Abstract
Spinal cord injury (SCI) affects millions of people worldwide. Neural progenitor cell (NPC) transplantation is a promising treatment for regenerating lost spinal cord tissue and restoring neurological function after SCI. We conducted a literature search and found that less than a quarter of experimental rodent cell and tissue transplantation studies have investigated anatomical outcomes at longer than 4 months post-transplantation. This is a critical topic to investigate, given that stem and progenitor cell therapies would need to remain in place throughout the lifetime of an individual. We sought to determine how commonly assessed anatomical outcomes evolve between early and far chronic time-points post-NPC transplantation. At either 8 weeks or 26 weeks following transplantation of NPCs into sites of cervical SCI, we evaluated graft neuronal density, astroglial cell density, graft axon outgrowth, and regeneration of host axon populations into grafts in male and female mice. We found that graft neuronal density does not change over time, but the numbers of graft-associated astrocytes and glial fibrillary acidic protein intensity is significantly increased in the far chronic phase compared with the early chronic time-point. In addition, graft axon outgrowth was significantly decreased at 26 weeks post-transplantation compared with 8 weeks post-transplantation. In contrast, corticospinal axon regeneration into grafts was not diminished over time, but rather increased significantly from early to far chronic periods. Interestingly, we found that graft neuronal density is significantly influenced by sex of the host animal, suggesting that sex-dependent processes may shape graft composition over time. Collectively, these results demonstrate that NPC transplants are dynamic and that commonly assessed outcome measures associated with graft efficacy evolve over the weeks to months post-transplantation into the spinal cord.
Keywords: cervical spinal cord injury, chronic, grafting, neural progenitor cells, transplantation
Introduction
Spinal cord injury (SCI) is a devastating event that typically results in lifelong neurological deficits, including paralysis, chronic sensory dysfunction and pain, and autonomic dysfunction. As of 2022, there are an estimated 294,000 Americans living with SCI, a figure that increases by 17,800 each year.1 To date, there are no proven effective U.S. Food and Drug Administration–approved therapeutic interventions that can even partially restore neurological function after SCI. Neural progenitor cell (NPC) transplantation is a promising therapeutic strategy to regenerate lost spinal cord tissue.2 Through decades of pre-clinical research, it has been shown that engrafted NPCs differentiate into glial cells and diverse subtypes of neurons,3–10 and that grafts support host axon regeneration and establish new synaptic connections with the injured host nervous system.6,9,11–18 Notably, transplantation of NPCs derived from human induced pluripotent stem cells is currently being evaluated in clinical trials for subacute SCI in Japan.19,20 Together, these observations highlight NPC transplantation as a promising therapeutic approach to restore neurological function following SCI.
Many experimental NPC transplantation studies over the past 40 years have examined outcomes associated with graft efficacy, such as neurogenesis, gliogenesis, graft axon outgrowth, and host axon regeneration into grafts. Most of these studies have focused on evaluating acute, subacute, and/or early chronic time-points following transplantation, with only about 21% (14/66) of studies evaluating outcomes after 4 months (Table 1).3,5,6,8–13,15,18,21–75 Long-term studies have examined anatomical and functional outcomes after transplantation, but few studies have compared how these outcomes change from early to far chronic post-grafting time-points. This is an important factor to consider in the design of clinically effective cell therapies, because the SCI environment evolves over time and there are differences between the early chronic and far chronic periods of SCI that may influence differentiation, function, or integration of NPC grafts. To investigate this question, we transplanted green fluorescent protein (GFP)+ E12.5 mouse spinal cord NPCs into sites of cervical SCI in adult C57BL/6 mice, allowing grafts to mature for either 8 weeks or 26 weeks, and evaluated histological outcomes.
Table 1.
Neural Stem and Progenitor Cell Transplantation Studies Categorized by the Study End-Point
| Time-point | Studies | Total number of studies |
|---|---|---|
| 0-7 days | Bregman and colleagues, 1998; Dumont and colleagues, 2018; Han and colleagues, 2002; Lepore and Fischer, 2005; Lepore and colleagues, 2004; Lu and colleagues, 2012; Poplawski and colleagues, 2020; Reier and colleagues, 1983; Theele and Reier, 1996; Theele and colleagues, 1996 | 11 |
| 8-14 days | Bernstein and colleagues, 1984; Ciciriello and colleagues, 2020; Han and colleagues, 2002; Houle and Reier, 1988; Ishii and colleagues, 2006; Kumamaru and colleagues, 2019; Lu and colleagues, 2012; Patel and Bernstein, 1983; Poplawski and colleagues, 2020; Reier and colleagues, 1983; Robinson and Lu, 2017; Theele and colleagues, 1996 | 12 |
| 15-30 days | Adler and colleagues, 2017; Bernstein and colleagues, 1984; Bernstein-Goral and Bregman, 1993, 1997; Bregman and Bernstein-Goral, 1991; Bregman and colleagues, 1998; Bregman and colleagues, 1989; Bregman and colleagues, 1993; Bregman and colleagues, 1997; Bregman and Reier, 1986; Broude and colleagues, 1999; Diener and Bregman, 1998b; Han and colleagues, 2002; Houle and Reier, 1988; Iarikov and colleagues, 2007; Ishii and colleagues, 2006; Jakeman and colleagues, 1989; Koffler and colleagues, 2019; Lepore and Fischer, 2005; Lepore and colleagues, 2004; Lu and colleagues, 2019; Medalha and colleagues, 2014; Patel and Bernstein, 1983; Pitonak and colleagues, 2022; Poplawski and colleagues, 2020; Reier and colleagues, 1986; Theele and colleagues, 1996; Zholudeva and colleagues, 2018 | 29 |
| 31-60 days | Bernstein and colleagues, 1984; Bernstein-Goral and Bregman, 1993; Bonner and colleagues, 2010; Bonner and colleagues, 2011; Bregman and Bernstein-Goral, 1991; Bregman and colleagues, 1993; Bregman and colleagues, 1997; Ceto and colleagues, 2020; Diener and Bregman, 1998a; Dulin and colleagues, 2018; Dumont and colleagues, 2018; Hayakawa and colleagues, 2022; Hou and colleagues, 2013; Houle and Reier, 1988, 1989; Ishii and colleagues, 2006; Itoh and Tessler, 1990; Jakeman and Reier, 1991; Jin and colleagues, 2016; Kadoya and colleagues, 2016; Kumamaru and colleagues, 2018; Lepore and Fischer, 2005; Lepore and colleagues, 2004; Lepore and colleagues, 2006; Lu and colleagues, 2012; Medalha and colleagues, 2014; Mitsui and colleagues, 2005; Ogawa and colleagues, 2002; Patel and Bernstein, 1983; Reier and colleagues, 1986; Reier and colleagues, 1983; Spruance and colleagues, 2018; Theele and Reier, 1996; Theele and colleagues, 1996; White and colleagues, 2010; Zhou and colleagues, 2022 | 36 |
| 61-120 days | Adler and colleagues, 2017; Bernstein and colleagues, 1984; Brock and colleagues, 2018; Ceto and colleagues, 2020; Coumans and colleagues, 2001; Diener and Bregman, 1998b; Gulino and colleagues, 2010; Horner and colleagues, 1996; Hunt and colleagues, 2017; Jakeman and colleagues, 1989; Kadoya and colleagues, 2016; Kim and colleagues, 2006; Lee and colleagues, 2014; Lu and colleagues, 2022; Nakamura and colleagues, 2005; Patel and Bernstein, 1983; Poplawski and colleagues, 2018; Reier and colleagues, 1986; Reier and colleagues, 1983; Reier and colleagues, 1992; Theele and Reier, 1996; White and colleagues, 2010 | 22 |
| Over 4 months | Bregman and colleagues, 1989; Bregman and Reier, 1986; Diener and Bregman, 1998a; Dumont and colleagues, 2018; Gulino and colleagues, 2010; Houle and Reier, 1988, 1989; Jakeman and Reier, 1991; Koffler and colleagues, 2019; Kumamaru and colleagues, 2019; Lepore and colleagues, 2006; Reier and colleagues, 1986; Reier and colleagues, 1983; Tessler and colleagues, 1988 | 14 |
A total of 66 studies3,5,6,8–13,15,18,21–75 utilizing rodent spinal cord–derived cells or tissue are included. Studies are categorized according to the post-transplantation time-points that animals were sacrificed. Note that some studies are listed in multiple rows; these studies assessed outcomes at multiple time-points.
