Abstract
Background:
Transcutaneous cervical vagus nerve stimulation (tcVNS) has emerged as a potential treatment strategy for patients with stress-related psychiatric disorders. Ghrelin is a hormone that has been postulated to be a biomarker of stress. While the mechanisms of action of tcVNS are unclear, we hypothesized that tcVNS reduces the levels of ghrelin in response to stress.
Methods:
Using a randomized double-blind approach, we studied the effects of tcVNS on ghrelin levels in individuals with a history of exposure to traumatic stress. Participants received either sham (n = 29) or active tcVNS (n = 26) after exposure to acute personalized traumatic script stress and mental stress challenges (public speech, mental arithmetic) over a three day period.
Results:
There were no significant differences in the levels of ghrelin between the tcVNS and sham stimulation groups at either baseline or in the absence of trauma scripts. However, tcVNS in conjunction with personalized traumatic scripts resulted in lower ghrelin levels compared to the sham stimulation group (265.2 ± 143.6 pg/ml vs 478.7 ± 349.2 pg/ml, P=0.01). Additionally, after completing the public speaking and mental arithmetic tests, ghrelin levels were found to be lower in the group receiving tcVNS compared to the sham group (293.3 ± 102.4 pg/ml vs 540.3 ± 203.9 pg/ml, P= 0.009).
Limitations:
Timing of ghrelin measurements, and stimulation of only left vagus nerve
Conclusion:
tcVNS decreases ghrelin levels in response to various stressful stimuli. These findings are consistent with a growing literature that tcVNS modulates hormonal and autonomic responses to stress.
Keywords: Stress, Transcutaneous cervical vagus nerve stimulation, Ghrelin
Introduction
Stress responses occur when individuals are faced with actual or perceived shifts in the internal or external environment to promote coping and adaptation (Joels and Baram, 2009; McEwen, 1998). These acute stress responses promote adaptive changes that prepare individuals for challenges; however, in patients with post-traumatic stress disorder (PTSD), the repeated and prolonged activation of these responses can cause detrimental effects (Juster et al., 2011; Lederbogen et al., 2011). There is a crucial need to identify novel biomarkers for assessing stress responses in patients with PTSD, which could also serve as therapeutic targets to modulate these responses.
Ghrelin is a peptide synthesized in the stomach and pancreas and is involved in the regulation of appetite, metabolism and food digestion (Lutter et al., 2008). Ghrelin likely mediates the relationship between stress, mood alterations, increased food consumption (especially for high fat diets) and obesity. In addition to the prominent role of ghrelin in eating behaviors and obesity, recent evidence suggests that ghrelin also plays an important role in the stress response (Bremner and Pearce, 2016; Yildiz et al., 2004). Plasma levels of ghrelin transiently increase during both acute and chronic stress exposures (Bali and Jaggi, 2016; Lutter et al., 2008; Zheng et al., 2009) and ghrelin mediates both stress-induced increases in food intake (Chuang et al., 2011), as well as behavioral responses to stress (Yildiz et al., 2004). The candidate mechanism by which therapeutic interventions could modulate stress responses.
Electrical stimulation of the vagus nerve has been shown to modulate vagal afferents through the nucleus tractus solitarus and affect brain areas that regulate stress responses, such as the amygdala, hippocampus and prefrontal cortex. This treatment modality appears to be effective in the treatment of PTSD (Aaronson et al., 2017; Cimpianu et al., 2017; George et al., 2005). However, widespread adaptation of vagal nerve stimulation (VNS) has been limited by the cost and potential for adverse events associated with implantable devices (Ramsay et al., 1994). Transcutaneous cervical VNS (tcVNS) has emerged as a convenient alternative to implantable devices for stimulation of the cervical portion of the vagus nerve (Adair et al., 2020) that may be particularly useful for stress-related psychiatric disorders due its effects in reducing sympathetic function and inflammation(Bremner et al., 2020b). We have previously shown that tcVNS reduces sympathetic (Gazi et al., 2020b; Gurel et al., 2020a; Gurel et al., 2020b; Gurel et al., 2020d; Gurel et al., 2020f) and Pituitary Adenylate Cyclase Activating Peptide (PACAP) responses to stress (Gurel et al., 2020c), enhances parasympathetic function (Gurel et al., 2020b), modulates brain areas involved in the stress response in traumatized individuals with and without PTSD (Wittbrodt et al., 2020), and reduces inflammatory (interleuken-6 (IL-6) and Interferon-γ (INF-γ)) responses to stress in PTSD (Bremner et al., 2020a).
Herein, we aimed to investigate the role of tcVNS on ghrelin levels in response to stressful stimuli in a group of patients with a history of psychological trauma. We hypothesized that plasma ghrelin levels increase in response to stressful stimuli and that these response are lower in those receiving tcVNS compared to sham controls.
