Abstract
To explore the differential regulation mechanism of heat stress on the egg production performance and egg quality of Jinding ducks, 200 Jinding ducks (360-day-old) in good health and with similar body weights and a normal appetite were selected and randomly divided into a control (normal temperature [NT]) group (20°C–25°C) and a heat stress (HS) group (32°C–36°C), with 4 replicates in each group and 25 ducks in each replicate. The pretrial period was 1 wk, and the formal trial period was 4 wk. At the end of the 4th wk, 12 duck eggs were collected from each replicate to determine egg quality. Pituitary and ovarian tissues of Jinding ducks were collected, transcriptome sequencing was performed to screen differentially expressed miRNAs and mRNAs related to high temperature and heat stress, and a competitive endogenous RNA regulatory network was constructed. The sequencing data were verified by qRT‒PCR method. The following results were obtained: (1) Compared with the NT group, the HS group had a significantly lower laying rate, total egg weight, average egg weight, total feed intake, and feed intake per duck (P < 0.01), an extremely significantly higher feed-to-egg ratio (P < 0.01), and a higher mortality rate. (2) Compared with the NT group, the HS group had an extremely significantly lower egg weight, egg yolk weight, eggshell weight, and eggshell strength (P < 0.01) and an extremely significantly lower yolk ratio and eggshell thickness (P < 0.01, P < 0.05); however, there was no significant difference in the egg shape index, Haugh unit or protein height (P > 0.05). (3) A total of 1,974 and 1,202 genes were identified in the pituitary and ovary, respectively, and there were 5 significantly differentially expressed miRNAs. The differentially expressed genes were involved in the arginine and proline metabolism pathways, ether lipid metabolism pathway, and drug metabolism–cytochrome P450 pathway, which are speculated to be related to the egg production performance of Jingding ducks under high-temperature heat stress. (4) Novel_221 may target the PRPS1 gene to participate in egg production performance; novel_168 and novel_289 may target PIGW; novel_289 may target Q3MUY2; and novel_289 and novel_208 may target PIGN or genes that may be related to high-temperature heat stress. (5) In pituitary tissue, upregulated novel_141 (center of the network) formed a regulatory network with HSPB1 and HSP30A, and downregulated novel_366 (center of the network) formed a regulatory network with the JIP1 gene. In ovarian tissue, downregulated novel_289 (center of the network) formed a regulatory network with the ZSWM7, ABI3, and K1C23 genes, novel_221 formed a regulatory network with the IGF1, BCL7B, SMC6, APOA4, and FARP2 genes, and upregulated novel_40 formed a regulatory network with the HA1FF10 gene. In summary, heat stress affects the production performance and egg quality of Jinding ducks by regulating the secretion of endocrine-related hormones and the release of neurotransmitters as well as the expression of miRNAs and mRNAs in pituitary and ovarian tissues. The miRNA‒mRNA regulatory network provides a theoretical basis for the molecular mechanism that regulates the stress response in pituitary and ovarian tissues, egg quality, and production performance under heat stress.
Key words: Jinding duck, heat stress, production performance, egg quality, miRNA‒mRNA regulatory network
INTRODUCTION
Laying ducks are more sensitive to heat stress, and their comfortable ambient temperature is generally 15°C to 25°C (Zeng et al., 2013). At 26°C to 32°C, laying ducks are in a sub-heat stress state; ambient temperatures that exceed 32°C induce stress, and physiological functions tend to be disrupted. Heat stress has a profound impact on poultry production performance. The decline in production performance is inextricably linked with a reduction in feed intake. The change in production performance is not merely an external manifestation but rather a result of a series of reactions to a continuous high-temperature environment. First, heat stress reduces feed intake, thus reducing egg production and egg quality, increasing the feed conversion rate, and greatly influencing metabolism, the endocrine system, and physiological function (Qaid and Al-Garadi, 2021). The production and metabolism of laying poultry are characterized by fast heat generation, high output, and a high metabolic rate, and poultry with high metabolic rates are highly prone to the negative effects of a high-temperature environment (Wasti et al., 2020). Heat stress inhibits the immune function of laying ducks, reducing the blood immunoglobulin concentration, the number of blood lymphocytes and macrophages, and phagocyte ability, and high temperature reduces cellular and humoral immunity to varying degrees, lowering disease resistance and increasing the mortality rate (Ma et al., 2014). Heat stress is an important factor in poultry productivity and causes economic losses in hot regions. There are several reasons for the decline in production performance. First, to adapt to a continuous high-temperature environment, poultry regulate their body temperature by increasing their water intake, reducing their feed intake and corresponding daily activities, and lowering their heat production to avoid secondary injury caused by heat stress (Saeed et al., 2019). However, it is difficult to achieve a balance between heat generation and heat dissipation. Studies have shown that for every 1°C increase in the breeding environment (Lu et al., 2007), the feed intake of species with a short growth cycle decreases by approximately 3.4%, and the feed intake of species with a long growth cycle decreases by approximately 1.7%. Second, after feed intake is suppressed, nutrient intake and digestion, and absorption capacity decrease, resulting in abnormal ovarian gene expression and reproductive hormone levels, thus affecting egg production, egg weight, egg quality, and blood biochemical indicators (Li et al., 2020). The adverse effect of heat stress on egg quality manifests as a longer production duration and cycle, causing greater economic losses to poultry production. Heat stress reduces the quality and yield of livestock and poultry products, primarily affecting egg yolk weight, eggshell thickness, ovary weight, and egg number in laying poultry (Zheng et al., 2022). He et al. (2019) found that at 37°C, the average daily weight gain decreased by 31.9%, and the average daily feed intake decreased by 15.8%. Sahin et al. (2018) showed that heat stress significantly increased the blood glucose and serum cholesterol of laying hens and significantly reduced egg production, feed intake, eggshell strength, eggshell weight, Haugh unit, and egg weight.
With continuous improvements in the scale and intensification of the laying duck industry, heat stress has become increasingly prominent and a technical bottleneck restricting the development of the laying duck industry. In recent years, many high-throughput sequencing technologies have been applied to investigate miRNA and heat stress. For example, through the high-throughput sequencing of 6 small RNA libraries of head kidney tissue of rainbow trout. Ma et al. (2019) identified 78 miRNAs expressed in different heat stress responses and found that important regulatory pathways and potential target genes were involved in heat stress, elucidating the heat stress regulatory mechanism of miRNAs in the head kidney of rainbow trout and supporting the development of the rainbow trout aquaculture industry. Wu et al. (2012) obtained 73 conserved miRNAs from the roots, stems, leaves, and flowers of ginseng and predicted 99 and 31 target genes of conserved and no conserved miRNA families, respectively, with many miRNAs showing tissue-specific expression. They identified 5 dehydration-responsive miRNAs and 10 heat-responsive miRNAs and discovered crosstalk among some stress-responsive miRNAs. These studies have led to a new understanding of the expression of miRNAs in different species and their specific regulatory functions. Zhu et al. (2019) compared the differential expression of miRNAs and mRNAs in laying hens under heat stress and under a normal temperature and identified 16 differentially expressed miRNAs and 502 differentially expressed genes (DEGs). They found that the response-related gene autophagy-related protein 9A might be controlled by the upregulated miRNAs gga-miR-92-5p, gga-miR-1618-5p, gga-miR-1737, and gga-miR-6557 in response to heat stress and revealed an increase in lipid metabolism in heat-stressed laying hens through analyses of DEGs. The level of heat stress directly affects the growth, development, and production performance of poultry. miRNAs are considered potential regulators of immune cytokines. Li et al. (2020) analyzed miRNAs in the spleen of heat-stressed poultry and found that some miRNAs involved in cytokine‒cytokine receptor interactions were differentially expressed between the control group and the heat-stressed group, including miR-34a and miR-449c, whose target genes are interleukin 2 (IL-2) and interleukin 12α (IL-12α). Their results indicated that miRNAs can directly or indirectly change the expression of immune cytokines and mediate the immunity of broiler chickens under acute heat stress. Tian et al. (2019) studied the morphological changes and miRNA expression profiles in 3 intestinal segments (duodenum, jejunum, and ileum) of Shaoxing ducks in response to high temperatures. Their results showed that heat stress caused different degrees of damage to these intestinal segments and that miRNAs were differentially expressed in the duodenum, jejunum, and ileum of ducks in the control and heat-stressed groups, differences that may have been related to heat-induced intestinal damage in ducks. Based on a review of many heat stress-related studies (Wu et al., 2012; Tian et al., 2019; Zhu et al., 2019; Li et al., 2020; Srikanth et al., 2020; Hu et al., 2021), miRNAs are closely related to heat stress, and increases in the internal and external temperatures of the body will affect the expression of miRNAs in the body to regulate target genes and target proteins, which in turn affect the state of the body during heat stress; additionally, the increase in ambient temperature determines the specificity, time sequence and concentration of miRNA expression. The reproductive process of animals is regulated by many factors, among which the hypothalamic-pituitary-ovarian axis plays a major role in regulation and is sensitive to temperature changes (Zhao et al., 2023). Heat stress affects the balance of the hypothalamic-pituitary-ovarian axis, and causes significant changes in female reproductive hormones such as gonadotropin-releasing hormone, follicle stimulating hormone, luteinizing hormone, and impaired ovarian function (An et al., 2020; Bei et al., 2020; Li et al., 2020), resulting in impaired or inhibited follicle development (Li et al., 2016), which affects its reproductive performance. In this study, Jinding ducks were used as research objects to explore the effect of high-temperature heat stress on the egg production performance and egg quality of laying ducks, aiming to provide a reference for research on the effects of heat stress on the egg production performance and egg quality of laying ducks. In addition, mRNAs and miRNAs in ovarian and pituitary tissues were analyzed to identify differentially expressed mRNAs and miRNAs after high-temperature heat stress, and the target genes of the differentially expressed mRNAs and miRNAs were predicted and functionally annotated to construct a miRNA‒mRNA interaction network diagram. This study not only lays a foundation for understanding the specific mechanism by which heat stress affects the production performance of Jinding ducks at the molecular level but also provides a scientific basis for how to alleviate the effect of heat stress on the egg production performance of Jinding ducks.