Methods
Ethics statement
Animal studies were performed in stringent compliance with the NIH (National Institutes of Health) Guidelines for Animal Care and Use of Laboratory Animals. NIH guidelines for laboratory animal care and safety were strictly followed. All experiments utilizing animals were approved by the Texas A&M University Institutional Animal Care and Use Committee. All efforts were made to minimize pain and distress.
Literature search
To generate the list of publications in Table 1, we performed a PubMed search on March 27, 2023, using the terms “fetal graft OR neural stem cell transplantation OR neural progenitor cell transplantation AND spinal cord injury,” retrieving approximately 1800 results. We manually screened search results to only include studies that utilized rat- or mouse-derived cells or tissue for transplantation into the adult rat or mouse central nervous system, resulting in 63 studies. We also added three additional studies from a previous publication by our group that included a literature search of spinal cord injury transplantation studies.76 A total of 66 citations meeting the above criteria are listed in Table 1. Studies were categorized by two independent reviewers (A.B. and J.N.D.) according to the survival times post-transplantation used in each study.
Animals
A total of 42 mice were used for this study, including thirty-six 8-10 week old C57BL/6 mice (n = 15 males, n = 21 females, #000664; Jackson Laboratories), and six 3-8 month old GFP+ males [C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ; #006567; Jackson Laboratories]. Male and female mice were used as experimental subjects for both treatment groups. Animals had free access to food and water throughout the study. All mice except for GFP males were group-housed in standard Plexiglas cages on a 12-h light/12-h dark cycle (light cycle = 6:00 a.m.-6:00 p.m.). GFP males were used as breeders to generate GFP+ embryos and were singly-housed with supplemental enrichment. Animal housing facilities had ambient temperature between 20-23°C and 30-70% humidity. Fifteen mice (eight males and seven females) were assigned to the 8-week group and 15 mice (seven males and eight females) were assigned to the 26-week group. The remaining 12 mice were used as breeders for embryo generation. One male mouse and one female mouse in the 8-week group died during surgery, leaving the final group size at n = 13 (seven males and six females). One female mouse from the 26-week group was perfused before the study end-point due to health concerns, leaving the final group size at n = 14 (seven males and seven females).
Neural progenitor cell preparation
Spinal cords from GFP+ mouse embryos (embryonic day 12.5 or E12.5) were dissected in ice-cold Hank's Buffered Salt Solution (HBSS; Gibco) on the day of grafting. The freshly harvested spinal cord tissue was then digested in trypsin and dissociated into a single-cell suspension of neural progenitor cells as previously described.10,76,77 Neural progenitor cells remained in neurobasal medium (Gibco) until grafting. Cell viability was assessed by trypan blue exclusion and confirmed to be >95% in all cases. Before grafting, neural progenitor cells were centrifuged at 3500 rpm for 4 min. Cells were then resuspended with HBSS to a final concentration of 500,000 cells/μL. All cells were kept on ice prior to resuspension or grafting.
Spinal cord injury and cell transplantation
Dorsal column lesions were performed at spinal cord cervical level 4 (C4). All surgeries were performed under deep anesthesia using a combination of ketamine (25 mg/kg), xylazine (5.8 mg/kg), acepromazine (0.25 mg/kg), and ≤1% inhaled isoflurane throughout the procedure. The skin along the upper back was shaved and scrubbed with betadine and 70% ethanol, then was incised using a scalpel blade and retracted using bulldog clips. The muscles overlying the spinal column were then incised and retracted. A C4 laminectomy was performed using rongeurs. Using a wire knife with an extruded diameter of 1.0-1.5 mm (McHugh Milieux, Downers Grove, IL), the dorsal column lesion was performed at the C4 region. The wire knife was lowered 0.8 mm into the spinal cord and raised to axotomize axons within the dorsal white matter. Using a Picospritzer III (General Valve Corporation), 2 μL of neural progenitor cells at a concentration of 500,000 cells per μL were immediately injected into the lesion cavity.
During injection, graft placement was assessed through the presence of green fluorescence using a fluorescence flashlight with Royal Blue LEDs (NightSea). Once neural progenitor cells were injected, the muscles were sutured with 4-0 Prolene sutures and skin was closed with stainless steel wound clips. A cocktail of 0.5 mg/mL banamine, 33 mg/mL ampicillin, and lactated Ringer's solution (Covetrus) was then injected subcutaneously at a volume of 0.5 mL into the recovering animals. Animals were allowed to recover in a warm resting area until fully bright and alert.
Cortical injections
Biotinylated dextran amine (BDA) was used to anterogradely label corticospinal axons in vivo. Cortical injections of BDA were performed at 2 weeks prior to the date of sacrifice (6 weeks post-SCI for the early chronic group, and 24 weeks post-SCI for the far chronic group). Surgeries were performed under deep anesthesia using a combination of ketamine, xylazine, acepromazine, and 1% vaporized isoflurane as described above. The skin on the dorsal surface of the head was shaved and scrubbed with betadine and 70% ethanol. Subjects were placed into a rodent stereotaxic apparatus using ear bars and a bite bar to stabilize the skull. Skin on the dorsal surface of the head was incised down the midline using a scalpel blade and retracted using bulldog clips to expose the skull. The intersection of bregma with the skull midline was used as a landmark to calculate relative injection coordinates.
Windows of the skull overlying the M1 and S1 cortical areas were removed with a dental drill to expose the brain surface; 10% w/v BDA (MW 10,000 kDa; Invitrogen) was injected into the cortex at injections sites listed in Supplementary Table S1 using a Nanoject III (Drummond). Injection coordinates that were over superficial blood vessels were skipped to avoid hemorrhaging in the brain. Pulled glass micropipettes were backfilled with prepared 10% BDA in sterile HBSS. A total volume of 3 μL (100 nL per injection site × 30 injection sites) was injected at a depth of 0.7 mm into the motor cortex, waiting at least 30 sec between each injection. Once all injections were completed, the incised skin was closed with wound clips, a cocktail of 0.5 mg/mL banamine, 33 mg/mL ampicillin, and lactated Ringer's solution was then injected subcutaneously at a volume of 0.5 mL into the recovering animals. Animals were allowed to recover in a warm resting area until fully bright and alert.
Tissue collection and immunohistochemistry
Tissue processing
Two weeks after BDA injections, animals were euthanized by anesthesia overdose and transcardially perfused with 0.1 M phosphate buffer (PB) followed by 4% paraformaldehyde (PFA) in 0.1 M PB. Spinal columns and brains were post-fixed in 4% PFA in 0.1 M PB overnight at 4°C, then cryopreserved in 30% sucrose in 0.1 M PB for at least 3 days at 4°C. Spinal cords were then removed and a 1-cm length of spinal cord centered around C4 was embedded in Tissue-Tek OCT compound (VWR) and frozen on dry ice. Fixed spinal cord tissue was then cut into 30 μm sagittal sections using a Leica cryostat. Sectioned tissue was stored in 24-well plates halfway filled with tissue collecting solution (TCS; comprised of tris-buffered saline [TBS] with ethylene glycol and glycerol for cryopreservation) at 4°C until use.