Methods
The present study was approved by the institutional review boards of Emory University, Georgia Institute of Technology, SPAWAR Systems Center Pacific, and the Department of Navy Human Research Protection Program (ClinicalTrials.gov # NCT02992899). All participants provided written informed consent before enrolling in the study. Participants included physically healthy adults between the ages of 18 and 70 with a history of psychological trauma. Patients were excluded if they had a diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, bulimia or anorexia, using the Structured Clinical Interview for DSM-5 Disorders. Additional exclusion criteria were current pregnancy, traumatic brain injury, meningitis, active implanted device, evidence or history of serious medical or neurological illness, such as cardiovascular, gastrointestinal, hepatic, renal, or other systemic illness; known carotid atherosclerosis, cervical vagotomy or positive toxicology screen.
Study design
The presents study was a randomized double-blind protocol spanning 3 days (ClinicalTrials.gov NCT02992899), with each subject receiving four administrations—two on the first day and one on each of the following two days—of either “active” tcVNS or “sham” stimulation. These administrations were not accompanied by any other form of stimulus to focus solely on the effects of tcVNS on human physiology.
Each participant provided his/her own traumatic experiences, and personalized voice recordings based on these experiences were presented as traumatic stress as previously described. The outline of the protocol is shown in Supplemental Figure. The first day included six traumatic stress prompts followed by immediate tcVNS or sham stimulation and two stimulation administrations without stress. Each of the second and third days included one stimulation administration without stress. The neutral scripts included descriptions of pleasant scenery to induce positive feelings to the subject. Stimulation with tcVNS or sham was applied immediately after the traumatic stress recording ended by the researcher from the left side of the neck. Participants also received two stimulation administrations (tcVNS or sham) without any stressor. Blood samples for measurements of ghrelin levels were obtained at baseline, after stimulation administrations without stressors and following the last stimulation was applied after the traumatic stress recording.
During day 2 and 3, participants underwent mental stress testing which included public speech and mental arithmetic tests. First, participants underwent a public speech task for which they were asked to provide a 2-min long defense statement in a scenario where they were accused of theft. After hearing the scenario details, they were given 2 min to prepare their defense and 2 min to present their statement. Stimulation was applied immediately after the public speech task. In the next step, subjects rested for 8 min in silence. Afterwards, participants were given another task for which they were required to answer series of arithmetic questions for 3 min. A researcher provided negative feedback for incorrect answers and delayed response times. A second stimulation was applied immediately after the arithmetic task. After two mental stressors and two stimulation administrations, the subjects were given a 90-min break. Blood samples for ghrelin measurements were taken at baseline and after the 90-min break following mental stress testing on day 2.
Blinding
Randomization into active tcVNS or sham groups was performed with pre-numbered devices by the manufacturer who were not involved in the research. Participants were randomly allocated to one of the two groups based on a statistician-developed computer-generated randomization table that assigned patients (1:1) with block stratification into tcVNS or sham groups. Random allocation was also carried out by personnel who did not take part in data collection or analyses. This was a double-blinded randomized study with both participants and researchers being blinded to the stimulus type. All statistical analyses were carried out by a biostatistician who did not take part in data collection or processing.
Transcutaneous cervical vagal nerve stimulation
Active tcVNS and sham stimuli were both administered using handheld GammaCore devices (ElectroCore, Basking Ridge, New Jersey, Supplemental Figure 2) as previously described (Gazi et al., 2020a; Gurel et al., 2020e). Briefly, stimulation was applied using collar, stainless steel electrodes with a conductive electrode gel that was placed on the left side of the neck over the carotid sheath. Active tcVNS devices produced an alternating current (AC) voltage signal which consisted of five 5 kHz sine bursts (1 ms of five sine waves; pulse width ¼ 40 ms) repeating at a rate of 25 Hz. The frequency of 25 Hz was chosen based on previous studies showing an optimized effect on autonomic function and other measures at this frequency. The sham devices produce an AC biphasic voltage signal consisting of 0.2 Hz square pulses (pulse width ¼ 5 s) eliciting a mild sensation. Both active and sham devices delivered 2 min of stimulation.
Plasma ghrelin
We measured the concentration of ghrelin in EDTA plasma using the MesoScale system (Meso Scale Diagnostics, Rockville, MD) system with a SECTOR Imager 2400. This system uses electrochemiluminescence for high sensitivity and broad dynamic range. Using the U-PLEX Human Ghrelin (total) assay (Catalog number, K1515XK) according to protocol supplied, the intra-assay CV was 4.3%.
Statistical analysis
Continuous variables are presented as means (standard deviation (SD)) and categorical variables as proportions (%). Differences between groups were assessed using the t-test for continuous variables and χ2 or Fischer exact tests for categorical variables where appropriate. Linear regression analysis was used to determine the bivariate association between ghrelin levels at baseline and after stimulation and baseline demographics. All analyses were conducted using Stata 14 (StataCorp., College Station, Texas). A P-value of < 0.05 was considered statistically significant. Effect sizes were calculated based on Cohen’s d for stimulation and post-stimulation intervals of changes in heart rate photoplethysmogram amplitude as described previously (Gurel et al., 2020e).
