Abstract

Shepherin I is a glycine- and histidine-rich antimicrobial peptide from the root of a shepherd’s purse, whose antimicrobial activity was suggested to be enhanced by the presence of Zn(II) ions. We describe Zn(II) and Cu(II) complexes of this peptide, aiming to understand the correlation between their metal binding mode, structure, morphology, and biological activity. We observe a logical sequence of phenomena, each of which is the result of the previous one: (i) Zn(II) coordinates to shepherin I, (ii) causes a structural change, which, in turn, (iii) results in fibril formation. Eventually, this chain of structural changes has a (iv) biological consequence: The shepherin I–Zn(II) fibrils are highly antifungal. What is of particular interest, both fibril formation and strong anticandidal activity are only observed for the shepherin I–Zn(II) complex, linking its structural rearrangement that occurs after metal binding with its morphology and biological activity.
Short abstract
The coordination of Zn(II) (but not that of Cu(II)) triggers the antifungal properties of shepherin I, a glycine- and histidine-rich antimicrobial peptide from the root of shepherd’s purse. Zn(II) binding causes a structural change, which triggers fibril formation. Fibrils are most likely relevant for establishing a new mechanism of fungal cell wall or membrane disruption.
Introduction
Antimicrobial peptides (AMPs) are small, usually polycationic peptides, isolated from natural sources, showing antibacterial, antiviral, antifungal, and even anticancer activity. As a result, AMPs represent promising alternative agents to overcome increasing antibiotic resistance problems.1 They are also known as host defense peptides due to being essential components of immune response of multicellular organisms.2 So far, more than 3500 AMPs have been reported and are diverse in biological source, activity, structure, and mechanism of action.3
Generally, AMPs have been separated into several groups based on their (i) biological source; (ii) peptide sequence; (iii) secondary structure; (iv) covalent bonding pattern; (v) biosynthesis route; (vi) molecular targets; (vii) antibacterial target; and (viii) high abundance of specific amino acids.4
It is not uncommon for antimicrobial peptides to be rich in one particular amino acid; the antimicrobial peptide database3 contains 117 specific “Xaa-rich” antimicrobial peptides; the most abundant ones are Pro-rich (56), Gly-rich (31), Arg-rich (20), and His-rich (15). Shepherin I is classified as both Gly-rich and His-rich.
The structural arrangement of AMPs is essential to understand their interaction mechanisms with biological targets. AMPs have three main modes of action: interaction with membrane phospholipids leading to their disruption,5,6 intracellular targeting in order to inhibit synthesis of nucleic acids, enzymes, and other crucial proteins,7 and also by less popular types such as molecular electroporation and sinking raft mechanisms8 (The first one uses electric potential to form a pore and disruption of the membrane; this mechanism is possible due to charged properties of peptides. The second one suggests that AMPs accumulate on the membrane and modify its shape; the aggregation of these peptides presses into the bilayer and sinking inside via transitional pore transporting them to the other side of the membrane.).
What is of particular interest in this work is the fact that both Zn(II)9,10 and Cu(II)1,11 are able to change the structure and increase the antimicrobial activity of antimicrobial peptides. Both metal ions are essential micronutrients and, being quite abundant in the human body (most commonly bound to other biomolecules), have to be considered an important AMP enhancement factor. Zinc(II), being bound to ca. 10% of all proteins, is 1 order of magnitude more abundant in the human body than copper, and is normally relatively nontoxic compared to other metals, e.g., copper.12,13
There are two metal-related activities of AMPs: (i) process named “nutritional immunity” which is based on binding metal ions essential for their virulence and life13 and (ii) when metal ions enhance antimicrobial activity of AMPs by changing their structure or/and charge.14 Examples of AMPs for which antimicrobial activity is metal-related are clavanins, pramlintide, calcitermin, or semenogelins.15−18
We focus on shepherin I, isolated from the roots of the plant Capsella bursa-pastoris and its antimicrobial activity in complexes with metal ions. We show how metal ions, such as Cu(II) and Zn(II) influence on the thermodynamic and structural properties of shepherin I, and we suggest the possible mechanisms of their antimicrobial action.
C. bursa-pastoris, also known as shepherd’s purse, is one of the most abundant flowering plants worldwide.19 Despite the fact that it is a plant that is well-known as a widespread and difficult to eradicate weed that grows massively in fields, pastures, and gardens, is has been used in medicine since ancient times.20 It has a huge pool of extremely valuable compounds such as flavonoids, sterols, vitamins, and metal ions necessary for health and well-being. Studies have shown its antihemorrhagic, antibacterial, anti-inflammatory, and anticancer properties.21 Alkaloids and flavonoids of C. bursa-pastoris show high antibiotic potencies and broad antimicrobial spectra. The plant’s antimicrobial properties were for a long time attributed mainly to sulforaphane, isothiocyanate compound, active against Gram-positive bacterium Bacillus anthracis and vancomycin-resistant Enterococci strains,20 until two novel peptides were isolated from this plant, which show activity against Gram-negative bacteria and fungi: shepherin I (Shep I) and shepherin II (Shep II).22
The sequence of shepherin I (Shep I) is quite uncommon and, for the same reason, easy to describe: It consists of 19 Gly, 8 His and 1 Tyr residue, or, in other words, almost 67.9% of its sequence are Gly residues and 28.6% are His residues (Figure 1). This peptide is also characterized by seven repeats of tripeptide GGH (Gly-Gly-His)–moreover six of them are adjacent.23
Figure 1.

Amino acid sequence of shepherin I isolated from C. bursa-pastoris.
Far-UV CD spectra of shepherin I showed no helical structure in 50% trifluoroethanol,22 but β-pleated sheet structures were observed in 60% TFE and 20 mM SDS.23
Glycine has no side chain and is too flexible to participate in the hydrogen bonds required for secondary structure such as α-helices and β-sheets, however, in some cases specific cross-strand pairing with aromatic residues can improve the stabilization of Gly residue in β sheets.24 Nevertheless, literature data indicate that Gly (and also Pro) residues are favored at several positions of some β-turn types.25,26
Previous studies show that the glycine-rich shepherin and its analogues are effective against Gram-negative bacteria including Escherichia coli, Pseudomonas putida, Pseudomonas syringae, and Serratia sp., and against yeast phase-fungi, like Candida albicans, Cryptococcus neoformans, and Saccharomyces cerevisiae. Moreover, the addition of 10 μM ZnCl2 improved the activity of Shep I (and its C-terminal amidated analogue) against some strains of C. albicans (including fluconazole-resistant strain).23
We focus on understanding the thermodynamic and structural properties of Shep I, and its Zn(II) and Cu(II) complexes, in order to understand the correlation with their antimicrobial properties and propose a potential mechanism of action.
