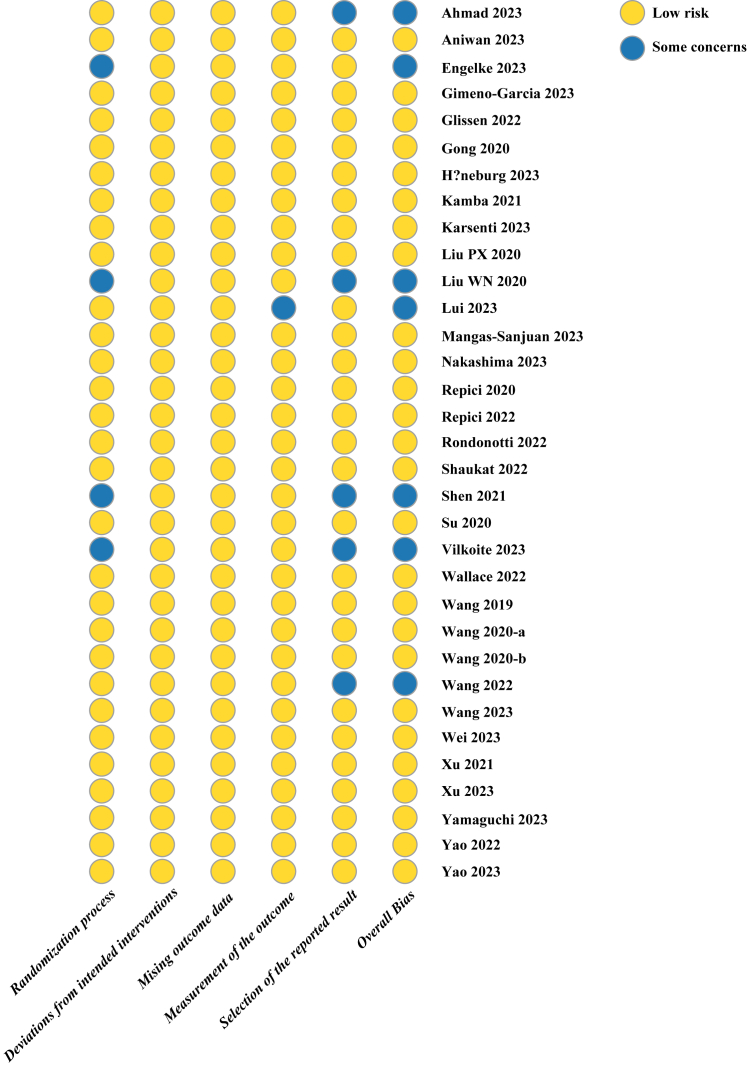
The revised Cochrane risk of bias (RoB 2) tool was employed to assess the risk of bias for the included studies. As shown in Fig. 3, seven out of the 33 trials exhibited at least one criterion for potential bias concerns, with issues primarily observed in the randomization process (n = 4), measurement of outcomes (n = 1), and selection of reported results (n = 5).
Fig. 3.
Risk of bias graph.
PMR, PDR, and PPC
An AI-aided colonoscopy showed a 52.5% decrease (19% risk difference) in the PMR (RR, 0.475; 95% CI, 0.294–0.768; Figure S1), a 23.8% increase (9.9% risk difference) in the PDR (RR, 1.238; 95% CI, 1.158–1.323; Figure S2), a 38.8% increase (37.1% rate difference) in PPC rate (IRR, 1.388; 95% CI, 1.270–1.517; Figure S3), and 0.271 more number of PPC (95% CI, 0.144–0.398; Figure S4) compared to standard colonoscopy (Table 2).
Table 2.
Summary of results.
| Studies | Effect size and 95% CI |
Heterogeneity |
Publication bias |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect size | 95% CI | P-value | I2 | 95% PI | P-value | Egger test | Missed study | Adjusted values | ||||||
| Adenoma outcomes | ||||||||||||||
| Adenoma miss rate (RR) | 7 | 0.495 | 0.390 | 0.627 | 0.00030 | 48.76% | 0.286 | 0.857 | 0.080 | 0.65 | NA | |||
| Adenoma detection rate (RR) | 29 | 1.242 | 1.159 | 1.332 | <0.0001 | 78.87% | 0.886 | 1.742 | <0.0001 | 0.00080 | 9 | 1.144 | 1.048 | 1.249 |
| Adenoma per colonoscopy (IRR) | 29 | 1.390 | 1.277 | 1.513 | <0.0001 | 86.24% | 0.909 | 2.126 | <0.0001 | 0.00040 | 5 | 1.311 | 1.184 | 1.451 |
| Adenoma per colonoscopy (WMD) | 19 | 0.202 | 0.144 | 0.259 | <0.0001 | 68.15% | −0.017 | 0.420 | 0.089 | 0.017 | 6 | 0.155 | 0.075 | 0.235 |
| Polyp outcomes | ||||||||||||||
| Polyp miss rate (RR) | 5 | 0.475 | 0.294 | 0.768 | 0.013 | 87.49% | 0.135 | 1.679 | <0.0001 | 0.57 | NA | |||
| Polyp detection rate (RR) | 24 | 1.238 | 1.158 | 1.323 | <0.0001 | 81.67% | 0.922 | 1.662 | <0.0001 | 0.0026 | 7 | 1.155 | 1.071 | 1.245 |
| Poly per colonoscopy (IRR) | 27 | 1.388 | 1.270 | 1.517 | <0.0001 | 91.99% | 0.892 | 2.160 | <0.0001 | 0.073 | NA | |||
| Poly per colonoscopy (WMD) | 10 | 0.271 | 0.144 | 0.398 | 0.00090 | 65.61% | −0.107 | 0.649 | 0.14 | 0.96 | NA | |||
| Procedure time | ||||||||||||||
| Insertion time (WMD) | 13 | −0.090 | −0.234 | 0.053 | 0.20 | 29.82% | −0.502 | 0.321 | 0.70 | 0.94 | NA | |||
| Inspection time (WMD) | 18 | 0.330 | 0.087 | 0.573 | 0.012 | 93.15% | −0.647 | 1.307 | <0.0001 | 0.68 | NA | |||
| Adverse event | ||||||||||||||
| Adverse event rate (RR) | 20 | 0.837 | 0.629 | 1.114 | 0.21 | 0.94% | 0.536 | 1.305 | 1.0 | 0.46 | NA | |||
CI, confidence interval; PI, prediction interval; RR, risk ratio; IRR, incidence rate ratios; WMD, weighted mean difference; NA, not applicable.
Substantial heterogeneity was observed for most polyp-related outcomes. The 95% prediction intervals crossed the no-effect line for PMR (0.135–1.679), PDR (0.922–1.662) and PPC rate (0.892–2.160), indicating the potential for negative intervention effects in future studies. Additionally, potential publication bias was identified for PDR (Figure S5). However, correcting for the possibility of publication bias would not change the conclusion.
