SUMMARY
Firefighters are at a 1.5 to 2 times greater risk of contracting certain types of cancers as compared to the general population. After preliminary studies, it was evident that contaminated turnout gear and ensemble elements could be linked to heightened cancer rates amongst firefighters. Compounds such as polycyclic aromatic hydrocarbons (PAHs), perfluorinated compounds, phenols, phthalates, brominated flame retardants, dioxins, volatile organic compounds, and many others are present in the contaminated gear, of which many are known carcinogens. A setup of headspace sampler-gas chromatograph-mass spectrometer was used to measure the off-gassing of the fabric samples taken from retired field-contaminated turnout jackets. The fabric samples were exposed to a specific temperature and allowed to equilibrate for a fixed time in the HS. A custom reference mix of phenols, phthalates and PAHs was put together to develop standard calibration curves. The compounds off-gassing from the outer shell, thermal liner and the moisture barrier were analyzed and the masses of certain marker compounds were calculated based of the standard calibration curves. The technique could be used as a screening method to thermally extract contaminants from field-contaminated firefighter turnout materials such as jackets, pants, gloves, and so on.
Keywords: contaminants, firefighting, gas-chromatography, headspace sampling, off-gassing, PAHs, phenols, phthalates
1. INTRODUCTION
1.1. Firefighting and chemicals involved
Firefighting is a profession that involves extreme complexity in terms of the conditions that firefighters face. The conditions vary largely depending on several factors such as type and quality of fuel, location of fire, enclosed area and ambient conditions such as wind. The firefighters must be prepared for the worst-case scenarios being flash fires, where temperatures could reach above 1000°C.1 With the heat arising from the fire, they are also exposed to several toxic chemicals from the smoke that gets generated from burning of combustible substances. The smoke from fires contains a plethora of gases, vapors, suspended liquids, and solid particulate matter. The chemical composition of the smoke completely depends on the vegetation/fuel that is burning, which means that structural and wildland firefighting have different chemical exposures. Complete combustion gives off carbon dioxide and water as the products, but it is the pyrolysis that leads to the generation of free radicals and toxic compounds.
In structural fires, plastics and polymers are major polluting elements. Thermal degradation of polymers releases methane, benzene, toluene, acrylonitrile, styrene, phenols, phthalates, polycyclic aromatic hydrocarbons (PAHs), perfluorooctanoic acid (PFOA), brominated flame retardants, urea, dioxins, volatile organic compounds (VOCs) and many other compounds.2,3 Burning of plastic specifically releases compounds such as hydrochloric acid, acrolein, hydrogen cyanide, and soot. The concern is that most of these chemicals are known to be potential carcinogens, thus severely affecting the health of the responders working closely at the incident. Other gases that have short-term acute effects are carbon dioxide, carbon monoxide, nitrogen oxides, sulfur oxides, and hydrogen cyanide which have very high levels for short periods of time.4 There are several factors that increase the risk of cancer among firefighters along with other health issues such as cardiac arrest, bronchitis, etc.5 Using contaminated gear is believed to be an issue and a potential source of exposure, since it is not frequently cleaned and it possibly off-gasses chemicals during storage. Exhaust of the fire engines and other response vehicles has carbon compounds that get directly inhaled by the firefighters during work and upon returning to the station. Also, the on-scene exposure to smoke and chemicals is of significant concern, especially in the absence of using appropriate personal protective equipment such as respiratory protection.6 There are places with high chemical exposure that are often overlooked. Gear and station uniforms stored in racks in the fire stations get contaminated frequently due to the smoke emission from the fire engines. Toxic gases and soot get deposited on the contaminated gear that goes unnoticed. On wearing contaminated gear while driving, there might be a transfer of contaminants onto the seat material, which could also be a possible source of further contamination on cleaned gear. Repeated dirty hand-to-mouth contact could give way to oral exposure of benzo(a) pyrene and dioxins that could cause cancer. Skin exposure to hydrocyanic acid and benzo(a) pyrene is dangerous and can be potentially carcinogenic. Exposure to phenols and formaldehyde can aggravate this effect.7
1.2. Off-gassing of toxic contaminants
After the turnout gear is exposed to various chemicals, smoke, soot, and particulates during fire suppression and overhaul, some of these compounds and particulates can be adsorbed onto the garments. Both, the volatiles and the semivolatiles that are adsorbed on the gear could off-gas repeatedly when the gear is used in hot conditions near a fire. The type and the amount of chemicals would vary depending on the temperature ranges present and the type of fire. There are several studies conducted to measure the VOCs after a fire incident.8,9 The volatile and semivolatile compounds tend to evaporate off the surface of the gear and ensemble elements. This study focuses on the semivolatile compounds, since they could keep off-gassing for longer periods of time as compared to the volatiles. A study by Fent et al showed that volatile compounds start to off-gas within 30 minutes of exposure.10
1.3. Thermal extraction using headspace sampler
Thermal extraction is particularly useful in removing compounds from fabrics that have lower boiling points. A headspace sampler (HS) is an instrument that has the capability to simulate off-gassing in solid or liquid matrices. The samples are placed in HS crimp-top glass vials and heated at a set temperature for a fixed amount of time. The instrument is built so that the vial can be equilibrated and shaken in the oven precisely with a 0.1°C accuracy. The best feature of this add-on is that it can be directly attached the gas chromatograph (GC) inlet via a heated transfer line. After a sample is heated, the gases evolving from the sample get collected in the headspace area between the sample and the vial cap. A needle precisely pierces through the sealed cap and transfers the gas to the transfer line through a heated loop. The heated transfer line then carries the gas into the GC inlet without any loss. One of the major advantages with using the HS is that there is very little sample preparation required. Any solid or liquid sample can be placed inside the glass vial and can be heated in the oven for analysis. Simulating the off-gassing and measuring the off-gassed air through any other method such as polymer sorption-liquid extraction or active/passive air sampling requires an elaborate set-up and complex sample preparation.
With previous studies of off-gassing of firefighter gear, it is known that the level of off-gassing of chemicals is largely dependent on the time after exposure/contamination that the fabric is sampled and the temperature of which the fabric was exposed.10 In respect to firefighting, the turnout jacket and pants are exposed to a lot of toxic chemicals in the fire scenario. Thereafter, the off-gassing depends on the conditions in which it is stored, transported and re-used during subsequent fire incidents. The HS can be used for a variety of applications depending on the temperature and time that is required. For example, if an outer shell material is exposed to a temperature of 200°C, it would simulate a possible temperature in a fire.1 A temperature of 200°C is also near the upper limit of the HS and is used as an extreme temperature to carry out maximum off-gassing of chemicals from the. There is a solid-gas equilibrium that gets formed inside the heated vial. When the equilibrium is reached, no more of the contaminants will get released from the fabric.