Results
The study cohort included 55 participants who underwent either tcVNS or sham stimulations. The mean (SD) age of the cohort was 33 (12) years; 55.9% were female, and 30.5% were African American. Table 1 compares the baseline characteristics between those receiving tcVNS compared to sham control group. There were no significant differences between the groups with respect to baseline sociodemographic characteristics (age, sex, race, educational level, marital status) or the prevalence of PTSD (Table 1).
Table 1.
Baseline characteristics of the study cohort stratified by treatment group (Sham vs tcVNS)
| Variable | Sham (N= 29) |
VNS (N= 26) |
P-value |
|---|---|---|---|
| Age, Mean (SD) | 34 (12) | 31 (10) | 0.23 |
| Female, N (%) | 16 (55.2) | 16 (61.5) | 0.62 |
| Black race, N (%) | 8 (28.6) | 10 (38.5) | 0.44 |
| BMI, Mean (SD) | 28.8 (5.3) | 27.0 (6.5) | 0.28 |
| Educational level, N (%) | |||
| College graduate | 18 (56.7) | 15 (57.3) | 0.71 |
| Marital Status, N (%) | 0.72 | ||
| Never married | 16 (59.3) | 17 (65.4) | |
| Married | 4 (14.8) | 5 (19.2) | |
| Divorced/separated | 7 (25.9) | 4 (15.3) | |
| PTSD, N (%) | 13 (44.8) | 14 (53.8) | 0.48 |
| Baseline ghrelin (pg/ml), Mean (SD) | 530.8 (336.2) | 432.3 (298.1) | 0.21 |
BMI= body mass index, PTSD= Post traumatic stress disorder
Correlates of Ghrelin Levels
Table 2 describes the bivariate correlates of baseline and post-stress ghrelin levels. There were no significant associations between baseline ghrelin levels and any of the sociodemographic characteristics (Table 2). However, post-stress ghrelin levels were found to be positively associated with older age (B 7.8, 95% CI 0.46, 15.29, P= 0.02). No other significant associations were present between post-stress ghrelin levels and other sociodemographic characteristics.
Table 2.
Bivariate analysis investigating the correlates of baseline and post-stress ghrelin
| Baseline Ghrelin | Post-stress Ghrelin | |
|---|---|---|
| Β (95% CI) | ||
| Age | 6.9 (−1.3, 15.9) | 7.8 (0.4, 15.2) |
| Female sex | 183.3 (−14.2, 381.2) | 82.3 (−113.2, 278.5) |
| Black race | −148.2 (−356.2, 61.3) | −128.2 (−340.7, 84.1) |
| BMI | −9.8 (−27.0, 7.4) | −2.27 (−19.7, 15.1) |
| Educational level | 55.2 (−41.3, 152.0) | 40.8 (−51.2, 132.9) |
| Marital Status | 101.0 (−11.3, 213.4) | 105.2 (−0.67, 211.9) |
| PTSD | 32.2 (−175.2, 240.6) | 62.3 (−134.5, 259.8) |
BMI= body mass index, PTSD= Post traumatic stress disorder
Modulation of Ghrelin with Vagal versus Sham Stimulation during Stress
Baseline ghrelin levels were not significantly different between the tcVNS and sham stimulation groups at either day 1 (Table 1, and Figure 1) or day 2 (Figure 2). In the absence of trauma scripts, there were no significant differences between the tcVNS and sham groups in the levels of ghrelin measured immediately after the termination of stimulation (Figure 1). However, when exposed to personalized traumatic scripts in conjunction with tcVNS, participants had significantly lower levels of ghrelin compared to the sham group (265.2 ± 143.6 pg/ml vs 478.7 ± 349.2, P=0.01, Figure 1). Similarly, as shown in Figure 2, plasma ghrelin levels measured 90 minutes after completing the public speaking and mental arithmetic stress tests on day 2 were significantly lower in the group receiving tcVNS compared to the sham group (293.3 ± 102.4 pg/ml vs 540.3 ± 203.9, P= 0.009).
Figure 1.