Immunohistochemistry
Immunohistochemical staining was divided into 2 days of staining. Sections were washed in TBS three times for 10 min each, then blocked in TBS containing 5% donkey serum (Lampire Biological Laboratories, #7332100) and 0.25% Triton-X-100 (Sigma-Aldrich) for 1 h at room temperature. Sections were then incubated with primary antibodies (Supplementary Table S2) diluted in blocking solution overnight at 4°C. The next day, sections were washed in TBS three times for 10 min each, then incubated with AlexaFluor-conjugated secondary antibodies (Jackson ImmunoResearch) in blocking solution for 2 h at room temperature (Supplementary Table S2). Finally, sections were washed in TBS three times for 10 min each, with the final wash containing 4′,6-diamidino-2-phenylindole (DAPI; 5 μg/mL, Sigma Aldrich, D9542). Sections were mounted to gelatin-coated slides, air-dried, rinsed in distilled water, and cover-slipped with Mowiol or Fluoromount mounting medium.
Image acquisition
Slides stained with fluorescent dyes were imaged in a dark room. All tissue for a given group was imaged using identical acquisition settings. Sagittal slides were imaged using a Nikon Eclipse upright fluorescent microscope equipped with a Prior Scientific XY motorized stage and a Zyla 4.2 PLUS monochrome camera (Andor). Nikon NIS-Elements software was used for image acquisition and XY stitching. Images were captured with a 10 × magnification objective. Images were exported as 8- or 16-bit TIFF files for analysis.
Image analysis
All image analysis was performed in a blinded fashion using ImageJ or FIJI software. GFP image channels were used to draw regions of interest (ROIs) around the border of graft for each individual image. Automated cell counting and axon tracing methods were always validated by manual counts and tracing. Sections that showed poor immunostaining or no NPC graft were excluded from analysis. One subject in the 26 weeks survival group had poor tissue quality and was excluded from all analysis except axon outgrowth quantification. One subject in the 8 weeks survival group was excluded from calcitonin gene-related peptide (CGRP)+ axon analysis because the quality of CGRP immunolabeling was poor.
Corticospinal axon quantification
Images of BDA immunoreactivity were first inverted (black on white) to visualize the axons more clearly. Axons within the graft were manually traced in ImageJ and the total length of axons per subject were divided by the total area of graft ROI for that subject.
NeuN quantification
NeuN+ cells within graft ROIs were first thresholded using the ImageJ Auto Local threshold function with the Bernsen's thresholding method in ImageJ.78 The Watershed function was applied to the images, then the Analyze Particles function was used to quantify the total number of NeuN+ cells within the graft. Total NeuN+ cells were normalized to graft volume to calculate cell density.
Axon outgrowth quantification
GFP fluorescence was overexposed in one channel while imaging so that fine GFP+ axons could be identified throughout the sagittally sectioned spinal cord. Graft ROIs were translated in 250-μm increments for 5 mm in both rostral and caudal directions. At each 250-μm increment, the total number of GFP+ axons crossing the leading edge of the ROI was manually counted. Data are represented as average axon outgrowth per section.
Sox9 quantification
Sox9+ nuclei within graft ROIs were thresholded with the Bernsen's thresholding method in ImageJ, and quantified using the Find Local Maxima function in ImageJ. Total Sox9+ cells were normalized to graft volume to calculate cell density.
Glial fibrillary acidic protein (GFAP) intensity quantification
Total pixel intensity of GFAP immunoreactivity was divided by the number of pixels in the ROI to obtain the mean gray value within the graft. The ROI was then enlarged by 250 microns and the graft ROI subtracted, and this process was repeated to measure the mean gray value in the area immediately surrounding the graft ROI.
CGRP+ axon quantification
CGRP immunoreactivity was quantified by applying the Phansalkar method of Auto Local Thresholding in ImageJ,79 then quantifying the number of thresholded pixels per graft ROI for each subject.
Statistical analysis
GraphPad Prism 8 (GraphPad Software, Inc.; La Jolla, CA) was used to perform statistical analysis. Statistical significance was defined as p < 0.05. Unpaired t-test was used for this analysis. Detailed statistical analysis results are provided in Supplementary Table S3.
Results
To investigate how outcomes evolve after NPC transplantation from the early chronic to the far chronic phase of graft maturation, we designed an experiment to transplant E12.5 GFP+ mouse NPCs into sites of C5 dorsal column lesion SCI (Fig. 1A). Experimental subjects were randomly assigned to either the early chronic group (survival time = 8 weeks after NPC transplantation) or the far chronic group (survival time = 26 weeks post-transplantation). Two weeks before the study end-point, all subjects received intracortical injections of biotinylated dextran amine into motor and somatosensory cortex to anterogradely label axons of the corticospinal tract (CST), which is important for recovery of fine motor function after SCI.80 Animals were sacrificed 2 weeks after cortical injections, and immunohistochemical outcomes were evaluated (Fig. 1A).
FIG. 1.
Graft neuronal density does not significantly change over time. (A) Experimental design for this study. Neural progenitor cells were isolated from E12.5 GFP+ mouse spinal cord tissue and transplanted immediately after C5 dorsal column spinal cord injury. Either 6 weeks later (“early chronic” group) or 24 weeks later (“far chronic” group), biotinylated dextran amine (BDA) was injected into bilateral motor and somatosensory cortices. Two weeks after BDA injections, animals were sacrificed for histology. (B) Images of NeuN immunoreactivity within grafts at (B) 8 weeks and (C) 26 weeks after transplantation into the injured host spinal cord. Graft/host boundaries are shown with dotted lines. (D) Quantification of NeuN+ cell density within grafts at 8 weeks or 26 weeks survival time. Blue data points represent male animals and magenta data points represent female animals. Data are mean ± standard error of the mean. 8 weeks: n = 13 mice; 26 weeks: n = 14 mice. Scale bars = 100 μm.
We first analyzed the density of neurons in grafts. Grafts at both 8 weeks and 26 weeks post-transplantation were filled with GFP+ cells, completely filled the lesion site, and were densely populated with neurons (Fig. 1B, 1C). Upon quantification of the total numbers of neurons in grafts, we did not identify any statistically significant differences between 8 weeks and 26 weeks treatment groups (Fig. 1D). This result demonstrates that grafts do not gain or lose a significant proportion of neurons from the early chronic to far chronic time-points after NPC transplantation. Interestingly, we noticed a trend in which male subjects appeared to exhibit higher graft neuronal density at both time-points post-transplantation (Fig. 1D; Supplementary Fig. S1). Upon stratification of data according to sex, we detected a statistically significant main effect of sex on graft neuronal density (p = 0.0145; Supplementary Fig. S1). Although multiple comparisons between males versus females did not reveal significant differences in group means, males trended toward higher graft neuronal density at 26 weeks post-grafting (p = 0.078; Supplementary Fig. S1). This suggests that sex-dependent factors, potentially related to immune response,10 may negatively impact graft neuronal survival in female animals at chronic time-points after grafting.