1.4. Scope of the research
This research investigates off-gassing of chemicals from retired field-contaminated gear. The study encompasses various parameters such as effect of temperature, difference in layers and age of the gear. The firefighter turnout jacket is a three-layer composite with the outer shell, moisture barrier, and thermal liner. The following analysis is vastly qualitative in nature, except for phenol and di-ethylhexyl phthalate (DEHP), for which reference calibration curves were prepared to enable quantitation. The study highlights the types of compounds off-gassing from various layers and the relative amounts. There are some important questions that this study intends to address:
Is there a difference in the compounds releasing from different layers of a field-contaminated jacket?
Does the age of the gear affect the type and amount of compounds that off-gas?
Could the HS be used to quantify the off-gassing levels of certain volatile and semivolatile compounds?
Very few studies have been undertaken to analyze the compounds off-gassing from actual retired contaminated gear. According to Fent et al, a variety of VOCs do off-gas from the gear after being exposed to a fire, but the semivolatiles must be researched further to determine if they get released as well.10 The volatilization of higher boiling compounds such as PAHs, of which many are known carcinogens, is not researched adequately. These chemicals are some of the most common fireground contaminants found in household products such as plastics, beauty products, etc.11
The International Agency for Research on Cancer (IARC) classified firefighting as a possibly carcinogenic profession. Compounds such as PAHs and phthalates are extremely toxic, of which some are known carcinogens. A method must be developed to identify and analyze the presence of these compounds in contaminated gear.6
2. MATERIALS AND METHODS
2.1. Materials
2.1.1. Chemical analysis
A custom calibration standard (referred to as “master mix”) of three phenols, three phthalates and four PAHs prepared in methylene chloride was purchased from Agilent Technologies. They were chosen based on their prevalence in structural fires and toxicity profiles.12 The mix had all the compounds (as seen in Table 1) at a concentration of 2000 ng/μL and was packaged in 2-mL amber colored vials and stored at room temperature. For calibration standards, n-hexane (gas chromatography grade, 99.9 + %, ACROS chemicals) was used.
TABLE 1.
Master mix compounds and their properties relevant to headspace sampler analysis13
| Compound | Boiling point (°C) | Volatile or semivolatile | Vapor pressure at 25°C (mm Hg) | Carcinogenicity level |
|---|---|---|---|---|
| Phenol | 182 | Volatile | 3.50E – 01 | Group 3 |
| 2,4,6-Trichlorophenol (2,4,6-TCP) | 246 | Volatile | 8.00E – 03 | Group 2B |
| Pentachlorophenol (PCP) | 310 | Semivolatile | 1.1E – 04 | Group 2B |
| Di-butyl phthalate (DBP) | 340 | Semivolatile | 2.00E – 05 | Group 3 |
| Benzyl butyl phthalate (BBP) | 370 | Semivolatile | 8.25E – 06 | Group 3 |
| Di-ethylhexyl phthalate (DEHP) | 384 | Semivolatile | 1/42E – 07 | Group 2B |
| Naphthalene (NA) | 218 | Volatile | 08.5E – 02 | Group 2B |
| Phenanthrene (PA) | 340 | Semivolatile | 1.21E – 04 | Group 3 |
| Pyrene (PY) | 404 | Semivolatile | 4.5E – 06 | Group 3 |
| Benzo[a] pyrene (BaP) | 495 | Semivolatile | 5.49E – 09 | Group 1 |
Note: The International Agency for Research on Cancer classifies substances to show whether they are suspected to cause cancer or not. It places the substances into four categories depending on the strength of evidence for their carcinogenicity. The categories are as follows: group 1—carcinogenic to humans, group 2A—probably carcinogenic to humans, group 2B—possibly carcinogenic to humans, and group 3—not classifiable as to its carcinogenicity to humans.14
2.1.2. Material assessment
Two sets of retired, field-contaminated firefighter turnout jackets (shown in Table 2) were donated by the Alachua County Fire Department (Florida, USA; Jackets A and B) and an additional jacket was donated by the Raleigh Fire Department (North Carolina, USA; Jacket C). Representative control samples of uncontaminated outer shell material were available, but unused thermal liner and moisture barrier materials were not available for analysis. Jackets A and B, being used for 18 months (1.5 years) are referred to as “1.5Y” gear and Jacket C being used for 10 years is referred to as “10Y” gear for convenience through this paper.
TABLE 2.
Specifications of retired turnout gear samples
| Retired tunout jacket A | Retired turnout jacket B | Retired turnout jacket C | |
|---|---|---|---|

|

|

|
|
| Manufacturer | Globe turnout gear | Globe turnout gear | Globe turnout gear |
| Date of manufacture | 02/2013 | 02/2013 | 05/2006 |
| Use life | ~18 months | ~18 months | ~10 years |
| Donated by | Alachua county fire department | Alachua county fire department | Raleigh fire department |
| Outer shell | Armor 7. gold | Armor 7.0 gold | 7.5 PBI Matrix gold |
| Moisture barrier | Glide gold two-layer | Glide gold two-layer | ARALITE quilt |
| Thermal liner | Gore® Crosstech Black 2F | Gore Crosstech Black 2F | Gore Crosstech |
2.2. GC-mass spectrometer chromatographic method development
Chromatographic analysis was conducted in the split mode with a 10:1 split ratio. The column used in the GC was an Agilent EPA 8270D, fused silica capillary column (30 m × 0.25 mm × 0.25 μm). An Agilent 5190–3136 UI Splitless Single Taper with glass wool liner was used in the GC inlet. The injection volume was 1 μL and the injection temperature was kept at 250°C with a helium flow rate of 1.2 mL/min. The oven gradient was set to begin at 40°C, increased to 280°C for 1 minute at a rate of 10°C/min, further increased to 300°C at 5°C/min for 1 minute. The total run time was 30 minutes. The mass spectrometer (MS) transfer line was kept at 280°C throughout the run. The MS quadrupole temperature was maintained at 230°C and the ion source temperature was kept at 150°C. The gain factor used was 1.00. The analysis was conducted in scan mode (35–550 amu) using electron impact ionization with an energy of 70 eV.
2.3. Headspace sampling method development
The sample introduction was achieved using Agilent 7697A HS connected to a 7890B GC and a 5977B MS system. The headspace oven temperature was maintained in the range of 100 to 200°C. The loop temperature was always maintained 10°C above the oven temperature and headspace transfer line temperature was always maintained 10°C above the loop temperature. The equilibration time was set to 30 minutes. The vials were made to shake at 100 shakes/min to account for movement of the fabric while in the oven. The temperatures chosen for this study were based on arbitrary values and upper limits of the instrument, where 100°C was the lower extraction temperature, whereas 200°C was the physical upper limit of the HS and used as the extreme temperature for maximum thermal extraction.
