Abstract
The mechanisms by which Escherichia coli cells survive exposure to the toxic electrophile N-ethylmaleimide (NEM) have been investigated. Stationary-phase E. coli cells were more resistant to NEM than exponential-phase cells. The KefB and KefC systems were found to play an important role in protecting both exponential- and stationary-phase cells against NEM. Additionally, RpoS and the DNA-binding protein Dps aided the survival of both exponential- and stationary-phase cells against NEM. Double mutants lacking both RpoS and Dps and triple mutants deficient in KefB and KefC and either RpoS or Dps had an increased sensitivity to NEM in both exponential- and stationary-phase cells compared to mutants missing only one of these protective mechanisms. Stationary- and exponential-phase cells of a quadruple mutant lacking all four protective systems displayed even greater sensitivity to NEM. These results indicated that protection by the KefB and KefC systems, RpoS and Dps can each occur independently of the other systems. Alterations in the level of RpoS in exponentially growing cells correlated with the degree of NEM sensitivity. Decreasing the level of RpoS by enriching the growth medium enhanced sensitivity to NEM, whereas a mutant lacking the ClpP protease accumulated RpoS and gained high levels of resistance to NEM. A slower-growing E. coli strain was also found to accumulate RpoS and had enhanced resistance to NEM. These data emphasize the multiplicity of pathways involved in protecting E. coli cells against NEM.
Within their natural environment, bacteria go through periods of rapid growth when nutrients are plentiful, but slow growth occurs as nutrients become limited and when waste products accumulate. The transitions between these two states can be mimicked in the laboratory by growth of bacteria in batch culture; early exponential phase represents when nutrients are plentiful, and stationary phase represents the nongrowing state. In addition, alterations in the genotype of cells and changes to the nutrient composition of the medium will affect the growth rate. Bacteria are subject to an array of stresses within their natural environment, and it has been demonstrated previously that stationary-phase cells survive these insults better than their exponential-phase counterparts (19). In Escherichia coli cells, this is, at least in part, due to the alternative sigma factor, RpoS, which accumulates in stationary-phase cells (23). RpoS is responsible for the activation of transcription of at least 30 genes, many of whose products encode proteins involved in protecting bacteria against stress (17). Regulation of RpoS levels in E. coli cells is complex and occurs at many levels (17, 24, 37). In rapidly growing exponential-phase cells, RpoS is maintained at a basal level both by low rates of synthesis and by a reduction in its half-life by severalfold compared with stationary-phase cells (24, 37). The short half-life of RpoS in rapidly growing exponential-phase cells is due to the degradation of RpoS by the ClpP protease system (33). However, it has been shown that RpoS can accumulate in exponential-phase cells when the growth rate is substantially reduced by either osmotic stress, glucose limitation, growth on a poor carbon source such as succinate, or growth at temperatures below 30°C (17, 23, 28, 30, 34).
Bacteria are exposed to toxic electrophiles both from within the cell and from their environment. In E. coli cells, the endogenous electrophile methylglyoxal is produced when bacteria are grown on a poor carbon source such as d-xylose in the presence of cAMP (1, 14, 18). Under these conditions, bacteria produce so much methylglyoxal that it is excreted into the environment. In addition, bacteria in the gut are likely to be exposed to electrophilic compounds from the diet. Methylglyoxal has also been found to accumulate during the cooking of food and is present in beverages such as coffee and wine (29). Electrophiles can be generated during the chlorination of poultry and are used as herbicides (36, 42). To understand the mechanisms by which E. coli cells defend themselves against toxic electrophiles, our research has focused on the electrophilic reagent N-ethylmaleimide (NEM). Survival of E. coli cells upon exposure to electrophiles such as methylglyoxal and NEM requires protective mechanisms. In exponential-phase cells, the tripeptide glutathione is central to these protective mechanism (4, 9, 14, 15, 27). Conjugation of the electrophile to glutathione is the first step in detoxification, and the resultant glutathione conjugate(s) activates either the KefB or KefC potassium channel or both (9, 14, 27). The nature of the glutathione adduct determines which potassium channel is activated (14). For example, KefB is activated by glutathione adducts formed from NEM and methylglyoxal, whereas KefC is activated by the NEM adduct but is only poorly activated by the adduct of methylglyoxal. The activation of KefB and KefC results in the rapid loss of potassium from the bacterial cell, via these channels, and this is balanced by an influx of sodium ions and protons (5, 13, 15). The influx of protons rapidly lowers the cytoplasmic pH of the E. coli cell, which protects the cell against the toxic effects of electrophiles (12, 13, 15). Exponential-phase cells of mutants lacking KefB and KefC are unable to acidify their cytoplasm upon electrophile addition and consequently lose viability.
We suggested previously that interference with the KefB and KefC systems could provide a novel antibacterial strategy since these systems play an important role in the protection of exponential-phase bacterial cells against stress (12–15). However, the most valuable targets for antibacterial therapy should be important for survival in both growing and stationary-phase bacteria. Previously we performed extensive studies of actively growing bacteria. Here we present data demonstrating that the KefB and KefC systems also play an important role in the protection of stationary-phase E. coli cells against the electrophile NEM. We also show that RpoS and the DNA-binding protein Dps provide additional protection against NEM to E. coli cells in both the exponential and stationary phases of growth. These data demonstrate the importance of the KefB and KefC systems, RpoS, and Dps in protecting actively growing and stationary-phase E. coli cells against stress.
MATERIALS AND METHODS
Bacterial strains and growth.
