Abstract
Cytauxzoon felis is a tick-borne piroplasmid hemoparasite that causes life-threatening disease in cats. Despite the critical role that ticks play in pathogen transmission, our knowledge regarding the C. felis life cycle remains limited to the feline hosts. Specific life stages of C. felis within the tick host have never been visualized microscopically and previous investigations have been limited to molecular detection by polymerase chain reaction (PCR). Sporozoites are the infectious stage of piroplasmids that are transmitted by ticks. In other tick-borne piroplasmids, sporozoite-based vaccines play a key role in disease prevention and management. We believe sporozoites have similar potential for cytauxzoonosis. Therefore, the objective of this study was to use different molecular and microscopic techniques to detect and evaluate C. felis sporozoites in tick salivary glands (SG). A total of 140 Amblyomma americanum adults that were fed on C. felis-infected cats as nymphs were included for this study. Specifically, dissected SGs were quartered and subjected to C. felis RT-PCR, RNAscope® in situ hybridization (ISH), histology, direct azure staining, and transmission electron microscopy (TEM). Cytauxzoon felis RT-PCR was also performed on half tick (HT) carcasses after SG dissection. Cytauxzoon felis RNA was detected in SGs of 17/140 ticks. Of these, 7/17 ticks had microscopic visualization via ISH and/or TEM. The remaining 10/17 ticks had only molecular detection of C. felis in SGs via RT-PCR without visualization. Cytauxzoon felis RNA was detected solely in HT carcasses via RT-PCR in 9/140 ticks. In ISH-positive tick SGs, hybridization signals were present in cytoplasms of SG acinar cells. TEM captured rare C. felis organisms with characteristic ultrastructural features of sporozoites. This study describes the first direct visualization of any developing stage of C. felis in ticks. Forthcoming studies should employ a combination of molecular and microscopic techniques to investigate the C. felis life cycle in A. americanum.
Keywords: Cytauxzoon felis, Sporozoites, In situ hybridization, Tick, Salivary glands
1. Introduction
Cytauxzoon felis is a tick-borne hemoprotozoan parasite that causes life-threatening disease in cats. The Cytauxzoon genus is classified under the phylum Apicomplexa, order Piroplasmida, and family Theileriidae. Since the first case of fatal cytauxzoonosis was published in 1976 in the United States, reports of C. felis and other Cytauxzoon spp. continue to emerge throughout the world, including Europe, South America, and Asia (Maia et al., 2013; Nentwig et al., 2018; Varshney et al., 2009; Willi et al., 2022; Zou et al., 2019). While tick vectors have not been identified for most Cytauxzoon spp., Amblyomma americanum is recognized as the primary vector for C. felis in the United States. Despite the crucial role that ticks play in pathogen transmission and development, our knowledge regarding the C. felis life cycle is primarily limited to the feline host. In closely related parasites, characterization of life stages in the vector have led to important breakthroughs in disease prevention and management (Goh et al., 2019; Han, 2000; Howick et al., 2019; Perry, 2016; Schetters, 2005). Understanding and utilizing these life stages of C. felis in A. americanum may allow us to overcome several major hurdles in cytauxzoonosis research, which include establishing an in vitro culture system and developing a vaccine.
It is presumed that the life cycle of C. felis in ticks mirrors its closest relative, Theileria, where sexual reproduction occurs in the tick midgut after a blood meal from an infected host. Kinetes then migrate to salivary glands where they undergo sporogony and mature into infective sporozoites (Jalovecka et al., 2018; Mehlhorn and Schein, 1993; Tarigo et al., 2013). Sporozoites represent an important interface between the tick and mammalian hosts. For this reason, they are frequently viewed as a key target in developing vaccines and therapeutics for other closely related apicomplexan parasites. In Theileria spp., tick-derived sporozoites have been widely used to produce vaccines, initiate in vitro culture (Baldwin et al., 1988; Brown et al., 1973), discover new therapeutics (Shaw, 1999) and investigate disease pathogenesis (Morrison et al., 1996; Ramsay et al., 2013; Shaw et al., 1991). To date, no stage of C. felis has been definitively identified in ticks, including sporozoites, which limits our ability to pursue similar avenues for Cytauxzoon research.
Traditional detection methods for Theileria sporozoites include direct staining of whole salivary glands, immunohistochemistry (IHC), or transmission electron microscopy (TEM). There is limited published data on this front in C. felis research. Microscopic features of whole salivary glands from C. felis-infected A. americanum were previously described, but sporozoites were not definitively identified (Nagamori, 2016). Transmission electron microscopy of C. felis-infected salivary glands has also been attempted with inconclusive results (personal communication with Dr. Mason Reichard, Oklahoma State University). To confirm the location of parasites, additional in situ molecular techniques like IHC may be required. IHC requires monoclonal antibodies or polyclonal antisera that specifically react with the pathogen (Ramos-Vara and Miller, 2014). Unfortunately, these resources are not readily available for C. felis. In situ hybridization (ISH) is a viable alternative detection method and target probes are easier to produce and customizable through many commercial manufacturers (Maes et al., 2014; Wang et al., 2012). Additionally, ISH was successfully used in previous studies to label C. felis in both feline and tick tissue sections (Khana et al., 2018; Yang et al., 2022).
The objective of this study was to detect C. felis in salivary glands of adult A. americanum ticks that were fed on C. felis-infected cats as nymphs. We evaluated four different microscopic techniques to visualize C. felis in this study – direct staining of whole salivary glands, histology, ISH, and TEM in adult ticks.
2. Materials and methods
2.1. Ticks
Amblyomma americanum nymphs were obtained from the Oklahoma State University (OSU) Tick Rearing Facility (Stillwater, OK, USA). Prior to SG evaluation, ticks were maintained as recommended by the tick rearing facility in a humidity chamber with 90–99% relative humidity at 20–23 °C and a 12 h light/12 h dark photoperiod.