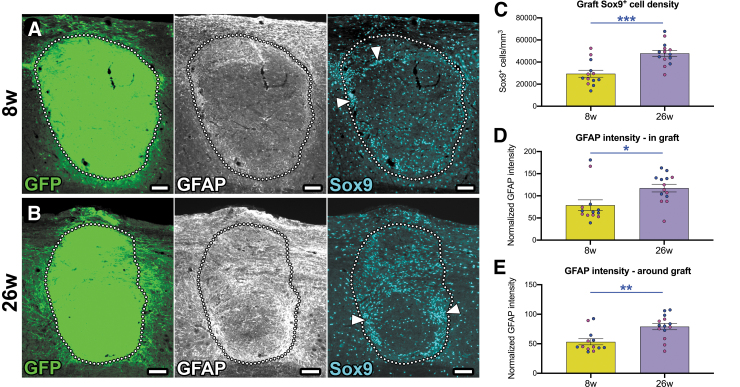
We next sought to determine how the abundances of astrocytes associated with NPC grafts might change from the early to far chronic period. Astrocytes in the spinal cord form a protective barrier around sites of SCI, known as the reactive glial boundary or historically as the “glial scar.”81,82 Transplantation of NPCs is thought to mitigate the formation of this reactive glial border, due to observations that GFAP immunoreactivity is decreased after transplantation of NPCs.10,43,67,83,84 However, this has not yet been assessed at early versus far chronic time-points. We therefore quantitatively assessed the presence of astrocyte markers in and around grafts at 8 weeks and 26 weeks post-transplantation. We found that grafts at both 8 weeks and 26 weeks post-transplantation were densely populated with Sox9+ astrocytes and GFAP+ astrocytic processes (Fig. 2A, 2B). Upon quantification, we found that Sox9+ cell density was significantly higher in far chronic grafts versus early chronic grafts (Fig. 2C). However, we did not determine whether these cells were graft-derived or host-derived, because it is difficult to perform colocalization analysis with GFP (as GFP signal is ubiquitous throughout the graft). Therefore, this observation of increased Sox9+ glial cell abundance in the 26 weeks group may be due in part to infiltration of grafts by host astrocytes, ongoing proliferation of graft astrocytes, or both.
FIG. 2.
Astroglial cell density within grafts increases from 8 weeks to 26 weeks post-transplantation. (A, B) Images of neural progenitor cell grafts at (A) 8 weeks and (B) 26 weeks following transplantation into the injured host spinal cord. Astrocytes in and around the grafts are immunolabeled with glial fibrillary acidic protein (GFAP) and Sox9 antibodies. Graft/host boundaries are shown with dotted lines. Arrowheads indicate regions with high Sox9+ cell density. (C) Quantification of the density of Sox9+ nuclei in grafts. Blue data points represent male animals and magenta data points represent female animals. (D, E) Quantification of GFAP immunoreactivity (D) in grafts and (E) in a 250-μm bordering region around grafts. *p = 0.045, **p = 0.0020, ***p = 0.0002 by unpaired, two-tailed Student's t test. Data are mean ± standard error of the mean. 8 weeks: n = 13 mice; 26 weeks: n = 14 mice. Scale bars = 100 μm.
We also observed that Sox9+ astrocytes were not evenly distributed throughout graft tissue; rather, grafts contained areas of high Sox9+ cell density, particularly around graft/host borders, as well as some areas relatively devoid of astrocytes (Fig. 2A, 2B). Additionally, we quantified the intensity of GFAP immunoreactivity in grafts (Fig. 2D) as well as within a 250-μm region immediately outside of the graft/host boundary (Fig. 2E).10 In each case, we found that GFAP intensity was significantly increased in 26 weeks grafts versus 8 weeks grafts. Together, these data suggest that astrocyte reactivity in and around NPC grafts increases from the early chronic to far chronic periods post-transplantation.
We also stratified experimental subjects by sex in order to determine whether there were any significant differences in graft astrocyte density between male and female subjects. We did not identify any significant main effects of sex on Sox9+ cell density or GFAP immunoreactivity in or around grafts (Supplementary Fig. S2). Hence, the biological sex of the host animal does not appear to influence the abundance of astrocytes in or around the graft.
We next evaluated graft axon outgrowth. Extension of graft-derived axons is widely agreed to be a requirement for graft/host neural relay formation.2 However, it is unclear whether graft-derived axon extension is maintained from early to far chronic time-points. GFP+ axons extending into the host spinal cord were manually quantified in 250-μm increments in the rostral and caudal directions. Grossly, we found that both groups had axons extending in both directions, on the order of less than 100 axons exiting the graft in each direction (Fig. 3A-D). Upon quantification, we found that the early chronic (8 weeks) group had significantly more axons emerging versus the 26 weeks group, especially within 750 μm from the graft (Fig. 3E). This result suggests that some, but not all, graft-derived axons may be eliminated over time from the early to far chronic periods post-transplantation. We did not identify any sex-dependent differences in graft axon outgrowth (Supplementary Fig. S3).
FIG. 3.
Graft axon outgrowth decreases from 8 weeks to 26 weeks post-transplantation. (A-D) Representative images of GFP+ graft-derived axon outgrowth at (A, B) 8 weeks post-transplantation and (C, D) 26 weeks post-transplantation. (E) Quantification of graft-derived GFP+ axon outgrowth at 250-μm intervals rostral and caudal to the graft. *p < 0.05, **p < 0.01 by two-way repeated measures Analysis of variance with Tukey's multiple comparisons test. All data are mean ± standard error of the mean. 8 weeks: n = 13 mice; 26 weeks: n = 15 mice. Scale bars = 100 μm (A, C), 50 μm (B, D).
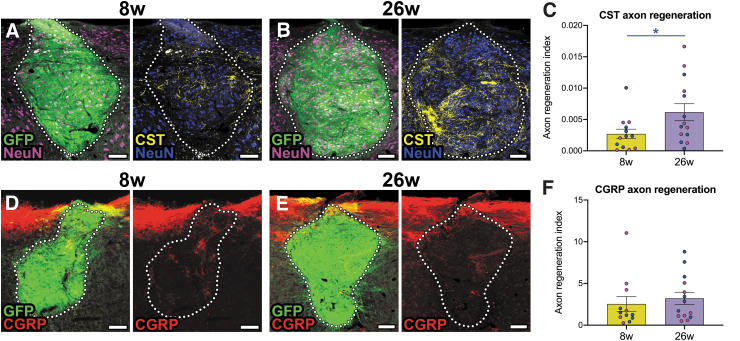
Finally, we assessed host axon regeneration into NPC grafts at early and far chronic time-points. We have previously shown that CST and CGRP+ axons spontaneously grow into grafts placed into sites of SCI.8,76 However, it is not clear whether this regenerative regrowth is maintained months after grafting. We first examined the density of BDA-labeled CST fibers in grafts. Although there is intrinsic variability in the degree of CST arborization from graft to graft, we found that there was significantly greater average CST axon density within 26 weeks grafts compared with 8 weeks grafts (Fig. 4A-C). This suggests that CST axon regeneration into, and arborization within, NPC grafts not only is stabilized but increases over time. We also examined growth of CGRP+ fibers into grafts (Fig. 4D, 4E). These nociceptive afferents normally relay pain and heat information, and we previously found that they regenerate exclusively into dorsal horn-like domains of NPC grafts.8 As with CST axons, we found that there was inter-animal variability in the degree of CGRP+ axon innervation into grafts. Overall, we did not detect any significant differences in CGRP+ axon growth into grafts between the early and far chronic time-points. Moreover, we failed to observe any significant effects of host animal sex on growth of either type of host axon into grafts (Supplementary Fig. S4).
FIG. 4.
Regeneration of host corticospinal but not calcitonin gene-related peptide (CGRP)+ axons into neural progenitor cell (NPC) grafts increases over time from 8 weeks to 26 weeks post-transplantation. (A, B) Representative images of corticospinal axon regeneration into NPC grafts at (A) 8 weeks and (B) 26 weeks post-transplantation into sites of cervical SCI. (C) Quantification of corticospinal axon density within grafts. *p = 0.0394 by unpaired, two-tailed Student's t test. Blue data points represent male animals and magenta data points represent female animals. (D, E) Representative images of CGRP+ axon regeneration into NPC grafts at (D) 8 weeks and (E) 26 weeks post-transplantation into sites of cervical SCI. (F) Quantification of corticospinal axon density within grafts. All data are mean ± standard error of the mean. 8 weeks: n = 12 mice; 26 weeks: n = 14 mice. Scale bars = 100 μm.