2.4. Sample trials of phenols, phthalates, and PAHs using the HS-GC-MS liquid injection system
A 50 ng/μL stock solution was prepared by pipetting 250 μL of the 2000 ng/μL master mix stock solution into a 10-mL volumetric flask and diluting with n-hexane. Further, a total of nine calibration solutions from 50 ng to 10 000 ng (as per mass-in vial, seen in Equation (1)) were prepared using two solutions: 50 ng/μL stock solution was used to prepare the 50, 100, 200, 500, and 1000 ng samples by pipetting 1, 2, 4, 10, and 20 μL, respectively into HS crimp-top vials. The 2000 ng/μL stock solution was used to prepare the 2000, 4000, 8000, and 10 000 ng samples by pipetting 1, 2, 4, and 5 μL, respectively into HS crimp-top vials. Every liquid calibration solution was spiked in a 20-ml HS crimp-top glass vial and run at 100°C and 200°C separately for an equilibration time of 30 minutes using the GC-MS method developed earlier. The mass in vial (ng) can be calculated by using the formula:
| (1) |
2.5. Quantitation of retired gear samples at 200°C for 30 minutes
After the retired field-contaminated samples were analyzed using the HS-GC-MS, the chromatogram was analyzed for the compounds with their respective peak areas. The area under the curve (peak area) is the amount of a compound detected as response. The response (y-axis) on the area-mass curve has no units. The masses of only those compounds were calculated which were found in both, the retired gear and the standard calibration curves.
Calculation of masses of compounds using the calibration curve equation at 200°C, 30 minutes was performed using the following straight-line equation:
| (2) |
where y = peak area, x = mass of compound (ng), m = slope of the line, and c = y-intercept
The peak areas (y) from the retired gear samples were then substituted in the calibration equations for HS liquid calibration at 200°C, 30 minutes (seen in Table 3), respectively to obtain the mass detected (x) values.
TABLE 3.
Calibration equations for phenol and di-ethylhexyl phthalate developed using headspace liquid injection
| Compound specification | Calibration equation |
|---|---|
| Phenol-200°C | Area = 1706x + 467 265 |
| DEHP-200°C | Area = 3953.92x − 3 256 495 |
2.6. Analysis of retired contaminated firefighter turnout jackets
Samples (5 cm × 5 cm) were cut from the turnout jackets at bottom back and chest area as shown in Figure 1. For convenience in the following analysis, the bottom back sampling site is referred to as BACK and the chest area sampling site is referred to as FRONT.
FIGURE 1.
Sampling locations on jackets
2.6.1. Specifications of the layers used in the trials
All the three layers—outer shell, thermal liner and moisture barrier (refer Figure 2)—were used in the study. For the outer shell, moisture barrier and thermal liner of the 1.5Y jackets, three replicates were used for the 100°C runs from the BACK sampling location. Additionally, three replicates from BACK sampling location and three replicates from FRONT sampling locations were used for the 200°C runs.
FIGURE 2.
Sample layers. A, outer shell, B, thermal liner, and C, moisture barrier
For the outer shell, moisture barrier, and thermal liner of the 10Y jacket, three replicates each were used for the 200°C runs from the BACK and FRONT sampling locations.
The samples were placed inside headspace crimp-top glass vials (seen in Figure 3) and run through the developed HS-GC-MS analytical method. A qualitative analysis was done using MS to identify the various compounds off-gassing at different conditions. The temperatures used for thermal extraction of the layers of the firefighter gear were 100°C and 200°C. The 200°C temperature was chosen arbitrarily, being a realistic condition in house fires and is also the upper limit of the headspace instrument. The 100°C was chosen as a lower extraction temperature.
FIGURE 3.
Samples in headspace vials. A, outer shell, B, thermal liner, C, moisture barrier
3. RESULTS AND DISCUSSIONS
3.1. Analysis of compounds using the HS liquid calibration at 200°C
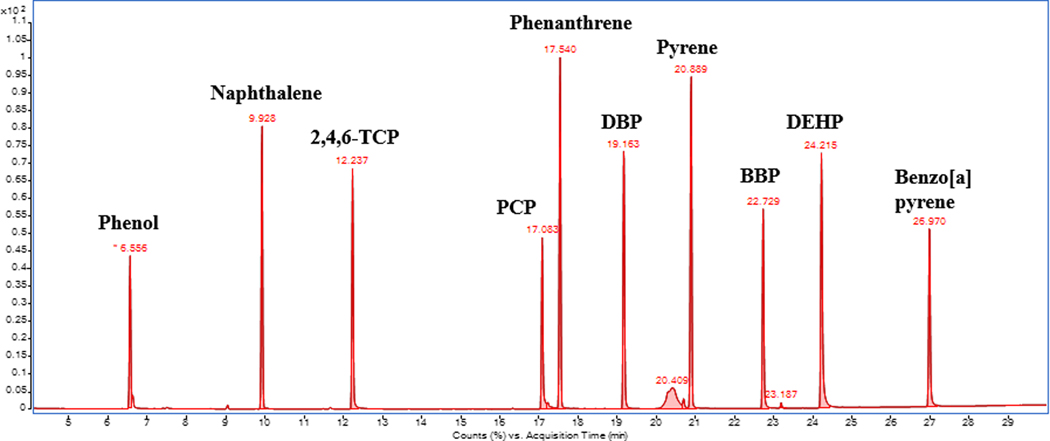
The HS liquid calibration was performed in the range of 50 to 10 000 ng in the vial to have reference values of responses for the fireground compounds. The responses achieved from analyzing pure liquid chemicals in the headspace vials was assumed to be the maximum amount of vaporization of the compound. Phenol and DEHP would be used as marker compounds, since they were found in field-contaminated retired gear. Figure 4 shows the compound peaks present in the master mix run using a HS injection at 200°C, 30 minutes. All the 10 compounds are detected and the peaks are separated with good resolution.
FIGURE 4.
Chromatogram of the compounds in the master mix using headspace liquid injection at 200°C for 30 minutes
The relevant compounds for calculating masses at 200°C were Phenol and DEHP, hence, it was important to know the calibration equation.