MATERIALS AND METHODS
Experimental Animals and Rearing
A total of 200 Jinding ducks (360-day-old) in good health and with similar body weight and a normal appetite were randomly divided into 2 groups (NT and HS) with 4 replicates in each group and 25 ducks in each replicate. The NT group was the control group, which was housed at an ambient temperature of 25°C ± 2°C, and the HS group was the high-temperature heat stress group, which was housed at an ambient temperature of 35°C ± 2°C. The pretrial period was 1 wk, and the formal trial period was 4 wk. The experiment was carried out at the Institute of Animal Husbandry and Veterinary Medicine, Fujian Academy of Agricultural Sciences (Fuzhou, Fujian, China). The ducks were raised in floor-rearing environments in strict accordance with Jinding duck-rearing management standards. Specifically, the light was fixed at 16 h/d, and the light intensity was 20 lx. The ducks were fed twice daily, i.e., once in the morning and once in the evening, and feed intake was recorded. The ducks had free access to water. Eggs were collected every morning, and the henhouse was cleaned regularly. Throughout the experiment, the ducks were fed formula feed 711 for laying ducks in the egg-producing period (Hualong Feedstuff Co., Ltd., Fuzhou, Fujian, China); the nutritional components are shown in Table 1.
Table 1.
Nutritional level of basal diet (%).
| Items | Content | Measured value |
|---|---|---|
| CP | ≥17.0 | 16.95 |
| Ca | 2.5–4.20 | 3.30 |
| TP | 0.35–1.00 | 0.69 |
| Nacl | 0.25–0.80 | 0.40 |
| Crude fiber | ≤6.0 | 3.47 |
| Ash | ≤17 | 11.88 |
| Met | 0.20–0.90 | 0.34 |
| Moisture | ≤13 | 11.98 |
Remarks: laying ducks laying period compound feed 711, product standard number: Q/FQHL 003-2018 feed production licence number: min feed permit (2020) 01352.
Production Performance
During the formal trial period, the number of eggs laid, deformed eggs, and deaths for each replicate were recorded every day, and the total egg weight for each replicate was recorded. The daily feed intake of each replicate was recorded and analyzed every day. Excel was used to analyze the laying rate, deformed egg rate, mortality rate, average egg weight, actual feed intake, and feed-to-egg ratio for each replicate.
Egg Quality
On the fourth weekend of the experiment, 12 duck eggs were collected from each replicate (n = 12); the eggs were weighed using an XH30002 electronic balance, and the weights were recorded. Vernier callipers were used to measure the transverse and longitudinal diameters of the eggs, and the egg shape index was calculated (egg shape index = longitudinal diameter/transverse diameter). Eggshell strength was measured using a TENOVO eggshell strength tester (KQ-1A, Tenovo International Co., Ltd., Beijing, China), and the yolk colour, albumen height, and Haugh unit of the duck eggs were measured using egg quality analyser (EA-01, Israel ORKA Food Technology Ltd., Bountiful, UT). Yolk weight and eggshell weight were acquired using an XH30002 electronic balance and then used to calculate the yolk ratio. Eggshell thickness at the tip, middle, and blunt ends was measured using a micrometer, and the average values were calculated.
Sample Collection, Total RNA Extraction, Library Construction, and HiSeq Sequencing
On the fourth weekend of the experiment, 1 Jinding duck was selected from each replicate, i.e., 4 individuals in the NT group and 4 individuals in the HS group, and sacrificed after fasting for 12 h. Pituitary and ovarian tissues were placed into cryopreservation tubes, which were immediately placed into liquid nitrogen after being labeled and numbered and then placed in a −80°C ultralow temperature freezer until being used for the transcriptome analysis.
Total RNA was extracted by the TRIzol method. RNA purity was checked using spectrophotometer (Nanophotometer, IMPLEN, CA). The RNA concentration was measured using RNA Assay Kit in Fluorometer (Qubit 2.0, Life Technologies, CA). RNA integrity was assessed using the RNA Assay Kit (Nano 6000,Agilent Technologies, CA).
Bioinformatics Analysis
Raw sequencing data were assessed for quality, filtered, and screened by sRNA length. The reference genome was obtained from Ensembl: www.ensembl.org/Anas_platyrhynchos/Info/Index,BGI_duck_1.0 (GCA_000355885.1). Known miRNAs were analyzed (mirdeep2_0_0_5), novel miRNAs were predicted (miREvo_v1.1), sRNAs were classified and annotated, miRNA copy numbers were converted to standard expression, and DEGs were analyzed (DEG Seq 1.2.2). Differentially expressed miRNAs were screened using the SAM package and subjected to cluster analysis. miRanda-3.3a, PITA, and RNAhybrid were used to predict the target genes of the differentially expressed miRNAs to obtain the corresponding relationship between miRNAs and target genes. Candidate target genes were subjected to Gene Ontology (Release2.12, GO, www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional annotation analyses.
RT‒PCR Validation
Primer Premier 6.0 and Beacon Designer 7.8 were used to design quantitative polymerase chain reaction (PCR) primers, which were then synthesized by Bioengineering Co., Ltd (Shanghai, China). The primer sequences are shown in Table 2, and the real-time PCR amplification system and reaction conditions: 20 μL system (template 2.0 μL, forward Primer 0.4 μL, reverse primer 0.4 μL, Genious 2X SYBR Green Fast qPCR Mix 10 μL, RoxII 0.4 μL, and ddH2O 6.8 μL). With the reaction condition:95℃ 3 min;45 cycles (95℃ 5 sec, 60℃ 30 sec). The Dissociation Stage used ABI QuantStudio 3 (Commonwealth, MA) to detect the relative expression of each target gene in the sample. Each sample was repeated 3 times, and the relative expression level of each gene was calculated using the 2−ΔΔCt method.
Table 2.
The primer sequences used in this study.
| Primer name | Primer sequences 5’-3’ |
|---|---|
| Phospholipids phosphatase 1-F | ACCACTGGAGCGATGTTCTA |
| Phospholipids phosphatase 1-R | GCAGGGTTGTATGGGAATCTTC |
| HFM1-F | GAGCAGGAACTCAACTTCATCT |
| HFM1-R | CTGGAGGTGACGAAGACAAC |
| peroxisomal-F | GGTGTTCAGTTTGCTCCCTTT |
| peroxisomal-R | GCAGCCCTCTCAATTTCTTCAT |
| galactosylcsulf-F | CAGCAGGAGAACGAGAAGATG |
| galactosylcsulf-R | TTCAAGTTGTAGCCCAGGATG |
| antizyme inhibitor 2-F | CGCTGCCGTGATAAACTCTG |
| antizyme inhibitor 2-R | TGGTAGACGAGGCTCTTCTTG |
| ACTB-F | GCTATGAACTCCCTGATGGC |
| ACTB-R | CAGGTCCTTACGGATGTCCAC |
| novel_90-F | CCGCGTAATGCCCCTAAAAATCCTTAT |
| novel_289-F | CGTGCTTGCTGTAGCTGAGCA |
| novel_221-F | CGTAAGCCTCGTGTCTCTGCAGT |
| novel_208-F | ATTGAGCACCGCGGTACCTG |
| U6-F | TTCGTGAAGCGTTCCATATTTT |
Statistical Analysis
The data were analyzed using SPSS19.0. One-way analysis of variance (ANOVA) was used to identify significant differences. P < 0.05 was considered a significant difference, and P < 0.01 was considered an extremely significant difference. Data are presented as the mean ± standard deviation (SD).