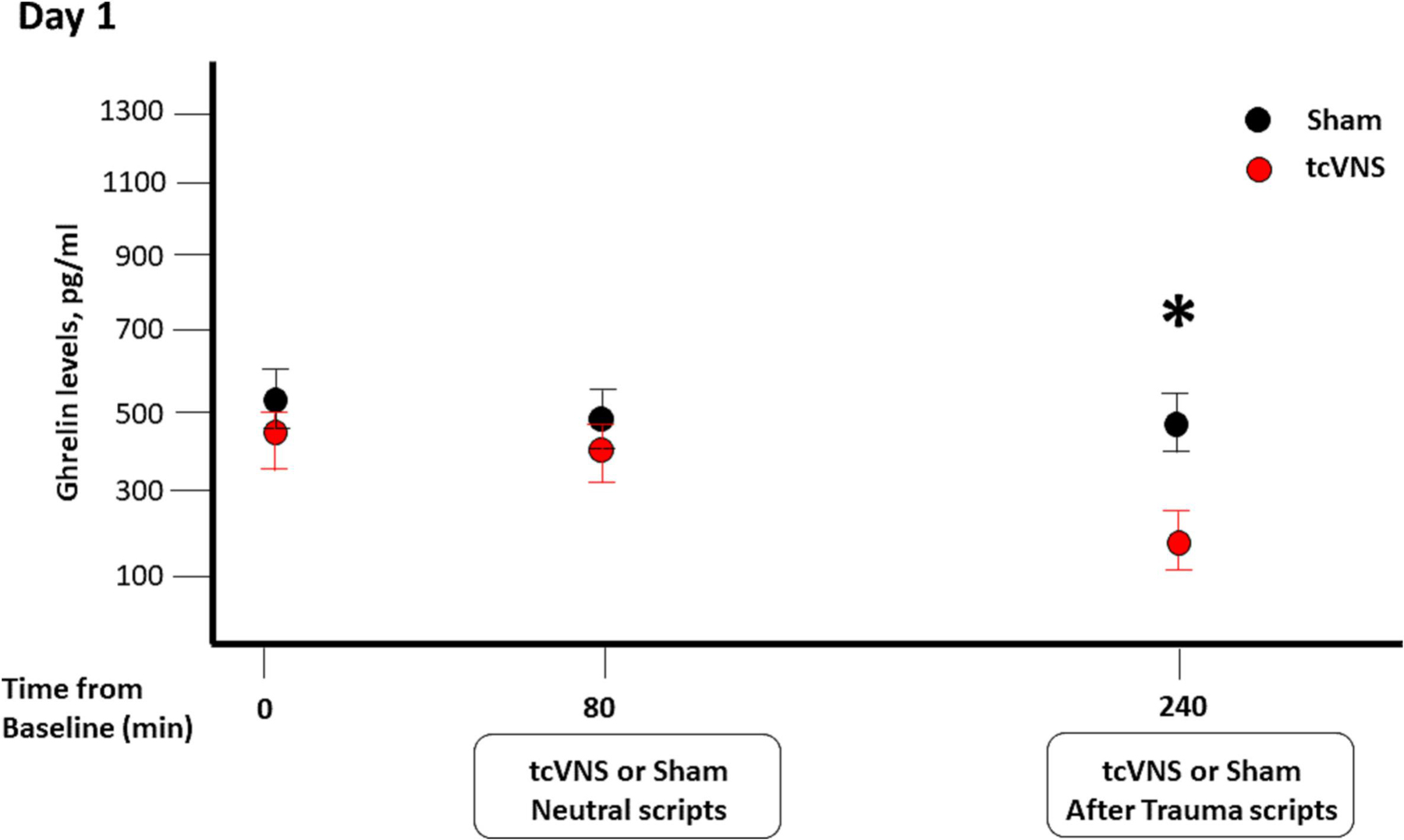
Effects of sham (black) or tcVNS (red) on ghrelin levels. Blood samples were collected at baseline, after stimulation following neutral scripts and after stimulation following trauma scripts.
Figure 2.