AMR, ADR, and APC
Individuals who intervened with the AI-aided systems experience a 50.5% decrease (17.5% risk difference) in AMR (RR, 0.495; 95% CI, 0.390–0.627; Figure S6), a 24.2% increase (7.4% risk difference) in ADR (RR, 1.242; 95% CI, 1.159–1.332; Figure S7), a 39% increase (19.9% rate difference) in APC rate (IRR, 1.390; 95% CI, 1.277–1.513; Figure S8), and 0.202 more number of APC (95% CI, 0.144–0.259; Figure S9) compared to standard colonoscopy (Table 2).
Substantial heterogeneity was observed for ADR and APC. Moreover, the 95% prediction intervals crossed the no-effect line for ADR (RR, 0.886–1.742) and APC (IRR, 0.909–2.126), indicating that AI-aided colonoscopy may not benefit adenoma detection in certain populations. Publication bias was identified for ADR (Figure S10) and APC (Figure S11 for rate ratio and Figure S12 for mean difference), yet it did not affect the overall conclusions.
Separate analysis for AMR, ADR, and APC
As shown in Table 3, the advanced AMR and sessile serrated lesion (SSL) miss rate in the AI-aided group was not statistically significant compared to the control group. Based on available data, there were no statistically significant interactions between AMR and pathology types.
Table 3.
Separate analysis for the primary outcomes.
| Studies | Effect size and 95% CI |
Heterogeneity |
Test for subgroup difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect size | 95% CI | P-value | I2 | 95% PI | P-value | |||||
| Adenoma miss rate (RR) | ||||||||||
| Pathology | ||||||||||
| Advanced adenoma miss rate (RR) | 4 | 0.702 | 0.127 | 3.876 | 0.56 | 32.28% | 0.015 | 33.429 | 0.32 | 0.59 |
| Sessile serrated lesion miss rate (RR) | 4 | 0.456 | 0.111 | 1.876 | 0.18 | 37.70% | 0.018 | 11.426 | 0.23 | |
| Adenoma detection rate (RR) | ||||||||||
| Pathology | ||||||||||
| Advanced adenoma | 13 | 1.132 | 1.016 | 1.261 | 0.028 | 38.33% | 0.804 | 1.593 | 0.59 | 0.64 |
| Sessile serrated lesion | 12 | 1.001 | 0.849 | 1.180 | 0.99 | 31.66% | 0.624 | 1.605 | 0.67 | |
| Non-neoplastic lesion | 7 | 1.163 | 0.976 | 1.386 | 0.079 | 50.71% | 0.764 | 1.771 | 0.13 | |
| Colorectal cancer | 8 | 1.441 | 0.702 | 2.955 | 0.27 | 62.30% | 0.203 | 10.237 | 0.40 | |
| Morphology | ||||||||||
| Polypoid | 6 | 1.230 | 1.050 | 1.441 | 0.020 | 63.37% | 0.768 | 1.969 | 0.46 | 0.27 |
| Non-polypoid | 8 | 1.419 | 1.204 | 1.671 | 0.0015 | 57.63% | 0.864 | 2.330 | 0.46 | |
| Size category | ||||||||||
| 0–5 mm | 9 | 1.269 | 1.133 | 1.421 | 0.0013 | 62.34% | 0.928 | 1.733 | 0.037 | 0.99 |
| 6–10 mm | 9 | 1.238 | 1.009 | 1.520 | 0.043 | 75.76% | 0.630 | 2.436 | 0.39 | |
| <10 mm | 3 | 1.308 | 0.878 | 1.950 | 0.10 | 52.20% | 0.163 | 10.488 | 0.34 | |
| >10 mm | 11 | 1.287 | 0.984 | 1.684 | 0.063 | 83.66% | 0.468 | 3.543 | 0.28 | |
| Location | ||||||||||
| Proximal colon | 7 | 1.187 | 1.134 | 1.242 | 0.00010 | 9.79% | 1.090 | 1.293 | 0.90 | 0.30 |
| Distal colon | 6 | 1.291 | 1.092 | 1.526 | 0.011 | 50.91% | 0.866 | 1.925 | 0.24 | |
| Adenoma per colonoscopy (WMD) | ||||||||||
| Pathology | ||||||||||
| Sessile serrated lesion | 4 | 0.001 | −0.030 | 0.032 | 0.94 | 6.76% | −0.076 | 0.077 | 0.91 | NA |
| Morphology | ||||||||||
| Polypoid | 5 | 0.131 | 0.076 | 0.185 | 0.0026 | 4.21% | 0.044 | 0.217 | 0.91 | 0.97 |
| Non-polypoid | 5 | 0.133 | −0.053 | 0.319 | 0.11 | 84.87% | −0.335 | 0.621 | 0.0012 | |
| Size category | ||||||||||
| 0–5 mm | 3 | 0.223 | −0.481 | 0.927 | 0.31 | 90.45% | −3.675 | 4.121 | 0.0060 | 0.027 |
| 6–10 mm | 3 | 0.031 | −0.017 | 0.079 | 0.11 | 7.29% | −0.154 | 0.216 | 0.68 | |
| <10 mm | 5 | 0.175 | 0.031 | 0.319 | 0.028 | 48.07% | −0.137 | 0.487 | 0.085 | |
| >10 mm | 6 | 0.017 | −0.005 | 0.040 | 0.10 | 13.61% | −0.024 | 0.059 | 0.78 | |
| Location | ||||||||||
| Proximal colon | 8 | 0.191 | 0.107 | 0.275 | 0.0010 | 63.19% | −0.019 | 0.402 | 0.00060 | 0.027 |
| Distal colon | 5 | 0.073 | −0.028 | 0.173 | 0.12 | 52.62% | −0.154 | 0.299 | 0.092 | |
| Adenoma per colonoscopy (IRR) | ||||||||||
| Pathology | ||||||||||
| Advanced adenoma | 19 | 1.057 | 0.856 | 1.304 | 0.59 | 76.86% | 0.411 | 2.716 | 0.47 | 0.012 |
| Sessile serrated lesion | 16 | 1.133 | 0.895 | 1.434 | 0.28 | 72.60% | 0.435 | 2.952 | 0.014 | |
| Non-neoplastic lesion | 14 | 1.632 | 1.389 | 1.916 | <0.0001 | 81.43% | 0.928 | 2.87 | <0.0001 | |
| Colorectal cancer | 4 | 2.061 | 0.196 | 21.625 | 0.40 | 52.11% | 0.005 | 808.073 | 0.25 | |
| Morphology | ||||||||||
| Polypoid | 13 | 1.453 | 1.262 | 1.673 | 0.00010 | 78.86% | 0.868 | 2.433 | 0.0023 | 0.80 |
| Non-polypoid | 13 | 1.517 | 1.18 | 1.95 | 0.0035 | 80.25% | 0.578 | 3.981 | 0.0019 | |
| Size category | ||||||||||
| 0–5 mm | 12 | 1.576 | 1.288 | 1.929 | 0.00040 | 89.00% | 0.783 | 3.173 | <0.0001 | 0.076 |
| 6–10 mm | 11 | 1.204 | 1.08 | 1.343 | 0.0035 | 24.57% | 0.93 | 1.559 | 0.61 | |
| <10 mm | 13 | 1.416 | 1.266 | 1.583 | <0.0001 | 77.27% | 0.982 | 2.041 | <0.0001 | |
| >10 mm | 13 | 1.223 | 0.979 | 1.528 | 0.072 | 64.18% | 0.59 | 2.534 | 0.29 | |
| Location | ||||||||||
| Proximal colon | 15 | 1.433 | 1.258 | 1.634 | <0.0001 | 78.73% | 0.895 | 2.296 | <0.0001 | 0.97 |
| Distal colon | 13 | 1.438 | 1.255 | 1.648 | 0.00010 | 72.36% | 0.922 | 2.243 | <0.0001 | |
CI, confidence interval; PI, prediction interval; RR, risk ratio; IRR, incidence rate ratios; WMD, weighted mean difference; NA, not applicable.