All bacterial strains used in this study were derivatives of E. coli K-12 and are described in Table 1. Exponential- and stationary-phase cultures were prepared as stated in the text in either K0 supplemented with 10 mM KCl (K10) (10) or M9 minimal medium. Unless stated otherwise, 0.2% (wt/vol) glucose was included as the carbon source. The medium was also supplemented with 0.4% (wt/vol) casein hydrolysate (CAS) and bases (adenine, cytosine, guanine, thymidine, and uracil) at 50 μg ml−1 as defined in the text. Cultures in K10 were prepared as follows. Stationary-phase cultures were prepared by growth from a single colony at 37°C and 300 rpm for ca. 16 h. Exponential-phase cultures were prepared by diluting an overnight culture 15-fold into fresh growth medium to give a starting optical density at 650 nm (OD650) of 0.05 to 0.1. The culture was then grown to an OD650 of 0.4. The stationary- and exponential-phase cultures were then diluted into fresh prewarmed medium (37°C) to give a starting OD650 of 0.04, and then either NEM or sodium acetate was added to the concentration defined in the text. For the viability experiments, 50-μl samples were removed from the cultures at the times defined in the text. Samples for the Western blots were prepared by filtering (4.5 cm, 0.45-μm pore size; Whatman) 2 × 20 ml of culture at defined times. For protein measurements, the samples were resuspended in 0.1 M NaOH.
TABLE 1.
Bacterial strains used in this study
| Straina | Genotype | Donor strain
|
|
|---|---|---|---|
| Name | Source | ||
| MJF274 | F−kdp thi rha lacI lacZ kup | ||
| MJF276 | MJF274, kefB kefC::Tn10 | ||
| MJF358 | MJF274, rpoS::kan | ZK1000 | A. Martinez |
| MJF359 | MJF276, rpoS::kan | ZK1000 | A. Martinez |
| MJF378 | MJF274, rpoS::Tn10 | RH90 | R. Hengge-Aronis |
| MJF371 | MJF274, dps::kan | ZK1058 | A. Martinez |
| MJF376 | MJF276, dps::kan | ZK1058 | A. Martinez |
| MJF381 | MJF378, dps::kan | ZK1058 | A. Martinez |
| MJF411 | MJF359, dps::cam | ZK1146 | R. Kolter |
| MJF413 | MJF276, dps::cam | ZK1146 | R. Kolter |
| Frag1 | F−thi rha gal lacZ | ||
| MJF372 | Frag1, rpoS::Tn10 | RH90 | R. Hengge-Aronis |
| MJF405 | Frag1, clpP::Tn9 | SG22098 | S. Gottesman |
| MSD462 | F−ΔlacU169 | ||
| MJF402 | MSD462, rpoS::Tn10 | RH90 | R. Hengge-Aronis |
| MJF385 | MSD462, clpP::Tn9 | SG22098 | S. Gottesman |
All strains were created by P1 transduction using the donor strains as indicated.
Exponential-phase cultures in M9 were prepared from a growth-arrested overnight culture (0.04% [wt/vol] glucose) as follows. The overnight culture was diluted into fresh prewarmed medium (37°C) to give an OD650 of 0.05 and then grown up to an OD650 of 0.3. Samples for the Western blots were harvested by centrifugation (4,500 × g, 15 min). An identical sample was filtered (4.5 cm, 0.45-μm pore size; Whatman) to determine the protein concentration, using the trichloroacetic acid (TCA) method (see below). The exponential-phase cells were then diluted into fresh prewarmed medium (37°C) to give a starting OD650 of 0.05, and NEM was added. All NEM additions were made from a freshly prepared 50 mM stock in 50% (vol/vol) ethanol. Viability experiments were conducted exactly as described previously (13–15). For viability experiments with cells grown in K10 medium, cells were diluted into K0 lacking all supplements, and recovery was conducted on K10 plates. Cells from M9 viability experiments were diluted into 0.9% (wt/vol) NaCl, and recovery was performed on LB plates. In both cases, samples had to be diluted at least 10-fold prior to spotting, as the neat sample did not recover due to the presence of NEM.
Western blots.
Western blotting was performed by a standard method (39). The cell pellets were resuspended in 100 μl of sodium dodecyl sulfate solubilization buffer (20% [vol/vol] glycerol, 2% [wt/vol] sodium dodecyl sulfate, 0.5 g of bromophenol blue liter−1, and 0.75 M β-mercaptoethanol in 0.125 M Tris-HCl [pH 6.8]), and incubated at 100°C for 10 min, and 30 μg of protein was loaded per well. The RpoS mouse monoclonal antibody was diluted 40,000-fold in 0.5% Marvel dissolved in PBS-T (15 mM phosphate buffer, 150 mM NaCl, 0.05% [vol/vol] Tween 20). The binding of the RpoS antibody was detected by using the SuperSignal ULTRA chemiluminescent substrate (Pierce, Rockford, Ill.). To eliminate problems with background, the blot was washed once with PBS-T prior to exposure.
Protein determinations.
For the M9-grown cultures, the samples were resuspended in ice-cold TCA to a final concentration of 0.5 M. The TCA-treated cultures were then filtered (4.5 cm; Whatman GF/F), washed with an equal volume of M9 medium (CAS-derived peptides that might interfere with the protein assay are soluble in the TCA and are therefore removed at this step), resuspended in 0.2 M NaOH, and left overnight at room temperature. Distilled water (2 ml) was added, and then the extract was filtered (4.5 cm, 0.45-μm pore size; Whatman) to remove debris. The total protein concentration of the extract was determined by using the Lowry method adapted for microtiter plates (25).
Reproducibility.
NEM is highly toxic, and variations in the actual survival data were observed between different days, although the trends were always the same. Therefore, it was important to test all strains for comparison, including the appropriate controls on the same day. Each graph represents data obtained from experiments conducted on the same day only and is representative of at least two experiments. Error bars represent the standard deviation from the mean for one experiment. At higher concentrations of NEM (above 0.3 mM), experiments were very reproducible and therefore results from different days could be averaged.
RESULTS
KefB and KefC play an important role in protecting stationary-phase cells against NEM.