RESULTS
The Effect of Heat Stress on the Production Performance and Egg Quality of Jinding Ducks
As shown in Table 3, compared with the NT group, the HS group had a significantly lower laying rate, total egg weight, average egg weight, feed intake, and feed intake per duck (P < 0.01), an extremely significantly higher feed-to-egg ratio (P < 0.01), and a higher mortality rate. As seen in Table 4, compared with the NT group, the HS group had an extremely significantly lower egg weight, yolk weight, eggshell weight, and eggshell strength (P < 0.01) and an extremely significantly lower yolk ratio and eggshell thickness (P < 0.05); there was no significant difference in egg shape index, Haugh unit or albumen height (P > 0.05).
Table 3.
The effect of heat stress on the production performance of Jinding ducks.
| Items | NT group | HS group | P value |
|---|---|---|---|
| laying rate(%) | 77.58 ± 1.07A | 67.80 ± 1.17B | <0.001 |
| Total egg weight(g) | 1,362.98 ± 21.59A | 1,153.32 ± 197.28B | <0.001 |
| Average egg weight(g) | 71.39 ± 2.72A | 67.16 ± 1.93B | <0.001 |
| Total feed intake(g) | 3,977.34 ± 512.51A | 3,565.14 ± 404.20B | <0.001 |
| Feed intake(g) | 161.96±20.51A | 144.23 ± 16.26B | 0.002 |
| Feed egg ratio(%) | 2.95 ± 0.35B | 3.17 ± 0.55A | 0.003 |
| Total mortality rate(%) | 2 | 7 |
In the same row, values with different large letter superscripts indicate extremely significant differences (P < 0.01), values with different small letter superscripts indicate significant differences (P < 0.05), and values with the same letter or no letter superscripts indicate no significant differences (P > 0.05).
Table 4.
The effect of heat stress on the egg quality of Jinding ducks.
| Items | NT group | HS group | P value |
|---|---|---|---|
| Yolk weight(g) | 23.05 ± 0.25A | 20.83 ± 0.24B | <0.001 |
| Shell weight(g) | 7.98 ± 0.08A | 7.43 ± 0.07B | <0.001 |
| Egg yolk ratio(%) | 30.96 ± 0.31a | 29.95 ± 0.27b | 0.017 |
| Egg-shaped index | 1.35±0.007 | 1.36±0.007 | 0.349 |
| Eggshell strength(kg/cm2) | 5.10 ± 1.03A | 4.53 ± 1.03B | 0.008 |
| Albumen height(mm) | 5.84 ± 0.15 | 5.69±0.18 | 0.516 |
| Haugh unit | 73.89 ± 1.36 | 72.91 ± 1.66 | 0.896 |
| Eggshell thickness(mm) | 0.378 ± 0.004a | 0.365 ± 0.005b | 0.030 |
In the same row, values with different large letter superscripts indicate extremely significant differences (P < 0.01), values with different small letter superscripts indicate significant differences (P < 0.05), and values with the same letter or no letter superscripts indicate no significant differences (P > 0.05).
Effect of High-Temperature Heat Stress on the Egg Production Performance of Jinding Ducks Based on Transcriptome Analyses
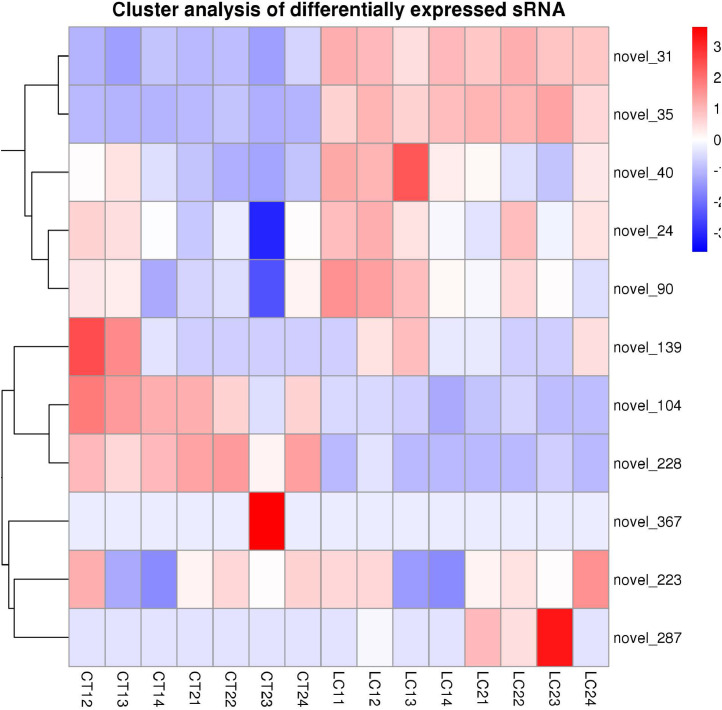
A total of 1,974 genes were identified in pituitary tissue, including 961 upregulated genes and 1,013 downregulated genes, and 5 significantly differentially expressed miRNAs were identified, including 3 upregulated miRNAs and 2 downregulated miRNAs. A total of 1,202 DEGs were identified in ovarian tissue, including 682 upregulated genes and 520 downregulated genes, and 5 significantly differentially expressed miRNAs were identified, including 2 upregulated miRNAs and 3 downregulated miRNAs. The results for some DEGs are shown in Table 5. Further analysis of differentially expressed miRNAs revealed that 10 were newly discovered unknown miRNAs (Table 6, Figure 1). Among them, novel_141, which was previously unknown and upregulated in pituitary tissue, was only expressed in the HS group, and novel_366, which was downregulated, was only expressed in the NT group, indicating that their expression may be conserved.
Table 5.
The results for some DEGs (part).
| Gene id | Difference multiple | P value | P value after correction | Gene name |
|---|---|---|---|---|
| 101795000 | −1.35 | 1.24e-13 | 1.91e-9 | CHGB |
| 119713657 | −0.77 | 2.91e-10 | 1.86e-6 | EIF3I |
| 113845674 | −1.55 | 3.61e-10 | 1.86e-6 | CFD |
| 119713479 | −2.23 | 5.07e-10 | 1.96e-6 | GKN2 |
| 119715377 | 3.41 | 1.33e-9 | 3.76e-6 | LOC119715377 |
| 101801469 | 1.36 | 1.46e-9 | 3.76e-6 | FAM13C |
| 101796982 | −1.47 | 2.28e-9 | 5.05e-6 | CYGB |
| 119715368 | 3.08 | 3.32e-9 | 6.41e-6 | LOC119715368 |
| 101797179 | −7.27 | 8.17e-9 | 1.40e-5 | LOC101797179 |
| 113844223 | −3.55 | 1.31e-7 | 2.03e-4 | INHA |
| 101800796 | −1.01 | 1.63e-7 | 2.29e-4 | NKAIN4 |
| 101798880 | 5.16 | 2.03e-7 | 2.61e-4 | HFM1 |
| 101793387 | 1.19 | 2.21e-7 | 2.63e-4 | COL5A1 |
| 101795227 | 0.72 | 2.69e-7 | 2.97e-4 | NABP1 |
| 101802315 | 1.94 | 3.45e-7 | 3.56e-4 | SLC26A8 |
| 101790644 | 0.87 | 5.17e-7 | 5.00e-4 | PRKG2 |
| 101800504 | −0.80 | 6.65e-7 | 5.91e-4 | YIPF1 |
| 101789452 | −1.82 | 6.88e-7 | 5.91e-4 | PENK |
| 101797242 | −1.27 | 7.45e-7 | 6.06e-4 | ISLR |
Table 6.
Results of differential miRNA expression analysis (part).
| sRNA | GCT1_readcount | CCT2_readcount | log2FoldChange | Pval | Padj |
|---|---|---|---|---|---|
| novel_104 | 184.8535346 | 72.10526 | 1.342027 | 0.017436 | 0.902362906 |
| novel_141 | 7.063182837 | 0 | 4.862618 | 0.045987 | 0.902362906 |
| novel_24 | 20,469.82117 | 10,258.43 | 0.996497 | 0.044913 | 0.902362906 |
| novel_31 | 2,887.49774 | 6,704.209 | −1.21479 | 0.041603 | 0.902362906 |
| novel_366 | 0 | 16.97477 | −7.08442 | 0.030006 | 0.902362906 |
| sRNA | GLC1_readcount | CLC2_readcount | log2FoldChange | Pval | Padj |
| novel_221 | 8.878923 | 35.05452 | −1.94293 | 0.027331 | 0.765281 |
| novel_289 | 0.168843 | 8.283575 | −4.81519 | 0.003852 | 0.269641 |
| novel_35 | 8,075.6 | 21,220.93 | −1.39387 | 0.018732 | 0.679557 |
| novel_40 | 9,577.726 | 4,475.477 | 1.09784 | 0.019416 | 0.679557 |
| novel_90 | 422.647 | 176.9023 | 1.255501 | 0.001037 | 0.145118 |
Figure 1.