Experimental Section
Materials and Methods
H2N-GYGGHGGHGGHGGHGGHGGHGHGGGGHG-COOH (shepherin I, Shep I) peptide (certified purity of 98%) was purchased from KareBay Biochem and used without further purification. Cu(II) and Zn(II) perchlorides were extra-pure products (Sigma-Aldrich); concentration of their stock solutions was determined by ICP–MS. The carbonate-free stock solution of 0.1 M NaOH (Sigma-Aldrich) was potentiometrically standardized with potassium hydrogen phthalate (Sigma-Aldrich). All of the samples were prepared with freshly doubly distilled water. The ionic strength (I) was adjusted to 0.1 M by the addition of NaClO4 (Sigma-Aldrich). All of the samples were weighted out using analytical scale Sartorius R200D.
Mass Spectrometry
High-resolution mass spectra were obtained on Bruker Apex FT-ICR and Shimadzu q-TOF LCMS 9030 spectrometers. Spectrometers were used for measurements on Cu(II) and Zn(II) complexes in the ranges of positive and negative values. The instrumental parameters were as follows: scan range m/z 150–2000; dry gas nitrogen; temperature 170 °C; capillary voltage 4500 V; ion energy 5 eV. The Cu(II) and Zn(II) complexes [(metal/ligand stoichiometry of 1:1) [ligand]tot = 100 μM] were prepared in a 50:50 MeOH/H2O mixture at pH 6. The samples were infused at a flow rate of 3 μL/min. Data were processed by application of the Bruker Compass DataAnalysis 4.0 program and the ACD/Spectrus Processor.
Potentiometry
Stability constants for proton and Cu(II) and Zn(II) complexes were calculated from titration curves carried out over the pH range of 2.50–12.00 at T = 298 K in a total volume of 3 mL. The pH-metric titrations were performed in 0.004 M HClO4 and 0.1 M NaClO4 ionic strength (both ligands are soluble in pure water solution). The potentiometric titrations were performed with a Metrohm 809 Titrando pH-meter titrator provided with Mettler-Toledo glass-body, micro combination pH electrode. The glass cell was equipped with a magnetic stirring system, a micro buret delivery tube, and an inlet–outlet tube for high-purity grade argon in order to maintain an inert atmosphere. Solutions were titrated with 0.1 M carbonate-free NaOH. The electrode were calibrated daily for hydrogen ion concentration by titrating HClO4 with alkaline solution in the same experimental conditions as above. Purities and the exact concentrations of the ligand solutions were determined by the Gran method.27 The ligand concentration was 0.5 mM. The metal-to-ligand ratio was 0.9:1 for metal complexes. The standard potential and the slope of the electrode couple were computed by means of the Glee program.28 HYPERQUAD2006 program was used for the stability constant calculations.29 The constants for hydrolysis of Cu(II) and Zn(II) ions were taken from literature.30,31 The speciation and competition diagrams were computed with the HySS program32 and drawn in OriginPro 2016 program.
Spectroscopy
The absorption spectra in the UV–vis region were recorded at 298 K on a Varian Cary300 Bio spectrophotometer in a 10 mm path length quartz cell. The spectral range was 200–800 nm. Circular dichroism (CD) spectroscopy experiments were performed on a Jasco V-750 spectropolarimeter at 298 K in a 10 mm quartz cell. The spectral range was 190–800 nm. Direct CD measurements (Θ) were converted to mean residue molar ellipticity (Δε) using the Jasco Spectra Manager
Far-UV circular dichroism (CD) spectra were recorded with a JASCO V-715 CD spectropolarimeter at the temperature of 293 K in 0.1 and 0.2 mm path length quartz cells. Every sample contained a peptide at a concentration of 0.3–0.4 mM and 0.9:1 of metal to ligand molar ratio. The samples were prepared in 4 mM HClO4 and 0.1 M NaClO4 ionic strength. In addition, the peptide titration by using of different concentrations of sodium dodecyl sulfate (SDS; [SDS] = from 10 to 200 mM) was measured. Far-UV CD spectra were recorded from 180 to 250 nm for ligands and complexes at selected pH. Appropriate amounts of NaOH or HClO4 solutions were added to change pH values.
Antimicrobial Activity Assay of Peptide and Peptide-Metal Ion Complex System
Antimicrobial activity was performed by using the broth microdilution method with spectrophotometric measurements. Peptide and peptide-metal ion systems were analyzed. Reference strains from the ATCC collection (E. coli 25922, Staphylococcus aureus 43300, Klebsiella pneumoniae 700603, Acinetobacter baumannii 19606, Pseudomonas aeruginosa 27853, Enterococcus faecalis 29212, and C. albicans 10231) were used. The experimental procedure followed the guidelines outlined in ISO standards 20776–1:201933 and 16256:2012,34 along with a modified Richard’s method.35−37
Stock peptide solution was prepared in distilled sterile water at four times concentration higher than the final one. Equimolar concentrations of Cu(II) and Zn(II) ions were added to the peptide. Serial dilutions of the peptide/peptide-metal ion solutions were prepared in 96-well microplates, covering a concentration range of 0.5– 1256 μg/mL. After 24 h incubation at 37 °C for bacteria or 25 °C for fungus, the bacterial and fungal suspensions were prepared to achieve a final inoculum density of 5 × 10q5 CFU/mL (for bacteria) and 0.5–2.5 × 105 CFU/mL (for fungus). A positive controls (TSB + strain) and negative controls (TSB) were also performed. Additionally, a solubility control for each peptide and peptide-metal ion system was also taken into account. To validate the assay, antibacterial/antifungal agents such as levofloxacin (A. baumannii 0.5 μg/mL, E. faecalis 4 μg/mL, P. aeruginosa 1 μg/mL, methicillin-resistant S. aureus (MRSA, 1 μg/mL), gentamicin (E. coli 4 μg/mL, K. pnemoniae 4 μg/mL), or amphotericin B (C. albicans 1 μg/mL), in accordance with the EUCAST examination, were tested against each strain. Microplates were incubated at 37 ± 1 or 25 ± 1 °C for 24 h on a shaker (500 rpm). After the incubation, spectrophotometric measurements were performed at 580 nm. The minimum inhibitory concentration that inhibits the growth of 50% microorganisms (MIC50) was determined by comparing the absorption results of the test samples with the positive control.