Acquisition feeding of A. americanum nymphs on C. felis-infected cats was performed as previously described (Thomas et al., 2018). Post-feeding, these nymphs were allowed to molt into adults. One hundred and forty of these ticks were evaluated. Eighty of the ticks were unfed (42 females and 38 males) and 60 ticks (30 females and 30 males) were placed into artificial feeding chambers for 12–72 h prior to SG dissection. Roughly equal numbers of males and females were included in each group to mimic the experimental tick transmission model (Thomas et al., 2018). Negative controls consisted of 36 unfed A. americanum adults (18 females, 18 males) that were purchased from OSU (fed on specific pathogen-free sheep as nymphs) and had not been acquisition-fed on C. felis-infected cats as nymphs. Feeding chambers were assembled following a previously described protocol with minor modifications (Fourie et al., 2019; Kröber and Guerin, 2007). Our modifications included the use of cat hair as an attachment stimulus and the feeding units were floated on a 37 °C water bath. Sheep blood (Hemostat laboratories, Dixon, CA, USA) was used for feeding and was changed every 12 h during the feeding period. Since either heat or feeding had been shown to stimulate sporogony (Lewengrub et al., 2011; Young et al., 1984), all ticks that were placed in the feeding chamber regardless of status of attachment were collected for evaluation. During collection, ticks were categorized as “attached” based on either physical attachment to the membrane at time of collection or if blood was present in the midgut during dissection.
Ticks were surface sterilized with 3% hydrogen peroxide, 5.25% sodium hypochlorite (household bleach) then 70% ethanol as previously described (Mani et al., 2012) and dissected for SG removal. Each pair of SG were quartered and placed separately in fixatives or reagents for RT-PCR, direct staining, histology preparation, and TEM (Fig. 1). After SGs were removed, the corresponding tick carcasses were also halved and stored in RNAlater™ (Invitrogen, Waltham, MA, USA) in −80°C until RNA extraction.
Fig. 1.

Experimental design. Each pair of Amblyomma americanum salivary glands (SG) were quartered and quarters were randomly subjected to one of four methods for the detection of C. felis: RT-PCR, direct azure staining, histology and in situ hybridization (ISH), and transmission electron microscopy. (RT-PCR: Reverse transcription polymerase chain reaction; H&E: Hemotoxylin and eosin stain).
2.2. C. felis reverse transcription PCR
Quartered SG and half tick (HT) carcasses from each tick were tested for the presence of C. felis 18S rRNA via two-step RT-PCR as previously described (Yang et al., 2022). Briefly, total RNA was extracted from tick tissues using the RNeasy Plus Micro kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 10 μl of extracted RNA using Taqman Reverse Transcription Reagents (Applied Biosystems, Foster City, CA, USA) and used as template in a SYBR™ green real-time PCR assay as previously described (Bio-Rad, Hercules, CA, USA). CFX96™ Manager Software was used to analyze amplification and melt curves (Schreeg et al., 2016).
2.3. RNAscope in situ hybridization and histology
2.3.1. Salivary gland processing for histology and ISH
Histologic sections of SG were prepared for standard histologic examination and RNAscope® ISH. Briefly, dissected SGs were fixed either in Bouin’s solution (n = 80) or 10% neutral-buffered formalin (NBF) (n = 60) for 24 h. Due to some non-specific background that was present in the Bouin’s fixed SGs during the ISH assays (Fig. 2), fixative was changed from Bouin’s solution to NBF. After fixation, SGs were washed in 70% ethanol, then embedded in molten Histogel™ in a cryomold until solidification. Histogel™ blocks with SG were then placed in tissue cassettes to undergo standard histologic processing, paraffin embedding, and sectioning. At least 12 serial sections (5 μm thick) were produced per SG for histologic examination or ISH. Specific usage and locations on the slides for each serial section are depicted in Fig. 3. Slide A was stained with hematoxylin and eosin (H&E) and slides B, C, D were used for ISH. If adequate tissue samples were not present on slide D, additional sections were used which may not be serial to the sections on the previous slide.
Fig. 2.

RNAscope ISH assays targeting 18S rRNA (red) and GAPDH mRNA (brown) of Cytauxzoon felis. (a, b) Positive controls using formalin-fixed paraffin embedded lung tissue from cats with cytauxzoonosis. Robust hybridization signals for C. felis schizonts are abundantly present within the pulmonary vein (asterisk) as well as scattered along the alveolar septa (arrows), (c–f) Salivary glands collected from non-infected Amblyomma americanum adults with respective ISH probe applied. No hybridization signals are observed within any SG acinar cells in all sections. Bouin’s-fixed SGs (c, d) had non-specific chromogen uptake along the salivary ductal linings (arrowheads), and this background staining was substantially reduced in formalin-fixed SGs (e, f).
Fig. 3.
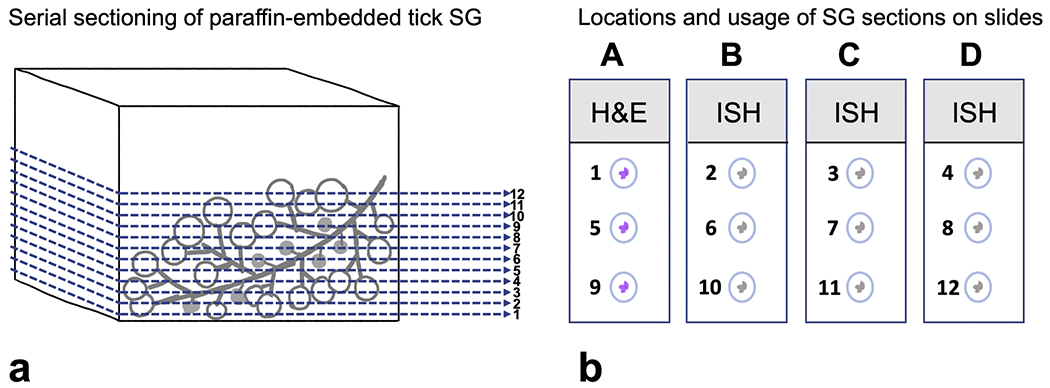
Representative illustration of histologic processing and usage of Amblyomma americanum salivary gland (SG). (a) Paraffin-embedded SG were sectioned sequentially, producing at least 12 sections per tissue block. The seriality of sections are illustrated in numerical order. (b) Serial sections were distributed on 4 slides (labeled A–D) and subjected to either routine H&E staining or ISH as shown. (H&E: Hemotoxylin and eosin; ISH: in situ hybridization).