3.2. Respective chromatograms of outer shell at 100 and 200°C
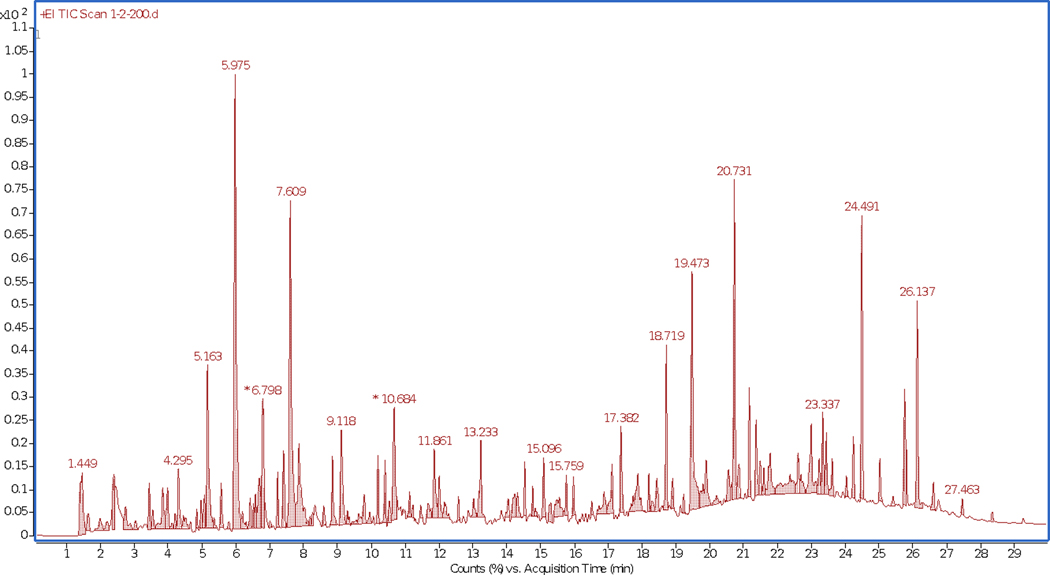
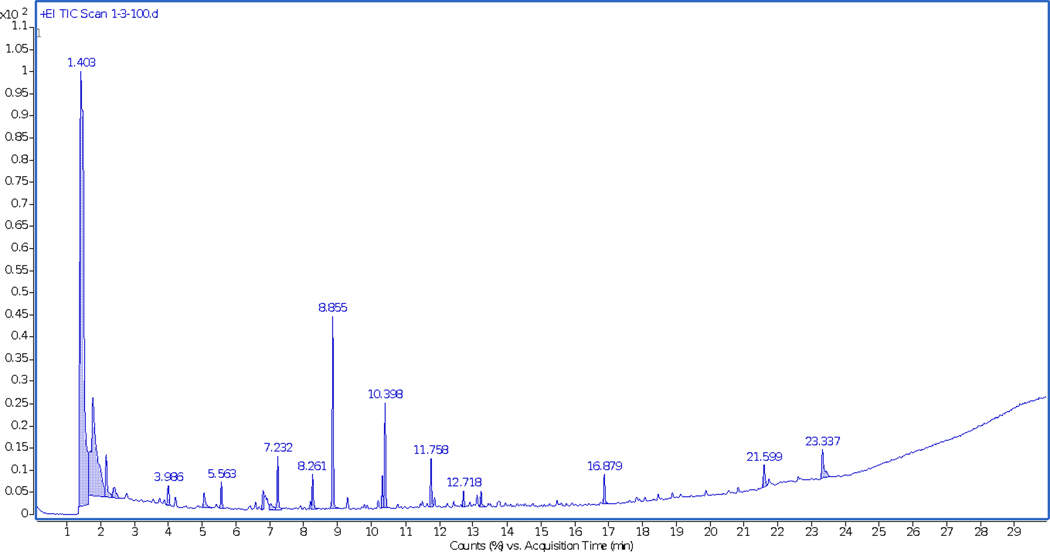
The following chromatograms depict the number of compounds off-gassing when fabric samples are exposed to a temperature of 100°C and 200°C. The samples used to demonstrate the difference are outer shell materials cut from the retired gear jackets. The higher number of peaks in the chromatograms of the 200°C (Figure 6) as compared to the chromatogram of the 100°C (Figure 5) run clearly depict the higher number of compounds emitting from the material at a higher temperature. There is a variability in the off-gassing of compounds at different temperatures, even though the fabric samples were taken from the same jacket. This is evident by the peaks appearing in Figure 5 being different from the peaks in Figure 6.
FIGURE 6.
Headspace-gas chromatograph-mass spectrometer chromatogram of retired gear outer shell analyzed using headspace sampler at 200°C
FIGURE 5.
Headspace-gas chromatograph-mass spectrometer chromatogram of retired gear outer shell samples analyzed using headspace sampler at 100°C
3.3. Overview of all the compounds off-gassing at different temperatures
All the compounds off-gassing at 100°C and 200°C from the various retired turnout jackets were noted in Tables 4–6. The data was separated on the basis of the layers tested at different conditions. Certain compounds were present in the unused reference outer shell materials. These are marked in Table 4 to clarify that the compounds did not get onto the gear due to external sources. Unused samples of moisture barrier and thermal liner were not available for analysis. Hence, some of the compounds listed in Tables 5 and 6 could be inherently present in the layers before it was exposed. There were some nondetects in the replicates analyzed for all the layers. The nondetects were considered as 0 and counted in the mean and the SD calculations. This did skew the data, but it was essential to provide a realistic value.
TABLE 4.
Overview of the levels of compounds off-gassing from the outer shell at various temperatures
| Jacket A and B-100°C | Jacket A and B-200°C | Jacket C-200°C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Compounds | Retention time (min) | Area | SD | % Prob | Area | SD | % Prob | Area | SD | % Prob |
| 1,2-Dichloroethane | 2.362 | - | - | - | - | - | - | 9.14E + 05 | 2.86E + 05 | 79 |
| 1H,1H,2H,2H-Perfluoro-1-decene | 2.374 | - | - | - | 3.72E + 06 | 1.91E + 06 | 85 | - | - | - |
| Dioxane | 2.854 | - | - | - | - | - | - | - | - | |
| N,N-Dimethylacetamide | 5.232 | - | - | - | 1.24E + 07 {6} | 6.42E + 06 | 89 | - | - | - |
| 1H,1H,2H,2H-Perfluorodecan-1-ol** | 5.963 | - | - | - | 2.66E + 07 | 1.94E + 07 | 99 | 3.17E + 07 | 2.87E + 06 | 99 |
| Phenol | 6.775 | 6.28E + 04 | 4.50E + 04 | 88 | 7.57E + 06 | 3.06E + 06 | 87 | 4.41E + 06 | 8.40E + 05 | 50 |
| Benzyl chloride | 7.403 | - | - | - | 2.71E + 06 | 2.11E + 06 | 76 | 1.78E + 06 | 6.38E + 04 | 60 |
| Acetophenone | 8.284 | 1.06E + 05 | 1.28E + 04 | 86 | 1.14E + 06 {6} | 7.37E + 05 | 85 | - | - | - |
| Nonanal** | 8.855 | 3.27E + 05 | 1.86E + 05 | 90 | 4.27E + 06 | 6.62E + 05 | 88 | - | - | - |
| Decanal** | 10.41 | - | - | - | 3.57E + 06 | 7.99E + 05 | 85 | - | - | - |
| Tetradecanoic acid | 17.37 | - | - | - | 7.91E + 06 {1} | 3.09E + 06 | 89 | 1.18E + 07 | 7.45E + 05 | 60 |
| 1-Chlorohexadecane | 18.719 | - | - | - | 1.33E + 07 | 2.45E + 06 | 79 | - | - | - |
| n-Hexadecanoic acid** | 19.485 | - | - | - | 2.25E + 07 | 5.70E + 06 | 89 | 2.60E + 07 | 3.39E + 05 | 77 |
| 1-Chlorooctadecane** | 20.731 | - | - | - | 2.20E + 07 | 3.81E + 06 | - | 2.37E + 07 | 1.35E + 06 | 65 |
| Triphenyl phosphate | 23.429 | - | - | - | - | - | - | 5.36E + 06 | 8.38E + 05 | 50 |
| Di-ethyl hexyl phthalate | 24.491 | - | - | - | 9.23E + 06 | 4.16E + 06 | 83 | 9.53E + 06 | 3.82E + 06 | 65 |
| Nonanedioic acid** | 25.76 | - | - | - | 6.18E + 05{7} | 4.04E + 06 | 94 | - | - | - |
| 1,4-Benzene dicarboxylic acid, bis(2-ethylhexyl ester) | 26.137 | - | - | - | 2.79E + 07{6} | 3.79E + 07 | 77 | - | - | - |
Note:
refers to the absence of the particular compound for that condition;
refers to the compound being inherently present in the uncontaminated outer shell material; and “{X}” refers to the number of nondetects for the calculation of that value.