The whole hierarchical cluster map of differentially expressed miRNAs was clustered by the value of log10 (TPM+1).
Cluster analysis based on log10 (TPM 1) value. The abscissa represents the fold change in miRNA expression in different experimental groups/different samples, the ordinate represents the statistically significant change in miRNA expression, and the scattered dots in the figure represent each miRNA. The red dots indicate significantly different miRNAs in the laying stage, and the blue dots indicate significantly different miRNAs in the brooding stage.
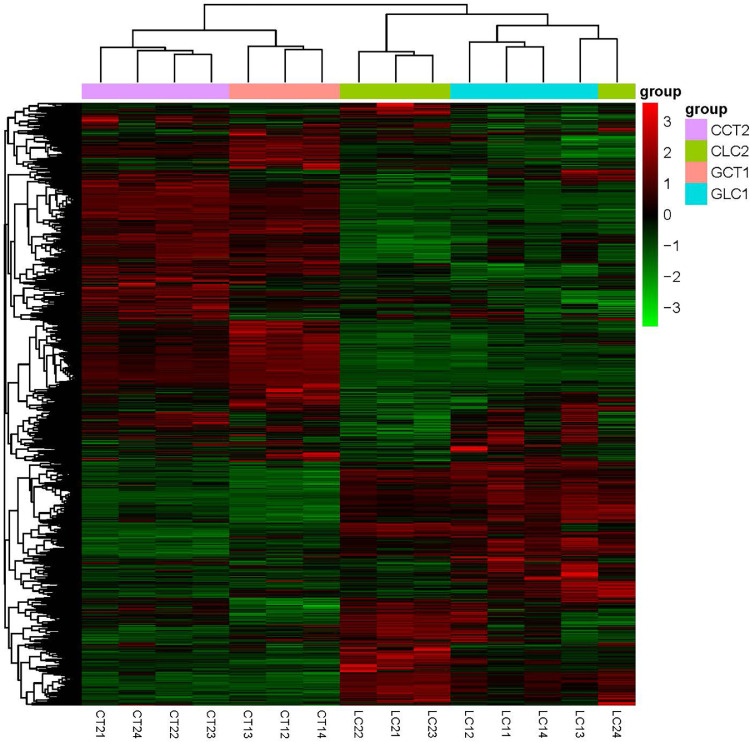
The FPKM (Fragments Per Kilobase of exon model per Million fragments mapped) values of the DEGs under different experimental conditions were used as the expression levels for hierarchical clustering analysis (Figure 2). Different colored regions identified different cluster groups. The genes within the same cluster group had similar expression patterns and therefore may have similar functions or participate in the same biological processes. The colors of different groups had different degrees of intensity, indicating that the genes had similar expression trends among the different samples and that the selected samples could be used for subsequent analysis.
Figure 2.
Differential gene clustering map.
The abscissa in the figure is the sample name, and the ordinate is the normalized value of the differential gene FPKM. The redder the color is, the higher the expression level; the greener the color is, the lower the expression level.
Using gene ontology (GO) (http://www.geneontology.org/) analysis, 1,504 DEGs between the GCT1 and CCT2 groups were annotated, and these genes were significantly enriched in 109 GO terms (P < 0.05), with high enrichment in transporter activity (GO:0005215), transmembrane transporter activity (GO:0022857) and cytoplasmic part (GO:0044444). A total of 806 DEGs between the GLC1 and CLC2 groups were annotated, and these genes were significantly enriched in 48 GO terms (P < 0.05), with high enrichment in protein-containing complex (GO:0032991), intracellular membrane-bound organelle (GO:0043231) and membrane-bound organelle (GO:0043227).
The target gene prediction results indicated 10 significantly differentially expressed miRNAs and a total of 2,702 target genes (Table 7). GO enrichment analysis was performed on the target genes of differentially expressed miRNAs. The annotation results indicated significant differences between the GLC1 and CLC2 groups for 94 GO terms, including calcium ion binding, calcium ion-dependent exocytosis, and other egg quality-related GO terms, in addition to significant differences in hexon binding, intermediate filament cytoskeleton, and cytoskeletal part.
Table 7.
Differential miRNA target gene prediction (part).
| miRNA | Target gene | Gene name |
|---|---|---|
| novel_104 | 101800229 | RHBT1 |
| novel_104 | 101791777 | MYB |
| novel_104 | 110353378 | HMX2 |
| novel_141 | 113845585 | HSPB1 |
| novel_141 | 101803760 | BCL7B |
| novel_141 | 119714097 | HSP30 |
| novel_141 | 119717823 | AIFM1 |
| novel_141 | 113845103 | BAX1B |
| novel_40 | 101795031 | MAIP1 |
| novel_40 | 101800967 | ESM1 |
| novel_40 | 101800185 | NUD14 |
| novel_40 | 101790544 | PSB7 |
| novel_40 | 119717560 | FYB2 |
| novel_40 | 101790497 | HA1F |
| novel_90 | 101790554 | VPS51 |
| novel_90 | 101794595 | MUL1 |
| novel_90 | 101792010 | PLCG1 |
| novel_221 | 101793597 | IGF1 |
| novel_221 | 101803760 | BCL7B |
Cluster profile software was used for the KEGG pathway enrichment analysis of DEGs (Table 8), and the results indicated that there were significant differences in the ribosome, oxidative phosphorylation, proteasome, arginine and proline metabolism, drug metabolism–cytochrome P450, glutathione metabolism, and histidine metabolism pathways. Among them, the arginine and proline metabolism pathways, ether lipid metabolism pathway, and drug metabolism–cytochrome P450 pathway may be related to the production performance of laying ducks under high-temperature heat stress. The downregulated genes involved were phospholipid phosphatase 1, galactosylceramide sulfotransferase isoform X1, amine oxidase, and aldehyde dehydrogenase family 1 member A3, among others. The upregulated genes involved were alkyl-dihydroxyacetone phosphate synthase (peroxisomal), delta-1-pyrroline-5-carboxylate synthase isoform X1, and aldehyde oxidase.
Table 8.
Kyoto Encyclopedia of Genes and Genomes enrichment list of differential genes.