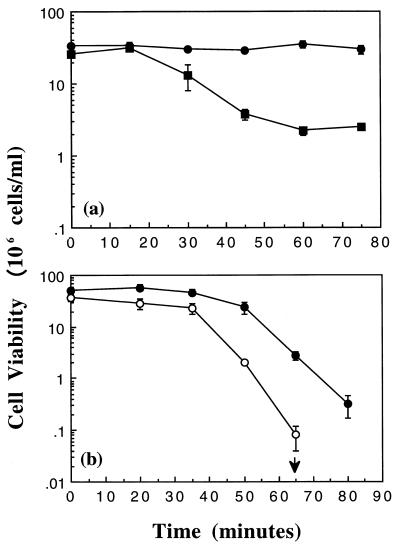
We have shown previously that the KefB and KefC potassium channels protect exponential-phase cells against the toxic effects of NEM (15). However, the role of these potassium channels in stationary-phase cells had not been established. Stationary-phase cells of MJF274 (KefB+ KefC+) and MJF276 (kefB kefC::Tn10) were diluted to the same starting cell density and then exposed to 0.2 mM NEM (Fig. 1a). The viability of stationary-phase cells of MJF274 remained constant throughout the 75-min exposure to 0.2 mM NEM. In contrast, the viability of stationary-phase cells lacking KefB and KefC was retained for up to 15 min and then declined. These results demonstrate that KefB and KefC have an important role in protection against NEM in stationary-phase cells. To investigate whether stationary-phase cells had increased resistance to NEM, exponential- and stationary-phase cells of MJF274 (KefB+ KefC+) were exposed to 0.2 mM NEM and cell viability was determined (data not shown). Under these conditions, both exponential- and stationary-phase cells of MJF274 remained viable for the 75-min exposure. However, when a higher concentration of NEM (0.4 mM) was used, both exponential- and stationary-phase cells of MJF274 retained almost complete viability for up to 35 min, after which their viability steadily declined (Fig. 1b). The decline in viability was more rapid for the exponential-phase cells of MJF274, and after 80 min of incubation with NEM, there were no surviving cells. In contrast, 3 × 105 stationary-phase cells survived 80 min of exposure to 0.4 mM NEM. It has also been shown that stationary-phase cells of MJF274 are more resistant than exponential-phase cells to the endogenous electrophile methylglyoxal (10a). These data demonstrate that stationary-phase cells are more resistant than exponential-phase cells to electrophiles.
FIG. 1.
Stationary-phase resistance against NEM. Cells were grown in K10 medium, and cell viability was determined exactly as described in Materials and Methods. (a) Stationary-phase cells of MJF274 (•) and MJF276 (kefB kefC::Tn10; ▪) were treated with 0.2 mM NEM at time zero. (b) Exponential (○)- and stationary (•)-phase cells of MJF274 were treated with 0.4 mM NEM at time zero. The arrow represents no viable cells by the next time point; error bars represent the standard deviation from the mean for one experiment.
RpoS and Dps protect exponential- and stationary-phase E. coli cells against NEM.
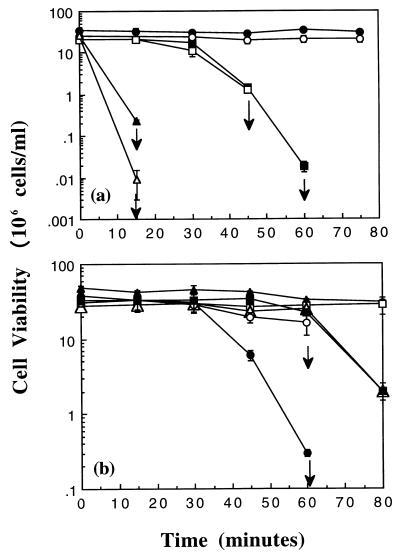
To investigate additional components involved in the protection of E. coli cells against NEM, we sought to determine the roles of the alternative sigma factor RpoS and of Dps, a DNA-binding protein first identified in starved bacterial cells (2, 17). We proposed previously that NEM damages bacterial cells by interacting with the nucleophilic centers of cellular macromolecules such as DNA (15). RpoS is known to regulate the expression of DNA repair enzymes; Dps is believed to bind to the DNA of the bacterial cell, with a lack of sequence specificity, and it has been found to protect cells against oxidative damage (2, 17, 26, 31, 38, 40). Strain MJF274 was transduced to Tetr and Kanr by using strains RH90 (rpoS::Tn10) and ZK1058 (dps::kan), respectively, to create MJF378 (MJF274, rpoS::Tn10) and MJF371 (MJF274, dps::kan) (Table 1). Exponential- and stationary-phase cells of MJF274, MJF378, and MJF371 were treated with 0.2 mM NEM, and cell viability was determined (Fig. 2a). Loss of either RpoS or Dps increased the sensitivity of both exponential- and stationary-phase E. coli cells to NEM, with a greater effect due to the loss of Dps than to that of RpoS. Under these conditions, exponential-phase cells of the mutant strains appeared to be slightly more sensitive to NEM than their stationary-phase counterparts (Fig. 2a). These data provided evidence that both RpoS and Dps play important roles in the protection of exponential- and stationary-phase E. coli cells against NEM. The greater sensitivity of the Dps-deficient mutant than of the strain lacking RpoS suggested that an RpoS-independent factor could regulate the level of Dps in both stationary- and exponential-phase cells.
FIG. 2.
RpoS and Dps protect exponential- and stationary-phase cells against NEM. Cells were grown in K10 medium, and cell viability of exponential (open symbols)- and stationary (closed symbols)-phase cells was determined exactly as described in Materials and Methods. (a) NEM was added at 0.2 mM to cells of MJF274 (•, ○), MJF378 (rpoS::Tn10; ▪, □), and MJF371 (dps::kan; ▴, ▵) at time zero; (b) 0.1 mM NEM was added to cells of MJF381 (rpoS::Tn10, dps::kan; ○, •), MJF371 (▵, ▴), and MJF378 (□, ▪) at time zero. The arrows represent no viable cells by the next time point; error bars represent the standard deviation from the mean for one experiment.
To confirm that protection by RpoS and that by Dps could occur independently, a double mutant lacking both RpoS and Dps was created by transduction of strain MJF378 to Kanr with strain ZK1058 as the donor to create strain MJF381 (MJF274, rpoS::Tn10 dps::kan; Table 1). Exponential- and stationary-phase cells of the double mutant lacking both RpoS and Dps were highly sensitive to 0.2 mM NEM and rapidly lost viability (data not shown). To monitor the loss of viability of cells of the double mutant, the concentration of NEM was reduced to 0.1 mM. At the lower NEM concentration (0.1 mM), stationary- and exponential-phase cells of the double mutant lacking RpoS and Dps displayed an increased sensitivity compared with cells of the single mutants lacking either RpoS or Dps alone (Fig. 2b). These data demonstrate that RpoS and Dps can provide protection to both exponential- and stationary-phase E. coli cells in the absence of each other. Exponential-phase cells of the double mutant lacking RpoS and Dps were more resistant to 0.1 mM NEM than stationary-phase cells of this strain, suggesting that these systems could have a greater contribution to survival against stress in cells of the latter condition (Fig. 2b).