TABLE 6.
Overview of the levels of compounds off-gassing from the thermal liner at various temperatures
| Jacket A and B-100°C | Jacket A and B-200°C | Jacket C-200°C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Compounds | Retention time (min) | SD | Area | % Prob | SD | Area | % Prob | SD | Area | % Prob |
| Benzaldehyde | 6.58 | - | - | - | - | - | - | 4.52E + 06 | 9.20E + 05 | 68 |
| Phenol | 6.775 | 1.22E + 05 | 9.63E + 04 | 76 | 1.13E + 07 | 2.28E + 06 | 77 | 9.08E + 06 | 2.01E + 06 | 70 |
| Octanal | 7.232 | 6.03E + 04 | 1.61E + 04 | 77 | 9.23E + 05 {2} | 5.61E + 05 | 76 | - | - | - |
| 1-methyl-2-pyrrolidinone | 7.932 | - | - | - | - | - | - | 6.16E + 07 | 9.61E + 06 | 82 |
| Acetophenone | 8.284 | 1.49E + 05 | 5.77E + 04 | 72 | 2.41E + 06 {1} | 1.52E + 06 | 78 | - | - | - |
| Nonanal | 8.855 | 2.87E + 05 | 6.49E + 04 | 73 | 1.64E + 06 | 7.39E + 05 | 82 | - | - | - |
| N,3-methyl,2-pyrrolidinone | 7.932 | - | - | - | - | - | - | 1.04E + 07 | 2.45E + 04 | 45 |
| Decanal | 10.41 | 1.50E + 05 | 5.52E + 04 | 79 | 2.68E + 06 {3} | 1.50E + 06 | 71 | - | - | - |
| 1-Phenoxy-propan-2-ol | 11.266 | - | - | - | - | - | - | 3.86E + 08 | 6.83E + 07 | 50 |
| Dodecanoic acid | 15.095 | - | - | - | 2.66E + 06 | 2.45E + 06 | 84 | - | - | - |
| Benzoic acid, 2-ethylhexyl ester | 16.878 | 5.36E + 04 | 2.74E + 04 | 72 | 2.49E + 06 {5} | 1.90E + 06 | - | - | - | - |
| Tetradecanoic acid | 17.37 | - | - | - | 6.66E + 06 | 1.17E + 06 | 77 | 1.85E + 07 | 7.98E + 06 | 75 |
| n-Hexadecanoic acid | 19.485 | - | - | - | 2.41E + 07 | 1.79E + 06 | 83 | 4.49E + 07 | 9.11E + 06 | 67 |
| Di-ethyl hexyl phthalate | 24.491 | - | - | - | 8.69E + 06 | 4.80E + 06 | 75 | 1.01E + 07 | 4.39E + 06 | 45 |
Note:
refers to the absence of the particular compound for that condition.
refers to the number of nondetects for the calculation of that value.
TABLE 5.
Overview of the levels of compounds off-gassing from the moisture barrier at various temperatures
| Jacket A and B-100°C | Jacket A and B-200°C | Jacket C-200°C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Compounds | Retention time (min) | Area | SD | % Prob | Area | SD | % Prob | Area | SD | % Prob |
| Tetrahydrofuran | 2.18 | - | - | - | 1.03E + 07 | 6.25E + 06 | 89 | 2.25E + 07 | 1.24E + 06 | 84 |
| 1,2-Dichloroethane | 2.362 | - | - | - | 4.62E + 07 | 1.23E + 07 | 98 | 5.39E + 07 | 1.54E + 06 | 93 |
| 1H,1H,2H,2H-Perfluorooctan-1-ol | 4.317 | - | - | 1.82E + 07 | 3.63E + 06 | 97 | - | - | ||
| N,N-Dimethylacetamide | 5.232 | 3.97E + 04 {1} | 2.42E + 04 | 80 | - | - | - | - | - | |
| Phenol | 6.775 | 1.66E + 05 | 4.49E + 04 | 75 | 5.16E + 07 | 1.37E + 07 | 69 | 1.06E + 08 | 1.06E + 08 | 55 |
| 1-chloro octane | 8.192 | - | - | - | 4.28E + 06 | 1.50E + 06 | 67 | - | - | - |
| Acetophenone | 8.284 | 1.05E + 05 | 4.93E + 04 | 70 | - | - | - | - | - | - |
| Nonanal | 8.855 | 1.05E + 05 | 2.76E + 04 | 70 | - | - | - | - | - | |
| Decanal | 10.41 | 4.64E + 04 {1} | 2.84E + 04 | 72 | - | - | - | - | - | - |
| 1-phenoxypropan-2-ol | 11.049 | - | - | - | 1.84E + 08 | 2.79E + 07 | 78 | 2.51E + 08 | 6.95E + 06 | 60 |
| Dodecanoic acid | 15.084 | - | - | - | 1.21E + 06 {4} | 1.44E + 06 | 79 | - | - | - |
| Tributyl phosphate | 16.078 | - | - | - | - | - | - | 1.16E + 07 | 5.92E + 05 | 96 |
| Benzoic acid, 2-ethylhexyl ester | 16.876 | - | - | - | 4.51E + 06 {1} | 3.67E + 06 | 80 | - | - | - |
| n-Hexadecanoic acid | 19.485 | - | - | - | 7.30E + 06 | 3.17E + 06 | 68 | - | - | - |
| Triphenyl phosphate | 23.429 | - | - | - | - | - | - | 7.46E + 06 | 9.25E + 05 | 79 |
| Di-ethyl hexyl phthalate | 24.491 | - | - | - | 4.07E + 06 | 3.01E + 06 | 70 | 5.73E + 06 | 1.52E + 06 | 62 |
Note:
refers to the absence of the particular compound for that condition.
refers to the number of nondetects for the calculation of that value.