2.3.2. RNAscope in situ hybridization assays
RNAscope® 2.5 HD red ISH assay (Advanced Cell Diagnostics; Newark, CA, USA) was performed on slides B and C using a proprietary antisense probe targeting C. felis 18S rRNA (Cf-18S-rRNA) as previously described. If C. felis was detected on slide B or C, RNAscope® 2.5 HD brown ISH assay was performed on slide D using a proprietary antisense probe targeting C. felis GAPDH mRNA (Cf-GAPDH). Positive controls consisted of formalin-fixed paraffin-embedded tissues from cats diagnosed with acute cytauxzoonosis, and negative controls consisted of SGs collected from A. americanum adults that had not been acquisition-fed on C. felis-infected cats as nymphs (Fig. 2). RNAscope® ISH assays were performed according to manufacturer’s guidelines with slight modifications after optimizing the pretreatment conditions for salivary glands in different fixatives. For NBF-fixed SG sections target retrieval time of 15 min and protease plus treatment of 20 min were used. For Bouin’s-fixed SG sections, target retrieval time of 10 min and protease plus treatment of 30 min were used.
2.4. Azure staining of salivary glands
To prepare SGs for direct staining, under a dissection microscope, quartered SG was spread out and air-dried on a clean glass slide (Fish-erbrand™ Premium Superfrost™). Dried SGs were then fixed with 100% methanol for 5 min and stained on the slide by covering the section with 0.1% aqueous azure solution (Sigma Aldrich, St. Louis, MO, USA) for 30 s to 1 min. Stained SG sections were then examined under a light microscope at 100X–400X magnification.
2.5. Transmission electron microscopy
Immediately after dissection, quartered SGs were stored in Karnovsky’s fixative (4% paraformaldehyde and 1% glutaraldehyde in 0.1 M sodium cacodylate buffer) at 4°C for up to 3 mo. Salivary glands were processed for TEM following standard procedures with slight modifications (Graham and Orenstein, 2007). Briefly, SGs were removed from the fixative, washed 3 times (10 min per wash) in 0.1 M sodium cacodylate buffer, then placed in 2% osmium tetroxide for one hour. Following osmication, SGs were washed again with 0.1 M sodium cacodylate buffer and subjected to serial dehydration in 70%, 90%, 95% and twice in 100% ethanol for 10 min each. SGs were sequentially submerged in 100% ethanol-Epon resin mixtures (2:1 ratio overnight, 1:1 ratio for 2 h, 1:2 ratio for 2 h, then 100% resin for 2 h), then flat embedded in resin with accelerator added in a small petri dish and polymerized in 70°C drying oven overnight. Specimens were then mounted onto blank blocks with Loctite adhesive and allowed to dry. Ultrathin sections (85 nm) were cut with a Leica EM UC7 ultramicrotome using a Diatome diamond knife, collected on 50 mesh formvar/carbon coated grids and post-stained with uranyl acetate and lead citrate. Sections were imaged in a Hitachi HT7800 transmission electron microscope operated at 80 kV.
2.6. Statistical analysis
Total numbers of C. felis-positive ticks via any molecular detection method (PCR in SG or HT, or ISH) were compared between the unfed group and the group placed in feeding chambers using Fisher’s Exact tests (Sokal and Rohlf, 1995). Significance level was set at 0.05 with a 95% confidence interval.
3. Results
A total of 140 ticks were evaluated for this study. Twenty-seven ticks (11 unfed, 16 exposed to feeding chamber) with positive test results are described in Table 1. Cytauxzoon felis RNA was detected in SGs of 17 ticks. Seven of these ticks had microscopic visualization via ISH and/or TEM. The remaining 10 ticks had only molecular detection of C. felis via RT-PCR without visualization. Nine additional ticks did not have C. felis detected in SGs but had C. felis RNA detected by RT-PCR solely in their HT carcasses after SG removal. One tick was positive for azure staining but C. felis was not detected via any molecular techniques.
Table 1.
Summary of Amblyomma americanum adults with positive Cytauxzoon felis test results in their salivary glands.
| Tick | F/M | SG PCR | HT PCR | SG ISH | Azure staining | TEM | A/NA | |
|---|---|---|---|---|---|---|---|---|
| Unfed | 1 | F | + | + | + | + | + | |
| 2 | F | − | + | − | + | |||
| 3 | F | − | + | − | − | |||
| 4 | F | + | + | − | − | − | ||
| 5 | F | − | + | − | − | |||
| 6 | F | + | − | − | − | |||
| 7 | F | − | − | + | − | |||
| 8 | M | + | − | + | + | − | ||
| 9 | M | − | + | − | − | |||
| 10 | M | − | + | − | − | |||
| 11 | M | + | − | − | − | |||
|
|
||||||||
| Feeding chamber | 12 | F | + | − | + | − | NA | |
| 13 | F | + | − | − | − | A | ||
| 14 | F | + | − | − | − | + | NA | |
| 15 | F | + | + | + | + | + | NA | |
| 16 | F | + | + | − | − | − | A | |
| 17 | F | + | + | + | − | NA | ||
| 18 | F | + | − | − | − | NA | ||
| 19 | F | + | − | − | − | NA | ||
| 20 | F | + | + | − | − | NA | ||
| 21 | M | − | − | − | + | NA | ||
| 22 | M | − | + | − | − | NA | ||
| 23 | M | + | + | − | − | A | ||
| 24 | M | − | + | − | − | NA | ||
| 25 | M | − | + | − | − | NA | ||
| 26 | M | − | + | − | − | NA | ||
| 27 | M | + | − | − | − | NA | ||
F: Female; M: Male; SG: Salivary glands; HT: Half tick; ISH: in situ hybridization; TEM: transmission electron microscopy; A: Attached; NA: Non-attached.
