Abstract
Background:
Multiple organ failure (MOF) is associated with poor outcomes and increased mortality in sepsis and trauma. There are limited data regarding MOF in patients after ruptured abdominal aortic aneurysm (rAAA) repair. We aimed to identify the contemporary prevalence and characteristics of patients with rAAA with MOF.
Methods:
We retrospectively reviewed patients with rAAA who underwent repair (2010-2020) at our multihospital institution. Patients who died within the first 2 days after repair were excluded. MOF was quantified by modified (excluding hepatic system) Denver, Sequential Organ Failure Assessment (SOFA) score, and Multiple Organ Dysfunction Score (MODS) for postoperative days 3 to 5 to determine the prevalence of MOF. MOF was defined as a Denver score of >3, dysfunction in two or more organ systems by SOFA score, or a MODS score of >8. Kaplan-Meier curves and log-rank testing were used to evaluate differences in 30-day mortality between multiple organ failure and patients without MOF. Logistic regression was used to assess predictors of MOF.
Results:
Of 370 patients with rAAA, 288 survived past two days (mean age, 73±10.1 years; 76.7% male; 44.1% open repair), and 143 had data for MOF calculation recorded. From postoperative days 3 to 5,41 (14.24%) had MOF by Denver, 26 (9.03%) by SOFA, and 39 (13.54%) by MODS criteria. Among these scoring systems, pulmonary and neurological systems were impacted most commonly. Among patients with MOF, pulmonary derangement occurred in 65.9% (Denver), 57.7% (SOFA), and 56.4% (MODS). Similarly, neurological derangement occurred in 92.3% (SOFA) and 89.7% (MODS), but renal derangement occurred in 26.8% (Denver), 23.1% (SOFA), and 10.3% (MODS). MOF by all three scoring systems was associated with increased 30-day mortality (Denver: 11.3% vs 41.5% [P < .01]; DOFA: 12.6% vs 46.2% [P < .01]; MODS: 12.5% vs 35.9% [P < .01]), as was MOF by any criteria (10.8% vs 35.7 %; P < .01). Patients with MOF were more likely to have a higher body mass index (55.9±26.6 vs 49.0±15.0; P = .011) and to have had a preoperative stroke (17.9% vs 6.0%; P = .016). Patients with MOF were less likely to have undergone endovascular repair (30.4% vs 62.1%; P < .001). Endovascular repair was protective against MOF (any criteria) on multivariate analysis (odds ratio, 0.23; 95% confidence interval, 0.08-0.64; P = .019) after adjusting for age, gender, and presenting systolic blood pressure.
Conclusions:
MOF occurred in only 9% to 14% of patients after rAAA repair, but was associated with a three-fold increase in mortality. Endovascular repair was associated with a reduced MOF incidence.
Keywords: Aortic aneurysm rupture, Abdominal aortic aneurysm, Multiple organ failure, Outcomes
Ruptured abdominal aortic aneurysms (rAAA) are highly fatal events associated with significant perioperative mortality.1,2 Individual organ failure is frequent and is associated with poor survival, and morbidity and mortality have been postulated to be related in part to multiple organ failure (MOF) after surgical repair of rAAA.3-5 MOF is a syndrome of widespread systemic inflammation that presents clinically as dysfunction across various organ systems. MOF may be the result of various etiologies, but has been associated with worse morbidity and mortality and a prolonged hospital course.6,7 The course of MOF has been studied in patients undergoing resuscitation for hemorrhagic shock and suggests that onset occurs early after the insult.8 Several scoring systems have been developed and validated to quantify MOF, including the Denver score, the Sequential Organ Failure Assessment (SOFA) score, and the Multiple Organ Dysfunction Score (MODS) score.9 These scores were primarily developed and validated on sepsis and trauma patients, but have been applied broadly to other fields.
There is a lack of literature characterizing MOF in the rAAA population, primarily consisting of small series. Most of this data also reflects practices prior to the current use of endovascular aortic repair (EVAR) options and EVAR-first strategies.3,10,11 We sought to use institutional data from a large multinetwork hospital system to review our experience with MOF after rAAA repair and characterize the incidence and outcomes associated with MOF in the context of modern rAAA treatment strategies.
METHODS
Study design.