For the detection rate of adenomas per patient, we found that AI-aided colonoscopy could identify more patients with advanced adenomas, polypoid and non-polypoid adenomas, diminutive (0–5 mm) and small adenomas (6–10 mm), proximal and distal adenomas. However, interaction analyses for subgroup differences determined that the performance of AI-aided colonoscopy in significantly improving ADR was confirmed irrespectively from adenoma pathology, morphology, size, and location.
For the number of APC, while more polypoid adenomas were detected via AI-aided colonoscopy, no significant interactions were found between APC and morphology. Tests for subgroup differences determined that the number of APC detected in the AI-aided colonoscopy group was significantly higher for small adenomas (<10 mm) and adenomas at the proximal colon.
Furthermore, for the rate of APC, no significant difference was observed between the AI-aided and control groups regarding the rate ratios of advanced adenomas, SSLs, colorectal cancers, and large adenomas (>10 mm). Of note, although AI-aided colonoscopy did not identify more patients with non-neoplastic lesions (RR, 1.163; 95% CI, 0.976–1.386), it showed a greater rate of non-neoplastic lesions per colonoscopy (IRR, 1.632; 95% CI, 1.389–1.916).
Procedure time, adverse events, and false alarms
As shown in Table 2, no statistically significant differences were observed between the AI-aided and standard colonoscopy groups for the insertion time (WMD, −0.090 min; 95% CI, −0.234 to 0.053 min; Figure S13). However, the inspection time was 20 s longer with AI-aided colonoscopy (WMD, 0.330 min; 95% CI, 0.087–0.573 min; Figure S14) than with standard colonoscopy.
In 17 out of the 20 included studies that reported data on adverse events, no adverse events occurred. There was no statistically significant difference in adverse event risk between the two groups (RR, 0.837; 95% CI, 0.629–1.114; Figure S15).
In eight trials, the reported number of false negatives was zero. However, these eight trials also reported false positives, ranging between 7.1% and 20.1%, and the pooled proportion of false positives on AI-aided colonoscopy was 12.2% (95% CI, 7.5%–16.9%; Figure S16).
Significant heterogeneity was observed for the inspection time, and the wide 95% prediction interval indicated that AI-aided colonoscopy might reduce inspection time by 39 s (95% prediction interval, −0.647 to 1.307 min) in some populations. Notably, no publication bias was detected.
Meta-regression analyses
Given the substantial heterogeneity in adenoma- and polyp-relevant outcomes, REML univariate meta-regression analyses with HK adjustment were performed to explore the sources of heterogeneity. As shown in Table S3, four aspects were explored as sources of heterogeneity. As for study characteristics, more involved centers were significantly associated with reduced differences in ADR, APC, PDR, and PPC.
Regarding patient characteristics, increased age was significantly associated with decreased differences in ADR, APC, PDR, and PPC. A higher proportion of symptomatic patients was significantly associated with increased differences in APC, PDR, and PPC. A higher proportion of fecal immunochemical test-positive patients were significantly associated with a reduced ADR difference. Moreover, a better bowel preparation, assessed by the Boston bowel preparation score, was significantly associated with increased inspection times and reduced ADR, PDR, and PPC differences. Additionally, a higher proportion of patients with adequate cleaning (Boston bowel preparation score >6) was significantly associated with reduced APC and PDR differences.
In terms of intervention characteristics, a higher proportion of patients who received anesthesia were significantly associated with an increased difference in PPC. Moreover, a higher proportion of patients who underwent colonoscopy in the morning were significantly associated with an increased difference in ADR, APC, and PPC. A longer inspection time was also significantly associated with a decreased difference in ADR, APC, PDR, and PPC.
As for endoscopist experience, a higher baseline ADR for standard colonoscopy was significantly associated with a reduced difference in ADR, APC, PDR, PPC, and inspection time. Similarly, a higher baseline PDR for routine colonoscopy was significantly associated with decreased ADR, APC, PDR, and PPC differences.
Subgroup analyses
Consistent with the meta-regression analyses, subgroup analyses also supported similar findings. Results from single-center studies showed more favorable outcomes compared to multiple-center studies. Additionally, endoscopists with low ADR (ADR <25%) appeared better than those reported with high ADR (ADR <25%). Moreover, bowel preparation and inspection time may also play a significant influencing role on the effect of AI-aided colonoscopy for polyp-relevant outcomes (Table S4).
Of note, subgroup analyses further determined that results obtained from China seem better than those reported in other countries; patients younger than screening (age <50 years) may benefit more from the AI-aided colonoscopy; overweight individuals (BMI >25) seem to benefit less from AI-aided colonoscopy.
Sensitivity analyses
The sensitivity analyses, detailed in Table S5, validated the consistency of the main findings. First, our results remained unchanged in leave-one-out analyses. Second, all conclusions were robust when only considering trials with data obtained by direct extraction. Third, results based on studies with a low risk of bias also suggest that the outcomes were robust, although the effect sizes became small.
Quality of evidence
Based on the GRADE approach, the overall quality of available evidence ranged from very low to moderate. The downgrade in the level of evidence for RCTs was primarily due to (1) the risk of bias of the included RCTs (assessed via the RoB 2 tool), (2) inconsistency attributed to the study (e.g., number of study centers and study region), and patient characteristics (e.g., age and indications for colonoscopy), (3) insufficient sample size and a small number of events, (4) the potential publication bias (Table S6).