Subsequently, 50 μL aliquots of a 1% (m/v) solution of 2,3,5-triphenyltetrazolium chloride (TTC) were added to each well. TTC is a redox indicator used to assess cellular respiration. The colorless TTC is oxidized to pink after reduction due to reactions in the respiratory chain, indicating microbial viability.
By combining the MIC50 results with the modified Richard’s method and the TTC indicator, the minimal bactericidal/fungicidal concentration (MBC/MFC) could be determined. MBC/MFC represents the lowest concentration of the antimicrobial agent required to kill the respective microbial strain, as evidenced by the absence of pink due to the lack of enzymatic reduction to red 1,3,5-triphenylformazan (TPF).
Neutral Red (NR) Cytotoxicity Uptake Assay
NR cytotoxicity uptake investigation was conducted for shepherin I–Zn(II) using human primary renal proximal tubule epithelial cells (RPTEC; ECACC 85011425). Following concentrations were chosen based on results obtained in antimicrobial activity assay: 1, 10, 75, and 125 μM. The experimental procedure followed ISO:10993 guidelines, specifically ISO:10993–5:200938 and ISO/IEC 17025:2005.39 The NR assay protocol from Nature Protocol was employed as a standardized approach.40
The experiment utilized MEMα supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and an appropriate amount of antibiotics (amphotericin B, gentamicin). Stock solution of the Shep I peptide and equimolar concentration of Zn(II) ions were prepared in water and subsequently diluted 100 times in the growth medium. Additionally, solutions of Zn(II) salts were tested to ensure the absence of any potential cytotoxic effects from the metal ions alone. Following the addition of the respective combinations of testing compounds and cells (at a density of 1 × 105 cells/mL) to the wells, the plates were incubated at 37 °C in a 5% CO2 environment for 48 and 72 h.
After removing the medium, each well received 100 μL of NR solution (40 μg/mL), followed by a 2 h incubation at 37 °C. Subsequently, the dye was removed, and the wells were rinsed with phosphate-buffered saline (PBS) and allowed to dry. Next, an NR destain solution (consisting of 1% glacial acetic acid, 50% 96% ethanol, and 49% deionized water, v/v) was added to each well. The plates were then shaken (30 min, 500 rpm) to extract NR from the cells and form a homogeneous solution. The absorbance was measured at 540 nm by using a microplate reader. Untreated cells were considered as a negative control, representing 100% potential cellular growth. Additionally, cells incubated with 1 μM staurosporine were used as a positive control for inducing cytotoxicity.
Atomic Force Microscopy (AFM) Imaging
AFM imaging was performed by using a Dimension V Veeco AFM instrument in the tapping mode with the SSS probe mounted. Morphology of the samples was verified just after their preparation; pH of the samples was set to the value of around 7.40. 30 μL aliquots of the samples were deposited on mica discs, and after a 30 min adsorption period, the samples were rinsed with Milli-Q water and dried. The mean width and height dimensions were calculated based on 50 individual profiles using Gwyddion, an open-source software for scanning probe microscopy data analysis.41
Results and Discussion
Shepherin I Protonation Constants
Based on a series of potentiometric titrations ten deprotonation constants (pKa) were established for Shep I (GYGGHGGHGGHGGHGGHGGHGHGGGGHG) (Table 1, Figure S1). The determined values are in agreement with those found in the literature for similar systems.42−48
Table 1. Deprotonation Constants for Shep I Peptide and Stability Constants for Its Complexes with Cu(II) and Zn(II) Ions in an Aqueous Solution of 4 mM HClO4 with I = 0.1 M NaClO4 at 298 Ka.
| Shep
I |
Shep
I–Cu(II) |
Shep
I–Zn(II) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| species | log βjkb | pKb,c | residue | species | log βjkd | pKae | species | log βjke | pKae |
| [H10L]9+ | 72.20(1) | 5.29 | His | ||||||
| [H9L]8+ | 66.91(2) | 5.76 | His | [CuH8L]9+ | 67.57(5) | ||||
| [H8L]7+ | 61.15(3) | 6.04 | His | [CuH7L]8+ | 63.21(2) | 4.36 | |||
| [H7L]6+ | 55.11(5) | 6.40 | His | [CuH6L]7+ | 58.22(2) | 4.99 | [ZnH6L]7+ | 54.09(3) | |
| [H6L]5+ | 48.71(6) | 6.54 | His | [CuH5L]6+ | 52.42(2) | 5.80 | [ZnH5L]6+ | 48.35(2) | 5.74 |
| [H5L]4+ | 42.17(4) | 6.97 | His | [CuH4L]5+ | 45.92(2) | 6.50 | [ZnH4L]5+ | 42.04(2) | 6.31 |
| [H4L]3+ | 35.20(3) | 7.21 | His | [CuH3L]4+ | 38.54(3) | 7.38 | [ZnH3L]4+ | 34.92(2) | 7.12 |
| [H3L]2+ | 27.99(2) | 8.03 | His | [CuH2L]3+ | 30.74(3) | 7.80 | [ZnH2L]3+ | 26.72(3) | 8.20 |
| [H2L]+ | 19.96(1) | 9.58 | NH2 | [CuHL]2+ | 21.57(4) | 9.17 | [ZnHL]2+ | 17.27(3) | 9.45 |
| [HL] | 10.38(1) | 10.38 | Tyr | [CuL]+ | 11.89(4) | 9.68 | [ZnL]+ | 7.59(3) | 9.68 |
| [CuH–1L] | 1.95(3) | 9.94 | |||||||
| [CuH–3L]2– | –20.00(3) | ||||||||
CL = 0.5 mM; molar ratio M/L = 0.9:1. The standard deviations are reported in parentheses as uncertainties on the last significant figure.
Protonation constants are presented as cumulative log βjk values. β(HjLk) = [HjLk]/([H]j[L]k), in which [L] is the concentration of the fully deprotonated peptide.
pKa = log β(HjLk) – log β(Hj – 1Lk).
Cu(II) and Zn(II) stability constants are presented as cumulative log βijk values. L stands for a fully deprotonated peptide ligand that binds Cu(II) and Zn(II) ions. β(MiHjLk) = [MiHjLk]/([M]i[H]j[L]k), where [L] is the concentration of the fully deprotonated peptide.
pKa = log β(MiHjLk) – log β(MiHj – 1Lk).