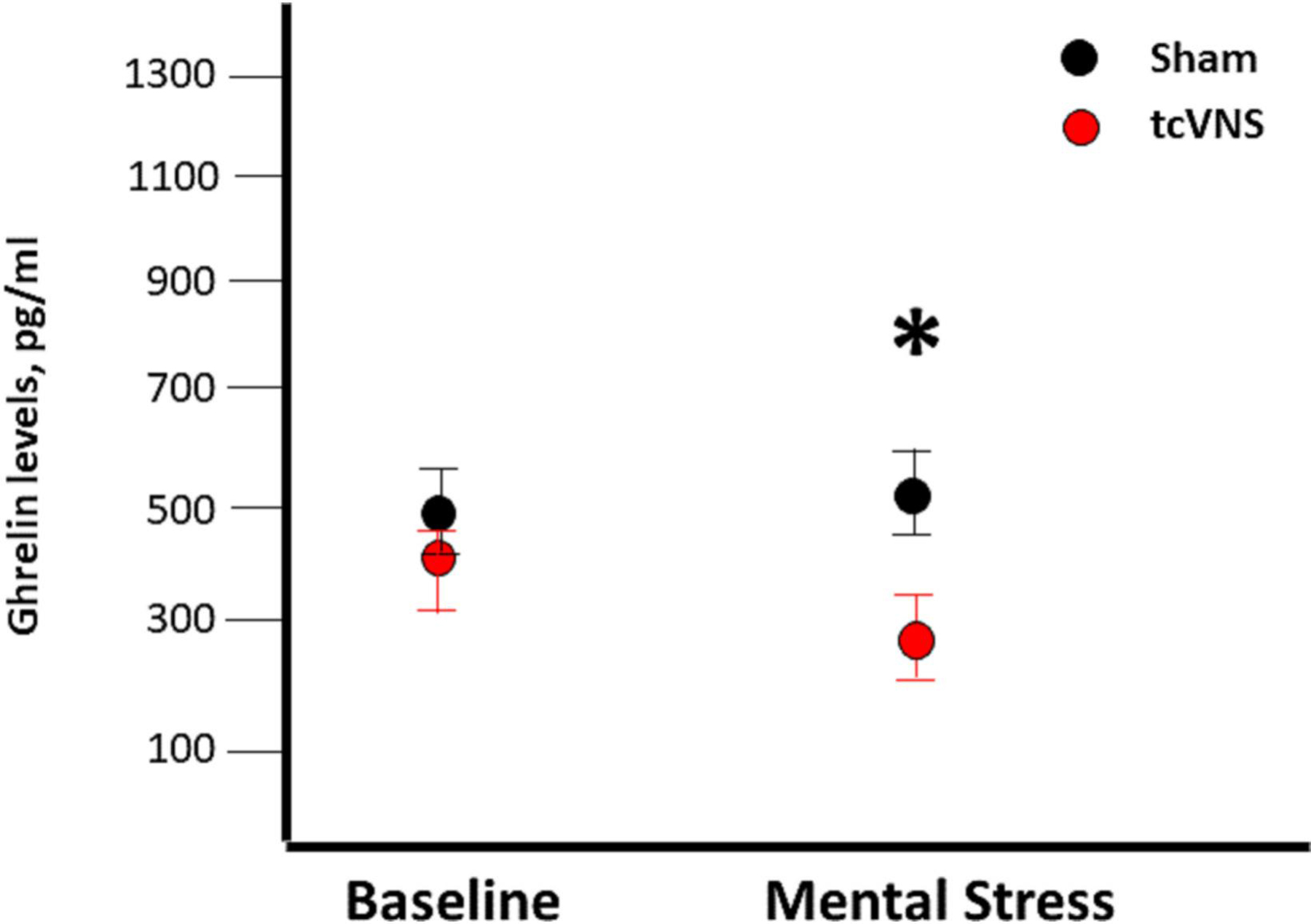
Effects of sham (black) or tcVNS (red) on ghrelin levels. Blood samples were collected at baseline, after the completion of public speaking and arithmetic mental stress.
Discussion
The findings from the present study showed for the first time that non-invasive tcVNS reduced the levels of ghrelin after stressful stimuli compared to sham stimulation. These findings were consistent after exposure to either trauma scripts or public and arithmetic stress tests. Of note, plasma levels of ghrelin were not significantly affected by vagal stimulation following exposure to neutral (non-traumatic) scripts. These findings suggest that ghrelin may have a role in the protective effects of tcVNS against stressful stimuli.
Neuromodulation represents novel treatment modalities for stress-related psychiatric disorders (Adair et al., 2020). Among these strategies, vagal nerve stimulation with implantable devices has shown to be effective in the treatment of depression and epilepsy (Kraus et al., 2013; Nemeroff et al., 2006). However, FDA-approved vagal stimulation for these diseases have historically involved surgical implantation to provide direct electrical stimulation of the vagus nerve (Aaronson et al., 2017; Bremner and Rapaport, 2017). The emergence of non-invasive devices for stimulation of the vagal nerve provides an attractive alternative as it reduces cost and the inconvenience associated with implanted devices (Bremner and Rapaport, 2017). However, the mechanisms of action of these devices and their efficacy have not been extensively studied. In the present study, we showed that ghrelin levels were lower in those undergoing tcVNS compared to sham controls in response to mental stress stimuli.
Ghrelin was discovered in 1999 as a hormone produced and secreted mostly by the stomach and was thought to act as a pro-hunger hormone as levels increased with starvation (Cummings et al., 2002). Further research, however, has revealed that ghrelin’s response to acute or chronic hunger states does not necessarily reflect a role of ghrelin in hunger, but rather a more prominent role as a stress response. This proposal has been further supported by observations that ghrelin levels are chronically higher in those with psychiatric illnesses such as major depressive disorder or bipolar disorder (Kurt et al., 2007; Ozsoy et al., 2015; Tuncel et al., 2016). More significantly, it has been shown that ghrelin could be a marker of treatment response in psychiatric disorders. Previous studies have demonstrated that a decrease in ghrelin levels were associated with treatment responses (Kurt et al., 2007; Lopez-Alarcon et al., 2020; Ozsoy et al., 2014; Ricken et al., 2017). These findings are consistent with the results of our study showing the association between lower ghrelin levels and responses to vagal stimulation during mental stress.