Relationship between the KefB and KefC systems, RpoS, and Dps in protecting exponential- and stationary-phase cells against NEM.
Having determined a role for RpoS and Dps in the protection of E. coli cells against NEM, we sought to investigate the relationship between these systems and the KefB and KefC potassium channels. Isogenic strains carrying mutations in KefB and KefC and either RpoS or Dps were created by transduction (Table 1). Stationary-phase cells of MJF274, MJF358 (MJF274, rpoS::kan), MJF371 (MJF274, dps::kan), MJF276 (kefB, kefC::Tn10), MJF359 (MJF276, rpoS::kan), and MJF376 (MJF276, dps::kan) were diluted to the same starting cell density and then exposed to 0.1 mM NEM (Fig. 3a). Cells of the triple mutant lacking KefB and KefC and either RpoS or Dps were more sensitive to NEM in the stationary phase of growth than cells lacking either the potassium channels, RpoS, or Dps alone. This was also the case for exponential-phase cells of the triple mutants lacking KefB and KefC and either RpoS or Dps (data not shown). These data provided strong evidence that the KefB and KefC systems, RpoS, and Dps play important roles in the protection of both exponential- and stationary-phase cells against NEM. However, they also suggest that each of these protective mechanisms can function independently. Cells of the triple mutant lacking KefB and KefC and Dps were slightly more sensitive to 0.1 mM NEM than the triple mutant lacking RpoS (Fig. 3a). This was even more apparent when the NEM concentration was increased to 0.2 mM (data not shown). These results were consistent with the data for the single mutants, where Dps appears to play a more dominant role in protection in the presence of 0.2 mM NEM (Fig. 2a), suggesting that the contribution of the protective system is determined by the concentration of NEM in the medium. We also observed that stationary- and exponential-phase cells of MJF358 (rpoS::kan) were always slightly more resistant to 0.1 mM NEM than cells of MJF378 (rpoS::Tn10) when the experiments were conducted on the same days (data not shown). However, the reason(s) behind this is not known, although the same observation was made when the role of RpoS in protection against the electrophilic anticancer drug mechloroethamine was analyzed (12a).
FIG. 3.
The KefB and KefC systems, RpoS, and Dps can provide protection against NEM independently. Cells were grown in K10 medium, and cell viability and Western blot assays were conducted with stationary-phase cells exactly as described in Materials and Methods. (a) NEM was added at 0.1 mM to cells of MJF274 (•), MJF276 (kefB kefC::Tn10; ▪), MJF358 (rpoS::kan; ⧫), MJF371 (dps::kan; ▴), MJF359 (kefB kefC::Tn10 rpoS::kan; ○), and MJF376 (kefB kefC::Tn10 dps::kan; □) at time zero, and cell viability was determined. (b) Western blot analysis using an RpoS monoclonal antibody. Lane 1, MJF274, no addition; lane 2, MJF274, 0.2 mM NEM (10 min); lane 3, MJF274, 0.2 mM NEM (30 min); lane 4, MJF276, no addition; lane 5, MJF276, 0.2 mM NEM (10 min); lane 6, MJF276, 0.2 mM NEM (30 min). Lanes 7 to 12 were the same except that sodium acetate was added to a final concentration of 50 mM instead of NEM. (c) NEM was added at 0.1 mM to cells of MJF359 (kefB kefC::Tn10 rpoS::kan; ○), MJF413 (kefB kefC::Tn10 dps::cam; □), and MJF411 (kefB kefC::Tn10 rpoS::kan dps::cam; ▵) at time zero, and cell viability was determined. The arrows represent no viable cells by the next time point; error bars represent the standard deviation from the mean for one experiment.
We have previously shown that activation of the KefB and KefC systems results in acidification of the cytoplasm and that this protects E. coli cells against the toxic effects of electrophiles such as NEM (15). It has also been proposed that acidification of the cytoplasm by weak acid addition to E. coli cells induces RpoS accumulation (32). To investigate whether RpoS was induced by the activation of the KefB and KefC systems under our experimental conditions, stationary-phase cells of MJF274 (KefB+ KefC+) and MJF276 (kefB kefC::Tn10) were treated with 0.2 mM NEM, and the level of RpoS was monitored by Western blotting using an RpoS monoclonal antibody (Fig. 3b). The amount of RpoS remained constant over a 30-min incubation in the presence of NEM irrespective of whether cells possessed KefB and KefC. This was also found to be the case for exponential-phase cells (data not shown). These data showed that under our experimental conditions, activation of the KefB and KefC systems did not protect by induction of RpoS. To confirm that the addition of weak acids could induce RpoS under our conditions, stationary-phase cells of MJF274 and MJF276 were exposed to 50 mM sodium acetate and the effect on RpoS levels was detected by Western blotting (Fig. 3b). Sodium acetate induced RpoS in both strains within 30 min of addition, demonstrating that weak acids can result in RpoS accumulation in our strains and growth medium.
The separate nature of the KefB and KefC systems, RpoS, and Dps was further supported by the creation of a quadruple mutant, MJF411, lacking KefB, KefC, RpoS, and Dps. Strain MJF411 was created by transduction of strain MJF359 (kefB kefC::Tn10 rpoS::kan) by using strain ZK1146 (dps::cam) as a donor (Table 1). To allow comparison with the appropriate triple mutants, strain MJF276 (kefB kefC::Tn10) was also transduced to Cmr by using ZK1146 as the donor, to give strain MJF413 (kefB kefC::Tn10 dps::cam; Table 1). Stationary-phase cells of the quadruple mutant were found to be highly sensitive to 0.1 mM NEM compared with cells of MJF413 and MJF359 (Fig. 3b). This was also found to be the case for exponential-phase cells of the quadruple mutant (data not shown). These data support the view that the KefB and KefC systems, RpoS, and Dps are important systems in NEM protection that can act independently of each other to protect cells against stress.