Discussion
Our study builds on a rich history of work in the field of neural tissue and cell transplantation. As noted in Table 1, there have been multiple published studies evaluating outcomes at several months after transplantation. One of the furthest-chronic studies was conducted by Lepore and colleagues57; they examined outcomes at up to 15 months post-transplantation and observed good graft survival, neurogenesis and gliogenesis, and formation of graft-derived synapses, however they did not assess host axon regeneration in this study.57 Many studies have evaluated survival, neuronal morphology, and even ingrowth of host CGRP+ and dorsal root axons into grafts.43,44,49,67,68,72 Bregman and colleagues found that fetal spinal cord tissue transplants supported regeneration of corticospinal axons up to 9 months after SCI/transplantation,28 although this study did not directly compare CST axon regeneration into grafts at early and far chronic time-points.
In this study, we have demonstrated that NPC grafts are not totally static after transplantation into the injured host spinal cord; in some aspects, they are dynamic. Some variables such as graft neuronal density remain unchanged over time. This is probably because neurons proliferate and differentiate early after transplantation, then the period of neurogenesis ends and neuron numbers are stable. However, other outcomes change over time. We found that astrocytes within and near graft tissue increased in number from 8 weeks to 26 weeks post-transplantation. This could possibly reflect an extended gliogenic period of transplanted NPCs, but could also indicate infiltration and reactivity of host astrocytes. These possibilities cannot be distinguished based on the current study design.
One key question to consider is whether there are processes endogenous to the injured spinal cord that contribute to increased astrogliosis over time. A great deal of work has shown that the cellular and molecular components of the “scar tissue” evolve from the subacute (∼1-2 weeks) to chronic (∼4-13 weeks) period.85–89 It is generally thought that the bulk of maturation of the astroglial layer occurs by about 28 days, after which point the scar stabilizes. However, there are scant data available on how the astroglial layer evolves from the early chronic to far chronic periods after SCI, such as the time frame observed in this study. In addition to changes in graft astrocyte population, we found that graft axon outgrowth is reduced from the early chronic to far chronic period. This could be due to developmental pruning, a process in which axons that fail to establish synaptic contacts, or whose synapses are weak, are eliminated.90,91 This could also be due to phagocytosis of graft-derived axons by host macrophages. Future work is needed to characterize the dynamic processes that contribute to the maintenance of graft axon outgrowth over time.
In addition to changes in these “intrinsic” properties of grafts, we also unexpectedly observed that host corticospinal axon regeneration into NPC grafts was actually highest in the far chronic group. This suggests that corticospinal axons, which regenerate into NPC grafts at 10-14 days post-transplantation,65 are not pruned back but may even continue to arborize and establish new synaptic connections throughout the lifetime of the animal. This is in contrast to what is known about the temporal dynamics of CST axon sprouting after injury. Using a single-collateral labeling technique, Lang and colleagues found that CST collaterals grew and became highly branched between 10 days to 3-4 weeks, but then the arborizations remained relatively static for up to 12 weeks post-injury.92 The time course of corticospinal remodeling is also similar when growth-promoting treatments are administered after SCI. For example, Geoffroy and colleagues demonstrated that after pyramidotomy, CST sprouting both in wild-type animals and following PTEN/SOCS3 deletion in cortical neurons is maxed out by 4 weeks post-treatment, and does not increase further by 7 weeks.93 Hence, our observations in the current study are unique in that they suggest graft-mediated mechanisms may promote ongoing growth and reorganization of corticospinal axons.
It is important to note here that despite our observation of a statistically significant difference between the two group means, inter-animal variability is very high in both groups (Fig. 4C). As with our previous findings,8 some grafts exhibit very little or no regeneration of CST axons, while others exhibit a great deal of regeneration. It is likely that the distributions of graft-derived postsynaptic targets for CST axons is the primary factor determining the extent of regeneration of CST axons and other host axon populations. Regardless, it is clear from our data that the CST does not “die back” after initial growth into grafts.
Biological sex is a critical source of variability in animal and human studies. We recently described a sex-dependent immune response in female mice receiving male NPC grafts.10 In this study, we observed significantly reduced graft neuronal density in female mice with male NPC grafts, but not other host/graft sex combinations, at 4 weeks post-transplantation compared with the neuronal density of intact spinal cord gray matter (p = 0.0281 by Dunnett's multiple comparisons test). In the current study, we observed a significant effect of biological sex of the host animal on graft neuronal density, with female subjects trending toward lower neuronal density than male subjects (Supplementary Fig. S1). The cells used in this study were not sex-restricted, but rather constituted a mixture of cells from male and female mouse embryos. Together, these observations indicate that pathological processes, probably arising from the female animal's immune response to the presence of engrafted male cells, could be driving the death of a subset of graft-derived neurons by 4-8 weeks post-transplantation into sites of SCI. This further highlights the need to consider biological sex in pre-clinical grafting studies, as well as ongoing and planned human trials. We did not observe any other significant sex-dependent differences in this study, suggesting that biological sex has a greater influence on graft neuronal survival than astrogliosis, axon outgrowth, or host axon regeneration.
One important factor to consider is that in the present study, we utilized a mild model of SCI, the dorsal column lesion. We selected this lesion model because 1) it axotomizes 98% of corticospinal axons and all ascending sensory afferent axons in the dorsal spinal cord,94 and 2) it supports good graft survival without requiring a scaffold or fibrin matrix.8,10,16,76 Hence, this model is well-suited for studies focused on corticospinal axon regeneration or forelimb functional recovery.8,83 It is possible that different outcomes would be observed if this study had been performed in a more severe model of SCI. For instance, a dorsal column lesion, which primarily injures white matter while sparing gray matter, is associated with formation of a reactive glial cell layer that is mitigated by NPC transplantation. Here, we showed that astrocyte density and reactivity increase over time in and around the NPC graft, suggesting that perhaps there is an ongoing inflammatory process driving reactive gliosis. However, it is very likely that the dynamics of reactive gliosis would be altered in a more severe lesion model such as a contusion, which injures primarily gray matter and disrupts vasculature leading to an immediate influx of blood-borne immune cells. Hence, findings of the present work may not necessarily translate to more severe SCI models. Further work is needed to explore the effects of lesion model and severity on outcomes after NPC transplantation.
Transparency, Rigor, and Reproducibility Summary
The study design was registered following completion of the study at the Open Data Commons for Spinal Cord Injury (https://odc-sci.org/data/887). The analysis plan was not formally pre-registered. A sample size of 15 mice per group for the 8 weeks group and 15 mice per group for the 26 weeks group was planned based on a power analysis with anticipated effect size of 1.5 for the primary outcome measure (axon outgrowth) calculated to yield 80% power to detect a statistically significant difference in SCI groups using a two-way repeated measures analysis of variance and p value <0.05, and taking into account attrition due to mortalities during surgery. Mice were randomly assigned to treatment group using a random number generator. Surgeries, tissue processing, and data analysis were performed by investigators blinded to treatment group. Tissue sections were stored at 4°C for 1-3 months prior to use. Experimental materials were analyzed in a single batch for each histological stain performed. The specificity of antibodies used for immunohistochemistry was confirmed using negative controls (no primary antibody) and comparison to historical results. No replication or external validation studies have been performed or are planned/ongoing at this time to our knowledge. Data from this study are available in a FAIR data repository (odc-sci.org). There is no analytic code associated with this study. No future use of these samples is possible because insufficient quantities remain. The authors agree to publish the manuscript using the Mary Ann Liebert Inc. “Open Access” option under appropriate license.