We conducted a retrospective cohort study review of all patients with rAAA who underwent repair at UPMC. Institutional review board approval for this study was obtained through the University of Pittsburgh Human Research Protection Office (#STUDY19070316). Reporting followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.12
We identified consecutive patients who underwent repair for rAAA at a multihospital integrated regional health care system centered on a quaternary referral hospital from 2010 to 2020. Patients with a previous history of aortic repair or rupture owing to traumatic injury were excluded. The study was landmarked at 2 days to include only patients who survived beyond this time. We based this threshold on previous studies of MOF in the trauma and injury literature that have used this method to control for survivor selection bias in the development of MOF and the difference in factors associated with immediate vs late mortality.1,13 We have also previously shown a bimodal distribution of rAAA mortality around this time period; immediate mortality was associated with hemorrhagic factors, whereas later mortality was associated with factors related to development of MOF.14 Landmarking the cohort resulted in a subset of patients that survived long enough to potentially develop MOF or to recover from the initial inflammatory insult of rAAA and surgical repair.
Data were collected from the electronic medical record and included demographic data (age, sex, race, ethnicity, presenting hospital), physiological parameters at presentation (presenting systolic blood pressure, presenting diastolic pressure, presenting heart rate), preoperative events (stroke, myocardial infarction), operative details (endovascular repair, duration of repair), and postoperative course (length of stay). Data about organ dysfunction—pulmonary, cardiac, nervous, hematological, hepatobiliary, and renal—were collected through postoperative day 5.
MOF.
MOF was defined using three previously published scoring systems: the Denver score, the SOFA score, and the MODS score (Supplementary Table I, online only).9 Given the missingness of data (Supplementary Table II, online only) regarding hepatobiliary function, we modified these scores following similar earlier publications.15-17 We defined MOF by the Denver criteria as a score of >3. MOF by the SOFA criteria was defined as dysfunction for two or more organs on a given day. MOF by MODS criteria was described as a score of ≥8.
Patients were identified as having MOF by the Denver criteria if they met the criteria for MOF by Denver score data available for day 3 to day 5. Similarly, MOF was identified with the SOFA and MODS scores. Because there was considerable overlap, we combined patients meeting any of the criteria as patients with MOF. Patients who did not meet the criteria for MOF or those with missing data were categorized as not having MOF. The primary outcome of our analysis was 30-day mortality.
Statistical analyses.
We used standard statistical tests to describe the entire cohort that met the selection criteria for our analysis and compared patients with MOF and patients without MOF. Continuous variables with parametric distribution were summarized as means with standard deviations and compared using t tests. Continuous variables with a nonparametric distribution were summarized using medians with interquartile ranges (IQR) and compared using the Kruskal-Wallis test. Categorical variables were summarized as frequencies and percentages and compared using the χ2 test.
We used standard statistical methods to describe the grade of dysfunction for individual organs according to the selected scoring systems in patients with MOF as per the given scoring method.
Kaplan-Meier survival analyses with log-rank testing were used to compare survival between patients without MOF and patients with MOF. Adjusted multivariate logistic regression models were used to generate adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for the primary outcome of 30-day mortality. For the model, variables with low missingness were chosen a priori and included age, body mass index (BMI), type of repair, and preoperative comorbidities such as chronic kidney disease, and operative characteristics (type of repair and need for transfer).
Logistic regression models were used to evaluate predictors of MOF, generating ORs. Only clinically relevant variables and low missingness were chosen for the model, initially on univariate analysis and then multivariate analysis.
As an exploratory analysis, we sought to compare differences between patients with MOF patients who experienced 30-day mortality and those who did not. This type of comparison is useful in elucidate factors strongly associated with mortality. A P value of <.05 determined significance for all analyses. Stata SE 15.1 (College Station, TX) was used to perform all statistical analyses.