Shepherin I–Metal Complexes
To investigate the precise stoichiometry, structural and thermodynamic properties of shepherin I–metal complexes set of experimental methods were used: mass spectrometry (MS), potentiometric titrations, and UV–visible and circular dichroism (CD) spectroscopy.
The mass spectra measurements revealed the stoichiometry of the metal complexes, indicating that only equimolar species were present in solution under the tested experimental conditions (Figures S2 and S3). The most intense signals (m/z) of each systems were identified and assigned to the appropriate species (Table S1). M/z values and isotopic distributions align perfectly with the simulated spectra.
Shep I–Cu(II) complex
Potentiometric measurements revealed the presence of 11 equimolar complex species for the Shep I–Cu(II) system in the pH range of 2.50–12.00. The complex distribution diagram and stability constants values are shown in Figure S4 and Table 1, respectively. A careful study of the obtained experimental potentiometric and spectroscopic results allows for a detailed thermodynamic and structural characterization of the formed species, showing the number and type of coordinated atoms from the peptides as described in the Supporting Information and summarized in Table S2.
At a physiological pH, Cu(II) is bound to a {2Nim} donor set. Deprotonations of subsequent imidazole residues, for which a decrease in the pKa value is observed (compared to the ligand, indicating the binding of the metal ion (Table 1)), and no significant changes in the UV–vis and CD spectra (Figure S5) may suggest the existence of several complex species in equilibrium, in each of which a maximum of two imidazole nitrogens are bound to copper(II). Such type of binding is referred to as polymorphic binding sites (metal can “move back and forth” along such regions). In case of Shep I, the regularly repeating GGH motif (GGHGGHGGHGGHGGHGGH) is an excellent candidate for this type of metal ion binding.42,45
The polymorphic binding mode is an interesting phenomenon used by nature to adjust the outcome of metal coordination to the current physiological requirements. It is observed here for Shep I–Cu(II) at physiological pH, and in numerous other cases, e.g., for Cu(II) complexes with a peptide from snake venom, which contains nine subsequent His residues.45
We compare the stability of copper complexes of the pHG peptide (from snake venom) to the peptide studied in this work, in which the His residues are separated by Gly repeats, on a competition plot, based on the complexes’ stability constants, which is a hypothetical situation in which equimolar amounts of the three reagents are mixed. The comparison shows that pHG has a higher affinity toward Cu(II) than Shep I (Figure 2). There are at least two reasons for such a big difference: (i) In pHG, Cu(II) is bound to 3 imidazole nitrogens, while in the case of Shep I to two nitrogens and (ii) most interestingly, a significant influence of metal ions on the helical structure formation has been observed in molecular dynamic simulations and also in the circular dichroism spectra for the pHG complex, which may orient the His side chains accordingly, making them more accessible for Cu(II) (which is not the case for Shep I, see the section “Impact of Metal Coordination on Shepherin I Structure”).45
Figure 2.
Competition plots for the Cu(II) complexes with Shep I (red) and pHG (cyan; Ac-EDDHHHHHHHHHG-NH2) peptides based on potentiometric data (Table 1 and for pHG taken from ref (45)).
Shep I–Zn(II) Complex
The stability constants for the Zn(II) complexes with Shep I were calculated on the basis of the titration curves recorded in the pH range of 2.50–10.00 (Figure S6). The pH-dependent complex species are described in detail in the SI and summarized in Table S3.
At physiological pH, where the [ZnH3L]4+ species dominate in solution, zinc(II) is bound by up to four histidine imidazoles (suggested on the basis of potentiometric titrations, Figure S6 and Table S3). It is also possible that the so-called polymorphic forms may occur in these complex species, where zinc(II) is bound to two different sets of {2Nim} that are in equilibrium (similar to the Shep I–Cu(II) complex species at pH around 7.40). However, such a coordination mode is also definitely less stable (as in the case of Cu(II) complexes) than that of the complex with the pHG peptide (Figure 3), in which His residues are not separated and bind zinc ion by different donor sets of {3Nim}.44,45
Figure 3.
Competition plots for the Zn(II) complexes with Shep I (red) and pHG (blue; Ac-EDDHHHHHHHHHG-NH2) peptides based on potentiometric data (Table 1 and for pHG taken from ref (45)).
Impact of Metal Coordination on Shepherin I Structure
Far-UV CD spectra of shepherin I show a β-sheet conformation at acidic pH (pH 3.50, and 5.50) (Figure 4). Above pH 7.50, characteristic spectra for random coils were observed.
Figure 4.
Far-UV CD spectra for Shep I in aqueous solution of 4 mM HClO4 with I = 0.1 M NaClO4 strongly depend on pH; optical path length = 0.1 mm. CL = 0.3 mM.
An interesting phenomenon was observed when Shep I was titrated with SDS at pH 5.50–quite surprisingly, the presence of 10 mM SDS increases the amount of β-sheet conformation, instead of inducing a structural rearrangement to an α-helical structure, what would have been expected from the helix-inducing SDS.49 The addition of additional SDS equivalents did not affect the structure (Figure 5).
Figure 5.
Far-UV CD spectra for Shep I in aqueous solution of 4 mM HClO4 titrated by addition of SDS solution at pH 5.50; optical path length = 0.2 mm. CL = 0.3 mM.
Both Cu(II) and Zn(II) slightly enhance the amount of the β-sheet structure in Shep I at acidic pH (Figure 6). At alkaline pH, random coil conformations were observed in both complexes. These results prove that the pH value, and not the addition of metal ions, is crucial for Shep I secondary structure.
Figure 6.

Comparison of far-UV CD spectra at 180–250 nm for shepherin I–metal complexes at acidic (5.50) and alkaline (9.50) pH in aqueous solution of 4 mM HClO4 with I = 0.1 M NaClO4; molar ratio M/L 0.9:1; the optical path length = 0.1 mm; CL = 0.3 mM. The dotted lines correspond to the spectra for the peptide.
In the presence of metal ions at pH 5.50, the positive maximum at ca. 197 nm became much more intense and may suggest the β-pleated sheet(s) stabilization (Figure 6, Table S4). However, the differences in the wavelength for the second maximum (shift from 222.8 to 228.8 nm, Table S4) may suggest that there may be (slightly) different conformations.23
At physiological pH, a mild turbidity was observed in potentiometric and spectroscopic studies; that is why, in order to check the structure/morphology of the metal complexes at pH 7.40 AFM imaging was used (discussed later in the text).