Previous studies have shown that ghrelin is transiently elevated by acute stress exposure (Raspopow et al., 2010; Rouach et al., 2007; Sinha et al., 2019), by short-term interpersonal stress (Jaremka et al., 2014), or in anticipation of laboratory stress (Raspopow et al., 2014). Similarly, exposure to chronic stress has shown to be associated with higher ghrelin levels in both humans and animal models with levels remaining elevated for extended periods of time even after the cessation of the stressful stimuli (Lutter et al., 2008; Yousufzai et al., 2018). In our study, we did not observe higher ghrelin levels after stressful stimuli with either trauma scripts or mental stress challenges (speaking and arithmetic). This lack of increase in ghrelin levels in response to stress could be attributed to the fact that we measured blood samples around 90 minutes after stressful stimuli. Ghrelin has a short half-life of between 10 and 31 minutes (Akamizu et al., 2004; Nagaya et al., 2001). Results from a recent meta-analysis indicate that ghrelin reaches its highest levels in the first 5 minutes following the stress intervention, and levels return to baseline after 45 minutes post-stress exposure (Bouillon-Minois et al., 2021).
Our study has a number of limitations. Blood samples for ghrelin measurements were collected 90 minutes after stressors and it is possible that the magnitude of the ghrelin response to tcVNS would vary at different time points post-stress exposure. Our study is also limited by the fact that only the left vagus nerve was stimulated per the study design. It is unclear if right sided vagal stimulation could also be associated with similar changes in ghrelin levels in response to mental stressors. We also did not evaluate the effects of blinding in either the tcVNS or sham groups. Psychometric measures were also not adopted in this study. Patients were also allowed to take their prescribed medications during the study period. While these medications could potentially confound the results of our study, we have previously shown that empirical evidence for relevant medications having a confounding effect on task performance remains sparse (Lanius et al., 2010). Therefore, future studies are needed to further confirm the results of our study.
In summary, we found that tcVNS modulates ghrelin levels in response to various stressful stimuli. Future work is needed to understand the longitudinal outcomes of tcVNS and whether ghrelin measurements could be utilized as a biomarker for assessment of response to neuromodulation treatments.
Supplementary Material
Highlights:
Ghrelin levels were similar between the tcVNS and sham groups at baseline
tcVNS and traumatic scripts resulted in lower ghrelin levels compared to sham group
With stress, ghrelin levels were lower in the tcVNS group than the sham group
Funding:
This work was sponsored by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Targeted Neuroplasticity Training (TNT) program through the Naval Information Warfare Center (NIWC) Cooperative Agreement No. N66001-16-4054. Dr. Moazzami received funding from the NIH (T32 HL130025).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of interest:
‘All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare that J.D.B has research funding support from ElectroCore LLC for the submitted work. Both active and sham stimulation devices used in this study were provided by ElectroCore free of charge. All remaining authors have no competing interests to report
References
- Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, Reimherr FW, Schwartz TL, Zajecka JM, 2017. A 5-Year Observational Study of Patients With Treatment-Resistant Depression Treated With Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am J Psychiatry 174, 640–648. [DOI] [PubMed] [Google Scholar]
- Adair D, Truong D, Esmaeilpour Z, Gebodh N, Borges H, Ho L, Bremner JD, Badran BW, Napadow V, Clark VP, Bikson M, 2020. Electrical stimulation of cranial nerves in cognition and disease. Brain Stimul 13, 717–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamizu T, Takaya K, Irako T, Hosoda H, Teramukai S, Matsuyama A, Tada H, Miura K, Shimizu A, Fukushima M, Yokode M, Tanaka K, Kangawa K, 2004. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol 150, 447–455. [DOI] [PubMed] [Google Scholar]
- Bali A, Jaggi AS, 2016. An Integrative Review on Role and Mechanisms of Ghrelin in Stress, Anxiety and Depression. Curr Drug Targets 17, 495–507. [DOI] [PubMed] [Google Scholar]