RESULTS
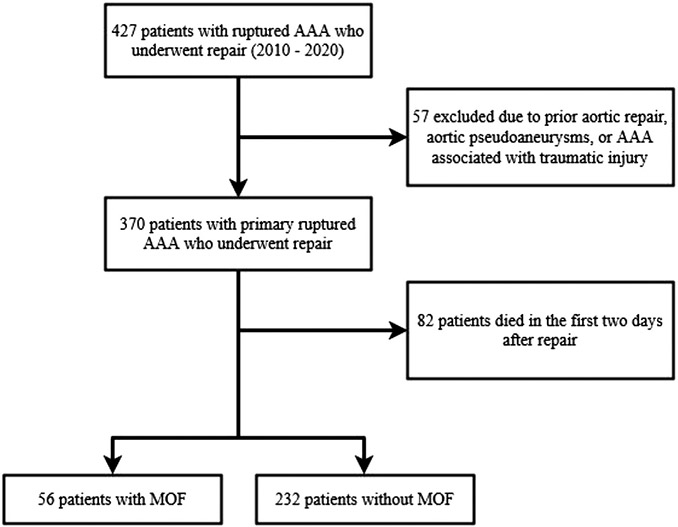
Of 370 patients with rAAA who underwent repair in our network from 2010 to 2020, 288 survived >2 days (Fig 1). From postoperative days 3 to 5, 41 (14.24%) had MOF by the Denver, 26 (9.03%) by SOFA, and 39 (13.54%) by MODS criteria. Overall, 19.4% of patients met the criteria for MOF by any scoring method (Supplementary Fig, online only). Patient demographics, physiological parameters, and operative techniques are summarized in Table I. There was no difference in age, race, and gender between patients with MOF and patients without MOF. There was no significant difference between the groups in terms of BMI (47.8 [44.2-60.4] patients with MOF vs patients without MOF 47.0 [39.7-54.3]; P = .076). Of note, a large proportion (77.4%) of our cohort had a BMI of >40 kg/m2. The distribution of comorbid cardiovascular conditions was similar between the groups, except that patients with MOF were more likely to have a history of stroke (17.9% patients with MOF vs 6.0% patients without MOF; P = .016). Both groups had similar rates of transfer from an outside hospital (patients with MOF, 69.6% vs patients without MOF, 64.2%; P = .44). The proportion of endovascular repair was lower in the patients with MOF group (patients with MOF, 30.4% vs patients without MOF, 62.1%; P < .01). Patients with MOF patients had a longer length of stay (patients with MOF: median, 15 days [IQR, 9-23 days] vs patients without MOF: median, 8 says [IQR, 5-11 days]; P < .01).
Fig 1.
Patient selection flow chart. AAA, abdominal aortic aneurysm; MOF, multiple organ failure.
Table I.
Patient characteristics of patients with and without multiple organ failure (MOF)
| Total (n = 288) | Without MOF (n = 232) | With MOF (n = 56) | P value | |
|---|---|---|---|---|
| Patient age, years | 72.0 [66.0-82.0] | 72.0 [66.0-82.0] | 73.5 [67.0-82.0] | .73 |
| <60 | 24 (8.3) | 21 (9.1) | 3 (5.4) | .64 |
| 60-80 | 175 (60.8) | 139 (59.9) | 36 (64.3) | |
| >80 | 89 (30.9) | 72 (31.0) | 17 (30.4) | |
| BMI | 47.5 [40.5-54.7] | 47.0 [39.7-54.3] | 47.8 [44.2-60.4] | .076 |
| BMI >40 kg/m2 | 223 (77.4) | 176 (75.9) | 47 (83.9) | .19 |
| Male sex | 221 (76.7) | 182 (78.4) | 39 (69.6) | .16 |
| White | 234 (81.3) | 189 (81.5) | 45 (80.4) | .85 |
| Confirmed non-Hispanic ethnicity | 194 (67.4) | 151 (65.1) | 43 (76.8) | .094 |
| Peripheral arterial disease | 13 (5.7) | 11 (5.9) | 2 (4.7) | .75 |
| COPD | 67 (32.1) | 56 (33.3) | 11 (26.8) | .42 |
| Hypertension | 112 (53.6) | 89 (53.0) | 23 (56.1) | .72 |
| Diabetes mellitus | 43 (18.6) | 36 (19.1) | 7 (16.3) | .66 |
| Smoking behavior | 125 (43.4) | 99 (42.7) | 26 (46.4) | .61 |
| Cerebrovascular disease | 24 (8.3) | 14 (6.0) | 10 (17.9) | .016a |
| Coronary artery disease | 112 (38.9) | 90 (38.8) | 22 (39.3) | .48 |
| Chronic kidney disease | 37 (12.8) | 25 (10.8) | 12 (21.4) | .088 |
| Transfer required | 188 (65.3) | 149 (64.2) | 39 (69.6) | .44 |
| Type of repair | <.001a | |||
| EVAR | 161 (55.9) | 144 (62.1) | 17 (30.4) | |
| Open | 127 (44.1) | 88 (37.9) | 39 (69.6) | |
| Length of stay | 9 [5-13.5] | 8 [5-11] | 15 [9-23] | <.001a |
BMI, Body mass index; COPD, chronic obstructive pulmonary disease; EVAR, endovascular aortic repair; PAD, peripheral arterial disease.
The data presented above are median (interquartile range) or number (%).
P < .05 was considered statistically significant.