Antimicrobial Activity
Do the thermodynamic stabilities and structural changes correlate with the antimicrobial properties of Shep I and its metal complexes? To answer this question, we carried out broth microdilution and TTC reduction tests. Both methods allowed determination of MIC50, representing the minimum inhibitory concentration at which microbial growth is inhibited for 50% of the tested microorganisms, as well as the MBC, which corresponds to the lowest concentration of a compound/complex required to eradicate a specific microorganism. The study encompassed six bacterial strains and one fungal strain. The comprehensive results of the antimicrobial activity assays are presented in Table 2, showing that, very surprisingly, the only compound for which any biological activity was detected was the Shep I–Zn(II) complex.
Table 2. In Vitro Antimicrobial Activity of Shepherin I with or without Cu(II)/Zn(II) Ions, Presented as MIC50 [μg/mL]a.
| C. albicans ATCC 10231 | E. coli ATCC 25922 | MRSA ATCC 43300 | P. aeruginosa ATCC 27853 | E. faecalis ATCC 29212 | K. pneumoniae ATCC 700603 | A. baumannii ATCC 19606 | |
|---|---|---|---|---|---|---|---|
| Shep I | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| Shep I–Cu(II) | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| Shep I–Zn(II) | 32 | n/d | n/d | n/d | n/d | n/d | n/d |
Significant values are bolded (n/d–not detected).
Shepherin I, in combination with zinc(II) ions, exhibits robust antifungal activity. The MIC50 values for Shep I–Zn(II) were determined to be 32 μg/mL. No antibacterial activity was observed within the tested concentration range, neither for shepherin I, nor for its complexes with metal ions.
Based on the antimicrobial activity results, the cytotoxicity of shepherin I in combination with zinc(II) ions was evaluated against human primary renal proximal tubule epithelial cells (RPTEC). The neutral red (NR) uptake assay was employed, which relies on the ability of living cells to incorporate and bind NR in their lysosomes. The cytotoxicity of the peptide-metal complex was evaluated at two time points: after 48 and 72 h of incubation with the cells. The results obtained from these experiments are presented in Table 3.
Table 3. Cytotoxicity [%] of Shep I–Zn(II) after 48/72 h of Incubation with RPTEC Cell Linea.
| cytotoxicity
[%] |
|||
|---|---|---|---|
| conc. [μM] | 48 h | 72 h | |
| Shep I–Zn(II) | 125 | 12 | 18 |
| 75 | 9 | 7 | |
| 10 | 9 | 0 | |
| 1 | 7 | 0 | |
Determined using the neutral red (NR) uptake assay.
The cytotoxicity assessment reveals that Shep I in combination with zinc(II) ions does not exhibit any significant cytotoxic effects on the RPTEC cell line within the tested concentration range. This finding suggests that the investigated peptide-metal ion complex, which has demonstrated potent antifungal activities, holds promise as a potential therapeutic agent with a favorable safety profile, exhibiting low cytotoxicity toward normal cellular function. The observed cytotoxicity level does not hinder the practical application of this peptide-metal complex.
These findings highlight the potential of Shep I in combination with zinc(II) ions as a promising effective agent for antifungal interventions. We were intrigued to find out why this particular complex, and not the copper(II) complex or the free ligand itself, shows very reasonable antifungal activity. We decided to take a literal “closer look” at this phenomenon, highlighting morphological changes that occur after Zn(II) is bound to Shep I under an atomic force microscope.
Morphology
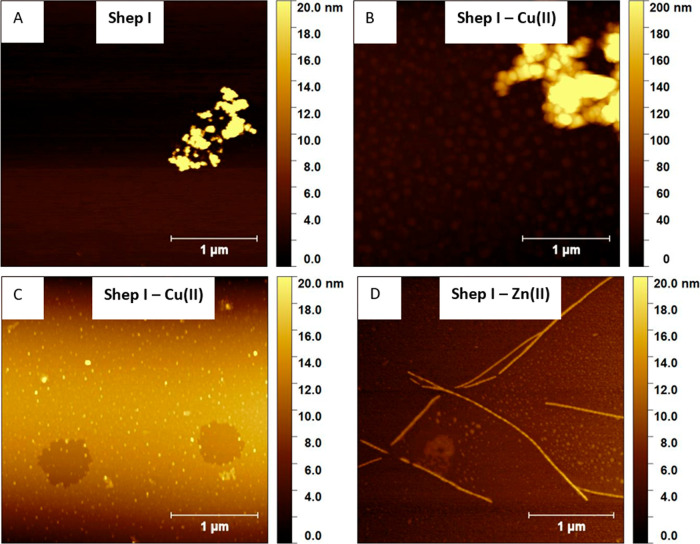
We analyzed the morphology of Shep I and its complexes with Cu(II) and Zn(II). The complexes became mildly turbid at pH 7.4, contrary to the free Shep I sample. Directly after preparation of the Shep I sample, AFM images showed the presence of aggregates that preferred to be associated together instead of being separated (Figure 7A) Similar aggregates were found in Shep I complexes with Cu(II), both in associated and separated forms (Figure 7B,C). Interestingly, for Shep I complexes with Zn(II), already at the initial time point, amyloid fibrils were visible with a mean width of 28.3 ± 5.1 nm and mean height of 6.1 ± 0.9 nm, with crossover distance equal to 46.0 ± 5.0 nm (Figure 7D).
Figure 7.
AFM images of Shep I and its complexes with Cu(II) and Zn(II) ions at pH 7.4.
These observations show that coordination of Zn(II) to Shep I triggers the formation of fibrillar structures, in contrast to Shep I alone or to its complex with Cu(II). This finding is in perfect agreement with the antifungal properties of the Shep I–Zn(II) complex, suggesting a correlation between the complex morphology and antimicrobial activity.
Conclusion
It is estimated that around 2 million people worldwide die each year as a result of fungal infections. C. albicans is the main pathogenic opportunistic fungus in humans and the most serious complication caused by this pathogen is systemic candidiasis, characterized by high mortality and neutropenia.50 In recent years, candidiasis in hospitalized patients infected with the SARS-CoV-2 virus (hospital secondary infection) has also become a severe problem.51
Commonly used antifungal drugs such as triazoles, pyrimidine analogues, echinocandins, and polyenes become less and less effective, especially when the infection involves C. albicans biofilm,52 therefore new drugs are being intensively sought. Commercially available groups of antifungal peptides include 1,3-β-glucan synthesis inhibitors (among them are the commercially available caspofungin, anidulafungin, and micafungin), cell wall chitin inhibitors, peptides which disrupt the fungal membrane and peptides that use more than one mode of action.53 The Shep I–Zn(II) complex, with a fibril-linked mode of action, may become a promising agent in the fight against candidiasis, provided that further studies elucidate the precise underlying mechanisms of their most likely fibril-related antifungal action.