Alterations in the RpoS level of exponential-phase cells affects NEM sensitivity.
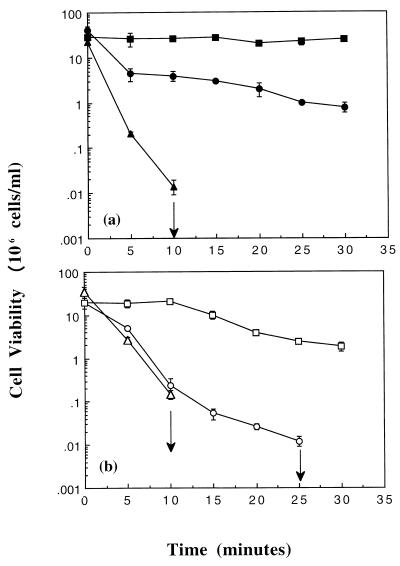
It has been demonstrated previously that RpoS levels are low in rapidly growing exponential-phase cells due to a reduction in its half-life compared to stationary-phase cells (23, 24, 33, 37). However, RpoS has been found to accumulate in exponential-phase cells when the growth rate has been substantially reduced (17, 23, 28, 30, 34). The data presented so far in this report clearly show a role for RpoS in protecting exponential-phase E. coli cells against electrophile damage. Our data suggested that growth in glucose-replete minimal medium must pose enough stress to cells to allow RpoS accumulation. To investigate this further, we analyzed the effect of enriching minimal medium with CAS on the level of RpoS and NEM sensitivity. Exponential-phase cells of Frag1 (RpoS+) were grown in M9 minimal medium in either the absence or presence of CAS and diluted to the same cell density in medium lacking CAS, and viability was determined in the presence of 0.3 mM NEM (Fig. 4a and b; the growth rates prior to NEM treatment were 0.7 and 1.1 h−1, respectively). Cells of Frag1 grown in the presence of CAS rapidly lost viability upon exposure to NEM, and by 30 min there were no viable cells remaining (Fig. 4b). In contrast, there were still 106 cells of Frag1 grown in the absence of CAS remaining after 30 min of exposure to NEM (Fig. 4a). A low level of RpoS could be detected in cells of Frag1 (Fig. 5a) upon Western blotting using an RpoS monoclonal antibody; however, due to the weak signal, no significant difference was observed when cells were grown in M9 supplemented with or without CAS (data not shown).
FIG. 4.
RpoS protects rapidly growing exponential-phase cells against NEM. Exponential-phase cells were grown in M9 medium, and cell viability was determined exactly as described in Materials and Methods. (a) NEM was added at 0.3 mM to cells of Frag1 (RpoS+; •). MJF405 (Frag1, clpP::Tn9; ▪), and MJF372 (Frag1, rpoS::Tn10; ▴) at time zero. (b) Cells were grown to exponential phase in M9 supplemented with 0.4% (wt/vol) CAS and then treated with 0.3 mM NEM at time zero (symbols same as panel a except open). The arrows represent no viable cells by the next time point; error bars represent the standard deviation from the mean for one experiment.
FIG. 5.
Effects of growth conditions and strain differences on RpoS levels. Exponential-phase cells were grown in M9 medium with the defined supplements, and the Western blot analyses were conducted exactly as described in Materials and Methods (where stated, CAS was added to 0.4% [wt/vol]). (a) Lane 1, Frag1; lane 2, MSD462 (exposure time, 10 times longer than in panel b). (b) Lane 1, Frag1; lane 2, MJF405 (Frag1, clpP::Tn9) with CAS; lane 3, MJF405; lane 4, MSD462 with CAS; lane 5, MSD462; lane 6, MSD462 with bases (adenine, cytosine, guanine, thymine and uracil; 50 μg.ml−1) and CAS; lane 7, MJF385 (MSD462, clpP::Tn9) with CAS; lane 8, MJF385; lane 9, stationary-phase MSD462 (positive control); lane 11, stationary-phase MJF402 (MSD462, rpoS::Tn10; negative control).
To confirm that the difference in sensitivity to NEM between Frag1 growing in the presence or absence of CAS was due to changes in RpoS, strain Frag1 was transduced to Tetr by using strain RH90 (rpoS::Tn10) as the donor to create strain MJF372 (Frag1, rpoS::Tn10) (Table 1). Cells of MJF372 were grown to exponential phase in M9 in either the absence or presence of CAS and then diluted into medium without CAS and exposed to 0.3 mM NEM (Fig. 4a and b; the growth rates of the RpoS mutant were unaltered compared with the parent strain under the same conditions, 1.1 and 0.7 h−1, respectively). Cells lacking RpoS were highly sensitive to NEM compared with cells of the parent strain (Fig. 4b). However, the addition of CAS did not increase the sensitivity of the RpoS-deficient cells to NEM but instead slightly enhanced survival compared to cells of the same strain grown at the slower growth rate (Fig. 4). These data confirmed the importance of RpoS in protecting relatively rapidly growing (growth rates of 0.7 and 1.1 h−1) exponential-phase cells of E. coli against NEM and also supported the view that changes in RpoS levels are responsible for the large increase in NEM sensitivity caused by supplementing the growth medium with CAS.
It has been shown previously that the ClpP protease is responsible for maintaining low levels of RpoS in rapidly growing exponential-phase cells (33). The importance of RpoS in the protection of exponential-phase cells against NEM was also further supported by the creation of a ClpP-deficient mutant of Frag1 by transduction (Table 1). Exponential-phase cells of MJF405 (Frag1, clpP::Tn9), which lacked the ability to produce the ClpP protease, accumulated high levels of RpoS (Fig. 5b) and were highly resistant to NEM (Fig. 4). However, increasing the growth rate of MJF405 from 0.7 to 1.0 h−1 by the addition of CAS slightly decreased the level of resistance to NEM (Fig. 4), although no difference in the RpoS level was detected (Fig. 5b). These data suggested that in this ClpP-deficient strain, an RpoS-independent factor can have an effect on NEM sensitivity.