3.3.1. Outer shell
Tables 4 summarize all the compounds off-gassing from the outer shell material at 100 and 200°C. The values from the replicates have been averaged and the values are averaged across the sampling sites as well for an overview of the outer shell. The area is displayed as a mean of all the replicates. The number of replicates (N) is six for Jackets A and B-100°C, twelve for Jackets A and B-200°C, and six for Jacket C-200°C. The %prob values represent the probability of match for a compound using the NIST 17 Mass spectral library. The NIST library compares the mass spectrum of the compound detected with the standard database of compounds stored in the software.
A wide spectrum of compounds was found to be off-gassing from the outer shell layer of the turnout jacket. 1H,1H,2H,2H-Per-fluorodecan-1-ol, which is a type of fluorinated compound, was found in the unused outer shell material. Fluorinated compounds are expected to be found, since firefighter gear is known to be coated with water repellent-fluorine based finishes. Compounds such as phenol, dioxane, acetophenone, and DEHP are known fireground contaminants, commonly found in household products and triphenyl phosphate is used as a fire-retardant chemical. The presence of these compounds in the outer shell material is justifiable and realistic since the gear was used for extended period in actual fire scenarios. Phenol and acetophenone were the only two compounds that were detected at both, 100 °C and 200°C from the outer shell layer.
3.3.2. Moisture barrier
Table 5 summarizes all the compounds off-gassing from the moisture barrier at 100 °C and 200°C. The values from the replicates have been averaged and the values are averaged across the sampling sites as well for an overview of the moisture barrier. The area is displayed as a mean of all the replicates. The number of replicates (N) is six for Jackets A and B-100°C, twelve for Jackets A and B-200°C, and six for Jacket C-200°C. The %prob values represent the probability of match for a compound using the NIST 17 Mass spectral library. The NIST library compares the mass spectrum of the compound detected with the standard database of compounds stored in the software.
A variety of compounds was found to be off-gassing from the moisture barrier. The moisture barrier layer is known to be treated and coated with chemicals to add functionality. The presence of a fluorinated compound- 1H,1H,2H,2H-Perfluorooctan-1-ol and a plasticizer-DEHP are relevant since these compounds could possibly be a part of the coating formulations. Only five out of the total sixteen compounds were detected at 100°C. This proves that 100°C is probably not a suitable temperature for performing thermal extraction of firefighter gear samples.
3.3.3. Thermal liner
Table 6 summarizes all the compounds off-gassing from the thermal liner material at 100 °C and 200°C. The values from the replicates have been averaged and the values are averaged across the sampling sites as well for an overview of the thermal liner. The area is displayed as a mean of all the replicates. The number of replicates (N) is six for Jackets A and B-100°C, twelve for Jackets A and B-200°C, and six for Jacket C-200°C. The %prob values represent the probability of match for a compound using the NIST 17 Mass spectral library. The NIST library compares the mass spectrum of the compound detected with the standard database of compounds stored in the software.
A wide range of compounds was found to be off-gassing from the thermal liner of the retired turnout jacket. Phenol and DEHP were detected, compounds that are commonly found in household products. 1-phenoxy-propanol-2-ol was detected, which is used in the manufacturing of paints and floor finishes.
3.4. Comparative analysis of off-gassed compounds at 200°C with different variables
Certain compounds were chosen as markers for comparison, based on the level of their toxicity and occurrence in retired jackets. The compounds chosen for outer shell, moisture barrier and thermal liner were phenol and DEHP. The values of the replicates were averaged and the mean responses were plotted. The error bars are plotted as SD values for the replicates.
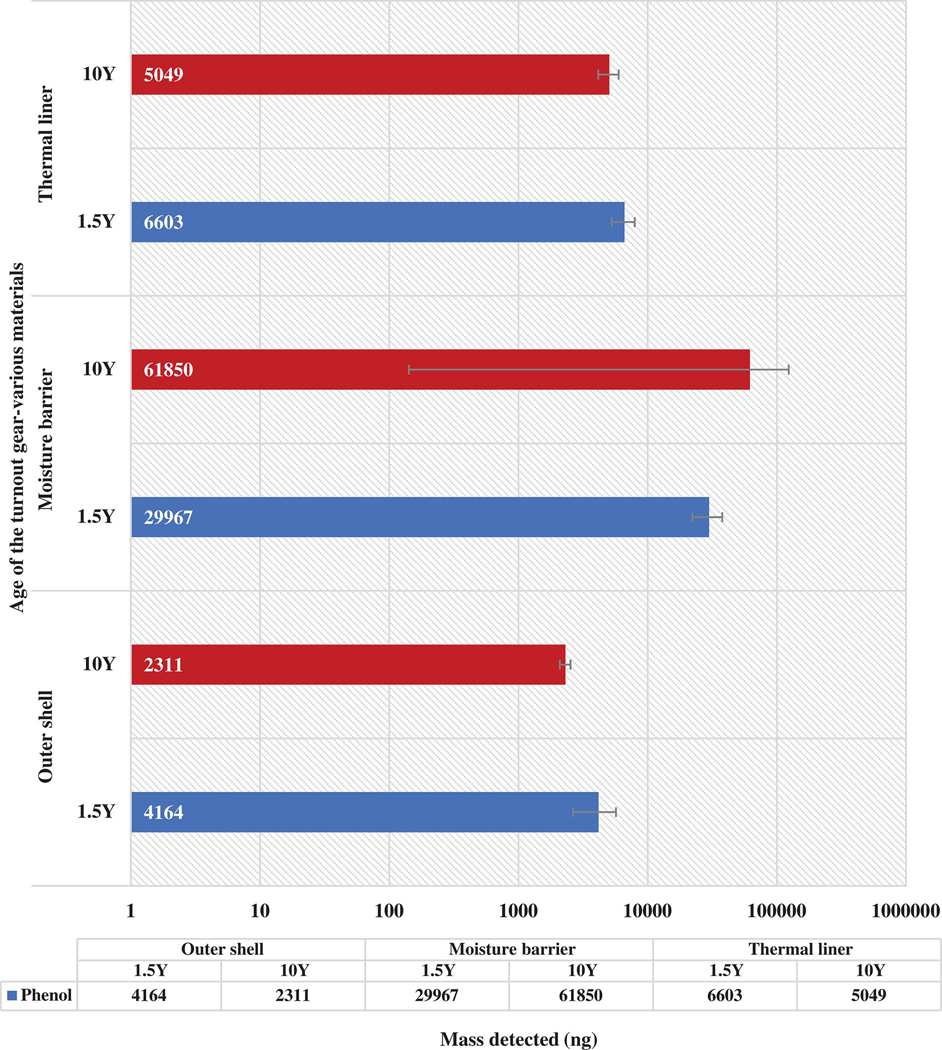
3.4.1. Off-gassing of phenol based on the age of the gear
Figure 7 shows the masses of phenol off-gassing from all the layers of the retired turnout jacket at 200°C. For the outer shell layer, the 1.5Y jacket had almost two times the mass as compared to the 10Y jacket. Phenol, being a relatively volatile compound, does off-gas at lower temperatures as well. Thus, the 1.5Y jacket being used for a shorter period before being retired, had a higher mass of phenol. For the moisture barrier layer, phenol emitted from the 10Y jacket was more than two times that of the 1.5Y jacket. Yet, the error bar for the 10Y jacket covers the entire range for the compound bar itself. This states that certain replicates had extremely low and extremely high masses detected. The replicates taken were not enough to conclude the mass for this condition. For the thermal liner layer, phenol off-gassing from the 1.5Y jacket had more than a 1000 ng higher mass as compared to the 10Y jacket.