| Path number | ITER | Differential gene ratio | Background gene number ratio | P value | P value after correction |
|---|---|---|---|---|---|
| apla03010 | ribosome | 31/593 | 107/5079 | 8.01e-7 | 1.20e-4 |
| apla00190 | oxidative phosphorylation | 30/593 | 123/5079 | 5.141e-5 | 0.00385746 |
| apla03050 | proteasome | 12/593 | 35/5079 | 3.77e-4 | 0.01883987 |
| apla00330 | Arginine and proline metabolism | 13/593 | 45/5079 | 0.001375994 | 0.05159979 |
| apla00071 | fatty acid oxidation | 8/593 | 32/5079 | 0.027190494 | 0.40441670 |
| apla00982 | Drug Metabolism CytochromeP450 | 7/593 | 27/5079 | 0.031411799 | 0.40441670 |
| apla00480 | glutathione metabolism | 10/593 | 45/5079 | 0.031547732 | 0.40441670 |
| apla00500 | starch and sucrose metabolism | 8/593 | 33/5079 | 0.032353337 | 0.40441670 |
| apla00340 | histidine metabolism | 6/593 | 23/5079 | 0.043884035 | 0.50635425 |
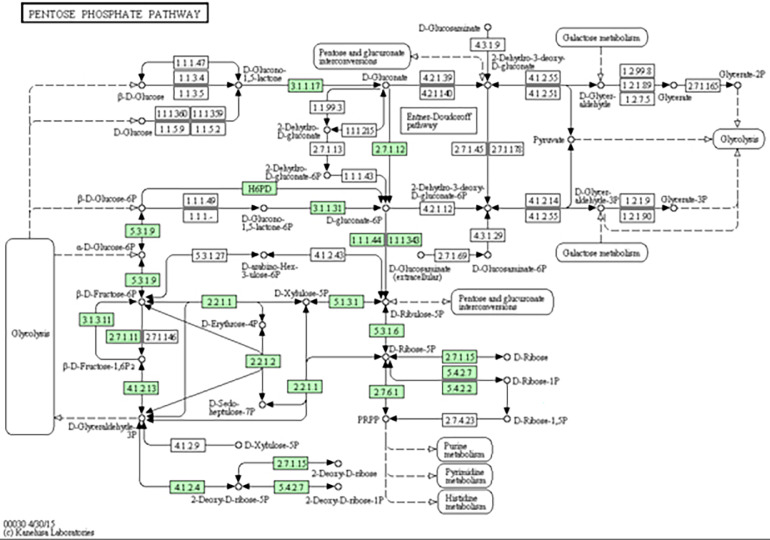
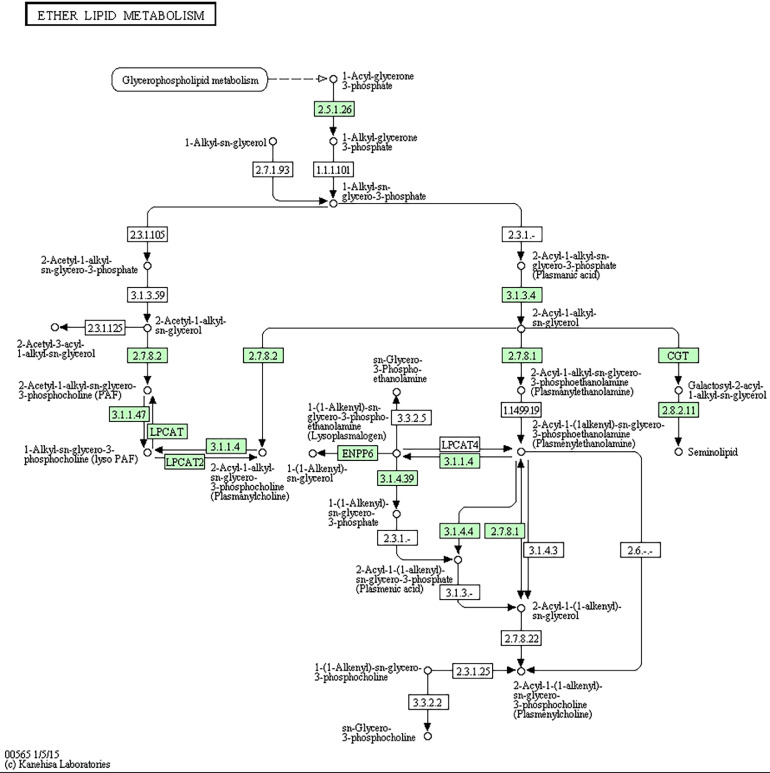
Kyoto Encyclopedia of Genes and Genomes annotation revealed that the target genes of differentially expressed miRNAs were involved in the phagosome pathway, the mRNA monitoring pathway, phototransduction, the pentose phosphate pathway, glycosylphosphatidylinositol-anchor biosynthesis, and ether lipid metabolism (P < 0.05). The activity of key enzymes in the pentose phosphate pathway (Figure 3) may be positively related to cholesterol biosynthesis in eggs. The pathway includes the target genes ATP-dependent 6-phosphofructokinase (PFKAL, corresponding to miRNAs novel_221 and novel_207), 6-phosphogluconolactonase (6PGL, corresponding to miRNAs novel_226, novel_234, novel_212, novel_396, novel_368, novel_208, novel_221 and novel_199), and ribose-phosphate pyrophosphokinase 1 (PRPS1, corresponding to the miRNA novel_221). These results suggest that the above miRNAs or target genes may be related to egg production performance. Ducks may reduce heat through ether lipid metabolism (Figure 4). This pathway includes the target genes phosphatidylinositol-glycan biosynthesis (PIGW, corresponding to miRNAs novel_168 and novel_289), phosphatidylinositol N-acetylglucosaminyltransferase subunit (Q3MUY2, corresponding to the miRNA novel_289), and glycosylphosphatidylinositol ethanolamine phosphate transferase 1 (PIGN, corresponding to miRNAs novel_289, apl-miR-11590-3p and novel_208). The above miRNAs or target genes may be related to high-temperature heat stress.
Figure 3.
Pentose phosphate pathway in this study.
Figure 4.
Ether lipid metabolism in this study.
Effect of High-Temperature Heat Stress on the Egg Production Performance of Jinding Duck Based on Integrated mRNA‒miRNA Transcriptome Analysis
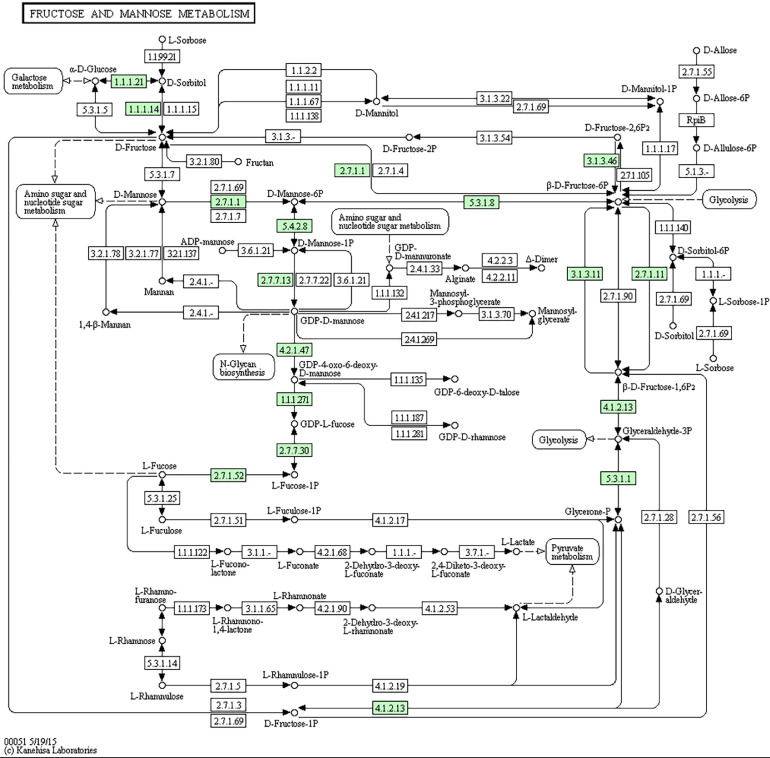
Kyoto Encyclopedia of Genes and Genomes enrichment analysis of candidate target genes identified the fructose and mannose metabolism pathways as significantly enriched (P < 0.05) (Figure 5). The DEGs involved were hexokinase-1, N-acetyl neuraminidase isoform X1, and L-fucose kinase isoform X1.
Figure 5.
Fructose and mannose metabolism in this study.
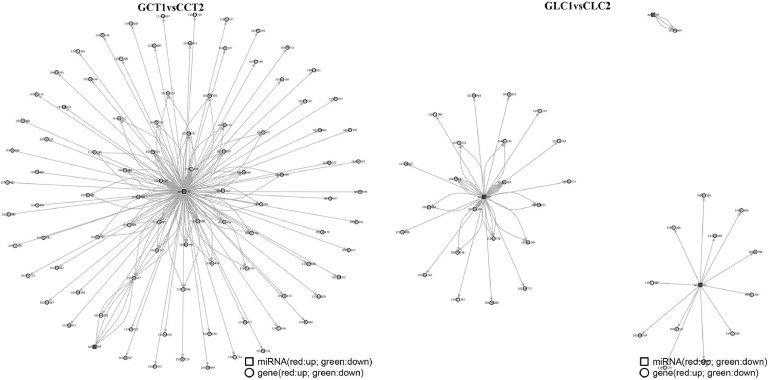
The interaction network between differentially expressed miRNAs and differentially expressed mRNAs was analyzed using Cytoscape software (https://cytoscape.org) (Figure 6). In pituitary tissue, the upregulated miRNA novel_141 (center of the network) formed a regulatory network with HSPB1, BCL7B, HSP30, and AIFM1, and the downregulated novel_366 (center of the network) formed a regulatory network with the JIP1 gene. In ovarian tissue, the downregulated novel_289 (center of the network) formed a regulatory network with ZSWM7, ABI3, and K1C23, novel_221 formed a regulatory network with IGF1, BCL7B, SMC6, APOA4, and FARP2, and the upregulated novel_40 formed a regulatory network with the HA1FF10 gene.
Figure 6.
The network diagram of the pituitary and ovarian differential miRNA-differential mRNA relationship in Cytoscape software.
QRT‒PCR Validation
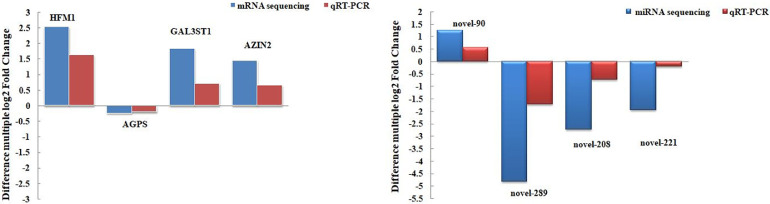
To verify the reliability of the high-throughput sequencing results, the relative expression of 3 upregulated genes, 1 upregulated miRNA, 1 downregulated gene, and 3 downregulated miRNAs was assessed in the ovaries of the NT group and HS group by qRT‒PCR method. The results showed (Figure 7) that the trends for the relative expression of upregulated and downregulated genes and miRNAs were consistent with the high-throughput sequencing results, indicating that the transcriptome analysis results were reliable.