Of the 26 ticks where we detected C. felis RNA, the results of the three molecular detection techniques did not always agree, and only three ticks were positive for C. felis RNA via all methods. A summary of the agreement between the three molecular assays is outlined in Table 2. No significant difference in C. felis RNA detection was found between the unfed ticks and the ticks that were placed in the feeding chambers (p = 0.12).
Table 2.
Summary of agreement between molecular detection of Cytauxzoon felis in salivary glands of Amblyomma americanum adults (SG RT-PCR, HT RT-PCR, and ISH).
| Total examined | Total negative | Total positives | SG PCR (+) HT PCR (+) ISH (+) |
SG PCR (+) HT PCR (+) ISH (−) |
SG PCR (+) HT PCR (−) ISH (−) |
SG PCR (+) HT PCR (−) ISH (+) |
SG PCR (−) HT PCR (+) ISH (−) |
SG PCR (−) HT PCR (−) ISH (+) |
SG PCR (−) HT PCR (+) ISH (+) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unfed | F | 42 | 35 | 7 | 1 | 1 | 1 | 0 | 3 | 1 | 0 |
| M | 38 | 34 | 4 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | |
| Total | 80 | 69 | 11 | 1 | 1 | 2 | 1 | 5 | 1 | 0 | |
|
|
|||||||||||
| Feeding chamber | F | 30 | 21 | 9 | 2 | 2 | 4 | 1 | 0 | 0 | 0 |
| M | 30 | 24 | 6 | 0 | 1 | 1 | 0 | 4 | 0 | 0 | |
| Total | 60 | 45 | 15 | 2 | 3 | 5 | 1 | 4 | 0 | 0 | |
F: Female; M: Male; SG: Salivary glands; HT: Half tick; ISH: in situ hybridization.
Light microscopy (H&E-stained SG histology and azure-stained whole SGs) did not reveal features that could be definitively associated with C. felis infection in tick SGs. However, we were able to partially characterize parasite morphology using TEM. Detailed results of each microscopic evaluation are described below.
3.1. Histology and RNAscope® ISH
Cytauxzoon felis 18S rRNA was detected in SGs via ISH in six out of 140 ticks (4.3%).
Hybridization signals were detected in rare SG acini. Within these infected acini, C. felis was not detected in every cell. In four ticks, signals were limited to single cells of the infected acini; while in the other two ticks, signals appeared to be present in 2–3 acinar cells. Positive signals were restricted to cytoplasm of the infected cells. We were able to corroborate the presence of C. felis by the detection of an additional ISH target (C. felis GAPDH mRNA) in four of six SGs that were positive for C. felis 18S rRNA by ISH. (Figs. 4, 5, and Supplemental Fig. 1). Failure to detect C. felis GAPDH mRNA in the remaining two ticks was correlated with the absence of the infected cells being captured in subsequent serial sections (Supplemental Fig. 1). Positive signals were not detected in any SG acinar cells from negative control ticks. Occasional chromogen uptake was noted in the tracheal and SG ductal linings of the Bouin’s-fixed SGs from both positive and negative ticks. This background staining was substantially reduced with NBF-fixed SGs (Fig. 2).
Fig. 4.

Bouin’s-fixed Amblyomma americanum salivary gland, capturing Cytauxzoon felis-infected acini using H&E corresponding with two RNAscope ISH probes (Cf-18SrRNA and Cf-GAPDH) in serial sections (arrows, 5–8). Locations on the slide of the demonstrated SG sections are indicated in the top left diagram. There is moderate non-specific background staining mainly along the salivary duct linings (arrowheads). 20X (H&E: Hemotoxylin and eosin; ISH: in situ hybridization).
Fig. 5.

NBF-fixed Amblyomma americanum salivary gland, capturing a Cytauxzoon felis-infected acinus using two RNAscope ISH probes (Cf-18SrRNA and Cf-GAPDH) in serial sections (arrows, 5–8). There is less background staining in the salivary ducts compared to Bouin’s-fixed samples. Locations on the slide of the demonstrated SG sections are indicated in the top left diagram. 20X (H&E: Hemotoxylin and eosin; ISH: in situ hybridization. NBF: neutral-buffered formalin).
When corresponding these ISH-positive cells with H&E-stained serial sections, the hybridization signals paralleled with acinar cells that have a hazy pale eosinophilic to amphiphilic cytoplasm and enlarged nuclei with dispersed chromatin (Fig. 4). No distinct nuclear features of parasites were observable in the cytoplasm of these infected cells. None of the affected acini appeared enlarged when compared to adjacent non-infected acini. These microscopic features were also more apparent on Bouin’s-fixed SGs compared to NBF-fixed SGs (Figs. 4 and 5).
Of the six SGs that were C. felis ISH-positive, three were unfed and three were exposed to the feeding chamber. The three ticks that were exposed to the feeding chamber were categorized as non-attached at the time of collection.