The degree of derangement for each organ system is summarized in Table II. Among these scoring systems, pulmonary and neurological systems were most commonly impacted. The pulmonary system was most frequently deranged in patients according to the Denver system, which does not account for neurological derangement. Among patients with MOF patients, pulmonary derangement occurred in 65.9% (Denver), 57.7% (SOFA), and 56.4% (MODS) of patients. Similarly, neurological derangement occurred in 92.3% (SOFA) and 89.7% (MODS) of patients, but renal derangement occurred in 26.8% (Denver), 23.1% (SOFA) and 10.3% (MODS) of patients. Hematological derangement and cardiac dysfunction were less commonly prevalent.
Table II.
Organ dysfunction distribution for patients with multiple organ failure (MOF) by each score
| Dysfunction | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Denver score (n = 41) | |||||
| Pulmonary, PaO2/FIO2, mm Hg | 4 (9.8) | 4 (9.8) | 6 (14.6) | 27 (65.9) | – |
| Renal creatinine, μmol/L | 4 (9.8) | 13 (31.7) | 13 (31.7) | 11 (26.8) | – |
| Cardiac inotropes | 15 (36.6) | 11 (26.8) | 11 (26.8) | 4 (9.8) | – |
| SOFA score (n = 26) | |||||
| Pulmonary, PaO2/FIO2, mm Hg | 1 (3.9) | 0 (0.0) | 4 (15.4) | 6 (23.1) | 15 (57.7) |
| Coagulation, platelet count, ×103/μL | 5 (19.2) | 3 (11.5) | 16 (61.5) | 2 (7.7) | 0 (0.0) |
| Cardiac, inotropes in μg/kg/min | 10 (38.5) | 1 (3.9) | 2 (7.7) | 10 (38.5) | 3 (11.5) |
| Renal creatinine, μmol/L | 5 (19.2) | 4 (15.4) | 7 (26.9) | 4 (15.4) | 6 (23.1) |
| Central nervous system, GCS | 1 (3.9) | 0 (0.0) | 0 (0.0) | 1 (3.9) | 24 (92.3) |
| MODS (n = 39) | |||||
| Pulmonary, PaO2/FIO2, mm Hg | 1 (2.6) | 3 (7.7) | 9 (23.1) | 4 (10.3) | 22 (56.4) |
| Renal creatinine, μmol/L | 5 (12.8) | 19 (48.7) | 12 (30.8) | 3 (7.7) | 0 (0.0) |
| Cardiovascular, PAR | 3 (7.7) | 14 (35.9) | 8 (20.5) | 9 (23.1) | 5 (12.8) |
| Coagulation, platelet count, ×103/μL | 4 (10.3) | 13 (33.3) | 10 (25.6) | 8 (20.5) | 4 (10.3) |
| Central nervous system, GCS | 4 (10.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 35 (89.7) |
FIO2, Fraction of inspired oxygen; GCS, Glasgow Coma Scale; MODS, Multiple Organ Dysfunction Score; PaO2, partial pressure of oxygen; PAR, pressure adjusted heart rate; SOFA, Sequential Organ Failure Assessment.
Values are number (%).
Overall, among the 288 patients in the primary cohort, the 30-day mortality was 16.0%. Including the complete cohort of 370 patients, the 30-day mortality in our cohort was 34.6%. On unadjusted Kaplan-Meier survival curves (Fig 2), MOF by all three scoring systems was associated with increased 30-day mortality (Denver: 11.3% vs 41.5% [P < .01]; SOFA: 12.6% vs 46.2% [P < .01]; MODS: 12.5% vs 35.9% [P < .01]), as was patients with MOF by any criteria (10.8% vs 35.7 %; P < .01). MOF remained an independent predictor of 30-day mortality on multivariate analysis (OR, 3.42; 95% CI, 1.47-7.96; P = .004) (Table III).
Fig 2.
Kaplan-Meier survival estimates for patient stratified by multiple organ failure (MOF) according to (A) Denver score, (B) Sequential Organ Failure Assessment (SOFA) score, (C) Multiple Organ Dysfunction Score (MODS) score, and (D) any score.
Table III.
Multivariate logistic regression model for 30-day mortality
| Variable | OR (95% CI) | P value |
|---|---|---|
| MOF | 3.42 (1.47-7.96) | .004a |
| Age | 1.10 (1.05-1.14) | <.001a |
| BMI | 0.99 (0.97-1.02) | .568 |
| Male sex | 1.59 (0.65-3.93) | .312 |
| White race | 0.75 (0.29-1.94) | .550 |
| Cerebrovascular disease | 2.89 (0.94-8.73) | .063 |
| Chronic kidney disease | 2.51 (0.95-6.67) | .064 |
| Coronary artery disease | 1.19 (0.55-2.58) | .654 |
| Transfer | 1.33 (0.58-3.04) | .499 |
| Endovascular repair | 0.57 (0.25-1.33) | .193 |
BMI, Body mass index; CI, confidence interval; OR, odds ratio; MOF, multiple organ failure.