Figure 7.
Fluorescent verification results of differentially expressed mRNAs and miRNAs.
DISCUSSION
Effects of High-Temperature Heat Stress on the Production Performance and Egg Quality of Jinding Ducks
The harm of high temperatures in summer to the production efficiency of laying poultry mainly manifests as reductions in feed intake and egg production performance. The high-temperature environment causes poultry to increase heat dissipation and reduce heat production to balance their body temperature and maintain a stable body state. A decreased capacity to take up and absorb nutrients adversely affects health and productivity (Lu, 2021). Heat stress can lead to a decrease in the egg production, laying rate, and average egg weight of laying poultry (Ren et al., 2007). Mack et al. (2013) showed that heat stress reduces egg production, egg weight, eggshell thickness, feed conversion rate, and egg quality and quantity of laying breeders and laying hens. In this study, heat stress significantly reduced the laying rate, total egg weight, average egg weight, feed intake, and feed intake per duck, significantly increased the feed-to-egg ratio, and increased the mortality rate of Jinding ducks, results that are consistent with the findings reported by Mack et al. (2013) and Mao et al. (2000).
Egg quality is affected by multiple factors, including heredity, hormones, stress, and age (Azmal et al., 2019). Heat stress affects the quality of eggs of laying hens, and an ambient temperature exceeding 28°C reduces the laying rate, eggshell thickness, and egg size in laying hens (An et al., 2017). The thickness, strength, and weight of eggshells reflect eggshell quality. Song et al. (2012) showed that heat stress significantly reduced egg weight and eggshell thickness. Ma et al. (2014) showed that heat stress reduced eggshell strength, eggshell thickness, and egg yolk color in laying ducks. The findings of this study are similar to those of the above studies: heat stress significantly reduced the eggshell thickness of Jinding ducks. The main reason may be that long-term exposure of laying ducks to heat stress caused unbalanced nutrient levels, affecting the absorption and intake of calcium and phosphorus and adversely affecting calcium deposition during eggshell formation. Albumen height is positively correlated with the Haugh unit and is proportional to the freshness of duck eggs (Sun et al., 2019). In this study, heat stress (extremely) significantly reduced yolk weight, yolk ratio, eggshell weight, and eggshell thickness, results that are consistent with the findings reported by Ma et al. (2014) and Song et al. (2012). Heat stress had no significant effect on the shape index, Haugh unit, or albumen height of duck eggs, which is consistent with the findings reported by Dong (2018).
Heat stress likely leads to eggshell thinning through 2 mechanisms. First, heat stress reduces the intake of calcium, phosphorus, and vitamins, which are the main substances that form eggshells, thus reducing eggshell thickness. Second, under heat stress, poultry must expel heat through more frequent respiration. However, frequent exhalation will lead to excessive exhalation of carbon dioxide from the lungs, resulting in an acid-base imbalance in the blood, causing respiratory alkalosis, which reduces the content of calcium and calcium bicarbonate in the blood, in turn leading to eggshell thinning (Wang, 2020).
The Molecular Mechanism of the Effect of High-Temperature Heat Stress on the Production Performance and Egg Quality of Jinding Duck Was Studied Based on Transcriptome Sequencing
The pituitary gland is an important endocrine organ that regulates animal growth, development, metabolism, and sexual function through a variety of hormones (Ooi et al., 2004). Zhang et al. (2019) conducted a genome-wide analysis of pituitary circRNAs in heat-stressed sows in summer and found that differentially expressed miRNAs in sows under heat stress were responses and adaptations to heat stress to regulate 5 specific genes. Becker et al. (2020) showed in a heat stress study of dairy cows that at excessively high temperatures beyond their thermoregulatory ability, heat stress affected various physiological and biochemical indicators of dairy cows, reducing their feed intake, immunity, and reproductive performance. Zeng et al. (2023) identified 493 differentially expressed miRNAs and 6,475 DEGs in pituitary tissue through a bioinformatics analysis of hypothalamic-pituitary-mammary gland (HPM) axis-related tissues in heat-stressed and normal dairy cows, constructed a competitive endogenous RNA network related to the heat stress response and lactation regulation in hypothalamus, pituitary, and mammary gland tissues, and found that most of the target genes of differentially expressed miRNAs were significantly enriched in the MAPK signaling pathway and hormone synthesis and secretion in the HPM axis. Heat stress-induced differences in RNA expression profiles in the hypothalamus, pituitary, and mammary gland tissues of the HPM axis may provide a molecular basis for regulating the stress response and lactation of heat-stressed dairy cows. Tang et al. (2022) identified a total of 514 differentially expressed miRNAs, including 442 known miRNAs and 72 novel miRNAs, in the ovarian tissue of heat-stressed and control rabbits and found that 23 differentially expressed miRNAs were significantly expressed in heat-stressed rabbits.
The results of this study showed that novel_141, which was previously unknown and upregulated in pituitary tissue, was only expressed in the HS group and that the downregulated novel_366 was only expressed in the NT group, indicating that their expression may be conserved. Heat stress affects the secretion of related hormones and the release of neurotransmitters by regulating the expression of pituitary and ovarian tissue-related genes and differentially expressed miRNAs, which may further affect the heat stress response and reproductive performance of Jinding ducks. In addition, calcium binding and calcium-dependent exocytosis in this study were significantly different in the ovarian tissues of the NT and HS groups, and it was speculated that they were related to the egg quality of Jinding ducks under heat stress.
Through a combined transcriptome and metabolome analysis, Srikanth et al. (2020) found that the arginine and proline metabolism, glutathione metabolism, and seleno-amino acid metabolism pathways were enriched in the chronic heat stress-induced oxidative stress response in pigs. Qian et al. (2022) showed that the DEGs in heat-stressed Clematis crass folia were significantly enriched in 23 pathways, including oxidative phosphorylation and phenylpropanoid biosynthesis. Li et al. (2016) studied the effect of heat stress on gene expression, steroid synthesis, and apoptosis of bovine granulosa cells and showed that steroidogenic factor 1 (SF-1) inhibited estrogen synthesis by regulating the expression of cytochrome P450 (CYP19A1) in ovarian follicles, there by reducing estrogen levels. In this study, under heat stress, Jinding ducks may inhibit estrogen synthesis by regulating the expression of cytochrome P450-related genes in ovarian follicles, thereby reducing the estrogen levels and consequently the production performance of Jinding ducks.
The Molecular Mechanism of the Effect of High-Temperature Heat Stress on the Production Performance and Egg Quality of Jinding Duck Was Analyzed Based on Transcriptome mRNA‒miRNA
Kyoto Encyclopedia of Genes and Genomes enrichment analysis of the candidate target genes found that the significantly differentially expressed pathways were the fructose and mannose metabolism pathways, involving DEGs such as hexokinase-1. Hexokinase has dual functions, i.e., phosphorylation and glucose sensing. Hexokinase can phosphorylate hexose, and hexose can enter glycolysis only after phosphorylation. Phosphorylated hexose participates in the glycolysis pathway and can provide cells with the energy required for life activities and precursor substances required for amino acid synthesis through glycolysis. Zhang (2021) showed that under high-temperature stress, most of the 6 hexokinase genes and 1 mannose-6-phosphate isomerase gene were upregulated, indicating that high-temperature stress upregulated the expression of metabolite synthesis-related genes, resulting in the accumulation of metabolites and that high-temperature stress can be alleviated by promoting the glycolysis pathway, which is speculated to be one of the strategies by which maize resists high-temperature stress. The results of this study indicate that 2 hexokinase genes, which were upregulated under high-temperature heat stress, might alleviate heat stress by participating in glycolysis.