3.2. Azure staining of whole salivary glands
Three of the unfed ticks and two of the ticks removed from the feeding chambers had rare enlarged salivary acini detected after azure staining (Fig. 6). When present, these swollen acini are 2–3 times in size compared to other acini in the same SG section. These enlarged acini appeared to have an increased cytoplasmic volume that is devoid of salivary granules. The cytoplasms of the swollen cells are pale blue with 1–4 dark blue nuclei. Enlarged acini were not noted in SGs from negative control ticks. Of the five ticks documented with these features, three were unfed and two were exposed to the feeding chamber. These three unfed ticks were all positive for C. felis via RT-PCR (SG or HT) and/or ISH. One SG from two ticks that were exposed to the feeding chamber was positive for C. felis via RT-PCR and/or ISH, and one was negative via all molecular detection methods. Of the two SGs that were exposed to the feeding chamber, none of the ticks were classified as attached to the membrane at the time of SG collection (Table 1).
Fig. 6.

Azure staining of tick salivary gland (SG) wet mounts. (a) SG from a negative control, non-infected Amblyomma americanum adult. (b-d) SG from ticks that were acquisition-fed on C. felis-infected cat as nymphs. Rare SG acini (arrows) were noted to be swollen, pale staining and lack salivary granules. 20X.
3.3. Transmission electron microscopy
Six SG quarters that tested positive for C. felis via SG RT-PCR were selected for TEM (three unfed, three exposed to the feeding chamber). Three of these six SGs contained rare, small ovoid protozoal organisms. One of these three ticks was in the unfed group and the other two were exposed to the feeding chamber. Neither of the ticks that were exposed to the feeding chamber were categorized as attached at the time of collection. The protozoal organisms were often found in E cells or within intercellular spaces. The host cells appear damaged with winkled nuclei or nuclear remnants (Fig. 7a). Organisms ranged from 0.5 to 0.8 μm in diameter, encircled by a single or double-layered membrane, with several variably sized electron-dense secretory organelles within the cytoplasm, and occasionally contained a single nucleus (Fig. 7b). Three negative control ticks were also evaluated via TEM and structures resembling protozoal organisms were not detected.
Fig. 7.

Representative electron micrographs of SG acinus with suspected Cytauxzoon felis organisms from an infected Amblyomma americanum adult. (a) Lower magnification (2000X) of an infected acinus. Infected cell is swollen and wrinkled, lack distinct salivary granules and host cell nucleus with fragments of nuclear debris. Multiple protozoal organisms are found scattered in cytoplasm and intercellular spaces (arrow), (b) High magnification (70,000X) of arrowed region in (a) capturing two suspect immature C. felis sporozoites. Parasites are ~500 nm in diameter, encircled by a double membrane (arrowheads), with intracytoplasmic secretory organelles (asterisk), and a nucleus (N).
4. Discussion
The life cycle of C. felis has not been characterized in the vector host and is thought to be similar to other piroplasmids. We demonstrated C. felis-infected SGs in A. americanum using several microscopic techniques: ISH, light microscopy (direct staining and histology) and TEM. The detection of ISH signals using probes targeting two separate RNA transcripts verifies the presence of C. felis in tick SGs. This finding was further supported by RT-PCR, light microscopy and TEM.
Despite being able to localize C. felis in tick SGs, it remained challenging to fully characterize the parasite morphology on light microscopy via direct azure staining or H&E. This finding contrasts with what has been described in other related piroplasmids, where infected salivary acinar cells are easily distinguishable under light microscopy. For instance, the Theileria-laden salivary acinar cells are often notably enlarged and contain many small parasitic nuclei (Haque et al., 2010; Tajeri et al., 2016; Voigt et al., 1995). These features were not observed in the SGs examined in the current study. It is possible that we were simply capturing the parasites at an early stage of sporogony that are difficult to directly visualize because only a few nuclei are present. Our TEM results supported this theory. Even with a low number of infected ticks and acini, we were able to find rare structures in C. felis-positive SGs with ultrastructural features that were suggestive of protozoal organisms. These features included double cellular membranes and intracytoplasmic secretory organelles (e.g. rhoptries or micronemes) that resembled those of Theileria and Babesia sporozoites (Guimarães, 1997; Mehlhorn and Schein, 1984; Schein et al., 1979). However, we did not find evidence of syncytium formation, such as multiple nuclei and cytoplasmic partitioning (Fawcett et al., 1982) as commonly described in later stages of sporogony.
The ultrastructural features of C. felis in ticks have never been reported and only life stages found in the feline host had been documented via TEM (Kier et al., 1987; Simpson et al., 1985). Although we were able to identify C. felis organisms via TEM, only rare organisms were found, presumably due to the low numbers of infected acini. Unfortunately, screening for rare events, such as this, using TEM can be extremely labor-intensive and expensive. In our study, the sections used for TEM were 85 nm thick. Therefore, to image the entire quartered SG section from a single tick (~0.5–1 mm3) would require processing and interpretation of 5000–10,000 sections. A targeted approach using correlative light and electron microscopy that is supplemented with in situ molecular techniques (e.g. ISH, IHC or immunofluorescence) should be explored to identify infected acini for ultrastructural imaging (De Boer et al., 2015; Osamura et al., 2000).