Discussion
According to our study, the 52.5% and 50.5% relative decrease in PMR and AMR, 23.8% and 24.2% relative increase in PDR and ADR, 38.8% and 39% relative increase in the rate of PPC and APC, as well as 0.271 more number of PPC and 0.202 more number of APC, respectively, underscore the benefit of employing of adding AI-aided systems during colonoscopy without significantly affecting the efficiency of the procedure, as evidenced by similar procedure times and adverse event rates in both groups.
With 33 studies involving 27,404 randomized patients, our meta-analysis is the most extensive to date in this field. Our sample size is far more than the latest study published on August 29, 2023, which included 21 randomized trials on 18,232 patients in their research.31 Our large sample size allowed for inferring potential benefits in detecting clinically significant, albeit infrequent, lesions like advanced adenomas and SSLs. The comprehensive size and nature of this study enhance the reliability of the evidence and its applicability in real-world clinical settings.34
Our study is the first to provide evidence regarding the significant contribution of AI-aided colonoscopy to the detection of more patients with advanced adenomas, which have the highest potential for malignancy. Consequently, identifying this factor could greatly reduce CRC risk.83 In other words, AI-aided colonoscopy is more effective in identifying people at a high risk of CRC.
Previous meta-analyses have also reported positive outcomes of AI-aided colonoscopy.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 However, owing to the limited sample size of each study, they often focused on small, non-advanced lesions, especially diminutive adenomas. Consistent with prior studies,18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 our study observed a significant increase in the detection of diminutive and small adenomas during AI-aided colonoscopy. Notably, diminutive and small lesions are often missed during colonoscopy screening.84 Previous studies have demonstrated that diminutive and small lesions show high-grade dysplasia and malignancy at rates between 0.6% and 5.6%.85,86 Moreover, an increased risk of metachronous cancer has been reported when three or more non-advanced diminutive adenomas are present.87,88 Although not all small lesions were up to the criteria for clinical treatment, their potential to develop CRC in the future cannot be ruled out. Overall, these positive detections could change the surveillance strategy for individuals, thus reducing the risk of interval CRC.
Despite the additional detection of advanced adenomas at a per-patient level (ADR), consistent with the latest study,31 our study failed to show an increase in the detection of advanced adenomas at a per-polyp level (APC). This discrepancy could be attributed to various reasons. One is that advanced lesions are larger and rarely overlooked by the human eye. Another is that the meta-analyses were underpowered to detect advanced adenomas, compared to conventional adenomas, owing to the low incidence of these lesions. Similarly, although AI-aided colonoscopy did not show an increase in the detection of non-neoplastic lesions at a per-patient level, we observed a greater number of non-neoplastic lesions at a per-polyp level. Such discrepancies are not unexpected, considering that not all AI-rescued missed neoplasia necessarily correspond to the only single neoplasia that characterizes a patient as neoplasia-bearing.
Of note, most findings of the latest study are in line with our results.31 Additionally, this study used several other key quality indicators as secondary endpoints to comprehensively assess the clinical utility of this emerging technology of colorectal neoplasia detection.89 The significant improvement of all these indicators in the current meta-analysis provides more compelling evidence for the potential of AI in colonoscopy. Moreover, the special subgroup and meta-regression analyses provide satisfactory explanations for the substantial heterogeneity among the outcomes, allowing for a better characterization of the patient population that is most likely to benefit from AI-aided colonoscopy.
Due to the higher rate of missed proximal adenoma detection,90 interval CRCs are more common in the proximal colon.91,92 To evaluate the accurate effect of AI-aided tools for colorectal adenoma detection with different characteristics, we conducted separate analyses based on the size, location, and morphology of adenomas. Our results indicated that AI-assisted colonoscopy has the potential to identify a greater number of APC for diminutive and small adenomas at the proximal colon, offering a potential method to decrease the incidence of proximal interval cancers.
Concerns regarding increased procedure time and complication risk with AI-aided colonoscopy were not substantially supported by our findings. Although there was a significant increase in the mean inspection time, the 20-s increased time might not be clinically significant. Thus, adding an AI-aided system will not significantly affect the colonoscopy workflow. The increased inspection time with AI-aided colonoscopy could be mainly attributed to improved lesion detection. Moreover, since the pooled proportion of false positives on AI-aided colonoscopy was 12% (95% CI, 7%–18%) in our study, the increased inspection time could also be partially attributed to the number of false positives.93
However, a concern arises about the increased rate of unnecessary resections for non-neoplastic lesions per colonoscopy, although without increasing the detection of patients with non-neoplastic lesions. Most of these resections were likely due to small hyperplastic polyps that are generally not associated with risk for interval CRC.85,94 Improved detection of these non-neoplastic lesions may ultimately result in overdiagnosis and overtreatment, raising concerns about the real benefit of AI-aided colonoscopy.95 However, recently published microsimulation studies suggested that the adoption of AI-aided tools has the potential to be cost-effective.96, 97, 98 Thus, the effect of such resections on the cost-effectiveness of AI-aided colonoscopy remains inconclusive and warrants further investigation.
An essential aspect of the clinical applications of AI-aided systems is understanding the factors influencing their effectiveness cost-effectively. To our knowledge, this is the first meta-analysis assessing and exploring the reasons behind heterogeneity. Similarly, this study is the first to provide evidence that endoscopists with lower ADR (or PDR), shorter inspection time, patients with lower BMI, and younger age will benefit more from AI-aided colonoscopy. Meanwhile, bowel preparation, anesthesia, and time of day also affect the effect of AI-aid colonoscopy to a certain extent.
Of note, results from China seem better than those reported in other countries, mainly attributed to the young age and low ADR for studies done in China. We have shown a negative correlation between age and the effect of AI-aided colonoscopy. Moreover, patients of younger age are known to have a lower risk of colorectal neoplasia, which may partly explain the low ADR in the control group for studies in China. Also, the low ADR may be attributed to genetic, dietary, lifestyle, and habitus differences, as well as the incidence of colorectal neoplasia between the different populations.60 Regarding studies coming from China, the indication of colonoscopy in the majority of patients was included for diagnostic purposes. This is problematic as true ADR should be accounted for screening colonoscopies only. This issue may explain the low ADR in the control group across the studies. Thus, the effect difference of AI-aided colonoscopy between China and other countries warrants further investigation via multicenter international trials.
This study has several limitations. First, the included studies varied in quality, but sensitivity analyses involving studies with a low risk of bias affirmed the robustness of the pooled results. Second, while the exclusion of studies with direct extraction data did not significantly alter the results in sensitivity analyses, it may reduce the strength of evidence. Third, potential publication bias, though addressed, could still affect the findings Fourth, some analyses did not reveal statistical differences, but caution is necessary due to the low statistical power. Finally, despite the identified associations in meta-regression and subgroup analyses, there might be unexplained confounding factors. Moreover, the associations identified in these studies may not translate to the individual level. Thus, some caution is warranted in extrapolating our findings in the subgroup and meta-regression analyses at individual patient levels.