- Bouillon-Minois JB, Trousselard M, Thivel D, Gordon BA, Schmidt J, Moustafa F, Oris C, Dutheil F, 2021. Ghrelin as a Biomarker of Stress: A Systematic Review and Meta-Analysis. Nutrients 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Gurel NZ, Jiao Y, Wittbrodt MT, Levantsevych OM, Huang M, Jung H, Shandhi MH, Beckwith J, Herring I, Rapaport MH, Murrah N, Driggers E, Ko Y-A, Alkhalaf ML, Soudan M, Song J, Ku BS, Shallenberger L, Hankus AN, Nye JA, Park J, Vaccarino V, Shah AJ, Inan OT, Pearce BD, 2020a. Transcutaneous vagal nerve stimulation blocks stress-induced activation of interleukin-6 and interferon-γ in posttraumatic stress disorder: A double-blind, randomized, sham-controlled trial. Brain Behav. Immun. Health 9, 100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Park J, Bikson M, Inan OT, 2020b. Application of non-invasive vagal nerve stimulation to stress-related psychiatric disorders. J. Pers. Med 10, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Pearce B, 2016. Neurotransmitter, neurohormonal, and neuropeptidal function in stress and PTSD., In: Wiley-Blackwell; (Ed.), Posttraumatic Stress Disorder: From Neurobiology to Treatment, Hoboken, New Jersey, pp. 181–232. [Google Scholar]
- Bremner JD, Rapaport MH, 2017. Vagus Nerve Stimulation: Back to the Future. Am J Psychiatry 174, 609–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM, 2011. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest 121, 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimpianu CL, Strube W, Falkai P, Palm U, Hasan A, 2017. Vagus nerve stimulation in psychiatry: a systematic review of the available evidence. J Neural Transm (Vienna) 124, 145–158. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ, 2002. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346, 1623–1630. [DOI] [PubMed] [Google Scholar]
- Gazi AH, Gurel NZ, Richardson KLS, Wittbrodt MT, Shah AJ, Vaccarino V, Bremner JD, Inan OT, 2020a. Digital Cardiovascular Biomarker Responses to Transcutaneous Cervical Vagus Nerve Stimulation: State-Space Modeling, Prediction, and Simulation. JMIR Mhealth Uhealth 8, e20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazi AH, Gurel NZ, Richardson KLS, Wittbrodt MT, Shah AJ, Vaccarino V, Bremner JD, Inan OT, 2020b. Digital cardiovascular biomarker responses to transcutaneous cervical vagus nerve stimulation: State-space modeling, prediction, and simulation. JMIR mHealth uHealth 8, e20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM, Howland R, Kling MA, Moreno F, Rittberg B, Dunner D, Schwartz T, Carpenter L, Burke M, Ninan P, Goodnick P, 2005. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry 58, 364–373. [DOI] [PubMed] [Google Scholar]
- Gurel NZ, Gazi AH, Scott KL, Wittbrodt MT, Shah AJ, Vaccarino V, Bremner JD, Inan OT, 2020a. Timing considerations for noninvasive Vagal Nerve Stimulation in clinical studies. AMIA Annual Symposium Proceedings 2019, 1061–1070. [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Huang M, Wittbrodt MT, Jung H, Ladd SL, Shandhi MH, Ko Y-A, Shallenberger L, Nye JA, Pearce B, Vaccarino V, Shah AJ, Bremner JD, Inan OT, 2020b. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul 13, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Jiao Y, Wittbrodt MT, Hankus A, Driggers EG, Shallenberger L, Murrah N, Huang M, Haffar A, Alkhalaf ML, Levantsevych OM, Nye JA, Vaccarino V, Shah AJ, Bremner JD, Inan OT, Pearce BD, 2020c. Effect of transcutaneous vagus nerve stimulation on the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) response to stress: A randomized, sham controlled, double blind pilot study. Comprehensive Psychoneuroendocrinology 4, 100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Wittbrodt MT, Jung H, Shandhi MH, Driggers EG, Ladd SL, Huang M, Ko Y-A, Shallenberger L, Becwith J, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Inan OT, Bremner JD, 2020d. Transcutaneous vagal nerve stimulation reduces sympathetic responses to stress in posttraumatic stress disorder: A double-blind, randomized, sham controlled trial. Neurobiol. Stress 13, e100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Wittbrodt MT, Jung H, Shandhi MMH, Driggers EG, Ladd SL, Huang M, Ko YA, Shallenberger L, Beckwith J, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Inan OT, Bremner JD, 2020e. Transcutaneous cervical vagal nerve stimulation reduces sympathetic responses to stress in posttraumatic stress disorder: A double-blind, randomized, sham controlled trial. Neurobiol Stress 13, 100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Wittbrodt WT, Jung H, Ladd SL, Shah AJ, Vaccarino V, Bremner JD, Inan OT, 2020f. Automatic detection of target engagement in transcutaneous cervical Vagal Nerve Stimulation for traumatic stress triggers. IEEE J. Biomed. Health Inform 24, 1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Belury MA, Andridge RR, Malarkey WB, Glaser R, Christian L, Emery CF, Kiecolt-Glaser JK, 2014. Interpersonal stressors predict ghrelin and leptin levels in women. Psychoneuroendocrinology 48, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Baram TZ, 2009. The neuro-symphony of stress. Nat Rev Neurosci 10, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trepanier L, Marin MF, Wan N, Sekerovic Z, Lord C, Fiocco AJ, Plusquellec P, McEwen BS, Lupien SJ, 2011. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Dev Psychopathol 23, 725–776. [DOI] [PubMed] [Google Scholar]