P < .05 was considered statistically significant.
On logistic regression modeling, chronic kidney disease was associated with a greater incidence of MOF (OR, 2.45; 95% CI, 1.06-5.67; P = .037), and EVAR was found to be protective against MOF (OR, 0.19; 95% CI, 0.09-0.39; P < .001) (Table IV).
Table IV.
Predictors of multiple organ failure (MOF) on logistic regression models
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.01 | 0.98-1.04 | .634 | 1.02 | 0.98-1.05 | .308 |
| Male | 0.63 | 0.33-1.21 | .164 | 0.87 | 0.41-1.84 | .712 |
| White | 0.93 | 0.45-1.95 | .849 | 0.88 | 0.39-1.98 | .749 |
| BMI >40 kg/m2 | 1.66 | 0.77-3.60 | .198 | 2.10 | 0.89-4.98 | .092 |
| Cerebrovascular disease | 3.39 | 1.42-8.09 | .006a | 6.19 | 2.23-17.15 | <.001a |
| Chronic kidney disease | 2.26 | 1.05-4.84 | .036a | 2.45 | 1.06-5.67 | .037a |
| EVAR | 0.27 | 0.14-0.50 | <.001a | 0.19 | 0.09-0.39 | <.001a |
BMI, Body mass index; CI, confidence interval; EVAR, endovascular aortic repair; HR, hazard ratio.
P < .05 was considered statistically significant.
The differences between 30-day survivors and nonsurvivors are summarized in Supplementary Table III (online only). Nonsurvivors were significantly older than survivors (82.0 years vs 70.0 years; P < .001). There were no differences in cardiovascular comorbid conditions between the two groups.
DISCUSSION
A large portion of the existing literature and understanding of MOF is derived from clinical entities such as sepsis and trauma.6,18 The association between MOF and extensive requirements for intensive care and hospital resources is well-established in trauma survivors.6,7 The epidemiology of MOF in rAAA domain remains unclear. Our analysis demonstrated an incidence of MOF in 9% to 14% of patients with rAAA who survived the initial postoperative period. In this subset of the rAAA patient population, a three-fold higher mortality rate was observed. Last, endovascular repair was associated with a lower incidence of MOF.
Much research effort, albeit nonvascular, has been generated by MOF, leading to an improved understanding of the mechanisms behind this syndrome. The syndrome is driven by inflammation in the absence of infection. Scoring systems have been devised to standardize definitions of MOF with validation efforts in the trauma literature.9 Crawford et al19 presented their work on the association of higher MOF scores with greater short-term morbidity and worse long-term survival after open mesenteric bypass for chronic mesenteric ischemia. Although this analysis provides valuable data about the derangements after this procedure, only elective operations were included, whereas vascular operations are frequently urgent and emergent. In the rAAA domain, MOF remains poorly characterized, and the little work published draws analogies to tenets of MOF in trauma patients. Tilney et al3 documented the apparent sequence of system failure in their 1974 experience with 18 patients as renal failure followed by pancreatic and pulmonary disease, culminating in gastrointestinal bleeding. Makar et al20 analyzed 14 patients and documented that patients with higher MODS scores were more likely to develop abdominal compartment hypertension. Laukonatous et al1 in their series of 197 patients, used the SOFA score at particular timepoints as predictive of 30-day mortality. Barakat et al21 published the UK experience after ruptures, demonstrating that MOF was more prevalent among patients who experienced in-hospital mortality, but a definition of MOF was not provided. Given that exploring MOF was not the primary aim of any of these studies, little data were present about this syndrome and its predictors. Moreover, no large series—retrospective or prospective—have been published recently to account for the modern advances in endovascular repair and improved understanding of this surgical emergency.3
Any individual organ failure after rAAA repair is associated with a cascade of events that predict poor survival.3 Hepatic dysfunction, acute kidney injury, and colonic ischemia individually predict higher early mortality after aortic rupture.4,5,22 The end-organ damage is driven by an inflammatory cytokine-mediated response rather than a direct ischemic reperfusion injury from aortic cross-clamping.11 Disruptions in the balance of proinflammatory and anti-inflammatory cytokines influence endothelial tone, leading to microvascular changes (such as edema, decreased tissue oxygenation, and intravascular volume depletion) and severe clinical manifestations, such as hypotension and shock.23 Organ-specific effects include impaired cardiac contractility, inflammatory infiltration at the lungs, ischemic impairment of the renal parenchyma, and hypoperfusion of the liver parenchyma.24 With an increasing number of organs experiencing failure, the mortality multiplies, and the involvement of three or more organs makes survival a rare outcome.25