The study findings suggest that AI-aided colonoscopy effectively improves the detection of colorectal neoplasia, especially adenomas, potentially mitigating the effects of suboptimal bowel preparation and short inspection time. Since small increments in colonoscopy quality could substantially affect net gains from any large-scale CRC screening program,99 the application of AI systems has the potential to maintain high quality and homogeneity of colonoscopies and further improve endoscopist performance in large screening programs and centers with high workloads.
This study also provides several implications for future research. (1) Although ADR is an important outcome of screening colonoscopy, wherein it is inversely related to CRC incidence and mortality,7,8 ADR remains a surrogate for CRC prevention. Concerning the long-term effectiveness of AI systems, clinical studies providing evidence on the AI-driven increased detection of colorectal neoplasia contributing to CRC prevention are lacking. Therefore, future studies with longitudinal follow-up are needed to confirm the potential benefit of AI-aided colonoscopy for the morbidity and mortality of CRC. (2) Although microsimulation studies suggested that adopting AI-aided tools may be cost-effective,96, 97, 98 further high-quality studies are needed to evaluate the cost-effectiveness of AI systems in different global regions to support their use in clinical practice. (3) Based on our subgroup analyses and meta-regression analyses, future studies should verify the performance of AI in different conditions, exploring the most applicable situation for AI-aided colonoscopy. (4) Further studies are required to improve the existing AI systems. Importantly, AI systems should undergo dedicated training with a large dataset of SSLs and advanced adenomas to increase the sensitivity and specificity for the detection of these high-risk precancerous lesions.100,101 Additionally, AI systems should undergo dedicated training with enough samples, including feces, fecal residue, wrinkled mucosa, and other false detections, to reduce false positives.
In conclusion, our study confirmed the role of AI-aided colonoscopy in safely improving the detection of colorectal neoplasia, particularly for low-risk lesions and advanced adenomas, without significant delays in procedure time. AI-aided colonoscopy might be most beneficial in endoscopists with lower ADRs and shorter inspection times and in younger patients with fair bowel preparation. Yet, to assess the long-term benefits and cost-effectiveness of reducing CRC, future studies are needed.
Contributors
Project development: Shenghan Lou, Fenqi Du, Wenjie Song, Binbin Cui, Yanlong Liu, and Peng Han. Data collection or management: Shenghan Lou, Fenqi Du, Wenjie Song, Yixiu Xia, Xinyu Yue, and Da Yang. Data analysis: Shenghan Lou, Fenqi Du, Wenjie Song, and Peng Han. Manuscript writing/editing: Shenghan Lou, Fenqi Du, Wenjie Song, Yixiu Xia, Xinyu Yue, Da Yang, Binbin Cui, Yanlong Liu, and Peng Han. Shenghan Lou, Fenqi Du, Wenjie Song, Binbin Cui, Yanlong Liu, and Peng Han have verified the underlying data. All authors read and approved the final version of the manuscript.
Data sharing statement
This systematic review extracted data from publicly available literature. Upon request, the authors can share these data with qualified researchers.
Declaration of interests
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102341.
Contributor Information
Binbin Cui, Email: cuibinbin@hrbmu.edu.cn.
Yanlong Liu, Email: liuyanlong@hrbmu.edu.cn.
Peng Han, Email: leospiv@hrbmu.edu.cn.
Appendix A. Supplementary data
Forest plot depicting the PMR without or with the intervention of AI-aided systems.
Forest plot depicting the PDR without or with the intervention of AI-aided systems.
Forest plot depicting the PPC rate without or with the intervention of AI-aided systems.
Forest plot depicting the mean difference for PPC without or with the intervention of AI-aided systems.
Funnel plot depicting the publication bias of PDR.
Forest plot depicting the AMR without or with the intervention of AI-aided systems.
Forest plot depicting the ADR without or with the intervention of AI-aided systems.
Forest plot depicting the APC rate without or with the intervention of AI-aided systems.
Forest plot depicting the mean difference for APC without or with the intervention of AI-aided systems.
Funnel plot depicting the publication bias of ADR.
Funnel plot depicting the publication bias of APC rate ratio.
Funnel plot depicting the publication bias of APC mean difference.
Forest plot depicting the insertion time without or with the intervention of AI-aided systems.
Forest plot depicting the inspection time without or with the intervention of AI-aided systems.
Forest plot depicting the adverse event risk without or with the intervention of AI-aided systems.
Forest plot depicting the false-positive rate without or with the intervention of AI-aided systems.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA A Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Davidson K.W., Barry M.J., Mangione C.M., et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021;325:1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 4.Zauber A.G., Winawer S.J., O'Brien M.J., et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winawer S.J., Zauber A.G., Ho M.N., et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H., Chang-Claude J., Jansen L., et al. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146:709–717. doi: 10.1053/j.gastro.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Kaminski M.F., Regula J., Kraszewska E., et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 8.Corley D.A., Jensen C.D., Marks A.R., et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–1306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao S., Wang S., Pan P., et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: a systematic review and meta-analysis. Gastroenterology. 2019;156:1661–1674.e11. doi: 10.1053/j.gastro.2019.01.260. [DOI] [PubMed] [Google Scholar]
- 10.Rex D.K., Cutler C.S., Lemmel G.T., et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 11.Anderson R., Burr N.E., Valori R. Causes of post-colonoscopy colorectal cancers based on world endoscopy organization system of analysis. Gastroenterology. 2020;158:1287–1299.e2. doi: 10.1053/j.gastro.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Berzin T.M., Topol E.J. Adding artificial intelligence to gastrointestinal endoscopy. Lancet. 2020;395:485. doi: 10.1016/S0140-6736(20)30294-4. [DOI] [PubMed] [Google Scholar]