- Kraus T, Kiess O, Hosl K, Terekhin P, Kornhuber J, Forster C, 2013. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal - a pilot study. Brain Stimul 6, 798–804. [DOI] [PubMed] [Google Scholar]
- Kurt E, Guler O, Serteser M, Cansel N, Ozbulut O, Altinbas K, Alatas G, Savas H, Gecici O, 2007. The effects of electroconvulsive therapy on ghrelin, leptin and cholesterol levels in patients with mood disorders. Neurosci Lett 426, 49–53. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Brewin CR, Bremner JD, Daniels JK, Friedman MJ, Liberzon I, McFarlane A, Schnurr PP, Shin L, Stein M, Vermetten E, 2010. Does neuroimaging research examining the pathophysiology of posttraumatic stress disorder require medication-free patients? J Psychiatry Neurosci 35, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wust S, Pruessner JC, Rietschel M, Deuschle M, Meyer-Lindenberg A, 2011. City living and urban upbringing affect neural social stress processing in humans. Nature 474, 498–501. [DOI] [PubMed] [Google Scholar]
- Lopez-Alarcon M, Zurita-Cruz JN, Torres-Rodriguez A, Bedia-Mejia K, Perez-Guemez M, Jaramillo-Villanueva L, Rendon-Macias ME, Fernandez JR, Martinez-Maronas P, 2020. Mindfulness affects stress, ghrelin, and BMI of obese children: a clinical trial. Endocr Connect 9, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM, 2008. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11, 752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 1998. Protective and damaging effects of stress mediators. N Engl J Med 338, 171–179. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K, 2001. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol 280, R1483–1487. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK, 2006. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 31, 1345–1355. [DOI] [PubMed] [Google Scholar]
- Ozsoy S, Besirli A, Abdulrezzak U, Basturk M, 2014. Serum ghrelin and leptin levels in patients with depression and the effects of treatment. Psychiatry Investig 11, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy S, Besirli A, Unal D, Abdulrezzak U, Orhan O, 2015. The association between depression, weight loss and leptin/ghrelin levels in male patients with head and neck cancer undergoing radiotherapy. Gen Hosp Psychiatry 37, 31–35. [DOI] [PubMed] [Google Scholar]
- Ramsay RE, Uthman BM, Augustinsson LE, Upton AR, Naritoku D, Willis J, Treig T, Barolat G, Wernicke JF, 1994. Vagus nerve stimulation for treatment of partial seizures: 2. Safety, side effects, and tolerability. First International Vagus Nerve Stimulation Study Group. Epilepsia 35, 627–636. [DOI] [PubMed] [Google Scholar]
- Raspopow K, Abizaid A, Matheson K, Anisman H, 2010. Psychosocial stressor effects on cortisol and ghrelin in emotional and non-emotional eaters: influence of anger and shame. Horm Behav 58, 677–684. [DOI] [PubMed] [Google Scholar]
- Raspopow K, Abizaid A, Matheson K, Anisman H, 2014. Anticipation of a psychosocial stressor differentially influences ghrelin, cortisol and food intake among emotional and non-emotional eaters. Appetite 74, 35–43. [DOI] [PubMed] [Google Scholar]
- Ricken R, Bopp S, Schlattmann P, Himmerich H, Bschor T, Richter C, Elstner S, Stamm TJ, Schulz-Ratei B, Lingesleben A, Reischies FM, Sterzer P, Borgwardt S, Bauer M, Heinz A, Hellweg R, Lang UE, Adli M, 2017. Ghrelin Serum Concentrations Are Associated with Treatment Response During Lithium Augmentation of Antidepressants. Int J Neuropsychopharmacol 20, 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N, Greenman Y, 2007. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology 32, 693–702. [DOI] [PubMed] [Google Scholar]
- Sinha R, Gu P, Hart R, Guarnaccia JB, 2019. Food craving, cortisol and ghrelin responses in modeling highly palatable snack intake in the laboratory. Physiol Behav 208, 112563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncel OK, Akbas S, Bilgici B, 2016. Increased Ghrelin Levels and Unchanged Adipocytokine Levels in Major Depressive Disorder. J Child Adolesc Psychopharmacol 26, 733–739. [DOI] [PubMed] [Google Scholar]
- Wittbrodt MT, Gurel NZ, Nye JA, Ladd S, Shandhi MMH, Huang M, Shah AJ, Pearce BD, Alam ZS, Rapaport MH, Murrah N, Ko Y-A, Haffer AA, Shallenberger LH, Vaccarino V, Inan OT, Bremner JD, 2020. Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimul 13, 1333–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J, 2004. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A 101, 10434–10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousufzai M, Harmatz ES, Shah M, Malik MO, Goosens KA, 2018. Ghrelin is a persistent biomarker for chronic stress exposure in adolescent rats and humans. Transl Psychiatry 8, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T, 2009. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol 296, R1358–1365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