The patient population presenting for rAAA repairs, similar to other vascular operations, is older, predominantly White, and has a high prevalence of comorbid cardiovascular diseases. Analysis of the Vascular Quality Initiative demonstrates a patient demographic and comorbid disease profile similar to our report.2 Moreover, approximately one-half of all patients with rAAA are eligible for endovascular repair.2 In our cohort, endovascular repair was less commonly used in patients who developed MOF; this may represent a selection bias with patients who have more complex anatomy and disease being reserved for open repair in the current decade, a trend associated with increased postoperative mortality and complication rates.26
Owing to the high prevalence of missing data in the retrospective analysis regarding MOF, earlier authors have similarly modified the originally published scores, used varying cut-offs, or used imputation methods.1,6,8,9 The cut-off of a score of >3 for the Denver MOF score has been used before in the trauma literature and was used similarly in our analysis.16 Varying definitions of MOF by SOFA score have been published. Some authors choose to exclude the central nervous system component; however, in light of missing data and lack of a standard definition, we decided to define MOF as organ dysfunction in two or more systems, but excluding the hepatic component.15,17 The original MODS score publication by Marshall et al27 categorized the score and focused on intensive care unit (ICU) mortality for each range. Later works have defined MOF by this system as a score of ≥5 on 2 consecutive days.16 We modified the definition of MOF to a score of 8 on any day owing to lack of longitudinal data, absent preexisting validated cut-offs in the rAAA population, and data suggesting this cut-off offers strong discriminative ability.16 It is important to note that these scores are continuous and their usefulness lies in identifying the onset of the syndrome of MOF and tracking its progression, rather than creating binary definitions for statistical analysis.9 The MODS and SOFA scores have been used in earlier analyses of MOF in patients with rAAA. The MODS score was used by Makar et al20 to define MOF as an outcome in their analysis, and the SOFA score was used by Laukontaus et al1 to classify MOF in an rAAA population managed with open repair. Our analysis reports that a subset of the patients with rAAA develop the syndrome of MOF, and mortality in this cohort was thrice as high as patients without documented evidence of postoperative MOF.
The development of MOF was seen in ≤14% of patients after rAAA operations in our analysis. Our data demonstrate that pulmonary and neurological dysfunction are prevalent in the MOF subpopulation, whereas hematological derangement and cardiac dysfunction are less common. Although a history of cerebrovascular disease was more prevalent in patients with MOF, consistent statistical differences in the neurological components of SOFA and MODS scores were not observed. This result suggests that the presentation was more reflective of the acute vascular insult, rather than chronic disease. A similar trend in cardiac and pulmonary dysfunction was observed in ICU patients from the Deutsche Interdisziplinäre Vereinigung für Intensiv-und Notfallmedizin or German Interdisciplinary Association for Intensive Care Medicine (DIVI) registry in Germany, but a relatively low rate of renal dysfunction was observed in our analysis.15 An analysis of American trends of MOF in ICU patients supports that pulmonary failure is common, but decreasing with time, along with cardiac dysfunction.6 This group of patients, albeit small, spends prolonged periods, requiring mechanical ventilation and a high degree of vigilant and costly care to survive MOF.6 The trauma literature suggests that resuscitation with high ratios of fresh frozen plasma to packed red blood cells offers a survival benefit and less ventilator dependence and presents a further avenue for research and optimization of management of patients with rAAA.28
Patients with rAAA may be treated with an open or endovascular approach. In the era of increasing familiarity with stent grafts, increasingly complex cases are chosen for open repair with limited representation in national databases.29 Open repair is more durable than a stent graft repair with greater freedom from reintervention and rupture, albeit with higher perioperative mortality, especially in cases of rupture.30,31 The Swedish experience suggests that almost 15% of open repairs are associated with mortality within 48 hours of repair.30 A recent 2021 meta-analysis corroborates that the postdischarge mortality difference between open and endovascular repair for rAAA is not significant.32 Endovascular repair is physiologically less stressful and is associated with a lesser inflammatory response.20 Lower cytokine levels and less severe clinical manifestations of inflammation-induced organ damage are observed after endovascular repair.20,33 Our analysis further demonstrates that EVAR is associated with less profound inflammation and, hence, lower rates of MOF. The judicious use of EVAR may also protect patients from the morbidity associated with MOF.