- 13.Byrne M.F., Shahidi N., Rex D.K. Will computer-aided detection and diagnosis revolutionize colonoscopy. Gastroenterology. 2017;153:1460–1464.e1. doi: 10.1053/j.gastro.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Byrne M.F., Chapados N., Soudan F., et al. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut. 2019;68:94–100. doi: 10.1136/gutjnl-2017-314547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinsard D.G., Mori Y., Misawa M., et al. Quality assurance of computer-aided detection and diagnosis in colonoscopy. Gastrointest Endosc. 2019;90:55–63. doi: 10.1016/j.gie.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Kalager M., Wieszczy P., Lansdorp-Vogelaar I., Corley D.A., Bretthauer M., Kaminski M.F. Overdiagnosis in colorectal cancer screening: time to acknowledge a blind spot. Gastroenterology. 2018;155:592–595. doi: 10.1053/j.gastro.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Mori Y., Bretthauer M., Kalager M. Hopes and hypes for artificial intelligence in colorectal cancer screening. Gastroenterology. 2021;161:774–777. doi: 10.1053/j.gastro.2021.04.078. [DOI] [PubMed] [Google Scholar]
- 18.Mohan B.P., Facciorusso A., Khan S.R., et al. Real-time computer aided colonoscopy versus standard colonoscopy for improving adenoma detection rate: a meta-analysis of randomized-controlled trials. eClinicalMedicine. 2020;29-30 doi: 10.1016/j.eclinm.2020.100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan C., Spadaccini M., Iannone A., et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:77–85.e6. doi: 10.1016/j.gie.2020.06.059. [DOI] [PubMed] [Google Scholar]
- 20.Barua I., Vinsard D.G., Jodal H.C., et al. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53:277–284. doi: 10.1055/a-1201-7165. [DOI] [PubMed] [Google Scholar]
- 21.Aziz M., Fatima R., Dong C., Lee-Smith W., Nawras A. The impact of deep convolutional neural network-based artificial intelligence on colonoscopy outcomes: a systematic review with meta-analysis. J Gastroenterol Hepatol. 2020;35:1676–1683. doi: 10.1111/jgh.15070. [DOI] [PubMed] [Google Scholar]
- 22.Shah S., Park N., Chehade N., et al. Effect of computer-aided colonoscopy on adenoma miss rates and polyp detection: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;38:162. doi: 10.1111/jgh.16059. [DOI] [PubMed] [Google Scholar]
- 23.Huang D., Shen J., Hong J., et al. Effect of artificial intelligence-aided colonoscopy for adenoma and polyp detection: a meta-analysis of randomized clinical trials. Int J Colorectal Dis. 2022;37:495–506. doi: 10.1007/s00384-021-04062-x. [DOI] [PubMed] [Google Scholar]
- 24.Deliwala S.S., Hamid K., Barbarawi M., et al. Artificial intelligence (AI) real-time detection vs. routine colonoscopy for colorectal neoplasia: a meta-analysis and trial sequential analysis. Int J Colorectal Dis. 2021;36:2291–2303. doi: 10.1007/s00384-021-03929-3. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Lu J., Yan J., Tan Y., Liu D. Artificial intelligence can increase the detection rate of colorectal polyps and adenomas: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:1041–1048. doi: 10.1097/MEG.0000000000001906. [DOI] [PubMed] [Google Scholar]
- 26.Shao L., Yan X., Liu C., Guo C., Cai B. Effects of ai-assisted colonoscopy on adenoma miss rate/adenoma detection rate: a protocol for systematic review and meta-analysis. Medicine. 2022;101 doi: 10.1097/MD.0000000000031945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Zhang X., Wu Q., Gu C., Wang Z. Artificial intelligence-aided colonoscopy for polyp detection: a systematic review and meta-analysis of randomized clinical trials. J Laparoendosc Adv Surg Tech. 2021;31:1143–1149. doi: 10.1089/lap.2020.0777. [DOI] [PubMed] [Google Scholar]
- 28.Aslam M.F., Bano S., Khalid M., et al. The effectiveness of real-time computer-aided and quality control systems in colorectal adenoma and polyp detection during colonoscopies: a meta-analysis. Ann Med Surg (Lond) 2023;85:80–91. doi: 10.1097/MS9.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashat M., Klair J.S., Singh D., Murali A.R., Krishnamoorthi R. Impact of real-time use of artificial intelligence in improving adenoma detection during colonoscopy: a systematic review and meta-analysis. Endosc Int Open. 2021;9:E513–E521. doi: 10.1055/a-1341-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivananthan A., Nazarian S., Ayaru L., et al. Does computer-aided diagnostic endoscopy improve the detection of commonly missed polyps? A meta-analysis. Clin Endosc. 2022;55:355–364. doi: 10.5946/ce.2021.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassan C., Spadaccini M., Mori Y., et al. Real-time computer-aided detection of colorectal neoplasia during colonoscopy : a systematic review and meta-analysis. Ann Intern Med. 2023;176:1209–1220. doi: 10.7326/M22-3678. [DOI] [PubMed] [Google Scholar]
- 32.Lou S., Yin X., Wang Y., Zhang Y., Xue Y. Laparoscopic versus open gastrectomy for gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Int J Surg. 2022;102 doi: 10.1016/j.ijsu.2022.106678. [DOI] [PubMed] [Google Scholar]
- 33.Aniwan S., Orkoonsawat P., Viriyautsahakul V., et al. The secondary quality indicator to improve prediction of adenoma miss rate apart from adenoma detection rate. Am J Gastroenterol. 2016;111:723–729. doi: 10.1038/ajg.2015.440. [DOI] [PubMed] [Google Scholar]
- 34.Gotzsche P.C. Why we need a broad perspective on meta-analysis. It may be crucially important for patients. BMJ. 2000;321:585–586. doi: 10.1136/bmj.321.7261.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 36.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi J., Luo D., Weng H., et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11(5):641–654. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 38.Sterne J., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 39.Egger M., Davey S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 41.Guyatt G.H., Oxman A.D., Schünemann H.J., Tugwell P., Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 42.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 43.IntHout J., Ioannidis J.P., Borm G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Röver C., Knapp G., Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015;15:99. doi: 10.1186/s12874-015-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.IntHout J., Ioannidis J.P., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7) doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley R.D., Higgins J.P., Deeks J.J. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 48.Higgins J.P., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 50.Mangas-Sanjuan C., de-Castro L., Cubiella J., et al. Role of artificial intelligence in colonoscopy detection of advanced neoplasias : a randomized trial. Ann Intern Med. 2023;176:1145–1152. doi: 10.7326/M22-2619. [DOI] [PubMed] [Google Scholar]
- 51.Yao L., Li X., Wu Z., Wang J., Luo C., et al. Effect of artificial intelligence on novice performed colonoscopy: a multicenter randomized controlled tandem study. Gastrointest Endosc. 2023;S0016-S5107(23) doi: 10.1016/j.gie.2023.07.044. 02795-02795[pii] [DOI] [PubMed] [Google Scholar]