FIGURE 7.
Comparison of phenol off-gassing at 200°C from the 1.5Y and 10Y gear
Both, the 1.5Y and 10Y jackets were retired turnout jackets that were stored in enclosed areas for an extended time. The compounds and the levels of compounds off-gassing from these jackets are not representative of the firefighting gear that is in regular use. The exposures to jackets used regularly would be different from the retired gear that is stored for a long period of time. Hence, further research must be done to assess the off-gassing of compounds and the risks associated with it by taking samples from jackets that have been recently exposed to contaminants. The values for the mass detected (ng) have been represented in the logarithmic scale, making it easier to depict visually.
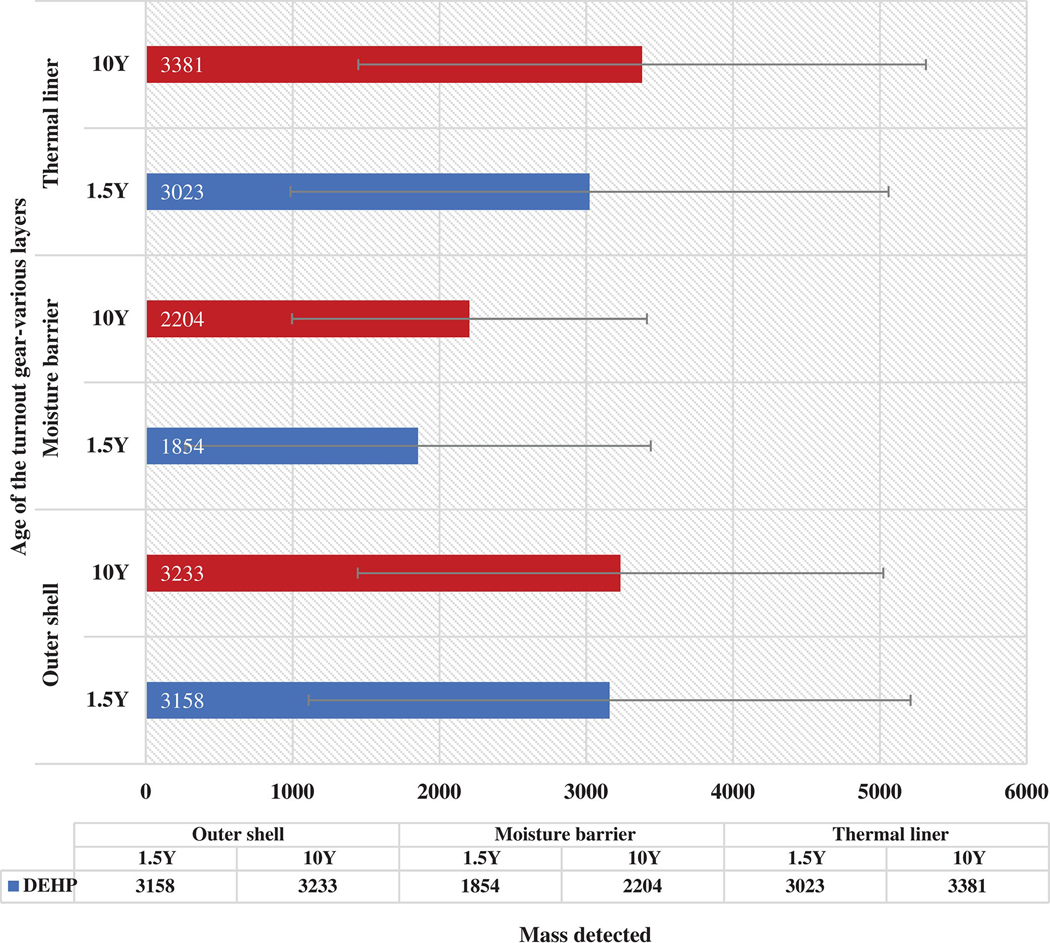
3.4.2. Off-gassing of DEHP based on age of the gear
Figure 8 shows the masses of DEHP off-gassing from the various layers of the turnout jacket at 200°C. For the outer shell material, the mass of DEHP was not significantly different for the 1.5Y and the 10Y jacket. For the moisture barrier and thermal liner, the mass of DEHP was similar for the 1.5Y and 10Y jackets. DEHP is a semivolatile compound having a boiling point of 384°C. Therefore, an exposure of 200°C for 30 minutes was possibly not enough for the complete volatilization of the compound from the layers that were analyzed on the HS. All the bars have large error bars, which implies that a wide range of masses off-gassed within replicates of the same layer. DEHP is a plasticizer that is commonly found in plastics and other synthetic materials. The levels of off-gassing from both the 1.5Y and 10Y jacket being similar, states that either the older (10Y) jacket was not exposed as compared to the 1.5Y. It could also be assumed that the 10Y jacket was effectively decontaminated and DEHP was removed from the jacket.
FIGURE 8.
Comparison of di-ethylhexyl phthalate off-gassing at 200°C from the 1.5Y and 10Y gear
Both, the 1.5Y and 10Y jackets were retired turnout jackets that were stored in enclosed areas for an extended time. The levels of DEHP off-gassing from these jackets does not directly correlate with the levels that would off-gas from a recently used gear. Further data must be collected by analyzing gear that has been exposed recently.