We found discordant results between our three molecular detection techniques: SG RT-PCR, HT RT-PCR and SG ISH. The majority (20/26) of the PCR-positive ticks (either SG or HT) were negative via ISH, and one PCR-negative tick (by both SG and HT) was positive via ISH. There are three primary explanations for these findings. First, the sensitivity for detecting C. felis probably varies between our testing modalities. PCR is generally thought to be more sensitive than ISH because it employs exponential amplification of RNA transcripts, whereas the detectability of ISH signals is directly dependent on the amount of targeted RNA present in the examined tissue sections (Atout et al., 2022; Biedermann et al., 2004). This could explain why most PCR-positive SGs were negative via ISH, as these SGs may have contained too few C. felis RNA targets to be detectable. Second, the difference in detection sensitivity is further compounded by the low percentage of infected acini in C. felis-positive tick SGs. Positive ISH signals were detected in only one acinus in three ticks, and two acini in the other three ticks. This translates to an acinar infection rate of approximately 1.2–4.0% as there were ~50–80 acini per examined SG section. Therefore, it is likely that some of the SG quarters from C. felis-infected ticks may not have contained any infected acini and yielded a false negative result. Third, the discordant PCR and ISH results in SG and HT carcasses may be due to asynchronous development of C. felis in ticks. C. felis RNA was detected solely in HT carcasses in nine ticks via RT-PCR. While it is possible that the SGs were falsely negative as discussed previously, some parasites may have remained in the midgut or other tick organs and have not invaded the SGs. C. felis RNA was also simultaneously detected in both HT carcasses and SG in seven ticks via PCR and/or SG ISH. This could be an indication that the parasites are developing asynchronously and are present in the tick concurrently in different life stages. The duration of feeding, which we were not able to assess for individual ticks, may have affected the timing of parasite development. This finding of asynchronous development has also been previously described for Theileria and Babesia in ticks (Guimarães, 1997; Karakashian et al., 1983; Mehlhorn and Warneckes, 1979). Further investigation, including localizing parasites in tick organs using ISH or TEM, should be explored to validate this theory.
Future studies should also evaluate the effects of feeding on C. felis sporogony. In Theileria spp., infected SGs appear more detectable in ticks that were partially fed when compared to unfed ticks, presumably due to rapid parasite replication with SG acinar cells (Schein and Friedhoff, 1978; Zapf and Schein, 1994). In the current study, we only examined unfed ticks and ticks that had been exposed to feeding chambers for a short period of time (12–72 h). While a subset of ticks in our study were placed in artificial feeding units, only three infected ticks had clear evidence of feeding (presence of blood in the midgut) visualized at the time of SG dissection. Even though C. felis was not detected in any of these ticks via ISH, our sample size was too small to assess whether feeding had any effects on our ability to detect C. felis in SGs.
We provided the first conclusive evidence of C. felis in tick SGs. Our ability to use ISH to identify and localize the parasites in tick organs and cells suggests that this is a useful platform to characterize the life cycle of C. felis in ticks. This would also serve as a basis to facilitating future studies involving sporozoites, including initiating in vitro culture or developing sporozoite-based vaccines against cytauxzoonosis.
Supplementary Material
Supplemental figure 1. Histology and ISH results from all six Cytauxzoon felis-ISH positive salivary glands from Amblyomma americanum adults. Left panel shows the locations of serial SG sections on slides used for H&E histology and ISH. Red squares reflect the specific SG sections demonstrated to the right. Positive C. felis ISH signals are indicated by arrows. (bar = 100 μm)
Acknowledgements
The authors would like to thank the technicians in the NCSU histology laboratory and Vector-borne Disease Diagnostic Laboratory for their technical support. We are also grateful for Cindy Yang for her assistance in the original artwork in the figures.
Funding
This research is funded by a charitable organization which wishes to remain anonymous. Stipend support for T.S. Yang was provided by the National Institute of Health T32OD011130.
Footnotes
CRediT authorship contribution statement
Tzushan S. Yang: Writing – original draft, Writing – review & editing, Investigation, Project administration. Mason V. Reichard: Investigation, Resources, Writing – review & editing. Jennifer E. Thomas: Investigation, Resources, Writing – review & editing. Laura S. Miller: Methodology, Writing – review & editing. Henry S. Marr: Investigation, Writing – review & editing. Michael Karounos: Investigation, Writing – review & editing. Aaron J. Bell: Methodology, Writing – review & editing. Adam J. Birkenheuer: Supervision, Investigation, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.ttbdis.2022.102056.
References
- Atout S, Shurrab S, Loveridge C, 2022. Evaluation of the suitability of RNAscope as a technique to measure gene expression in clinical diagnostics: a systematic review. Mol. Diagn. Ther 26, 19–37. 10.1007/S40291-021-00570-2/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin CL, Black SJ, Brown WC, Conrad PA, Goddeeris BM, Kinuthia SW, Lalor PA, Machugh ND, Morrison WI, Morzaria SP, Naessens J, Newson J, 1988. Bovine T Cells, B Cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect. Immun 56, 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann K, Dandachi N, Trattner M, Vogl G, Doppelmayr H, Moré E, Staudach A, Dietze O, Hauser-Kronberger C, 2004. Comparison of real-time PCR signal-amplified in situ hybridization and conventional PCR for detection and quantification of human papillomavirus in archival cervical cancer tissue. J. Clin. Microbiol 42, 3758–3765. 10.1128/JCM.42.8.3758-3765.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CGD, Stagg DA, Purnell RE, Kanhai GK, Payne RC, 1973. Infection and transformation of bovine lymphoid cells in vitro by infective particles of Theileria parva. Nature 245, 101–103. 10.1038/245101a0. [DOI] [PubMed] [Google Scholar]