Based on the differential expression of miRNAs and their candidate target genes in pituitary and ovarian tissues of Jinding ducks in the HS group and NT group, 3 miRNA‒target gene regulatory networks were constructed to better visualize the regulatory relationship between miRNAs and their candidate target genes in high-temperature heat stress and normal temperature processes. Specifically, in pituitary tissue, the upregulated miRNA novel_141 (center of the network) formed a regulatory network with HSPB1, BCL7B, HSP30, and AIFM1, and the downregulated novel_366 (center of the network) formed a regulatory network with the JIP1 gene. Heat shock proteins (HSPs) are a class of highly conserved stress response chaperone proteins that can be synthesized in response to various stresses (including heat stress) and participate in various cellular processes (such as protein folding, protein trafficking, and protein complex assembly/disassembly and degradation) to maintain cell homeostasis (Zininga et al., 2018). Many studies have shown that HSPs are also involved in biological functions such as inflammation, immunity, cell differentiation, and antioxidation (Bolhassani and Agi, 2019; Das et al., 2019). Heat shock proteins are classified into 6 families based on molecular mass, structure, and function: small HSPs (sHSPs), HSP40, HSP60, HSP70, HSP90, and large HSPs. HSPB1 and HSP30 are important members of the sHSP family (Bolhassani and Agi, 2019). Studies have shown that HSP30 in fish participates in the stress response and maintains homeostasis under heat stress and stimulation (Zarate and Bradley, 2003; Tomalty et al., 2015). HSPB1 prevents apoptosis by protecting cells from heat shock, apoptotic effectors, oxidative stress, and local ischemia (Acunzo et al., 2012). Chu et al. (2022) showed that HSPB1 was significantly highly expressed in the liver of small yellow croaker under high temperatures, indicating that HSPB1 plays an important role in the response to temperature stress. In ovarian tissue, upregulated novel_40 formed a regulatory network with the HA1FF10 gene, downregulated novel_289 (center of the network) formed a regulatory network with ZSWM7, ABI3, and K1C23, and novel_221 formed a regulatory network with IGF1, BCL7B, SMC6, APOA4, and FARP2. During follicle development, cells such as small theca cells and granulosa cells can secrete insulin-like growth factor 1 (IGF1) (Yuan et al., 2018). The expression of IGF1 in granulosa cells and ovarian theca cells stimulates their proliferation (Onagbesan and Peddie, 1995; Armstrong and Hogg, 1996; Onagbesan et al., 1999). In this study, downregulated novel_221 in ovarian tissue formed a regulatory network with the IGF1 gene, indicating that under high-temperature heat stress, the proliferation of ovarian granulosa cells may be inhibited by downregulating IGF1 gene expression, which results in a decline in the production performance of Jinding ducks. Therefore, further research on novel_141 and novel_221 and their target genes HSPB1, HSP30, and IGF1 should be conducted.
CONCLUSIONS
In summary, heat stress regulates the secretion of endocrine-related hormones and the release of neurotransmitters, and the expression of miRNAs and mRNAs in pituitary and ovarian tissues may affect the production performance and egg quality of Jinding ducks under heat stress. The established miRNA‒mRNA regulatory networks provide a theoretical basis for the molecular mechanisms that regulate the stress response in pituitary and ovarian tissues, egg quality, and production performance under heat stress. The regulatory relationships between miRNAs and predicted target genes and the effects of various candidate miRNAs that are involved in and mediate thermal stress in the pituitary and ovary need to be further verified.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of Fujian Province (2020J01348), the Public Welfare Project of Fujian Province (2022R1026008, 2021R1026005, 2020R10260016, 2020R1026008), the 5511 Collaborative Innovation Project of Fujian Province (XTCXGC2021008), and the Scientific and Technological Innovation Team Project of the Fujian Academy of Agricultural Sciences (CXTD2021006-2).
Data Availability Statement: The datasets supporting the conclusions of this article are included within the article. All datasets are available from the corresponding author upon reasonable request.
Author Contributions: Qingwu Xin, Li Li performed the experiments, analyzed the data, and drafted the manuscript. These 2 contributed equally to this work. Bangzhe Zhao, Wenli Shi, Xiaona Hao, Linli Zhang, Zhongwei Miao, and Zhiming Zhu helped in animal experiments and laboratory analysis. Qinlou Huang and Nenzhu Zheng contributed to the experimental design and supervised the study. All the authors have read and approved the final manuscript.
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Acunzo J., Katsogiannou M., Rocchi P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int. J. Biochem. Cell Biol. 2012;44:1622–1631. doi: 10.1016/j.biocel.2012.04.002. [DOI] [PubMed] [Google Scholar]
- An G.H., Chen X.W., Li C., Zhang L., Wei M.F., Chen J.J., Ma Q., Yang D.F, Wang J. Pathophysiological changes in female rats with estrous cycle disorder induced by long-term heat stress. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/4701563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An T.T., Zhang J.J., Yang J.S., Lin Y.X., Yang F.P., Xin S.J., Wang S.Q., Dai G.J. Effects of dietary vitamin C on laying performance, egg quality and blood biochemical indexes of laying hens under high temperature condition. Jiangsu Agricult. Sci. 2017;45:117–120. [Google Scholar]
- Armstrong D.G., Hogg C.O. Insulin-like growth factor I (IGF-I), IGF-II and type-I IGF receptor gene expression in the ovary of the laying hen. J. Reprod. Fertil. 1996;106:101–106. doi: 10.1530/jrf.0.1060101. [DOI] [PubMed] [Google Scholar]
- Azmal S.A., Bhuiyan A.A., Omar A.I., Ma S., Sun C.H., Han Z.D., Zhang M., Zhao S.H., Li S.J. Novel polymorphisms in RAPGEF6 gene associated with egg-laying rate in Chinese Jing Hong chicken using genome-wide SNP scan. Genes. 2019;10:384. doi: 10.3390/genes10050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C.A., Collier R.J., Stone A.E. Invited review: physiological and behavioural effects of heat stress in dairy cows. Dairy Sci. 2020;103:6751–6770. doi: 10.3168/jds.2019-17929. [DOI] [PubMed] [Google Scholar]
- Bei M.Y., Wang Q., Yu W.S., Lu H., Yu J. Effects of heat stress on ovarian development and the expression of HSP genes in mice. J. Therm. Biol. 2020;89 doi: 10.1016/j.jtherbio.2020.102532. [DOI] [PubMed] [Google Scholar]
- Bolhassani A., Agi E. Heat shock proteins in infection. Clin. Chim. Acta. 2019;498:90–100. doi: 10.1016/j.cca.2019.08.015. [DOI] [PubMed] [Google Scholar]
- Chu T.Q., Liu F., Chen H.L., Zhan W., Wang M.J., Qin G.C., Lou B., Xu W.T. Response of hsp90b1 and hspb1 to temperature stress in the liver of Larimichthys polyactis. J. Agricult. Biotechnol. 2022;30:528–538. [Google Scholar]
- Das J.K., Xiong X.F., Ren X.C., Yang J.M., Song J.X. Heat shock proteins in cancer immunotherapy. J. Oncol. 2019;2019:1–9. doi: 10.1155/2019/3267207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z.X. Master’s Degree Thesis. Zhongkai College of Agricultural Engineering; Guangzhou, Guangdong: 2018. The effects of heat stress on laying performance and tissue damage of laying ducks. [Google Scholar]
- He S.P., Li S., Arowolo M.A., Yu Q.F., Chen F., Hu R.Z., He J.H. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim. Sci. J. 2019;90:401–411. doi: 10.1111/asj.13161. [DOI] [PubMed] [Google Scholar]
- Hu Q.D., Qian R.J., Zhang Y.J., Zhang X.L., Ma X.H., Zheng J. Physiological and gene expression changes of Clematis crassifolia and Clematis cadmia in response to heat stress. Front Plant Sci. 2021;12:421. doi: 10.3389/fpls.2021.624875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.P., Tong Q., Shi Z.X., Li H., Wang Y., Li B.M., Yan G.Q., Chen H., Zheng W.C. Effects of chronic heat stress and ammonia concentration on blood parameters of laying hens. Poult. Sci. 2020;99:3784–3792. doi: 10.1016/j.psj.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Y., Gao H., Tian Z., Wu Y., Wang Y.Z., Fang Y., Lu L., Han Y., Wu S.S., Haq I., Zeng S.M. Effects of chronic heat stress on granulosa cell apoptosis and follicular atresia in mouse ovary. J. Anim. Sci. Biotechnol. 2016;7:57. doi: 10.1186/s40104-016-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wu J., Luo M., Sun Y., Wang G.L. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulose cells. Cell Stress Chaperones. 2016;21:467–475. doi: 10.1007/s12192-016-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.H., Q. Yu Z., Chen Z. Effect of heat stress on mitogen-activated protein kinases in the hypothalamic-pituitary-gonadal axis of developing Wenchang chicks. Poult. Sci. 2020;99:567–577. doi: 10.3382/ps/pez499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Song Y.P., Bao X.Y., Zhang J.Q. The mediation of miR-34a/miR-449c for immune cytokines in acute cold/heat-stressed broiler chicken. Animals. 2020;10:2168. doi: 10.3390/ani10112168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.Q. Jiangxi Agricultural University; Nanchang: 2021. Study on the Effect and Mechanism of Electrolyzed-reduced Rich-hydrogen Water on Relieving Chronic Heat Stress in Laying Hens. [Google Scholar]