4. CONCLUSION
The data collected and analyzed from over 100 trials gave an insight into the thermal extraction of both volatile and semivolatile compounds from retired firefighting turnout jackets. This study clearly focuses on the thermal extraction of the compounds in the form of off-gassing of compounds from the turnout gear. A temperature of 200°C and an equilibration time of 30 minutes was found to be a suitable condition to perform the thermal extraction of compounds from a contaminated firefighting turnout jacket. It was seen that there was a difference in the amounts that off-gassed between different sampling sites, different layers and service life of the gear. The exploratory research was successful in developing a simple, yet unique method to identify the off-gassing of various types of compounds that are present in firefighters’ gear. The data generated from the study is important and significant, since it directly relates to real-world contamination of firefighter gear and paves a way to further analyze the health risks associated with the chemicals that firefighters are exposed to. Compounds such as DEHP are known 2B carcinogens, whereas fluoro-compounds, phenols, dioxanes, and acids are toxic and pose a threat to the firefighters. The presence of these compounds found through the research justifies that a structural fire contains a mixture of compounds that is unique to each fire. Having the data from control calibration runs, the mass values for marker compounds such as phenol and DEHP were obtained as well. Having an exact value of the masses/ concentrations of toxic compounds, from the firefighter gear is crucial in evaluating the health and occupational risks associated with firefighting and having a better understanding of the conditions and methods to tackle them.
5. FUTURE WORK
This study was exploratory in nature and defined a method to be able to analyze real-world samples that included field-contaminated firefighter jackets. However, the conditions used in the study were limited and could be expanded to include a wider range of temperatures to understand the levels of off-gassing of certain compounds. A temperature profile of commonly found fireground contaminants could be developed that would be helpful in identifying the risks associated with fighting a fire at various conditions. The method developed through this research could also be used to analyze off-gassing from firefighter pants, gloves, hoods, and other ensemble elements. Firefighters’ gear from various geographical regions, service life could be studied to mark differences in compounds that are detected. This could be compared to the materials used in construction, nature of the fires, techniques used in firefighting, and so on. The current headspace GC method could also be used to identify the off-gassing from wildland firefighters’ gear, since their exposures are drastically different as compared to a structural firefighter.
Currently, the only method to decontaminate the turnout gear is to wash with a suitable surfactant in a washer-extractor with about 40% of the contaminants removed from the gear after being washed in accordance to NFPA 1851 Standard on Selection, Care, and Maintenance of PPE for Structural and Proximity Firefighting.15 The turnout gear gets damaged if the conditions of washing are intensified.16,17 This is concerning since it is not completely effective in removing the contaminants and damages the gear as well. The current research on off-gassing concludes that headspace sampling could be used as a screening method to thermally extract the compounds from the contaminated gear. The 200°C condition was found to be useful to assess a variety of compounds off-gassing from all the three layers of the turnout jacket. Yet, compounds such as PAHs which are high boiling and highly carcinogenic were not found to off-gas at high temperatures (200°C). For such compounds, a conventional washing with specific surfactants and other conditions will have to be used. Several other compounds such as dioxins, acids, phthalates, and so on were found to off-gas from the gear and the toxicity of every compound must be further studied in detail to understand the risks associated with them. Since this study proves that a lot of these compounds are present as contaminants in the turnout gear, there could be a risk associated with the dermal exposure through the skin. Hence, not just off-gassed chemicals, but dermal exposure from the outer shell, thermal liner and the moisture barrier to the skin must be studied as well to have a thorough understanding of the risks associated through firefighters’ suits.
ACKNOWLEDGEMENTS
This study was funded as part of the Federal Emergency Management Agency’s (FEMA) Assistance to Firefighters Grant Program, reference number: EMW-2017-FP-00601
Funding information
Federal Emergency Management Agency, Grant/Award Number: EMW-2017-FP-00601
REFERENCES
- 1.Fang JB and Breese JN. Fire development in residential basement rooms. National Bureau of Standards (currently NIST), NBSIR; 80–2120; 1980; Gaithersburg, MD. [Google Scholar]
- 2.Ackerman MY (2011). Thermal decomposition of selected flame resistant clothing materials. AATCC Review: the magazine of the textile dyeing, printing, and finishing industry, 11(3), 35–43. [Google Scholar]
- 3.Baxter CS, Hoffman JD, Knipp MJ, Reponen T, Haynes EN. Exposure of firefighters to particulates and polycyclic aromatic hydrocarbons. J Occup Environ Hyg. 2014;11(7):D85–D91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals. Vol 3. Washington DC: National Academies Press; 2003. [Google Scholar]
- 5.Guidotti TL, Clough VM. Occupational health concerns of firefighting. Annu Rev Public Health. 1992;13(1):151–171. [DOI] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer. IARC working group on the evaluation of carcinogenic risks to humans. Painting, firefighting, and shiftwork. IARC Monograph on Evaluating Carcinogenic Risks to Humans; 2010; 98 (9), p. 764. [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DA, Harrison TR, Yang F, Wendorf Muhamad J, Morgan SE. Firefighter perceptions of cancer risk: Results of a qualitative study. Am J Ind Med. 2017;60:644–650. [DOI] [PubMed] [Google Scholar]
- 8.Queensland Fire and Rescue Service (QFRS): Firefighter exposures to airborne contaminants during extinguishment of simulated office room fires. In: Kirk KM, Ridgway M, Splawinski Z, et al. (Research Report 2011–02). QFRS Scientific Branch; 2011. [Google Scholar]
- 9.Austin CC, Wang D, Ecobichon DJ, Dussault G. Characterization of volatile organic compounds in smoke at municipal structural fires. J Toxicol Environ Health A. 2001;63(6):437–458. [DOI] [PubMed] [Google Scholar]
- 10.Fent KW, Evans DE, Booher D, et al. Volatile organic compounds off-gassing from firefighters’ personal protective equipment ensembles after use. J Occup Environ Hyg. 2015;12(6):404–414. [DOI] [PubMed] [Google Scholar]
- 11.European Society of Human Reproduction and Embryology. https://medicalxpress.com/news/2018-12-chemicals-personal-household-products-linked.html
- 12.J Occup Environ Hyg. 2014;11(7):D85–D91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/ [DOI] [PubMed]
- 14.International Agency for Research on Cancer (IARC) Identification of Carcinogenic Hazards to Humans. https://monographs.iarc.fr/agents-classified-by-the-iarc/
- 15.National Fire Protection Association, Codes and Standards. www.nfpa.org/codes-and-standards/aa-codes-and-standards/list-of-codes-and-standards/details?code=1851
- 16.PPE Cleaning Validation Project Interim Report. Fire prevention research foundation interim report. August 2, 2017. [Google Scholar]
- 17.Cinnamon ML. Post Use Analysis of Firefighter Turnout Gear-Phase III [Master’s Thesis]. University of Kentucky. http://knowledge.uky.edu/cgi/viewcomment/egi?article=1004&context=mat_etds [Google Scholar]