- De Boer P, Hoogenboom JP, Giepmans BNG, 2015. Correlated light and electron microscopy: ultrastructure lights up! Nat. Method 12 (6), 503–513. 10.1038/nmeth.3400, 201512. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Büscher G, Doxsey S, 1982. Salivary gland of the tick vector of east coast fever. III. The ultrastructure of sporogony in Theileria parva. Tissue Cell 14, 183–206. 10.1016/0040-8166(82)90017-9. [DOI] [PubMed] [Google Scholar]
- Fourie JJ, Evans A, Labuschagne M, Crafford D, Madder M, Pollmeier M, Schunack B, 2019. Transmission of Anaplasma phagocytophilum (Foggie, 1949) by Ixodes ricinus (Linnaeus, 1758) ticks feeding on dogs and artificial membranes. Parasit. Vector 12, 1–10. 10.1186/sl3071-019-3396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh YS, McGuire D, Rénia L, 2019. Vaccination with sporozoites: models and correlates of protection. Front. Immunol 10.3389/fimmu.2019.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L, Orenstein JM, 2007. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat. Protoc 2, 2439–2450. 10.1038/nprot.2007.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães AM, 1997. Ultrastructure of sporogony in Babesia equi in salivary glands of adult female Boophilus microplus ticks. Parasitol. Res 84, 69–74. 10.1007/s004360050359. [DOI] [PubMed] [Google Scholar]
- Han YS, 2000. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 19, 6030–6040. 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Jyoti Singh NK, Rath SS, 2010. Prevalence of Theileria annulata infection in Hyalomma anatolicum anatolicum in Punjab state, India. J. Parasit. Dis 34, 48–51. 10.1007/S12639-010-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howick VM, Russell AJC, Andrews T, Heaton H, Reid AJ, Natarajan K, Butungi H, Metcalf T, Verzier LH, Rayner JC, Berriman M, Herren JK, Billker O, Hemberg M, Talman AM, Lawniczak MKN, 2019. The malaria cell atlas: single parasite transcriptomes across the complete Plasmodium life cycle. Science 365 (1979). 10.1126/science.aaw2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalovecka M, Hajdusek O, Sojka D, Kopacek P, Malandrin L, 2018. The complexity of piroplasms life cycles. Front. Cell Infect. Microbiol 8, 248. 10.3389/fcimb.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakashian SJ, Rudzinska MA, Spielman A, Lewengrub S, Piesman J, Shoukrey N, 1983. Ultrastructural studies on sporogony of Babesia microti in salivary gland cells of the tick Ixodes dammini. Cell Tissue Res. 231, 275–287. [DOI] [PubMed] [Google Scholar]
- Khana DB, Peterson DS, Stanton JB, Schreeg ME, Birkenheuer AJ, Tarigo JL, 2018. Genetic conservation of Cytauxzoon felis antigens and mRNA expression in the schizont life-stage. Vet. Parasitol 263, 49–53. 10.1016/j.vetpar.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Kier AB, Wagner JE, Kinden DA, 1987. The pathology of experimental cytauxzoonosis. J. Comp. Pathol 97, 415–432. 10.1016/0021-9975(87)90020-X. [DOI] [PubMed] [Google Scholar]
- Kröber T, Guerin PM, 2007. In vitro feeding assays for hard ticks. Trend. Parasitol 23, 445–449. 10.1016/j.pt.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Lewengrub S, Rudzinska MA, Piesman J, Spielman A, Zung J, 2011. The influence of heat on the development of Babesia microti in unfed nymphs of Ixodes dammini. Can. J. Zool 67, 1510–1515. 10.1139/Z89-215. [DOI] [Google Scholar]
- Maes RK, Langohr IM, Wise AG, Smedley RC, Thaiwong T, Kiupel M, 2014. Beyond H&E: integration of nucleic acid-based analyses into diagnostic pathology. Vet. Pathol 51, 238–256. 10.1177/0300985813505878. [DOI] [PubMed] [Google Scholar]
- Maia LMP, Cerqueira A, de MF, de Barros Macieira D, de Souza AM, Moreira NS, da Silva AV, Messick JB, Ferreira RF, Almosny NRP, 2013. Cytauxzoon felis and “Candidatus Mycoplasma haemominutum” coinfection in a Brazilian domestic cat (Felis catus). Revista Brasileira de Parasitologia Veterinária 22, 289–291. 10.1590/sl984-29612013000200049. [DOI] [PubMed] [Google Scholar]
- Mani RJ, Reichard MV, Morton RJ, Kocan KM, Clinkenbeard KD, 2012. Biology of Francisella tularensis subspecies holarctica live vaccine strain in the tick vector Dermacentor variabilis. PLoS One 7, e35441. 10.1371/journal.pone.0035441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H, Schein E, 1993. The piroplasms: “A long story in short” or “Robert Koch has seen it. Eur. J. Protistol 29, 279–293. 10.1016/S0932-4739(11)80371-8. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H, Schein E, 1984. The Piroplasms: life cycle and sexual stages. Adv. Parasitol 23, 27–103. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H, Warneckes M, 1979. Electron-Microscopic Studies on Theileria ovis Rodhain, 1916: development of kinetes in the gut of the vector tick, Rhipicephulus evertsi evertsi Neumann, 1897, and their transformation within cells of the salivary glands. J. Parasitol 26, 377–385. [DOI] [PubMed] [Google Scholar]
- Morrison WI, Machugh ND, Lalor PA, 1996. Pathogenicity of Theileria parva is influenced by the host cell type infected by the parasite. Infect. Immun 64, 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamori Y, 2016. Transmission of Cytauxzoon felis By Amblyomma americanum: Engorgement Weight of Nymphs and Attachment Time of Adults for Transmission to Domestic Cats. Oklahoma State University. [Google Scholar]
- Nentwig A, Meli ML, Schrack J, Reichler IM, Riond B, Gloor C, Howard J, Hofmann-Lehmann R, Willi B, 2018. First report of Cytauxzoon sp. infection in domestic cats in Switzerland: natural and transfusion-transmitted infections. Parasit. Vect 11, 1–13. 10.1186/sl3071-018-2728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osamura RY, Itoh Y, Matsuno A, 2000. Applications of plastic embedding to electron microscopic immunocytochemistry and in situ hybridization in observations of production and secretion of peptide hormones. J. Histochem. Cytochem 48, 885–891. 10.1177/002215540004800701. [DOI] [PubMed] [Google Scholar]