To investigate further the link between RpoS and protection against NEM, we sought to analyze cells of another E. coli strain, MSD462 (RpoS+). The growth rate of cells of MSD462 in minimal medium is substantially lower and the level of RpoS is significantly higher than that of cells of Frag1 (Table 1 and Fig. 5, respectively). As predicted from the high RpoS levels, cells of MSD462 were more resistant to NEM than cells of Frag1 under the same growth conditions (Table 2). In addition, the RpoS levels of cells of MSD462 were virtually the same whether cells had been grown in the presence or absence of CAS (Fig. 5b), and this correlated with the very similar NEM sensitivity data (Table 2). However, growth of MSD462 cells in medium enriched further by the addition of both CAS and bases significantly reduced RpoS levels (Fig. 5b) and drastically increased sensitivity to NEM (Table 2). As observed with the RpoS− and ClpP-deficient mutants of Frag1, cells of MJF402 (MSD462, rpoS::Tn10) had an increased sensitivity to NEM, whereas cells of MJF385 (MSD462, clpP::Tn9) accumulated RpoS (Fig. 5b) and gained resistance to NEM compared with cells of the parent strain (Table 2). These findings confirmed the interrelationship of RpoS accumulation and sensitivity to NEM in exponentially growing cells.
TABLE 2.
Relationship between growth rate and NEM sensitivity
| Strain | Phenotypea | Growth conditionb | Avg growth rate (h−1) ± SD | Avg T90 (min) ± SDc |
|---|---|---|---|---|
| MSD462 | Clp+ | −CAS | 0.5 ± 0 | 43.5 ± 12 (6) |
| MJF385 | Clp− | −CAS | 0.5 ± 0 | 82.7 ± 1.7 (3) |
| MSD462 | Clp+ | +CAS | 0.8 ± 0 | 33.8 ± 9.4 (12) |
| MJF402 | Clp+ | +CAS | 0.8 ± 0 | 9.3 ± 3.4 (3) |
| MJF385 | Clp− | +CAS | 0.7 ± 0.1 | 60.5 ± 8.8 (4) |
| FRAG1 | Clp+ | −CAS | 0.7 ± 0 | 27.7 ± 6.4 (3) |
| MSD462 | Clp+ | +CAS + bases | 1.1 ± 0 | 6.3 ± 1.2 (5) |
| Frag1 | Clp+ | +CAS | 1.1 ± 0.1 | 6.6 ± 3 (12) |
All cells were RpoS+ except MJF402 (MSD462, rpoS::Tn10).
Exponential-phase cultures were grown in M9 medium with the defined supplements. CAS and bases (adenine, thymine, cytosine, guanine, and uracil) were added to 0.4% (wt/vol) and 50 μg/ml, respectively.
NEM viability experiments were conducted exactly as described in Materials and Methods. T90 is the time taken for 90% of the cells to die upon exposure to 0.3 mM NEM. The data are average values of the number of experiments given in parentheses.
DISCUSSION
The data presented here demonstrate that stationary-phase E. coli cells are more resistant than exponential-phase cells to electrophiles. Resistance to NEM was found to be dependent on the KefB and KefC potassium channels, RpoS, and Dps. All four systems were required for protection against stress both in actively growing and in stationary-phase cells. By the analysis of single, double, triple, and quadruple mutants, we provide strong evidence that the individual protective mechanisms can function in the absence of each other. However, it is likely that these systems are not completely independent and that Dps is still responsible for some of the RpoS-regulated protection, since the expression of Dps is known to be affected by RpoS (3, 17). The greater sensitivity of the Dps mutant than of the RpoS-deficient strains in the presence of 0.2 mM NEM suggests the strategic importance of the Dps protein for cell survival. NEM is thought to damage bacterial cells by interacting with the nucleophilic centers of macromolecules such as DNA, and hence protection by Dps is likely to involve binding to the DNA to shield it from NEM attack (2, 26). However, it is also possible that Dps is an activator of gene expression and is responsible for the induction of other proteins involved in protection against NEM (2). The finding that Dps can still provide some protection to cells against NEM even in the absence of RpoS in both stationary- and exponential-phase cells provides evidence that some other factor(s) in the cell must be able to regulate dps expression. It has been shown previously that the dps promoter can be activated by OxyR in growing cells and by integration host factor in stationary-phase cells (3). Recently, it has been demonstrated that OxyR is produced in exponential-phase cells of E. coli during aerobic growth (16). Consistent with this finding, exponential-phase cells of an OxyR-deficient mutant of Salmonella typhimurium were found to be highly sensitive to NEM, and an OxyR(Con) mutant that expresses the OxyR regulon constitutively was highly resistant to NEM (7). It is also possible that OxyR regulates Dps in RpoS-deficient stationary-phase cells, since it has been demonstrated that in the absence of RpoS, OxyR accumulates in E. coli cells in this phase of growth (16).
RpoS is clearly important for survival of E. coli cells upon exposure to NEM. While the role of RpoS in the expression of Dps can in part account for this, other gene products of the RpoS regulon must also be important since protection is observed even in the absence of Dps. Three candidates known to be regulated by RpoS are the DNA repair proteins Ada, AidB, and exonuclease III (22, 31, 35, 38, 40). Preliminary data suggest that exonuclease III could play a role, since mutants unable to make this protein were sensitive to both NEM and another electrophile, methylglyoxal (15a). The role of Ada is less clear, since we found that Ada was not induced by NEM but preinduction of this system by RpoS could protect cells against NEM (15a, 38). Alternatively, NEM may interact with the cell to produce oxidative damage, and therefore the RpoS-regulated katE gene, encoding hydroperoxidase HPII, may play an important role (31). RpoS has also been found to regulate the accumulation of certain fatty acids, and hence membrane permeability could be altered by the presence or absence of RpoS (41). However, RpoS was not found to influence the entry of NEM into E. coli cells (26a).