Our study of MOF has some limitations. First, our analysis is retrospective and hence cannot account for residual confounding and variations in clinical data collection. This trend is consistent with previous analyses highlighting the high missingness in the data needed to calculate MOF and the high degree of imputations required to compensate for them.34 Hence, we could not establish a true group of patients without MOF owing to data limitations. Second, compared with the trauma literature, the number of patients included in our analysis is limited owing to the rare nature of the pathology and decreasing incidence.35 However, our analysis presents the first granular description of a large number of patients with rAAA who develop MOF using institutional data.
CONCLUSIONS
MOF develops in a subset of patients after successful rAAA repair and is associated with considerable short-term mortality. Endovascular repair may benefit against the high mortality associated with this syndrome. Further prospective efforts with more robust data and follow-up are necessary to truly understand the natural history of MOF and identify potential intervention targets.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: Single-center retrospective cohort study
Key Findings: We assessed 288 patients with abdominal aortic aneurysm rupture. Multiple organ failure (MOF) was observed in 9% to 14% of patients, depending on the scoring system used. Patients with MOF had worse survival (10.8% vs 35.7%; P < .01). This association was also observed on adjusted analysis (odds ratio, 3.42; 95% confidence interval, 1.47-7.96; P = .004).
Take Home Message: Although MOF is observed in only 9%-14% of patients after repair of abdominal aortic aneurysm rupture, it is associated with a three-fold increase in mortality.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Presented at the Thirty-Sixth Annual Meeting of the Eastern Vascular Society, Philadelphia, PA, September 29-October 1, 2022.
Additional material for this article may be found online at www.jvascsurg.org.
REFERENCES
- 1.Laukontaus SJ, Lepäntalo M, Hynninen M, Kantonen I, Pettilä V. Prediction of survival after 48-h of intensive care following open surgical repair of ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2005;30:509–15. [DOI] [PubMed] [Google Scholar]
- 2.D’Oria M, Hanson KT, Shermerhorn M, et al. Editor’s choice - short term and long term outcomes after endovascular or open repair for ruptured infrarenal abdominal aortic aneurysms in the vascular quality initiative. Eur J Vasc Endovasc Surg 2020;59:703–16. [DOI] [PubMed] [Google Scholar]
- 3.Tilney NL, Bailey GL, Morgan AP. Sequential system failure after rupture of abdominal aortic aneurysms: an unsolved problem in postoperative care. Ann Surg 1973;178:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dovzhanskiy DI, Bischoff MS, Wilichowski CD, Rengier F, Klempka A, Böckler D. Outcome analysis and risk factors for postoperative colonic ischaemia after aortic surgery. Langenbeck’s Arch Surg 2020;405:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermreck AS, Proberts KS, Thomas JH. Severe jaundice after rupture of abdominal aortic aneurysm. Am J Surg 1977;134:745–8. [DOI] [PubMed] [Google Scholar]
- 6.Sauaia A, Moore EE, Johnson JL, et al. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J Trauma Acute Care Surg 2014;76:582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewar DC, Mackay P, Balogh Z. Epidemiology of post-injury multiple organ failure in an Australian trauma system. ANZ J Surg 2009;79:431–6. [DOI] [PubMed] [Google Scholar]
- 8.Minei JP, Cuschieri J, Sperry J, et al. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med 2012;40:1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fröhlich M, Wafaisade A, Mansuri A, et al. Which score should be used for posttraumatic multiple organ failure? - Comparison of the MODS, Denver- and SOFA- Scores. Scand J Trauma Resusc Emerg Med 2016;24:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz DJ, Stanley JC, Zelenock GB. Operative mortality rates for intact and ruptured abdominal aortic aneurysms in Michigan: an eleven-year statewide experience. J Vasc Surg 1994;19:804–15; discussion: 816-7. [DOI] [PubMed] [Google Scholar]
- 11.Bown MJ, Nicholson ML, Bell PR, Sayers RD. Cytokines and inflammatory pathways in the pathogenesis of multiple organ failure following abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2001;22:485–95. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. [DOI] [PubMed] [Google Scholar]
- 13.Bown MJ, Cooper NJ, Sutton AJ, et al. The post-operative mortality of ruptured abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2004;27:65–74. [DOI] [PubMed] [Google Scholar]