- Perry BD, 2016. The control of East Coast fever of cattle by live parasite vaccination: a science-to-impact narrative. One Health 2, 103. 10.1016/J.ONEHLT.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Vara JA, Miller MA, 2014. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry-the red, brown, and blue technique. Vet. Pathol 51, 42–87. 10.1177/0300985813505879. [DOI] [PubMed] [Google Scholar]
- Ramsay JD, Ueti MW, Johnson WC, Scoles GA, Knowles DP, Mealey RH, 2013. Lymphocytes and macrophages are infected by Theileria equi, but T cells and B cells are not required to establish infection in vivo. PLoS One 8, e76996. 10.1371/journal.pone.0076996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein E, Friedhoff KT, 1978. Light microscopic studies on the development of Theileria annulata (Dschunkowsky and Luhs, 1904) in Hyalomma anatolicum excavatum (Koch, 1844). II. The development in haemolymph and salivary glands (author’s transl). Z. Parasitenkd 56, 287–303. 10.1007/bf00931721. [DOI] [PubMed] [Google Scholar]
- Schein E, Mehlhorn H, Voigt WP, 1979. Electron microscopical studies on the development of Babesia canis (Sporozoa) in the salivary glands of the vector tick Dermacentor reticulatus. Acta Trop. 36, 229–241. [PubMed] [Google Scholar]
- Schetters T, 2005. Vaccination against canine babesiosis. Trend. Parasitol 21, 179–184. 10.1016/j.pt.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Schreeg ME, Marr HS, Griffith EH, Tarigo JL, Bird DM, Reichard M.v., Cohn LA, Levy MG, Birkenheuer AJ, 2016. PCR amplification of a multi-copy mitochondrial gene (cox3) improves detection of Cytauxzoon felis infection as compared to a ribosomal gene (18S). Vet. Parasitol 225, 123–130. 10.1016/j.vetpar.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Shaw MK, 1999. Theileria parva: sporozoite entry into Bovine Lymphocytes is not dependent on the parasite cytoskeleton. Exp. Parasitol 92, 24–31. [DOI] [PubMed] [Google Scholar]
- Shaw MK, Tilney LG, Musoke AJ, 1991. The entry of Theileria parva sporozoites into bovine lymphocytes: evidence for MHC class I involvement. J. Cell Biol 113, 87–101. 10.1083/jcb.113.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CF, Harvey JW, Lawman P, J M, Murray J, Kocan AA, Carlisle JW, 1985. Ultrastructure of schizonts in the liver of cats with experimentally induced cytauxzoonosis. Am. J. Vet. Res 46, 384–390. [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ, 1995. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd. ed. W.H. Freeman and Company, 10.2307/2343822. [DOI] [Google Scholar]
- Tajeri S, Razmi G, Haghparast A, 2016. Establishment of an artificial tick feeding system to study Theileria lestoquardi infection. PLoS One 11, e0169053. 10.1371/journal.pone.0169053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarigo JL, Scholl EH, Bird DMK, Brown CC, Cohn LA, Dean GA, Levy MG, Doolan DL, Trieu A, Nordone SK, Felgner PL, Vigil A, Birkenheuer AJ, 2013. A novel candidate vaccine for cytauxzoonosis inferred from comparative apicomplexan genomics. PLoS One 8, e71233. 10.1371/journal.pone.0071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JE, Ohmes CM, Payton ME, Hostetler JA, Reichard M.v., 2018. Minimum transmission time of Cytauxzoon felis by Amblyomma americanum to domestic cats in relation to duration of infestation, and investigation of ingestion of infected ticks as a potential route of transmission. J. Feline Med. Surg 20, 67–72. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney JP, Deshmukh VV, Chaudhary PS, 2009. Fatal cytauxzoonosis in a kitten. Intas Polivet 10, 392–393. [Google Scholar]
- Voigt WP, Mwaura SN, Njihia GM, Nyaga SG, Young AS, 1995. Detection of Theileria parva in the salivary glands of Rhipicephalus appendiculatus: evaluation of staining methods. Parasitol. Res 81, 74–81. 10.1007/BF00932420. [DOI] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y, 2012. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Molecul. Diagnost 14, 22–29. 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi B, Meli ML, Cafarelli C, Gilli UO, Kipar A, Hubbuch A, Riond B, Howard J, Schaarschmidt D, Regli W, Hofmann-Lehmann R, 2022. Cytauxzoon europaeus infections in domestic cats in Switzerland and in European wildcats in France: a tale that started more than two decades ago. Parasit. Vect 15, 1–17. 10.1186/S13071-021-05111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TS, Reichard M.v., Marr HS, Cohn LA, Nafe L, Whitehurst N, Birkenheuer AJ, 2022. Direct injection of Amblyomma americanum ticks with Cytauxzoon felis. Ticks Tick Borne Dis. 13, 101847 10.1016/J.TTBDIS.2021.101847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AS, Leitch BL, Mutugi JJ, 1984. Some factors controlling the stimulation of sporogony of Theileria parva in its tick vector Rhipicephalus appendiculatus. Int. J. Parasitol 14, 97–102. 10.1016/0020-7519(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Zapf F, Schein E, 1994. New findings in the development of Babesia (Theileria) equi (Laveran, 1901) in the salivary glands of the vector ticks, Hyalomma species. Parasitol. Res 80, 543–548. 10.1007/bf00933000. [DOI] [PubMed] [Google Scholar]
- Zou FC, Li Z, Yang JF, Chang JY, Liu GH, Lv Y, Zhu XQ, 2019. Cytauxzoon felis infection in domestic cats, Yunnan Province, China, 2016. Emerg. Infect. Dis 25, 353–354. 10.3201/eid2502.181182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Histology and ISH results from all six Cytauxzoon felis-ISH positive salivary glands from Amblyomma americanum adults. Left panel shows the locations of serial SG sections on slides used for H&E histology and ISH. Red squares reflect the specific SG sections demonstrated to the right. Positive C. felis ISH signals are indicated by arrows. (bar = 100 μm)