- 52.Wang L., Feng H., Chen W., Luan F. Artificial intelligence - based colorectal polyp diagnostic system can increase the detection rate of polyps: a prospective randomized controlled study. Chin J Gastroenterol. 2022;27:163–167. [Google Scholar]
- 53.Gimeno-García A.Z., Hernández Negrin D., Hernández A., et al. Usefulness of a novel computer-aided detection system for colorectal neoplasia: a randomized controlled trial. Gastrointest Endosc. 2023;97:528–536.e1. doi: 10.1016/j.gie.2022.09.029. [DOI] [PubMed] [Google Scholar]
- 54.Liu P., Wang P., Glissen Brown J.R., et al. The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study. Therap Adv Gastroenterol. 2020;13 doi: 10.1177/1756284820979165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vilkoite I., Tolmanis I., Meri H.A., et al. The role of an artificial intelligence method of improving the diagnosis of neoplasms by colonoscopy. Diagnostics. 2023;13 doi: 10.3390/diagnostics13040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W.N., Zhang Y.Y., Bian X.Q., et al. Study on detection rate of polyps and adenomas in artificial-intelligence-aided colonoscopy. Saudi J Gastroenterol. 2020;26:13–19. doi: 10.4103/sjg.SJG_377_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamba S., Tamai N., Saitoh I., et al. Reducing adenoma miss rate of colonoscopy assisted by artificial intelligence: a multicenter randomized controlled trial. J Gastroenterol. 2021;56:746–757. doi: 10.1007/s00535-021-01808-w. [DOI] [PubMed] [Google Scholar]
- 58.Hüneburg R., Bucksch K., Schmeißer F., et al. Real-time use of artificial intelligence (CADEYE) in colorectal cancer surveillance of patients with Lynch syndrome-A randomized controlled pilot trial (CADLY) United European Gastroenterol J. 2023;11:60–68. doi: 10.1002/ueg2.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen P., Li W.Z., Li J.X., et al. Real-time use of a computer-aided system for polyp detection during colonoscopy, an ambispective study. J Dig Dis. 2021;22:256–262. doi: 10.1111/1751-2980.12985. [DOI] [PubMed] [Google Scholar]
- 60.Wang P., Berzin T.M., Glissen Brown J.R., et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68:1813–1819. doi: 10.1136/gutjnl-2018-317500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engelke C., Graf M., Maass C., et al. Prospective study of computer-aided detection of colorectal adenomas in hospitalized patients. Scand J Gastroenterol. 2023:1–6. doi: 10.1080/00365521.2023.2212309. [DOI] [PubMed] [Google Scholar]
- 62.Wang P., Liu P., Glissen Brown J.R., et al. Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Gastroenterology. 2020;159:1252–1261.e5. doi: 10.1053/j.gastro.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 63.Yamaguchi D., Shimoda R., Miyahara K., et al. Impact of an artificial intelligence-aided endoscopic diagnosis system on improving endoscopy quality for trainees in colonoscopy: a prospective, randomized, multicenter study. Dig Endosc. 2023 doi: 10.1111/den.14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su J.R., Li Z., Shao X.J., et al. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: a prospective randomized controlled study (with videos) Gastrointest Endosc. 2020;91:415–424.e4. doi: 10.1016/j.gie.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 65.Wallace M.B., Sharma P., Bhandari P., et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 2022;163:295–304.e5. doi: 10.1053/j.gastro.2022.03.007. [DOI] [PubMed] [Google Scholar]
- 66.Ahmad A., Wilson A., Haycock A., et al. Evaluation of a real-time computer-aided polyp detection system during screening colonoscopy: AI-DETECT study. Endoscopy. 2023;55:313–319. doi: 10.1055/a-1966-0661. [DOI] [PubMed] [Google Scholar]
- 67.Wei M.T., Shankar U., Parvin R., et al. Evaluation of computer aided detection during colonoscopy in the community (AI-SEE): a multicenter randomized clinical trial. Am J Gastroenterol. 2023;118:1841. doi: 10.14309/ajg.0000000000002239. [DOI] [PubMed] [Google Scholar]
- 68.Rondonotti E., Di Paolo D., Rizzotto E.R., et al. Efficacy of a computer-aided detection system in a fecal immunochemical test-based organized colorectal cancer screening program: a randomized controlled trial (AIFIT study) Endoscopy. 2022;54:1171–1179. doi: 10.1055/a-1849-6878. [DOI] [PubMed] [Google Scholar]
- 69.Repici A., Badalamenti M., Maselli R., et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020;159:512–520.e7. doi: 10.1053/j.gastro.2020.04.062. [DOI] [PubMed] [Google Scholar]
- 70.Karsenti D., Tharsis G., Perrot B., et al. Effect of real-time computer-aided detection of colorectal adenoma in routine colonoscopy (COLO-GENIUS): a single-centre randomised controlled trial. Lancet Gastroenterol Hepatol. 2023;8:726. doi: 10.1016/S2468-1253(23)00104-8. [DOI] [PubMed] [Google Scholar]
- 71.Yao L., Zhang L., Liu J., et al. Effect of an artificial intelligence-based quality improvement system on efficacy of a computer-aided detection system in colonoscopy: a four-group parallel study. Endoscopy. 2022;54:757–768. doi: 10.1055/a-1706-6174. [DOI] [PubMed] [Google Scholar]
- 72.Wang P., Liu X., Berzin T.M., et al. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol Hepatol. 2020;5:343–351. doi: 10.1016/S2468-1253(19)30411-X. [DOI] [PubMed] [Google Scholar]
- 73.Gong D., Wu L., Zhang J., et al. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol Hepatol. 2020;5:352–361. doi: 10.1016/S2468-1253(19)30413-3. [DOI] [PubMed] [Google Scholar]
- 74.Glissen Brown J.R., Mansour N.M., Wang P., et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: a United States multicenter randomized tandem colonoscopy study (CADeT-CS trial) Clin Gastroenterol Hepatol. 2022;20:1499–1507.e4. doi: 10.1016/j.cgh.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 75.Lui T., Hang D.V., Tsao S., et al. Computer-assisted detection versus conventional colonoscopy for proximal colonic lesions: a multicenter, randomized, tandem-colonoscopy study. Gastrointest Endosc. 2023;97:325–334.e1. doi: 10.1016/j.gie.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 76.Aniwan S., Mekritthikrai K., Kerr S.J., et al. Computer-aided detection, mucosal exposure device, their combination, and standard colonoscopy for adenoma detection: a randomized controlled trial. Gastrointest Endosc. 2023;97:507–516. doi: 10.1016/j.gie.2022.09.023. [DOI] [PubMed] [Google Scholar]
- 77.Shaukat A., Lichtenstein D.R., Somers S.C., et al. Computer-aided detection improves adenomas per colonoscopy for screening and surveillance colonoscopy: a randomized trial. Gastroenterology. 2022;163:732–741. doi: 10.1053/j.gastro.2022.05.028. [DOI] [PubMed] [Google Scholar]
- 78.Nakashima H., Kitazawa N., Fukuyama C., et al. Clinical evaluation of computer-aided colorectal neoplasia detection using a novel endoscopic artificial intelligence: a single-center randomized controlled trial. Digestion. 2023;104:1–9. doi: 10.1159/000528085. [DOI] [PubMed] [Google Scholar]