Data Availability
All data generated or analyzed during this study are included in this published article and the supplementary files. Raw data will be made available upon request, within two weeks of the request. Additionally, data from this study are available in a FAIR data repository, the Open Data Commons for Spinal Cord Injury (odc-sci.org) under http://doi.org/10.34945/F5XK5Q, and can be accessed at https://odc-sci.org/data/887.
Supplementary Material
Acknowledgments
We thank Amy Leonards and Zachary Cantu for research support, and Amy Chen for helpful scientific discussion.
Authors' Contributions
A.B. performed experiments, analyzed data, and wrote the manuscript.
A.T. performed animal surgeries.
J.J. performed immunohistochemistry and data analysis.
K.V. performed immunohistochemistry.
J.N.D. conceived of the study, analyzed data, and wrote the manuscript.
Funding Information
We gratefully acknowledge funding from the National Institutes of Health (R01NS116404 to J.N.D.), Mission Connect, a project of the TIRR Foundation (J.N.D.), and the Texas A&M University College of Science Undergraduate Research Opportunities Program (to A.B.). We thank the Texas A&M University LAUNCH Undergraduate Research Scholars program for supporting A.B. during this project.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. National Spinal Cord Injury Statistical Center. Traumatic Spinal Cord Injury Facts and Figures at a Glance. Birmingham, AL; 2022. Available from: https://msktc.org/sites/default/files/SCI-Facts-Figs-2022-Eng-508.pdf [Last access September 10, 2023]. [Google Scholar]
- 2. Fischer I, Dulin JN, Lane MA. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat Rev Neurosci 2020;21:366–383; doi: 10.1038/s41583-020-0314-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han SS, Kang DY, Mujtaba T, et al. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp Neurol 2002;177(2):360–375; doi: 10.1006/exnr.2002.7995 [DOI] [PubMed] [Google Scholar]
- 4. Lepore AC, Han SS, Tyler-Polsz CJ, et al. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol 2004;1(2):113–126; doi: 10.1017/s1740925x04000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol 2005;194(1):230–242; doi: 10.1016/j.expneurol.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 6. Lu P, Wang Y, Graham L, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012;150(6):1264–1273; doi: 10.1016/j.cell.2012.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zholudeva LV, Iyer N, Qiang L, et al. Transplantation of neural progenitors and V2a interneurons after spinal cord injury. J Neurotrauma 2018;35(24):2883–2903; doi: 10.1089/neu.2017.5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dulin JN, Adler AF, Kumamaru H, et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat Commun 2018;9(1):84; doi: 10.1038/s41467-017-02613-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumamaru H, Lu P, Rosenzweig ES, et al. Regenerating corticospinal axons innervate phenotypically appropriate neurons within neural stem cell grafts. Cell Rep 2019;26(9):2329–2339 e4; doi: 10.1016/j.celrep.2019.01.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pitonak M, Aceves M, Amar Kumar P, et al. Effects of biological sex mismatch on neural progenitor cell transplantation for spinal cord injury in mice. Nature Commun 2022;13(1):5380; doi: 10.1038/s41467-022-33134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. White TE, Lane MA, Sandhu MS, et al. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Experimental neurology 2010;225(1):231–236; doi: 10.1016/j.expneurol.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonner JF, Connors TM, Silverman WF, et al. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci 2011;31(12):4675–4686; doi: 10.1523/JNEUROSCI.4130-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hou S, Tom VJ, Graham L, et al. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J Neurosci 2013;33(43):17138–17149; doi: 10.1523/JNEUROSCI.2851-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dougherty BJ, Gonzalez-Rothi EJ, Lee KZ, et al. Respiratory outcomes after mid-cervical transplantation of embryonic medullary cells in rats with cervical spinal cord injury. Exp Neurol 2016;278:22–26; doi: 10.1016/j.expneurol.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kadoya K, Lu P, Nguyen K, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med 2016;22(5):479–487; doi: 10.1038/nm.4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adler AF, Lee-Kubli C, Kumamaru H, et al. Comprehensive monosynaptic rabies virus mapping of host connectivity with neural progenitor grafts after spinal cord injury. Stem Cell Reports 2017;8(6):1525–1533; doi: 10.1016/j.stemcr.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dulin JN, Adler AF, Kumamaru H, et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nature communications 2018;9(1):84; doi: 10.1038/s41467-017-02613-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zholudeva LV, Iyer N, Qiang L, et al. Transplantation of neural progenitors and V2a interneurons after spinal cord injury. J Neurotrauma 2018; doi: 10.1089/neu.2017.5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugai K, Sumida M, Shofuda T, et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen Ther 2021;18:321–333; doi: 10.1016/j.reth.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsuji O, Sugai K, Yamaguchi R, et al. Concise Review: Laying the Groundwork for a First-In-Human Study of an Induced Pluripotent Stem Cell-Based Intervention for Spinal Cord Injury. Stem Cells 2019;37(1):6–13; doi: 10.1002/stem.2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adler AF, Lee-Kubli C, Kumamaru H, et al. Comprehensive monosynaptic rabies virus mapping of host connectivity with neural progenitor grafts after spinal cord injury. Stem Cell Reports 2017;8(6):1525–1533; doi: 10.1016/j.stemcr.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernstein, JJ, Patel U, Kelemen, M, et al. Ultrastructure of fetal spinal cord and cortex implants into adult rat spinal cord. J Neurosci Res 1984;11(4):359–372; doi: 10.1002/jnr.490110404 [DOI] [PubMed] [Google Scholar]
- 23. Bernstein-Goral H, Bregman BS. Spinal cord transplants support the regeneration of axotomized neurons after spinal cord lesions at birth: a quantitative double-labeling study. Exp Neurol 1993;123(1):118–132; doi: 10.1006/exnr.1993.1145 [DOI] [PubMed] [Google Scholar]
- 24. Bernstein-Goral H, Bregman BS. Axotomized rubrospinal neurons rescued by fetal spinal cord transplants maintain axon collaterals to rostral CNS targets. Exp Neurol 1997;148(1):13–25; doi: 10.1006/exnr.1997.6640 [DOI] [PubMed] [Google Scholar]
- 25. Bonner, J.F., Blesch, A., Neuhuber, B., et al. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res 2010;88(6):1182–1192; doi: 10.1002/jnr.22288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bregman, BS, Bernstein-Goral H. 1991. Both regenerating and late-developing pathways contribute to transplant-induced anatomical plasticity after spinal cord lesions at birth. Exp Neurol 1991;112(1):49–63; doi: 10.1016/0014-4886(91)90113-q [DOI] [PubMed] [Google Scholar]
- 27. Bregman, B.S., Broude, E., McAtee, M., et al. Transplants and neurotrophic factors prevent atrophy of mature CNS neurons after spinal cord injury. Exp Neurol 1998;149(1):13–27; doi: 10.1006/exnr.1997.6669 [DOI] [PubMed] [Google Scholar]
- 28. Bregman BS, Kunkel-Bagden E, McAtee M, et al. Extension of the critical period for developmental plasticity of the corticospinal pathway. J Comp Neurol 1989;282(3):355–370; doi: 10.1002/cne.902820304 [DOI] [PubMed] [Google Scholar]
- 29. Bregman BS, Kunkel-Bagden E, Reier PJ, et al. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol 1993;123(1):3–16; doi: 10.1006/exnr.1993.1136 [DOI] [PubMed] [Google Scholar]