- 14.Reitz KM, Phillips AR, Tzeng E, Makaroun MS, Leeper CM, Liang NL. Characterization of immediate and early mortality after repair of ruptured abdominal aortic aneurysm. J Vasc Surg 2022;76:1578–87.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bingold TM, Lefering R, Zacharowski K, et al. Individual organ failure and concomitant risk of mortality differs according to the type of admission to ICU - a retrospective study of SOFA score of 23,795 patients. PLoS One 2015;10:e0134329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauaia A, Moore EE, Johnson JL, Ciesla DJ, Biffl WL, Banerjee A. Validation of postinjury multiple organ failure scores. Shock 2009;31:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fröhlich M, Lefering R, Probst C, et al. Epidemiology and risk factors of multiple-organ failure after multiple trauma: an analysis of 31,154 patients from the TraumaRegister DGU. J Trauma Acute Care Surg 2014;76:921–7; discussion: 927-8. [DOI] [PubMed] [Google Scholar]
- 18.Fan B, Klatt J, Moor MM, et al. Prediction of recovery from multiple organ dysfunction syndrome in pediatric sepsis patients. Bioinformatics 2022;38(Suppl 1):i101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawford JD, Kahn TA, Scali ST, Giles KA, Berceli SA, Huber TS. Characterization and association with outcomes of ischemia-reperfusion events after open mesenteric bypass for chronic mesenteric ischemia. J Vasc Surg 2019;70:e93–4. [DOI] [PubMed] [Google Scholar]
- 20.Makar RR, Badger SA, O’Donnell ME, Loan W, Lau LL, Soong CV. The effects of abdominal compartment hypertension after open and endovascular repair of a ruptured abdominal aortic aneurysm. J Vasc Surg 2009;49:866–72. [DOI] [PubMed] [Google Scholar]
- 21.Barakat HM, Shahin Y, Din W, et al. Perioperative, postoperative, and long-term outcomes following open surgical repair of ruptured abdominal aortic aneurysm. Angiology 2020;71:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aziz F, Patel M, Ortenzi G, Reed AB. Incidence of postoperative deep venous thrombosis is higher among cardiac and vascular surgery patients as compared with general surgery patients. Ann Vasc Surg 2015;29:661–9. [DOI] [PubMed] [Google Scholar]
- 23.Biradar V, Moran JL. SIRS, sepsis and multiorgan failure. In: Fitridge R, Thompson M, editors. Mechanisms of vascular disease: a reference book for vascular specialists. University of Adelaide Press; 2011. [PubMed] [Google Scholar]
- 24.Asim M, Amin F, El-Menyar A. Multiple organ dysfunction syndrome: contemporary insights on the clinicopathological spectrum. Qatar Med J 2020;2020:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Prognosis in acute organ-system failure. Ann Surg 1985;202:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomy BJ, Devlin J, Lala S, et al. Comparison of contemporary and historical outcomes of elective and ruptured open abdominal aortic aneurysm repair. J Vasc Surg 2022;75:543–51. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995;23:1638–52. [DOI] [PubMed] [Google Scholar]
- 28.Spoerke N, Michalek J, Schreiber M, et al. ; Trauma Outcomes Group. Crystalloid resuscitation improves survival in trauma patients receiving low ratios of fresh frozen plasma to packed red blood cells. J Trauma 2011;71(2 Suppl 3):S380–3. [DOI] [PubMed] [Google Scholar]
- 29.Woodford C, El Khoury R, Ramirez JL, et al. Capturing the complexity of open abdominal aortic surgery in the endovascular era. J Vasc Surg 2022;76(6):1520–6. [DOI] [PubMed] [Google Scholar]
- 30.Sari H, David G, Carl-Magnus W. Time distribution of mortality after ruptured abdominal aortic aneurysm repair. Ann Vasc Surg 2022;86:313–9. [DOI] [PubMed] [Google Scholar]
- 31.Yei K, Mathlouthi A, Naazie I, Elsayed N, Clary B, Malas M. Long-term outcomes associated with open vs endovascular abdominal aortic aneurysm repair in a medicare-matched database. JAMA Netw Open 2022;5:e2212081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kontopodis N, Galanakis N, Ioannou CV, Tsetis D, Becquemin JP, Antoniou GA. Time-to-event data meta-analysis of late outcomes of endovascular versus open repair for ruptured abdominal aortic aneurysms. J Vasc Surg 2021;74:628–38.e4. [DOI] [PubMed] [Google Scholar]
- 33.Swartbol P, Truedsson L, Norgren L. The inflammatory response and its consequence for the clinical outcome following aortic aneurysm repair. Eur J Vasc Endovasc Surg 2001;21:393–400. [DOI] [PubMed] [Google Scholar]
- 34.Braasch MC, Halimeh BN, Guidry CA. Availability of multiple organ failure score components in surgical patients. Surg Infect 2022;23:178–82. [DOI] [PubMed] [Google Scholar]
- 35.Abdulameer H, Taii HA, Al-Kindi SG, Milner R. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg 2019;69:378–84.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.