- 79.Xu L., He X., Zhou J., et al. Artificial intelligence-assisted colonoscopy: a prospective, multicenter, randomized controlled trial of polyp detection. Cancer Med. 2021;10:7184–7193. doi: 10.1002/cam4.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang P., Liu X.G., Kang M., et al. Artificial intelligence empowers the second-observer strategy for colonoscopy: a randomized clinical trial. Gastroenterol Rep (Oxf) 2023;11 doi: 10.1093/gastro/goac081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Repici A., Spadaccini M., Antonelli G., et al. Artificial intelligence and colonoscopy experience: lessons from two randomised trials. Gut. 2022;71:757–765. doi: 10.1136/gutjnl-2021-324471. [DOI] [PubMed] [Google Scholar]
- 82.Xu H., Tang R., Lam T., et al. Artificial intelligence-assisted colonoscopy for colorectal cancer screening: a multicenter randomized controlled trial. Clin Gastroenterol Hepatol. 2023;21:337–346.e3. doi: 10.1016/j.cgh.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Brenner H., Hoffmeister M., Stegmaier C., et al. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585–1589. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahmad O.F., Soares A.S., Mazomenos E., et al. Artificial intelligence and computer-aided diagnosis in colonoscopy: current evidence and future directions. Lancet Gastroenterol Hepatol. 2019;4:71–80. doi: 10.1016/S2468-1253(18)30282-6. [DOI] [PubMed] [Google Scholar]
- 85.Vleugels J., Hassan C., Senore C., et al. Diminutive polyps with advanced histologic features do not increase risk for metachronous advanced colon neoplasia. Gastroenterology. 2019;156:623–634.e3. doi: 10.1053/j.gastro.2018.10.050. [DOI] [PubMed] [Google Scholar]
- 86.Jeong Y.H., Kim K.O., Park C.S., et al. Risk factors of advanced adenoma in small and diminutive colorectal polyp. J Kor Med Sci. 2016;31:1426–1430. doi: 10.3346/jkms.2016.31.9.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim N.H., Jung Y.S., Lee M.Y., et al. Risk of developing metachronous advanced colorectal neoplasia after polypectomy in patients with multiple diminutive or small adenomas. Am J Gastroenterol. 2019;114:1657–1664. doi: 10.14309/ajg.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 88.Kim J.Y., Kim T.J., Baek S.Y., et al. Risk of metachronous advanced neoplasia in patients with multiple diminutive adenomas. Am J Gastroenterol. 2018;113:1855–1861. doi: 10.1038/s41395-018-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rex D.K. Key quality indicators in colonoscopy. Gastroenterol Rep (Oxf) 2023;11 doi: 10.1093/gastro/goad009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pickhardt P.J., Nugent P.A., Mysliwiec P.A., Choi J.R., Schindler W.R. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352–359. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 91.Brenner H., Hoffmeister M., Arndt V., et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 92.Nakagawa-Senda H., Hori M., Matsuda T., Ito H. Prognostic impact of tumor location in colon cancer: the Monitoring of Cancer Incidence in Japan (MCIJ) project. BMC Cancer. 2019;19:431. doi: 10.1186/s12885-019-5644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hassan C., Badalamenti M., Maselli R., et al. Computer-aided detection-assisted colonoscopy: classification and relevance of false positives. Gastrointest Endosc. 2020;92:900–904.e4. doi: 10.1016/j.gie.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 94.von Renteln D., Barkun A.N. Increasing detection rates for diminutive adenomas: are we on the right track. Gut. 2016;65:1056–1057. doi: 10.1136/gutjnl-2016-311555. [DOI] [PubMed] [Google Scholar]
- 95.Zimmermann-Fraedrich K., Rösch T. Artificial intelligence and the push for small adenomas: all we need. Endoscopy. 2023;55:320–323. doi: 10.1055/a-2038-7078. [DOI] [PubMed] [Google Scholar]
- 96.Sekiguchi M., Igarashi A., Toyoshima N., et al. Cost-effectiveness analysis of computer-aided detection systems for colonoscopy in Japan. Dig Endosc. 2023;35:891. doi: 10.1111/den.14532. [DOI] [PubMed] [Google Scholar]
- 97.Thiruvengadam N.R., Coté G.A., Gupta S., et al. An evaluation of critical factors for the cost-effectiveness of real-time computer-aided detection: sensitivity and threshold analyses using a microsimulation model. Gastroenterology. 2023;164:906–920. doi: 10.1053/j.gastro.2023.01.027. [DOI] [PubMed] [Google Scholar]
- 98.Barkun A.N., von Renteln D., Sadri H. Cost-effectiveness of artificial intelligence-aided colonoscopy for adenoma detection in colon cancer screening. J Can Assoc Gastroenterol. 2023;6:97–105. doi: 10.1093/jcag/gwad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robertson D.J., Kaminski M.F., Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut. 2015;64:982–990. doi: 10.1136/gutjnl-2014-308076. [DOI] [PubMed] [Google Scholar]
- 100.Aziz M., Desai M., Hassan S., et al. Improving serrated adenoma detection rate in the colon by electronic chromoendoscopy and distal attachment: systematic review and meta-analysis. Gastrointest Endosc. 2019;90:721–731.e1. doi: 10.1016/j.gie.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 101.Kolb J.M., Molmenti C.L., Patel S.G., Lieberman D.A., Ahnen D.J. Increased risk of colorectal cancer tied to advanced colorectal polyps: an untapped opportunity to screen first-degree relatives and decrease cancer burden. Am J Gastroenterol. 2020;115:980–988. doi: 10.14309/ajg.0000000000000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot depicting the PMR without or with the intervention of AI-aided systems.
Forest plot depicting the PDR without or with the intervention of AI-aided systems.
Forest plot depicting the PPC rate without or with the intervention of AI-aided systems.
Forest plot depicting the mean difference for PPC without or with the intervention of AI-aided systems.
Funnel plot depicting the publication bias of PDR.
Forest plot depicting the AMR without or with the intervention of AI-aided systems.
Forest plot depicting the ADR without or with the intervention of AI-aided systems.
Forest plot depicting the APC rate without or with the intervention of AI-aided systems.
Forest plot depicting the mean difference for APC without or with the intervention of AI-aided systems.
Funnel plot depicting the publication bias of ADR.
Funnel plot depicting the publication bias of APC rate ratio.
Funnel plot depicting the publication bias of APC mean difference.
Forest plot depicting the insertion time without or with the intervention of AI-aided systems.
Forest plot depicting the inspection time without or with the intervention of AI-aided systems.
Forest plot depicting the adverse event risk without or with the intervention of AI-aided systems.
Forest plot depicting the false-positive rate without or with the intervention of AI-aided systems.