- 30. Bregman BS, McAtee M, Dai HN, et al. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol 1997;148(2):475–494; doi: 10.1006/exnr.1997.6705 [DOI] [PubMed] [Google Scholar]
- 31. Bregman BS, Reier PJ. Neural tissue transplants rescue axotomized rubrospinal cells from retrograde death. J Comp Neurol 1986;244(1):86–95; doi: 10.1002/cne.902440107 [DOI] [PubMed] [Google Scholar]
- 32. Brock JH, Graham L, Staufenberg E, et al. Rodent neural progenitor cells support functional recovery after cervical spinal cord contusion. J Neurotrauma 2018;35(9):1069–1078; doi: 10.1089/neu.2017.5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Broude E, McAtee M, Kelley MS, et al. Fetal spinal cord transplants and exogenous neurotrophic support enhance c-Jun expression in mature axotomized neurons after spinal cord injury. Exp Neurol 1999;155(1):65–78; doi: 10.1006/exnr.1998.6964 [DOI] [PubMed] [Google Scholar]
- 34. Ceto S, Sekiguchi KJ, Takashima Y, et al. Neural stem cell grafts form extensive synaptic networks that integrate with host circuits after spinal cord injury. Cell Stem Cell 2020;27(3):430–440.e5; doi: 10.1016/j.stem.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ciciriello AJ, Smith DR, Munsell MK, et al. Acute implantation of aligned hydrogel Tubes Supports Delayed Spinal Progenitor Implantation. ACS Biomater Sci Eng 2020;6(10):5771–5784; doi: 10.1021/acsbiomaterials.0c00844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coumans JV, Lin TT, Dai HN, et al. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci 2001;21(23):9334–9344; doi: 10.1523/JNEUROSCI.21-23-09334.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diener PS, Bregman BS, 1998a. Fetal spinal cord transplants support growth of supraspinal and segmental projections after cervical spinal cord hemisection in the neonatal rat. J Neurosci 1998;18(2):779–93; doi: 10.1523/JNEUROSCI.18-02-00779.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diener PS, Bregman BS. 1998b. Fetal spinal cord transplants support the development of target reaching and coordinated postural adjustments after neonatal cervical spinal cord injury. J Neurosci 1998;18(2):763–78; doi: 10.1523/JNEUROSCI.18-02-00763.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dumont CM, Munsell MK, Carlson MA, et al. Spinal progenitor-laden bridges support earlier axon regeneration following spinal cord injury. Tissue Eng Part A 2018;24(21-22):1588–1602; doi: 10.1089/ten.TEA.2018.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gulino R, Litrico L, Leanza G, 2010. Long-term survival and development of fetal ventral spinal grafts into the motoneuron-depleted rat spinal cord: role of donor age. Brain Res 2010;1323:41–47; doi: 10.1016/j.brainres.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 41. Hayakawa K, Jin Y, Bouyer J, et al. Transplanting neural progenitor cells into a chronic dorsal column lesion model. Biomedicines 2022;10(2):350; doi: 10.3390/biomedicines10020350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horner PJ, Reier PJ, Stokes BT, 1996. Quantitative analysis of vascularization and cytochrome oxidase following fetal transplantation in the contused rat spinal cord. J Comp Neurol 1996;364(4):690–703; doi: [DOI] [PubMed] [Google Scholar]
- 43. Houle JD, Reier PJ. Transplantation of fetal spinal cord tissue into the chronically injured adult rat spinal cord. J Comp Neurol 1988;269(4):535–547; doi: 10.1002/cne.902690406 [DOI] [PubMed] [Google Scholar]
- 44. Houle JD, Reier PJ. Regrowth of calcitonin gene-related peptide (CGRP) immunoreactive axons from the chronically injured rat spinal cord into fetal spinal cord tissue transplants. Neurosci Lett 1989. Sep 11;103(3):253–258; doi: 10.1016/0304-3940(89)90108-0 [DOI] [PubMed] [Google Scholar]
- 45. Hunt M, Lu P, Tuszynski MH. 2017. Myelination of axons emerging from neural progenitor grafts after spinal cord injury. Exp Neurol 2017;296:69–73; doi: 10.1016/j.expneurol.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 46. Iarikov DE, Kim BG, Dai HN, et al. Delayed transplantation with exogenous neurotrophin administration enhances plasticity of corticofugal projections after spinal cord injury. J Neurotrauma 2007;24(4):690–702; doi: 10.1089/neu.2006.0172 [DOI] [PubMed] [Google Scholar]
- 47. Ishii K, Nakamura M, Dai H, et al. Neutralization of ciliary neurotrophic factor reduces astrocyte production from transplanted neural stem cells and promotes regeneration of corticospinal tract fibers in spinal cord injury. J Neurosci Res 2006;84(8):1669–1681; doi: 10.1002/jnr.21079 [DOI] [PubMed] [Google Scholar]
- 48. Itoh Y, Tessler A. Regeneration of adult dorsal root axons into transplants of fetal spinal cord and brain: a comparison of growth and synapse formation in appropriate and inappropriate targets. J Comp Neurol 1990;302(2):272–293; doi: 10.1002/cne.903020207 [DOI] [PubMed] [Google Scholar]
- 49. Jakeman LB, Reier PJ. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J Comp Neurol 1991;307(2):311–334; doi: 10.1002/cne.903070211 [DOI] [PubMed] [Google Scholar]
- 50. Jakeman LB, Reier PJ, Bregman BS, et al. Differentiation of substantia gelatinosa-like regions in intraspinal and intracerebral transplants of embryonic spinal cord tissue in the rat. Exp Neurol 1989;103(1):17–33; doi: 10.1016/0014-4886(89)90181-7 [DOI] [PubMed] [Google Scholar]
- 51. Jin Y, Bouyer J, Shumsky JS, et al. Transplantation of neural progenitor cells in chronic spinal cord injury. Neuroscience 2016;320:69–82; doi: 10.1016/j.neuroscience.2016.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim BG, Dai HN, Lynskey JV, et al. Degradation of chondroitin sulfate proteoglycans potentiates transplant-mediated axonal remodeling and functional recovery after spinal cord injury in adult rats. J Comp Neurol 2006;497(2):182–198; doi: 10.1002/cne.20980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koffler J, Zhu W, Qu X, et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med 2019;25(2):263–269; doi: 10.1038/s41591-018-0296-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kumamaru H, Lu P, Rosenzweig ES, et al. Activation of intrinsic growth state Enhances host axonal regeneration into neural progenitor cell grafts. Stem Cell Reports 2018;11(4):861–868; doi: 10.1016/j.stemcr.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee KZ, Lane MA, Dougherty BJ, et al. Intraspinal transplantation and modulation of donor neuron electrophysiological activity. Exp Neurol 2014;251:47–57; doi: 10.1016/j.expneurol.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lepore AC, Han SS, Tyler-Polsz CJ, et al. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol 2004;1(2):113-126; doi: 10.1017/s1740925x04000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lepore AC, Neuhuber B, Connors TM, et al. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience 2006;139(2):513–530; doi: 10.1016/j.neuroscience.2005.12.043 [DOI] [PubMed] [Google Scholar]
- 58. Lu P, Freria CM, Graham L, et al. Rehabilitation combined with neural progenitor cell grafts enables functional recovery in chronic spinal cord injury. JCI Insight 2022;7(16):e158000; doi: 10.1172/jci.insight.158000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu P, Gomes-Leal W, Anil S, et al. Origins of Neural Progenitor Cell-Derived Axons Projecting Caudally after Spinal Cord Injury. Stem Cell Reports 2019;13(1):105–114; doi: 10.1016/j.stemcr.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Medalha CC, Jin Y, Yamagami T, et al. Transplanting neural progenitors into a complete transection model of spinal cord injury. J Neurosci Res 2014;92(5):607–618; doi: 10.1002/jnr.23340 [DOI] [PubMed] [Google Scholar]