- Lu Q., Wen J., Zhang H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult. Sci. 2007;86:1059–1064. doi: 10.1093/ps/86.6.1059. [DOI] [PubMed] [Google Scholar]
- Ma F., Liu Z., Huang J.Q., Li Y.J., Kang Y.J., Liu X.X., Wang J.F. High-throughput sequencing reveals microRNAs in response to heat stress in the head kidney of rainbow trout (Oncorhynchus mykiss) Funct. Integrat. Genom. 2019;19:775–786. doi: 10.1007/s10142-019-00682-3. [DOI] [PubMed] [Google Scholar]
- Ma X.Y., Lin Y.C., Zhang H.X., Chen W., Wang S., Ruan D., Jiang Z.Y. Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Anim. Reproduct. Sci. 2014;145:182–190. doi: 10.1016/j.anireprosci.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Mack L.A., J.N. Felver-Gant R.L.Dennis, Cheng H.W. Genetic variations alter production and behavioural responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013;92:285–294. doi: 10.3382/ps.2012-02589. [DOI] [PubMed] [Google Scholar]
- Mao L.Y., He W.G., Xu X.Q. Effects of common stress factors on laying performance of ducks. Hum. Anim. Husband. Vet. 2000;06:9–11. [Google Scholar]
- Onagbesan O.M., Vleugels B., Buys N., Bruggeman V., Safi M., Decuypere E. Insulin-like growth factors in the regulation of avian ovarian functions. Domest. Anim. Endocrinol. 1999;17:299–313. doi: 10.1016/s0739-7240(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Onagbesan O.M., Peddie M.J. Effects of insulin-like growth factor I and interactions with transforming growth factor alpha and LH on proliferation of chicken granulosa cells and production of progesterone in culture. J. Reproduct. Fertil. 1995;104:259–265. doi: 10.1530/jrf.0.1040259. [DOI] [PubMed] [Google Scholar]
- Ooi G.T., Tawadros N., Escalona R.M. Pituitary cell lines and their endocrine applications. Mol. Cell. Endocrinol. 2004;228:1–21. doi: 10.1016/j.mce.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Qaid M.M., Al-Garadi M.A. Protein and amino acid metabolism in poultry during and after heat stress: a review. Animals. 2021;11:1167. doi: 10.3390/ani11041167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian R.J., Hu Q.D., Ma X.H., Zhang X.L., Ye Y.J., Liu H.J., Gao H.D., Zheng J. Comparative transcriptome analysis of heat stress responses of Clematis lanuginosa and Clematis crassifolia. BMC Plant Biol. 2022;22:138. doi: 10.1186/s12870-022-03497-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.L., Zhao J.S., Zhu G.H., Cheng M., Li A.J. Effect of heat stress on laying hens. Shandong Anim. Husband. Vet. 2007;06:48–50. [Google Scholar]
- Saeed M., Abbas G., Alagawany M., Kamboh A.A., Abd EI-Hack M.E., Khafaga A.F., Chao S. Heat stress management in poultry farms: a comprehensive overview. J. Therm. Biol. 2019;84:414–425. doi: 10.1016/j.jtherbio.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Sahin N., Hayirli A., Orhan C., Tuzcu M., Komorowski J.R., Sahin K. Effects of the supplemental chromium form on performance and metabolic profile in laying hens exposed to heat stress. Poult. Sci. 2018;97:1298–1305. doi: 10.3382/ps/pex435. [DOI] [PubMed] [Google Scholar]
- Song Z.G., Liu L., Sheikhahmadi A., Jiao C.H., Lin H. Effect of heat exposure on gene expression of feed intake regulatory peptides in laying hens. J. Biomed. Biotechnol. 2012;1155:484869–484877. doi: 10.1155/2012/484869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth K., Park J.E., Ji S.Y., Kim K.H., Lee Y.K., Kumar H., Kim M., Baek Y.C., Kim H., Jang G.W., Choi B.H., Lee S.D. Genome-wide transcriptome and metabolome analyses provide novel insights and suggest a sex-specific response to heat stress in pigs. Genes. 2020;11:1–19. doi: 10.3390/genes11050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.J., Liu J.N., Yang N., Xu G.Y. Egg quality and egg albumen property of domestic chicken, duck, goose, turkey, quail, and pigeon. Poult. Sci. 2019;98:4516–4521. doi: 10.3382/ps/pez259. [DOI] [PubMed] [Google Scholar]
- Tang L.P., Bai X., Xie X.H., Chen G.H., Jia X.B., Lei M., Li C.Y., Lai S.J. Negative effects of heat stress on ovarian tissue in female rabbit. Front. Vet. Sci. 2022;14 doi: 10.3389/fvets.2022.1009182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalty K.M.H., Meek M.H., Stephens M.R., Rincon G., Fangue N.A., May B.P., Baerwald M.R. Transcriptional response to acute thermal exposure in juvenile chinook salmon determined by RNAseq. G3: Genes Genom. Genet. 2015;5:1335–1349. doi: 10.1534/g3.115.017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Li G.Q., Bu X.C., Shen J.D., Tao Z.R., Chen L., Zeng T., Du X., Lu L.Z. Changes in morphology and miRNAs expression in small intestines of Shaoxing ducks in response to high temperature. Mol. Biol. Rep. 2019;46:3843–3856. doi: 10.1007/s11033-019-04827-2. [DOI] [PubMed] [Google Scholar]
- Wang S.J. Harm and prevention countermeasures of heat stroke in poultry in hot season. Poult. Sci. 2020;5:33–34. [Google Scholar]
- Wasti S., Sah N., Mishra B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. 2020;10:1266. doi: 10.3390/ani10081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Wang M.Z., Ma Y.M., Yuan L.C., Lu S.F. High-throughput sequencing and characterization of the small RNA transcriptome reveal features of novel and conserved microRNAs in Panax ginseng. PLoS One. 2012;7:e44385. doi: 10.1371/journal.pone.0044385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G.Q., Chen C.M., Jiang Y., Zhang J.J., Tang H.M., Liu H.P. Research progress on the correlation between insulin growth factor and ovarian function. Hun. J. Tradit.l Chin. Med. 2018;230:179–181. [Google Scholar]
- Zarate J., Bradley T.M. Heat shock proteins are not sensitive indicators of hatchery stress in salmon. Aquaculture. 2003;223:175–187. [Google Scholar]
- Zeng H.F., Xia H.B., Wang X.L., Wang Y., Fang J., Li S.J., Zhai Y.F., Han Z.Y. Comprehensive profiling of ceRNA (circRNA-miRNA-mRNA) networks in hypothalamic-pituitary-mammary gland axis of dairy cows under heat stress. Int. J. Mol. Sci. 2023;24:888. doi: 10.3390/ijms24010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T., Li G.Q., Wang D.Q., Li J.J., Tian Y., Chen L., Huang A.X., Shi F.X., Shen J.D., Tao Z.R., Lu L.Z. Polymorphism of heat shock protein 90 gene and its association with laying performance in laying ducks. Chin. J. Anim. Sci. 2013;03:12–15. [Google Scholar]
- Zhang H.J., Hu B.Y., Xiong J.L., Chen T., Xi Q.Y., Luo J.Y., Jiang Q.Y., Sun J.J., Zhang Y.L. Genome wide analysis of circular RNA in pituitaries of normal and heat-stressed sows. BMC Genom. 2019;20:1013. doi: 10.1186/s12864-019-6377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.N. Zhengzhou University; Henan, Zhengzhou: 2021. Analysis of New Maize Variety Zhengdan 309 Heat Stress Response Mechanism Based on Transcriptomics and Metabolomics. [Google Scholar]
- Zhao J.B., Pan H.B., Liu Y., Yang H., Shi H.M., Ge C.R. Interacting networks of the hypothalamic-pituitary-ovarian axis regulate layer hens performance. Genes. 2023;14:141. doi: 10.3390/genes14010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.H., Xie T., Li S.L., Wang W., Wang Y.J., Cao Z.J., Yang H.J. Effects of selenium as a dietary source on performance, inflammation, cell damage, and reproduction of livestock induced by heat stress: a review. Front. Immunol. 2022;12:6002. doi: 10.3389/fimmu.2021.820853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.H., Liao R.R., Wu N., Zhu G.S., Tu Y.Y., Yang C.S. Integrating miRNA and mRNA expression profiles in plasma of laying hens associated with heat stress. Mol. Biol. Rep. 2019;46:2779–2789. doi: 10.1007/s11033-019-04724-8. [DOI] [PubMed] [Google Scholar]
- Zininga T., Ramatsui L., Shonhai A. Heat shock proteins as immunomodulants. Molecules. 2018;23:2846–2863. doi: 10.3390/molecules23112846. [DOI] [PMC free article] [PubMed] [Google Scholar]