The finding that cells of a triple mutant lacking both KefB and KefC and either RpoS or Dps exhibited an enhanced sensitivity, and cells of the quadruple mutant lacking all four systems displayed an even greater sensitivity to NEM, provided evidence for the separate nature of these protective mechanisms. The activation of KefB and KefC results in acidification of the cytoplasm, and it has been demonstrated previously that RpoS is induced by the addition of weak acids that lower the intracellular pH (32). However, we have shown that protection by KefB and KefC can occur in the absence of RpoS, suggesting that if RpoS is induced by the activation of these channels, it is not responsible for all of the KefB-KefC-mediated protection. We also found that under the conditions of our experiments, RpoS did not accumulate after activation of the KefB and KefC systems by NEM, although induction of RpoS did occur after weak acid addition. These data provide evidence that the protection by the KefB and KefC systems does not result from an increase in the level of RpoS.
The data presented in this report also highlight the fact that clearly targeting one or more of these stress protective mechanisms would create a very effective antibacterial strategy. It would also be difficult for bacterial cells to develop resistance, since these mechanisms can act independently of each other. The KefB-KefC-like systems have been detected in all gram-negative bacteria tested to date, and Dps homologs have also been detected in both gram-negative and gram-positive bacteria (2, 6, 8). Although RpoS homologs have not been identified in gram-positive bacteria, changes associated with stationary phase have been observed. For example, in cells of the human pathogen Staphylococcus aureus, an alternative sigma factor, ςB, is believed to be regulated in response to growth phase, and in cells of Bacillus subtilis, a similar sigma factor is responsible for the expression of a wide array of stress genes (21). It has been demonstrated by other workers that in S. typhimurium, RpoS regulates virulence genes and mutations in RpoS lead to avirulence (11, 20). From our experiments, interference with both RpoS and either the potassium channels or Dps would greatly sensitize bacteria to the environment of the host and lead not only to the inability to cause disease but also to bacterial cell death.
It had been shown previously that RpoS plays an important role in protecting stationary-phase cells against certain types of stress (17, 19). RpoS has also been found to play a role in exponential-phase cells where the growth rate is substantially reduced (17, 23, 28, 30, 34). The data presented here point to the importance of RpoS in relatively rapidly growing exponential-phase cells (growth rate ranging from 0.5 to 1.1 h−1) compared to previous studies where the growth rates were much lower. Even in cells of Frag1 where the RpoS level is barely detectable by Western blot analysis, deletion of RpoS dramatically sensitizes cells to NEM. These data demonstrate that low levels of RpoS that accumulate in rapidly growing exponential-phase cells can have a profound effect on cell survival in the presence of stress. Thus, for many studies in the laboratory that use growing cells, and in the environment where cells achieve submaximum growth rates, RpoS will be a major determinant of the survival potential of the cell. In conclusion, these data demonstrate the complexity of protective mechanisms against the electrophile NEM in both actively growing and stationary-phase E. coli cells. The importance of these systems in both phases of growth for survival against stress suggests that one or more of these mechanisms could be targeted in the design of novel antibiotics.
ACKNOWLEDGMENTS
G.P.F. is a Wellcome Trust Toxicology Fellow, Y.N. was supported by a Wellcome Trust Visiting Fellowship, R.I.C. is supported by the BBSRC, I.R.B. is a Wellcome Trust Research Leave Fellow, and the group is supported by a Wellcome Trust Programme grant.
We acknowledge Neil Hunter for preliminary experiments on stationary-phase resistance and thank Karen Sutherland and Vanessa Santana for technical assistance. Thanks also go to Susan Gottesman, Regina Hengge-Aronis, Roberto Kolter, and Asuncion Martinez for the provision of donor strains and to Nancy Thompson for the RpoS monoclonal antibody.
REFERENCES
- 1.Ackerman R S, Cozzarelli N R, Epstein W. Accumulation of toxic concentrations of methylglyoxal by wild-type Escherichia coli K-12. J Bacteriol. 1974;119:357–362. doi: 10.1128/jb.119.2.357-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almirón M, Link A J, Furlong D, Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli cells. Genes Dev. 1992;6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- 3.Altuvia S, Almiron M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and ςs in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 4.Apontoweil P, Berends W. Isolation and characterization of glutathione-deficient mutants of Escherichia coli K12. Biochim Biophys Acta. 1975;399:10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- 5.Bakker E P, Mangerich W E. N-ethylmaleimide induces K+-H+ antiport activity in Escherichia coli K-12. FEBS Lett. 1982;140:177–180. doi: 10.1016/0014-5793(82)80888-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Helmann J D. Bacillus subtilis MrgA is a Dps (PexB) homologue: evidence for metalloregulation of an oxidative-stress gene. Mol Microbiol. 1995;18:295–300. doi: 10.1111/j.1365-2958.1995.mmi_18020295.x. [DOI] [PubMed] [Google Scholar]
- 7.Christman M F, Morgan R W, Jacobson F S, Ames B N. Positive control of a regulon for defences against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 8.Douglas R M, Roberts J A, Munro A W, Richie G Y, Lamb A J, Booth I R. The distribution of homologues of the Escherichia coli KefC K+-efflux system in other bacterial species. J Gen Microbiol. 1991;137:1999–2005. doi: 10.1099/00221287-137-8-1999. [DOI] [PubMed] [Google Scholar]
- 9.Elmore M J, Lamb A J, Ritchie G Y, Douglas R M, Munro A, Gajewska A, Booth I R. Activation of potassium efflux from Escherichia coli by glutathione metabolites. Mol Microbiol. 1990;4:405–412. doi: 10.1111/j.1365-2958.1990.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 10.Epstein W, Kim B S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971;108:639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Evans, G., G. P. Ferguson, and I. R. Booth. Unpublished data.
- 11.Fang C F, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. The alternative ς factor KatF (RpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson G P, Chacko A D, Lee C, Booth I R. The activity of the high-affinity potassium uptake system Kdp sensitizes cells of Escherichia coli towards methylglyoxal. J Bacteriol. 1996;178:3957–3961. doi: 10.1128/jb.178.13.3957-3961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Ferguson, G. P., A. Larrson, and B. Mannervik. Unpublished data.