- 61. Mitsui T, Shumsky JS, Lepore AC, et al. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci 2005;25(42):9624–9636; doi: 10.1523/JNEUROSCI.2175-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nakamura M, Okano H, Toyama Y, et al. Transplantation of embryonic spinal cord-derived neurospheres support growth of supraspinal projections and functional recovery after spinal cord injury in the neonatal rat. J Neurosci Res 2005;81(4):457–468; doi: 10.1002/jnr.20580 [DOI] [PubMed] [Google Scholar]
- 63. Ogawa Y, Sawamoto K, Miyata T, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res 2002;69(6):925–933; doi: 10.1002/jnr.10341 [DOI] [PubMed] [Google Scholar]
- 64. Patel U, Bernstein JJ. Growth, differentiation, and viability of fetal rat cortical and spinal cord implants into adult rat spinal cord. J Neurosci Res 1983;9(3):303–310; doi: 10.1002/jnr.490090307 [DOI] [PubMed] [Google Scholar]
- 65. Poplawski GHD, Kawaguchi R, van Niekerk E, et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature 2020;581(7806):77–82; doi: 10.1038/s41586-020-2200-5 [DOI] [PubMed] [Google Scholar]
- 66. Poplawski GHD, Lie R, Hunt M, et al. Adult rat myelin enhances axonal outgrowth from neural stem cells. Sci Transl Med 2018;10(442):eaal2563; doi: 10.1126/scitranslmed.aal2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reier PJ, Bregman BS, Wujek JR. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol 1986;247(3):275–296; doi: 10.1002/cne.902470302 [DOI] [PubMed] [Google Scholar]
- 68. Reier PJ, Perlow MJ, Guth L. Development of embryonic spinal cord transplants in the rat. Brain Res 1983;312(2):201–219; doi: 10.1016/0165-3806(83)90137-2 [DOI] [PubMed] [Google Scholar]
- 69. Reier PJ, Stokes BT, Thompson FJ, et al. Fetal cell grafts into resection and contusion/compression injuries of the rat and cat spinal cord. Exp Neurol 1992. Jan;115(1):177–188; doi: 10.1016/0014-4886(92)90245-l [DOI] [PubMed] [Google Scholar]
- 70. Robinson J, Lu P. Optimization of trophic support for neural stem cell grafts in sites of spinal cord injury. Exp Neurol 2017;291:87–97; doi: 10.1016/j.expneurol.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 71. Spruance VM, Zholudeva LV, Hormigo KM, et al. Integration of transplanted neural precursors with the injured cervical spinal cord. J Neurotrauma 2018;35(15):1781–1799; doi: 10.1089/neu.2017.5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tessler A, Himes BT, Houle J, et al. Regeneration of adult dorsal root axons into transplants of embryonic spinal cord. J Comp Neurol 1988;270(4):537–548; doi: 10.1002/cne.902700407 [DOI] [PubMed] [Google Scholar]
- 73. Theele D.P, Reier, PJ.. Immunomodulation with intrathymic grafts or anti-lymphocyte serum promotes long-term intraspinal allograft survival. Cell Transplant 1996;5(2):243–255; doi: 10.1177/096368979600500213 [DOI] [PubMed] [Google Scholar]
- 74. Theele DP, Schrimsher GW, Reier PJ. Comparison of the growth and fate of fetal spinal iso- and allografts in the adult rat injured spinal cord. Exp Neurol 1996;142(1):128–143; doi: 10.1006/exnr.1996.0184 [DOI] [PubMed] [Google Scholar]
- 75. Zhou J, Wu Y, Tang Z, et al. Alginate hydrogel cross-linked by Ca(2+) to promote spinal cord neural stem/progenitor cell differentiation and functional recovery after a spinal cord injuryhh. Regen Biomater 2022;9:rbac057; doi: 10.1093/rb/rbac057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aceves M, Tucker A, Chen J, et al. Developmental stage of transplanted neural progenitor cells influences anatomical and functional outcomes after spinal cord injury in mice. Commun Biol 2023;6(1):544; doi: 10.1038/s42003-023-04893-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Letchuman S, Tucker A, Miranda D, et al. Transcription factor Hb9 is expressed in glial cell lineages in the developing mouse spinal cord. eNeuro 2022;9(6); doi: 10.1523/ENEURO.0214-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bernsen, J. Dynamic thresholding of gray-level images. In Proceedings of the 8th International Conference on Pattern Recognition, Paris; 1986. [Google Scholar]
- 79. Phansalkar N, More S, Sabale A, et al. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. Int Conf Commun Signal Processing Kerala India 2011:218–220; doi: 10.1109/ICCSP.2011.5739305 [DOI] [Google Scholar]
- 80. Serradj N, Agger SF, Hollis ER, 2nd. Corticospinal circuit plasticity in motor rehabilitation from spinal cord injury. Neurosci Lett 2017;652:94–104; doi: 10.1016/j.neulet.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 81. Cregg JM, DePaul MA, Filous AR, et al. Functional regeneration beyond the glial scar. Exp Neurol 2014;253:197–207; doi: 10.1016/j.expneurol.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci 2004;5(2):146–156; doi: 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- 83. Kadoya K, Lu P, Nguyen K, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med 2016;22(5):479–487; doi: 10.1038/nm.4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Houle J. The structural integrity of glial scar tissue associated with a chronic spinal cord lesion can be altered by transplanted fetal spinal cord tissue. J Neurosci Res 1992;31(1):120–130; doi: 10.1002/jnr.490310117 [DOI] [PubMed] [Google Scholar]
- 85. Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun 2019;10(1):3879; doi: 10.1038/s41467-019-11707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fitch MT, Doller C, Combs CK, et al. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci 1999;19(19):8182–8198; doi: 10.1523/JNEUROSCI.19-19-08182.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li Y, Raisman G. Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp Neurol 1995;134(1):102–111; doi: 10.1006/exnr.1995.1041 [DOI] [PubMed] [Google Scholar]
- 88. Li C, Wu Z, Zhou L, et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct Target Ther 2022;7(1):65; doi: 10.1038/s41392-022-00885-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tamaru T, Kobayakawa K, Saiwai H, et al. Glial scar survives until the chronic phase by recruiting scar-forming astrocytes after spinal cord injury. Exp Neurol 2023;359:114264; doi: 10.1016/j.expneurol.2022.114264 [DOI] [PubMed] [Google Scholar]
- 90. Faust TE, Gunner G, Schafer DP. Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS. Nat Rev Neurosci 2021;22(11):657–673; doi: 10.1038/s41583-021-00507-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nagappan-Chettiar S, Yasuda M, Johnson-Venkatesh EM, et al. The molecular signals that regulate activity-dependent synapse refinement in the brain. Curr Opin Neurobiol 2023;79:102692; doi: 10.1016/j.conb.2023.102692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lang C, Guo X, Kerschensteiner M, et al. Single collateral reconstructions reveal distinct phases of corticospinal remodeling after spinal cord injury. PLoS One 2012;7(1):e30461; doi: 10.1371/journal.pone.0030461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Geoffroy CG, Meves JM, Kim HJM, et al. Targeting PTEN but not SOCS3 resists an age-dependent decline in promoting axon sprouting. iScience 2022;25(11):105383; doi: 10.1016/j.isci.2022.105383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Weidner N, Ner A, Salimi N, et al. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A 2001;98(6):3513–3518; doi: 10.1073/pnas.051626798 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and the supplementary files. Raw data will be made available upon request, within two weeks of the request. Additionally, data from this study are available in a FAIR data repository, the Open Data Commons for Spinal Cord Injury (odc-sci.org) under http://doi.org/10.34945/F5XK5Q, and can be accessed at https://odc-sci.org/data/887.