Once again, it becomes clear that there is a significant and underestimated effect of metal coordination on antimicrobial activity. Often, metal binding causes a morphological and/or structural change that triggers a different mode of action, enhancing, or even initiating antimicrobial properties of AMPs.
Recently, we have described a similar effect of Zn(II)-triggered structural change of the antidiabetic (and also antifungal) pramlintide.16 In that case, binding of Zn(II) to the N-terminal amine group and to the imidazole of His18 resulted in a kink of the peptide, which triggered the formation of fibrils, which turned out to have antifungal properties. Although the phenomenon itself was fascinating, the applicable potential of such a complex (with a 256 μM MIC50) was marginal. In the case of shepherin I, we observe a similar scheme of Zn(II) coordination–structural change–fibril formation–anticandidal activity; however, in this case, a considerable potential is definitely present (32 μM MIC50). To the best of our knowledge, in the literature, there is very little information both on (i) how the nature and location of Zn(II) binding residues influence the properties of fibril self-assembly54 as well as on (ii) the antifungal mode of action of amyloid fibrils; they may, as in the case of serum albumin amyloid, disrupt the fungal membrane or cell wall, via interactions with the candidal Als3 cell wall protein.55 Further investigations that aim to explain the mechanism of the fibrils being antifungal and to explore their potential against other clinically relevant fungal strains are absolutely necessary.
Acknowledgments
The work was supported by the National Science Centre (UMO-2017/26/E/ST5/00364 - M.R-Ż. and UMO-2021/41/B/ST4/02654 - J.W.)
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.3c03409.
pH-dependent species distributions, distribution diagrams for Shep I–Cu(II) and Shep I–Zn(II) systems in aqueous solution of 4 mM HClO4, pH-dependent spectra, m/z values for Shep I ligand and complexes with Cu(II) and Zn(II) ions, mass spectra (ESI-MS), pH-dependent spectra, potentiometric and spectroscopic data for proton and Shep I–Cu(II) system, Stability constants, positive band maxima in far-UV CD spectroscopy and a detailed description of the coordination of Cu(II) and Zn(II) ions with Shep I (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Łoboda D.; Kozłowski H.; Rowińska-Zyrek M. Antimicrobial Peptide-Metal Ion Interactions-a Potential Way of Activity Enhancement. New J. Chem. 2018, 42, 7560–7568. 10.1039/C7NJ04709F. [DOI] [Google Scholar]
- Zasloff M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Wang G.; Li X.; Wang Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44 (D1), D1087–D1093. 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulahoum H.; Ghorbani Zamani F.; Timur S.; Zihnioglu F. Metal Binding Antimicrobial Peptides in Nanoparticle Bio-Functionalization: New Heights in Drug Delivery and Therapy. Probiotics Antimicrob Proteins 2020, 12 (1), 48–63. 10.1007/s12602-019-09546-5. [DOI] [PubMed] [Google Scholar]
- Gidalevitz D.; Ishitsuka Y.; Muresan A. S.; Konovalov O.; Waring A. J.; Lehrer R. I.; Lee K. Y. C. Interaction of Antimicrobial Peptide Protegrin with Biomembranes. Proc. Natl. Acad. Sci. U. S. A. 2003, 100 (11), 6302–6307. 10.1073/pnas.0934731100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D. I.; Prenner E. J.; Vogel H. J. Tryptophan- and Arginine-Rich Antimicrobial Peptides: Structures and Mechanisms of Action. Biochimica et Biophysica Acta (BBA) - Biomembranes 2006, 1758 (9), 1184–1202. 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Erdem Büyükkiraz M.; Kesmen Z. Antimicrobial Peptides (AMPs): A Promising Class of Antimicrobial Compounds. J. Appl. Microbiol. 2022, 132 (3), 1573–1596. 10.1111/jam.15314. [DOI] [PubMed] [Google Scholar]
- Miteva M.; Andersson M.; Karshikoff A.; Otting G. Molecular Electroporation: A Unifying Concept for the Description of Membrane Pore Formation by Antibacterial Peptides, Exemplifed with NK-Lysin. FEBS Lett. 1999, 462 (1–2), 155–158. 10.1016/S0014-5793(99)01520-3. [DOI] [PubMed] [Google Scholar]
- Donaghy C.; Javellana J. G.; Hong Y.-J.; Djoko K.; Angeles-Boza A. M. The Synergy between Zinc and Antimicrobial Peptides: An Insight into Unique Bioinorganic Interactions. Molecules 2023, 28 (5), 2156. 10.3390/molecules28052156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydengård V.; Andersson Nordahl E.; Schmidtchen A. Zinc Potentiates the Antibacterial Effects of Histidine-Rich Peptides against Enterococcus Faecalis. FEBS Journal 2006, 273 (11), 2399–2406. 10.1111/j.1742-4658.2006.05246.x. [DOI] [PubMed] [Google Scholar]
- Portelinha J.; Duay S. S.; Yu S. I.; Heilemann K.; Libardo M. D. J.; Juliano S. A.; Klassen J. L.; Angeles-Boza A. M. Antimicrobial Peptides and Copper(II) Ions: Novel Therapeutic Opportunities. Chem. Rev. 2021, 121 (4), 2648–2712. 10.1021/acs.chemrev.0c00921. [DOI] [PubMed] [Google Scholar]
- Sandstead H. Requirements and Toxicity of Essential Trace Elements, Illustrated by Zinc and Copper. Am. J. Clin. Nutr. 1995, 61 (3), 621S–624S. 10.1093/ajcn/61.3.621S. [DOI] [PubMed] [Google Scholar]
- Wątły J.; Potocki S.; Rowińska-Żyrek M. Zinc Homeostasis at the Bacteria/Host Interface-From Coordination Chemistry to Nutritional Immunity. Chem.—Eur. J. 2016, 22 (45), 15992–16010. 10.1002/chem.201602376. [DOI] [PubMed] [Google Scholar]