- 13.Ferguson G P, Mclaggan D, Booth I R. Potassium channel activation by glutathione-S-conjugates in Escherichia coli: protection against methylglyoxal is mediated by cytoplasmic acidification. Mol Microbiol. 1995;17:1025–1033. doi: 10.1111/j.1365-2958.1995.mmi_17061025.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson G P, Munro A W, Douglas R M, Mclaggan D, Booth I R. Activation of potassium channels during metabolite detoxification in Escherichia coli. Mol Microbiol. 1993;9:1297–1303. doi: 10.1111/j.1365-2958.1993.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson G P, Nikolaev Y, Mclaggan D, Maclean M, Booth I R. Survival during exposure to the electrophilic reagent N-ethylmaleimide in Escherichia coli: role of KefB and KefC potassium channels. J Bacteriol. 1997;179:1007–1012. doi: 10.1128/jb.179.4.1007-1012.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Ferguson, G. P., Y. Nikolaev, B. Sedgewick, and I. R. Booth. Unpublished data.
- 16.Gonzalez-Flencha B, Demple B. Transcriptional regulation of the Escherichia coli oxyR gene as a function of cell growth. J Bacteriol. 1997;179:6181–6186. doi: 10.1128/jb.179.19.6181-6186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hengge-Aronis R. Survival of hunger and stress: the role of RpoS in early stationary phase gene regulation in E. coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 18.Hopper D J, Cooper R A. The regulation of Escherichia coli methylglyoxal synthase: a new control site in glycolysis? FEBS Lett. 1971;13:213–216. doi: 10.1016/0014-5793(71)80538-0. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins D E, Schultz J E, Matin A. Starvation induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988;170:3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowarz L, Coynault C, Robbe-Saule V, Norel F. The Salmonella typhimurium katF (rpoS) gene: cloning, nucleotide sequence, and regulation of spvR and spvABCD virulence plasmid genes. J Bacteriol. 1994;176:6852–6860. doi: 10.1128/jb.176.22.6852-6860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kullik I, Gianchino P. The alternative sigma factor ςB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 22.Landini P, Hajec L I, Volkert M R. Structure and transcriptional regulation of Escherichia coli adaptive response gene aidB. J Bacteriol. 1994;176:6583–6589. doi: 10.1128/jb.176.21.6583-6589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 24.Lange R, Hengee-Aronis R. The cellular concentration of the ςS subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 25.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurements with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Martinez A, Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol. 1997;179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Mclaggan, D., and I. R. Booth. Unpublished data.
- 27.Meury J, Kepes A. Glutathione and the gated potassium channels of E. coli. EMBO J. 1982;1:339–343. doi: 10.1002/j.1460-2075.1982.tb01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muffler A, Traulsen D D, Lange R, Hengge-Aronis R. Posttranscriptional osmotic regulation of the ςS subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1996;178:1607–1613. doi: 10.1128/jb.178.6.1607-1613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagoa M, Fujita Y, Sugimura T. Methylglyoxal in beverages and foods: its mutagenicity and carcinogenicity. 1984. Presented at the IARC Meeting, Lyon, France. [PubMed] [Google Scholar]
- 30.Notely L, Ferenci T. Induction of RpoS-dependent functions in glucose-limited continuous culture: what level of nutrient limitation induces the stationary phase of Escherichia coli? J Bacteriol. 1996;178:1465–1468. doi: 10.1128/jb.178.5.1465-1468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sak B D, Eisenstark A, Touati C. Exonuclease III and the catalase hydroperoxidase II in E. coli are both regulated by the katF gene product. Proc Natl Acad Sci USA. 1989;86:3271–3275. doi: 10.1073/pnas.86.9.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schellhorn H E, Stones V L. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J Bacteriol. 1992;174:4769–4776. doi: 10.1128/jb.174.14.4769-4776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheweder T, Lee K, Lomovskaya O, Matin A. Regulation of Escherichia coli starvation sigma factor (s) by ClpXP protease. J Bacteriol. 1996;178:470–476. doi: 10.1128/jb.178.2.470-476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sledjeski D D, Gupta A, Gottesman S. The small RNA, DsrA, is essential for the low-temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J. 1996;15:3993–4000. [PMC free article] [PubMed] [Google Scholar]
- 35.Smirnova G V, Oktyabrsky O N, Moshonkina E V, Zakirova N V. Induction of the alkylation-inducible aidB gene of Escherichia coli by cytoplasmic acidification and N-ethylmaleimide. Mutat Res. 1994;314:51–56. doi: 10.1016/0921-8777(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 36.Stevens K L, Wilson R E, Friedman M. Inactivation of a tetrachloride mutagen from stimulated processing water. J Agric Food Chem. 1995;43:2424–2427. [Google Scholar]
- 37.Takayanagi Y, Tanaka K, Takahashi H. Structure of the 5′ upstream region and the regulation of the rpoS gene of Escherichia coli. Mol Gen Genet. 1994;243:525–531. doi: 10.1007/BF00284200. [DOI] [PubMed] [Google Scholar]
- 38.Taverna P, Sedgwick B. Generation of an endogenous DNA-methylating agent by nitrosation in Escherichia coli. J Bacteriol. 1996;178:5105–5111. doi: 10.1128/jb.178.17.5105-5111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Towbin H, Staehelin T, Gordon G. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkert M R, Hajec L I, Matijasevic Z, Fang F C, Prince R. Induction of the Escherichia coli aidB gene under oxygen limited conditions requires a functional rpoS (katF) gene. J Bacteriol. 1994;176:7638–7645. doi: 10.1128/jb.176.24.7638-7645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang A Y, Cronan J E. The growth-phase dependent synthesis of cyclopropane fatty acids in Escherichia coli is the result of an RpoS(KatF)-dependent promoter plus enzyme stability. Mol Microbiol. 1994;11:1009–1017. doi: 10.1111/j.1365-2958.1994.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 42.Zablotowicz R M, Hoagland R E, Locke M A, Hickey W J. Glutathione S-transferase and metabolism of glutathione conjugates by rhizosphere bacteria. Appl Environ Microbiol. 1995;61:1054–1060. doi: 10.1128/aem.61.3.1054-1060.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]