- Som A.; Yang L.; Wong G. C. L.; Tew G. N. Divalent Metal Ion Triggered Activity of a Synthetic Antimicrobial in Cardiolipin Membranes. J. Am. Chem. Soc. 2009, 131 (42), 15102–15103. 10.1021/ja9067063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.; Matera-Witkiewicz A.; Mikołajczyk A.; Wieczorek R.; Rowińska-Żyrek M. Chemical “Butterfly Effect” Explaining the Coordination Chemistry and Antimicrobial Properties of Clavanin Complexes. Inorg. Chem. 2021, 60 (17), 12730–12734. 10.1021/acs.inorgchem.1c02101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek D.; Dzień E.; Wątły J.; Matera-Witkiewicz A.; Mikołajczyk A.; Hajda A.; Olesiak-Bańska J.; Rowińska-Żyrek M. Zn(II) Binding to Pramlintide Results in a Structural Kink, Fibril Formation and Antifungal Activity. Sci. Rep 2022, 12 (1), 20543. 10.1038/s41598-022-24968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellotti D.; Toniolo M.; Dudek D.; Mikołajczyk A.; Guerrini R.; Matera-Witkiewicz A.; Remelli M.; Rowińska-Żyrek M. Bioinorganic Chemistry of Calcitermin - the Picklock of Its Antimicrobial Activity. Dalton Transactions 2019, 48 (36), 13740–13752. 10.1039/C9DT02869B. [DOI] [PubMed] [Google Scholar]
- Dudek D.; Miller A.; Hecel A.; Kola A.; Valensin D.; Mikołajczyk A.; Barcelo-Oliver M.; Matera-Witkiewicz A.; Rowińska-Żyrek M. Semenogelins Armed in Zn(II) and Cu(II): May Bioinorganic Chemistry Help Nature to Cope with Enterococcus Faecalis ?. Inorg. Chem. 2023, 62, 14103. 10.1021/acs.inorgchem.3c02390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery T.; Lynch K. M.; Arendt E. K. Natural Antifungal Peptides/Proteins as Model for Novel Food Preservatives. Compr Rev. Food Sci. Food Saf 2019, 18 (5), 1327–1360. 10.1111/1541-4337.12480. [DOI] [PubMed] [Google Scholar]
- Choi W. J.; Kim S. K.; Park H. K.; Sohn U. D.; Kim W. Anti-Inflammatory and Anti-Superbacterial Properties of Sulforaphane from Shepherd’s Purse. Korean Journal of Physiology & Pharmacology 2014, 18 (1), 33. 10.4196/kjpp.2014.18.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Snafi A. E. The Chemical Constituents and Pharmacological Effects of Capsella bursa-pastoris - A Review. Int. J. Pharmacol. Toxicol. 2015, 5 (2), 76–81. [Google Scholar]
- Park C. J.; Park C. B.; Hong S.-S.; Lee H.-S.; Lee S. Y.; Kim S. C. Characterization and CDNA Cloning of Two Glycine- and Histidine-Rich Antimicrobial Peptides from the Roots of Shepherd’s Purse, Capsella Bursa-Pastoris. Plant Mol. Biol. 2000, 44 (2), 187–197. 10.1023/A:1006431320677. [DOI] [PubMed] [Google Scholar]
- Remuzgo C.; Oewel T. S.; Daffre S.; Lopes T. R. S.; Dyszy F. H.; Schreier S.; Machado-Santelli G. M.; Teresa Machini M. Chemical Synthesis, Structure-Activity Relationship, and Properties of Shepherin I: A Fungicidal Peptide Enriched in Glycine-Glycine-Histidine Motifs. Amino Acids 2014, 46 (11), 2573–2586. 10.1007/s00726-014-1811-2. [DOI] [PubMed] [Google Scholar]
- Merkel J. S.; Regan L. Aromatic Rescue of Glycine in β Sheets. Fold Des 1998, 3 (6), 449–456. 10.1016/S1359-0278(98)00062-5. [DOI] [PubMed] [Google Scholar]
- Trevino S. R.; Schaefer S.; Scholtz J. M.; Pace C. N. Increasing Protein Conformational Stability by Optimizing β-Turn Sequence. J. Mol. Biol. 2007, 373 (1), 211–218. 10.1016/j.jmb.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E. G.; Thornton J. M. A Revised Set of Potentials for β-Turn Formation in Proteins. Protein Sci. 1994, 3 (12), 2207–2216. 10.1002/pro.5560031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gran G.; Dahlenborg H.; Laurell S.; Rottenberg M. Determination of the Equivalent Point in Potentiometric Titrations. Acta Chem. Scand. 1950, 4, 559–577. 10.3891/acta.chem.scand.04-0559. [DOI] [Google Scholar]
- Gans P. GLEE, a New Computer Program for Glass Electrode Calibration. Talanta 2000, 51 (1), 33–37. 10.1016/S0039-9140(99)00245-3. [DOI] [PubMed] [Google Scholar]
- GANS P.; SABATINI A.; VACCA A. Investigation of Equilibria in Solution. Determination of Equilibrium Constants with the HYPERQUAD Suite of Programs. Talanta 1996, 43 (10), 1739–1753. 10.1016/0039-9140(96)01958-3. [DOI] [PubMed] [Google Scholar]
- Petitt L.IUPAC Stability Constants Database. Chem. Int. 2001, 23 ( (1), ). 10.1515/ci.2001.23.1.18b. [DOI] [Google Scholar]
- Arena G.; Cali R.; Rizzarelli E.; Sammartano S. Thermodynamic Study on the Formation of the Cupric Ion Hydrolytic Species. Thermochim. Acta 1976, 16 (3), 315–321. 10.1016/0040-6031(76)80024-X. [DOI] [Google Scholar]
- Alderighi L.; Gans P.; Ienco A.; Peters D.; Sabatini A.; Vacca A. Hyperquad Simulation and Speciation (HySS): A Utility Program for the Investigation of Equilibria Involving Soluble and Partially Soluble Species. Coord. Chem. Rev. 1999, 184 (1), 311–318. 10.1016/S0010-8545(98)00260-4. [DOI] [Google Scholar]
- ISO 20776-1:2019: Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices - Part 1: Broth Micro-Dilution Reference Method for Testing the in Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases; International Organization for Standardization, 2019.
- ISO 16256:2021: Clinical Laboratory Testing and in Vitro Diagnostic Test Systems - Broth micro-dilution Reference Method for Testing the in Vitro Activity of Antimicrobial Agents against Yeast Fungi Involved in Infectious Diseases; International Organization for Standardization, 2021.
- Gabrielson J.; Hart M.; Jarelöv A.; Kühn I.; McKenzie D.; Möllby R. Evaluation of Redox Indicators and the Use of Digital Scanners and Spectrophotometer for Quantification of Microbial Growth in Microplates. J. Microbiol Methods 2002, 50 (1), 63–73. 10.1016/S0167-7012(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Francisco F. L.; Saviano A. M.; Pinto T. de J. A.; Lourenço F. R. Development, Optimization and Validation of a Rapid Colorimetric Microplate Bioassay for Neomycin Sulfate in Pharmaceutical Drug Products. J. Microbiol Methods 2014, 103, 104–111. 10.1016/j.mimet.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Sabaeifard P.; Abdi-Ali A.; Soudi M. R.; Dinarvand R. Optimization of Tetrazolium Salt Assay for Pseudomonas Aeruginosa Biofilm Using Microtiter Plate Method. J. Microbiol Methods 2014, 105, 134–140. 10.1016/j.mimet.2014.07.024. [DOI] [PubMed] [Google Scholar]
- ISO 10993-5:2009: Biological Evaluation of Medical Devices; Part 5: Tests for in Vitro Cytotoxicity; International Organization for Standardization, 2009.
- ISO/IEC 17025:2017: General Requirements for the Competence of Testing and Calibration Laboratories; International Organization for Standardization, 2017.
- Repetto G.; del Peso A.; Zurita J. L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc 2008, 3 (7), 1125–1131. 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- Nečas D.; Klapetek P.. Gwyddion: An Open-Source Software for SPM Data Analysis. Open Physics 2012, 10 ( (1), ). 10.2478/s11534-011-0096-2. [DOI] [Google Scholar]
- Wątły J.; Simonovsky E.; Wieczorek R.; Barbosa N.; Miller Y.; Kozlowski H. Insight into the Coordination and the Binding Sites of Cu 2+ by the Histidyl-6-Tag Using Experimental and Computational Tools. Inorg. Chem. 2014, 53 (13), 6675–6683. 10.1021/ic500387u. [DOI] [PubMed] [Google Scholar]
- Hecel A.; Wątły J.; Rowińska-Żyrek M.; Świątek-Kozłowska J.; Kozłowski H. Histidine Tracts in Human Transcription Factors: Insight into Metal Ion Coordination Ability. JBIC Journal of Biological Inorganic Chemistry 2018, 23 (1), 81–90. 10.1007/s00775-017-1512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wątły J.; Hecel A.; Wieczorek R.; Rowińska-Żyrek M.; Kozłowski H. Poly-Gly Region Regulates the Accessibility of Metal Binding Sites in Snake Venom Peptides. Inorg. Chem. 2022, 61 (36), 14247–14251. 10.1021/acs.inorgchem.2c02584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wątły J.; Simonovsky E.; Barbosa N.; Spodzieja M.; Wieczorek R.; Rodziewicz-Motowidlo S.; Miller Y.; Kozlowski H. African Viper Poly-His Tag Peptide Fragment Efficiently Binds Metal Ions and Is Folded into an α-Helical Structure. Inorg. Chem. 2015, 54 (16), 7692–7702. 10.1021/acs.inorgchem.5b01029. [DOI] [PubMed] [Google Scholar]
- Perinelli M.; Guerrini R.; Albanese V.; Marchetti N.; Bellotti D.; Gentili S.; Tegoni M.; Remelli M. Cu(II) Coordination to His-Containing Linear Peptides and Related Branched Ones: Equalities and Diversities. J. Inorg. Biochem 2020, 205, 110980. 10.1016/j.jinorgbio.2019.110980. [DOI] [PubMed] [Google Scholar]
- Lakatos A.; Gyurcsik B.; Nagy N. V.; Csendes Z.; Wéber E.; Fülöp L.; Kiss T. Histidine-Rich Branched Peptides as Cu(II) and Zn(II) Chelators with Potential Therapeutic Application in Alzheimer’s Disease. Dalton Trans. 2012, 41 (6), 1713–1726. 10.1039/C1DT10989H. [DOI] [PubMed] [Google Scholar]
- Rola A.; Palacios O.; Capdevila M.; Valensin D.; Gumienna-Kontecka E.; Potocki S. Histidine-Rich C-Terminal Tail of Mycobacterial GroEL1 and Its Copper Complex--The Impact of Point Mutations. Inorg. Chem. 2023, 62 (18), 6893–6908. 10.1021/acs.inorgchem.2c04486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A. K.; Tiwari C.; Ray S.; Holden S.; Armstrong D. A.; Rosengren K. J.; Rodger A.; Panwar A. S.; Martin L. L. Secondary Structure Transitions for a Family of Amyloidogenic, Antimicrobial Uperin 3 Peptides in Contact with Sodium Dodecyl Sulfate. ChemPlusChem 2022, 87 (1), e202100408. 10.1002/cplu.202100408. [DOI] [PubMed] [Google Scholar]
- Macias-Paz I. U.; Pérez-Hernández S.; Tavera-Tapia A.; Luna-Arias J. P.; Guerra-Cárdenas J. E.; Reyna-Beltrán E. Candida Albicans the Main Opportunistic Pathogenic Fungus in Humans. Rev. Argent. Microbiol. 2023, 55 (2), 189–198. 10.1016/j.ram.2022.08.003. [DOI] [PubMed] [Google Scholar]
- Didbaridze T.; Ratiani L.; Labadze N.; Maziashvili T. Prevalence and Prognosis of Candidiasis among Covid-19 Patients: Data from ICU Department. Int. J. Prog. Sci. Technol. 2021, 26, 36–39. 10.52155/ijpsat.v26.1.2940. [DOI] [Google Scholar]
- Costa-de-Oliveira S.; Rodrigues A. G. Candida Albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8 (2), 154. 10.3390/microorganisms8020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejuk A.; Leng Q.; Begum M. D.; Woodle M. C.; Scaria P.; Chou S.-T.; Mixson A. J. Peptide-Based Antifungal Therapies against Emerging Infections. Drugs Future 2010, 35 (3), 197. 10.1358/dof.2010.35.3.1452077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovsky E.; Miller Y. Controlling the Properties and Self-Assembly of Helical Nanofibrils by Engineering Zinc-Binding β-Hairpin Peptides. J. Mater. Chem. B 2020, 8 (33), 7352–7355. 10.1039/D0TB01503B. [DOI] [PubMed] [Google Scholar]
- Gong J.; Bing J.; Guan G.; Nobile C.; Huang G. The Als3 Cell Wall Adhesin Plays a Critical Role in Human Serum Amyloid A1-Induced Cell Death and Aggregation in Candida albicans. Antimicrob. Agents Chemother. 2020, 64 (6), 00024-20. 10.1128/AAC.00024-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.